1. Introduction
The integration of chemotherapy and immunotherapy, collectively termed chemoimmunotherapy, has emerged as a transformative approach in cancer treatment, leveraging the cytotoxic effects of chemotherapeutic agents and the tumor-specific targeting capabilities of the immune system [
1,
2,
3]. Among chemoimmunotherapeutic agents, therapeutic antibodies such as trastuzumab and sacituzumab or antibody–drug conjugates (ADCs) such as trastuzumab-emtansine (T-DM1) have demonstrated significant clinical efficacy by selective antigen-directed delivery [
4,
5,
6]. However, the therapeutic potential of ADCs remains limited by critical challenges such as intratumoral antigen heterogeneity, dynamic antigen loss, and the inability to fully activate the host immune system [
6,
7,
8]. These limitations often result in incomplete tumor eradication, the development of resistance, and suboptimal long-term outcomes [
9,
10,
11].
To overcome these obstacles, recent strategies have focused on combining ADCs with immune effector cells, such as natural killer (NK) cells, to enhance the therapeutic synergy between direct cytotoxicity and immune-mediated tumor elimination [
12,
13,
14,
15]. NK cells possess intrinsic tumor-killing capabilities and can mediate antibody-dependent cellular cytotoxicity (ADCC) in the presence of therapeutic antibodies [
12,
14,
16]. Among the various NK cell platforms, the NK-92 cell line is a well-established, clinically approved “off-the-shelf” therapeutic recognized for its consistent expansion, potent cytotoxic activity, and lack of inhibitory killer immunoglobulin-like receptors (KIRs), which minimizes the risk of graft-versus-host disease [
17,
18]. NK-92 cells are deficient in FcγRIII (CD16), which impairs IgG-binding and limits antibody- or ADC-driven antitumor activity [
15,
19].
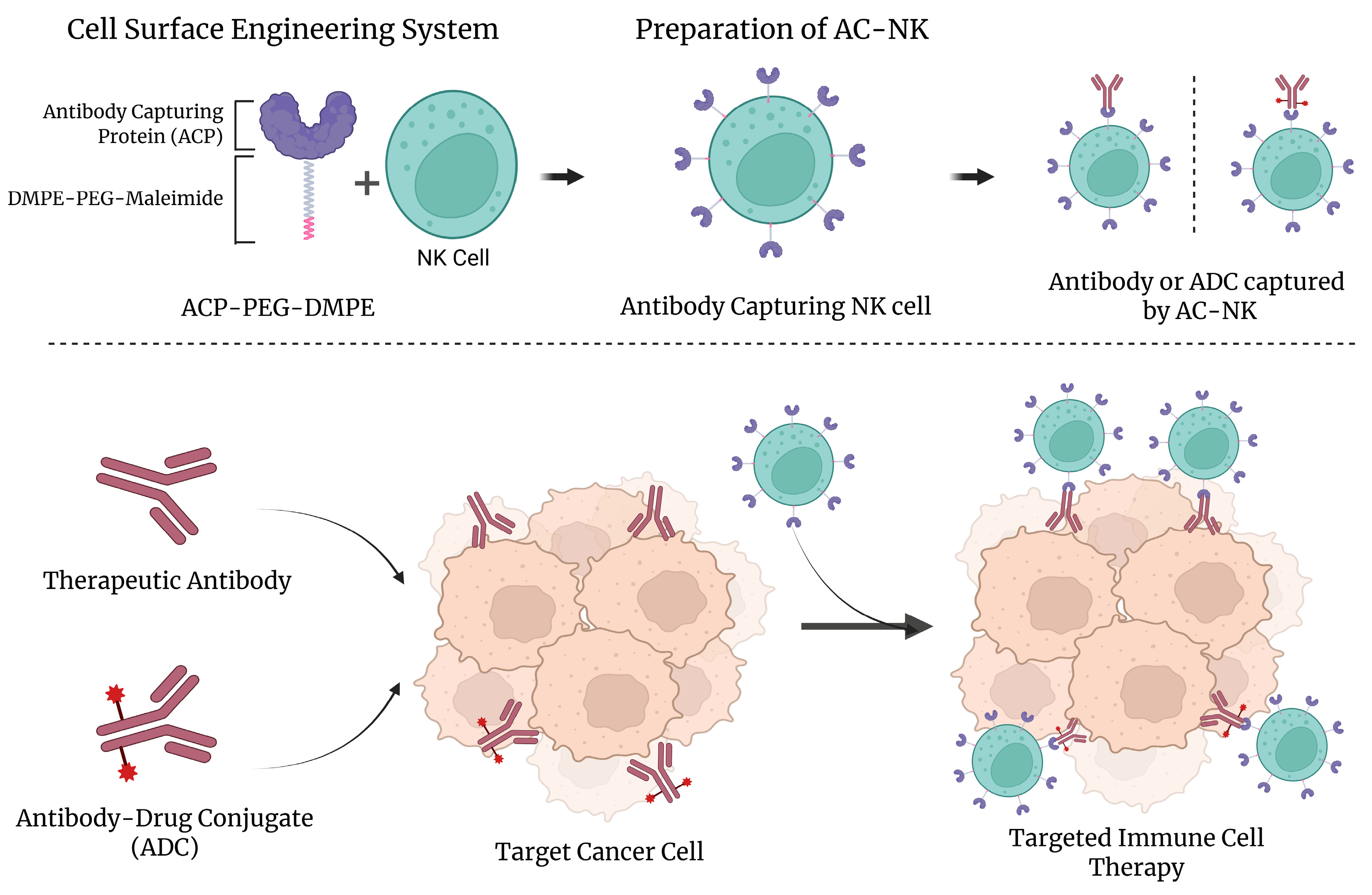
We developed a lipid-based antibody-capturing protein (ACP) platform that rapidly and non-genetically transforms natural killer (NK) cells into programmable antibody-guided immune effectors. This process relies on the spontaneous insertion of a hydrophobic lipid–polymer conjugate, DMPE-PEG-maleimide, into the NK cell membrane, allowing for covalent anchoring of thiolated Protein A (ACP) via maleimide–thiol coupling [
13,
14,
16]. Once immobilized on the cell surface, ACP selectively binds to the Fc region of therapeutic IgG antibodies or antibody–drug conjugates (ADCs), equipping NK cells with antibody-directed tumor-targeting ability. This strategy enables the modular, reversible, and efficient surface functionalization of immune cells without compromising membrane integrity or cellular viability. Unlike traditional genetic engineering methods, which require an extended processing time and pose risks such as insertional mutagenesis, our approach offers a simple and scalable alternative for functionalizing immune effector cells.
To evaluate the performance of our system, we conjugated ACP-modified NK cells (AC-NKs) with clinically relevant therapeutic agents, including trastuzumab (TZ), trastuzumab-emtansine (T-DM1), and sacituzumab (SZ). These AC-NKs effectively recognized and eliminated HER2-positive and Trop-2-positive cancer cells through the combined action of the NK cells and the surface-bound antibodies or ADCs. Compared to the conventional co-treatment of NK cells with antibodies, AC-NKs demonstrated enhanced tumor targeting, improved the membrane localization of therapeutic antibodies, and upregulated the expression of CD107a, indicating active immune degranulation. Furthermore, the ACP-based anchoring system enabled the flexible, sequential, or simultaneous binding of multiple antibodies, allowing for customizable and multi-antigen targeting. This tunable surface engineering platform has the potential to support personalized immunotherapy by adapting antibody loading levels to match specific therapeutic needs.
Unlike previous one-step methods that directly anchor ADCs on immune cells, our ACP system introduces a modular protein-based binding interface, which enhances stability and adaptability while supporting broader clinical applications [
12]. Herein, we established a proof-of-concept demonstrating that ACP stably and reversibly delivers therapeutic antibodies to NK cells, enhancing target binding and cytotoxicity in vitro without genome editing. These in vitro data support the feasibility of a modular approach compatible with the rapid and scalable production of off-the-shelf immune cell products. These findings support the ability of ACP-mediated surface engineering to unlock the combined efficacy of current ADC and NK cell therapies. By reversibly and antigen-independently conjugating therapeutic antibodies to immune cells, this strategy could advance chemoimmunotherapy for solid tumors.
2. Materials and Methods
2.1. Materials
All therapeutic antibodies included trastuzumab (TZ), sacituzumab (SZ), and trastuzumab emtansine (T-DM1) (Selleckchem, Houston, TX, USA). DMPE-PEG-maleimide (Molecular weight = 2 kDa) and Protein A-thiol were obtained from Nanocs (New York, NY, USA) and Protein Mods (Waunakee, WI, USA), respectively. Proteins were fluorescently labeled with NHS-fluorescein or NHS-rhodamine (Thermo Fisher Scientific, Waltham, MA, USA). Cell culture used Dulbecco’s modified Eagle’s medium (DMEM) and RPMI 1640 (Luscience, Hanam, Republic of Korea) supplemented with recombinant human interleukin-2 (IL-2; Lonza, Walkersville, MD, USA). Viability and apoptosis were assessed with Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Kumamoto, Japan) and the PE Annexin V/7-AAD kit (BioLegend, San Diego, CA, USA), respectively. FITC-conjugated anti-human CD107a and APC-conjugated anti-human CD56 and CD244 were obtained from BD biosciences (San Jose, CA, USA) and Biolegend (San Diego, CA, USA), respectively. For the Western blot assay, anti-rabbit IgG, anti-6 × His tag, and HRP-conjugated secondary antibodies were sourced from Cell Signaling Technology (Danvers, MA, USA). Enzyme-linked immunosorbent assays (ELISA) employed the ClinMax™ Human Granzyme B kit (ACRO Biosystems, Seoul, Republic of Korea) and the Human Perforin kit (Invitrogen, Carlsbad, CA, USA). Unless otherwise specified, all reagents were analytical grade. Confocal laser scanning microscopy (CLSM; Carl Zeiss, Oberkochen, Germany) and flow cytometry analyses (FACS Calibur and FACSymphony, Becton Dickinson, Franklin Lakes, NJ, USA) were performed at Kangwon National University (Chuncheon, Republic of Korea).
2.2. Production of a PEG-DMPE Conjugated with Antibody-Capturing Protein
To generate antibody-capturing protein (ACP) conjugated with PEG-DMPE, DMPE-PEG-maleimide was dissolved in distilled water and incubated at 60 °C until completely dissolved. Thiol-modified protein A was dissolved separately in PBS (phosphate-buffered saline, Welgene, Gyeongsan, Republic of Korea). The two components were mixed at a molar ratio of 10:1 (DMPE-PEG-maleimide: Protein A) to promote conjugation via maleimide–thiol bonding. The reaction was maintained at 4 °C with gentle agitation overnight. After thiol–maleimide coupling, unreacted excess DMPE-PEG-maleimide was eliminated from Protein A-PEG-DMPE through dialysis against cold 1× PBS twice. The concentration of the resulting DMPE-PEG-protein A conjugate was quantified using a DC protein assay kit (Bio-Rad, Hercules, CA, USA).
2.3. Cell Culture
SK-BR-3 and Calu-3 (HER2-positive) and MDA-MB-231 (HER2-negative) were obtained from the Korea Cell Line Bank (KCLB, Seoul, Republic of Korea) and maintained per the supplier’s protocol. SK-BR-3 and MDA-MB-231 cells were grown in RPMI 1640 containing 10% FBS (MilliporeSigma, St. Louis, MO, USA) and antibiotics (100 U/mL penicillin, 100 µg/mL streptomycin). Calu-3 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) with the same concentrations of supplements. All adherent cell lines were cultured at 37 °C in a humidified atmosphere containing 5% CO2 and passaged every 2–3 days when approximately 80% confluence was reached. The NK-92 human natural killer cell line was purchased from the American Type Culture Collection Center (ATCC, Manassas, VA, USA) and maintained according to ATCC guidelines. Cells were maintained in α-MEM containing 20% FBS, 1% penicillin–streptomycin, 4 mM folic acid, 20 mM inositol, 0.02 mM 2-mercaptoethanol, and 100 U/mL recombinant human IL-2. Suspension cultures of NK-92 cells were cultured under the same temperature and carbon dioxide conditions as the cancer cell lines and passaged every 2 days to maintain optimal cell density.
2.4. Optimization of Surface Modification Condition for Production of Antibody-Capturing NK Cell (AC-NK) Using ACP-PEG-DMPE
Rhodamine was conjugated with an antibody-capturing protein to optimize the modification of NK cells using ACP-PEG-DMPE. Rhodamine-conjugated ACP-PEG-DMPE was mixed with NK cells (1 × 106 cells) at varying concentrations of ACP-PEG-DMPE for 15 min at room temperature (RT). We also prepared AC-NKs using 40 μg of ACP-PEG-DMPE for 10 to 40 min at RT.
2.5. Evaluation of Cytotoxicity and Growth in ACP-PEG-DMPE Treated NK Cells
To assess the effect of ACP-PEG-DMPE on NK cell activity during the generation of antibody-capturing NK (AC-NK) cells, we assessed both the cytotoxicity of C3-PEG-DMPE on NK-92 cells and the proliferative capacity of AC-NKs. NK-92 cells were cultured with 0 (Control group), 10, 20, or 40 μg of C3-PEG-DMPE at 37 °C in a 5% CO2 atmosphere for 24 h to evaluate the cytotoxic effects. The AC-NKs were prepared by mixing NK cells with various amounts of ACP-PEG-DMPE for 15 min under RT. The AC-NKs were then maintained under the same culture conditions for 48 h. The viability and proliferation of NK cells after modification were measured using the CCK-8 kit following the manufacturer’s protocol. The absorbance at 450 nm was detected using a microplate reader (Biotek, Winooski, VT, USA).
2.6. Confirmation of the Antibody Capturing of AC-NKs and the Formation of AC-NKs
We synthesized rhodamine-tagged ACP-PEG-DMPE to examine its surface localization on NK cells. Rhodamine-labeled ACP-PEG-DMPE was used to treat NK cells for 15 min at RT. The AC-NKs were mixed with FITC-labeled Trastuzumab at RT for 15 min and then washed with 1× PBS. After detachment, all samples were fixed in 4% PFA (paraformaldehyde) prepared in 1× PBS. They were stored at 4 °C until FACS and confocal laser scanning microscope (CLSM) analyses. The fluorescence of NK cells with rhodamine-labeled ACP-PEG-DMPE and FITC-labeled Trastuzumab was analyzed by flow cytometry and CLSM at the core facility of Kangwon National University (Chuncheon, Republic of Korea).
2.7. Characterization of AC-NKs with Trastuzumab
The availability of NK cell receptors after the AC-NKs were bound with trastuzumab (TZ) was analyzed using APC-labeled CD56 and CD244(2B4). Each antibody was applied onto AC-NKs bound with TZ, following the manufacturer’s recommendations. The presence of CD56 and CD244 on AC-NKs bound with TZ was determined using FPLC (Fast Protein Liquid Chromatography) and analyzed using FlowJo software v10.10.0.
2.8. Verification of Specific Binding of AC-NKs to Trastuzumab That Recognized HER2 Positive Cancer Cells
All cancer cells, including Calu-3, SK-BR-3, and MDA-MB-231, were labeled with Cell Tracker Blue CMAC for evaluation of the HER2-positive cancer cell targeting ability of the AC-NKs to bind with TZ. They were plated at 5 × 104 cells per well 24 h before treatment. The cancer cells were seeded in wells at a density of 5 × 104 cells/well 24 h prior to treatment. AC-NKs were prepared using rhodamine-conjugated ACP-PEG-DMPE. All cancer cells were incubated with 5 μg of FITC-labeled trastuzumab (TZ) for 30 min and then washed with MEM α (minimum essential medium alpha) twice. After washing, each cancer cell was co-incubated with AC-NKs for 30 min. The location of FITC-TZ and AC-NKs was observed using CLSM. All CLSM images were analyzed using ImageJ software v2.16.0.
To quantify the number of remaining AC-NKs after co-incubation, all cancer cells and NK cells were stained using CMAC (blue) and CMTPX (Red), respectively. Then, 5 μg of trastuzumab was used to treat all cancer cells for 30 min. After incubation, the cells were washed with MEM α twice. Each cancer cell was mixed with NK or AC-NKs at an E:T (effector-to-target) of 10:1 for 30 min. Unbound NK cells were washed with 1× PBS, and the collected remaining cells were analyzed using flow cytometry.
2.9. In Vitro Anti-Cancer Efficacy of AC-NKs via Capturing Trastuzumab (TZ)
To determine the anti-cancer availability of AC-NKs with antibodies, trastuzumab (TZ) was used to treat cancer cells, and then AC-NKs were treated. HER2-positive cancer cells (SK-BR-3, Calu-3, and BT-474), and HER2-negative cancer cells (MDA-MB-231), were labeled with Cell Tracker Blue CMAC. All cancer cells were seeded at 5 × 104 cells/well in a 24-well plate and incubated for 24 h at 37 °C and 5% CO2. Then, they were incubated with 5 μg of TZ for 1 h at 37 °C and 5% CO2 and washed with MEM α. They were co-incubated with NK cells and AC-NKs at an E:T ratio of 10:1 for 30 min. After co-incubation, unbound NK cells and AC-NKs were washed with MEM α in all treatment groups, and the remaining cancer cells bound with effect cells were further incubated for 24 h. To analyze cancer cell death, an annexin V assay kit was processed according to the manufacturer’s recommended protocol and analyzed using flow cytometry at the core facility of Kangwon National University (Chuncheon, Republic of Korea).
2.10. The Mechanism of AC-NKs’ Anti-Cancer Effect Through Capturing Trastuzumab (TZ)
The synergetic anti-cancer effect of the AC-NKs and TZ was quantified by the expression level of CD107a in HER2-positive cancer cells, such as SK-BR-3, Calu-3, and BT-474, and a HER2-negative cancer cell, including MDA-MB-231. Cancer cells were plated at 5 × 104 cells per well in a 48-well plate and cultured at 37 °C and 5% CO2 for 24 h. Then, 5 μg of TZ was used to treat all cancer cells for 1 h at 37 °C and 5% CO2, and these were then washed with MEM α. They were co-cultured with NK cells or AC-NKs at an E:T ratio of 1:1 for 30 min. After incubation, unbound NK and AC-NKs were washed with MEM α in all treatment groups. FITC-conjugated anti-CD107a was used to treat the remaining cells. After 2 h, all cells in each well were collected and stained with APC-labeled CD56 antibody for NK cell identification. Fluorescence was measured by flow cytometry at the Kangwon National University core facility (Chuncheon, Republic of Korea).
2.11. In Vitro Determination of Anti-Cancer Efficacy of AC-NKs Through Antibody Capturing
To assess the synergetic anti-cancer effect of AC-NKs via antibody capturing, we treated trastuzumab-emtansine (T-DM1) and sacituzumab (SZ) into HER2-positive cancer cells, such as BT-474 and SK-BR-3, and TROP-2-positive cancer cells, including MCF-7 and SK-BR-3, respectively. All cancer cells were stained with Cell Tracker Blue CMAC, seeded at 5 × 104 cells per well in 24-well plates, and then incubated for 24 h under cell culture conditions (37 °C and 5% CO2). Then, 5 μg of T-DM1 and SZ were used to treat HER2-positive cancer cells and TROP-2-positive cancer cells, respectively. They were then co-cultured with NK cells and AC-NKs at an E:T ratio of 10:1 for 30 min. After co-incubation, unbound NK cells and AC-NKs were washed with MEM α in all treatment groups, and then the remaining cells were further cultured for 24 h. To analyze cancer cell death, an annexin V assay kit was processed according to the manufacturer’s recommended protocol and analyzed using flow cytometry at the core facility of Kangwon National University (Chuncheon, Republic of Korea).
2.12. Statistical Analysis
All data are shown as mean ± SD (standard deviation). Comparisons used Student’s t-test. * p < 0.05, ** p < 0.01, and # p < 0.001 values were considered statistically significant.
4. Discussion
Cell surface engineering is increasingly being utilized to enhance the efficacy of immune cells and impart novel functions. However, conventional chemical and physical modification strategies can disrupt cell membrane structure and inhibit cellular activity, limiting their translational utility [
12,
16,
25]. To address these limitations, lipid–polymer structures, in which hydrophobic lipid anchors are inserted into the cell membrane and hydrophilic polymer spacers provide functional moieties to the extracellular environment, have been widely reported [
26,
27]. These systems enhance biocompatibility, reduce nonspecific interactions, and enable modular, reproducible assembly suitable for therapeutic applications. Among the lipid anchors, 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (DMPE) was selected due to its strong hydrophobic anchorage to cell membranes and compatibility with immune cells [
14,
16]. The phosphor ethanolamine head group of DMPE enables efficient conjugation of functional proteins, supporting modular and reproducible assembly [
28,
29]. Polyethylene glycol (PEG) was conjugated to DMPE to form a lipid–polymer composite that increases solubility and enables modular functionalization. Furthermore, adjustment of PEG chain length establishes a defined nanoscale separation from the plasma membrane, thereby reducing geometric interference and improving accessibility for additional functional moieties.
In this study, we selected PEG with a molecular weight of 2kDa to prepare PEG-DMPE, according to a previous study showing balanced membrane insertion efficiency while minimizing steric hindrance [
12,
30]. By introducing a thiol-modified protein A (antibody capture protein, ACP) onto this PEG-DMPE scaffold, we produced an AC platform that enables noncovalent capture of therapeutic antibodies and antibody–drug conjugates on the immune cell surface. Furthermore, we selected protein A, rather than protein G, as the Fc-binding module in this study. A protein A binds with high affinity to human IgG1, IgG2, and IgG4, but with weaker binding to IgG3, whereas protein G binds to all four human IgG subclasses, including IgG3 [
31,
32]. Since the therapeutic antibodies evaluated here (trastuzumab, T-DM1, and sacituzumab) are human IgG1, both antibody binding proteins are compatible. However, protein A avoids albumin binding, which is present in some protein G variants, and the construct used in this study provides a multivalent Fc-binding domain that facilitates high-density IgG capture in mobile membranes [
33,
34]. These characteristics motivated the use of Protein A in this AC platform.
These results indicate that AC-NKs preserve the antibody-mediated tumor-targeting function and enhance antigen-specific recognition by NK cells. Unlike the co-treatment of unmodified NK cells with therapeutic antibodies, which showed a low anti-cancer effect in both HER2-positive and Trop-2 positive cells, the co-treatment of AC-NKs with a therapeutic antibody such as trastuzumab (TZ), trastuzumab-emtansine (T-DM1), and Sacituzumab (SZ) improved their anti-cancer efficacy in HER2-positive and Trop-2 positive cells. The lipid-based anchoring method could allow therapeutic antibodies to move dynamically along the NK cell membrane after being captured by an antibody-capturing protein on the surface of NK cells. Compared with unmodified NK cells treated with TZ, T-DM1, or SZ, AC-NK exhibited significantly stronger target cell recognition and cytotoxicity as evidenced by increased CD107a expression. These data are consistent with previous reports demonstrating the tumor-selective activity of ADCs immobilized directly or via an intermediate scaffold. Similarly, a previous study using T-DM1-modified NK cells demonstrated no cytotoxicity against HER2-negative cells [
12,
13,
14].
The ACP-mediated surface modification platform offers reversibility, tunability, and adaptability, allowing the temporal control of immune cell activation. Because the antibody capture protein is anchored via noncovalent lipid insertion, antibody loading and unloading can be dynamically regulated under physiological conditions. By adjusting the concentration and incubation time of ACP or antibodies, the degree of surface functionalization can be precisely tuned to optimize therapeutic efficacy and minimize off-target effects. This flexibility supports the sequential or simultaneous targeting of multiple tumor antigens and enables rapid retargeting in response to antigen changes, which are important for overcoming tumor heterogeneity and immune evasion. In addition, the ACP platform is cost-effective, safe, and compatible with existing cell manufacturing workflows. It requires only a simple, mild culture step without complex infrastructure or extended cell culture, significantly reducing the time and cost associated with production. By avoiding permanent genetic modifications, the risks of insertional mutagenesis or unintended genomic alterations are avoided, providing a safer, more accessible, and clinically scalable approach for developing next-generation immune cell therapies
The ACP-based surface engineering system offers a promising solution to the major limitations of current cancer immunotherapy, particularly for solid tumors characterized by antigenic heterogeneity and immunosuppressive microenvironments, by enabling rapid, reversible, and multi-antigen functionalization without genetic manipulation. The efficacy of AC-NK, demonstrated in both HER2-positive and Trop-2-positive cancer models, demonstrates its potential as a modular, off-the-shelf immunotherapy platform. By simply exchanging therapeutic antibodies or ADCs, the system can be readily tailored to target a wide range of malignancies, including those with dynamic or mixed antigen expression. The scalability, safety, and flexibility of the ACP system position it as a next-generation immunotherapy approach with strong translational potential. Future studies will focus on extending this strategy to primary NK cells and T cells, assessing in vivo persistence and biodistribution, and applying it to combination therapies including immune checkpoint blockade or CAR engineering. Overall, our findings demonstrate that ACP-mediated surface engineering represents a versatile and clinically viable platform for personalized antibody-directed cell-based immunotherapy.
5. Conclusions
We reported a one-step, non-genetic method that uses a lipid-anchored antibody-capture protein to rapidly and reversibly load therapeutic antibodies or antibody–drug conjugates onto NK cell surfaces. By immobilizing thiolated protein A via DMPE-PEG-mediated membrane insertion, we generated antibody-captured NK cells (AC-NKs) that retained cytotoxicity while acquiring antigen-specific targeting function. This modular approach preserved NK cell viability and enhanced tumor selectivity without the genetic or chemical modification of the therapeutics. When co-administered with clinically approved drugs such as trastuzumab (TZ), trastuzumab-emtansine (T-DM1), or sacituzumab (SZ), AC-NKs exhibited significantly enhanced antitumor efficacy in HER2- and Trop-2-positive tumor models. This platform enables multi-antigen targeting and tunable antibody loading, providing the flexibility to overcome tumor heterogeneity and immune evasion mechanisms. These results demonstrate that this ACP system can serve as a universal interface to rapidly transform immune cells into programmable tumor-targeting therapeutics. Importantly, this approach is broadly applicable to other immune effector cells, including T cells, dendritic cells, and CAR-engineered cells, and is compatible with a variety of therapeutic antibodies and ADCs currently in development. Within the limitations of this in vitro study, ACP-based surface engineering enables NK cells to bind therapeutic antibodies and ADCs in an antigen-dependent manner. Translational studies require the in vivo evaluation of persistence, biodistribution, safety, and the impact of endogenous IgG and other serum proteins that can occupy protein A and induce background or sink effects. Strategies able to mitigate these effects (e.g., ex vivo preloading, Fc binding affinity modulation, and ACP density control) are therefore needed. Therefore, this study presents the use of AC-NKs as a modular platform with translational potential that requires further preclinical evaluation rather than a clinically validated tool.