Abstract
Background/Objectives: The skin is a protective barrier against pathogens such as herpes simplex virus type 1 (HSV-1), which causes recurrent and highly prevalent skin infections worldwide. The increasing resistance of HSV-1 to conventional treatments has driven the search for new therapeutic alternatives. In this context, the essential oil of Lippia graveolens (EOL) has demonstrated promising antiviral activity; however, its high volatility limits direct skin application. To overcome this, polymeric nanoparticles (NPs) loaded with EOL were developed to improve its availability and antiviral efficacy. Methods: Nanoformulations were prepared by nanoprecipitation, and their antiviral activity against HSV-1 was evaluated using the plaque reduction assay. The effect of the nanoformulations on skin barrier integrity was assessed using an ex vivo porcine skin model and non-invasive techniques. Results: The NP-EOL exhibited physicochemical properties favorable for skin deposition, including a particle size around 200 nm, a polydispersity index of ≤ 0.2, and negative zeta potential. Moreover, NP-EOL showed 1.85-fold higher antiviral activity against HSV-1 compared with free EOL, while also reducing cytotoxicity in Vero cells. Conclusions: Results demonstrated that the NPs promoted skin hydration without altering pH or transepidermal water loss, suggesting they do not disrupt skin homeostasis. This study supports the potential of NP-based systems as effective topical delivery vehicles for EOL, representing a promising therapeutic alternative against HSV-1 skin infections.
1. Introduction
The skin plays a crucial role in protecting the body against environmental factors. This protective function is primarily attributed to the stratum corneum (SC), the outermost layer of the epidermis. The SC is composed of corneocytes that are surrounded by ceramides, fatty acids, and cholesterol; together they form a lamellar hydrophobic structure that limits transepidermal water loss and prevents the entrance of harmful substances and pathogenic microbes. In addition, various components such as enzymes, protease inhibitors, immune cells (e.g., Langerhans cells), and antimicrobial peptides contribute to the homeostasis of the SC barrier [1]. However, the integrity of the skin barrier can be compromised by diverse factors such as trauma, burns, skin disorders, and underlying medical conditions leading to cutaneous infections caused by pathogens (i.e., bacteria, fungi, and viruses) that penetrate this barrier [2].
Herpes simplex virus type 1 (HSV-1) is an enveloped, double-stranded DNA virus that causes recurrent vesicular eruptions, most commonly in the orofacial region (i.e., cold sores) [3]. HSV-1 is highly contagious, and it is estimated that 3.7 billion people under the age of 50 are infected with the virus. The main antiherpetic treatments used to accelerate the healing of blisters and relieve symptoms such as pain, itching, or burning sensations are acyclic nucleosides, including acyclovir and derivatives (i.e., famciclovir and valacyclovir) [4]. Depending on the severity of the symptoms and the host’s immune status, the antiviral therapy can be administered either systemically or topically [5,6]. However, one of the central challenges associated with oral antiviral treatments is their low bioavailability caused by the limited absorption of the drug through the gastrointestinal tract. In the case of acyclovir, some patients have exhibited absorption rates as low as 10–27% of the administered dose. This low bioavailability requires higher doses, which can lead to adverse effects such as nausea, vomiting, headache, irritability, and even renal injury [7]. On the other hand, for topical treatment, acyclovir is not a good candidate for passive permeation due to its polarity (logP = −1.76 at pH 7), which limits its partitioning into the lipid matrix of the SC [8]. In addition, resistance of HSV-1 to these antiviral treatments has become a significant concern, particularly in immunocompromised patients undergoing long-term therapies [9]. Currently, there is growing interest in the development of new antiviral agents with alternative mechanisms of action that can replace or complement existing therapies, for example, essential oils (EOs).
EOs are complex mixtures of terpenoids, ketones, aldehydes, and esters that have been approved for use in the food, cosmetic, and pharmaceutical industries [10]. Various studies have demonstrated their beneficial biological properties, including inhibitory activity against HSV-1 [11,12]. Specifically, oregano has shown promising antiviral properties. The chemical composition of oregano EO includes hydrocarbon sesquiterpenes (β-caryophyllene), hydrocarbon monoterpenes (ρ-cymene, β-thujene), and oxygenated monoterpenes (carvacrol and thymol), the latter being the major constituents as they represent about 20–60% of the total composition [13,14,15]. The antiviral activity of oregano EO (Lippia graveolens) against HSV-1 was reported by Pilau et al. [16], who determined a 50% inhibitory concentration (IC50) of 99.6 μg/mL. Later, another study demonstrated that specific components of oregano EO like β-caryophyllene (IC50 = 0.25 μg/mL), ρ-cymene (IC50 > 0.1%, equivalent to >1000 μg/mL), thymol, and carvacrol (IC50 = 7 μM, equivalent to 1.1 μg/mL) display antiviral activity against the virus [17]. Although EOs hold great potential as therapeutic alternatives, their use is limited due to their physicochemical characteristics. For example, they are volatile at room temperature and susceptible to degradation in the presence of oxygen, light, and humidity, moreover their application in aqueous-based formulations is challenging because of their nonpolar nature [18].
To approach these limitations, nanoencapsulation can result in an efficient strategy to deliver active compounds including EO whose chemical characteristics influence their usage. Specifically, polymeric nanoparticles (NPs) have been extensively studied for topical use because their nanometric size increases residence time and enhances direct contact with the skin. Moreover, their polymeric composition enables the controlled release of EO onto the stratum corneum (SC), improving their availability and consequently, the efficacy of the active compounds [19]. Also, it has been shown that NPs with sizes around 200 nm tend to accumulate in hair follicles and skin folds [20]. For these reasons, NPs represent a promising tool for the topical delivery of EOs in the treatment of HSV-1 related infections. In a study by Kalita et al. [21], chloramphenicol and lemongrass EOs were co-encapsulated in a poly-ε-caprolactone NPs for topical application in the treatment of burns co-infected with bacteria and fungi. The nanoformulation exhibited enhanced in vitro antimicrobial activity against 22 microbial pathogens. Additionally, the NPs facilitated penetration into the injured dermal tissue and accelerated the wound healing process.
Therefore, the aim of this study was to evaluate the in vitro antiviral activity against HSV-1 using a polymeric NP formulation loaded with Lippia graveolens EO (EOL). The investigation also measured the effect of the NPs on the biophysical properties of the skin, in order to determine the potential use of the formulation as a topical treatment for dermal infections with HSV-1.
2. Materials and Methods
2.1. Materials
Lippia graveolens was collected in Coahuila, Mexico. The chemical composition and the proportion of each component of the EO were analyzed by González-Moreno et al., [22] using a gas chromatography–mass spectrometry (GC-MS) and gas chromatography with flame ionization detection (GC-FID). The Vero cell line (ATCC CCL-81) and the HSV-1 strain KOS (ATCC VR-1493D) virus were kindly donated from the Department of Genetics, School of Medicine, UANL. Dimethylsulfoxide (DMSO) and carvacrol (CRV) (≥98% grade GC) were obtained from Sigma Aldrich (St. Louis, MO, USA). Methanol was purchased from Fisher Chemical (Waltham, MA, USA), while acetone and isopropyl alcohol were obtained from Tedia (Fairfield, OH, USA). All solvents were of analytical grade. Distilled water was obtained from a Milli-Q water purification system (Boston, MA, USA). The nanoparticle-forming polymer, Eudragit® L100 (copolymer of methacrylic acid-methyl methacrylate (1:1)), was kindly obtained from Helm (Naucalpan, State of Mexico, Mexico). Dulbecco Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and the rest of the reagents used for the in vitro tests were from Gibco, Grand Island, NY, USA.
2.2. Preparation and Characterization of EO-Loaded NP
The NPs were prepared by the nanoprecipitation method as described by Fessi et al. [23]. Briefly, an organic phase was prepared with 25 mg of Eudragit® L100 in 15 mL of acetone–isopropanol mixture (1:1). Then, 50 mg of EOL was added to the solution. Subsequently, the organic phase was injected using a syringe into the aqueous phase containing 20 mL of distilled water under magnetic stirring. Diffusion of the organic phase into the aqueous phase induced the aggregation polymer and encapsulation of EOL into nanoparticles (NP-EOL). Finally, the organic solvents were removed through two purification techniques: (i) dialysis using regenerated cellulose membrane Spectrum/Por®4 (Spectrum Laboratories, Rancho Dominguez, CA, USA) and (ii) evaporation under reduced pressure (Laborota 4003 control, Heidolph Instruments, Schwabach, Germany). EOL-loaded NPs purified by dialysis were identified as NP-EOL-D, while those purified by evaporation were identified as NP-EOL-R. Unloaded NPs, without EOL, were obtained following the same procedure described above. Similarly, the NPs without EOL purified by dialysis were identified as NP-BCO-D, and those purified by evaporation as NP-BCO-R.
The physicochemical characterization of formulations was carried out in terms of particle size, polydispersity index (PDI), zeta potential, and pH (Conductronic, pH120 model, Puebla, Puebla, Mexico). The mean particle size and PDI were measured at a 90-degree scattering angle using dynamic light scattering, while the zeta potential was measured by laser Doppler microelectrophoresis. Both measurements were performed using a Zetasizer Nano-ZS90 (Malvern Instruments, Worcestershire, UK), in triplicate at 25 °C. In addition, morphological examination of NPs was performed using a scanning electron microscope (JEOL JSM-7401F, Kyoto, Japan). Samples of dried NPs were dispersed in water and then, drops of the dispersion were placed on metallic studs and coated with gold.
The NP-EOL-R and the NP-EOL-D formulations were stored at 25 ± 2 °C under 40–60% relative humidity conditions. The stability of the formulations was evaluated by determining particle size, PDI, and zeta potential.
2.3. Fourier-Transform Infrared Analysis
Fourier-transform infrared (FT-IR) spectroscopy was performed using a Frontier FT-IR spectrometer (PerkinElmer, Waltham, MA, USA) to establish interactions between the raw material used for NPs formulation (Eudragit® L100 polymer and EOL). Additionally, the nanoformulations (NP-BCO-D, NP-BCO-R, NP-EOL-D and NP-EOL-R) were examined. The polymer and EOL were evaluated directly, while the NPs were centrifuged and the resulting pellet was placed in a desiccator. Spectra were examined over wavenumber range of 4000 to 400 cm−1.
2.4. Cell Viability Assessment
Cell viability was determined using the Mosmann method with slight modifications [24]. For the assay, Vero cells were cultured in 96-well plates at a density of 1.5 × 104 cells/well in DMEM with 10% FBS and 1% DMSO. After 24 h, the cells were supplemented with different concentrations of EOL, CRV, NP-EOL-R, NP-EOL-D, NP-BCO-R, and NP-BCO-D and incubated for 48 h at 37 °C and 5% CO2. Vero cells added to only DMEM with 10% FBS and 1% DMSO were used as negative controls. After 48 h incubation, cell viability was determined by adding 10 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution at a concentration of 5 mg/mL per well, followed by incubation for 3 h at 37 °C in a 5% CO2 atmosphere. The culture medium was removed, and 100 μL of DMSO was added. Absorbance was measured at 540 nm using a Thermo Scientific Model 357 microplate reader (Waltham, MA, USA). The cytotoxic concentration 50 (CC50) was determined as the concentration of NP-EOL, NP-BCO, CRV, and EOL required to reduce cell viability by 50%, relative to the viability of untreated cells (negative control). Experiments were performed in quadruplicate.
2.5. Evaluation of Antiherpetic Activity In Vitro
A viral plaque reduction assay was performed to determine the IC50 according to the methodology described by Silva Mares et al. [25]. Briefly, confluent monolayers of Vero cells in 24-well culture plates were incubated with 25 plaque forming units (PFUs) of HSV-1 for 1 h at 37 °C. The supernatant was discarded, and fresh medium supplemented with 0.32% IgG and 1% DMSO was added. Subsequently, concentrations of 11, 22, and 44 μg/mL of NP-EOL, NP-BCO, CRV, and EOL were evaluated; these concentrations were selected based on prior cytotoxicity results, representing low, medium, and high values that were non-toxic. The cells were incubated for 48 h. Finally, the cells were fixed with methanol and stained with Giemsa reagent (CTR Scientific, Monterrey, Nuevo Leon, Mexico). The IC50 was determined as the concentration at which a 50% reduction in PFU formation was observed compared with the 0% reduction in the negative control (infected cells). All assays were performed in triplicate. The selectivity index (SI) of each sample was calculated as the ratio of CC50 to IC50.
2.6. Ex Vivo Biophysical Evaluation of Skin Treated with EOL-Loaded NP
The porcine ear skin was obtained from the R.E.T.S.A. slaughterhouse in Monterrey, NL, Mexico. After cleaning and the removal of subcutaneous fat, the skin was frozen at −4 °C for up to four weeks before use.
For the experiment, the skin was thawed and hydrated for 30 min. Subsequently, it was mounted in a modified Franz diffusion cell, consisting of a donor compartment and a receptor compartment, with the porcine skin placed between them. The receptor compartment contained 15 mL of phosphate-buffered saline (PBS) at pH 7.4 and was maintained under magnetic stirring (300 rpm) at 36.0 ± 0.5 °C. The donor compartment (with an area of 2.54 cm2) was filled with 1 mL of the treatment of NP-EOL or NP-BCO and covered to prevent evaporation. The formulations were in contact with the skin for 4, 6, and 8 h. After exposure time, the formulations were removed from the skin surface by gently patting with cotton. Subsequently, the effect of the formulations was evaluated in terms of transepidermal water loss (TEWL), skin surface pH, stratum corneum water content (SCWC), as well as the melanin and erythema index, and these were measured on the pig skin with the respective Tewameter TM300, pH 905, Corneometer CM825, and Mexameter MX 18 probes, respectively, all connected to a MPA5 system (Courage & Khazaka, Köln, Germany). Measurements were carried out in quintuplicate, in a draft-free room, with controlled temperature (25 ± 2 °C) and relative humidity (30–40%).
2.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism software version 7.0 (San Diego, CA, USA). One-way ANOVA, followed by a multiple comparison test, was conducted to compare the means of more than two groups (p < 0.05).
3. Results
3.1. Preparation and Characterization of EOL-Loaded Nanoparticles
The NP-EOL-R and NP-EOL-D formulation were obtained using the nanoprecipitation technique established by Fessi et al. [23]. The physicochemical characteristics of the nanoformulations are presented in Table 1.

Table 1.
Physicochemical characterization of EOL-loaded NPs (Mean ± SD, n = 3).
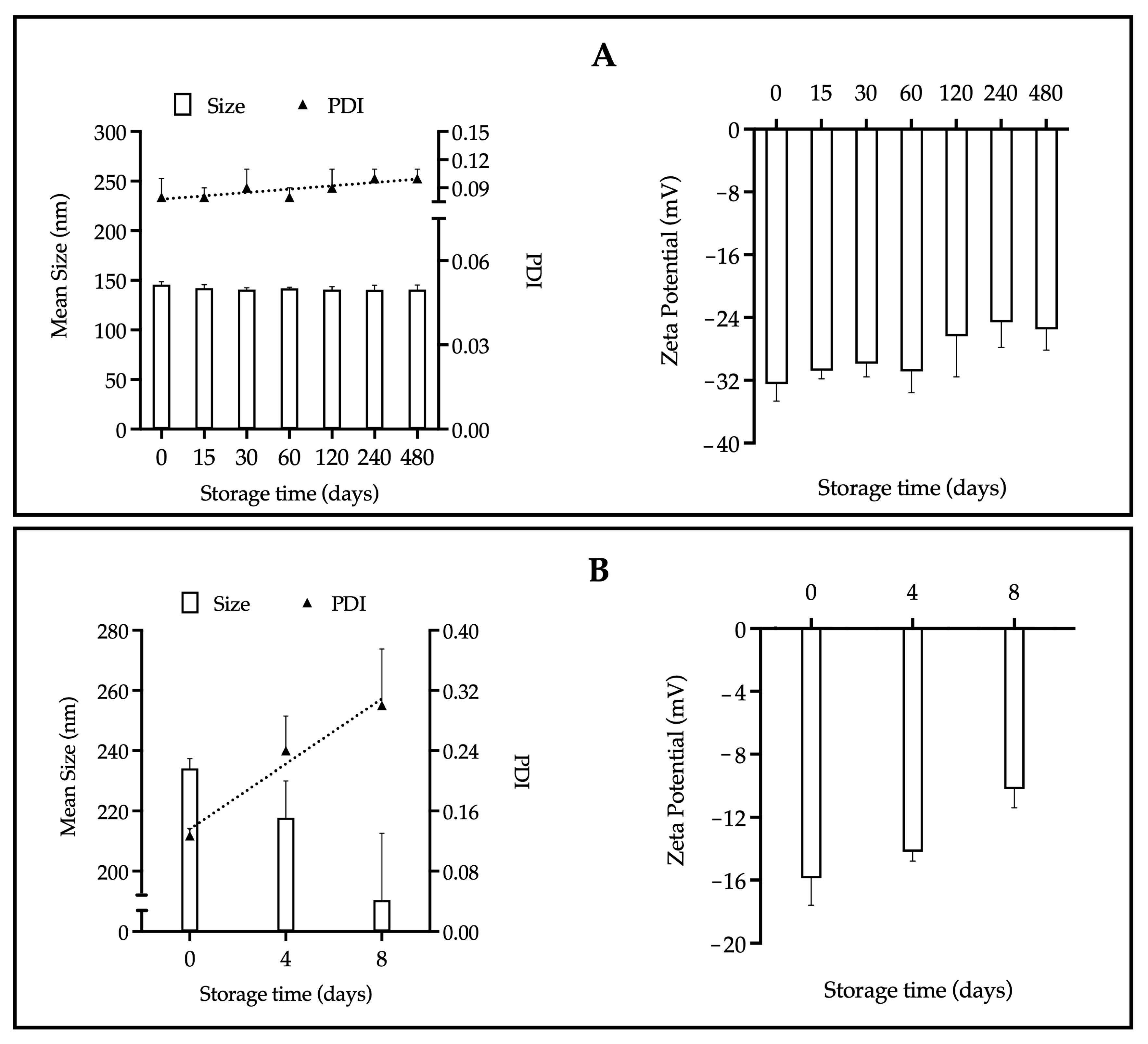
As shown in Figure 1, the stability parameters of the EOL-loaded NPs: particle size, polydispersity index (PDI), and zeta potential, were evaluated over a period of 480 days at 25 °C under relative humidity conditions of 40–60%.
Figure 1.
Stability of the NP-EOL-R (A) and NP-EOL-D (B) as a function of particle size, PDI and zeta potential (Mean ± SD, n = 3). The dashed line corresponds to a visual guide to indicate the general direction of the data.
3.2. Fourier-Transform Infrared Spectroscopy Analysis of NP-EOL Components
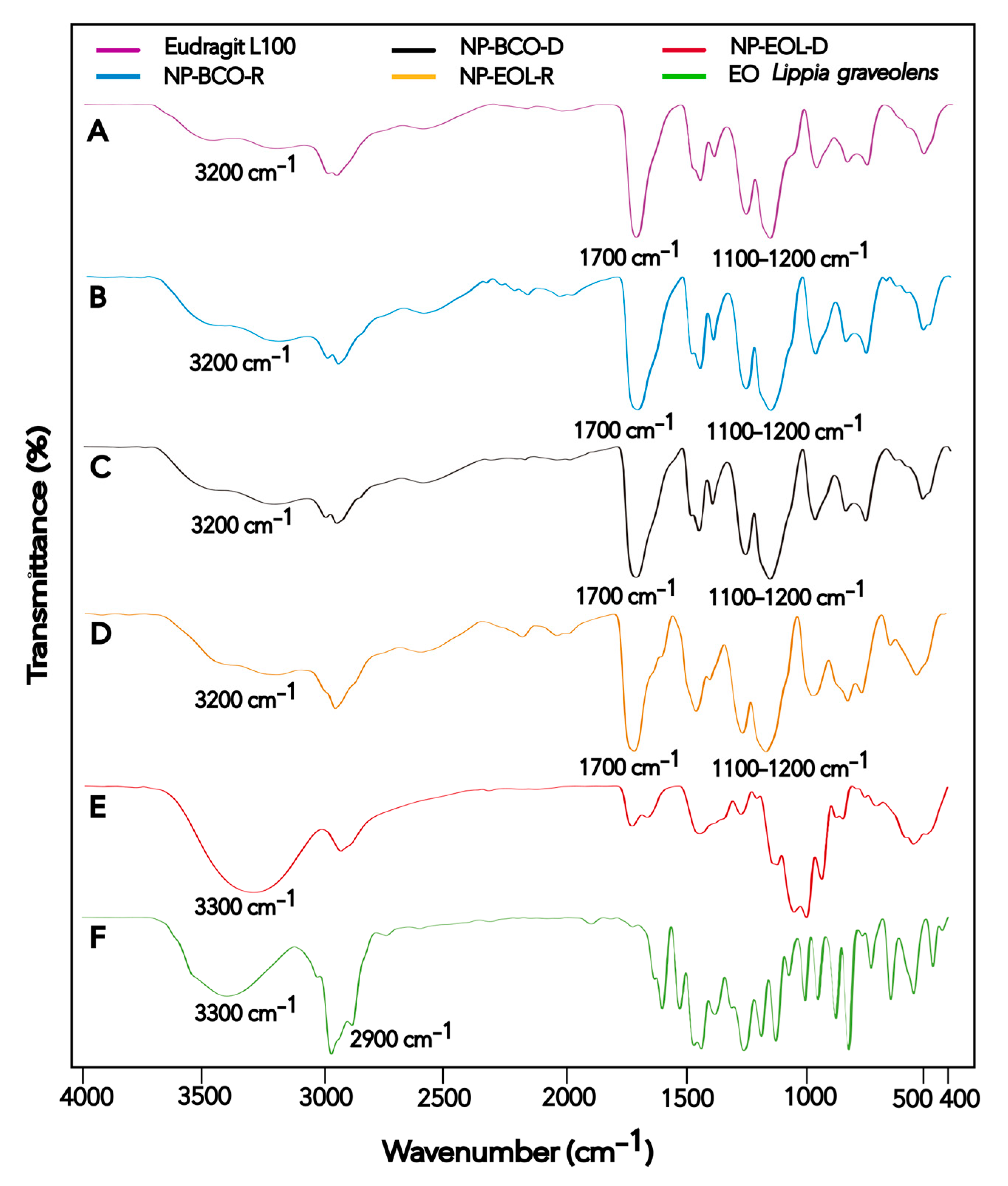
FT-IR spectroscopy was used to characterize the Eudragit® L100 polymer, Lippia graveolens EO, EOL-loaded NPs, and unloaded NPs, in order to analyze the interactions between the NP components. The spectrum of each material is shown in Figure 2.
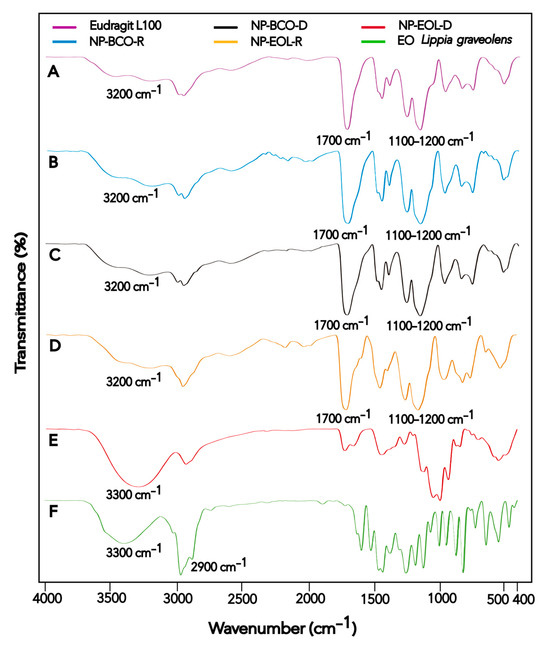
Figure 2.
FT-IR spectra of Eudragit® L100 (A), NP-BCO-R (B), NP-BCO-D (C), NP-EOL-R (D), NP-EOL-D (E), and EO Lippia graveolens (F).
The morphology, size, and homogeneity of NP-EOL-R were further analyzed by SEM. Representative micrographs are presented in Figure 3.

Figure 3.
Evaluation of the size and homogeneity of nanoparticles loaded with Lippia graveolens essential oil, obtained by the nanoprecipitation technique and purified through evaporation, using scanning electron microscopy (SEM) at a magnification of ×30,000.
3.3. Cytotoxicity Against Vero Cells and in Vitro Antiherpetic Activity
The cytotoxic effects of NP-EOL, NP-BCO, EOL, and CRV are shown in Table 2. NP-BCO formulations purified by dialysis and reduced pressure evaporation exhibited no cytotoxicity toward Vero cells at the tested concentrations (>200 μg/mL). The CC50 values for NP-EOL-R, NP-EOL-D, EOL, and CRV were 71.89, 57.16, 84.81, and 83.32 μg/mL, respectively.

Table 2.
Cytotoxic effect against Vero cell lines, using MTT assay, and antiherpetic activity of polymeric nanoparticles loaded with EOL.
The antiherpetic activity of NP-EOL, NP-BCO, EOL, and CRV was evaluated using a plaque reduction assay. NP-EOL-R showed the highest antiviral efficacy, with an IC50 value of 25.83 μg/mL, compared with NP-EOL-D with an IC50 of 36.60 μg/mL. In contrast, EOL and CRV inhibited HSV-1 with IC50 values of 47.74 and 27.55 μg/mL, respectively. The SI calculated for NP-EOL-R, NP-EOL-D, EOL, and CRV was 2.78, 1.56, 1.78, and 3.02, respectively.
3.4. Ex Vivo Biophysical Evalaution of Skin Treated with EOL-Loaded Nanoparticles
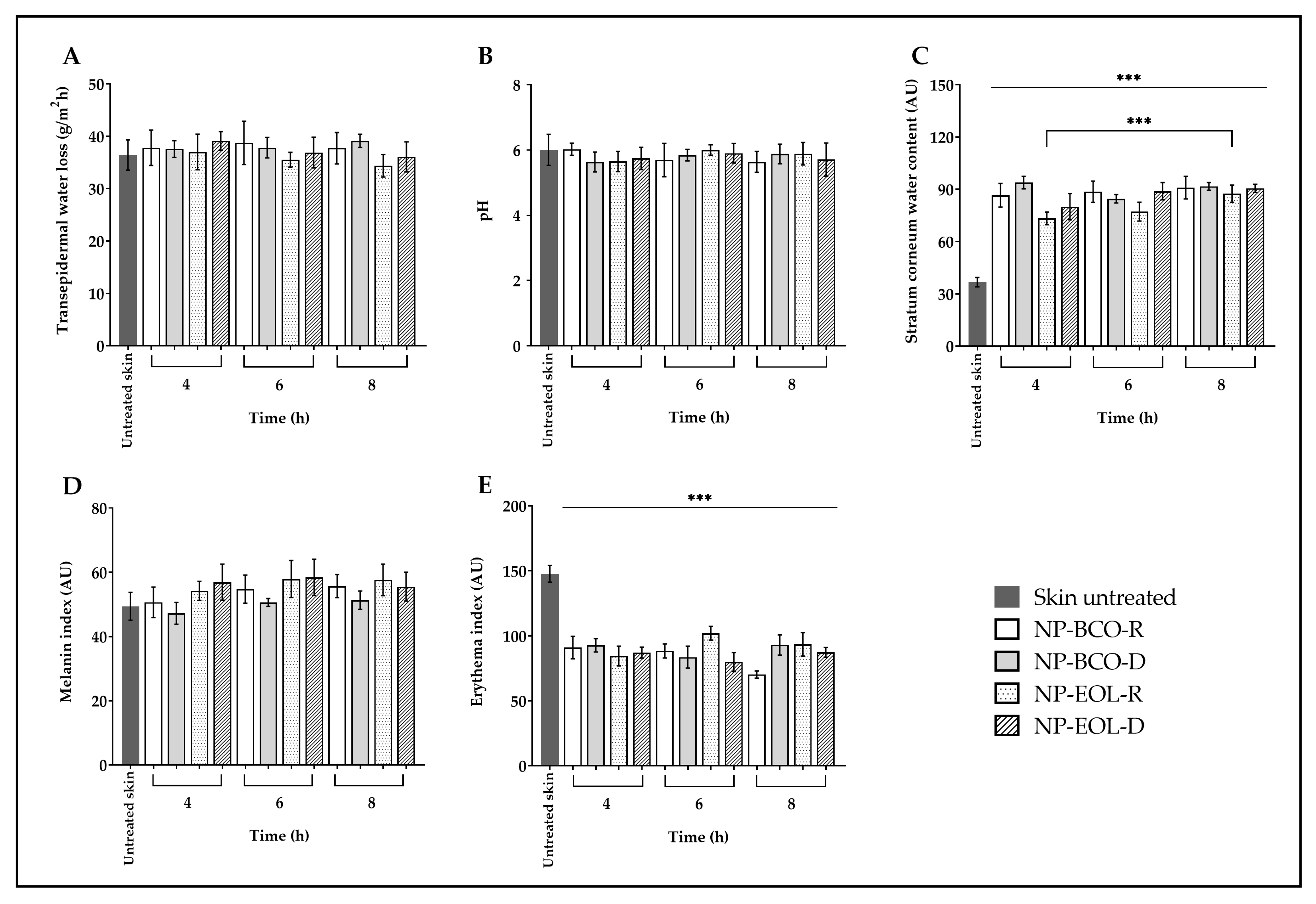
The biophysical parameters of porcine skin were evaluated before the application of the nanoformulations and after 4, 6 and 8 h of contact time (Figure 4). In the case of untreated skin, TEWL showed no significant changes after exposure to the nanoformulations (baseline value 36.59 ± 2.42 g/m2h; Figure 4A). Similarly, the surface pH of the untreated skin (6.09 ± 0.54) remained unchanged following the treatment (Figure 4B). However, a statistically significant increase was observed in the SCWC of treated skin compared with the untreated controls (baseline: 36.04 ± 2.75 AU), after 4, 6 and 8 h of application (Figure 4C). In the case of NP-EOL-R, SCWC values nearly doubled. Regarding the melanin index, the values obtained after 4, 6 and 8 h of exposure to the nanoformulations were like those of the untreated skin (baseline: 50.43 ± 5.59 AU; Figure 4D). Finally, the erythema index decreased significantly after contact with the nanoformulations at all tested time points, compared with untreated skin (baseline: 149.55 ± 6.44 AU; Figure 4E).
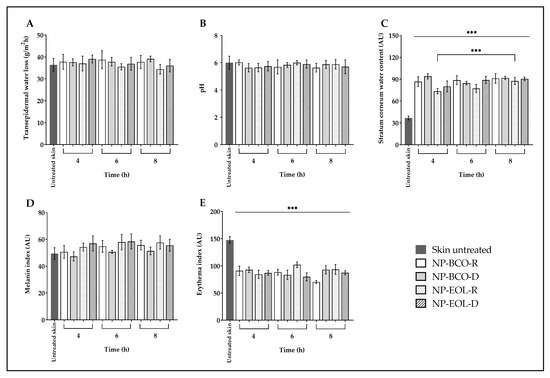
Figure 4.
Ex vivo biophysical effect on transepidermal water loss (A), pH (B), stratum corneum water content (C), melanin (D), and erythema (E) after the contact of EOL-loaded NPs compared with untreated skin (mean ± SD, n = 5). *** Indicates significant differences p < 0.001, compared to untreated skin.
4. Discussion
In this study, NPs were developed with the aim of improving the topical delivery of oregano EO for the treatment of HSV-1. The NPs were formulated using the nanoprecipitation technique established by Fessi et al., which is widely employed for the encapsulation of hydrophobic compounds such as EOs [26]. This technique is distinguished by its simplicity, rapidity, and versatility, as it is compatible with a broad range of polymers and solvents, offering flexibility in material selection depending on the intended application [27]. Among the commonly used polymeric materials are polymethylmethacrylate derivatives, such as Eudragit® L100. This polymer has been approved by the FDA for the delivery of active compounds targeted to the skin due to its ability to dissolve at pH 6. Frequently, in skin disorders besides inflammation and epidermal lesions, the pH tends to shift to a near neutral environment, this favors the release of the active compound [28,29]. Dong et al. [30], evaluated the skin penetration and release of dexamethasone utilizing a Eudragit® L100-based NP through electron paramagnetic resonance studies. The results showed an in vitro release efficiency and skin penetration of dexamethasone in comparison with commercial cream; the experiments were both conducted on intact and barrier-disrupted skin at pH values above 6.
Once the NPs were obtained, their physicochemical characterization was carried out based on particle size, PDI, and zeta potential (Table 1). The NP-EOL-R formulation exhibited a size of 145.89 ± 5.89 nm. This result is consistent with that reported by Zhang et al. [31], who prepared a Eudragit® L100-based NP loaded with Piper nigrum L. EO using nanoprecipitation followed by solvent removal under reduced pressure, obtaining particles with a size of 178 nm. On the other hand, the NP-EOL-D formulations showed a particle size of 232.1 ± 13.50 nm, in an agreement with the findings of Silva-Flores et al. [32], who encapsulated essential oils from Rosmarinus officinalis and Lavandula dentata, obtaining particle sizes ranging from 227 to 231 nm using the nanoprecipitation technique, followed by purification using dialysis. In our study, the particle size difference, of approximately 80 nm between NP-EOL-R and NP-EOL-D, is attributed to the process used to purify the NPs. Several studies have reported that solvent evaporation rate can influence the physicochemical characteristics of NPs, including their morphology, particle size, and release profile [33,34]. During evaporation, the application of reduced pressure increases the solvent removal rate, generating density differences between the surface and the core of the particles. This phenomenon induces rapid structural relaxation, preventing the full extension of polymer chains and resulting in their contraction, which leads to smaller particles. Conversely, the slower solvent removal rate achieved by dialysis allows sufficient time for polymer chain rearrangement before the particle solidification occurs [35].
The particle sizes obtained for NP-EOL-R and NP-EOL-D fall within the 200 nm range. Donalisio et al. [36], demonstrated that chitosan nanospheres loaded with acyclovir, with a particle size of 200 nm, exhibited 13-fold higher in vitro antiviral activity against HSV-1 compared with non-encapsulated acyclovir. Furthermore, in vitro permeation studies showed that the NPs improved acyclovir deposition in the SC when compared with a commercial cream. In the present study, both NPs formulations exhibited a suitable particle size for deposition in the SC and within skin folds, making them appropriate candidates for the topical delivery of EOs.
The PDI is associated with particle size distribution within a population of NPs. In the Zetasizer Nano-ZS90 (Malvern Instruments), the PDI values range from 0 to 1, where the ones closer to 0 indicate a uniform particle size distribution. In this study, the formulations presented a PDI ≤ 0.2 (Table 1), which falls within the range considered acceptable for polymer-based nanoparticle applications [37]. A homogeneous particle size distribution is a critical attribute that ensures consistent NPs interactions (e.g., bioadhesion and EO release) across the skin surface and with viral particles, potentially enhancing antiviral treatment efficacy.
In this study, the electrokinetic properties of nanoformulations were evaluated. The zeta potential is based on the measurement of the electrochemical equilibrium at the interface (Stern and diffuse layers) surrounding the dispersed NPs [38]. The zeta potential of NP-EOL-R was −32.47 ± 2.18 mV, whereas NP-EOL-D exhibited a value of −15.90 ± 1.70 mV. The negative values indicate that negative charges dominate the diffuse double layer of the NPs, which is attributed to the anionic nature of the Eudragit® L100 polymer due to the ionization of carboxylic end groups exposed on the surface of the polymer chains [28]. The difference in the magnitude of the zeta potential between NP-EOL-R and NP-EOL-D may be due to the higher surface-to-volume ratio of the smaller NP (NP-EOL-R), which favors a greater interaction of the surface charges with ions in the surrounding medium. Moreover, studies on the electrical double layer in low ionic strength media such as distilled water have shown a less compact structure, facilitating more interactions between smaller particles and the medium, thereby increasing the zeta potential [39]. Therefore, this increase in interactions for smaller NPs may explain why the zeta potential value of NP-EOL-R was approximately twice that of NP-EOL-D.
A NPs suspension is considered stable when it maintains its initial physical characteristics (e.g., size, PDI, zeta potential) over a defined period. Visual appearance, such as the absence of aggregates, is another relevant parameter for assessing stability [40]. In colloidal systems, instability may occur when the absolute value of the zeta potential is low, indicating that attractive Van der Waals forces overcome repulsive forces, thus promoting particle aggregation. In contrast, when particles exhibit a high surface charge density or an absolute zeta potential equal to or greater than ± 30 mV, sufficient electrostatic repulsion is generated to prevent coalescence and promote formulation stability [38]. According to the guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), the storage stability of nanomedicines must be evaluated for a minimum of 12 months [41,42]. Figure 1 shows the stability of the nanoformulations stored at 25 °C and 40–60% relative humidity; these conditions were chosen based on the recommendations of current antiviral treatments. In this study, NP-EOL-R (Figure 1A) showed no changes in physical characteristics (size, PDI, zeta potential) over 480 days. In contrast, NP-EOL-D (Figure 1B) exhibited an increase in PDI from 0.1 to 0.3 after 8 days of storage, suggesting increased heterogeneity in particle size. Also, the zeta potential of NP-EOL-D decreased to −10 mV, indicating a reduction in electrostatic repulsion forces, which compromised the stability of the formulation and correlated with aggregate formation and phase separation. These results indicate that the storage conditions employed affected the integrity of NP-EOL-D. In contrast, colloidal systems with zeta potentials above ± 30 mV, such as NP-EOL-R, exhibited greater stability. This is consistent with the findings of Zhang et al. [31], who developed a Eudragit® L100-based nanoparticle formulation loaded with Black pepper EO, which showed a zeta potential of −57 mV and remained stable for five weeks under various temperature conditions.
As part of the physicochemical characterization of the nanoformulations, the encapsulation efficiency percentage (%EE) was determined using a validated GC-FID method. The %EE values for NP-EOL-R and NP-EOL-D were 16.23 and 29.61%, respectively. These results were lower than those reported by previous reports [22], likely due to differences in the polymer employed or the purification method used.
The FT-IR spectra obtained for the raw materials and nanoformulations are presented in Figure 2. The spectrum of the Eudragit® L100 polymer (Figure 2A) is consistent with the one reported by Saadallah et al. [43]. In the FT-IR spectra, two bands were observed, one near 1156.02 cm−1 and another in the region of 1255.91 cm−1, both characteristic of C–O stretching vibrations of esters. Additionally, a band appeared around 1711.61 cm−1 corresponding to C=O stretching vibrations of carboxylic acids, and another at 3202.42 cm−1 associated with hydroxyl group (O–H) vibrations. These signals are characteristic of acrylic copolymers. The signals observed in the Eudragit® L100 polymer spectrum were also present in the spectra of NP-BCO-R and NP-BCO-D (Figure 2B and Figure 2C, respectively), confirming that the structural characteristics of the polymer were preserved in both nanoformulations. On the other hand, the EOL spectrum (Figure 2F) showed a broad band around 3369.87 cm−1 and a peak at 2959.55 cm−1, which are characteristic of O–H and C–H stretching vibrations, respectively. These signals are consistent with those reported by Gutierrez et al. [44]. The O–H and C–H groups detected in the EOL spectrum may be attributed to the presence of CRV, a component with a phenolic functional group that is found in high proportion in EOL. The spectrum of NP-EOL-R (Figure 2D) was similar to that of NP-BCO-R, supporting the inference that the EOL was successfully encapsulated. Moreover, no new bands corresponding to the formation of new compounds were observed, indicating that only physical or weak chemical interactions occurred between the EOL and the polymeric chains, and that no chemical reactions took place among the NP components. In contrast, the spectrum of NP-EOL-D (Figure 2E) differed from that of NP-BCO-D, this finding suggests that some EOL components may have been retained or adsorbed on the polymer surface [26].
As will be discussed later, NP-EOL-R exhibited favorable biological effects, which could offer a promising alternative for the treatment of HSV-1 infections. In consequence, it was also analyzed by SEM to assess the NPs morphology, size, and homogeneity in the size particles. As illustrated in Figure 3, the NPs exhibited a spherical shape and smooth surface. No particle aggregates were observed, and the particle size (within the 200 nm range) was consistent with the hydrodynamic diameter measured for NP-EOL-R (145.89 ± 5.89 nm).
To evaluate the antiherpetic activity, Vero cells were used as this cell line was approved by the National Committee for Clinical Laboratory Standards for susceptibility tests against various viral infections, including HSV-1 [45]. To explore the potential use of nano-encapsulated Lippia graveolens EO as an antiviral agent, the toxicity of the formulation was evaluated in vitro utilizing Vero cells. Cytotoxicity was assessed using the MTT assay for the treatments NP-BCO-R, NP-BCO-D, NP-EOL-R, NP-EOL-D, EOL, and its major component, CRV. As shown in Table 2, at concentrations below 200 µg/mL, NP-BCO purified by reduced pressure evaporation and dialysis did not exhibit cytotoxic effects on Vero cells. This finding aligns with Slavkova et al. [46], who evaluated the cytotoxic effects of a Eudragit® L100 NP on the viability of the human keratinocyte HaCaT cell line, determining that the NPs did not reduce cell viability or exhibit toxic effects. Regarding the EOL, a CC50 of 84.81 ± 1.36 µg/mL was obtained. Previously, Gómez et al. [47], reported a comparable cytotoxicity for Lippia graveolens EO in the HEK293 cell line, with a CC50 of 90.0 ± 18.1 µg/mL. For CRV, a CC50 of 83.32 ± 1.17 µg/mL was observed, which is similar to the value reported by Nakamura et al. [48], who obtained a CC50 of 86 ± 1.41 µg/mL. Finally, the CC50 of NP-EOL-R was 71.89 ± 0.41 µg/mL, while that of NP-EOL-D was 57.16 ± 0.86 µg/mL, the latter showing greater cytotoxicity. In a previous study conducted by the same research group, the compounds present in the EOL were identified [22]. Therefore, the difference in cytotoxicity between the two nanoformulations could be related to the encapsulation of EOL components in different proportions depending on the purification method employed. It is worth noting that the reduced pressure evaporation method favored the encapsulation of compounds such as caryophyllene oxide and butylated hydroxyanisole, unlike dialysis. In a study conducted by Damayantii et al. [49], the in silico toxicity of food preservatives was evaluated, and it was demonstrated that butylated hydroxyanisole exhibited moderate toxicity. Furthermore, another study reported that caryophyllene oxide inhibits the mitochondrial electron transport chain, which could affect cell viability and therefore modify toxicity [50]. However, further studies are needed to establish the cause of the observed toxic effects. Based on the cytotoxicity results in Vero cells, at concentrations of 11, 22, and with 44 µg/mL of NP-BCO-R, NP-BCO-D, NP-EOL-R, and NP-EOL-D, EOL and CRV were selected for subsequent antiviral assays.
The antiviral activity of the nanoformulations against HSV-1 was evaluated using the plaque reduction assay, a widely accepted method to test susceptibility of viruses that can induce cytopathic effects [51]. These effects manifest as structural and functional alterations in infected cells such as cell rounding, enlargement, granulation, syncytia formation, loss of adherence to the substrate, and cell lysis [52,53]. Cellular damage caused by viral replication manifests in the formation of clear zones or plaques in the cell monolayer that become evident after staining the cells. Thus, the plaque reduction assay allows for the determination of the IC50 of a substance based on its ability to reduce the number of plaques formed. The IC50 values of the tested treatments are presented in Table 2. NP-BCO purified by reduced pressure evaporation and dialysis did not show in vitro antiviral activity against HSV-1 at any of the concentrations tested. In contrast, free CRV exhibited an IC50 of 27.55 ± 1.66 µg/mL, a value lower than that reported by Pilau et al. [16], who obtained an IC50 of 48.6 µg/mL. The IC50 of EOL was 47.74 ± 2.80 µg/mL, which is approximately half the value reported by Pilau et al. (IC50 de 99 μg/mL) [16]. Some studies suggest that the antiviral activity of EOs and their components is due to their lipophilic nature, which can induce alterations in viral particles (disruption of the viral envelope or capsid disintegration) and interfere with viral binding to the host-cell receptors [17,54]. Gilling et al. [55], reported that oregano EO at 4% (v/v) and CRV at 0.5% (v/v) caused an increase in the particle size of murine norovirus, capsid disintegration, and the subsequent loss of viral infectivity. In another study, pretreatment of HSV-1 virions with CRV at 100 µM resulted in a disruption of 79% of the viral envelope [56]. Another proposed mechanism involves the inhibition of the interaction between the virus and host-cell receptors. In this context, thymol, another major component of EOL, has also been documented for its antiviral properties. In an in silico study, Cura et al. [57] evaluated the interaction of thymol with HSV-1 and showed that hydrogen bonds were formed between thymol and the viral glycoproteins gB (i.e., amino acids GLN416, LEU228, and GLU42) and gD (i.e., amino acids PRO172 and ARG174); these interactions could interfere with viral adhesion and penetration into hosts cells, thereby preventing the early steps of the infection process. On the other hand, the antiherpetic activity of NP-EOL-R showed an IC50 of 25.83 ± 1.53 μg/mL, compared with 36.60 ± 2.41 μg/mL for NP-EOL-D, demonstrating greater antiviral effectiveness in the nanoparticles purified by reduced pressure evaporation. This difference could be related to the following factors: (i) The purification method influenced the profile of encapsulated compounds, as reflected in the %EE values obtained (i.e., 16.92% for NP-EOL-R and 29.61% for NP-EOL-D). This variation suggests that each purification method favored the differential encapsulation of active components, thereby impacting the observed antiviral activity. (ii) The surface characteristics of the nanoparticles (zeta potential) may affect their interaction with the virus. In this regard, Yadavalli et al. [58], reported that polyanionic molecules can interact with the cationic domains of viral glycoproteins (gB, gC). As noted previously, NP-EOL-R exhibited an absolute zeta potential nearly twofold greater than that of NP-EOL-D. This higher negative surface charge may contribute to enhanced electrostatic interactions with the virus, thereby facilitating the release of EOL and its interaction with the viral envelope. However, no experimental evidence is currently available to confirm this mechanism. Therefore, additional studies including release profiling, cellular uptake assays, and antiviral activity evaluation at different stages of the viral replication cycle are required to elucidate the mechanism responsible for the greater activity observed.
The SI is a parameter that indicates a compound’s ability to inhibit a virus without significantly affecting host cells. It is calculated as the ratio between the cytotoxic concentration (CC50) and the effective antiviral concentration (IC50). A SI value equal to or greater than 4 suggests effective antiviral activity with low cytotoxicity, making the compound suitable for therapeutic use [59]. The SI values obtained in this study are presented in Table 2. As shown, the EOL exhibited a SI value of 1.78, while NP-EOL-R and NP-EOL-D had values of 2.78 and 1.56, respectively. These results indicate that encapsulating EOL in nanoparticles, combined with solvent removal by reduced pressure evaporation, increased the SI value, thereby improving both the safety and efficacy of EOL as an antiviral agent. Although the SI value obtained for NP-EOL-R did not reach the reference threshold (SI ≥ 4), it is important to emphasize that this formulation improved selectivity compared with the free EO. Nevertheless, the SI value of below 4 highlights the need to optimize the nanoformulation. In this regard, adjustments in the preparation conditions (e.g., solvents and polymer in the organic phase) and in the purification method could modify the profile of encapsulated and non-encapsulated compounds. Such modifications may reduce the contribution of components responsible for immediate cytotoxicity, thereby improving the balance between antiviral efficacy and safety.
The in vitro assays conducted in this study allowed for a controlled, rapid, and cost-effective evaluation of the antiviral potential of EOL encapsulated in NPs, as well as their effects on cell viability. However, the validation of these results in HSV-1 in vivo infection models is required, since such models offer a more complete perspective on efficacy, dosage, pharmacokinetics, and safety within a more complex biological system.
On the other hand, evaluating the effect of topical treatments using a porcine skin model is a versatile, efficient, and predictive approach that supports the development of new formulations. Because porcine skin is akin to human skin in various morphophysiological characteristics, such as vascular organization, lipid film composition, epidermal and SC thickness, and follicular structure, it is a suitable option for studying skin damage, wound healing, and drug delivery [60]. Another advantage is that porcine skin can be obtained as a slaughterhouse by-product, which helps address ethical concerns related to animal experimentation while also reducing experimental costs [61].
Biophysical parameters such as TEWL, pH, water content, and the erythema and melanin index are among the most used indicators to assess the skin barrier function.
TEWL measurement is widely used in dermatological research as an indicator of skin barrier integrity as it calculates the amount of water that diffuses from the dermis and epidermis through SC to the skin surface [62]. When the skin is damaged, as in the case of skin disorders or burns, TEWL levels may increase, indicating impaired barrier function [63].
As shown in Figure 4A, TEWL values did not differ significantly from untreated skin following the application of NP-EOL-R, NP-EOL-D, NP-BCO-R, and NP-BCO-D on the skin surface for 4, 6, and 8 h. These findings suggest that the water-based composition of the nanoformulations did not compromise the skin’s hydrolipidic barrier. Similar results were reported by Saraiva et al. [64], who demonstrated that in vivo application of chitosan nanoparticles loaded with Helichrysum italicum EO on human skin did not significantly alter TEWL.
Another important parameter in maintaining skin barrier function is pH. In humans, the skin has an acidic pH ranging from 4.1 to 5.8, depending on the body site. Skin pH influences various functions, including keratinocyte differentiation and the formation and function of the lipid bilayer [65]. As shown in Figure 4B, skin pH values did not change significantly after contact with the nanoformulations for 4, 6, and 8 h. These findings are consistent with ex vivo studies by Silva-Flores et al. [66], who reported that the application of Eudragit® EPO nanoparticles loaded with Rosmarinus officinalis and Lavandula dentata EO caused no significant changes in skin pH on porcine skin. Previous studies have shown that formulations with an acidic pH help maintain the skin’s natural acid mantle. In the present study, the applied nanoformulations did not alter skin pH, suggesting that they preserve the acidic environment essential for microbiome balance, thereby creating unfavorable conditions for the growth of pathogenic bacteria and fungi.
It is well known that the water content of the SC (SCWC) plays a crucial role in the appearance and health of the skin, as it influences its elasticity, flexibility, and functional properties such as active ingredient permeation. The SC normally contains between 10 and 30% water [67]. However, various factors, including environmental exposure, surfactant use, solar radiation, or skin disorders, can reduce the SCWC to below 10%, affecting the enzymatic activity required for normal desquamation. This may lead to decreased flexibility and a dry, scaly appearance of the skin [68]. Figure 4C shows the SCWC values following contact of the nanoformulations with the surface of porcine skin. A statistically significant increase in SCWC levels was observed compared with untreated skin. This increase may be attributed to the natural moisturizing factor, which is composed of amino acids and their derivatives, such as pyrrolidone carboxylic acid and urocanic acid, as well as lactates, urea, and electrolytes. These components possess hydroscopic properties that may help retain the water present in the nanoformulations, thereby contributing to skin hydration [69]. Furthermore, given that the nanoformulations are an aqueous suspension containing more than 99% (w/w) water, this characteristic may, at least in part, account for the increase in the SCWC observed after their application. Notably, the SCWC values increased progressively with longer contact times of NP-EOL-R with the skin, suggesting that the nanoformulation may promote sustained SC hydration, which is promising for the development of topical systems with prolonged activity.
On the other hand, skin color changes can be evaluated as indicators of skin barrier integrity. Human skin color is primarily determined by the presence of melanin, hemoglobin, carotenoids, and bilirubin [70]. Melanin not only contributes to pigmentation but also protects the skin against oxidative stress induced by UV radiation, air pollutants, and chemical compounds [71,72]. Melanin content varies with sex, age, ethnicity, lifestyle, environmental conditions, and exposure to certain substances [73,74]. In an in vivo study, the melanin index of the skin was evaluated using a Mexameter probe after applying a cream containing Olea europaea leaf extract. These results indicated no significant changes in pigmentation after two months of treatment [75]. These findings are consistent with the results shown in Figure 4D, where no statistically significant differences in the melanin index were observed between the skin treated with the nanoformulations and untreated skin, suggesting that the formulations do not alter melanin content on the skin surface. Moreover, the melanin index remained unchanged for up to 8 h post-application, indicating that the nanoformulations do not induce changes in skin melanin levels even after prolonged contact.
Regarding hemoglobin, which is present in red blood cells circulating through blood vessels, it is associated with the red coloration of the skin because is strongly reflects longer wavelengths while predominantly absorbing radiation in the shorter wavelength range (corresponding to blue) [76]. Under certain conditions, such as contact with allergens, viral infections, or increased body temperature, vasodilation is triggered, increasing blood flow to the superficial capillaries of the skin. The accumulation of hemoglobin in these areas manifests as skin redness, known as erythema [77]. Alam et al. [78], used the same Mexameter probe in an in vivo model to evaluate the erythema index after applying a cream containing Echinacea purpurea extract. Their results showed no increase in erythema levels during the study period. As shown in Figure 4E, a statistically significant decrease in erythema index values was observed following the application of the nanoformulations compared with untreated skin. This result may suggest that the deposition of nanoparticles on the skin surface, due to their nanometric size and solid nature, favors the dispersion of incident light. Puentes Ossa et al. [79], reported that conjugated fluorene silica nanoparticles sized between 100 and 125 nm are capable of scattering light and exhibit low absorption coefficients within the spectral range 300–800 nm, which encompasses the wavelengths used by the Mexameter (568–880 nm). Therefore, the amount of radiation absorbed by hemoglobin would be modified by the presence of the NPs, resulting in an apparent decrease in erythema values.
To our knowledge, the antiviral activity of EOL in NPs has not been previously reported.
5. Conclusions
In the present study, NP-EOL were obtained with adequate physicochemical characteristics for topical use. The results suggest that nanoencapsulation combined with purified evaporation under reduced pressure potentiated the antiherpetic activity of Lippia graveolens EO by about two times, and an improvement in the security profile. The biophysical evaluation in skin demonstrated that the nanoformulations favors SC hydration without compromising the skin barrier. Therefore, NP-EOL is a promising alternative for topic treatment against viral infections caused by HSV-1.
Author Contributions
Methodology, N.N.E.-C., R.Á.-R., D.A.S.-M., L.A.P.-L., C.L.-R., J.G.B.-G. and S.A.G.-R.; validation, R.Á.-R.; formal analysis, N.N.E.-C., R.Á.-R., D.A.S.-M. and S.A.G.-R.; investigation, N.N.E.-C., R.Á.-R. and S.A.G.-R.; writing—original draft, N.N.E.-C.; writing—review and editing, D.A.S.-M., L.A.P.-L., C.L.-R., C.R.-M. and J.G.B.-G.; visualization, L.A.P.-L., C.L.-R., C.R.-M. and J.G.B.-G.; supervision, C.R.-M. and S.A.G.-R.; project administration, R.Á.-R. and S.A.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Autónoma de Nuevo León/Programa de Apoyo a la Ciencia, Tecnología e Innovation, grant numbers 40-BQ-2023 and 66ByQ-2024.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
N.N.E.-C. is thankful to the CONAHCYT (SECIHTI) Mexico Program of Scholarships for Postgraduate Study 792777.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HSV-1 | herpes simplex virus type 1 |
| NPs | polymeric nanoparticles |
| SC | stratum corneum |
| EO | essential oil |
| CRV | carvacrol |
| NP-EOL-R | Lippia graveolens essential oil-loaded nanoparticles purified by evaporation |
| NP-EOL-D | Lippia graveolens essential oil-loaded nanoparticles purified by dialysis |
| NP-BCO-R | nanoparticles without essential oil purified by evaporation |
| NP-BCO-D | nanoparticles without essential oil purified by dialysis |
| PDI | polydispersity index |
| FT-IR | Fourier-transform infrared spectroscopy |
| SEM | scanning electron microscopy |
| IC50 | inhibitory concentration 50 |
| CC50 | cytotoxic concentration 50 |
| SI | selectivity index |
| TEWL | transepidermal water loss |
| SCWC | stratum corneum water content |
References
- Madawi, E.A.; Al Jayoush, R.A.; Rawas-Qalaji, M.; Ei Thu, H.; Khan, S.; Sohail, M.; Mahmood, A.; Hussain, Z. Polymeric nanoparticles as tunable nanocarriers for targeted delivery of drugs to skin tissues for treatment of topical skin diseases. Pharmaceutics 2023, 15, 657. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Hurjui, L.L.; Branisteanu, D.C.; Serban, D.N.; Branisteanu, D.E.; Dima, N.; Serbanm, I.L. Skin—A vast organ with immunological function (review). Exp. Ther. Med. 2020, 20, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, D.; Koe, K.H.; Maharajan, M.K.; Panda, S. A comprehensive overview of epidemiology, pathogenesis and the management of herpes labialis. Viruses 2023, 15, 225. [Google Scholar] [CrossRef] [PubMed]
- Almansorri, A.K.; Al-Shirifi, H.M.H.; Al-Musawi, S.; Ahmed, B.B. Investigation of the inhibition activity of aluminium oxide nanoparticles for herpes simplex type 1. Arch. Razi Inst. 2023, 78, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Center for Drug Evaluation and Research. Recurrent Herpes Labialis: Developing Drugs for Treatment and Prevention; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recurrent-herpes-labialis-developing-drugs-treatment-and-prevention (accessed on 15 July 2025).
- Piperi, E.; Papadopoulou, E.; Georgaki, M.; Dovrat, S.; Illan, M.B.; Nikitakis, N.G.; Yarom, N. Management of oral herpes simplex virus infections: The problem of resistance. A narrative review. Oral Dis. 2023, 30, 877–894. [Google Scholar] [CrossRef]
- Fekete, G.L.; Fekete, L.; Ancuceanu, R.; Ianoși, S.L.; Drăgănescu, M.; Brihan, I. Acyclovir-induced immune thrombocytopenia: Case report and review of the literature. Exp. Ther. Med. 2020, 20, 3417–3420. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of enhancing skin permeability of acyclovir through sterile topical lyophilized wafer on self-dissolving microneedle-treated skin. Dose Response Int. J. 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Andreu, S.; Ripa, I.; Bello-Morales, R.; López-Guerrero, J.A. Valproic acid and its amidic derivatives as new antivirals against alphaherpesviruses. Viruses 2020, 12, 1356. [Google Scholar] [CrossRef]
- Predoi, D.; Groza, A.; Iconaru, S.L.; Predoi, G.; Barbuceanu, F.; Guegan, R.; Motelica-Heino, M.S.; Cimpeanu, C. Properties of basil and lavender essential oils adsorbed on the surface of hydroxyapatite. Materials 2018, 11, 652. [Google Scholar] [CrossRef]
- Orchard, A.; Vuuren, S.V. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid. Based Complement. Altern. Med. 2017, 1, 4517971. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Medina-Romero, Y.M.; Hernandez-Hernandez, A.B.; Rodriguez-Monroy, M.A.; Canales-Martínez, M.M. Essential oils of Bursera morelensis and Lippia graveolens for the development of a new biopesticides in postharvest control. Sci. Rep. 2021, 11, 20135. [Google Scholar] [CrossRef]
- Alam, P.; Imran, M.; Ali, A.; Majid, H. Cananga odorata (Ylang-Ylang) essential oil containing nanoemulgel for the topical treatment of scalp psoriasis and dandruff. Gels 2024, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Rodríguez, S.E.; López-Rivera, R.J.; García-Márquez, E.; Estarrón-Espinosa, M.; Espinosa-Andrews, H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: Impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci. Biotechnol. 2019, 28, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Pilau, M.R.; Alves, S.H.; Weiblen, R.; Arenhart, S.; Cueto, A.P.; Lovato, L.T. Antiviral activity of the Lippia graveolens (Mexican oregano) essential oil and its main compound carvacrol against human and animal viruses. Braz. J. Microbiol. 2011, 42, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Badgujar Vaishnavi, C.; Shinde Ananya, J.; Nehete Jitendra, Y.; Bhambar Rajendra, S. Strategies to improve stability of essential oils. J. Pharm. Sci. Res. 2021, 13, 416–425. [Google Scholar]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent advances in nanomaterials for dermal and transdermal applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Skin penetration and distribution of polymeric nanoparticles. J. Control. Release 2004, 99, 53–62. [Google Scholar] [CrossRef]
- Kalita, S.; Kandimalla, R.; Devi, B.; Kalita, B.; Kalita, K.; Deka, M.; Kataki, A.C.; Sharma, A.; Kotoky, J. Dual delivery of chloramphenicol and essential oil by poly-ε-caprolactone–pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Adv. 2017, 7, 1749–1758. [Google Scholar] [CrossRef]
- González-Moreno, B.J.; Galindo-Rodríguez, S.A.; Rivas-Galindo, V.M.; Pérez-López, L.A.; Granados-Guzmán, G.; Álvarez-Román, R. Enhancement of strawberry shelf life via a multisystem coating based on Lippia graveolens essential oil loaded in polymeric nanocapsules. Polymers 2024, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.B.S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Silva-Mares, D.; Rivas-Galindo, V.M.; Salazar-Aranda, R.; Pérez-Lopez, L.A.; Waksman De Torres, N.; Pérez-Meseguer, J.; Torres-Lopez, E. Screening of north-east Mexico medicinal plants with activities against herpes simplex virus and human cancer cell line. Nat. Prod. Res. 2019, 33, 1531–1534. [Google Scholar] [CrossRef]
- Albuquerque, P.M.; Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Martins Perez, M.T.; Manzato, L. Biotechnological applications of nanoencapsulated essential oils: A review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Álvarez Román, R.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Ofridam, F.; Lebaz, N.; Gagniére, É.; Mangin, D.; Elaissari, A. Polymethylmethacrylate derivatives Eudragit® E100 and L100: Interactions and complexation with surfactants. Polym. Adv. Technol. 2021, 32, 379–390. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for skin applications: Where do we stand? Angew. Chem. Int. Ed. 2022, 134, e202107960. [Google Scholar] [CrossRef]
- Dong, P.; Sahle, F.F.; Lohan, S.B.; Saeidpour, S.; Albrecht, S.; Teutloff, C.; Bodmeier, R.; Unbehauen, M.; Wolff, C.; Haag, R.; et al. pH-sensitive Eudragit® L 100 nanoparticles promote cutaneous penetration and drug release on the skin. J. Control. Release 2019, 295, 214–222. [Google Scholar] [CrossRef]
- Zhang, M.; Qiu, B.; Sun, M.; Wang, Y.; Wei, M.; Gong, Y.; Yan, M. Preparation of Black pepper (Piper nigrum L.) essential oil nanoparticles and its antitumor activity on triple negative breast cancer in vitro. J. Food Biochem. 2022, 46, e14406. [Google Scholar] [CrossRef]
- Silva Flores, P.G. Desarrollo y Evaluación Dermatocinética de Nanopartículas con Aceites Esenciales para Su aplicación en Piel. Doctoral Thesis, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México, 2019. [Google Scholar]
- Dai, X.; Qiang, X.; Hils, C.; Schmalz, H.; Gröschel, A.H. Frustrated microparticle morphologies of a semicrystalline triblock terpolymer in 3D soft confinement. ACS Nano 2021, 15, 1111–1120. [Google Scholar] [CrossRef]
- Chung, T.W.; Huang, Y.Y.; Tsai, Y.L.; Liu, Y.Z. Effects of solvent evaporation rate on the properties of protein-loaded PLLA and PDLLA microspheres fabricated by emulsion-solvent evaporation process. J. Microencapsul. 2002, 19, 463–471. [Google Scholar] [CrossRef]
- Allison, S.D. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic) acid microparticle drug delivery systems. J. Pharm. Sci. 2007, 97, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-loaded chitosan nanospheres from nano-emulsion templating for the topical treatment of herpesviruses infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Enginering 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Chu, B.; Biriukov, D.; Bischoff, M.; Předota, M.; Roke, S.; Marchioro, A. Evolution of the electrical double layer with electrolyte concentration probed by second harmonic scattering. Faraday Discuss. 2023, 246, 407–425. [Google Scholar] [CrossRef]
- Wang, B.; LvYe, J.; Yang, S.; Shi, Y.; Chen, Q. Critical review of food colloidal delivery system for bioactive compounds: Physical characterization and application. Foods 2024, 13, 2596. [Google Scholar] [CrossRef]
- Dikpati, A.; Maio, V.D.P.; Ates, E.; Greffard, K.; Bertrand, N. Studying the stability of polymer nanoparticles by size exclusion chromatography of radioactive polymers. J. Control. Release 2024, 369, 394–403. [Google Scholar] [CrossRef]
- Muthu, M.S.; Feng, S.S. Pharmaceutical stability aspects of nanomedicines. Nanomedicine 2009, 4, 857–860. [Google Scholar] [CrossRef]
- Saadallah, M.S.; Hamid, O.A. Eudragit® L100 nanoparticles for transdermal delivery of rosuvastatin calcium. J. Excip. Food Chem. 2022, 13, 80–93. [Google Scholar]
- Gutiérrez-Pacheco, M.M.; Ortega-Ramírez, L.A.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Lizardi-Mendoza, J.; Miranda, R.; Ayala-Zavala, J.F. Individual and combined coatings of chitosan and Carnauba wax with oregano essential oil to avoid water loss and microbial decay of fresh cucumber. Coatings 2020, 10, 614. [Google Scholar] [CrossRef]
- Leary, J.J.; Wittrock, R.; Sarisky, R.T.; Weinberg, A.; Levin, M.J. Susceptibilities of herpes simplex viruses to penciclovir and acyclovir in eight cell lines. Antimicrob. Agents Chemother. 2002, 46, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Lazov, C.; Spassova, I.; Kovacheva, D.; Pencheva-El Tibi, I.; Stefanova, D.; Tzankova, V.; Petrov, P.D.; Yoncheva, K. Formulation of budesonide-loaded polymeric nanoparticles into hydrogels for local therapy of atopic dermatitis. Gels 2024, 10, 79. [Google Scholar] [CrossRef]
- Velandia, S.A.; Quintero, E.; Stashenko, E.E.; Ocazionez, R.E. Actividad antiproliferativa de aceites esenciales de plantas cultivadas en colombia. Acta Biológica Colomb. 2017, 23, 189–198. [Google Scholar] [CrossRef]
- Nakamura de Vasconcelos, S.S.; Caleffi-Ferracioli, K.R.; Hegeto, L.A.; Baldin, V.P.; Nakamura, C.V.; Stefanello, T.F.; Freitas Gauze, G.; Yamazaki, D.A.S.; Scodro, R.B.L.; Siqueira, V.L.D.; et al. Carvacrol activity & morphological changes in Mycobacterium tuberculosis. Future Microbiol. 2018, 13, 877–888. [Google Scholar]
- Damayanti, S.; Permana, J.; Tjahjono, D.H. The use of computational chemistry to predict toxicity of antioxidants food additives and its metabolites as a reference for food safety regulation. Sch. Res. Libr. 2015, 7, 174–181. [Google Scholar]
- Monzote, L.; Stamberg, W.; Staniek, K.; Gille, L. Toxic effects of carvacrol, caryophyllene oxide, and ascaridole from essential oil of Chenopodium ambrosioides on mitochondria. Toxicol. Appl. Pharmacol. 2009, 240, 337–347. [Google Scholar] [CrossRef]
- Landry, M.L.; Stanat, S.; Biron, K.; Brambilla, D.; Britt, W.; Jokela, J.; Chou, S.; Drew, W.L.; Erice, A.; Gilliam, B.; et al. A standardized plaque reduction assay for determination of drug susceptibilities of cytomegalovirus clinical isolates. Antimicrob. Agents Chemother. 2000, 44, 688–692. [Google Scholar] [CrossRef]
- Dolskiy, A.A.; Grishchenko, I.V.; Yudkin, D.V. Cell cultures for virology: Usability, advantages, and prospects. Int. J. Mol. Sci. 2020, 21, 7978. [Google Scholar] [CrossRef]
- Akkutay-Yoldar, Z.; Yoldar, M.T.; Akkaş, Y.B.; Şurak, S.; Garip, F.; Turan, O.; Ekizoğlu, B.; Yüca, O.C.; Özkul, A.; Ünver, B. A web-based artificial intelligence system for label-free virus classification and detection of cytopathic effects. Sci. Rep. 2025, 15, 5904. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Dobreva, A.; Doynovska, R.; Krastev, D.; Mileva, M. Antiviral activity of Rosa damascena Mill. and Rosa alba L. essential oils against the multiplication of herpes simplex virus type 1 strains sensitive and resistant to acyclovir. Biology 2021, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Antiviral efficacy and mechanisms of action of oregano essential oil and its primary component carvacrol against murine norovirus. J. Appl. Microbiol. 2014, 116, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.L.; Chuang, H.S.; Lee, M.H.; Wei, C.L.; Lin, C.F.; Tsai, Y.C. Inhibition of herpes simplex virus type 1 by thymol-related monoterpenoids. Planta Med. 2012, 78, 1636–1638. [Google Scholar] [CrossRef]
- Cura Bermudez, P.M. Análisis In Silico de la Interacción de los Compuestos Mayoritarios del Aceite Esencial de Orégano (Lippia sp.) con las Proteínas de Envoltura del Herpes Simple Tipo 1 y Evaluación Toxicológica. Bachelor’s Thesis, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México, 2024. [Google Scholar]
- Yadavalli, T.; Mallick, S.; Patel, P.; Koganti, R.; Shukla, D.; Date, A.A. Pharmaceutically acceptable carboxylic acid-terminated polymers show activity and selectivity against HSV-1 and HSV-2 and synergy with antiviral drugs. ACS Infect. Dis. 2020, 6, 2926–2937. [Google Scholar] [CrossRef]
- Reichling, J. Antiviral and virucidal properties of essential oils and isolated compounds—A scientific approach. Planta Med. 2022, 88, 587–603. [Google Scholar] [CrossRef]
- Hwang, J.H.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.M. Ex vivo live full-thickness porcine skin model as a versatile in vitro testing method for skin barrier research. Int. J. Mol. Sci. 2021, 22, 657. [Google Scholar] [CrossRef]
- Uhm, C.; Jeong, H.; Hyon, S.; Hwang, J.S.; Lim, K.M.; Nam, K.T. Comparison of structural characteristics and molecular markers of rabbit skin, pig skin, and reconstructed human epidermis for an ex vivo human skin model. Toxicol. Res. 2023, 39, 477–484. [Google Scholar] [CrossRef]
- Klotz, T.; Maddern, G.; Caplash, Y.; Wagstaff, M. Devices measuring transepidermal water loss of the skin: A systematic review protocol of measurement properties. Skin Res. Technol. 2021, 28, 497–539. [Google Scholar] [CrossRef]
- Green, M.; Kashetsky, N.; Feschuk, A.; Maibach, H.I. Transepidermal water loss (TEWL): Environment and pollution—A systematic review. Skin Health Dis. 2022, 2, e104. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Crespo, A.M.; Vaz, F.; Filipe, M.; Santos, D.; Jacinto, T.A.; Paiva-Santos, A.C.; Rodrigues, M.; Ribeiro, M.P.; Coutinho, P.; et al. Development and characterization of thermal water gel comprising Helichrysum italicum essential oil-loaded chitosan nanoparticles for skin care. Cosmetics 2023, 10, 8. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin barrier function: The interplay of physical, chemical, and immunologic properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Silva-Flores, P.G.; Galindo-Rodríguez, S.A.; Pérez-López, L.A.; Álvarez-Román, R. Development of essential oil-loaded polymeric nanocapsules as skin delivery systems: Biophysical parameters and dermatokinetics ex vivo evaluation. Molecules 2023, 28, 7142. [Google Scholar] [CrossRef] [PubMed]
- Logger, J.G.M. General introduction and thesis outline. In Noninvasive Assessment of Healthy and Inflamed Skin; Universitair Medisch Centrum Utrecht: Utrecht, The Netherlands, 2024. [Google Scholar]
- Bari, D.S.; Ali, Z.K.; Hameed, S.A.; Aldosky, H.Y.Y. Evaluation of the effect of several moisturizing creams using the low frequency electrical susceptance approach. J. Electr. Bioimpedance 2024, 15, 4–9. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, Y.; Kim, S.S.; Min Ju, H.; Baek, J.H.; Park, C.S.; Lee, D.H. Inhibitory effect of corn silk on skin pigmentation. Molecules 2014, 19, 2808–2818. [Google Scholar] [CrossRef]
- Maddaleno, A.S.; Camargo, J.; Mitjans, M.; Vinardell, M.P. Melanogenesis and melasma treatment. Cosmetics 2021, 8, 82. [Google Scholar] [CrossRef]
- Cho, Y.H. Codonopsis pilosula extract protects melanocytes against H2O2-induced oxidative stress by activating autophagy. Cosmetics 2021, 8, 67. [Google Scholar] [CrossRef]
- Lim, T.W.; Lee, M.H. A study of skin color by melanin index according to sex, age, site and skin phototype in koreans. Ann. Dermatol. 2002, 14, 71. [Google Scholar] [CrossRef]
- Dave, B.H.; Thomas, P.S.; Joshi, P.B.; Shah, P.S. Prevalence of oral melanin pigmentation among children of 4-14 years of age and its association with passive smoking. J. South. Asian Assoc. Pediatr. Dent. 2020, 3, 19–22. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Natasha, J.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract—Containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef]
- Setaro, M.; Sparavigna, A. Quantification of erythema using digital camera and computer-based colour image analysis: A multicentre study. Skin Res. Technol. 2002, 8, 84–88. [Google Scholar] [CrossRef]
- Abdlaty, R.; Hayward, J.; Farrell, T.; Fang, Q. Skin erythema and pigmentation optical assessment techniques: Review article. Photodiagnosis Photodyn. Ther. 2020, 33, 102127. [Google Scholar] [CrossRef]
- Yotsawimonwat, S.; Rattanadechsakul, J.; Rattanadechsakul, P.; Okonogi, S. Skin improvement and stability of Echinacea purpurea dermatological formulations. Int. J. Cosmet. Sci. 2010, 32, 340–346. [Google Scholar] [CrossRef]
- Puentes Ossa, A.; Rodriguez Patarroyo, D.J.; Salamanca Bernal, J.A. Computational modeling of light scattering in polymer nanoparticles for optical characterization. Cienc. E Ing. Neogranadina 2024, 34, 63–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).