Non-Competitive Binding of Isatuximab and Daratumumab to CD38: Implications for Targeted Therapy in Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Reagents and BM Samples
2.2. Assessment of Daratumumab and Isatuximab Binding
2.3. CD38 Recovery Assay
2.4. Viability Assay
2.5. Migration and Adhesion Assays
2.6. Statistics
3. Results
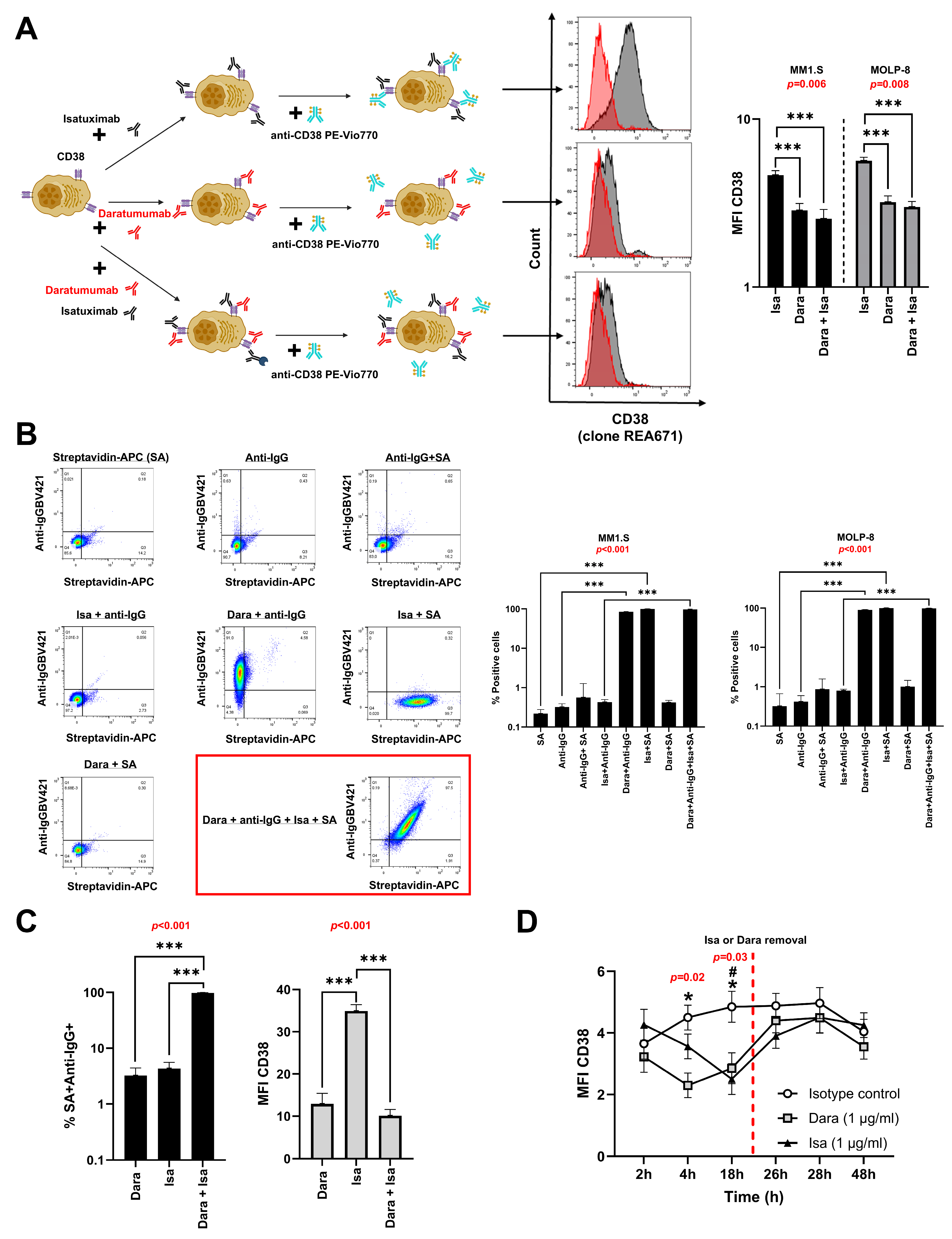
3.1. Isatuximab and Daratumumab Do Not Compete for Binding to CD38 on MM Cell Lines
3.2. Isatuximab and Daratumumab Do Not Compete for Binding of CD38 on BM Cells from MM Patients
3.3. CD38 Expression Rapidly Recovers on MM Cells Following Antibody Removal
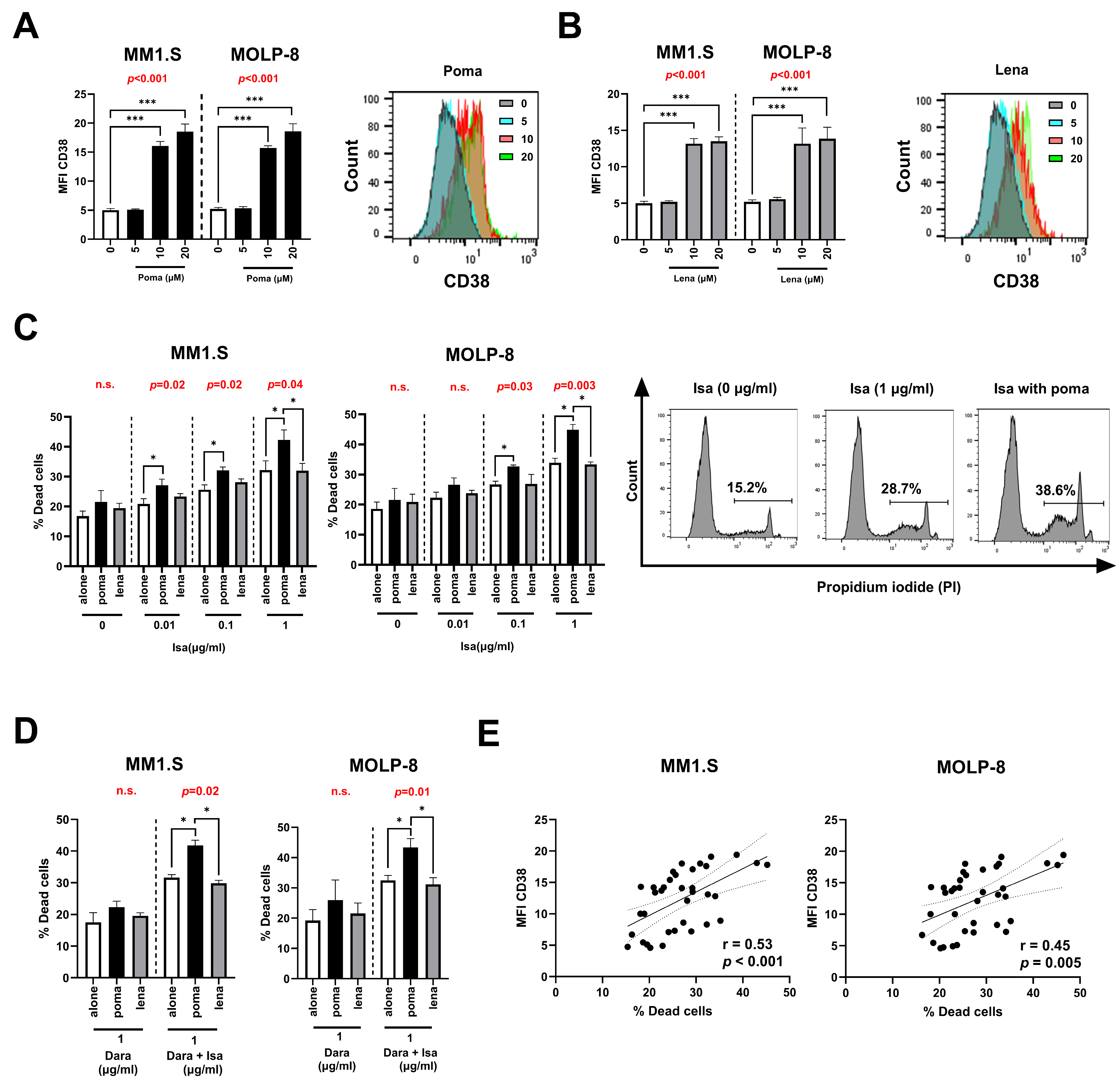
3.4. Pomalidomide Increases CD38 Levels and Enhance Isatuximab Apoptosis in MM Cell Lines
3.5. Isatuximab Interferes with In Vitro Migration and Cell Adhesion of MM Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| IMiDs | Immunomodulatory drugs |
| PIs | Proteasome inhibitors |
| RRMM | Relapsed/refractory MM |
| BM | Bone marrow |
| MFI | Mean fluorescence intensity |
| RT | Room temperature |
| RBC | Red blood cell |
| PI | Propidium iodide |
| CFSE | Carboxyfluorescein-succinimidyl ester |
| VLA-4 | Very late antigen-4 |
| VCAM-1 | Vascular-cell adhesion molecule-1 |
| Treg | Regulatory T cell |
| BSA | Bovine serum albumin |
| RFUs | Relative fluorescence units |
| IQR | Interquartile range |
| ANOVA | Analysis of variance |
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Skerget, S.; Penaherrera, D.; Chari, A.; Jagannath, S.; Siegel, D.S.; Vij, R.; Orloff, G.; Jakubowiak, A.; Niesvizky, R.; Liles, D.; et al. Comprehensive molecular profiling of multiple myeloma identifies refined copy number and expression subtypes. Nat. Genet. 2024, 56, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, Z.X.; Xie, H.; Xie, P.L. Dimethyl Fumarate Augments Anticancer Activity of Angstrom Silver Particles in Myeloma Cells through NRF2 Activation. Adv. Ther. 2025, 8, 2400363. [Google Scholar]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef]
- Morandi, F.; Horenstein, A.L.; Costa, F.; Giuliani, N.; Pistoia, V.; Malavasi, F. CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma. Front. Immunol. 2018, 9, 2722. [Google Scholar] [CrossRef]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-de-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 597959. [Google Scholar] [CrossRef]
- Li, Y.; Yang, R.; Chen, L.; Wu, S. CD38 as an Immunomodulator in Cancer. Future Oncol. 2020, 16, 2853–2861. [Google Scholar] [CrossRef]
- Van de Donk, N.W.; Janmaat, M.L.; Mutis, T.; Lammerts van Bueren, J.J.; Ahmadi, T.; Sasser, A.K.; Lokhorst, H.M.; Parren, P.W. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 2016, 270, 95–112. [Google Scholar] [CrossRef]
- Liu, Q.; Kriksunov, I.A.; Graeff, R.; Munshi, C.; Lee, H.C.; Hao, Q. Crystal Structure of Human CD38 Extracellular Domain. Structure 2005, 13, 1331–1339. [Google Scholar] [CrossRef]
- Van de Donk, N.W.C.J.; Richardson, P.G.; Malavasi, F. CD38 antibodies in multiple myeloma: Back to the future. Blood 2018, 131, 13–29. [Google Scholar] [CrossRef]
- Gozzetti, A.; Ciofini, S.; Simoncelli, M.; Santoni, A.; Pacelli, P.; Raspadori, D.; Bocchia, M. Anti CD38 monoclonal antibodies for multiple myeloma treatment. Hum. Vaccin. Immunother. 2022, 18, 2052658. [Google Scholar] [CrossRef]
- Hogan, K.A.; Chini, C.C.S.; Chini, E.N. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front. Immunol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, X.; Liu, Y.; Tang, S.; Miao, J.; Zhou, Q.; Chen, S. IL-1β-induced NF-κB activation down-regulates miR-506 expression to promotes osteosarcoma cell growth through JAG1. Biomed. Pharmacother. 2017, 95, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, H.; Sun, Z.; Zhang, J. Inhibition of Multiple Myeloma Growth by Wogonin Involves Mitochondrial Apoptosis and G2/M Cycle Arrest. Int. J. Pharmacol. 2024, 20, 166–173. [Google Scholar] [CrossRef]
- Matas-Céspedes, A.; Vidal-Crespo, A.; Rodriguez, V.; Villamor, N.; Delgado, J.; Giné, E.; Roca-Ho, H.; Menéndez, P.; Campo, E.; López-Guillermo, A.; et al. The Human CD38 Monoclonal Antibody Daratumumab Shows Antitumor Activity and Hampers Leukemia–Microenvironment Interactions in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2017, 23, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Vaisitti, T.; Aydin, S.; Rossi, D.; Cottino, F.; Bergui, L.; D’Arena, G.; Bonello, L.; Horenstein, A.L.; Brennan, P.; Pepper, C.; et al. CD38 increases CXCL12-mediated signals and homing of chronic lymphocytic leukemia cells. Leukemia 2010, 24, 958–969. [Google Scholar] [CrossRef]
- Zucchetto, A.; Vaisitti, T.; Benedetti, D.; Tissino, E.; Bertagnolo, V.; Rossi, D.; Bomben, R.; Dal Bo, M.; Del Principe, M.I.; Gorgone, A.; et al. The CD49d/CD29 complex is physically and functionally associated with CD38 in B-cell chronic lymphocytic leukemia cells. Leukemia 2012, 26, 1301–1312. [Google Scholar] [CrossRef]
- Martin, T.G.; Corzo, K.; Chiron, M.; Velde, H.V.; Abbadessa, G.; Campana, F.; Solanki, M.; Meng, R.; Lee, H.; Wiederschain, D.; et al. Therapeutic Opportunities with Pharmacological Inhibition of CD38 with Isatuximab. Cells 2019, 12, 1522. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Martino, E.A.; Derudas, D.; Rossi, E.; Stefanoni, P.; Mangiacavalli, S.; Zamagni, E.; Offidani, M.; Furlan, A.; Quinto, A.M.; Della Pepa, R.; et al. Efficacy and Prognostic Indicators of Isatuximab, Pomalidomide, and Dexamethasone (IsaPd) in Daratumumab-Refractory Multiple Myeloma Patients: A Multicenter Real-World Study. Hematol. Oncol. 2025, 43, e70042. [Google Scholar]
- Becnel, M.R.; Horowitz, S.B.; Thomas, S.K.; Iyer, S.P.; Patel, K.K.; Manasanch, E.E.; Weber, D.M.; Kaufman, G.P.; Lee, H.C.; Orlowski, R.Z. Descriptive Analysis of Isatuximab Use Following Prior Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 20–21. [Google Scholar] [CrossRef]
- Mikhael, J.; Belhadj-Merzoug, K.; Hulin, C.; Vincent, L.; Moreau, P.; Gasparetto, C.; Pour, L.; Spicka, I.; Vij, R.; Zonder, J.; et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J. 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- De Weers, M.; Tai, Y.-T.; van der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.H.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W.; et al. Daratumumab, a Novel Therapeutic Human CD38 Monoclonal Antibody, Induces Killing of Multiple Myeloma and Other Hematological Tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Deckert, J.; Wetzel, M.-C.; Bartle, L.M.; Skaletskaya, A.; Goldmacher, V.S.; Vallée, F.; Zhou-Liu, Q.; Ferrari, P.; Pouzieux, S.; Lahoute, C.; et al. SAR650984, A Novel Humanized CD38-Targeting Antibody, Demonstrates Potent Antitumor Activity in Models of Multiple Myeloma and Other CD38+ Hematologic Malignancies. Clin. Cancer Res. 2014, 20, 4574–4583. [Google Scholar] [CrossRef] [PubMed]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.C.J.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, L.; Acharya, C.; An, G.; Wen, K.; Qiu, L.; Munshi, N.C.; Tai, Y.-T.; Anderson, K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin. Cancer Res. 2017, 23, 4290–4300. [Google Scholar] [CrossRef]
- Kitadate, A.; Kobayashi, H.; Abe, Y.; Narita, K.; Miura, D.; Takeuchi, M.; Matsue, K. Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica 2020, 105, e37–e40. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Anderson, K.C. Targeting CD38 alleviates tumor-induced immunosuppression. Oncotarget 2017, 8, 112166–112167. [Google Scholar] [CrossRef]
- Zhu, C.; Song, Z.; Wang, A.; Srinivasan, S.; Yang, G.; Greco, R.; Theilhaber, J.; Shehu, E.; Wu, L.; Yang, Z.-Y.; et al. Isatuximab Acts Through Fc-Dependent, Independent, and Direct Pathways to Kill Multiple Myeloma Cells. Front. Immunol. 2020, 11, 1771. [Google Scholar] [CrossRef]
- Lammerts van Bueren, J.; Jakobs, D.; Kaldenhoven, N.; Roza, M.; Hiddingh, S.; Meesters, J.; Voorhorst, M.; Gres-nigt, E.; Wiegman, L.; Ortiz Buijsse, A.; et al. Direct in Vitro Comparison of Daratumumab with Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood 2014, 124, 3474. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [PubMed]
- Lee, H.T.; Kim, Y.; Park, U.B.; Jeong, T.J.; Lee, S.H.; Heo, Y.-S. Crystal structure of CD38 in complex with daratumumab, a first-in-class anti-CD38 antibody drug for treating multiple myeloma. Biochem. Biophys. Res. Commun. 2021, 536, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Malerba, E.; Leone, P.; Prete, M.; Terragna, C.; Cavo, M.; Racanelli, V. Drug resistance in multiple myeloma: Soldiers and weapons in the bone marrow niche. Front. Oncol. 2022, 12, 973836. [Google Scholar] [CrossRef]
- Ghose, J.; Viola, D.; Terrazas, C.; Caserta, E.; Troadec, E.; Khalife, J.; Gunes, E.G.; Sanchez, J.; McDonald, T.; Marcucci, G.; et al. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology 2018, 7, e1486948. [Google Scholar] [CrossRef]
- Oberle, A.; Brandt, A.; Alawi, M.; Langebrake, C.; Janjetovic, S.; Wolschke, C.; Schütze, K.; Bannas, P.; Kröger, N.; Koch-Nolte, F.; et al. Long-term CD38 saturation by daratumumab interferes with diagnostic myeloma cell detection. Haematologica 2017, 102, e368–e370. [Google Scholar] [CrossRef]
- Boxhammer, R.; Steidl, S.; Endell, J. Effect of IMiD compounds on CD38 expression on multiple myeloma cells: MOR202, a human CD38 antibody in combination with pomalidomide. J. Clin. Oncol. 2015, 33, 8588. [Google Scholar] [CrossRef]
- Lopez-Girona, A.; Mendy, D.; Ito, T.; Miller, K.; Gandhi, A.K.; Kang, J.; Karasawa, S.; Carmel, G.; Jackson, P.; Ab-basian, M.; et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia 2012, 26, 2326–2335. [Google Scholar] [CrossRef]
- Gertz, M.A. Pomalidomide and myeloma meningitis. Leuk. Lymphoma 2013, 54, 681–682. [Google Scholar] [CrossRef]
- Leleu, X.; Delea, T.; Guyot, P.; Moynahan, A.; Singh, E.; Tekle, C.; Lin, P.L. Updated Results from a Matching-Adjusted Indirect Comparison of Efficacy Outcomes for Isatuximab Plus Pomalidomide and Dexamethasone Versus Daratumumab Plus Pomalidomide and Dexamethasone in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood 2023, 142, 6732. [Google Scholar] [CrossRef]
- Leleu, X.; Martin, T.; Weisel, K.; Schjesvold, F.; Iida, S.; Malavasi, F.; Manier, S.; Min, C.-K.; Ocio, E.M.; Pawlyn, C.; et al. Anti-CD38 antibody therapy for patients with relapsed/refractory multiple myeloma: Differential mechanisms of action and recent clinical trial outcomes. Ann. Hematol. 2022, 101, 2123–2137. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuna-Gómez, R.; López-Pardo, J.; Mulet, M.; Nomdedéu, J.; Cantó, E.; Ortin, R.; Cayuela, Á.S.; Ortiz, M.À.; Guinart-Cuadra, A.; Vidal, S. Non-Competitive Binding of Isatuximab and Daratumumab to CD38: Implications for Targeted Therapy in Multiple Myeloma. Pharmaceutics 2025, 17, 1278. https://doi.org/10.3390/pharmaceutics17101278
Osuna-Gómez R, López-Pardo J, Mulet M, Nomdedéu J, Cantó E, Ortin R, Cayuela ÁS, Ortiz MÀ, Guinart-Cuadra A, Vidal S. Non-Competitive Binding of Isatuximab and Daratumumab to CD38: Implications for Targeted Therapy in Multiple Myeloma. Pharmaceutics. 2025; 17(10):1278. https://doi.org/10.3390/pharmaceutics17101278
Chicago/Turabian StyleOsuna-Gómez, Rubén, Jordi López-Pardo, Maria Mulet, Josep Nomdedéu, Elisabet Cantó, Rosa Ortin, Ángela Sánchez Cayuela, Ma Àngels Ortiz, Albert Guinart-Cuadra, and Silvia Vidal. 2025. "Non-Competitive Binding of Isatuximab and Daratumumab to CD38: Implications for Targeted Therapy in Multiple Myeloma" Pharmaceutics 17, no. 10: 1278. https://doi.org/10.3390/pharmaceutics17101278
APA StyleOsuna-Gómez, R., López-Pardo, J., Mulet, M., Nomdedéu, J., Cantó, E., Ortin, R., Cayuela, Á. S., Ortiz, M. À., Guinart-Cuadra, A., & Vidal, S. (2025). Non-Competitive Binding of Isatuximab and Daratumumab to CD38: Implications for Targeted Therapy in Multiple Myeloma. Pharmaceutics, 17(10), 1278. https://doi.org/10.3390/pharmaceutics17101278







