Formulation of Self-Emulsifying Microemulsion for Acemetacin Using D-Optimal Design: Enteric-Coated Capsule for Targeted Intestinal Release and Bioavailability Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening of Components for ACM-SEME
2.3. Construction of Pseudo-Ternary Phase Diagram
2.4. Experimental Design for Optimization of Liquid ACM-SEME
2.5. Preparation of Acemetacin Self-Micro-Emulsifying Drug Delivery Systems
2.6. Characterization of the Prepared Liquid ACM-SEME
2.6.1. Thermodynamic Stability Study
Centrifugation Test
Heating-Cooling Cycle
Freezing–Thawing Test
2.6.2. Globule Size and Polydispersity Index Determination
2.6.3. Determination of ACM Content in Liquid ACM-SEME
2.6.4. In Vitro Release of Liquid ACM-SEME
2.6.5. Percentage Transmittance (T%)
2.6.6. Zeta Potential of the Optimized Liquid ACM-SEME Formulation
2.6.7. Drying of Liquid ACM-SEME Optimized Formulation
2.6.8. Characterization of the Dried ACM-SEME Optimized Formulation
Morphological Analysis of Dried ACM-SEME Optimized Formulation
FTIR Determination
X-Ray Diffraction (XRD)
Differential Scanning Calorimetry
Capsule Filling and Coating
In Vitro Release Study of Eudragit L100-Coated Capsules Filled with Spray-Dried ACM-SEME
Effect of ACM on the Noxious Time Threshold on Rats
Ex Vivo Permeation Study
3. Results and Discussion
3.1. Screening and Selection of ACM-SEME Components
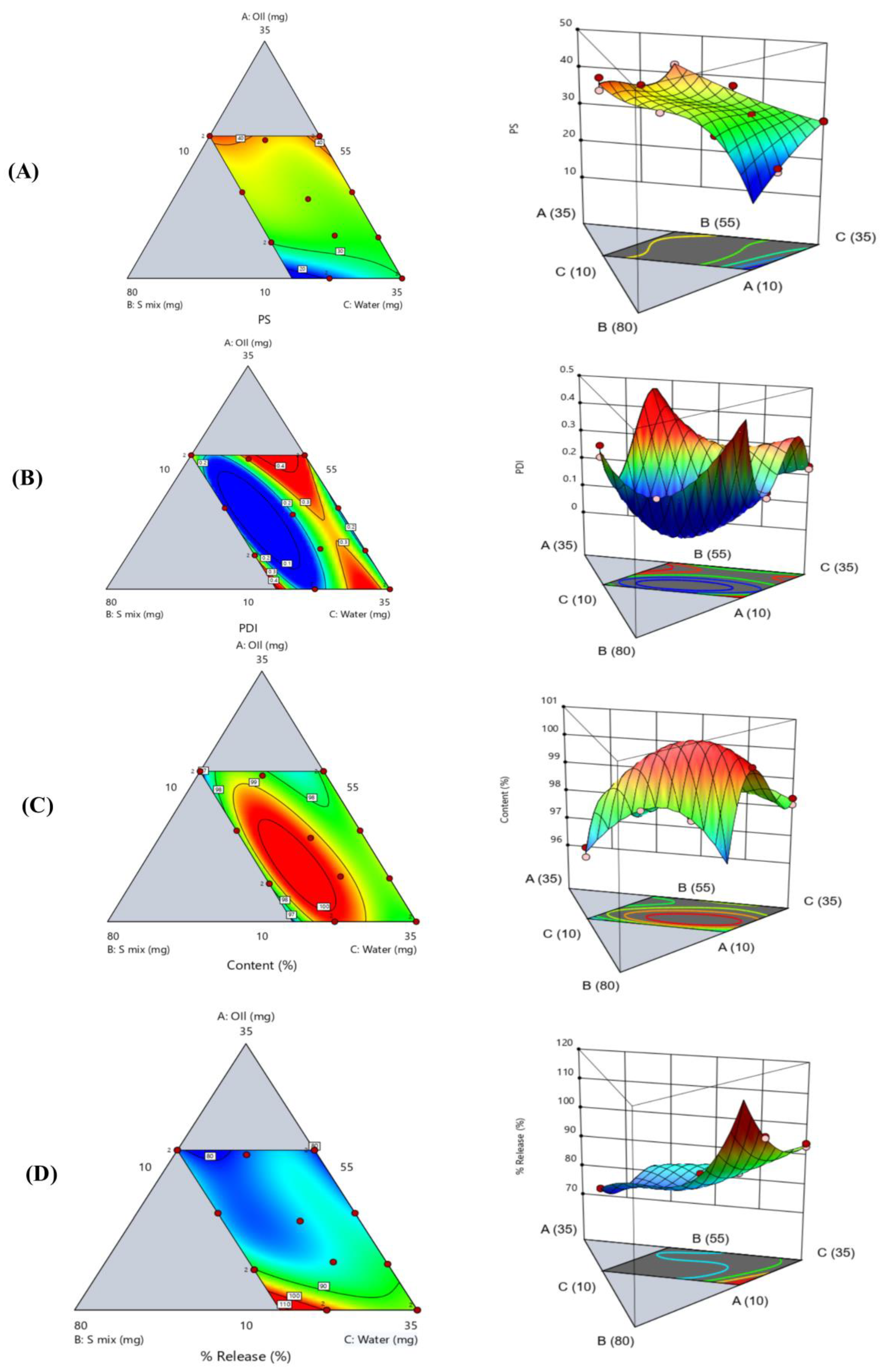
3.2. Pseudo-Ternary Phase Diagram and Microemulsion Region
3.3. Thermodynamic Stability of Liquid ACM-SEME
3.4. Experimental Design for Optimization of Liquid ACM-SEME
3.5. Globule Size and Polydispersity Index
3.6. ACM Content in Liquid ACM-SEME
3.7. In Vitro Release of Liquid ACM-SEME
3.8. Percentage Transmittance (T%) and Zeta Potential
3.9. FTIR Spectral Results and Interpretation
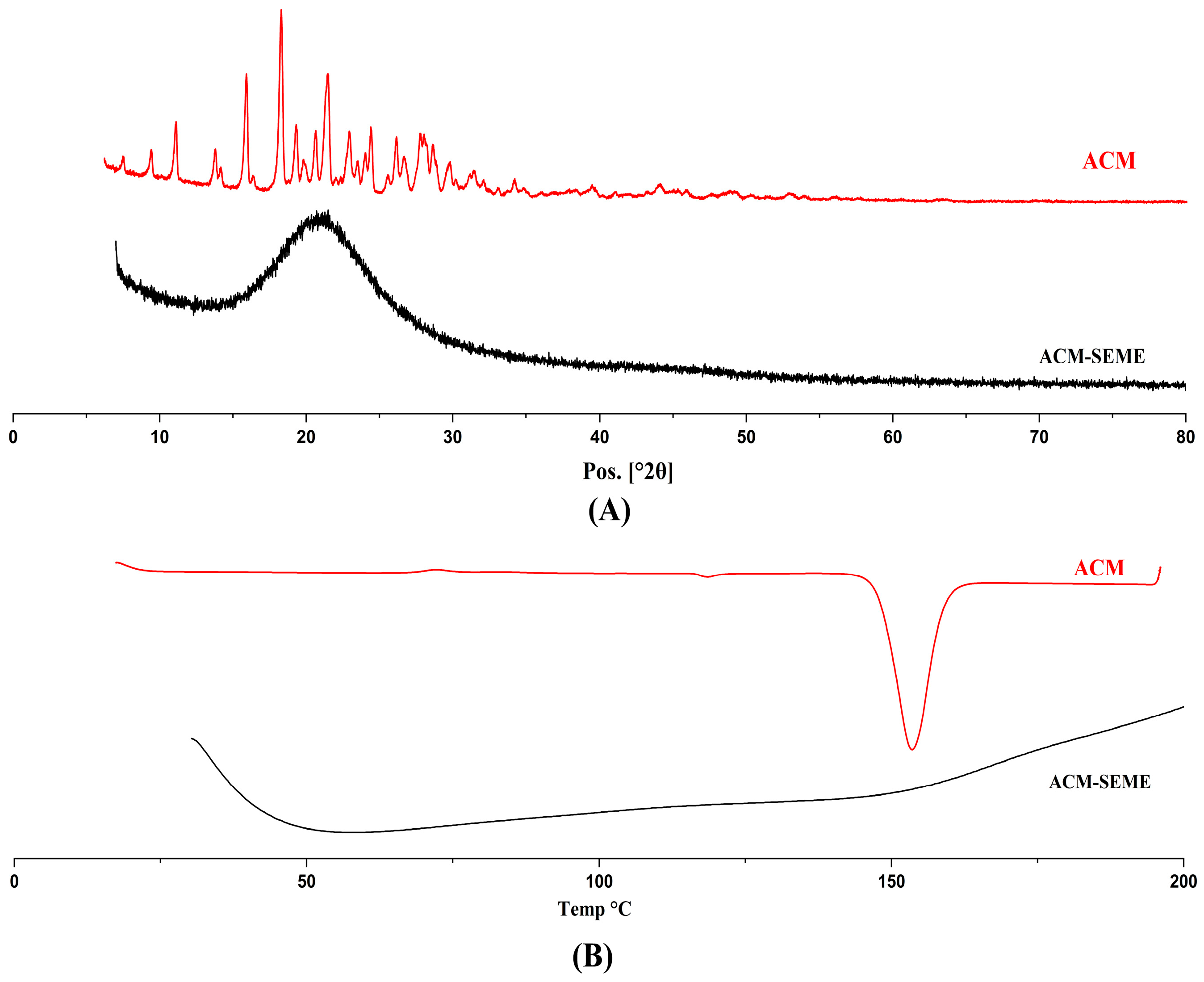
3.10. XRD and DSC
3.11. Morphological Analysis of Dried ACM-SEME Optimized Formulation
3.12. In Vitro Release Study of Eudragit L100-Coated Capsules Filled with Spray-Dried ACM-SEME
3.13. Effect of ACM on the Noxious Time Threshold on Rats
3.14. Ex Vivo Permeation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACM | Acemetacin |
| SEME | Self-Emulsifying Microemulsion |
| UV | Ultraviolet |
| NSAID | Non-steroidal anti-inflammatory drug |
| COX | Cyclooxygenase |
| PEG400 | Polyethylene Glycol 400 |
| PEG600 | Polyethylene Glycol 600 |
| PS | Particle size |
| PDI | Polydispersity Index |
| FTIR | Fourier Transform Infrared Spectroscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
| XRD | X-ray diffraction |
| DSC | Differential Scanning calorimetry |
| 3D | Three-Dimensional |
| SD | Standard Deviation |
| PBS | Phosphate-Buffered saline |
| USP | United States pharmacopeia |
References
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Chaudhry, W.R.; Akbar, A. Effects of non-steroidal anti-inflammatory drugs (NSAIDs) and gastroprotective NSAIDs on the gastrointestinal tract: A narrative review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Chang, C.-H.; Chen, H.-C.; Lin, J.-W.; Kuo, C.-W.; Shau, W.-Y.; Lai, M.-S. Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: A nationwide case-crossover study in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 763–771. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, P.; Wang, Y.; Jiang, X.; Fu, Q. Co-Amorphization of Acemetacin with Basic Amino Acids as Co-Formers for Solubility Improvement and Gastric Ulcer Mitigation. Pharmaceutics 2024, 16, 745. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Derry, S.; McQuay, H.J. Single dose oral acemetacin for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2009, 2019, CD007589. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharani, H.; Al-Kinani, K. Formulation and in vitro Evaluation of Acemetacin Nanosuspension. Iraqi J. Pharm. Sci. 2024, 33, 133–146. [Google Scholar] [CrossRef]
- Gelbrich, T.; Haddow, M.; Griesser, U. Acemetacin monohydrate. Cryst. Struct. Commun. 2007, 63, o451–o453. [Google Scholar] [CrossRef]
- Pattewar, S.; Kasture, S.; Pande, V.; Sharma, S. Self microemulsifying drug delivery system: A lipid based drug delivery system. Int. J. Pharm. Sci. Res. 2016, 7, 443. [Google Scholar] [CrossRef]
- Feng, W.; Qin, C.; Abdelrazig, S.; Bai, Z.; Raji, M.; Darwish, R.; Chu, Y.; Ji, L.; Gray, D.A.; Stocks, M.J. Vegetable oils composition affects the intestinal lymphatic transport and systemic bioavailability of co-administered lipophilic drug cannabidiol. Int. J. Pharm. 2022, 624, 121947. [Google Scholar] [CrossRef]
- Ande, N.S.; Sonone, B.K.; Bakal, L.R.; Ajmire, V.P.; Sawarkar, S.H. Role of surfactant and co-surfactant in microemulsion: A review. Res. J. Pharm. Technol. 2022, 15, 4829–4834. [Google Scholar] [CrossRef]
- Uttreja, P.; Karnik, I.; Adel Ali Youssef, A.; Narala, N.; Elkanayati, R.M.; Baisa, S.; Alshammari, N.D.; Banda, S.; Vemula, S.K.; Repka, M.A. Self-Emulsifying Drug Delivery Systems (SEDDS): Transition from Liquid to Solid—A Comprehensive Review of Formulation, Characterization, Applications, and Future Trends. Pharmaceutics 2025, 17, 63. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; El-Din, A.S.S.; Hassan, H.M.; Sayed, A.M.; Alhadrami, A.H.; Rateb, M.E.; Naguib, D.M. Development and evaluation of a self-nanoemulsifying drug delivery system for sinapic acid with improved antiviral efficacy against SARS-CoV-2. Pharmaceutics 2023, 15, 2531. [Google Scholar] [CrossRef]
- Al-Tamimi, D.J.; Hussein, A.A. Formulation and characterization of self-microemulsifying drug delivery system of tacrolimus. Iraqi J. Pharm. Sci. 2021, 30, 91–100. [Google Scholar] [CrossRef]
- Jones, B.; Allen-Moyer, K.; Goos, P. A-optimal versus D-optimal design of screening experiments. J. Qual. Technol. 2021, 53, 369–382. [Google Scholar] [CrossRef]
- Hussein, A.A. Preparation and evaluation of liquid and solid self-microemulsifying drug delivery system of mebendazole. Iraqi J. Pharm. Sci. 2014, 23, 89–100. [Google Scholar] [CrossRef]
- Abdullah, T.M.; Khalid, K.A.-K. Topical propranolol hydrochloride nanoemulsion: A promising approach drug delivery for infantile hemangiomas. Iraqi J. Pharm. Sci. 2023, 32, 300–315. [Google Scholar] [CrossRef]
- Muhammed, S.A.; Al-Kinani, K.K. Formulation and in vitro evaluation of meloxicam as a self-microemulsifying drug delivery system. F1000Research 2023, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, L.; Zhang, Y.; Li, X.; Liu, C.; Jiang, B.; Xu, J.; Sun, Z. Formation of whey protein isolate nanofibrils by endoproteinase GluC and their emulsifying properties. Food Hydrocoll. 2019, 94, 71–79. [Google Scholar] [CrossRef]
- Jabir, S.A.; Rajab, N.A. Preparation, In-vitro, Ex-vivo, and Pharmacokinetic Study of Lasmiditan as Intranasal Nanoemulsion-based In Situ Gel. Pharm. Nanotechnol. 2025, 13, 239–253. [Google Scholar] [CrossRef]
- Bansode, S.T.; Kshirsagar, S.J.; Madgulkar, A.R.; Bhalekar, M.R.; Bandivadekar, M.M. Design and development of SMEDDS for colon-specific drug delivery. Drug Dev. Ind. Pharm. 2016, 42, 611–623. [Google Scholar] [CrossRef]
- Abd-Alhammid, S.N.; Kadhum, R.W. Process Factors Affecting the Preparation and Characterization of Dutasteride Nanosuspension. Iraqi J. Pharm. Sci. 2025, 34, 35–48. [Google Scholar]
- Al-Ameri, A.A.F.; Al-Gawhari, F.J. Formulation development of meloxicam binary ethosomal hydrogel for topical delivery: In vitro and in vivo assessment. Pharmaceutics 2024, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Rajab, N.A.; Sulaiman, H.T. Olmesartan Medoxomil Nanomicelle Using Soluplus for Dissolution Enhancement: Preparation, In-vitro and Ex-vivo Evaluation. Iraqi J. Pharm. Sci. 2025, 34, 47–59. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Kouchak, M.; Fatahiasl, J.; Angali, K.A.; Ramezani, Z.; Amini, M.; Dorkoosh, F.A.; Handali, S. Effects of coating layer and release medium on release profile from coated capsules with Eudragit FS 30D: An in vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, H.J. Eudragit L-100 for enteric coating of hard gelatine capsules: Formulation and evaluation. Issues Biol. Sci. Pharm. Res. 2015, 3, 14–20. [Google Scholar] [CrossRef]
- McConnell, E.L.; Short, M.D.; Basit, A.W. An in vivo comparison of intestinal pH and bacteria as physiological trigger mechanisms for colonic targeting in man. J. Control. Release 2008, 130, 154–160. [Google Scholar] [CrossRef]
- Çağlar, E.Ş.; Okur, M.E.; Aksu, B.; Üstündağ Okur, N. Transdermal delivery of acemetacin loaded microemulsions: Preparation, characterization, in vitro—Ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy. J. Dispers. Sci. Technol. 2024, 45, 662–672. [Google Scholar] [CrossRef]
- Attari, Z.; Bhandari, A.; Jagadish, P.; Lewis, S. Enhanced ex vivo intestinal absorption of olmesartan medoxomil nanosuspension: Preparation by combinative technology. Saudi Pharm. J. 2016, 24, 57–63. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; AlOmrani, A.H.; B Yassin, A.E. Novel sulpiride-loaded solid lipid nanoparticles with enhanced intestinal permeability. Int. J. Nanomed. 2014, 9, 129–144. [Google Scholar] [CrossRef]
- Hokkala, E.; Strachan, C.J.; Agopov, M.; Järvinen, E.; Semjonov, K.; Heinämäki, J.; Yliruusi, J.; Svanbäck, S. Thermodynamic solubility measurement without chemical analysis. Int. J. Pharm. 2024, 653, 123890. [Google Scholar] [CrossRef] [PubMed]
- Vertzoni, M.; Alsenz, J.; Augustijns, P.; Bauer-Brandl, A.; Bergström, C.A.S.; Brouwers, J.; Müllerz, A.; Perlovich, G.; Saal, C.; Sugano, K.; et al. UNGAP best practice for improving solubility data quality of orally administered drugs. Eur. J. Pharm. Sci. 2022, 168, 106043. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Alshehri, S.; Alsarra, I.A. Solubility and Thermodynamics Data of Cabozantinib Malate in Various Aqueous Solutions of Dimethyl Sulfoxide at Different Temperatures. Molecules 2023, 28, 7805. [Google Scholar] [CrossRef]
- Zargar-Shoshtari, S.; Wen, J.; Alany, R.G. Formulation and physicochemical characterization of imwitor 308 based self microemulsifying drug delivery systems. Chem. Pharm. Bull. 2010, 58, 1332–1338. [Google Scholar] [CrossRef]
- Chaudhari, S.P.; Dugar, R.P. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Syed, A.H.; Sevoo, T.; Kanesen, K. Synergistic influence of nanoparticles and surfactants on interfacial tension reduction, wettability alteration and stabilization of oil-in-water emulsion. J. Pet. Sci. Eng. 2020, 186, 106779. [Google Scholar] [CrossRef]
- Abd Sisak, M.A.; Daik, R.; Ramli, S. Study on the effect of oil phase and co-surfactant on microemulsion systems. Malays. J. Anal. Sci. 2017, 21, 1409–1416. [Google Scholar] [CrossRef]
- Tartari, A.P.S.; Jacumazo, J.; Lorenzett, A.K.P.; Freitas, R.A.d.; Mainardes, R.M. Development and Characterization of Silibinin-Loaded Nanoemulsions: A Promising Mucoadhesive Platform for Enhanced Mucosal Drug Delivery. Pharmaceutics 2025, 17, 192. [Google Scholar] [CrossRef]
- Casler, M.D. Fundamentals of experimental design: Guidelines for designing successful experiments. Agron. J. 2015, 107, 692–705. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method-A review. J. Build. Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The influence of nanoparticle properties on oral bioavailability of drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef]
- Dapčević Hadnađev, T.; Dokić, P.; Krstonošić, V.; Hadnađev, M. Influence of oil phase concentration on droplet size distribution and stability of oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2013, 115, 313–321. [Google Scholar] [CrossRef]
- Tripathi, S.; Bhattacharya, A.; Singh, R.; Tabor, R.F. Rheological behavior of high internal phase water-in-oil emulsions: Effects of droplet size, phase mass fractions, salt concentration and aging. Chem. Eng. Sci. 2017, 174, 290–301. [Google Scholar] [CrossRef]
- Rowe, E.L. Effect of Emulsifier Concentration and Type on the Particle Size Distribution of Emulsions. J. Pharm. Sci. 1965, 54, 260–264. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Nazzal, S.; Nutan, M.; Palamakula, A.; Shah, R.; Zaghloul, A.; Khan, M. Optimization of a self-nanoemulsified tablet dosage form of Ubiquinone using response surface methodology: Effect of formulation ingredients. Int. J. Pharm. 2002, 240, 103–114. [Google Scholar] [CrossRef]
- Sousa, A.M.; Pereira, M.J.; Matos, H.A. Oil-in-water and water-in-oil emulsions formation and demulsification. J. Pet. Sci. Eng. 2022, 210, 110041. [Google Scholar] [CrossRef]
- Dai, B.; Leal, L.G. The mechanism of surfactant effects on drop coalescence. Phys. Fluids 2008, 20, 040802. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Chen, Y.; Narayan, S.; Dutcher, C.S. Phase-dependent surfactant transport on the microscale: Interfacial tension and droplet coalescence. Langmuir 2020, 36, 14904–14923. [Google Scholar] [CrossRef]
- McClements, D.J.; Dungan, S.R. Factors that affect the rate of oil exchange between oil-in-water emulsion droplets stabilized by a nonionic surfactant: Droplet size, surfactant concentration, and ionic strength. J. Phys. Chem. 1993, 97, 7304–7308. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Abbas, S.; Eric, K.; Xia, S.; Zhang, X. Modified SPI improves the emulsion properties and oxidative stability of fish oil microcapsules. Food Hydrocoll. 2015, 51, 108–117. [Google Scholar] [CrossRef]
- Kommuru, T.R.; Gurley, B.; Khan, M.A.; Reddy, I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Tang, B.; Cheng, G.; Gu, J.-C.; Xu, C.-H. Development of solid self-emulsifying drug delivery systems: Preparation techniques and dosage forms. Drug Discov. Today 2008, 13, 606–612. [Google Scholar] [CrossRef]
- Pharmacopeia, U. USP32/NF27: United States Pharmacopeia and National Formulary; The United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2009; pp. 80–86. [Google Scholar]
- Pouton, C.W. Lipid formulations for oral administration of drugs: Non-emulsifying, self-emulsifying and ‘self-microemulsifying’drug delivery systems. Eur. J. Pharm. Sci. 2000, 11, S93–S98. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, E.; Rabiti, D.; Murillo Moreno, J.S.; Can Karaca, A.; Van der Meeren, P. Oil phase solubility rather than diffusivity determines the release of entrapped amino acids and di-peptides from water-in-oil-in-water emulsions. Molecules 2022, 27, 394. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Goo, Y.T.; Won, Y.H.; Hong, S.H.; Choi, J.Y.; Sin, G.H.; Kim, C.H.; Jung, H.M.; Choi, Y.W. Optimization of a solidified micelle formulation for enhanced oral bioavailability of atorvastatin calcium using statistical experimental design. Pharm. Dev. Technol. 2023, 28, 479–491. [Google Scholar] [CrossRef]
- Yeom, D.W.; Song, Y.S.; Kim, S.R.; Lee, S.G.; Kang, M.H.; Lee, S.; Choi, Y.W. Development and optimization of a self-microemulsifying drug delivery system for atorvastatin calcium by using D-optimal mixture design. Int. J. Nanomed. 2015, 10, 3865–3877. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of advanced emulsion technology in the food industry: A review and critical evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Linke, A.; Weiss, J.; Kohlus, R. Factors determining the surface oil concentration of encapsulated lipid particles: Impact of the emulsion oil droplet size. Eur. Food Res. Technol. 2020, 246, 1933–1943. [Google Scholar] [CrossRef]
- Patel, A.R.; Vavia, P.R. Preparation and in vivo evaluation of SMEDDS (self-microemulsifying drug delivery system) containing fenofibrate. AAPS J. 2007, 9, E344–E352. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Majithiya, R.J.; Umrethia, M.L.; Murthy, R.S. Design and development of microemulsion drug delivery system of acyclovir for improvement of oral bioavailability. AAPS Pharmscitech 2006, 7, E172–E177. [Google Scholar] [CrossRef] [PubMed]
- Rich, P.R.; Breton, J. FTIR studies of the CO and cyanide adducts of fully reduced bovine cytochrome c oxidase. Biochemistry 2001, 40, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Thayyil, M.S.; Sivaramakrishnan, P.A.; Sulaiman, M.K.; Hussan, K.P.S.; Panicker, C.Y.; Nair, K.L. Dielectric spectroscopic studies in supercooled liquid and glassy states of Acemetacin, Brucine and Colchicine. Journal of Non-Crystalline Solids 2019, 508, 33–45. [Google Scholar] [CrossRef]
- Khan, Y.; Durrani, S.; Siddique, M.; Mehmood, M. Hydrothermal synthesis of alpha Fe2O3 nanoparticles capped by Tween-80. Mater. Lett. 2011, 65, 2224–2227. [Google Scholar] [CrossRef]
- Nanjwade, B.K.; Patel, D.J.; Udhani, R.A.; Manvi, F.V. Functions of lipids for enhancement of oral bioavailability of poorly water-soluble drugs. Sci. Pharm. 2011, 79, 705–727. [Google Scholar] [CrossRef]
- Zhuo, X.; Sener, Z.; Kabedev, A.; Zhao, M.; Arnous, A.; Leng, D.; Foderà, V.; Löbmann, K. Mechanisms of Drug Solubility Enhancement Induced by β-Lactoglobulin-Based Amorphous Solid Dispersions. Molecular Pharmaceutics 2023, 20, 5206–5213. [Google Scholar] [CrossRef]
- Corrie, L.; Ajjarapu, S.; Banda, S.; Parvathaneni, M.; Bolla, P.K.; Kommineni, N. HPMCAS-Based Amorphous Solid Dispersions in Clinic: A Review on Manufacturing Techniques (Hot Melt Extrusion and Spray Drying), Marketed Products and Patents. Materials 2023, 16, 6616. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2203. [Google Scholar] [CrossRef]
- Qiao, M.; Zhang, L.; Ma, Y.; Zhu, J.; Xiao, W. A novel electrostatic dry coating process for enteric coating of tablets with Eudragit® L100-55. Eur. J. Pharm. Biopharm. 2013, 83, 293–300. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Prebeg, Ž.; Kurjaković, N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100-55 and Eudragit® S100 combinations. J. Control. Release 1999, 58, 215–222. [Google Scholar] [CrossRef]
- Okur, M.E.; Karayıldırım, Ç.K. Central possible antinociceptive mechanism of naringin. Istanb. J. Pharm. 2021, 51, 204–211. [Google Scholar] [CrossRef]
- He, C.-X.; He, Z.-G.; Gao, J.-Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Araya, H.; Tomita, M.; Hayashi, M. The novel formulation design of O/W microemulsion for improving the gastrointestinal absorption of poorly water soluble compounds. Int. J. Pharm. 2005, 305, 61–74. [Google Scholar] [CrossRef]
- Ruan, L.P.; Chen, S.; Yu, B.Y.; Zhu, D.N.; Cordell, G.A.; Qiu, S.X. Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur. J. Med. Chem. 2006, 41, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Wu, S.; Ju, B.; Zhu, D.; Yan, Y.; Wang, M.; Hu, J. Preparation and evaluation of microemulsion-based transdermal delivery of Cistanche tubulosa phenylethanoid glycosides. Mol. Med. Rep. 2017, 15, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Schumann, L.V.; Bunjes, H. Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics 2022, 14, 2464. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Villarreal, O.; Rojas-González, L.; Bernad-Bernad, M.J.; Miranda-Calderón, J.E. Self-emulsifying drug delivery system for praziquantel with enhanced ex vivo permeation. J. Pharm. Innov. 2023, 18, 525–537. [Google Scholar] [CrossRef]
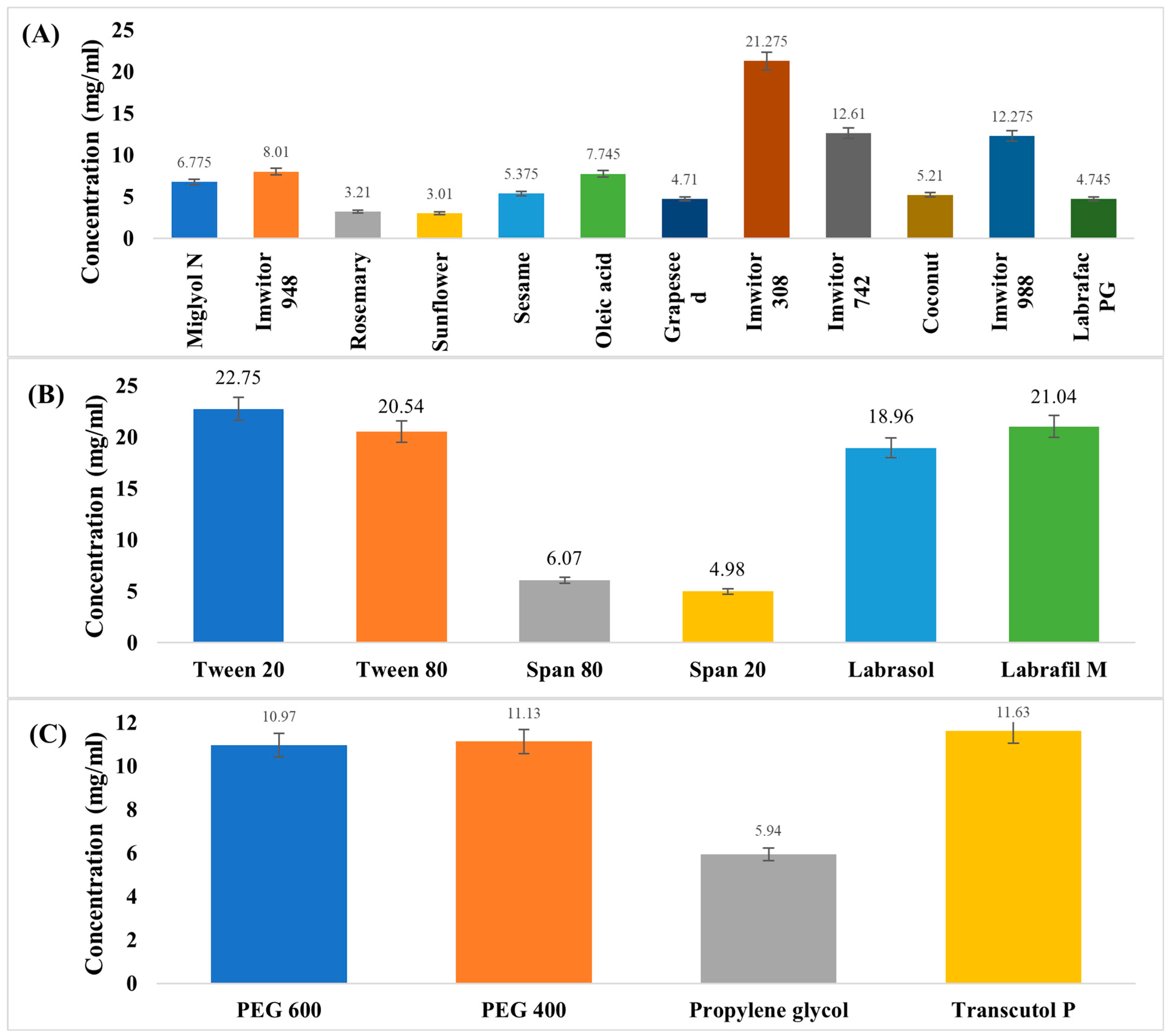


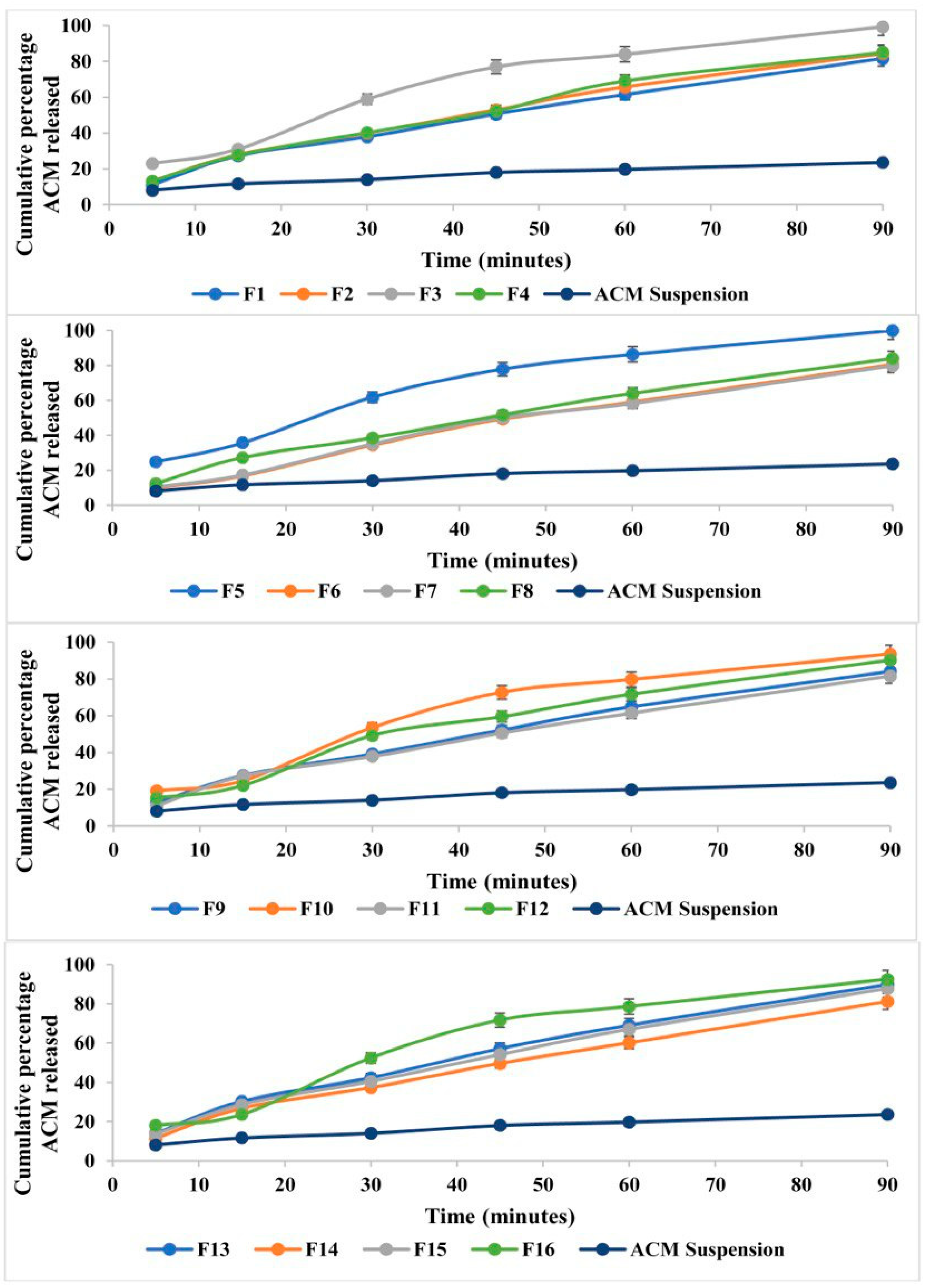


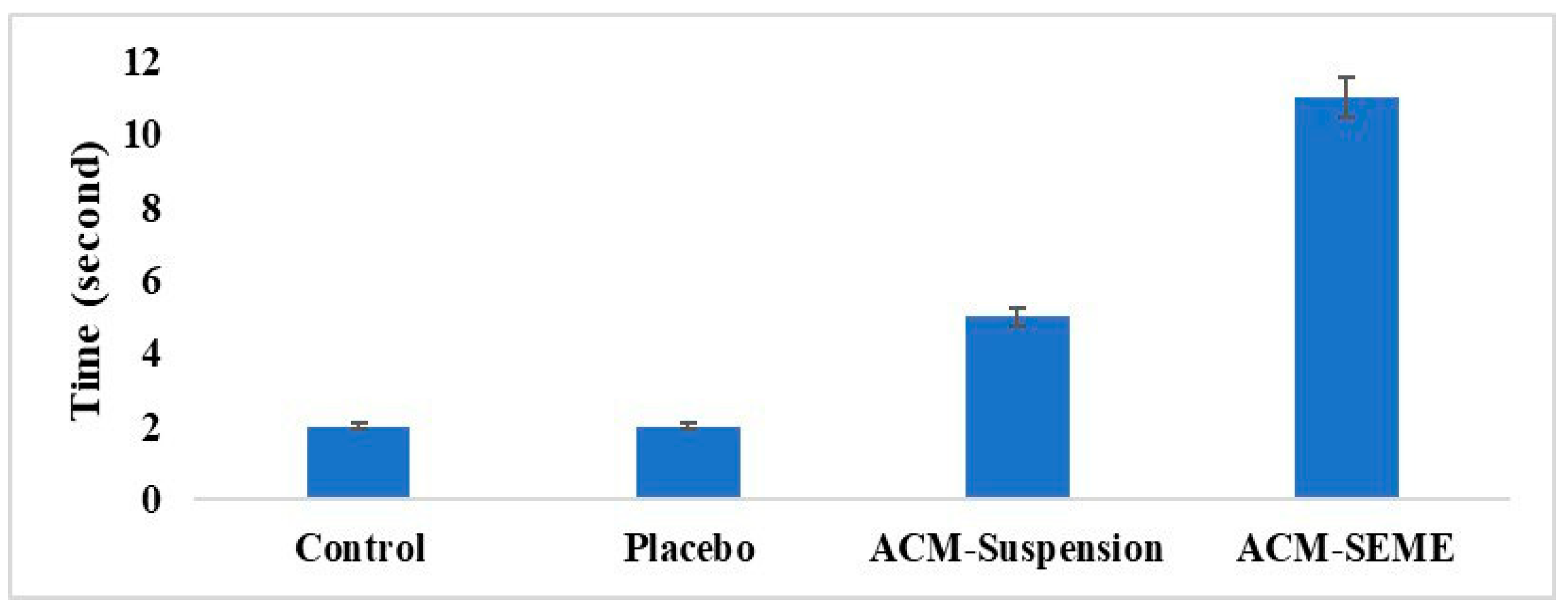
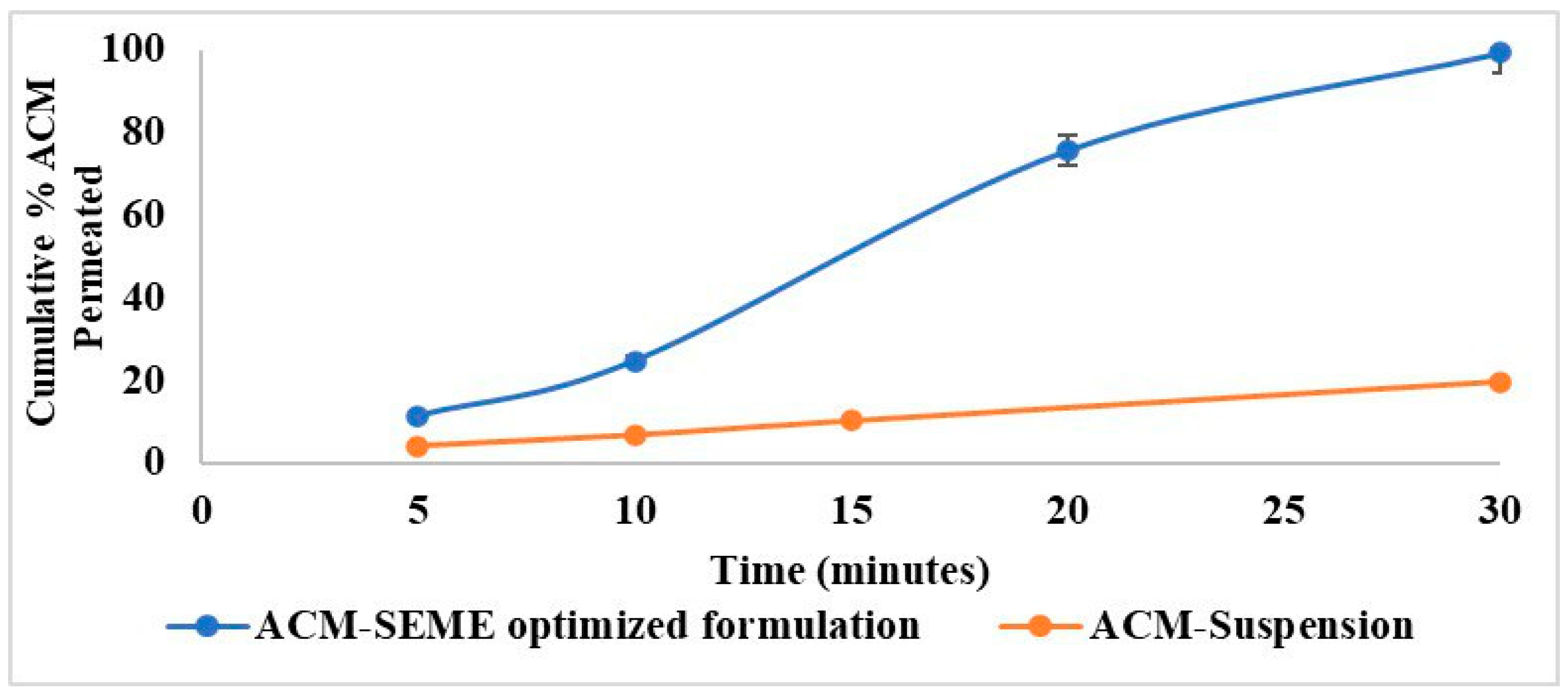
| Formulation | Imwitor 308 Oil % w/w | Smix % w/w | Water % w/w | PS (nm) | PDI | ACM Content % | % Released After 90 min | T% |
|---|---|---|---|---|---|---|---|---|
| F1 | 25 | 65 | 10 | 40.03 ± 2.04 | 0.29 ± 0.01 | 96.56 ± 0.57 | 81.63 ± 0.25 | 99.31 ± 0.24 |
| F2 | 18 | 59 | 22 | 34.09 ± 1.34 | 0.2 ± 0.02 | 99.49 ± 0.59 | 84.39 ± 0.55 | 99.54 ± 0.11 |
| F3 * | 10 | 62 | 28 | 20.21 ± 0.58 | 0.16 ± 0.01 | 99.61 ± 0.23 | 99.349 ± 0.54 | 99.77 ± 0.35 |
| F4 | 15 | 59 | 27 | 32.85 ± 0.98 | 0.23 ± 0.03 | 99.4 ± 0.14 | 85.03 ± 0.67 | 99.77 ± 0.21 |
| F5 * | 10 | 62 | 28 | 21.26 ± 0.74 | 0.18 ± 0.02 | 99.75 ± 0.14 | 99.91 ± 0.24 | 99.77 ± 0.15 |
| F6 | 25 | 55 | 20 | 41.95 ± 1.25 | 0.32 ± 0.01 | 98.21 ± 0.16 | 80.57 ± 0.65 | 99.77 ± 0.12 |
| F7 | 25 | 55 | 20 | 42.36 ± 1.24 | 0.31 ± 0.03 | 98.54 ± 0.18 | 79.71 ± 0.32 | 99.54 ± 0.14 |
| F8 | 19 | 55 | 26 | 37.75 ± 1.32 | 0.18 ± 0.02 | 98.64 ± 0.54 | 83.97 ± 0.78 | 99.77 ± 0.18 |
| F9 | 19 | 65 | 16 | 35.5 ± 0.85 | 0.16 ± 0.01 | 98.31 ± 0.65 | 84.18 ± 0.57 | 99.77 ± 0.16 |
| F10 | 10 | 55 | 35 | 30.01 ± 0.75 | 0.22 ± 0.02 | 98.31 ± 0.33 | 93.53 ± 0.86 | 99.54 ± 0.36 |
| F11 | 25 | 65 | 10 | 43.25 ± 1.23 | 0.33 ± 0.01 | 96.9 ± 0.25 | 81.63 ± 0.63 | 99.54 ± 0.24 |
| F12 | 14 | 65 | 21 | 30.69 ± 0.89 | 0.27 ± 0.03 | 98.09 ± 0.65 | 90.34 ± 0.98 | 99.54 ± 0.19 |
| F13 | 14 | 65 | 21 | 30.65 ± 1.01 | 0.28 ± 0.02 | 98.21 ± 0.46 | 89.92 ± 0.46 | 99.77 ± 0.32 |
| F14 | 25 | 60 | 15 | 39.35 ± 1.36 | 0.31 ± 0.2 | 98.31 ± 0.47 | 81.208 ± 0.78 | 99.77 ± 0.24 |
| F15 | 14 | 55 | 31 | 31.39 ± 0.87 | 0.19 ± 0.1 | 98.72 ± 0.87 | 87.79 ± 0.96 | 99.31 ± 0.34 |
| F16 | 10 | 55 | 35 | 30.09 ± 0.57 | 0.23 ± 0.01 | 98.09 ± 0.45 | 92.47 ± 0.85 | 99.77 ± 0.25 |
| PS | PDI | ACM Content | % Released | |
|---|---|---|---|---|
| Model | Special Quartic | Cubic | Cubic | Special Quartic |
| p-value | <0.0001 | 0.0003 | <0.0001 | <0.0001 |
| F-value | 56.26 | 29.18 | 46.71 | 208.16 |
| R2 | 0.9847 | 0.9777 | 0.9859 | 0.9958 |
| Adjusted R2 | 0.9672 | 0.9442 | 0.9648 | 0.9910 |
| Predicted R2 | 0.8629 | 0.8260 | 0.8797 | 0.9212 |
| Adeq Precision | 23.0750 | 13.6299 | 22.7263 | 42.5470 |
| The coded equation for the dependent variable | ||||
| Response | Equation | |||
| PS | PS = +56.42 A − 31.41 B + 29.87 C + 84.88 AB − 13.74 AC + 36.99 BC − 699.26 A2BC + 985.06 AB2C + 190.48 ABC2 | |||
| PDI | PDI = +1.21 A + 24.90 B + 0.2249 C − 48.69 AB − 1.94 AC − 44.90 BC + 49.94 ABC + 27.21 AB(A-B) − 0.7593 AC(A-C) − 23.50 BC(B-C) | |||
| ACM content | ACM content = 99.3596 A − 86.5521 B + 98.1989 C + 340.7 AB − 1.17886 AC + 333.557 BC − 316.671 ABC − 209.148 AB(A-B) − 5.06269 AC(A-C) + 158.716 BC(B-C) | |||
| Cumulative percentage released | Cumulative percentage released = +76.53 A + 260.59 B + 92.90 C − 285.58 AB − 12.80 AC − 193.84 BC + 1172.86 A2BC − 1740.66 AB2C − 5.77 ABC2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abduljaleel, Z.Z.; Al-Kinani, K.K. Formulation of Self-Emulsifying Microemulsion for Acemetacin Using D-Optimal Design: Enteric-Coated Capsule for Targeted Intestinal Release and Bioavailability Enhancement. Pharmaceutics 2025, 17, 1270. https://doi.org/10.3390/pharmaceutics17101270
Abduljaleel ZZ, Al-Kinani KK. Formulation of Self-Emulsifying Microemulsion for Acemetacin Using D-Optimal Design: Enteric-Coated Capsule for Targeted Intestinal Release and Bioavailability Enhancement. Pharmaceutics. 2025; 17(10):1270. https://doi.org/10.3390/pharmaceutics17101270
Chicago/Turabian StyleAbduljaleel, Zaineb Z., and Khalid K. Al-Kinani. 2025. "Formulation of Self-Emulsifying Microemulsion for Acemetacin Using D-Optimal Design: Enteric-Coated Capsule for Targeted Intestinal Release and Bioavailability Enhancement" Pharmaceutics 17, no. 10: 1270. https://doi.org/10.3390/pharmaceutics17101270
APA StyleAbduljaleel, Z. Z., & Al-Kinani, K. K. (2025). Formulation of Self-Emulsifying Microemulsion for Acemetacin Using D-Optimal Design: Enteric-Coated Capsule for Targeted Intestinal Release and Bioavailability Enhancement. Pharmaceutics, 17(10), 1270. https://doi.org/10.3390/pharmaceutics17101270






