A Scoping Review of Recent Developments in Cellulose-Derived Hydrogels for Dental Applications
Abstract
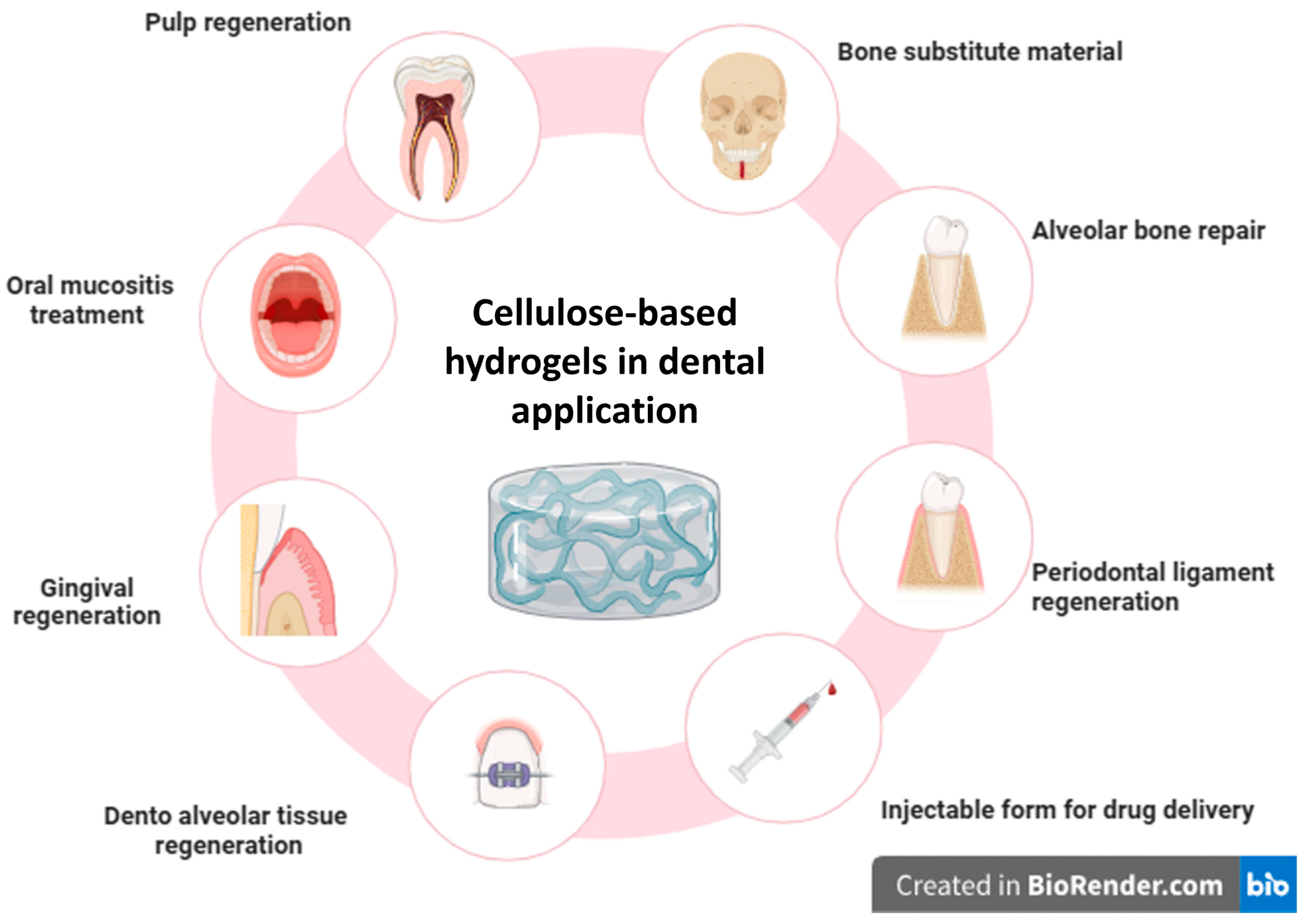
1. Introduction
2. Materials and Methods
2.1. Protocol Registration and Research Question
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Data Sources and Search Strategy
2.4. Choosing the Sources of Evidence
2.5. Data Items and Data Charting Process
2.6. Critical Appraisal of Each Evidence Source
2.7. Results Synthesis
3. Results
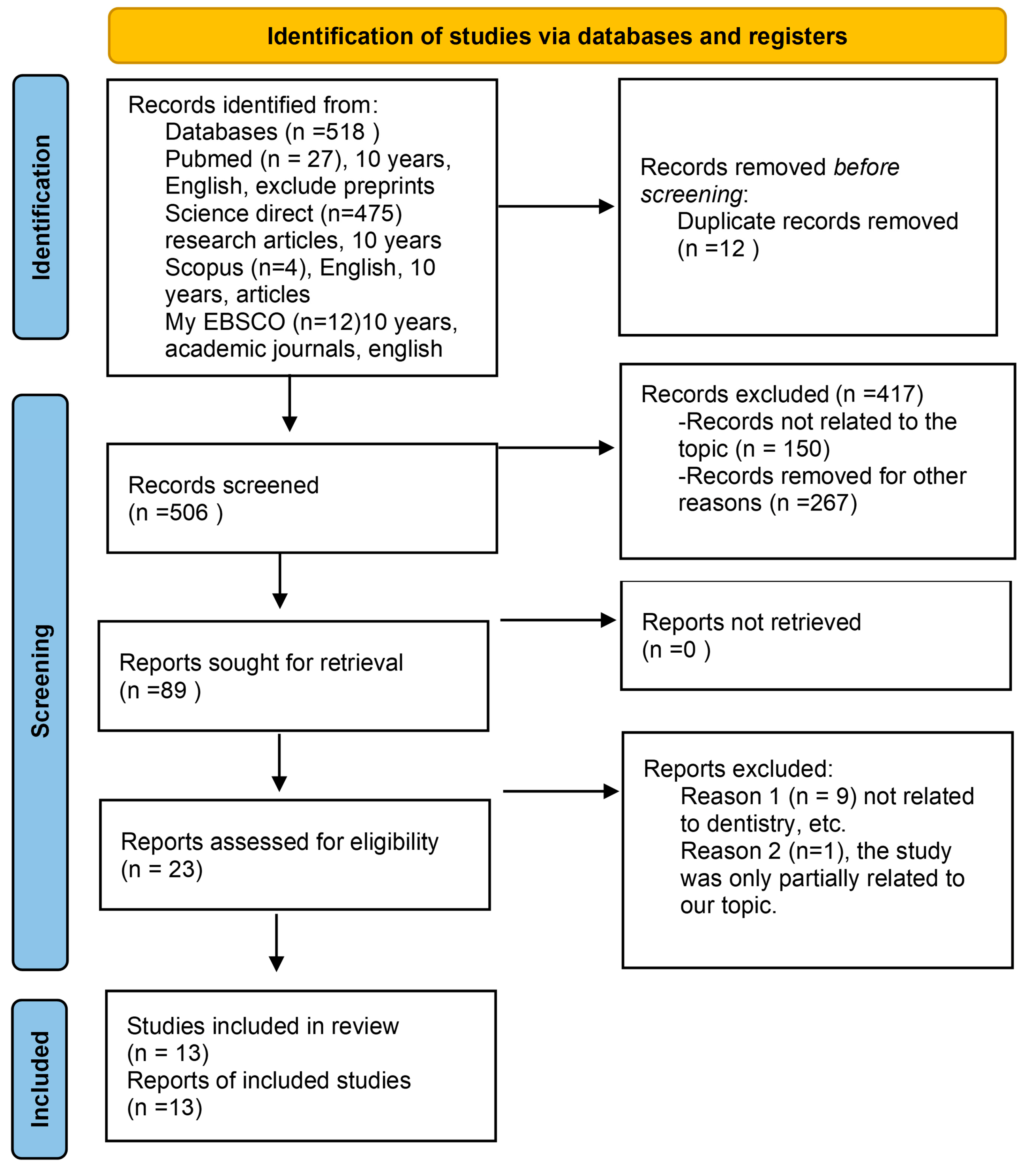
3.1. Study Selection and Data Charting
3.2. Features of the Source of Evidence
| Author (s), Year | Study Type | Material/ Scaffold Composition | Objective | Study Medium | Methods of Analysis | Outcomes | Dental Applications | Limitations |
|---|---|---|---|---|---|---|---|---|
| Rad et al., 2019 [24] | In vitro | CAox-PULLGEL 1 + BG-NPs 2 | Dentin regeneration | hDPSCs 3 | SEM 4 Cell culture assays | ↑ 5 Cell adhesion, proliferation, ALP 6 activity, calcium deposition, odontogenic differentiation | Regenerative endodontics | In vitro |
| Divband et al., 2021 [26] | In vitro | Chitosan, biguanidine, CMC 7 + VEGF 8 and BMP-2 9 | Osteogenic development of hDPSCs | hDPSCs | qRT-PCR 10, Western blot MTT 11 assay | ↑ Proliferation and osteogenic differentiation | Regenerative endodontics | In vitro |
| Malik et al., 2020 [32] | In vitro | Thyroxine-loaded chitosan/CMC-HA 12 | Periodontal regeneration via angiogenesis | CAM 13-MC3T3-E1 14 | SEM 15 FTIR 16 Cytotoxicity assays CAM 17 assay | ↑ Angiogenic activity, non-toxic to cells | Periodontal regeneration | In vitro |
| Huerta et al., 2020 [33] | In vitro | CNF 18 scaffolds (HIUS 19 processed) | Cytocompatibility for periodontal therapy | Human gingival fibroblasts | Cell viability and proliferation assay | ↑ Cell viability, proliferation | Periodontal therapy | In vitro |
| Amoli et al., 2024 [34] | In vitro | pNIPAM 20-methylcellulose microgels | Drug delivery in dentoalveolar engineering | hDPSCs | Hydrodynamic size, VPTT 21, drug loading and cytocompatibility | Effective copolymerization, drug delivery | Drug delivery in dento-alveolar tissue | In vitro |
| Zeeshan et al., 2018 [35] | In vitro | CH 22-HPMC 23 + BG 24 + ZNO 25 | Alveolar bone repair | MC3T3-E1 | SEM FTIR Mechanical tests Cell viability assays | Good biocompatibility, ↑ osteoblast differentiation | Alveolar bone repair | In vitro |
| Pagano et al., 2019 [36] | In vitro and ex vivo | NaCMC 26 + CH 27 thermosensitive gel | Oral mucositis and drug delivery | Porcine mucosa | Rheological test, ex vivo mucoadhesion Drug release | ↑ Mucoadhesion, sustained drug release | Oral mucositis | Limited polymer range No human testing |
| Lu et al., 2021 [37] | In vitro and in vivo (rats) | COF-HEC 28 iodine hydrogel | Sustained iodine release for periodontitis | Rat periodontal cells, artificial saliva | TGA 29 Titration Molecular docking | Sustained iodine release ↓ 30 bone resorption in vivo Comparable efficacy to minocycline ointment in treating periodontitis. | Periodontitis | Rat model—not performed on human |
| Liu et al., 2014 [38] | In vitro | Si-HPMC 31 hydrogel composite CPC 32 | Mechanical property evaluation | No cells | Injectability, compressive strength, SEM, XRD 33, FTIR. | ↑ Cohesion, mechanical strength, porosity | Bone substitute material for clinical application | Air bubble entrapment Delayed CDHA 34 formation |
| Dalir et al., 2022 [27] | In vitro | CS 35 + OCNCs 36 + MTA 37 hydrogel | Injectable regenerative scaffold | hDPSCs | Gelation time FTIR SEM MTT assay ALP activity ARS 38 staining | ↑ ALP activity, differentiation, proliferation, and functional calcium nodules | Regenerative endodontic | In vitro and short-term study |
| Teti et al., 2015 [25] | In vitro | CMC-HA 39 hydrogel | Osteogenic/odontogenic differentiation of DPSCs | hDPSCs | MTT assay RTPCR 40 EM 41 analysis | ↑ Biocompatibility, cell adhesion, osteogenic, and odontogenic differentiation | Dental pulp/periodontal regeneration | Small sample size No in vivo validation |
| Tritean et al., 2024 [39] | In vitro | CSFa 42 + BNC 43 + SeNPsK 44 hydrogel | -Biological and mechanical evaluation | HGF-1 45 | DLS 46 ELISA 47 Antimicrobial assays | ↑ Anti-inflammatory, antimicrobial, and antioxidant activity | Dental implants, periodontal | No in vivo study |
| Srisura et al., 2024 [40] | In vitro | CMC + CaP 48 + MeHA 49 hydrogel | Injectable scaffold for bone regeneration | MC3T3-E1 | DLS FTIR NMR 50 TEM 51 and SEM | ↑ Stability, biocompatibility ↑ Injectability ↑ Osteoblast proliferation and mineralization | Injectable scaffold for bone regeneration | No in vivo testing |
3.3. Results of Outcomes
3.3.1. Primary Outcomes
3.3.2. Secondary Outcomes
3.3.3. Tertiary Outcomes
3.4. Synthesis of Results
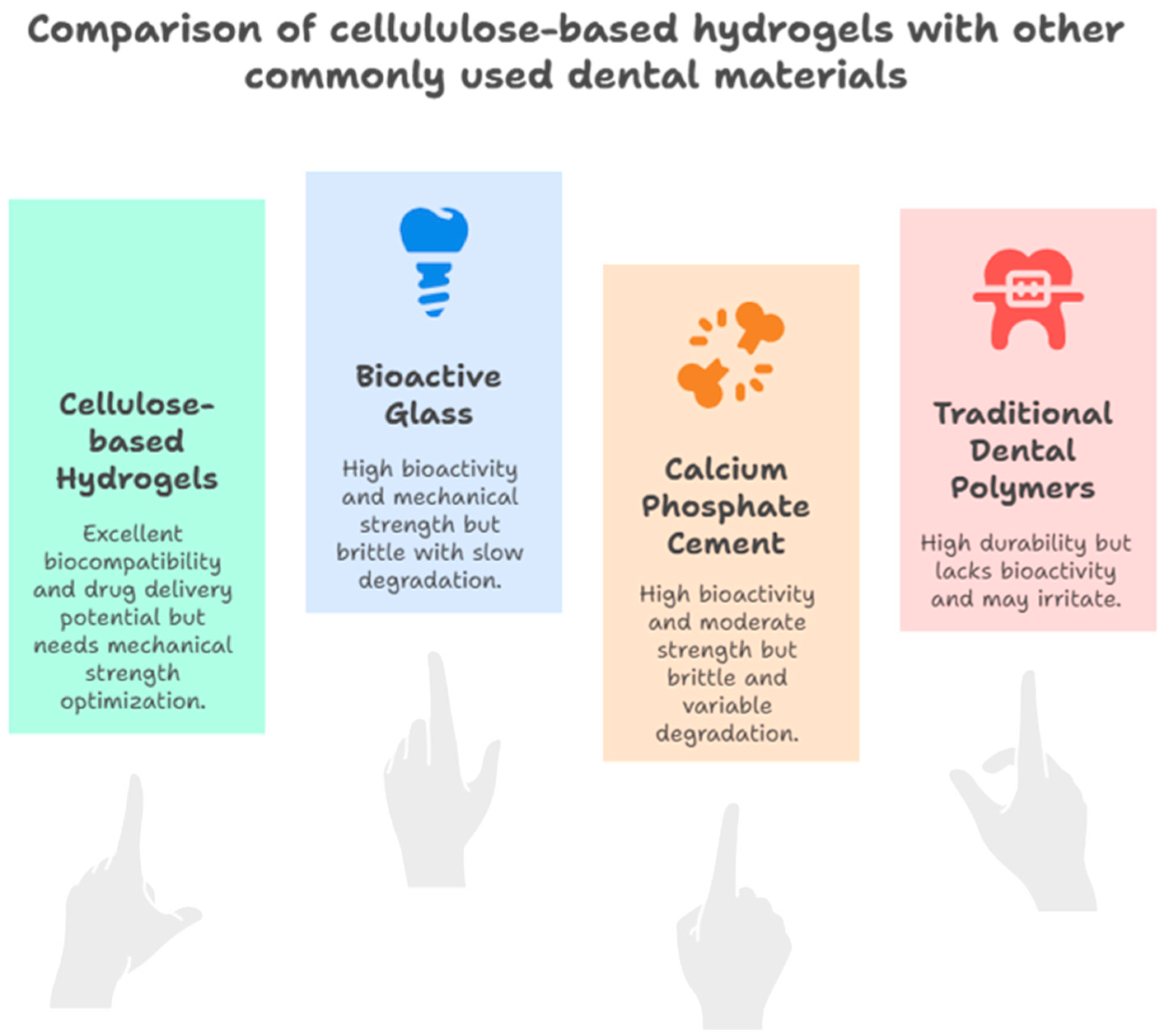
4. Discussion
4.1. Critical Analysis of the Included Studies
4.1.1. Biocompatibility and Cellular Response
4.1.2. Variability in Methodology
4.1.3. Mechanical and Structural Limitations
4.1.4. Interaction with Oral Bacteria
4.1.5. Biocompatibility and Degradation
4.1.6. Cytotoxicity
4.1.7. Swelling Effects
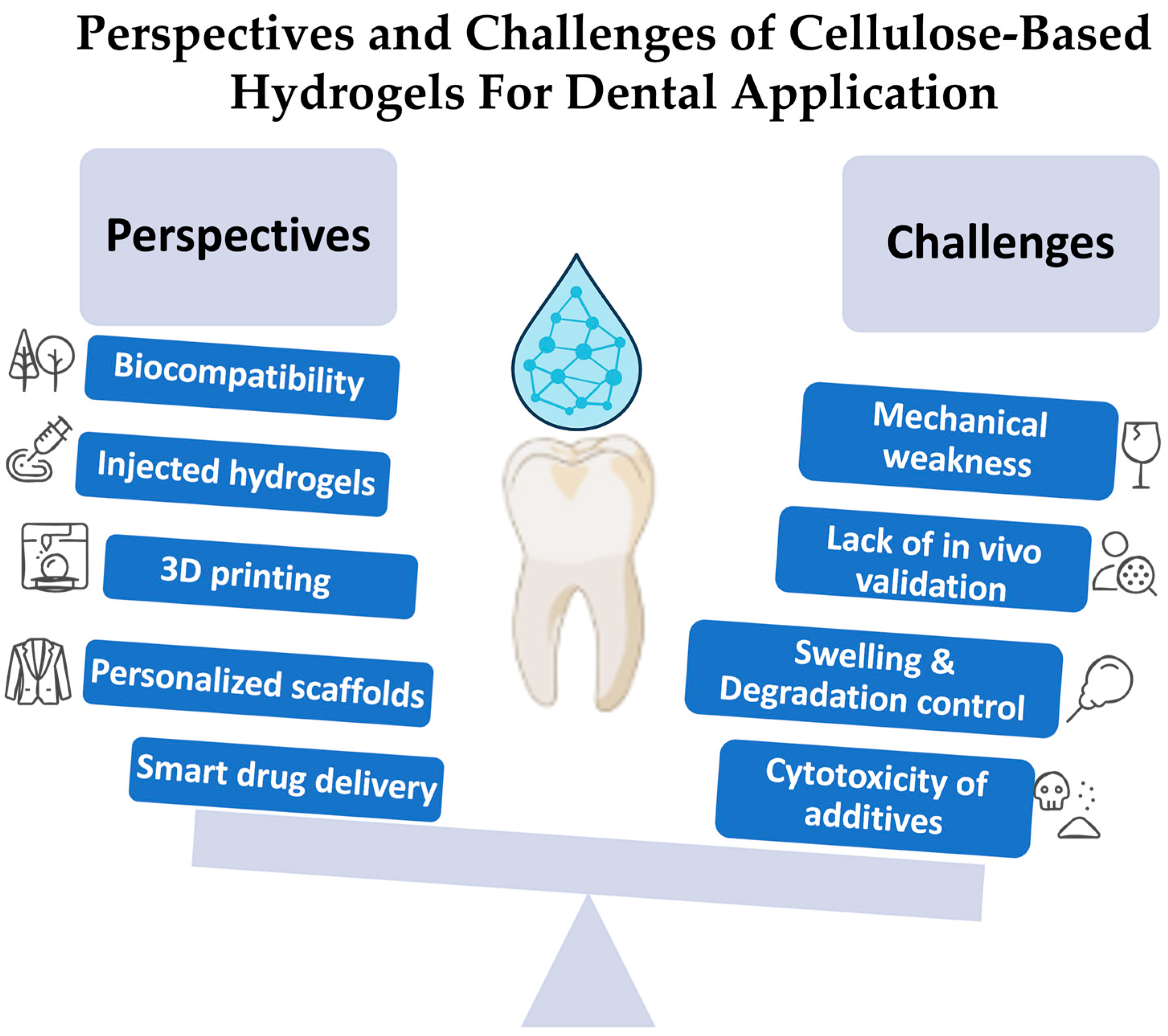
4.1.8. Authors’ Perspectives, Opinion, Limitations, and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BG-NPs | Bioactive glass nanoparticles |
| B | Functional ions like boron |
| DPSCs | Dental pulp stem cells |
| DSPP | Dentin sialo phosphoprotein |
| OPN | Osteopontin |
| ALP | Alkaline phosphatase |
| EDX | Energy-dispersive X-ray spectroscopy |
Appendix A
| Author (s), Year | Study Model | Compressive Strength (MPa) | Modulus (Elastic/Young/Hydrogel) | Degradation (Mass Loss %) | Cell Viability (%) | Porosity (%) | Controls (Ct) Used |
|---|---|---|---|---|---|---|---|
| Rad et al., 2019 [24] | In vitro (hDPSCs 1 on CA 2/ox-PULL 3/GEL + B-BG-NPs 4 scaffolds) | 0.40 ± 0.03 | ~0.5 MPa 5 (with BG-NPs 6) | ~28%–37% (30 days) | 120% vs Ct 7 (↑ 8 proliferation) | 87.5–94.5% | Scaffolds without bioactive glass nanoparticles (BG-NPs) |
| Divband et al., 2021 [26] | In vitro (injectable CMC 9/CSG 10 hydrogels with growth factor loading) | 3.2–4.3 ± 0.5 | 3.2–5.5 MPa | Degradation ↑ with porosity | ~90–100% viable (high biocompatibility) | High (interconnected) | Cells without hydrogel; base hydrogel without growth factors |
| Malik et al., 2020 [32] | In vitro (thyroxine-loaded chitosan/CMC/HA hydrogels 11/aerogels) | Not specified | Hydrogel modulus in kPa 12 range | Stable (swelling-dependent) | ~95–100% (cytocompatible) | Highly porous aerogels | Blank hydrogel without thyroxine; ex vivo tissue without hydrogel |
| Huerta et al., 2020 [33] | In vitro (gingival fibroblasts on LL-CNF 13 and HL-CNF 14 aerogels) | Compressive strength not provided; ~264 Pa 15 storage modulus | ~264 Pa storage modulus | Stable aerogels | ~95–236% (viability upto 236% at day 11 VS control)- Highly biocompatible | Highly porous aerogels | Glass cover slips seeded with gingival fibroblasts |
| Amoli et al., 2024 [34] | In vitro | Not directly given | ↑ modulus with Si-HPMC 16 | Slower degradation with Si-HPMC | ~100% (good biocompatibility) | Porosity measured gravimetrically | Cells alone without microgels (no-microgel control) |
| Zeeshan et al., 2018 [35] | In vitro | 0.27–0.45 | Modulus from stress–strain slope | 7–21% (28 days) | >90% viable (pre-osteoblasts) | Porous, BG load-dependent 17 | Scaffolds without BG (no bioactive glass) |
| Pagano et al., 2019 [36] | In vitro and ex vivo | Improved with HA | Not clearly reported | Gradual degradation | ↑ HDPSC proliferation and mineralization | Porous hydrogels with HA | Hydrogels without hyaluronic acid (HA) |
| Lu et al., 2021 [37] | In vitro and in vivo (rats) | 0.32–0.98 | 50–70 MPa | 3–23% (28 days) | High viability in vitro and in vivo | Porosity 22–70% | Negative control: saline; positive: minocycline ointment; test: calcium phosphate cement (CPC) scaffold |
| Liu et al., 2014 [38] | In vitro (Si-HPMC composite CPCs 18) | CPC compressive strength (wet) | Young’s modulus measured | Degradation slower with Si-HPMC | High viability (~95–100%) | 23–71% | Calcium phosphate cement (CPC) alone |
| Dalir et al., 2022 [27] | In vitro (injectable CS 19/OCNC 20 hydrogels with MTA 21) | Not specified | Hydrogel modulus reported (exact numeric value not present) | Degradation dependent on porosity | >90% viability (cytocompatible) | Highly porous injectable gels | Chitosan/oxidized cellulose nanocrystal (CS/OCNC) hydrogel without mineral trioxide aggregate (MTA); tissue culture plate |
| Teti et al., 2015 [25] | In vitro | Not specified | Elastic hydrogel behavior (numeric value not specified) | Slow degradation | ↑ HDPSCs viability vs. TCP 22 | High porosity (hydrogel) | Tissue culture plastic (TCP) without scaffold |
| Tritean et al., 2024 [39] | In vitro | Not reported | Hydrogel modulus (numeric value not specified) | Biodegradable | >90% viability | Porous hydrogel | Plain hydrogel without functionalization |
| Srisura et al., 2024 [40] | In vitro | Not reported | Hydrogel modulus (exact numeric value not present) | Biodegradable | High viability (~95–100%) | Porous aerogels | Hydrogel without calcium phosphate (CaP) nanoparticles |
| Study (Authors, Year) | Model System (Cell Line/Primary/In Vivo/Others) | Sample Size (Per Assay as Reported) | Controls Used (Name) |
|---|---|---|---|
| Rad et al., 2019 [24] | Primary hDPSCs 1, in vitro | Cell assays in triplicate (n = 3); ALP 2/calcium n = 4 | Scaffolds without BG-NPs 3; medium-only |
| Divband et al., 2021 [26] | Primary hDPSCs, in vitro | MTT 4/viability in triplicate (n = 3) | Untreated cells (cells + medium) |
| Malik et al., 2020 [32] | MC3T3 5 cells in vitro + CAM assay (ex vivo) | CAM 6 bead counts n = 3; in vitro triplicates | Control hydrogel (no thyroxine); untreated wells |
| Huerta et al., 2020 [33] | Primary oral/gingival cells, in vitro | Assays performed in duplicate | Blank glass/untreated scaffolds |
| Amoli et al., 2024 [34] | Primary hDPSCs, in vitro | DNA 7 and viability assays in triplicate (n = 3) | Control microgel formulations; medium-only |
| Zeeshan et al., 2018 [35] | MC3T3-E1 cell line, in vitro | Cell assays in triplicates | Tissue culture plastic (TCP 8); untreated cells |
| Pagano et al., 2019 [36] | In vitro release + ex vivo porcine buccal mucosa | Gelling/release/mucoadhesion n = 3 | Blank formulations; porcine mucosa baseline |
| Lu et al., 2021 [37] | In vitro hDPSCs + in vivo rat periodontal defect model | Animal groups n = 3–6; in vitro triplicates | Saline control; minocycline ointment |
| Liu et al., 2014 [38] | In vitro (cement materials only) | Setting times mean of 3; mechanical properties mean of 6 | Control CPC 9 (no Si-HPMC 10) |
| Dalir et al., 2022 [27] | Primary hDPSCs on cellulose scaffold, in vitro | Cell assays (MTT, ALP, ARS 11) carried out in triplicate (n = 3) | Untreated HDPSCs (medium only); blank scaffolds without bioactive glass particles |
| Teti et al., 2015 [25] | Primary hDPSCs, in vitro | Proliferation, viability, ALP assays performed in triplicate (n = 3) | Negative control: cells cultured on tissue culture plastic (TCP); scaffold without hydroxyapatite additive |
| Tritean et al., 2024 [39] | Primary hDPSCs, in vitro | qRT-PCR 12, viability, mineralization assays all reported in triplicate (n = 3) | Blank scaffold without active components; untreated cells |
| Srisura et al., 2024 [40] | Primary hDPSCs, in vitro | Cytotoxicity/viability assays/ qRTPCR/Western blot, ALP assay, Alizarin red assay all conducted in triplicate (n = 3) | Multiple controls: (1) cells alone without hydrogels (negative control); (2) GAPDH 13 as house-keeping control in qRT-PCR; (3) β-actin 14 as loading control in Western blot. |
References
- Maeda, H. Aging and Senescence of Dental Pulp and Hard Tissues of the Tooth. Front. Cell Dev. Biol. 2020, 8, 605996. [Google Scholar] [CrossRef]
- Guiglia, R.; Musciotto, A.; Compilato, D.; Procaccini, M.; Russo, L.; Ciavarella, D.; Muzio, L.; Cannone, V.; Pepe, I.; D’Angelo, M.; et al. Aging and Oral Health: Effects in Hard and Soft Tissues. Curr. Pharm. Des. 2010, 16, 619–630. [Google Scholar] [CrossRef]
- Guo, K.; Wang, Y.; Feng, Z.-X.; Lin, X.-Y.; Wu, Z.-R.; Zhong, X.-C.; Zhuang, Z.-M.; Zhang, T.; Chen, J.; Tan, W.-Q. Recent Development and Applications of Polydopamine in Tissue Repair and Regeneration Biomaterials. Int. J. Nanomed. 2024, 19, 859–881. [Google Scholar] [CrossRef]
- Najman, S.; Stojanović, S.; Živković, J.; Najdanović, J.; Radenković, M.; Vasiljević, P.; Ignjatović, N. Applications Of Biomaterials In Regenerative Medicine And Tissue Engineering—Concepts And Perspective. Contemp. Mater. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Oleksy, M.; Dynarowicz, K.; Aebisher, D. Advances in Biodegradable Polymers and Biomaterials for Medical Applications—A Review. Molecules 2023, 28, 6213. [Google Scholar] [CrossRef] [PubMed]
- Saha, H.; Halder, J.; Rizmi, R.K.B.M.; Hossain, S.; Alam, M.; Azad, H.K.M.; Rahman, M.Z. Recent Advancements in Nanostructured Biomaterials for Biomedical Applications and Regenerative Medicine. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2024; pp. 255–275. [Google Scholar]
- Guo, X.; Li, J.; Wu, Y.; Xu, L. Recent Advancements in Hydrogels as Novel Tissue Engineering Scaffolds for Dental Pulp Regeneration. Int. J. Biol. Macromol. 2024, 264, 130708. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Borjas, A.; Bonto, A.; Ursu, A.V.; Dupont, M.; Roche, J.; Delattre, C. Exploring Novel Applications for Hydrogels Derived from Modified Celluloses. Polymers 2024, 16, 530. [Google Scholar] [CrossRef]
- Kamini; Puri, D. Hydrogel-Based Drug Delivery Systems—A Review. Polym. Technol. Mater. 2024, 63, 2213–2236. [Google Scholar] [CrossRef]
- Kaur, H.; Gogoi, B.; Sharma, I.; Das, D.K.; Azad, M.A.; Pramanik, D.D.; Pramanik, A. Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications. Mol. Pharm. 2024, 21, 4827–4848. [Google Scholar] [CrossRef]
- Ornaghi, H.L.; Monticeli, F.M.; Agnol, L.D. A Review on Polymers for Biomedical Applications on Hard and Soft Tissues and Prosthetic Limbs. Polymers 2023, 15, 4034. [Google Scholar] [CrossRef]
- Al-Moameri, H.; Rasool, D.A.; Nahi, Z.M. A Review Of Polymer-Based Materials Used In Biomaterials For Medical Applications. J. Eng. Sustain. Dev. 2022, 26, 1–13. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Litvinova, T. Biomaterials and Agents: Pharmaceutical and Biomedical Applications in Dental Research. Pharmaceutics 2024, 16, 894. [Google Scholar] [CrossRef]
- Han, F.; Chen, S.; Wang, F.; Liu, M.; Li, J.; Liu, H.; Yang, Y.; Zhang, H.; Liu, D.; He, R.; et al. High-Conductivity, Self-Healing, and Adhesive Ionic Hydrogels for Health Monitoring and Human-Machine Interactions Under Extreme Cold Conditions. Adv. Sci. 2025, 12, 2412726. [Google Scholar] [CrossRef]
- Lv, J.; Pan, L.; Zhu, Y.; Zhang, X.; Yang, L.; Wang, X.; Yin, X. Thermo-Responsive Hydrogel Evaporator via In-Situ Chromogenic Strategy for High-Efficiency Solar Desalination with Robust Salt Resistance. Chem. Eng. J. 2025, 516, 164026. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Peshkov, Y.; Potapov, A.; Gribanova, Y.; Shikhaliev, K.; Ippolitov, Y.; Freitas, R.O.; Mahdy, I.A.; Mahdy, M.A.; et al. Biomimetic Organomineral Layers with Antibacterial Properties Based on Di/Tetrahydroquinolinediol and Nanocrystalline Hydroxyapatite Deposited on Enamel Surface. Biomater. Sci. 2025, 13, 2444–2461. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mathur, A. Analysis of Tissue Engineering for the Scaffolds in Dentistry. In Proceedings of the 2022 Fourth International Conference on Emerging Research in Electronics, Computer Science and Technology (ICERECT), Mandya, India, 26 December 2022; IEEE: New York, NY, USA, 2022; pp. 1–6. [Google Scholar]
- Li, X.; Jiang, G.; Wang, G.; Zhou, J.; Zhang, Y.; Zhao, D. Promising Cellulose–Based Functional Gels for Advanced Biomedical Applications: A Review. Int. J. Biol. Macromol. 2024, 260, 129600. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef] [PubMed]
- Bhaladhare, S.; Das, D. Cellulose: A Fascinating Biopolymer for Hydrogel Synthesis. J. Mater. Chem. B 2022, 10, 1923–1945. [Google Scholar] [CrossRef] [PubMed]
- Rzhepakovsky, I.; Piskov, S.; Avanesyan, S.; Sizonenko, M.; Timchenko, L.; Anfinogenova, O.; Nagdalian, A.; Blinov, A.; Denisova, E.; Kochergin, S.; et al. Composite of Bacterial Cellulose and Gelatin: A Versatile Biocompatible Scaffold for Tissue Engineering. Int. J. Biol. Macromol. 2024, 256, 128369. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef]
- Sevari, S.P.; Shahnazi, F.; Chen, C.; Mitchell, J.C.; Ansari, S.; Moshaverinia, A. Bioactive Glass-containing Hydrogel Delivery System for Osteogenic Differentiation of Human Dental Pulp Stem Cells. J. Biomed. Mater. Res. Part A 2020, 108, 557–564. [Google Scholar] [CrossRef]
- Rad, R.M.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of Human Dental Pulp Stem Cells Behavior on a Novel Nanobiocomposite Scaffold Prepared for Regenerative Endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar] [CrossRef]
- Teti, G.; Salvatore, V.; Focaroli, S.; Durante, S.; Mazzotti, A.; Dicarlo, M.; Mattioli-Belmonte, M.; Orsini, G. In Vitro Osteogenic and Odontogenic Differentiation of Human Dental Pulp Stem Cells Seeded on Carboxymethyl Cellulose-Hydroxyapatite Hybrid Hydrogel. Front. Physiol. 2015, 6, 297. [Google Scholar] [CrossRef]
- Divband, B.; Aghazadeh, M.; Al-qaim, Z.H.; Samiei, M.; Hussein, F.H.; Shaabani, A.; Shahi, S.; Sedghi, R. Bioactive Chitosan Biguanidine-Based Injectable Hydrogels as a Novel BMP-2 and VEGF Carrier for Osteogenesis of Dental Pulp Stem Cells. Carbohydr. Polym. 2021, 273, 118589. [Google Scholar] [CrossRef]
- Abdolahinia, E.D.; Alipour, M.; Aghazadeh, M.; Hassanpour, M.; Ghorbani, M.; Aghazadeh, Z. An Injectable Chitosan-Based Hydrogel Reinforced by Oxidized Nanocrystalline Cellulose and Mineral Trioxide Aggregate Designed for Tooth Engineering Applications. Cellulose 2022, 29, 3453–3465. [Google Scholar] [CrossRef]
- Aryal AC, S.; Islam, M.S.; Samsudin, A.R. Investigation of the Effect of a Time Delay on the Characteristics and Survival of Dental Pulp Stem Cells from Extracted Teeth. Arch. Oral Biol. 2020, 119, 104896. [Google Scholar] [CrossRef]
- Yamada, T.; Ezura, Y.; Hayata, T.; Moriya, S.; Shirakawa, J.; Notomi, T.; Arayal, S.; Kawasaki, M.; Izu, Y.; Harada, K.; et al. β2 Adrenergic Receptor Activation Suppresses Bone Morphogenetic Protein (BMP)-Induced Alkaline Phosphatase Expression in Osteoblast-Like MC3T3E1 Cells. J. Cell. Biochem. 2015, 116, 1144–1152. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.H.; Shahzadi, L.; Batool, R.; Safi, S.Z.; Khan, A.S.; Khan, A.F.; Chaudhry, A.A.; Rehman, I.U.; Yar, M. Thyroxine-Loaded Chitosan/Carboxymethyl Cellulose/Hydroxyapatite Hydrogels Enhance Angiogenesis in in-Ovo Experiments. Int. J. Biol. Macromol. 2020, 145, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Formation of Cellulose Nanofiber Scaffold with Low and High Lignin Content and Their Cytocompatibility with Gingival Fibroblast Cells. Ultrason. Sonochemistry 2020, 64, 104759. [Google Scholar] [CrossRef] [PubMed]
- Amoli, M.S.; Yang, H.; Anand, R.; EzEldeen, M.; Aktan, M.K.; Braem, A.; Jacobs, R.; Bloemen, V. Development and Characterization of Colloidal PNIPAM-Methylcellulose Microgels with Potential Application for Drug Delivery in Dentoalveolar Tissue Engineering Strategies. Int. J. Biol. Macromol. 2024, 262, 129684. [Google Scholar] [CrossRef]
- Zeeshan, R.; Mutahir, Z.; Iqbal, H.; Ali, M.; Iqbal, F.; Ijaz, K.; Sharif, F.; Shah, A.T.; Chaudhry, A.A.; Yar, M.; et al. Hydroxypropylmethyl Cellulose (HPMC) Crosslinked Chitosan (CH) Based Scaffolds Containing Bioactive Glass (BG) and Zinc Oxide (ZnO) for Alveolar Bone Repair. Carbohydr. Polym. 2018, 193, 9–18. [Google Scholar] [CrossRef]
- Pagano, C.; Giovagnoli, S.; Perioli, L.; Tiralti, M.C.; Ricci, M. Development and Characterization of Mucoadhesive-Thermoresponsive Gels for the Treatment of Oral Mucosa Diseases. Eur. J. Pharm. Sci. 2020, 142, 105125. [Google Scholar] [CrossRef]
- Lu, S.; Ren, X.; Guo, T.; Cao, Z.; Sun, H.; Wang, C.; Wang, F.; Shu, Z.; Hao, J.; Gui, S.; et al. Controlled Release of Iodine from Cross-Linked Cyclodextrin Metal-Organic Frameworks for Prolonged Periodontal Pocket Therapy. Carbohydr. Polym. 2021, 267, 118187. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Rethore, G.; Khairoun, K.; Pilet, P.; Tancret, F.; Bouler, J.-M.; Weiss, P. A Novel Injectable, Cohesive and Toughened Si-HPMC (Silanized-Hydroxypropyl Methylcellulose) Composite Calcium Phosphate Cement for Bone Substitution. Acta Biomater. 2014, 10, 3335–3345. [Google Scholar] [CrossRef]
- Tritean, N.; Dimitriu, L.; Dima, Ș.-O.; Ghiurea, M.; Trică, B.; Nicolae, C.-A.; Moraru, I.; Nicolescu, A.; Cimpean, A.; Oancea, F.; et al. Bioactive Hydrogel Formulation Based on Ferulic Acid-Grafted Nano-Chitosan and Bacterial Nanocellulose Enriched with Selenium Nanoparticles from Kombucha Fermentation. J. Funct. Biomater. 2024, 15, 202. [Google Scholar] [CrossRef]
- Srisura, P.; Pinyakit, Y.; Ngoensawat, U.; Yuntasiri, P.; Putri, K.N.A.; Chanamuangkon, T.; Phoolcharoen, W.; Intasanta, V.; Hoven, V.P. Carboxymethyl Cellulose-Stabilized Calcium Phosphate Particles for Injectable Hydrogel-Based Bone Tissue Engineering. Soft Matter 2024, 20, 8824–8834. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and Biomaterials for Dental Alveolar Tissue Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef]
- Talaat, W.; Aryal AC, S.; Al Kawas, S.; Samsudin, A.R.; Kandile, N.G.; Harding, D.R.; Ghoneim, M.M.; Zeiada, W.; Jagal, J.; Aboelnaga, A.; et al. Nanoscale Thermosensitive Hydrogel Scaffolds Promote the Chondrogenic Differentiation of Dental Pulp Stem and Progenitor Cells: A Minimally Invasive Approach for Cartilage Regeneration. Int. J. Nanomed. 2020, 15, 7775–7789. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional Cellulose-Based Hydrogels for Biomedical Applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In Situ-Forming Injectable Hydrogels for Regenerative Medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Smriti Aryal, A.C.; Islam, M.S. Potential Role of BMP7 in Regenerative Dentistry. Int. Dent. J. 2024, 74, 901–909. [Google Scholar] [CrossRef]
- Salama, M.A.; Ismail, A.A.; Islam, M.S.; Aghila Rani, K.G.; Al Kawas, S.; Samsudin, A.R.; Smriti Aryal, A.C. Impact of Bone Morphogenetic Protein 7 and Prostaglandin Receptors on Osteoblast Healing and Organization of Collagen. PLoS ONE 2024, 19, e0303202. [Google Scholar] [CrossRef]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, e2103691. [Google Scholar] [CrossRef]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-Containing Antimicrobial Membrane Based on Chitosan-TPP Hydrogel for the Treatment of Wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, P.; Kozlovskaya, V.; Nijampatnam, B.; Rojas, E.M.; Pukkanasut, P.; Inman, D.; Dolmat, M.; Law, A.C.; Schormann, N.; Deivanayagam, C.; et al. Hydrogel-Encapsulated Biofilm Inhibitors Abrogate the Cariogenic Activity of Streptococcus Mutans. J. Med. Chem. 2023, 66, 7909–7925. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Lu, Y.; Li, M.; Liu, S.; Yang, Y.; Song, Y.; Chen, S.; Kang, J.; Dong, A.; et al. A Microenvironment-Responsive Graphdiyne-Iron Nanozyme Hydrogel with Antibacterial and Anti-Inflammatory Effect for Periodontitis Treatment. Adv. Healthc. Mater. 2024, 2403683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, X.; Cheng, B.; Yin, M.; Hou, Z.; Li, X.; Liu, K.; Tie, C.; Yin, M. Degradation-Kinetics-Controllable and Tissue-Regeneration-Matchable Photocross-Linked Alginate Hydrogels for Bone Repair. ACS Appl. Mater. Interfaces 2022, 14, 21886–21905. [Google Scholar] [CrossRef]
- Shu, Q. Biodegradable Materials in Medical Applications: Research Progress. Appl. Comput. Eng. 2025, 126, 182–187. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and Biodegradable Hydrogels: Gelation, Biodegradation and Biomedical Applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wei, E.; Wu, L.; Wang, Y.; Lin, Q.; Wu, N.; Chen, H.; Tang, N. Novel Biomaterials for Wound Healing and Tissue Regeneration. ACS Omega 2024, 9, 32268–32286. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.; Venkatesh, K.V.; Nandini, V.; Sihivahanan, D.; Alamoudi, A.; Bahammam, H.A.; Bahammam, S.A.; Zidane, B.; Bahammam, M.A.; Chohan, H.; et al. Evaluating the Effect of Tideglusib-Loaded Bioactive Glass Nanoparticles as a Potential Dentine Regenerative Material. Materials 2022, 15, 4567. [Google Scholar] [CrossRef] [PubMed]
- Ghilan, A.; Nita, L.E.; Pamfil, D.; Simionescu, N.; Tudorachi, N.; Rusu, D.; Rusu, A.G.; Bercea, M.; Rosca, I.; Ciolacu, D.E.; et al. One-Step Preparation of Carboxymethyl Cellulose—Phytic Acid Hydrogels with Potential for Biomedical Applications. Gels 2022, 8, 647. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Kim, J. Swelling Behavior of Polyacrylamide–Cellulose Nanocrystal Hydrogels: Swelling Kinetics, Temperature, and PH Effects. Materials 2019, 12, 2080. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic Biomass: A Sustainable Platform for the Production of Bio-Based Chemicals and Polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryal A C, S.; Islam, M.S.; Mohammed, M.M.; Abu-Nada, L.; Abdulhameed, E.A.; Narasimhan, S.; Pattanaik, S.; Lim, G.S. A Scoping Review of Recent Developments in Cellulose-Derived Hydrogels for Dental Applications. Pharmaceutics 2025, 17, 1252. https://doi.org/10.3390/pharmaceutics17101252
Aryal A C S, Islam MS, Mohammed MM, Abu-Nada L, Abdulhameed EA, Narasimhan S, Pattanaik S, Lim GS. A Scoping Review of Recent Developments in Cellulose-Derived Hydrogels for Dental Applications. Pharmaceutics. 2025; 17(10):1252. https://doi.org/10.3390/pharmaceutics17101252
Chicago/Turabian StyleAryal A C, Smriti, Md Sofiqul Islam, Marwan Mansoor Mohammed, Lina Abu-Nada, Elaf Akram Abdulhameed, Sangeetha Narasimhan, Snigdha Pattanaik, and Ghee Seong Lim. 2025. "A Scoping Review of Recent Developments in Cellulose-Derived Hydrogels for Dental Applications" Pharmaceutics 17, no. 10: 1252. https://doi.org/10.3390/pharmaceutics17101252
APA StyleAryal A C, S., Islam, M. S., Mohammed, M. M., Abu-Nada, L., Abdulhameed, E. A., Narasimhan, S., Pattanaik, S., & Lim, G. S. (2025). A Scoping Review of Recent Developments in Cellulose-Derived Hydrogels for Dental Applications. Pharmaceutics, 17(10), 1252. https://doi.org/10.3390/pharmaceutics17101252









