Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges
Abstract
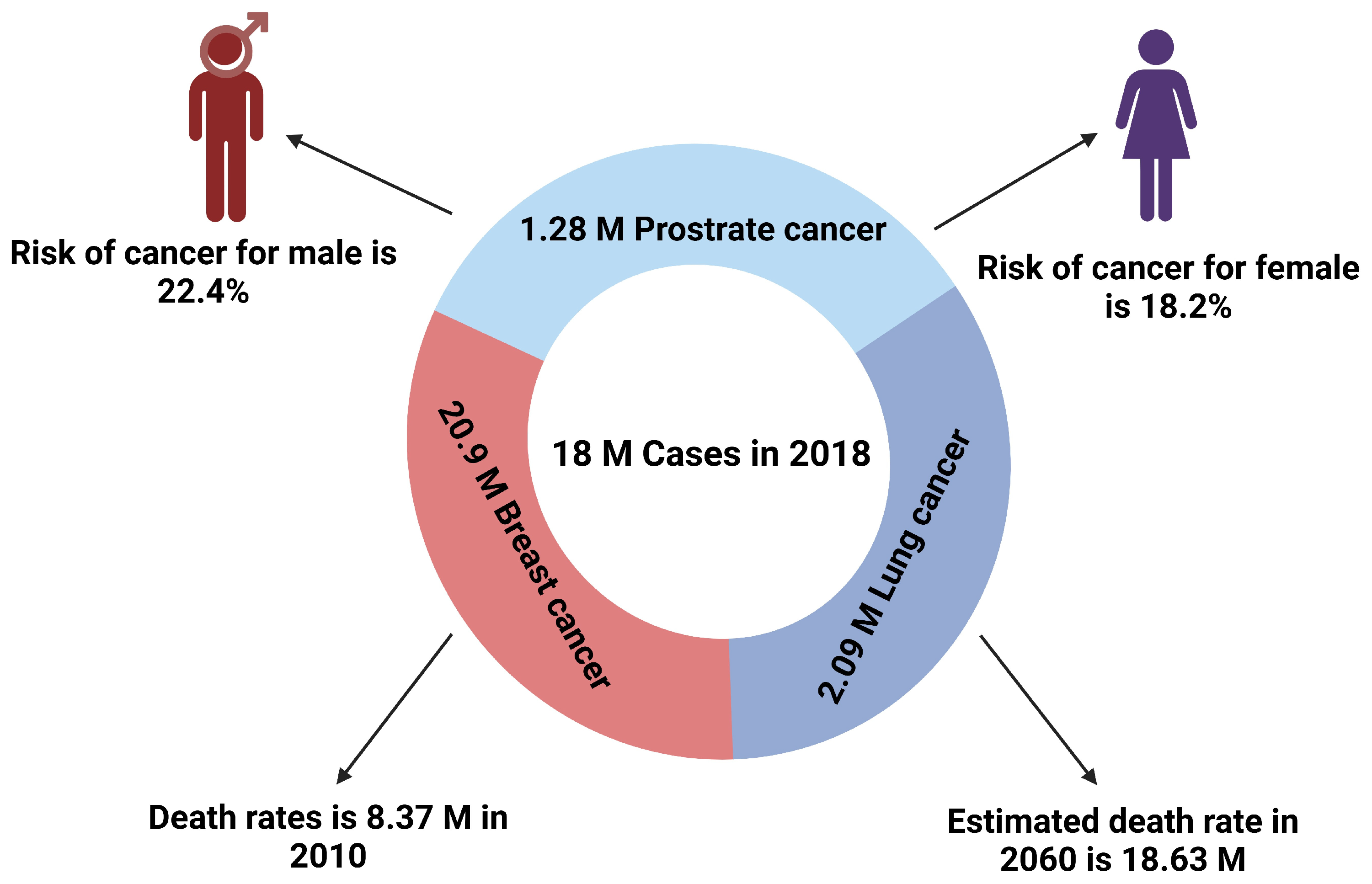
1. Introduction
| Cancer Type | Common Treatments | 5-Year Survival Rate (Today) | 5-Year Survival Rate (20 Years Ago) |
|---|---|---|---|
| Breast cancer | Surgery, Chemotherapy, Radiation, Hormone Therapy, Immunotherapy | 91% | 77% |
| Prostate cancer | Surgery, Radiation, Hormone Therapy, Chemotherapy | 98% | 87% |
| Lung cancer | Surgery, Chemotherapy, Radiation, Immunotherapy, Targeted Therapy | 25% | 16% |
| Colon cancer | Surgery, Chemotherapy, Radiation, Immunotherapy | 65% | 58% |
2. Nanotechnology Approach in Cancer Therapy
2.1. Dendrimers
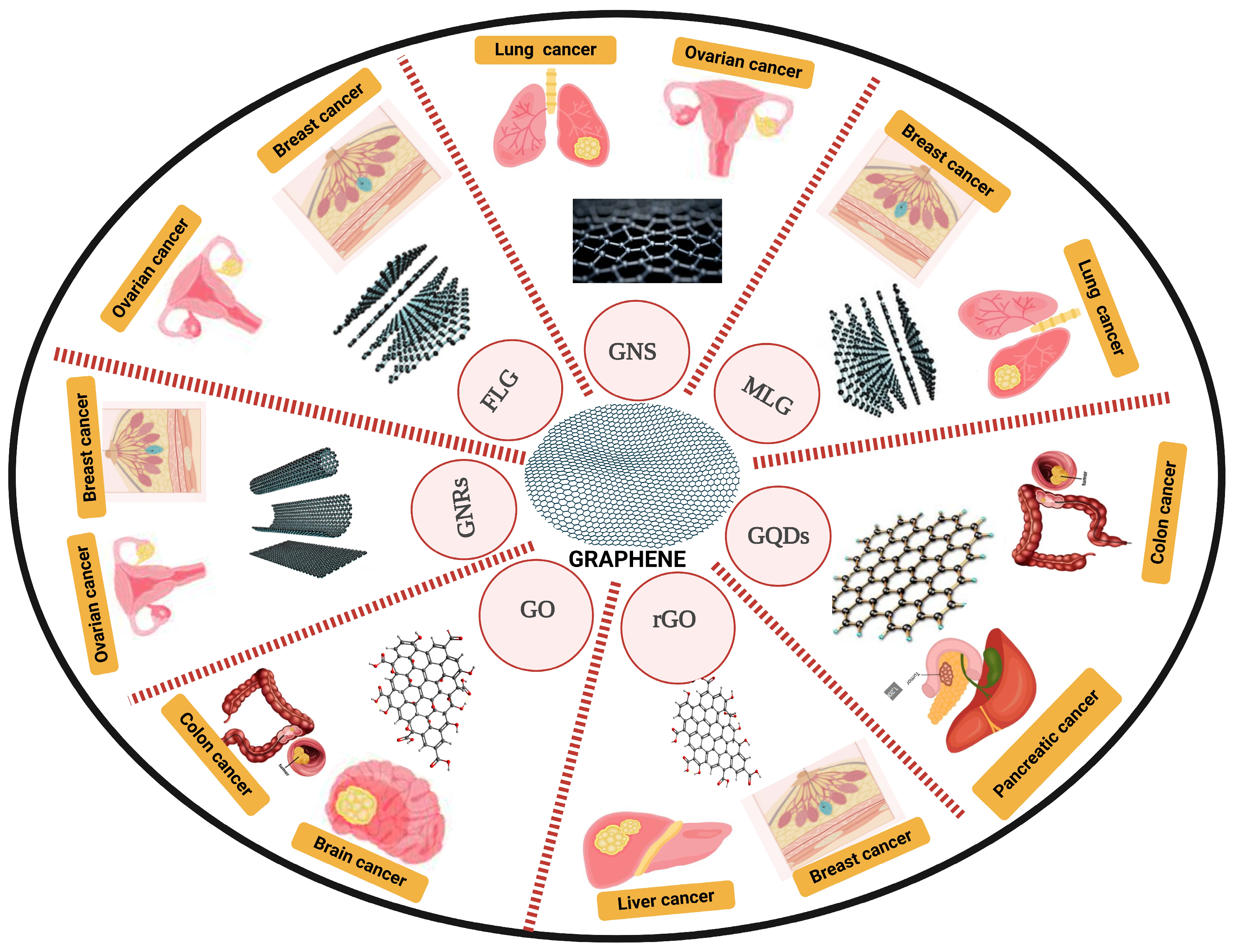
2.2. Graphene
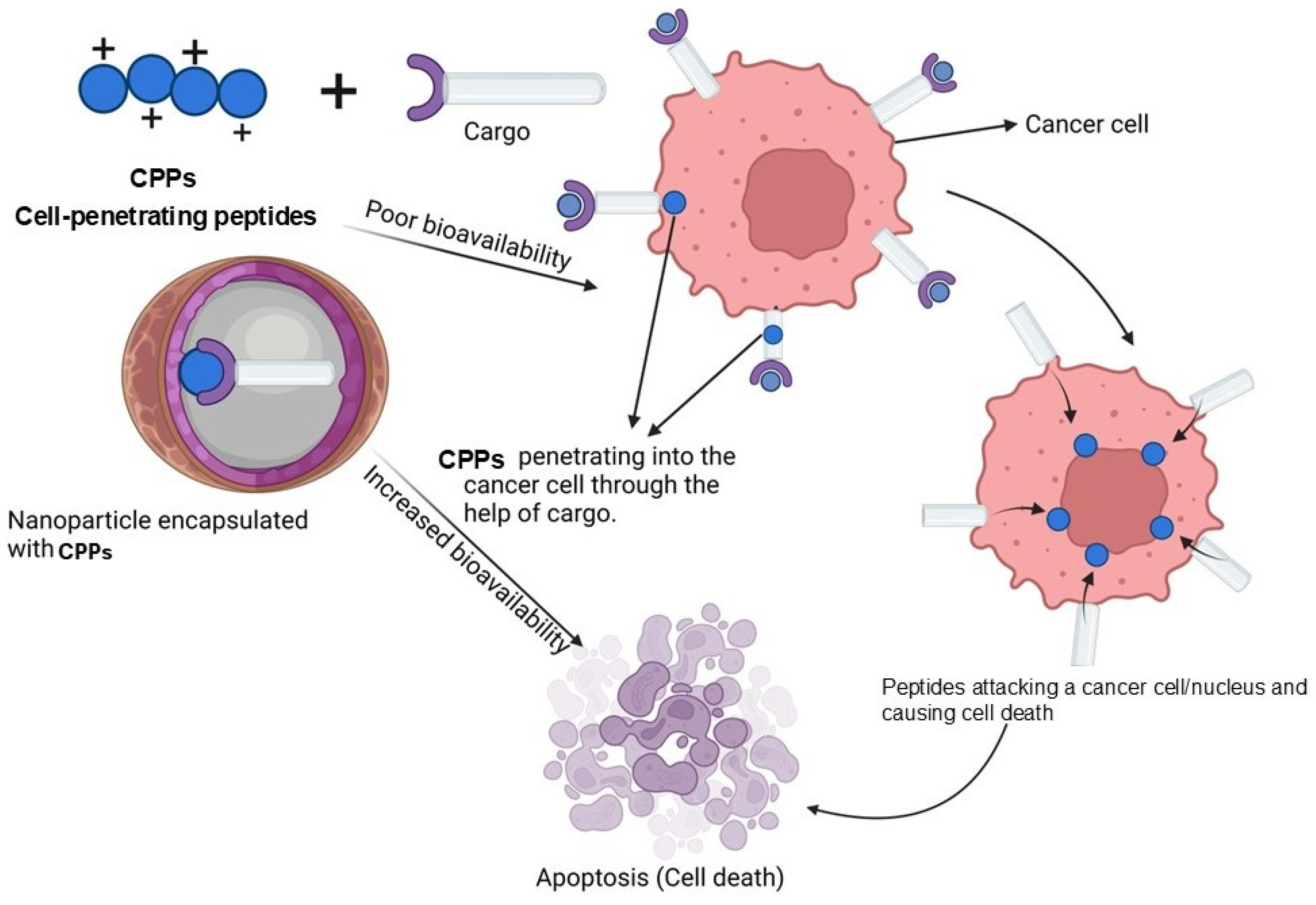
2.3. Cell-Penetrating Peptide (CPP) Nanoparticles
2.4. Antibody Conjugated Nanoparticles
2.5. Lipid Nanoparticles
2.6. Magnetic Nano-Materials in the Treatment of Cancer
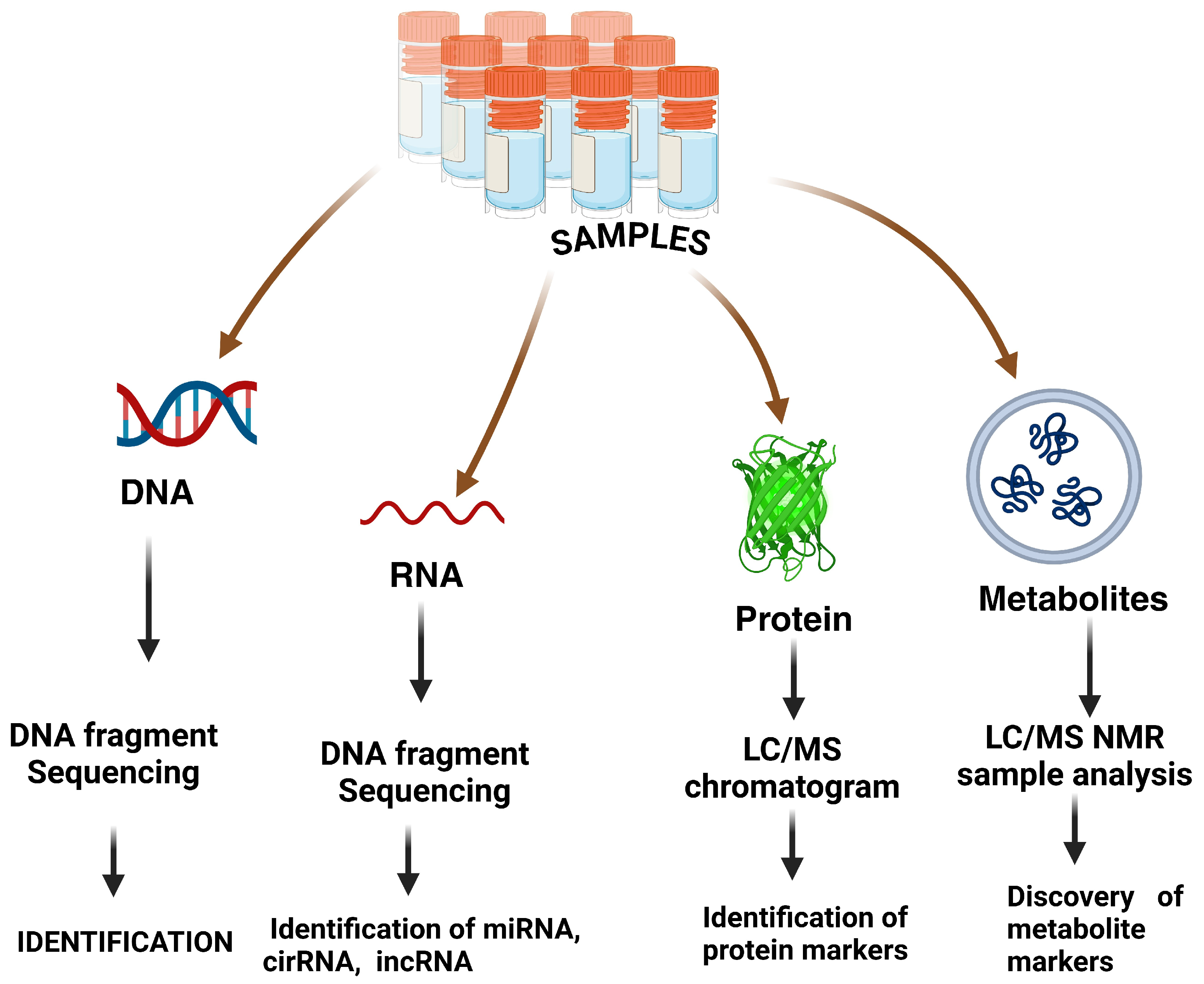
3. Nano-Omics
4. Environmental Factors and Outcomes for Patients in Rural Areas with the Progression of New Anti-Tumor Interventions
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Shan, C.-W.; Chen, Z.; Han, G.-C.; Feng, X.-Z.; Kraatz, H.-B. Electrochemical immuno-biosensors for the detection of the tumor marker alpha-fetoprotein: A review. Talanta 2024, 271, 125638. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Al-Imran, M.; Akter, S.; Mozumder, M.A.S.; Bhuiyan, R.J.; Rahman, T.; Ahmmed, M.J.; Bhuiyan, R.J.; Mozumder, M.A.S.; Akter, S.; Hossen, M.E. Evaluating Machine Learning Algorithms for Breast Cancer Detection: A Study on Accuracy and Predictive Performance. Am. J. Eng. Technol. 2024, 6, 22–33. [Google Scholar] [CrossRef]
- Owens, O.L.; Leonard, M.; Singh, A. Efficacy of Alexa, Google Assistant, and Siri for supporting informed prostate cancer screening decisions for African-American Men. J. Cancer Educ. 2023, 38, 1752–1759. [Google Scholar] [CrossRef]
- Rhanime, E. An Examination of Lung Cancer Treatment Characteristics on Lung Cancer Patients with Co-Existing Heart Disease. Bachelor’s Thesis, University of Central Florida, Orlando, FL, USA, 2022. [Google Scholar]
- Williams, E.D. Healthcare Leadership Perceptions of Screening for Social Risk Factors, Toward Colorectal Cancer Screening Uptake. Ph.D. Thesis, Franklin University, Columbus, OH, USA, 2023. [Google Scholar]
- Clancy, E. ACS Report Shows Prostate Cancer on the Rise, Cervical Cancer on the Decline; Renal & Urology News; Haymarket Media, Inc.: London, UK, 2023. [Google Scholar]
- Sathishkumar, K.; Chaturvedi, M.; Das, P.; Stephen, S.; Mathur, P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J. Med. Res. 2022, 156, 598–607. [Google Scholar]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer nanotechnology: A new revolution for cancer diagnosis and therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 69, pp. 52–68. [Google Scholar]
- Kong, X.; Gao, P.; Wang, J.; Fang, Y.; Hwang, K.C. Advances of medical nanorobots for future cancer treatments. J. Hematol. Oncol. 2023, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Sarella, P.N.K.; Vipparthi, A.K.; Valluri, S.; Vegi, S.; Vendi, V.K. Nanorobotics: Pioneering drug delivery and development in pharmaceuticals. Res. J. Pharm. Dos. Forms Technol. 2024, 16, 81–90. [Google Scholar] [CrossRef]
- Shapira, P.; Youtie, J.; Kay, L. National innovation systems and the globalization of nanotechnology innovation. J. Technol. Transf. 2011, 36, 587–604. [Google Scholar] [CrossRef]
- Shin, S.; Ko, H.; Kim, C.H.; Yoon, B.K.; Son, S.; Lee, J.A.; Shin, J.M.; Lee, J.; Song, S.H.; Jackman, J.A.; et al. Curvature-sensing peptide inhibits tumour-derived exosomes for enhanced cancer immunotherapy. Nat. Mater. 2023, 22, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Andoh, V.; Ocansey, D.K.W.; Naveed, H.; Wang, N.; Chen, L.; Chen, K.; Mao, F. The advancing role of nanocomposites in cancer diagnosis and treatment. Int. J. Nanomed. 2024, 19, 6099–6126. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Di Petrillo, A.; Gatto, G.; Pilia, L.; Kumar, A. Nanoparticles in cancer diagnosis and treatment. Materials 2023, 16, 5354. [Google Scholar] [CrossRef] [PubMed]
- Ale, Y.; Nainwal, N. Progress and Challenges in the Diagnosis and Treatment of Brain Cancer Using Nanotechnology. Mol. Pharm. 2023, 20, 4893–4921. [Google Scholar] [CrossRef]
- Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Review of the role of nanotechnology in overcoming the challenges faced in oral cancer diagnosis and treatment. Molecules 2023, 28, 5395. [Google Scholar] [CrossRef] [PubMed]
- Alrushaid, N.; Alam Khan, F.; Al-Suhaimi, E.A.; Elaissari, A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics 2023, 15, 1025. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-targeted nanocarrier for cancer therapy. Front. Pharmacol. 2022, 12, 800481. [Google Scholar] [CrossRef]
- Leporatti, S. Thinking about enhanced permeability and retention effect (EPR). J. Pers. Med. 2022, 12, 1259. [Google Scholar] [CrossRef]
- Prieložná, J.; Mikušová, V.; Mikuš, P. Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles. Int. J. Pharm. X 2024, 8, 100281. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric nanoparticles for delivery of natural bioactive agents: Recent advances and challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, S.K.; Koushki, K.; Izadi, O.; Penson, P.E.; Sukhorukov, V.N.; Kesharwani, P.; Sahebkar, A. Advancements in curcumin-loaded PLGA nanoparticle delivery systems: Progressive strategies in cancer therapy. J. Drug Target. 2024, 32, 1207–1232. [Google Scholar] [CrossRef]
- Oryani, M.A.; Nosrati, S.; Javid, H.; Mehri, A.; Hashemzadeh, A.; Karimi-Shahri, M. Targeted cancer treatment using folate-conjugated sponge-like ZIF-8 nanoparticles: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 1377–1404. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [PubMed]
- Ghazal, H.; Waqar, A.; Yaseen, F.; Shahid, M.; Sultana, M.; Tariq, M.; Bashir, M.K.; Tahseen, H.; Raza, T.; Ahmad, F. Role of nanoparticles in enhancing chemotherapy efficacy for cancer treatment. Next Mater. 2024, 2, 100128. [Google Scholar] [CrossRef]
- Zeng, L.; Gowda, B.J.; Ahmed, M.G.; Abourehab, M.A.; Chen, Z.S.; Zhang, C.; Li, J.; Kesharwani, P. Advancements in nanoparticle-based treatment approaches for skin cancer therapy. Mol. Cancer 2023, 22, 10. [Google Scholar] [CrossRef]
- Mishra, S.; Bhatt, T.; Kumar, H.; Jain, R.; Shilpi, S.; Jain, V. Nanoconstructs for theranostic application in cancer: Challenges and strategies to enhance the delivery. Front. Pharmacol. 2023, 14, 1101320. [Google Scholar] [CrossRef]
- Naumenko, E.; Guryanov, I.; Gomzikova, M. Drug Delivery Nano-Platforms for Advanced Cancer Therapy. Sci. Pharm. 2024, 92, 28. [Google Scholar] [CrossRef]
- Sultana, Z.; Jamil, Z.; Samanta, A.; Alam, S.S.M.; Ali, S.; Hoque, M. Current status, challenges, and future perspective of nanomedicine-based cancer immunotherapy. In Nanomedicine in Cancer Immunotherapy; Academic Press: Cambridge, MA, USA, 2024; pp. 495–516. [Google Scholar]
- Al-Taie, A.; Özcan Bülbül, E. A paradigm use of monoclonal antibodies-conjugated nanoparticles in breast cancer treatment: Current status and potential approaches. J. Drug Target. 2024, 32, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sandbhor, P.; Palkar, P.; Bhat, S.; John, G.; Goda, J.S. Nanomedicine as a multimodal therapeutic paradigm against cancer: On the way forward in advancing precision therapy. Nanoscale 2024, 16, 6330–6364. [Google Scholar] [CrossRef]
- Kesharwani, P.; Xie, L.; Banerjee, S.; Mao, G.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3, 4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf. B Biointerfaces 2015, 136, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.; Kumar, A. Analytical Model to Deduce the Conformational and Dynamical Behavior in Dendrimers: A Review. Polymers 2024, 16, 1918. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Talhami, M.; Hassanein, A.; Mahdi, E.; Maryam, A.E.; Hassan, M.K.; Altaee, A.; Das, P.; Hawari, A.H. Advances in functionalization and conjugation mechanisms of dendrimers with iron oxide magnetic nanoparticles. Nanoscale 2024, 16, 13331–13372. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P.; Porwal, M.; Sur, S.; Verma, A. Dendrimer as nanocarrier for drug delivery and drug targeting therapeutics: A fundamental to advanced systematic review. Int. J. Polym. Mater. Polym. Biomater. 2024, 73, 310–332. [Google Scholar] [CrossRef]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Kesharwani, P.; Tekade, R.K.; Jain, N.K. Generation dependent cancer targeting potential of poly (propyleneimine) dendrimer. Biomaterials 2014, 35, 5539–5548. [Google Scholar] [CrossRef] [PubMed]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.-F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, Y.; Sun, Y. Dendrimer Applications for Cancer Therapies. J. Phys. Conf. Ser. 2021, 1948, 012205. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Abdelghani, A.A.; El-Sadany, S.K.; Overy, D.P.; Kerr, R.G. Antimicrobial and anticancer activities of organoiron melamine dendrimers capped with piperazine moieties. Eur. Polym. J. 2016, 82, 307–323. [Google Scholar] [CrossRef]
- Lim, J.; Lo, S.-T.; Hill, S.; Pavan, G.M.; Sun, X.; Simanek, E.E. Antitumor activity and molecular dynamics simulations of paclitaxel-laden triazine dendrimers. Mol. Pharm. 2012, 9, 404–412. [Google Scholar] [CrossRef]
- Ambekar, R.S.; Choudhary, M.; Kandasubramanian, B. Recent advances in dendrimer-based nanoplatform for cancer treatment: A review. Eur. Polym. J. 2020, 126, 109546. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, X.; Hu, Q.; Wu, Y.; Yu, F.; He, C.; Qian, Y.; Han, Y.; Tang, J.; Hu, H. Novel manganese and polyester dendrimer-based theranostic nanoparticles for MRI and therapy of breast cancer. J. Mater. Chem. B 2023, 11, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Davoodikia, B.; Hamedani, M.P.; Saffari, M.; Ebrahimi, S.E.S.; Hamzeh, M.S.; Hashemi, S.; Ardestani, M.S.; Ghoreishi, S.M. Synthesis of novel nano-radiotracer for in-vivo bone imaging: 99mTc-citric acid based PEG dendrimer and its conjugation with alendronate. Arab. J. Chem. 2022, 15, 104060. [Google Scholar] [CrossRef]
- Dey, A.D.; Bigham, A.; Esmaeili, Y.; Ashrafizadeh, M.; Moghaddam, F.D.; Tan, S.C.; Yousefiasl, S.; Sharma, S.; Maleki, A.; Rabiee, N.; et al. Dendrimers as nanoscale vectors: Unlocking the bars of cancer therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 86, pp. 396–419. [Google Scholar]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, K.; Hu, Q.; Yao, Q.; Shen, Y.; Yu, G.; Tang, G. Targeted co-delivery of PTX and TR3 siRNA by PTP peptide modified dendrimer for the treatment of pancreatic cancer. Small 2017, 13, 1602697. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cheng, Y.; Xu, T.; Wang, X.; Wen, L.-P. Targeting cancer cells with biotin–dendrimer conjugates. Eur. J. Med. Chem. 2009, 44, 862–868. [Google Scholar] [CrossRef]
- Patri, A.K.; Myc, A.; Beals, J.; Thomas, T.P.; Bander, N.H.; Baker, J.R. Synthesis and in vitro testing of J591 antibody—Dendrimer conjugates for targeted prostate cancer therapy. Bioconjug. Chem. 2004, 15, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Maiti, P. Nanomedicine and versatile therapies for cancer treatment. MedComm 2022, 3, e163. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Huang, Y.; Lian, B.; Ma, C.; Han, L.; Chen, Y.; Wu, S.; Li, N.; Zhang, W.; et al. A Self-Assembling Amphiphilic Peptide Dendrimer-Based Drug Delivery System for Cancer Therapy. Pharmaceutics 2021, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Bitan-Cherbakovsky, L.; Libster, D.; Aserin, A.; Garti, N. Complex dendrimer–lyotropic liquid crystalline systems: Structural behavior and interactions. J. Phys. Chem. B 2011, 115, 11984–11992. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Wang, Y.; Blake, S.; Yu, M.; Mei, L.; Wang, H.; Shi, J. RNA nanotechnology-mediated cancer immunotherapy. Theranostics 2020, 10, 281. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. The therapeutic efficacy of dendrimer and micelle formulations for breast cancer treatment. Pharmaceutics 2020, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Oyeniyi, Y.; Abdurahma, A. Fabrication and design of dendrimers for cancer chemotherapy. Bio Res. 2016, 14, 926–935. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, Y.; Li, Q.; Liu, W.; Dong, A.; Zhang, L. 2D nanomaterial-based 3D hydrogels for anti-infection therapy. J. Mater. Chem. B 2024, 12, 916–951. [Google Scholar] [CrossRef]
- Anegbe, B.; Ifijen, I.H.; Maliki, M.; Uwidia, I.E.; Aigbodion, A.I. Graphene oxide synthesis and applications in emerging contaminant removal: A comprehensive review. Environ. Sci. Eur. 2024, 36, 15. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.-U.; Hahn, J.R.; Ryu, S.; You, N.-H. Highly water-dispersible methylpyridinium salt functionalized reduced graphene oxide and poly (vinyl alcohol) composites. Compos. Part B Eng. 2024, 271, 111142. [Google Scholar] [CrossRef]
- Doan, L.; Nguyen, T.T.T.; Tran, K.; Huynh, K.G. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Chitosan, Polyethylene Glycol, Polyvinyl Alcohol, and Polyvinylpyrrolidone as Methylene Blue Adsorbent Beads. Polymers 2024, 16, 1839. [Google Scholar] [CrossRef] [PubMed]
- Virk, V.; Deepak, H.; Taneja, K.; Srivastava, R.; Giri, S. Amelioration in nanobiosensors for the control of plant diseases: Current status and future challenges. Front. Nanotechnol. 2024, 6, 1310165. [Google Scholar] [CrossRef]
- Huang, K.J.; Li, J.; Wu, Y.Y.; Liu, Y.M. Amperometric immunobiosensor for α-fetoprotein using Au nanoparticles/chitosan/TiO2-graphene composite based platform. Bioelectrochemistry 2013, 90, 1823. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Lee, Y.T.; Tseng, H.R.; Zhu, Y.; You, S.; Agopian, V.G.; Yang, J.D. Alpha-fetoprotein: Past, present, and future. Hepatol. Commun. 2024, 8, e0422. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Jorge, P.A. Applications of Electrochemical Impedance Spectroscopy in Disease Diagnosis—A Review. Sens. Actuators Rep. 2024, 8, 100205. [Google Scholar] [CrossRef]
- Zheng, R.; Wu, A.; Li, J.; Tang, Z.; Zhang, J.; Zhang, M.; Wei, Z. Progress and outlook on electrochemical sensing of lung cancer biomarkers. Molecules 2024, 29, 3156. [Google Scholar] [CrossRef]
- Wang, C.W.; Weaver, S.D.; Boonpattrawong, N.; Schuster-Little, N.; Patankar, M.; Whelan, R.J. A revised molecular model of ovarian cancer biomarker CA125 (MUC16) enabled by long-read sequencing. Cancer Res. Commun. 2024, 4, 253–263. [Google Scholar] [CrossRef]
- Milella, M.; Rutigliano, M.; Lasorsa, F.; Ferro, M.; Bianchi, R.; Fallara, G.; Crocetto, F.; Pandolfo, S.D.; Barone, B.; D’amati, A.; et al. The role of MUC1 in renal cell carcinoma. Biomolecules 2024, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Kuru, C.İ.; Ulucan-Karnak, F.; Yilmaz-Sercinoglu, Z. Cancer diagnosis via functionalized nanomaterial-based biosensors. In Functionalized Nanomaterials for Biosensing and Bioelectronics Applications; Woodhead Publishing: Sawston, UK, 2024; pp. 251–270. [Google Scholar]
- Cui, Q.; Li, J.; Li, Y.; Tang, L.; Li, K.; Li, T.; Chen, X.; Zhang, Z.; Zhang, G.J. Sensitive and rapid detection of bacterial endotoxin with a functional carbon nanotube field-effect transistor biosensor. Talanta 2024, 266, 125035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, Q.; Xu, J. Research progress in non-precious metal oxide/compound-based electrodes for non-enzymatic electrochemical glucose sensor applications. Mater. Sci. Semicond. Process. 2024, 181, 108643. [Google Scholar] [CrossRef]
- Balayan, S.; Islam, M.S.; Bhattacharjee, S.; Banik, S.; Mishra, A.; Ashaduzzaman, M.; Tiwari, A. Programmable electroanalysis enabling computable bioelectronics. Chem. Eng. J. 2024, 487, 150392. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Shi, J.; Jiang, Z.; Zhang, C.Y. Graphene-based nanomaterials for cancer therapy and anti-infections. Bioact. Mater. 2022, 14, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Bin Hussein, M.Z.; Fakurazi, S.; Twyman, L.J. Graphene oxide-gallic acid nanodelivery system for cancer therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Lima-Sousa, R.; de Melo-Diogo, D.; Alves, C.G.; Costa, E.C.; Ferreira, P.; Louro, R.O.; Correia, I.J. Hyaluronic acid functionalized green reduced graphene oxide for targeted cancer photothermal therapy. Carbohydr. Polym. 2018, 200, 93–99. [Google Scholar] [CrossRef]
- Deng, X.; Liang, H.; Yang, W.; Shao, Z. Polarization and function of tumor-associated macrophages mediate graphene oxide-induced photothermal cancer therapy. J. Photochem. Photobiol. B Biol. 2020, 208, 111913. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mohammad-Rezaei, R.; Jahanban-Esfahlan, R.; Massoumi, B.; Abbasian, M.; Jafarizad, A.; Jaymand, M. A de novo theranostic nanomedicine composed of PEGylated graphene oxide and gold nanoparticles for cancer therapy. J. Mater. Res. 2020, 35, 430–441. [Google Scholar] [CrossRef]
- Lakshmanamoorthy, K.; Prabhu, S.; Ravikumar, V.; Manivannan, S. Effect of Ionic Liquid Anions in Tunning the Morphology and Size of Ag in rGO-Ag Nanocomposites: Anticancer Activity of the Composites Against A549 Lung Cancer Cells. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3417–3428. [Google Scholar] [CrossRef]
- Dou, R.; Du, Z.; Bao, T.; Dong, X.; Zheng, X.; Yu, M.; Yin, W.; Dong, B.; Yan, L.; Gu, Z. The polyvinylpyrrolidone functionalized rGO/Bi2S3 nanocomposite as a near-infrared light-responsive nanovehicle for chemo-photothermal therapy of cancer. Nanoscale 2016, 8, 11531–11542. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Ghule, A.V.; Ling, Y.-C. Multimodal Imaging and Phototherapy of Cancer and Bacterial Infection by Graphene and Related Nanocomposites. Molecules 2022, 27, 5588. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.W.; Li, J.; Pu, K. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Luo, T.; Qu, J.; Chen, W.R. Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem. Biomaterials 2021, 265, 120421. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.W.; Sunwoo, S.-H.; Hong, Y.J.; Koo, J.H.; Kim, J.H.; Baik, S.; Hyeon, T.; Kim, D.-H. Soft bioelectronics based on nanomaterials. Chem. Rev. 2021, 122, 5068–5143. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, G.; Wen, X.; Li, F.; Ji, X.; Li, Q.; Wu, M.; Cheng, Q.; Yu, Y.; Tang, J.; et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J. Control. Release 2020, 326, 131–139. [Google Scholar] [CrossRef]
- Shaheen, F.; Aziz, M.H.; Fakhar-e-Alam, M.; Atif, M.; Fatima, M.; Ahmad, R.; Hanif, A.; Anwar, S.; Zafar, F.; Abbas, G.; et al. An in vitro study of the photodynamic effectiveness of GO-ag nanocomposites against human breast Cancer cells. Nanomaterials 2017, 7, 401. [Google Scholar] [CrossRef]
- Kumar, P.S.; Padmalaya, G.; Elavarasan, N.; Sreeja, B. GO/ZnO nanocomposite-as transducer platform for electrochemical sensing towards environmental applications. Chemosphere 2023, 313, 137345. [Google Scholar] [CrossRef] [PubMed]
- Melo, B.L.; Lima-Sousa, R.; Alves, C.G.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Chitosan-based injectable in situ forming hydrogels containing dopamine-reduced graphene oxide and resveratrol for breast cancer chemo-photothermal therapy. Biochem. Eng. J. 2022, 185, 108529. [Google Scholar] [CrossRef]
- Yin, F.; Hu, K.; Chen, Y.; Yu, M.; Wang, D.; Wang, Q.; Yong, K.-T.; Lu, F.; Liang, Y.; Li, Z. SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics 2017, 7, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, C.; Zheng, D.; Guo, Q.; Maierhaba, M.; Xue, L.; Zeng, X.; Wu, Y.; Gao, W. An original cuproptosis-related genes signature effectively influences the prognosis and immune status of head and neck squamous cell carcinoma. Front. Genet. 2023, 13, 1084206. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.S.; Lee, S.S.; Barocas, D.A.; Tosoian, J.J. Evaluation of blood and urine based biomarkers for detection of clinically-significant prostate cancer. Prostate Cancer Prostatic Dis. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Ruzgar, S.T.; Sanli, S.; Keles, N.A.; Ayden, A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; et al. Magnetic nanoparticle-based electrochemical sensing platform using ferrocene-labelled peptide nucleic acid for the early diagnosis of colorectal cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Florek, K.; Mendyka, D.; Gomułka, K. Vascular Endothelial Growth Factor (VEGF) and Its Role in the Cardiovascular System. Biomedicines 2024, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; A Aljabali, A.; Tambuwala, M.M. Bioinspired and green synthesis of silver nanoparticles for medical applications: A green perspective. Appl. Biochem. Biotechnol. 2024, 196, 3636–3669. [Google Scholar] [CrossRef] [PubMed]
- Abdelkawi, A.; Slim, A.; Zinoune, Z.; Pathak, Y. Surface modification of metallic nanoparticles for targeting drugs. Coatings 2023, 13, 1660. [Google Scholar] [CrossRef]
- Shin, M.C.; Zhang, J.; Min, K.A.; Lee, K.; Byun, Y.; David, A.E.; He, H.; Yang, V.C. Cell-penetrating peptides: Achievements and challenges in application for cancer treatment. J. Biomed. Mater. Res. Part A 2014, 102, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Singh, P.; Kumar, M.; Pandey, S.K.; Sharma, R.K.; Wangoo, N. A lysine-rich cell penetrating peptide engineered multifunctional gold nanoparticle-based drug delivery system with enhanced cellular penetration and stability. J. Mater. Sci. 2022, 57, 16842–16857. [Google Scholar] [CrossRef]
- Shadmani, N.; Makvandi, P.; Parsa, M.; Azadi, A.; Nedaei, K.; Mozafari, N.; Poursina, N.; Mattoli, V.; Tay, F.R.; Maleki, A.; et al. Enhancing Methotrexate Delivery in the Brain by Mesoporous Silica Nanoparticles Functionalized with Cell-Penetrating Peptide using in Vivo and ex Vivo Monitoring. Mol. Pharm. 2023, 20, 1531–1548. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Xie, X.; Cai, X.; Zhang, H.; Gong, W.; Wang, Z.; Mei, X. PEGylated liposomes with NGR ligand and heat-activable cell-penetrating peptide—Doxorubicin conjugate for tumor-specific therapy. Biomaterials 2014, 35, 4368–4381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-Y.; Zhang, W.; Liu, H.; Jiang, J.-J.; Wang, W.-J.; Jia, Z.-Y. Cell-Penetrating peptide-modified graphene oxide nanoparticles loaded with rictor siRNA for the treatment of triple-negative breast cancer. Drug Des. Dev. Ther. 2021, 15, 4961–4972. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Deng, B.; Zhao, H.; Li, L.; Fang, Y.; Li, S.; Deng, X.; Chen, J. Design, Synthesis, and Bioevaluation of Novel Enzyme-Triggerable Cell Penetrating Peptide-Based Dendrimers for Targeted Delivery of Camptothecin and Cancer Therapy. J. Med. Chem. 2022, 65, 5850–5865. [Google Scholar] [CrossRef]
- Majeed, S.; Saravanan, M.; Danish, M.; Zakariya, N.A.; Ibrahim, M.N.M.; Rizvi, E.H.; NisaAndrabi, S.U.; Barabadi, H.; Mohanta, Y.K.; Mostafavi, E. Bioengineering of green-synthesized TAT peptide-functionalized silver nanoparticles for apoptotic cell-death mediated therapy of breast adenocarcinoma. Talanta 2023, 253, 124026. [Google Scholar] [CrossRef]
- Khan, S.; Vahdani, Y.; Hussain, A.; Haghighat, S.; Heidari, F.; Nouri, M.; Bloukh, S.H.; Edis, Z.; Babadaei, M.M.N.; Ale-Ebrahim, M.; et al. Polymeric micelles functionalized with cell penetrating peptides as potential pH-sensitive platforms in drug delivery for cancer therapy: A review. Arab. J. Chem. 2021, 14, 103264. [Google Scholar] [CrossRef]
- Xu, J.; Khan, A.R.; Fu, M.; Wang, R.; Ji, J.; Zhai, G. Cell-penetrating peptide: A means of breaking through the physiological barriers of different tissues and organs. J. Control. Release 2019, 309, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ji, Z.; Zhang, F.; Luo, P.; Zhang, H.; Zhou, J.; Cheng, H.; Ding, Y. Charge reversal hairpin peptide modified synergy therapeutic nanoplatforms for tumor specific drug shuttling. Biomater. Sci. 2022, 10, 4889–4901. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zhang, L.; Chen, P. Membrane Internalization Mechanisms and Design Strategies of Arginine-Rich Cell-Penetrating Peptides. Int. J. Mol. Sci. 2022, 23, 9038. [Google Scholar] [CrossRef]
- Kadari, A.; Pooja, D.; Gora, R.H.; Gudem, S.; Kolapalli, V.R.M.; Kulhari, H.; Sistla, R. Design of multifunctional peptide collaborated and docetaxel loaded lipid nanoparticles for antiglioma therapy. Eur. J. Pharm. Biopharm. 2018, 132, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Gao, Y.; Song, C.; Ouyang, Z.; Li, C.; Mignani, S.; Majoral, J.-P.; Shi, X.; Shen, M. Impact of molecular rigidity on the gene delivery efficiency of core–shell tecto dendrimers. J. Mater. Chem. B 2021, 9, 6149–6154. [Google Scholar] [CrossRef] [PubMed]
- Matijass, M.; Neundorf, I. Cell-penetrating peptides as part of therapeutics used in cancer research. Med. Drug Discov. 2021, 10, 100092. [Google Scholar] [CrossRef]
- Wu, Y.; Angelova, A. Recent uses of lipid nanoparticles, cell-penetrating and bioactive peptides for the development of brain-targeted nanomedicines against neurodegenerative disorders. Nanomaterials 2023, 13, 3004. [Google Scholar] [CrossRef]
- Nam, S.H.; Park, J.; Koo, H. Recent advances in selective and targeted drug/gene delivery systems using cell-penetrating peptides. Arch. Pharmacal Res. 2023, 46, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Zorko, M.; Langel, Ü. Cell-penetrating peptides. In Cell Penetrating Peptides; Humana: New York, NY, USA, 2022; pp. 3–32. [Google Scholar]
- Kardani, K.; Bolhassani, A. Exploring novel and potent cell penetrating peptides in the proteome of SARS-CoV-2 using bioinformatics approaches. PLoS ONE 2021, 16, e0247396. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Navarro, M. Advances in peptide-mediated cytosolic delivery of proteins. Adv. Drug Deliv. Rev. 2021, 171, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.C.; Scott, C.J. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy. Drug Discov. Today: Technol. 2018, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Farahavar, G.; Abolmaali, S.S.; Gholijani, N.; Nejatollahi, F. Antibody-guided nanomedicines as novel breakthrough therapeutic, diagnostic and theranostic tools. Biomater. Sci. 2019, 7, 4000–4016. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wu, F.G.; Chen, X. Antibody-incorporated nanomedicines for cancer therapy. Adv. Mater. 2022, 34, 2109210. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An overview of antibody conjugated polymeric nanoparticles for breast cancer therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef] [PubMed]
- Hammood, M.; Craig, A.W.; Leyton, J.V. Impact of endocytosis mechanisms for the receptors targeted by the currently approved antibody-drug conjugates (ADCs)—A necessity for future ADC research and development. Pharmaceuticals 2021, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Gulati, N.; Awasthi, R.; Arora, V.; Singh, S.K.; Kumar, S.; Gupta, G.; Dua, K.; Pahwa, R.; Dureja, H. Monoclonal antibodies and antibody-drug conjugates as emerging therapeutics for breast cancer treatment. Curr. Drug Deliv. 2024, 21, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Z.; Li, C.; Duan, G.; Wang, K.; Li, Q.; Tao, T. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed. Pharmacother. 2018, 106, 275–284. [Google Scholar] [CrossRef]
- Taheri-Ledari, R.; Qazi, F.S.; Saeidirad, M.; Maleki, A. A diselenobis-functionalized magnetic catalyst based on iron oxide/silica nanoparticles suggested for amidation reactions. Sci. Rep. 2022, 12, 14865. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, D.W.; Jones, V.O.; Choi, Y.; Ferry, V.E.; Geller, M.A.; Azarin, S.M. Sonosensitizer-Functionalized Graphene Nanoribbons for Adhesion Blocking and Sonodynamic Ablation of Ovarian Cancer Spheroids. Adv. Healthc. Mater. 2021, 10, 2001368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma-Walia, N.; Kapoor, S.; Tandon, S. Antibody-targeted nanoparticles for cancer treatment. NanoBioMedicine 2020, 8, 35–65. [Google Scholar]
- Sharma, T.; Kaur, J.; Prajapati, B.; Jain, A. Lipid-Based Excipients: The Holy Grail in Pharmaceutical Product Development. In Lipid-Based Drug Delivery Systems; Jenny Stanford Publishing: Singapore, 2024; pp. 1–26. [Google Scholar]
- Sambhakar, S.; Saharan, R.; Narwal, S.; Malik, R.; Gahlot, V.; Khalid, A.; Najmi, A.; Zoghebi, K.; Halawi, M.A. Exploring LIPIDs for their potential to improves bioavailability of lipophilic drugs candidates: A review. Saudi Pharm. J. 2023, 31, 101870. [Google Scholar]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current advances in lipid nanosystems intended for topical and transdermal drug delivery applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Quach, H.; Le, T.-V.; Nguyen, T.-T.; Nguyen, P.; Nguyen, C.K.; Dang, L.H. Nano-Lipids Based on Ginger Oil and Lecithin as a Potential Drug Delivery System. Pharmaceutics 2022, 14, 1654. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Guan, S.; Witte, F. The therapeutic potential of MSC-EVs as a bioactive material for wound healing. Eng. Regen. 2021, 2, 182–194. [Google Scholar] [CrossRef]
- Guevara, M.L.; Persano, F.; Persano, S. Advances in lipid nanoparticles for mRNA-based cancer immunotherapy. Front. Chem. 2020, 8, 589959. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Vicario-De-La-Torre, M.; Caballo-González, M.; Vico, E.; Morales-Fernández, L.; Arriola-Villalobos, P.; Heras, B.D.L.; Benítez-Del-Castillo, J.M.; Guzmán, M.; Millar, T.; Herrero-Vanrell, R.; et al. Novel nano-liposome formulation for dry eyes with components similar to the preocular tear film. Polymers 2018, 10, 425. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef] [PubMed]
- Le, N.T.T.; Du Cao, V.; Nguyen, T.N.Q.; Le, T.T.H.; Tran, T.T.; Thi, T.T.H. Soy lecithin-derived liposomal delivery systems: Surface modification and current applications. Int. J. Mol. Sci. 2019, 20, 4706. [Google Scholar] [CrossRef]
- Sundar, S.K.; Parikh, J.K. Advances and trends in encapsulation of essential oils. Int. J. Pharm. 2023, 635, 122668. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Hasham, R.; Abd Rasid, Z.I.; Noor, N.M. Formulation and characterization of nanostructured lipid carrier encapsulate lemongrass oil using ultrasonication technique. Chem. Eng. Trans. 2021, 83, 475–480. [Google Scholar]
- Coc, L.M.C.; Lacatusu, I.; Badea, N.; Barbinta-Patrascu, M.E.; Meghea, A. Effective lipid nanocarriers based on linseed oil for delivery of natural polyphenolic active. J. Nanomater. 2021, 2021, 8853941. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salim, N.; Wui, W.T.; Basri, M.; Rahman, M.B.A. Optimization of quercetin loaded palm oil ester based nanoemulsion formulation for pulmonary delivery. J. Oleo Sci. 2018, 67, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.-P.; Shi, F.; Zhao, J.-H.; Liu, Y.; Wang, Z.; Zhang, Y.-T. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012, 7, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, G.; Muralidaran, Y.; Sridharan, B.; Ganesh, R.N.; Viswanathan, P. Evaluation of garlic oil in nano-emulsified form: Optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem. Toxicol. 2017, 105, 203–213. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Mollazadeh, H.; Afshari, A.R. Pomegranate seed oil: A comprehensive review on its therapeutic effects. Int. J. Pharm. Sci. Res. 2016, 7, 430. [Google Scholar]
- Charman, W.N. Lipid Vehicle and Formulation Effects on Intestinal Lymphatic Drug Transport. In Lymphatic Transport of Drugs; Routledge: London, UK, 2019; pp. 113–179. [Google Scholar]
- Hauss, D.J.; Fogal, S.E.; Ficorilli, J.V.; Price, C.A.; Roy, T.; Jayaraj, A.A.; Keirns, J.J. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J. Pharm. Sci. 1998, 87, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Conejos-Sánchez, I.; Griffin, B.T.; O’Driscoll, C.M.; Alonso, M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, S.; Arora, S.; Samsonraj, R.; Govindaiah, P.; Vuree, S. Lipid Based Nanoparticles for Treatment of Cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Xu, C.; Wei, J.; Liu, Y.; Cun, X.; Yu, Q.; Tang, X.; Yin, S.; Zhang, Z.; et al. Tumor-targeted chemoimmunotherapy with immune-checkpoint blockade for enhanced anti-melanoma efficacy. AAPS J. 2019, 21, 18. [Google Scholar] [CrossRef]
- Mei, K.-C.; Liao, Y.-P.; Jiang, J.; Chiang, M.; Khazaieli, M.; Liu, X.; Wang, X.; Liu, Q.; Chang, C.H.; Zhang, X.; et al. Liposomal delivery of mitoxantrone and a cholesteryl indoximod prodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano 2020, 14, 13343–13366. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.R.; Paliwal, R.; Pal, H.C.; Saxena, A.K.; Sharma, P.R.; Gupta, P.N.; Agrawal, G.P.; Vyas, S.P. Estrogen-anchored pH-sensitive liposomes as nanomodule designed for site-specific delivery of doxorubicin in breast cancer therapy. Mol. Pharm. 2012, 9, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E. Liposomal anthracyclines in metastatic breast cancer: Clinical update. Oncologist 2003, 8, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Elamir, A.; Ajith, S.; Al Sawaftah, N.; Abuwatfa, W.; Mukhopadhyay, D.; Paul, V.; Al-Sayah, M.H.; Awad, N.; Husseini, G.A. Ultrasound-triggered herceptin liposomes for breast cancer therapy. Sci. Rep. 2021, 11, 7545. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-Q.; Chen, M.-S.; Zhou, X.; Guo, L.-M.; Zhu, J.-J.; Wang, R.; Zhang, X.-X.; Gan, Y. Chitosan oligosaccharide modified liposomes enhance lung cancer delivery of paclitaxel. Acta Pharmacol. Sin. 2021, 42, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.S.; Patil, S.M.; Shukla, S.K.; Kulkarni, N.S.; Gupta, V.; Kunda, N.K. Pulmonary delivery of osimertinib liposomes for non-small cell lung cancer treatment: Formulation development and in vitro evaluation. Drug Deliv. Transl. Res. 2022, 12, 2474–2487. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Lo, P.Y.; Cho, E.C.; Zheng, J.H.; Li, M.; Huang, J.H.; Lee, K.C. Integration of PEG and PEI with graphene quantum dots to fabricate pH-responsive nanostars for colon cancer suppression in vitro and in vivo. FlatChem 2022, 31, 100320. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, W.; Yang, Y.; Wang, Q. Gemcitabine-loaded RGD modified liposome for ovarian cancer: Preparation, characterization and pharmacodynamic studies. Drug Des. Dev. Ther. 2019, 13, 3281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, H.; Chen, S. Aptamer (AS1411)-conjugated liposome for enhanced therapeutic efficacy of miRNA-29b in ovarian cancer. J. Nanosci. Nanotechnol. 2020, 20, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef]
- Soni, N.K.; Sonali, L.; Singh, A.; Mangla, B.; Neupane, Y.R.; Kohli, K. Nanostructured lipid carrier potentiated oral delivery of raloxifene for breast cancer treatment. Nanotechnology 2020, 31, 475101. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, Y.; Wu, Z.; Zhang, L.; He, D.; Li, X.; Wang, Z. Synergistic combination therapy of lung cancer: Cetuximab functionalized nanostructured lipid carriers for the co-delivery of paclitaxel and 5-demethylnobiletin. Biomed. Pharmacother. 2019, 118, 109225. [Google Scholar] [CrossRef]
- Kamel, A.E.; Fadel, M.; Louis, D. Curcumin-loaded nanostructured lipid carriers prepared using Peceol™ and olive oil in photodynamic therapy: Development and application in breast cancer cell line. Int. J. Nanomed. 2019, 14, 5073. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Mohamad, N.E.; Abu, N.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. Antitumor and anti-metastatic effects of citral-loaded nanostructured lipid carrier in 4T1-induced breast cancer mouse model. Molecules 2020, 25, 2670. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.H.; Alcantara, K.P.; Bulatao, B.P.I.; Sorasitthiyanukarn, F.N.; Muangnoi, C.; Nalinratana, N.; Vajragupta, O.; Rojsitthisak, P. Chitosan-coated nanostructured lipid carriers for transdermal delivery of tetrahydrocurcumin for breast cancer therapy. Carbohydr. Polym. 2022, 288, 119401. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, A.; Annu; Biswas, L.; Verma, A.K.; Sahoo, P.K.; Baboota, S.; Ali, J. Ribociclib nanostructured lipid carrier aimed for breast cancer: Formulation optimization, attenuating in vitro specification, and in vivo scrutinization. BioMed Res. Int. 2022, 2022, 6009309. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.; Rehman, M.U.; Asim, M.H.; Mahmood, A.; Ijaz, M.; Alamgeer; Irfan, H.M.; Anwar, F.; Ali, M.Y. Calycosin-loaded nanostructured lipid carriers: In-vitro and in-vivo evaluation for enhanced anti-cancer potential. J. Drug Deliv. Sci. Technol. 2022, 67, 102957. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Harisa, G.I.; Shahba, A.A.; Alanazi, F.K.; Qamar, W. Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer. Molecules 2023, 28, 448. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Pi, C.; Zhao, S.; Fu, S.; Yang, H.; Zheng, X.; Zhang, X.; Zhao, L.; Wei, Y. Oral co-delivery nanoemulsion of 5-fluorouracil and curcumin for synergistic effects against liver cancer. Expert Opin. Drug Deliv. 2020, 17, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.E.M.; Lemos, J.d.A.; Fernandes, R.S.; Silva, J.d.O.; Ottoni, F.M.; Townsend, D.M.; Rubello, D.; Alves, R.J.; Cassali, G.D.; Ferreira, L.A.M.; et al. Enhanced antitumor efficacy of lapachol-loaded nanoemulsion in breast cancer tumor model. Biomed. Pharmacother. 2021, 133, 110936. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; Fahmy, U.A.; Alfaleh, M.A.; Asfour, H.Z. Formulation design, statistical optimization, and in vitro evaluation of a naringenin nanoemulsion to enhance apoptotic activity in a549 lung cancer cells. Pharmaceuticals 2020, 13, 152. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Bawadud, R.S.; Gashlan, H.M. Incorporation of docetaxel and thymoquinone in borage nanoemulsion potentiates their antineoplastic activity in breast cancer cells. Sci. Rep. 2020, 10, 18124. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Tang, S.; Yu, A.; Wang, A.; Tang, W.; Jia, H.; Oupický, D. Nanoemulsion-Assisted siRNA Delivery to Modulate the Nervous Tumor Microenvironment in the Treatment of Pancreatic Cancer. ACS Appl. Mater. Interfaces 2022, 14, 10015–10029. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Varghese, J.; Vidyalakshmi, I.S.; Thomas, R.K. Magnetic Nanoparticles and Its Biomedical Applications. In Magnetic Nanoparticles: A New Platform for Drug Delivery; Joshy, K.S., Sabu, T., Thakur, V.K., Eds.; Springer: Singapore, 2021; pp. 1–30. [Google Scholar]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release 2021, 335, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Farzin, A.; Etesami, S.A.; Quint, J.; Memic, A.; Tamayol, A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv. Healthc. Mater. 2020, 9, e1901058. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Tertis, M.; Cristea, C. Implication of magnetic nanoparticles in cancer detection, screening and treatment. Magnetochemistry 2019, 5, 55. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Gholami, A.; Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Chiang, W.-H.; Parvin, N. Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab. Rev. 2020, 52, 205–224. [Google Scholar] [PubMed]
- Cheng, H.W.; Tsao, H.Y.; Chiang, C.S.; Chen, S.Y. Advances in magnetic nanoparticle-mediated cancer immune-theranostics. Adv. Healthc. Mater. 2021, 10, 2001451. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, C.; Baiyee, Z.M.; Shao, Z.; Ciucci, F. Nonstoichiometric oxides as low-cost and highly-efficient oxygen reduction/evolution catalysts for low-temperature electrochemical devices. Chem. Rev. 2015, 115, 9869–9921. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.W.; Booijink, R.; Pater, L.; Wols, I.; Vrynas, A.; Storm, G.; Prakash, J.; Bansal, R. Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles (FGF2-SPIONs) ameliorate hepatic stellate cells activation in vitro and acute liver injury in vivo. J. Control. Release 2020, 328, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.L.; Gupta, M.K.; Johnson, B.N.; McAlpine, M.C. 3D printed bionic nanodevices. Nano Today 2016, 11, 330–350. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Kostarelos, K.; Mallick, P.; Dive, C.; Hadjidemetriou, M. Nano-omics: Nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. Nat. Rev. Clin. Oncol. 2022, 19, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Dalal, N.; Jalandra, R.; Sharma, M.; Prakash, H.; Makharia, G.K.; Solanki, P.R.; Singh, R.; Kumar, A. Omics technologies for improved diagnosis and treatment of colorectal cancer: Technical advancement and major perspectives. Biomed. Pharmacother. 2020, 131, 110648. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Diaz, A.K.; Shaw, T.I.; Li, Y.; Niu, M.; Cho, J.-H.; Paugh, B.S.; Zhang, Y.; Sifford, J.; Bai, B.; et al. Deep multiomics profiling of brain tumors identifies signaling networks downstream of cancer driver genes. Nat. Commun. 2019, 10, 3718. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.; Rydz, E.; Peters, C.E. Inequalities in environmental cancer risk and carcinogen exposures: A scoping review. Int. J. Environ. Res. Public Health 2023, 20, 5718. [Google Scholar] [CrossRef] [PubMed]
- Nagar, A.; Madamanchi, D.; Nair, G.R.; Revikumar, A.; Ray, S.; Vajjala, S.M.; S, A.B.; Shivale, S. Barriers to Cancer Diagnosis and Treatment: A Pilot Qualitative Study of Patient and Practitioner Perspectives in Rural India. Cureus 2024, 16, e67249. [Google Scholar] [CrossRef]
- Deo, S.V.; Pramanik, R.; Chaturvedi, M.; Nath, A.; Ghosh, J.; Das Majumdar, S.K.; Salins, N.; Kadayaprath, G.; Garg, P.K.; Chaturvedi, A.; et al. Telemedicine and cancer care in India: Promises, opportunities and caveats. Future Sci. OA 2022, 8, FSO821. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Gao, J.; Wen, L. Pro-death or pro-survival: Contrasting paradigms on nanomaterial-induced autophagy and exploitations for cancer therapy. Acc. Chem. Res. 2019, 52, 3164–3176. [Google Scholar] [CrossRef] [PubMed]
- Veiga, N.; Diesendruck, Y.; Peer, D. Targeted nanomedicine: Lessons learned and future directions. J. Control. Release 2023, 355, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, X.; Wang, C.; Zhao, Z.; Zhang, S.; Wang, Y.; Sun, B.; Luo, C.; He, Z. Molecularly engineered carrier-free co-delivery nanoassembly for self-sensitized photothermal cancer therapy. J. Nanobiotechnol. 2021, 19, 282. [Google Scholar] [CrossRef] [PubMed]



| Dendrimers | Conjugated Molecules | Drug | Type of Cancer | Conditions | Ref. |
|---|---|---|---|---|---|
| Plain dendrimers | |||||
| Poly (propylene imine) dendrimer (PPI) | Chemical conjugation | Melphalan | Breast | In vitro | [47,48] |
| Polyether dendrimers | D-glucose amine | Methotrexate (MTX) | Gliomas (malignant cancer) | In vitro | [49] |
| Poly-l-lysine dendrimers (PLL) | - | DOX | Lung and ovarian | In vitro and in vivo | [50] |
| Melamine dendrimer | Organoiron | Piperazine moieties | Breast | In vitro | [51] |
| Triazine dendrimer | - | Paclitaxel | Prostate | In vivo and in vitro | [52] |
| Poly (amidoamine) PAMAM dendrimer | Enzyme linked aptamer | Au-nanoparticles and prostate-specific antigen (PSA) | Prostate | In vitro | [53] |
| Polyester dendrimer | Manganese | Hypericin | Breast | In vitro | [54] |
| Citric acid dendrimer | Polyethylene glycol (PEG) | Alendronate | Bone | In vitro | [55] |
| Dendrimers based on macromolecular monomers | |||||
| Peptide dendrimer | Tetra-peptide sequence Gly-Phe-Leu-Gly (GFLG) | DOX | Ovarian | In vivo and in vitro | [53] |
| Carbohydrate dendrimer | D glucosamine | Methotrexate | Glioma | In vitro | [56] |
| PEG dendrimers | Di sulfide linkage | PTX and TR3 siRN | Pancreatic | In vitro and in vivo | [57] |
| Surface engineered dendrimers | |||||
| Acetylated dendrimer | Fluorescein isothiocyanate (FITC) | Biotin | Breast | In vitro | [58] |
| Antibody conjugated dendrimer | J591 anti-PSMA (prostate-specific membrane antigen), fluorophores | - | Prostate | In vitro | [59] |
| Folate conjugated dendrimer | PAMAM | MTX | Cervical and ovarian | In vitro | [60] |
| SiRNA conjugated dendrimer | PAMAM | - | Breast | In vivo | [61] |
| Dendrimers with special property | |||||
| Amphiphilic dendrimer | Peptide | DOX | Breast | In vivo and in vitro | [62] |
| Liquid crystalline dendrimer | Lyotropic | DOX | Ovarian | In vivo and in vitro | [63] |
| Hybrid dendrimer | Lipid | Paclitaxel | Ovarian | In vivo and in vitro | [64] |
| Micellar dendrimer | Micelles | Paclitaxel | Breast | In vivo | [65] |
| Multilingual dendrimer | Functional group | Methotrexate (MTX) | Cervical | In vivo | [66] |
| Graphene-Based Nanomaterials | Properties | Size (nm) | Type of Cancer | Type of Applications | Ref. |
|---|---|---|---|---|---|
| Bare graphene-based nanomaterial | |||||
| Graphene quantum dots (GQDs) | PTA | 41 | Cervical, (HeLa), breast | Drug delivery | [82] |
| Graphene oxide (GO) | Sharp edges/ROS | 10–120 | Liver | Drug delivery and imaging | [83] |
| Reduced graphene oxide (rGO) | Sharp edges/ROS | 42 | Liver | Drug delivery | [84] |
| Modified GBNMs | |||||
| GO-PEG | PTA | 10–120 | Osteosarcoma | Phytothermal therapy (PTT) | [85] |
| rGO-HA | PTA | 220–240 | Liver | PTT | [86] |
| GO-PEG-FA/GNPs-DOX | PTA/drug carrier | 10–120 | Human breast | PTT/chemotherapy | [87] |
| Silver nanostructure-reduced graphene oxide composites (rGO-Ag) | PTA/drug carrier | 40–50 | Lung | PTT/drug delivery | [88] |
| PVP-rGO/Bi2S3@DOX | PTA/RSs carrier | 10–120 | Cervical | PTT/radiotherapy | [89] |
| PAH/FA/PEG/GO/siRNA | PTA/gene carrier | 10–120 | Pancreatic | PTT/gene therapy | [90] |
| GO-(HPPH)-PEG-HK | PS/carrier | 10–120 | Synergized immunotherapy | PDT/immunotherapy | [91] |
| rGO/MTX/SB | PTA/drug carrier | 40–50 | Metastatic | PTT/chemotherapy/immunotherapy | [92] |
| GP/AgNW/doped/GP/IrOx | Transparent bioelectronics | - | Colon | PTT/PDT/detecting/chemotherapy | [93] |
| N-GQD/HMSN/C3N4/PEG-RGD | PTA | - | - | PTT/PDT/imaging | [94] |
| GO-Ag nanocomposites | Sharp edges/ROS | ~1.2 | Human breast | Membrane disruption/oxidative stress/Ag2+ | [95] |
| GO-ZnO nanocomposites | Sharp edges/ROS | - | All type of cancer | Membrane disruption/oxidative stress | [96] |
| DOPA-rGO | Chitosan carrier | 750–1000 | Breast | Chemo-photothermal therapy | [97] |
| PAH/FA/PEG/GO/siRNA | PTA/gene carrier | 10–100 | Pancreatic | PTT/gene therapy | [98] |
| CPP-Conjugated/Modified Nanoparticles | Drug | Type of Cancer | Condition | Ref. |
|---|---|---|---|---|
| CPP-modified lipid nano-capsule | Paclitaxel | GL261, glioma brain tumor | In vivo | [105] |
| Lysine-rich CPP conjugated with AuNPs | Doxorubicin | Anticancer activity | In vivo | [106] |
| Mesoporous silica nanoparticles conjugated with CPP | Methotrexate | Brain | In vitro | [107] |
| PEGlyated liposomes with CPP | Doxorubicin | Human breast adenocarcinoma cells and human fibrosarcoma cells | In vitro | [108] |
| CPP-modified graphene oxide nanoparticles | Small interfering SiRNA | Breast cancer | In vivo | [109] |
| Dendrimers conjugated with CPP | Camptothecin | Leukemia | In vivo | [110] |
| Silver nanoparticles conjugated with CPP | - | Breast adenocarcinoma | In vivo | [111] |
| PEG-PLA-PMs-HE-CPP | Paclitaxel | Breast cancer | In vivo | [112] |
| BSA nanoparticle conjugated with CPP(KALA) | Doxorubicin (DOX) Indocyanine green (ICG) | Inhibits tumor activity | Both in vivo and in vitro | [113] |
| Graphene oxide conjugated with CPP | Doxorubicin | Breast cancer | In vitro | [114] |
| Arginine-rich CPPs | Doxorubicin loaded liposomes | Ovarian cancer | In vitro | [115] |
| Solid lipid nanoparticle conjugated with CPP | Docetaxel | Glioblastoma multiforme | In vivo | [116] |
| PLGA-PEG with CPP | Paclitaxel | Hepatic cancer | In vivo | [117] |
| Solid lipid nanoparticles surface modified with cyclic peptides | Paclitaxel and naringenin | Glioblastoma multiforme | Both in vivo and in vitro | [118] |
| Polymersomes conjugated with selective CPP | MTX | Lung cancer | In vivo | [119] |
| Conjugated Nano Lipids | Drug | Type of Cancer | Condition | Ref. |
|---|---|---|---|---|
| Liposomes | ||||
| PEGylated liposomes | Doxorubicin | Breast | In vitro | [157] |
| Charge-reversal cell-penetrating peptide-modified liposomes | Paclitaxel | Melanoma | In vitro | [158] |
| pH-responsive liposomes | Mitoxantrone | Breast and renal | In vitro | [159] |
| Estrogen receptor-anchored pH-sensitive liposomes | Doxorubicin | Breast | In vitro | [160] |
| Anthracycline-conjugated liposomes | Daunorubicin, Doxorubicin, Epirubicin | Breast | In vitro | [161] |
| Herceptin liposomes | Doxorubicin | Breast | In vivo and in vitro | [162] |
| Chitosan oligosaccharide-modified liposomes | Paclitaxel | Lung | In vivo and in vitro | [163] |
| Osimertinib-encapsulated liposomes | Osimertinib | Non small-cell lung | In vitro | [164] |
| PEGlyated cationic liposomes | Kinesin spindle protein (KSP) siRNA/paclitaxel | Ovarian | In vivo and in vitro | [165] |
| RGD-modified liposomes | Gemcitabine (GEM) | Ovarian | In vitro | [166] |
| Aptamer-conjugated liposomes | miRNA-29b | Ovarian | In vitro | [167] |
| Nano lipid carrier | ||||
| Topical nanostructured lipid carrier | Quercetin and resveratrol | Skin | In vivo and in vitro | [168] |
| Nanostructured lipid carrier | Raloxifene | Breast | In vitro | [169] |
| Cetuximab functionalized nanostructure lipid carrier | Paclitaxel and 5-Demethylnobiletin (DMN) | Lung | In vivo | [170] |
| Nanostructured lipid carrier | Curcumin | Breast | In vitro | [171] |
| Citral-loaded nanostructured lipid | - | Breast | In vivo | [172] |
| Chitosan-coated nanostructure lipid carrier | Tetrahydro curcumin | Breast | In vitro | [173] |
| Hyaluronic acid-modified nanostructured lipid carrier | Kaempferol | Non small-lung | In vitro | [174] |
| Nanostructured lipidic carriers (NLCs) | Ribociclib (RBO) | Breast | In vitro and in vivo | [175] |
| Calycosin-loaded NLCs | Calycosin | Breast | In vitro and in vivo | [176] |
| Gefitinib-loaded NLCs | Gefitinib (GEF) | Metastatic lung | In vivo | [177] |
| Nano-emulsions | ||||
| Novel nano-emulsion | 5-fluorouracil and Curcumin | Lung | In vivo | [178] |
| LAP-loaded nano-emulsion (NE-LAP) | Lapachol (LAP) | Breast | In vitro | [179] |
| Naringenin nano-emulsions | Naringenin (NAR) | Lung | In vivo | [180] |
| Borage oil based nano-emulsion (B-NE) | Docetaxel (DTX) and thymoquinone (TQ) | Breast | In vitro | [181] |
| Nano-emulsion-assisted siRNA | - | Pancreatic | In vivo and in vitro | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puttasiddaiah, R.; Basavegowda, N.; Lakshmanagowda, N.K.; Raghavendra, V.B.; Sagar, N.; Sridhar, K.; Dikkala, P.K.; Bhaswant, M.; Baek, K.-H.; Sharma, M. Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges. Pharmaceutics 2025, 17, 70. https://doi.org/10.3390/pharmaceutics17010070
Puttasiddaiah R, Basavegowda N, Lakshmanagowda NK, Raghavendra VB, Sagar N, Sridhar K, Dikkala PK, Bhaswant M, Baek K-H, Sharma M. Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges. Pharmaceutics. 2025; 17(1):70. https://doi.org/10.3390/pharmaceutics17010070
Chicago/Turabian StylePuttasiddaiah, Rachitha, Nagaraj Basavegowda, Nityashree Kyathegowdanadoddi Lakshmanagowda, Vinay Basavegowda Raghavendra, Niju Sagar, Kandi Sridhar, Praveen Kumar Dikkala, Maharshi Bhaswant, Kwang-Hyun Baek, and Minaxi Sharma. 2025. "Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges" Pharmaceutics 17, no. 1: 70. https://doi.org/10.3390/pharmaceutics17010070
APA StylePuttasiddaiah, R., Basavegowda, N., Lakshmanagowda, N. K., Raghavendra, V. B., Sagar, N., Sridhar, K., Dikkala, P. K., Bhaswant, M., Baek, K.-H., & Sharma, M. (2025). Emerging Nanoparticle-Based Diagnostics and Therapeutics for Cancer: Innovations and Challenges. Pharmaceutics, 17(1), 70. https://doi.org/10.3390/pharmaceutics17010070










