Preparation of pH-Responsive Tanshinone IIA-Loaded Calcium Alginate Nanoparticles and Their Anticancer Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Calcium Alginate Nanoparticles
2.3. Pharmaceutical Characterization
Encapsulation Efficiency and Loading Efficiency
Encapsulation efficiency (%) = (weight of loaded drug/weight of total drug) × 100
2.4. Cell Culture
2.5. CCK 8 Assay
2.6. Flow Cytometry Analysis
2.7. Western Blots Assay
2.8. Cell Migration Assay
2.9. Measurement of Intracellular Ca2+ Level
2.10. Confocal Imaging of Cells
2.11. Fourier Transform Infrared (FTIR)
2.12. Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM)
2.13. HPLC Analysis to Measure the TanIIA Contents
2.14. Solubility Determination
2.15. Statistical Analysis
2.16. RNA Extraction and Transcriptome Sequencing
2.17. Sequence Assembly, Functional Annotation and Classification
3. Results
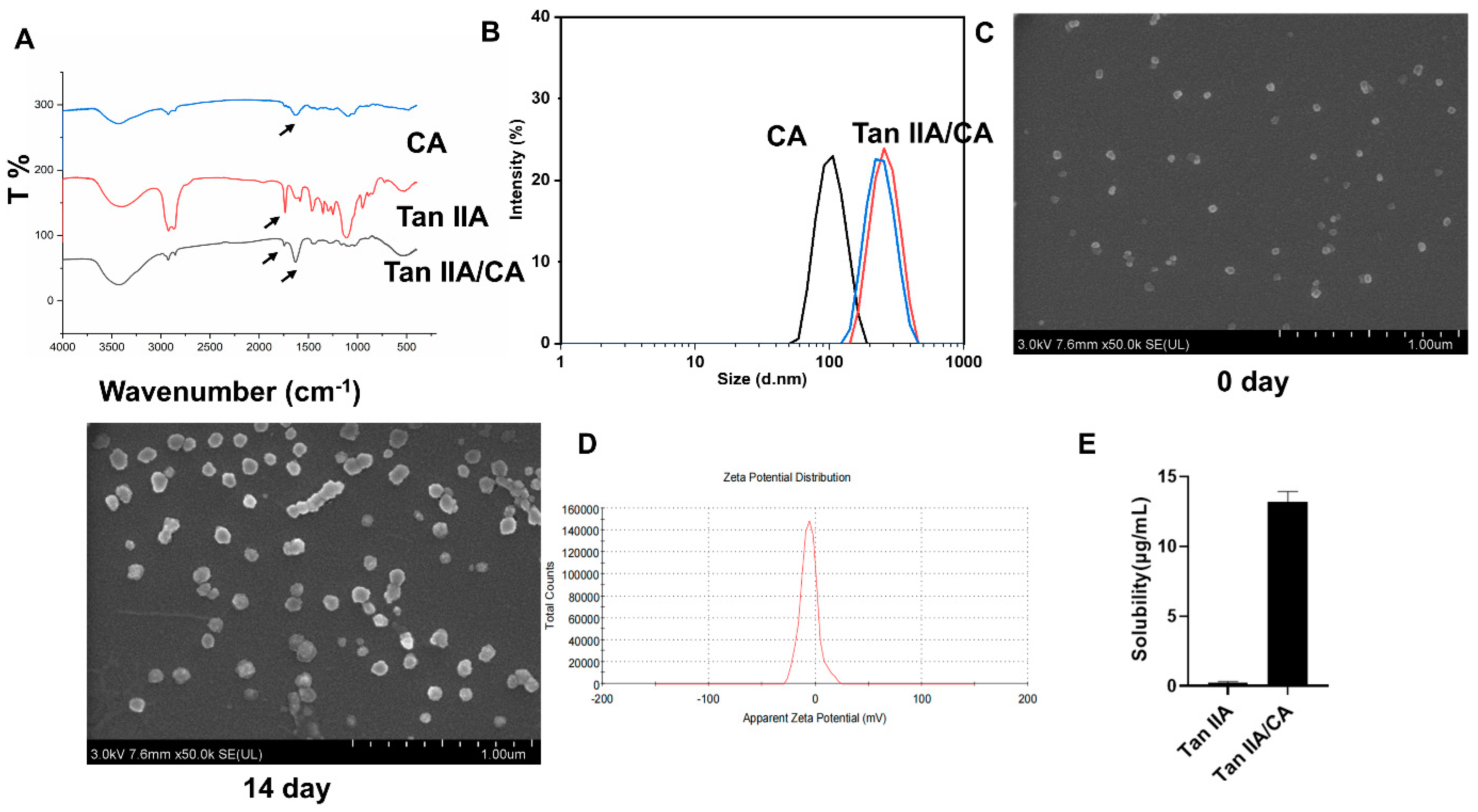
3.1. Characterization of Nanoparticles
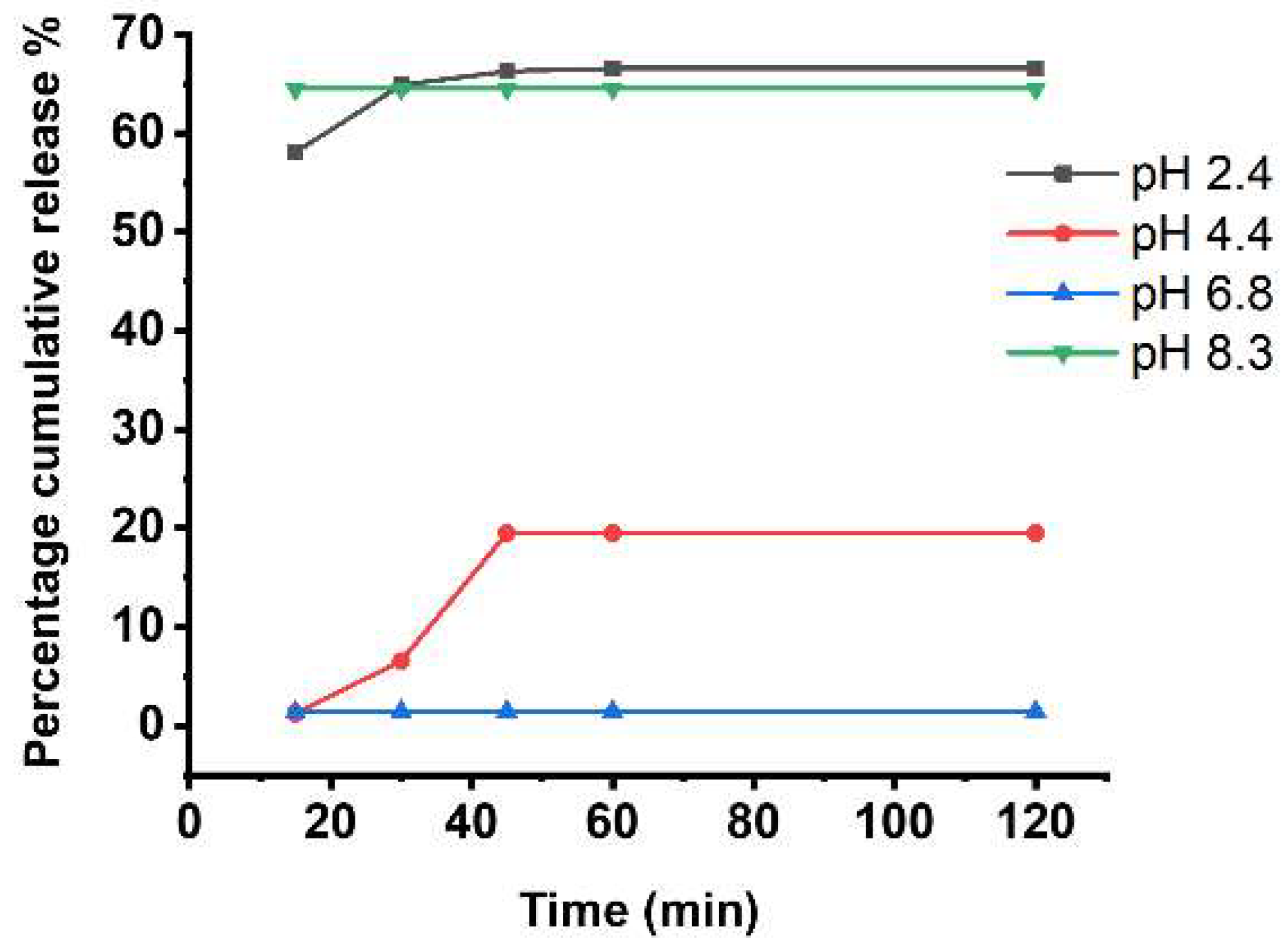
3.2. Drug Encapsulation and Release In Vitro
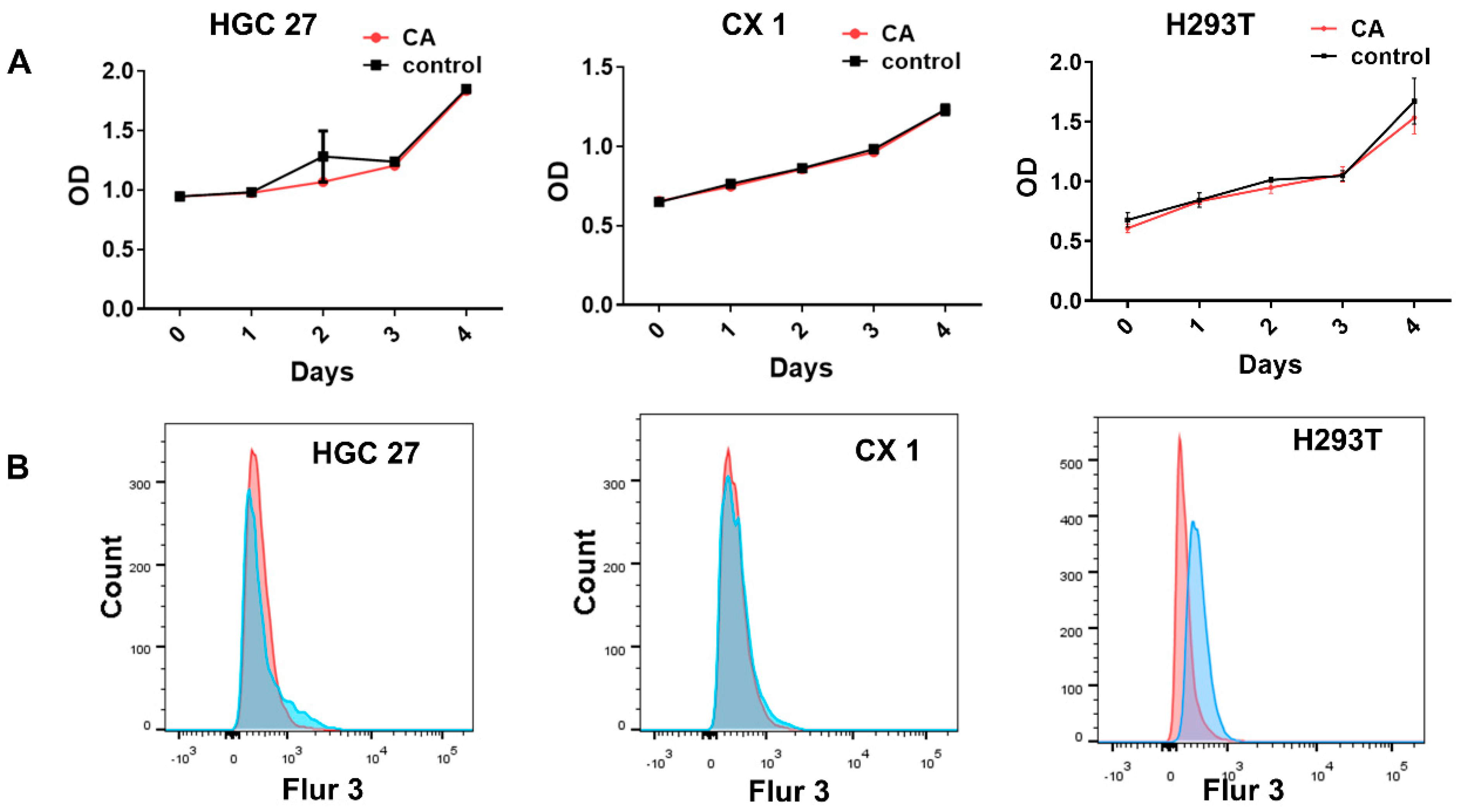
3.3. Nontoxic Properties
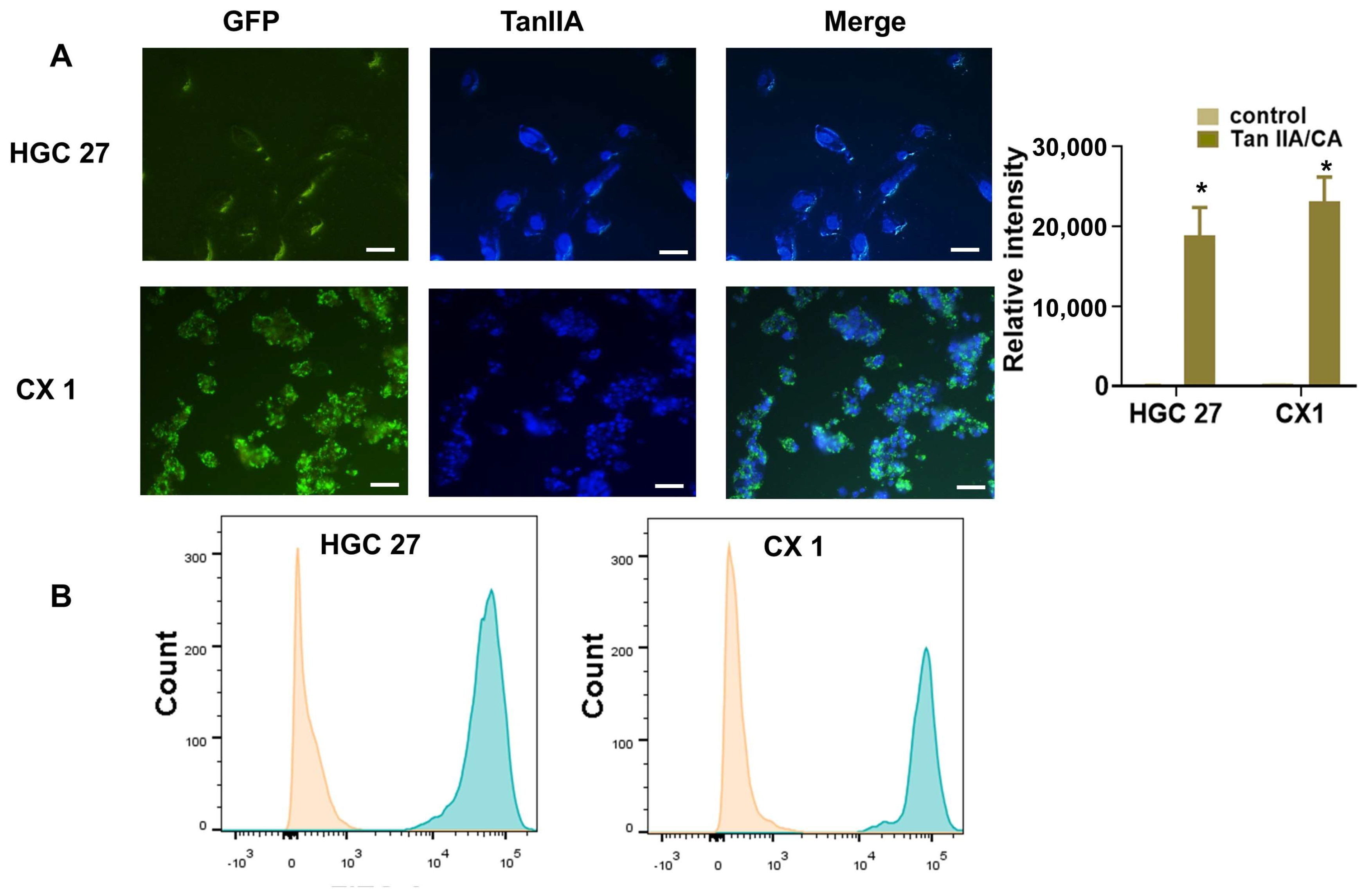
Drug Uptake Ability In Vitro
3.4. Anticancer Ability In Vitro
3.5. Transcriptomic Analyses
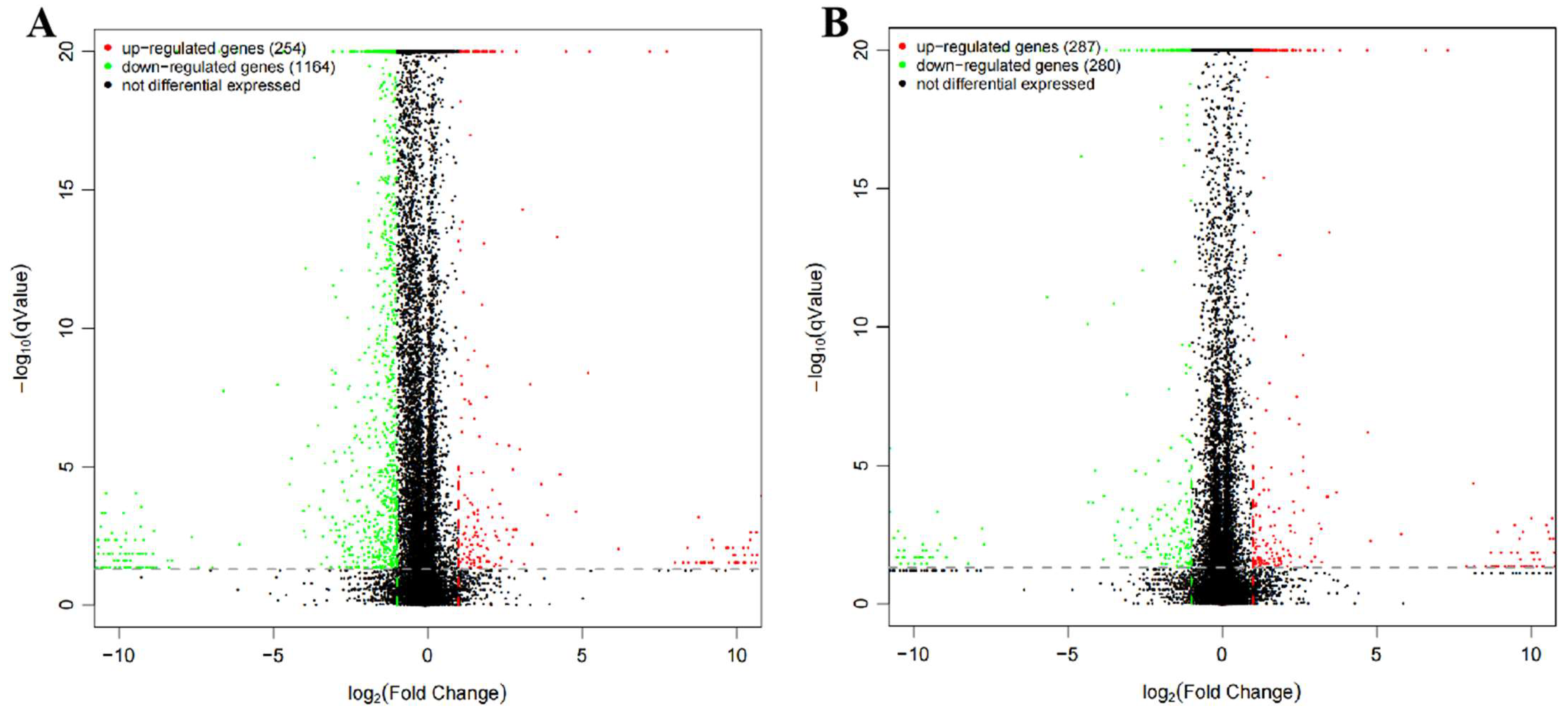
3.6. Differentially Expressed Genes (DEGs)
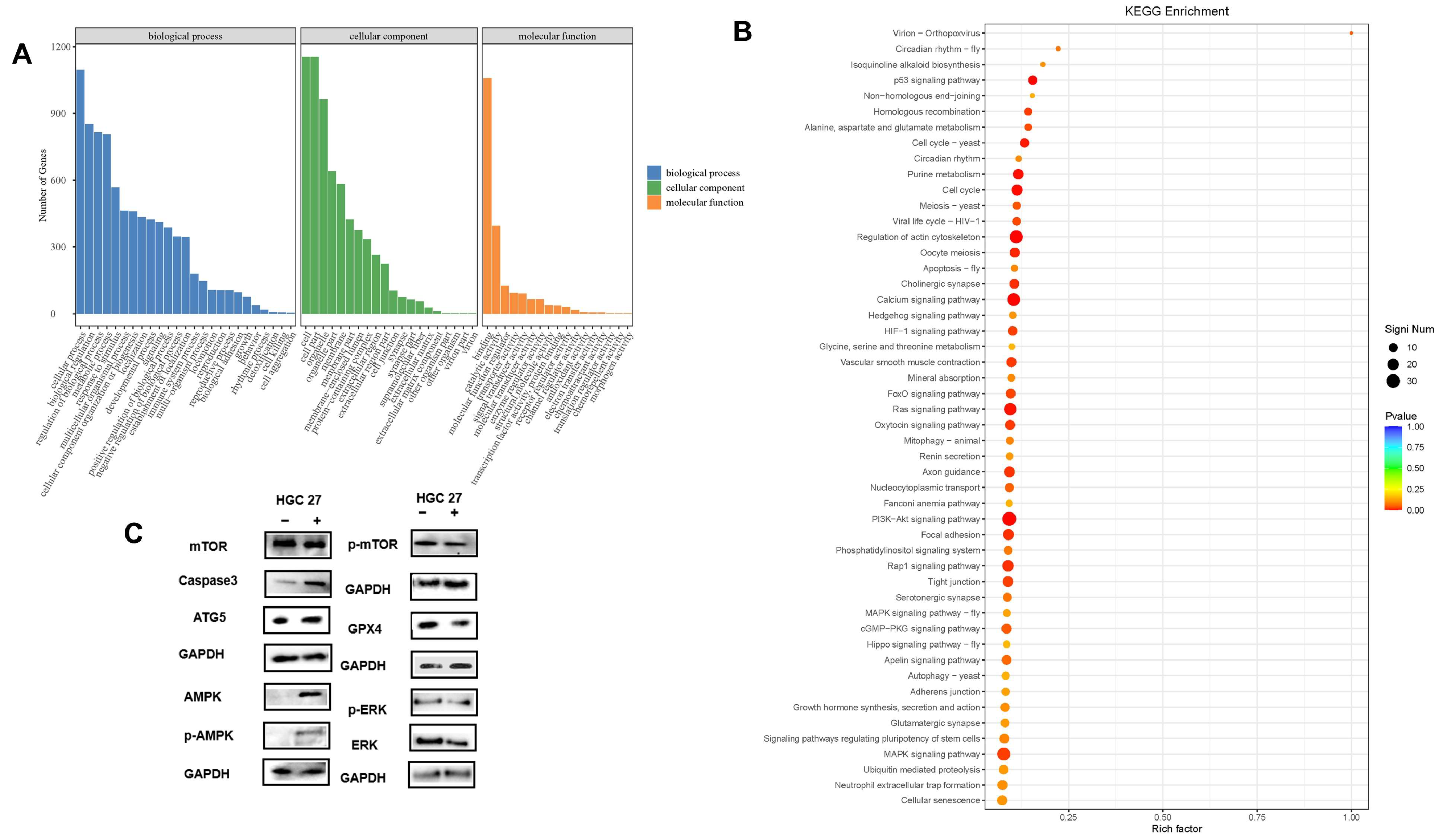
3.7. Gene Ontology (GO) Analyses and KEGG Pathway Analyses of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Gao, J. Novel anticancer drugs approved in 2020. Drug Discov. Ther. 2021, 15, 44–47. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Yang, C.; Mu, Y.; Li, S.; Zhang, Y.; Liu, X.; Li, J. Tanshinone IIA: A Chinese herbal ingredient for the treatment of atherosclerosis. Front. Pharmacol. 2023, 14, 1321880. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, X.; Tian, X.; Zhang, J.; Xue, S.; Jiang, Y.; Liu, T.; Wang, X.; Sun, Q.; Hong, Y.; et al. Tanshinone I inhibits doxorubicin-induced cardiotoxicity by regulating Nrf2 signaling pathway. Phytomedicine 2022, 106, 154439. [Google Scholar] [CrossRef]
- Lai, Z.; He, J.; Zhou, C.; Zhao, H.; Cui, S. Tanshinones: An Update in the Medicinal Chemistry in Recent 5 Years. Curr. Med. Chem. 2021, 28, 2807–2827. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, P.; Zou, Y.; Yuan, L.; Xu, Y.; Zhang, X.; Luo, X.; Zhang, Z. Tanshinone IIA and hepatocellular carcinoma: A potential therapeutic drug. Front. Oncol. 2023, 13, 1071415. [Google Scholar] [CrossRef]
- Guo, R.; Li, L.; Su, J.; Li, S.; Duncan, S.E.; Liu, Z.; Fan, G. Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases. Drug Des. Dev. Ther. 2020, 14, 4735–4748. [Google Scholar] [CrossRef]
- Li, W.; Huang, T.; Xu, S.; Che, B.; Yu, Y.; Zhang, W.; Tang, K. Molecular Mechanism of Tanshinone against Prostate Cancer. Molecules 2022, 27, 5594. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Y.Y.; Chen, H.; Wu, Y.C.; Zhang, L. Tanshinone I attenuates the malignant biological properties of ovarian cancer by inducing apoptosis and autophagy via the inactivation of PI3K/AKT/mTOR pathway. Cell Prolif. 2020, 53, e12739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pan, Y.; Wang, Y.; Wang, B.; Yan, Y.; Qu, Y.; Ke, X. Tanshinones induce tumor cell apoptosis via directly targeting FHIT. Sci. Rep. 2021, 11, 12217. [Google Scholar] [CrossRef]
- Ashour, A.A.; Ramadan, A.A.; Abdelmonsif, D.A.; El-Kamel, A.H. Enhanced oral bioavailability of Tanshinone IIA using lipid nanocapsules: Formulation, in-vitro appraisal and pharmacokinetics. Int. J. Pharm. 2020, 586, 119598. [Google Scholar] [CrossRef]
- Meng, Z.; Meng, L.; Wang, K.; Li, J.; Cao, X.; Wu, J.; Hu, Y. Enhanced hepatic targeting, biodistribution and antifibrotic efficacy of tanshinone IIA loaded globin nanoparticles. Eur. J. Pharm. Sci. 2015, 73, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, C.; Lenon, G.B.; Xue, C.C.L.; Li, W. Preparation and characterisation of solid dispersions of tanshinone IIA, cryptotanshinone and total tanshinones. Asian J. Pharm. Sci. 2017, 12, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Zhang, Q.; Li, H.; Ma, W.C.; Zhang, N.; Jin, H.; Mao, S.J. Development of intravenous lipid emulsion of tanshinone IIA and evaluation of its anti-hepatoma activity in vitro. Int. J. Pharm. 2012, 424, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Bhaladhare, S.; Bhattacharjee, S. Chemical, physical, and biological stimuli-responsive nanogels for biomedical applications (mechanisms, concepts, and advancements): A review. Int. J. Biol. Macromol. 2023, 226, 535–553. [Google Scholar] [CrossRef]
- Fugolin, A.P.P.; Huynh, B.; Rajasekaran, S.P. Innovations in the Design and Application of Stimuli-Responsive Restorative Dental Polymers. Polymers 2023, 15, 3346. [Google Scholar] [CrossRef]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Jiang, C.; Xu, K.; Xu, T.; Yu, X.; Fang, J.; Yang, Y.; Dai, X. Advances in Hydrogels for Meniscus Tissue Engineering: A Focus on Biomaterials, Crosslinking, Therapeutic Additives. Gels 2024, 10, 114. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Kirindage, K.; Jeon, H.N.; Han, E.J.; Jayasinghe, A.M.K.; Ahn, G. Preparation of microspheres by alginate purified from Sargassum horneri and study of pH-responsive behavior and drug release. Int. J. Biol. Macromol. 2022, 202, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Z.; Wang, F.; Hong, L.; Deng, L.; Zhong, J.; Wang, Z.; Cui, W. Colon-Targeted Adhesive Hydrogel Microsphere for Regulation of Gut Immunity and Flora. Adv. Sci. 2021, 8, e2101619. [Google Scholar] [CrossRef]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Wan Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of Black Seed Oil in Alginate Beads as a pH-Sensitive Carrier for Intestine-Targeted Drug Delivery: In Vitro, In Vivo and Ex Vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles-An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Volic, M.; Pajic-Lijakovic, I.; Djordjevic, V.; Knezevic-Jugovic, Z.; Pecinar, I.; Stevanovic-Dajic, Z.; Veljovic, D.; Hadnadjev, M.; Bugarski, B. Alginate/soy protein system for essential oil encapsulation with intestinal delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef]
- Chu, J.N.; Traverso, G. Foundations of gastrointestinal-based drug delivery and future developments. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Sun, C.Y.; Wu, C.J.; Wu, C.C.; Wu, V.; Lin, F.H. CharXgen-Activated Bamboo Charcoal Encapsulated in Sodium Alginate Microsphere as the Absorbent of Uremic Toxins to Retard Kidney Function Deterioration. Int. J. Mol. Sci. 2020, 21, 1257. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Li, J.; Lu, Y.; Li, H.; Zhuang, J.; Ren, X.; Wang, M.; Sun, C. Tanshinone IIA: New Perspective on the Anti-Tumor Mechanism of A Traditional Natural Medicine. Am. J. Chin. Med. 2022, 50, 209–239. [Google Scholar] [CrossRef] [PubMed]
- Roach, P.J. AMPK → ULK1 → autophagy. Mol. Cell. Biol. 2011, 31, 3082–3084. [Google Scholar] [CrossRef]
- Hsu, C.C.; Peng, D.; Cai, Z.; Lin, H.K. AMPK signaling and its targeting in cancer progression and treatment. Semin. Cancer Biol. 2022, 85, 52–68. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef] [PubMed]



| Particles | Encapsulation Efficiency % | Loading Capacity % |
|---|---|---|
| DOX/CA | 57.8 ± 5.6 | 46.5 ± 3.2 |
| Tan IIA/CA | 50.2 ± 5.1 | 42.4 ± 4.5 |
| Sample | HT1 | HT2 | HT3 | H1 | H2 | H3 |
| Total Reads Count (#) | 55,590,646 | 51,585,078 | 55,087,862 | 51,282,474 | 56,343,348 | 53,812,911 |
| Total Bases Count (bp) | 8,018,322,967 | 7,455,267,812 | 773,679,538 | 7,466,638,761 | 8,170,208,380 | 7,818,423,571 |
| Average Read Length (bp) | 144.24 | 144.52 | 144.45 | 145.6 | 145.01 | 145.3 |
| Q10 Bases Count (bp) | 8,001,423,705 | 7,439,545,492 | 772,048,462 | 7,449,007,281 | 8,151,318,468 | 7,800,162,875 |
| Q10 Bases Ratio (%) | 99.79% | 99.79% | 99.7% | 99.76% | 99.77% | 99.7% |
| Q20 Bases Count (bp) | 7,952,826,783 | 7,394,373,331 | 767,360,026 | 7,397,235,782 | 8,096,391,117 | 7,746,813,450 |
| Q20 Bases Ratio (%) | 99.18% | 99.18% | 99.1% | 99.07% | 99.10% | 99.1% |
| Q30 Bases Count (bp) | 7,798,359,715 | 7,251,470,951 | 752,491,533 | 7,231,616,645 | 7,920,864,107 | 7,576,240,376 |
| Q30 Bases Ratio (%) | 97.26% | 97.27% | 97.2% | 96.85% | 96.95% | 96.92% |
| N Bases Count (bp) | 433,618 | 367,761 | 400,689 | 362,907 | 438,470 | 400,689 |
| N Bases Ratio (%) | 0.01% | 0.00% | 0.00% | 0.00% | 0.01% | 0.00% |
| GC Bases Count (bp) | 4,261,930,436 | 3,997,774,689 | 412,985,256 | 4,012,761,140 | 4,300,578,456 | 415,666,980 |
| GC Bases Ratio (%) | 53.15% | 53.62% | 53.41% | 53.74% | 52.64% | 53.72% |
| Sample | CT1 | CT2 | CT3 | C1 | C2 | C3 |
| Total Reads Count (#) | 44,685,862 | 49,494,306 | 47,090,084 | 52,192,284 | 53,704,402 | 52,948,343 |
| Total Bases Count (bp) | 6,479,365,295 | 7,172,275,399 | 6,825,820,347 | 7,500,961,916 | 7,773,077,912 | 7,637,019,914 |
| Average Read Length (bp) | 145 | 144.91 | 144.96 | 143.72 | 144.74 | 144.23 |
| Q10 Bases Count (bp) | 6,461,773,661 | 7,151,595,460 | 6,806,684,561 | 7,480,328,311 | 7,754,647,673 | 7,617,487,992 |
| Q10 Bases Ratio (%) | 99.73% | 99.71% | 99.71% | 99.72% | 99.76% | 99.76% |
| Q20 Bases Count (bp) | 6,409,958,787 | 7,091,490,487 | 6,750,724,637 | 7,419,732,123 | 7,701,413,745 | 7,560,572,934 |
| Q20 Bases Ratio (%) | 98.93% | 98.87% | 98.97% | 98.92% | 99.08% | 99.10% |
| Q30 Bases Count (bp) | 6,245,530,856 | 6,900,969,339 | 6,573,250,098 | 7,226,834,821 | 7,532,886,563 | 7,379,860,692 |
| Q30 Bases Ratio (%) | 96.39% | 96.22% | 96.95% | 96.35% | 96.91% | 96.61% |
| N Bases Count (bp) | 312,621 | 367,599 | 340,110 | 381,956 | 386,130 | 384,043 |
| N Bases Ratio (%) | 0.00% | 0.01% | 0.00% | 0.01% | 0.00% | 0.00% |
| GC Bases Count (bp) | 3,524,789,488 | 3,844,896,391 | 3,684,842,940 | 4,075,942,614 | 4,132,313,249 | 4,104,127,932 |
| GC Bases Ratio (%) | 54.40% | 53.61% | 53.82% | 54.34% | 53.16% | 54.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, T.; Wang, J.; Ma, Y.; Huang, Y.; Yoon, S.; Mu, L.; Li, R.; Wang, X.; Zhang, L.; Li, P.; et al. Preparation of pH-Responsive Tanshinone IIA-Loaded Calcium Alginate Nanoparticles and Their Anticancer Mechanisms. Pharmaceutics 2025, 17, 66. https://doi.org/10.3390/pharmaceutics17010066
Ren T, Wang J, Ma Y, Huang Y, Yoon S, Mu L, Li R, Wang X, Zhang L, Li P, et al. Preparation of pH-Responsive Tanshinone IIA-Loaded Calcium Alginate Nanoparticles and Their Anticancer Mechanisms. Pharmaceutics. 2025; 17(1):66. https://doi.org/10.3390/pharmaceutics17010066
Chicago/Turabian StyleRen, Tianying, Jing Wang, Yingxin Ma, Yichen Huang, Somy Yoon, Lijun Mu, Ru Li, Xuekun Wang, Lina Zhang, Pan Li, and et al. 2025. "Preparation of pH-Responsive Tanshinone IIA-Loaded Calcium Alginate Nanoparticles and Their Anticancer Mechanisms" Pharmaceutics 17, no. 1: 66. https://doi.org/10.3390/pharmaceutics17010066
APA StyleRen, T., Wang, J., Ma, Y., Huang, Y., Yoon, S., Mu, L., Li, R., Wang, X., Zhang, L., Li, P., & Ji, L. (2025). Preparation of pH-Responsive Tanshinone IIA-Loaded Calcium Alginate Nanoparticles and Their Anticancer Mechanisms. Pharmaceutics, 17(1), 66. https://doi.org/10.3390/pharmaceutics17010066







