The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential
Abstract
1. Introduction
2. Pentacyclic Triterpenoids
3. Molecular Mechanisms of Pentacyclic Triterpenoids in NSCLC
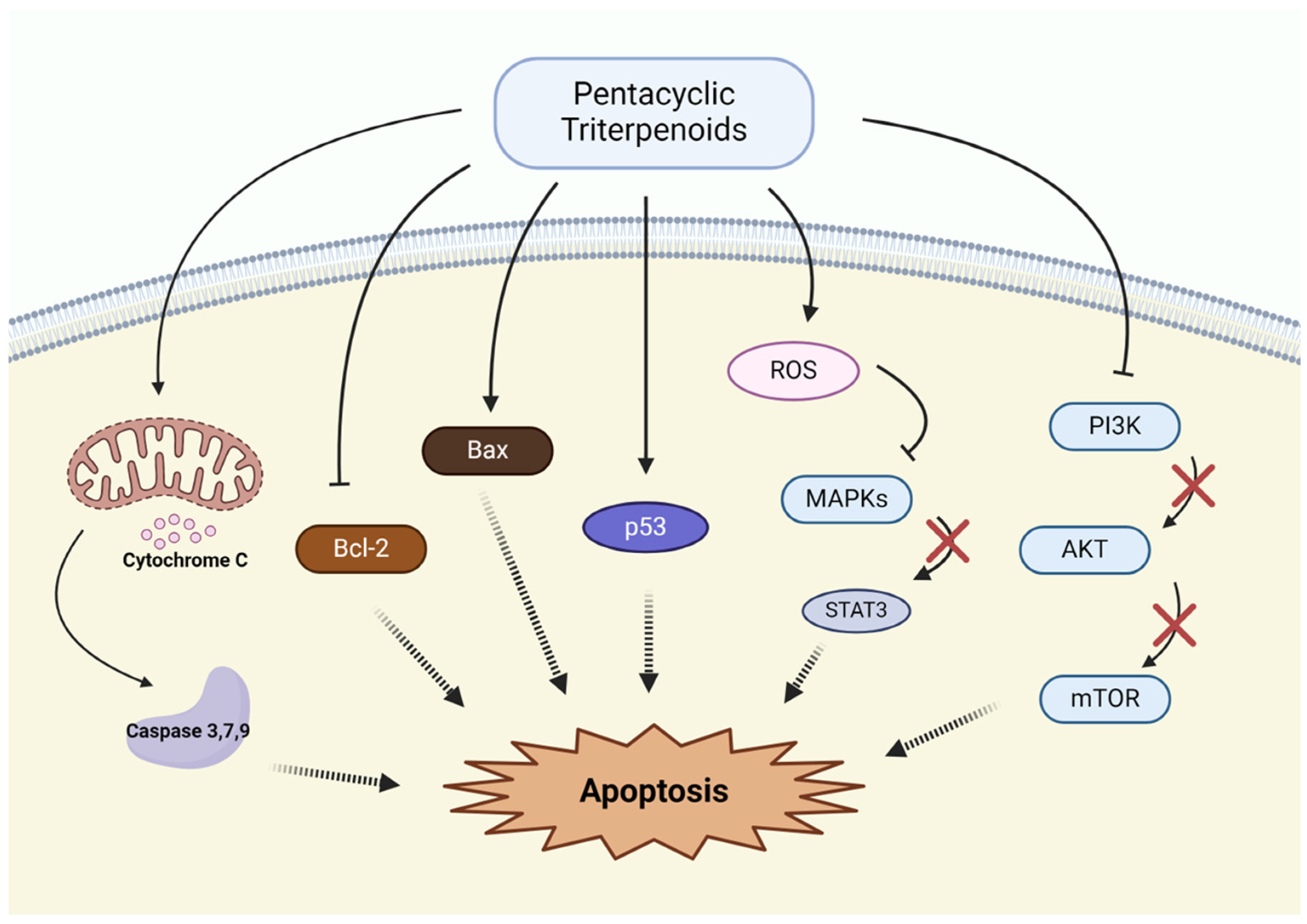
3.1. Induction of Apoptosis
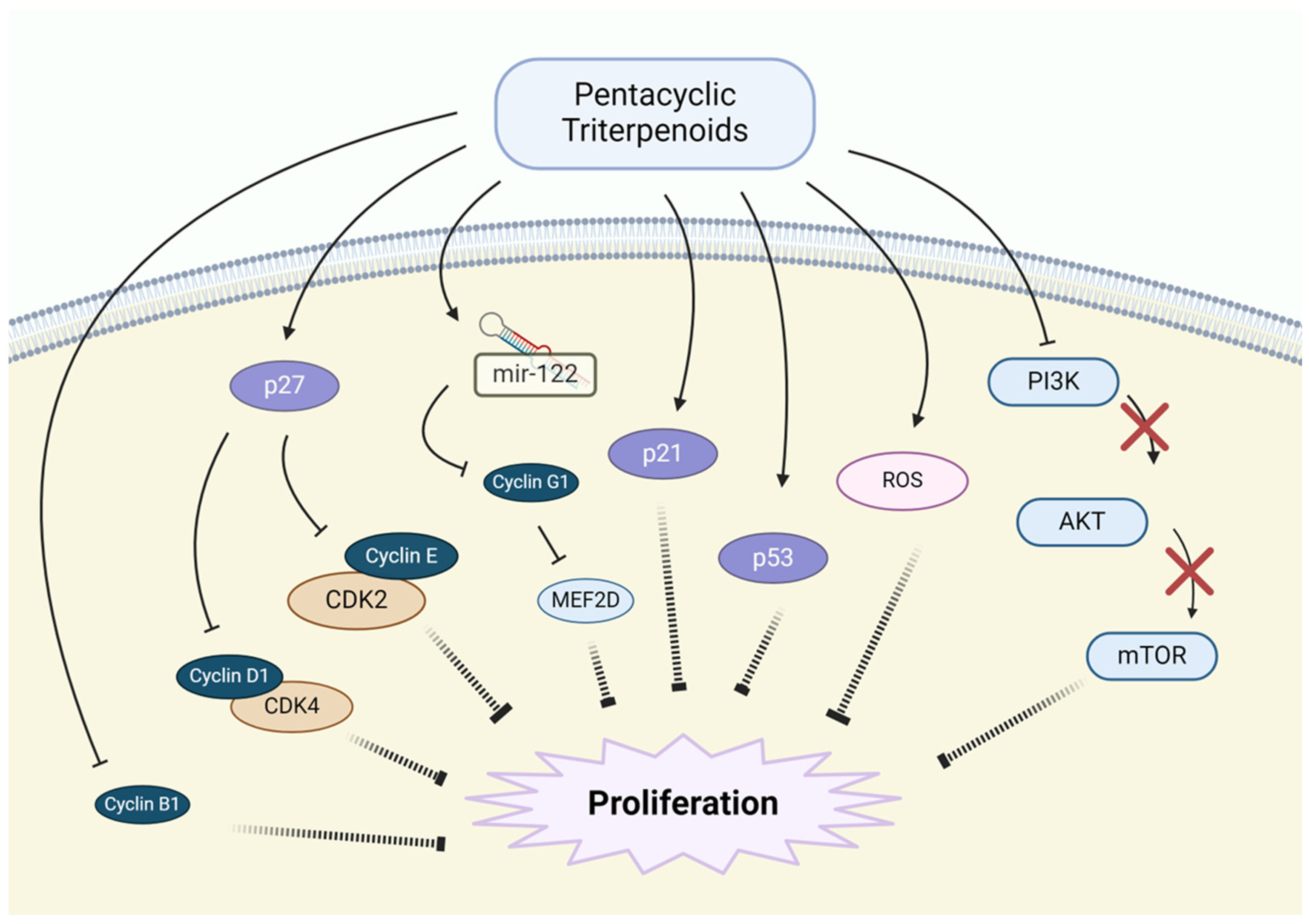
3.2. Inhibition of Proliferation and Cell Growth
3.3. Inhibition of Angiogenesis
3.4. Suppression of Metastasis
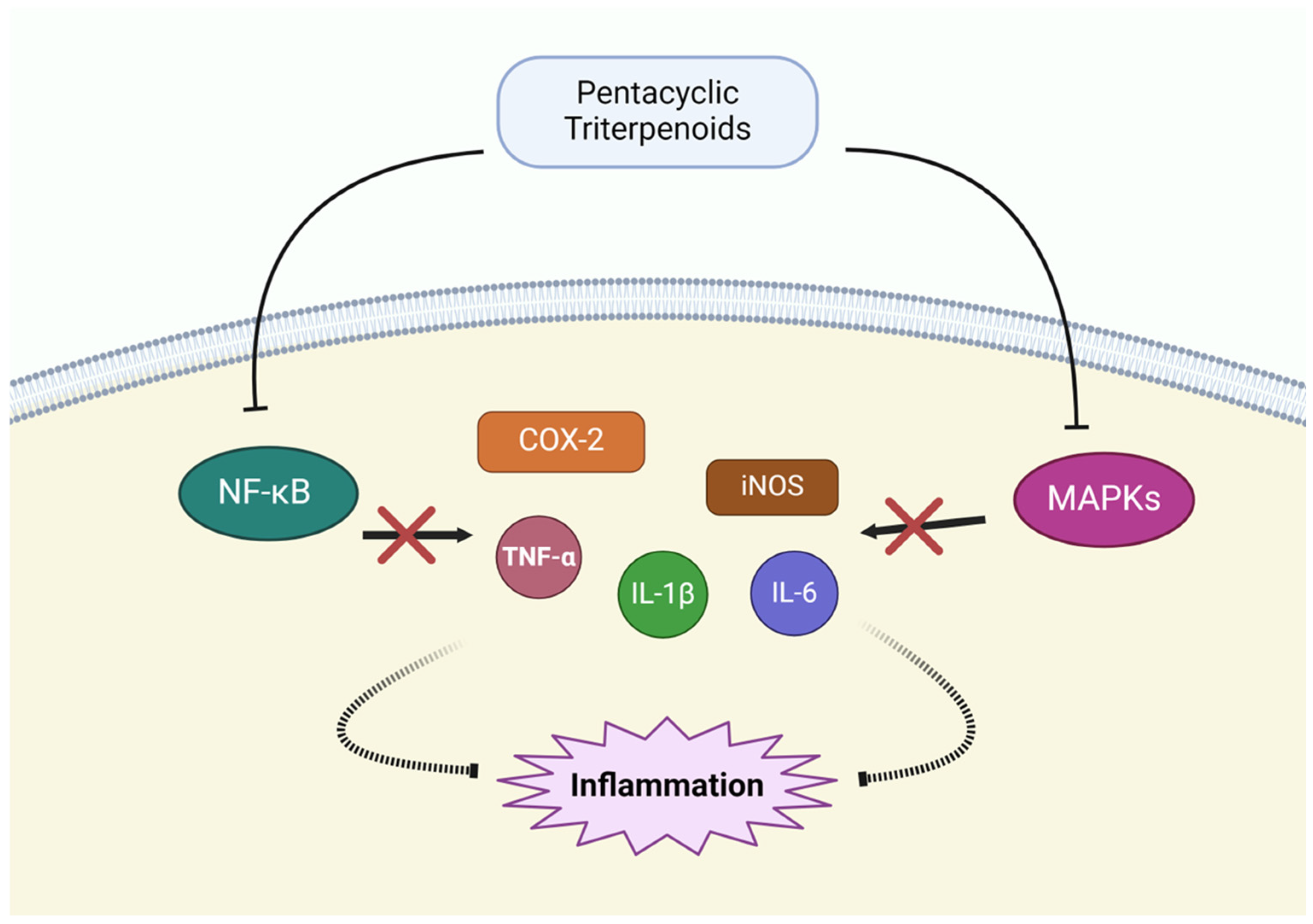
3.5. Modulation of Inflammatory Pathways
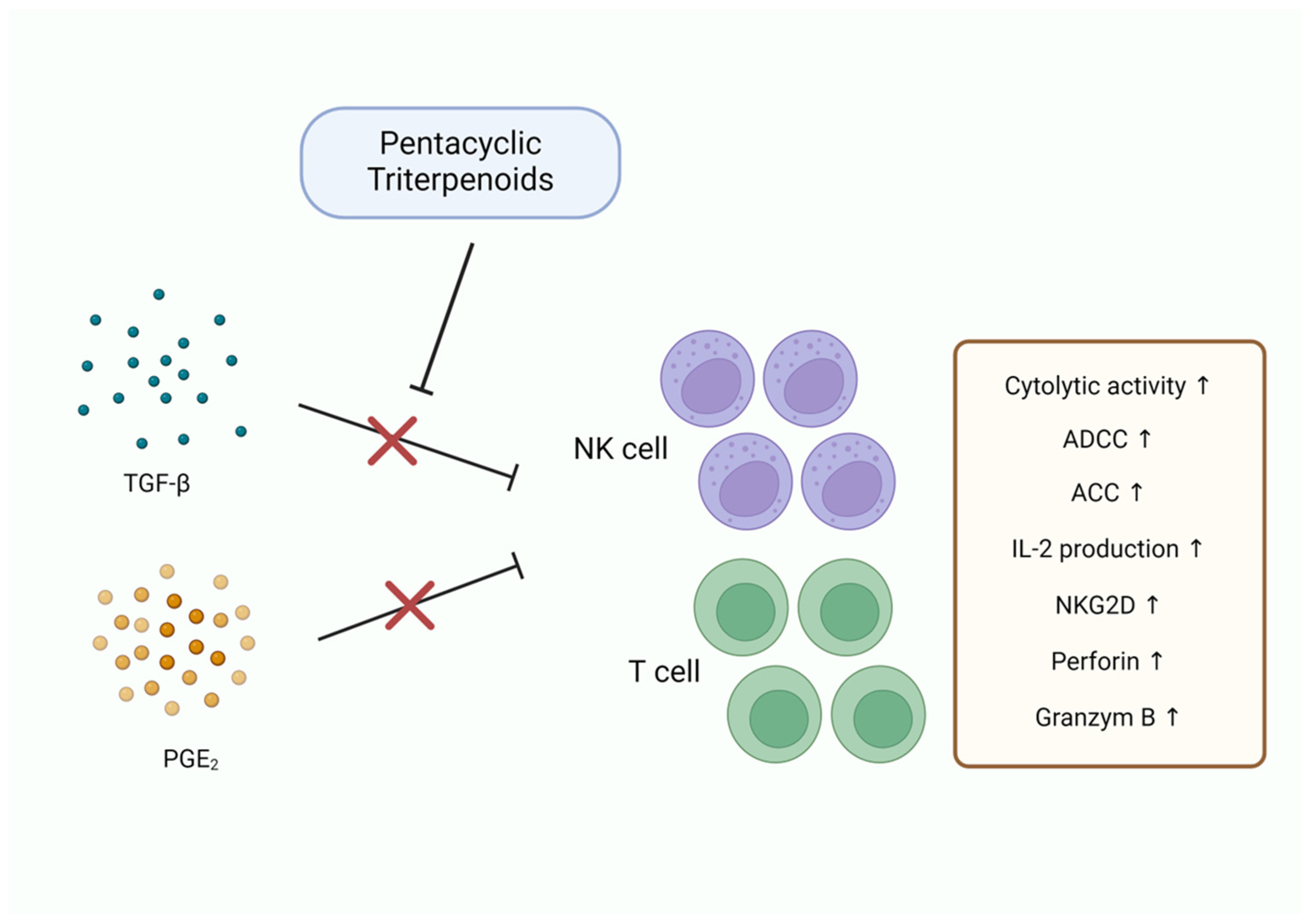
3.6. Immune Modulation
4. Improving the Therapeutic Potential of Pentacyclic Triterpenoids in NSCLC Applications and Future Directions
4.1. Optimizing Drug Formulation and Delivery
4.2. Combination Strategies to Overcome Chemoresistance
4.3. Targeting the TME
4.4. Personalized Medicine and Biomarker Development
4.5. Clinical Trials and Translational Research
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ferlay, J.E.M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today/en (accessed on 11 September 2024).
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Popper, H.H. Progression and metastasis of lung cancer. Cancer Metastasis Rev. 2016, 35, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Winslow, M.M.; Sage, J. Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 2021, 13, e13122. [Google Scholar] [CrossRef] [PubMed]
- Shanker, M.; Willcutts, D.; Roth, J.A.; Ramesh, R. Drug resistance in lung cancer. Lung Cancer Targets Ther. 2010, 1, 23–36. [Google Scholar]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef]
- Basumallik, N.; Agarwal, M. Small Cell Lung Cancer. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ray, M.R.; Jablons, D.; He, B. Lung cancer therapeutics that target signaling pathways: An update. Expert Rev. Respir. Med. 2010, 4, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Karachaliou, N.; Mayo, C.; Costa, C.; Magrí, I.; Gimenez-Capitan, A.; Molina-Vila, M.A.; Rosell, R. KRAS mutations in lung cancer. Clin. Lung Cancer 2013, 14, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Mogi, A.; Kuwano, H. TP53 mutations in nonsmall cell lung cancer. BioMed Res. Int. 2011, 2011, 583929. [Google Scholar] [CrossRef] [PubMed]
- Mathiot, L.; Nigen, B.; Goronflot, T.; Hiret, S.; Doucet, L.; Pons-Tostivint, E.; Bennouna, J.; Denis, M.G.; Herbreteau, G.; Raimbourg, J. Prognostic Impact of TP53 Mutations in Metastatic Nonsquamous Non–small-cell Lung Cancer. Clin. Lung Cancer 2024, 25, 244–253.e242. [Google Scholar] [CrossRef] [PubMed]
- Fumarola, C.; Bonelli, M.A.; Petronini, P.G.; Alfieri, R.R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 2014, 90, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC). Thorac. Cancer 2020, 11, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sabri, N.; Li, J.; Li, W.X. Role of STAT3 in lung cancer. Jak-Stat 2014, 3, e999503. [Google Scholar] [CrossRef]
- Mohrherr, J.; Haber, M.; Breitenecker, K.; Aigner, P.; Moritsch, S.; Voronin, V.; Eferl, R.; Moriggl, R.; Stoiber, D.; Győrffy, B. JAK–STAT inhibition impairs K-RAS-driven lung adenocarcinoma progression. Int. J. Cancer 2019, 145, 3376–3388. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Scott, W.J.; Howington, J.; Feigenberg, S.; Movsas, B.; Pisters, K. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines. Chest 2007, 132, 234S–242S. [Google Scholar] [CrossRef]
- García-Fernández, C.; Fornaguera, C.; Borrós, S. Nanomedicine in Non-Small Cell Lung Cancer: From Conventional Treatments to Immunotherapy. Cancers 2020, 12, 1609. [Google Scholar] [CrossRef]
- Škarda, J.; Hajdúch, M.; Kolek, V. Drug resistance in lung cancer. Cancer Ther. 2008, 6, 377–388. [Google Scholar]
- Liu, W.-J.; Du, Y.; Wen, R.; Yang, M.; Xu, J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol. Ther. 2020, 206, 107438. [Google Scholar] [CrossRef] [PubMed]
- Gkolfinopoulos, S.; Mountzios, G. Recent clinical trials of immunotherapy in non-small-cell lung cancer. Immunotherapy 2019, 11, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 196. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S. Chemotherapy Resistance in Lung Cancer. In Lung Cancer and Personalized Medicine: Current Knowledge and Therapies; Ahmad, A., Gadgeel, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–209. [Google Scholar]
- Nishio, K.; Nakamura, T.; Koh, Y.; Suzuki, T.; Fukumoto, H.; Saijo, N. Drug resistance in lung cancer. Curr. Opin. Oncol. 1999, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Kamble, S.M.; Goyal, S.N.; Patil, C.R. Multifunctional pentacyclic triterpenoids as adjuvants in cancer chemotherapy: A review. RSC Adv. 2014, 4, 33370–33382. [Google Scholar] [CrossRef]
- Banerjee, J.; Samanta, S.; Ahmed, R.; Dash, S.K. Bioactive Pentacyclic Triterpenes Trigger Multiple Signalling Pathways for Selective Apoptosis Leading to Anticancer Efficacy: Recent Updates and Future Perspectives. Curr. Protein Pept. Sci. 2023, 24, 820–842. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Liu, J.; Sun, Z.; Silverstein, R.; Zou, M.; Finkel, T.; Bugge, T.H.; Leppla, S.H.; Liu, S. A potent tumor-selective ERK pathway inactivator with high therapeutic index. PNAS Nexus 2022, 1, pgac104. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Connolly, J.D. Triterpenoids. Nat. Prod. Rep. 2013, 30, 1028–1065. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yin, X.; Kou, C.; Thimmappa, R.; Hua, X.; Xue, Z. Classification, biosynthesis and biological function of triterpene esters in plants. Plant Commun. 2024, 5, 100845. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Wei, B.; Zhu, D.; Yu, B. Site-selective CH hydroxylation of pentacyclic triterpenoids directed by transient chiral pyridine-imino groups. Nat. Commun. 2020, 11, 4371. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- JC Furtado, N.A.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Aderibigbe, B.A. Potential Pharmacological Properties of Triterpene Derivatives of Ursolic Acid. Molecules 2024, 29, 3884. [Google Scholar] [CrossRef] [PubMed]
- Similie, D.; Minda, D.; Bora, L.; Kroškins, V.; Lugiņina, J.; Turks, M.; Dehelean, C.A.; Danciu, C. An Update on Pentacyclic Triterpenoids Ursolic and Oleanolic Acids and Related Derivatives as Anticancer Candidates. Antioxidants 2024, 13, 952. [Google Scholar] [CrossRef]
- Adtani, P.N.; Narasimhan, M.; Girija, D.M. In vitro anticancer activity of a pentacyclic triterpenoid via the mitochondrial pathway in bone-invasive oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2021, 25, 313–321. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ anti-cancer effects: Focus on autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Q.; Sun, L.; Zhou, Y.-Q.; Liu, J.-J.; Mo, W.-B.; Cheng, K.-G. Pentacyclic triterpene-amino acid derivatives induced apoptosis and autophagy in tumor cells, affected the JNK and PI3K/AKT/mTOR pathway. Bioorganic Med. Chem. 2023, 94, 117478. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszen, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini-Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Fernandes, J.; Castilho, R.O.; da Costa, M.R.; Wagner-Souza, K.; Kaplan, M.A.C.; Gattass, C.R. Pentacyclic triterpenes from Chrysobalanaceae species: Cytotoxicity on multidrug resistant and sensitive leukemia cell lines. Cancer Lett. 2003, 190, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Qayum, A.; Kumar, A.; Khare, V.; Sharma, P.R.; Andotra, S.S.; Singh, S.K.; Koul, S.; Gupta, P.N. Improved efficacy of cisplatin in combination with a nano-formulation of pentacyclic triterpenediol. Mater. Sci. Eng. C 2016, 68, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, S.; Chen, Z.; Chen, A.T.; Ma, J.; Deng, G.; Xu, W.; Zhou, J.; Yu, Z.-Q.; Yao, G.; et al. Synergistic chemotherapy for breast cancer and breast cancer brain metastases via paclitaxel-loaded oleanolic acid nanoparticles. Mol. Pharm. 2020, 17, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Kutkowska, J.; Strzadala, L.; Rapak, A. Synergistic activity of sorafenib and betulinic acid against clonogenic activity of non-small cell lung cancer cells. Cancer Sci. 2017, 108, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre-Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A review on preparation of betulinic acid and its biological activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Fernandes, S.; Vieira, M.; Prudêncio, C.; Ferraz, R. Betulinic acid for glioblastoma treatment: Reality, challenges and perspectives. Int. J. Mol. Sci. 2024, 25, 2108. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.M.; Dehelean, C.A.; Peev, C.; Aluas, M.; Zupkó, I.; Kása, P., Jr.; Alexa, E. Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell lines. Nat. Prod. Res. 2012, 26, 968–974. [Google Scholar] [CrossRef]
- Akao, T. Differences in the metabolism of glycyrrhizin, glycyrrhetic acid and glycyrrhetic acid monoglucuronide by human intestinal flora. Biol. Pharm. Bull. 2000, 23, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.-C.; Shyu, M.-H.; Yen, G.-C. Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Manouchehri, F.; Bonsaii, M. Study on the interaction of glycyrrhizin and glycyrrhetinic acid with RNA. J. Photochem. Photobiol. B Biol. 2012, 111, 27–34. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Zuo, J.; Meng, T.; Wang, Y.; Tang, W. A review of the antiviral activities of glycyrrhizic acid, glycyrrhetinic acid and glycyrrhetinic acid monoglucuronide. Pharmaceuticals 2023, 16, 641. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J. Biologically active pentacyclic triterpenes and their current medicine signification. J. Appl. Biomed. 2003, 1, 7–12. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Valdés, K.; Morales, J.; Rodríguez, L.; Günther, G. Potential use of nanocarriers with pentacyclic triterpenes in cancer treatments. Nanomedicine 2016, 11, 3139–3156. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin. B 2017, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qin, Y.; Ma, H.; Zheng, X.; Zhou, R.; Sun, S.; Huang, Y.; Duan, Q.; Liu, W.; Wu, P. Downregulating NF-κB signaling pathway with triterpenoids for attenuating inflammation: In vitro and in vivo studies. Food Funct. 2019, 10, 5080–5090. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Xia, S. Lupeol triterpene exhibits potent antitumor effects in A427 human lung carcinoma cells via mitochondrial mediated apoptosis, ROS generation, loss of mitochondrial membrane potential and downregulation of m-TOR/PI3Ksol; AKT signalling pathway. J. BUON 2018, 23, 635–640. [Google Scholar] [PubMed]
- Bhatt, M.; Patel, M.; Adnan, M.; Reddy, M.N. Anti-metastatic effects of lupeol via the inhibition of MAPK/ERK pathway in lung cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2021, 21, 201–206. [Google Scholar] [CrossRef]
- Babu, T.S.; Michael, B.P.; Jerard, C.; Vijayakumar, N.; Ramachandran, R. Study on the anti metastatic and anticancer activity of triterpene compound lupeol in human lung cancer. Apoptosis 2019, 5, 16. [Google Scholar]
- Ertorun, I.; Arpa, Ş.K.; Temel, H.E. Comparison of apoptotic effects of lupeol on A549 and C6 cell lines. Eur. J. Life Sci. 2024, 3, 21–30. [Google Scholar] [CrossRef]
- Hsu, T.-I.; Chen, Y.-J.; Hung, C.-Y.; Wang, Y.-C.; Lin, S.-J.; Su, W.-C.; Lai, M.-D.; Kim, S.-Y.; Wang, Q.; Qian, K.; et al. A novel derivative of betulinic acid, SYK023, suppresses lung cancer growth and malignancy. Oncotarget 2015, 6, 13671. [Google Scholar] [CrossRef] [PubMed]
- Kutkowska, J.; Strzadala, L.; Rapak, A. Hypoxia increases the apoptotic response to betulinic acid and betulin in human non-small cell lung cancer cells. Chem. Biol. Interact. 2021, 333, 109320. [Google Scholar] [CrossRef]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Abid Ali, S.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef]
- Mihoub, M.; Pichette, A.; Sylla, B.; Gauthier, C.; Legault, J. Bidesmosidic betulin saponin bearing L-rhamnopyranoside moieties induces apoptosis and inhibition of lung cancer cells growth in vitro and in vivo. PLoS ONE 2018, 13, e0193386. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, K.A.; Rocha, G.d.G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic acid initiates apoptosis in non-small cell lung cancer cell lines and reduces metastasis of a B16F10 melanoma model in vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Chen, C.-J.; Shih, Y.-L.; Yeh, M.-Y.; Liao, N.-C.; Chung, H.-Y.; Liu, K.-L.; Lee, M.-H.; Chou, P.-Y.; Hou, H.-Y.; Chou, J.-S.; et al. Ursolic acid induces apoptotic cell death through AIF and endo G release through a mitochondria-dependent pathway in NCI-H292 human lung cancer cells in vitro. In Vivo 2019, 33, 383–391. [Google Scholar] [CrossRef]
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the beneficial effects of ursolic acid against lung cancer. Molecules 2022, 27, 7466. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Lin, C.-Y.; Tsai, C.-W.; Yin, M.-C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. Vitr. 2011, 25, 1274–1280. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Wang, C.; Xu, W.-T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.-N.; Fu, Z.-R.; Wang, Y.; Jin, C.-H. 18β-Glycyrrhetinic acid has anti-cancer effects via inducing apoptosis and G2/M cell cycle arrest, and inhibiting migration of A549 lung cancer cells. OncoTargets Ther. 2021, 14, 5131–5144. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, M.; Wang, Q.; Lu, T.; Luo, P.; Chen, L.; Xia, F.; Pang, H.; Shen, S.; Cheng, G.; et al. Glycyrrhetinic acid inhibits non-small cell lung cancer via promotion of Prdx6-and caspase-3-mediated mitochondrial apoptosis. Biomed. Pharmacother. 2024, 173, 116304. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Y.; Chu, Y.-L.; Jiang, Z.-B.; Chen, X.-M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell. Physiol. Biochem. 2014, 33, 375–388. [Google Scholar] [CrossRef]
- Singh, J.; Hussain, Y.; Meena, A.; Sinha, R.A.; Luqman, S. Asiatic acid impedes NSCLC progression by inhibiting COX-2 and modulating PI 3 K signaling. FEBS Lett. 2024, 598, 3036–3052. [Google Scholar] [CrossRef]
- Kim, K.B.; Kim, K.; Bae, S.; Choi, Y.; Cha, H.J.; Kim, S.Y.; Lee, J.H.; Jeon, S.H.; Jung, H.J.; Ahn, K.J.; et al. MicroRNA-1290 promotes asiatic acid-induced apoptosis by decreasing BCL2 protein level in A549 non-small cell lung carcinoma cells. Oncol. Rep. 2014, 32, 1029–1036. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Liu, W.; Zhang, C.; Li, Y.; Hou, J.; Yu, Y.; Li, G.; Wang, Q. Combination of betulinic acid and EGFR-TKIs exerts synergistic anti-tumor effects against wild-type EGFR NSCLC by inducing autophagy-related cell death via EGFR signaling pathway. Respir. Res. 2024, 25, 215. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-Y.; Cao, D.; Ren, Q.-N.; Zhang, S.-S.; Zhou, N.-N.; Mai, S.-J.; Feng, B.; Wang, H.-Y. Combination treatment with inhibitors of erk and autophagy enhances antitumor activity of betulinic acid in non–small-cell lung cancer in vivo and in vitro. Front. Pharmacol. 2021, 12, 684243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Zhang, Y.-J.; Han, J.-C. RETRACTED ARTICLE: Betulin inhibits lung carcinoma proliferation through activation of AMPK signaling. Tumor Biol. 2014, 35, 11153–11158. [Google Scholar] [CrossRef]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Li, D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol. Cell. Biochem. 2015, 400, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-L.; Kuo, P.-L.; Lin, C.-C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.Y.; Sp, N.; Lee, J.-M.; Jang, K.-J. Antitumor effects of ursolic acid through mediating the inhibition of STAT3/PD-L1 signaling in non-small cell lung cancer cells. Biomedicines 2021, 9, 297. [Google Scholar] [CrossRef]
- Mendes, V.I.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M.; Chen, N.; Ma, A.; Zhu, C.; Zhao, R.; Jiang, M.; Zhou, J.; Ye, L.; Fu, H.; et al. Glycyrrhetinic acid induces G1-phase cell cycle arrest in human non-small cell lung cancer cells through endoplasmic reticulum stress pathway. Int. J. Oncol. 2015, 46, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, R.; Yang, R.; Xiao, Y.; Yan, J.; Zheng, C.; Xiao, W.; Huang, C.; Wang, Y. Licorice extract inhibits growth of non-small cell lung cancer by down-regulating CDK4-Cyclin D1 complex and increasing CD8+ T cell infiltration. Cancer Cell Int. 2021, 21, 529. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, W.; Chen, Y.; Liu, X.; Wang, J.; Qin, X.; Yuan, D.; Yu, T.; Chen, G.; Mi, Y.; et al. Glycyrrhizin suppresses the growth of human NSCLC cell line HCC827 by downregulating HMGB1 level. BioMed Res. Int. 2018, 2018, 6916797. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, J.; Zhao, C.; Zhao, L.; Feng, B. Antiproliferative, cell-cycle dysregulation effects of novel asiatic acid derivatives on human non-small cell lung cancer cells. Chem. Pharm. Bull. 2013, 61, 1015–1023. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Q.; Yu, M.; Qi, X.; Wang, G.; Xiao, L.; Yi, Q.; Jin, W. Ursolic acid sensitizes radioresistant NSCLC cells expressing HIF-1α through reducing endogenous GSH and inhibiting HIF-1α. Oncol. Lett. 2017, 13, 754–762. [Google Scholar] [CrossRef]
- He, D.H.; Chen, Y.F.; Zhou, Y.L.; Zhang, S.B.; Hong, M.; Yu, X.; Wei, S.F.; Fan, X.Z.; Li, S.Y.; Wang, Q.; et al. Phytochemical library screening reveals betulinic acid as a novel Skp2-SCF E3 ligase inhibitor in non–small cell lung cancer. Cancer Sci. 2021, 112, 3218–3232. [Google Scholar] [CrossRef]
- Liu, K.; Guo, L.; Miao, L.; Bao, W.; Yang, J.; Li, X.; Xi, T.; Zhao, W. Ursolic acid inhibits epithelial–mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells. Anti-Cancer Drugs 2013, 24, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-F.; Guo, N.-N.; Chu, J.; Jin, S.; Yang, B.; Li, J.; Zhang, T.; Guo, J.-T.; Chen, L.; Liang, C.-Y. Glycyrrhizin treatment inhibits proliferation and invasive potential of lung cancer cells. Int. J. Clin. Exp. Med. 2016, 9, 10592–10596. [Google Scholar]
- Cui, Q.; Ren, J.; Zhou, Q.; Yang, Q.; Li, B. Effect of asiatic acid on epithelial-mesenchymal transition of human alveolar epithelium A549 cells induced by TGF-β1. Oncol. Lett. 2019, 17, 4285–4292. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Narożna, M.; Krajka-Kuźniak, V. Anti-Cancer Potential of Synthetic Oleanolic Acid Derivatives and Their Conjugates with NSAIDs. Molecules 2021, 26, 4957. [Google Scholar] [CrossRef]
- Li, C.; Chen, J.; Yuan, W.; Zhang, W.; Chen, H.; Tan, H. Preventive effect of ursolic acid derivative on particulate matter 2.5-induced chronic obstructive pulmonary disease involves suppression of lung inflammation. IUBMB Life 2020, 72, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Kao, T.-C.; Lo, W.-H.; Yen, G.-C. Glycyrrhizic acid and 18β-glycyrrhetinic acid modulate lipopolysaccharide-induced inflammatory response by suppression of NF-κB through PI3K p110δ and p110γ inhibitions. J. Agric. Food Chem. 2011, 59, 7726–7733. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-Z.; Liang, G.-B.; Li, M.-S.; Fang, Y.-L.; Zhao, S.-F.; Zhou, M.-M.; Liao, Z.-X.; Sun, J.; Wang, H.-S. Synthesis and discovery of asiatic acid based 1, 2, 3-triazole derivatives as antitumor agents blocking NF-κB activation and cell migration. MedChemComm 2019, 10, 584–597. [Google Scholar] [CrossRef]
- Yi, J.-E.; Obminska-Mrukowicz, B.; Yuan, L.-Y.; Yuan, H. Immunomodulatory effects of betulinic acid from the bark of white birch on mice. J. Vet. Sci. 2010, 11, 305–313. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory activity of betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Ogasawara, M.; Yamasaki-Yashiki, S.; Hamada, M.; Yamaguchi-Miyamoto, T.; Kawasuji, T.; Honda, H.; Yanagibashi, T.; Ikutani, M.; Watanabe, Y.; Fujimoto, R.; et al. Betulin Attenuates TGF-β1-and PGE2-Mediated Inhibition of NK Cell Activity to Suppress Tumor Progression and Metastasis in Mice. Biol. Pharm. Bull. 2022, 45, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Raphael, T.; Kuttan, G. Effect of naturally occurring triterpenoids ursolic acid and glycyrrhizic acid on the cell-mediated immune responses of metastatic tumor-bearing animals. Immunopharmacol. Immunotoxicol. 2008, 30, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Lian, G.-Y.; Wang, Q.-M.; Tang, P.M.-K.; Zhou, S.; Huang, X.-R.; Lan, H.-Y. Combination of asiatic acid and naringenin modulates NK cell anti-cancer immunity by rebalancing Smad3/Smad7 signaling. Mol. Ther. 2018, 26, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Cox, G.; Jones, J.; Walker, R.; Steward, W.; O’Byrne, K.J. Angiogenesis and non-small cell lung cancer. Lung Cancer 2000, 27, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar]
- Piotrowski, I.; Kulcenty, K.; Suchorska, W. Interplay between inflammation and cancer. Rep. Pract. Oncol. Radiother. 2020, 25, 422–427. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [PubMed]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The role of tumor inflammatory microenvironment in lung cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Zhou, Z.; Cao, J.; Guo, Q. Recent Advances of Natural Pentacyclic Triterpenoids as Bioactive Delivery System for Synergetic Biological Applications. Foods 2024, 13, 2226. [Google Scholar] [CrossRef] [PubMed]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for delivery of pentacyclic triterpenoids in anticancer therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef] [PubMed]
- Milan, A.; Mioc, A.; Prodea, A.; Mioc, M.; Buzatu, R.; Ghiulai, R.; Racoviceanu, R.; Caruntu, F.; Şoica, C. The optimized delivery of triterpenes by liposomal nanoformulations: Overcoming the challenges. Int. J. Mol. Sci. 2022, 23, 1140. [Google Scholar] [CrossRef] [PubMed]
- Nistor, M.; Rugina, D.; Diaconeasa, Z.; Socaciu, C.; Socaciu, M.A. Pentacyclic triterpenoid phytochemicals with anticancer activity: Updated studies on mechanisms and targeted delivery. Int. J. Mol. Sci. 2023, 24, 12923. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery systems for birch-bark triterpenoids and their derivatives in anticancer research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Sève, P.; Dumontet, C. Chemoresistance in non-small cell lung cancer. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 73–88. [Google Scholar] [CrossRef]
- Chang, A. Chemotherapy, chemoresistance and the changing treatment landscape for NSCLC. Lung Cancer 2011, 71, 3–10. [Google Scholar] [CrossRef]
- Van der Deen, M.; De Vries, E.G.; Timens, W.; Scheper, R.J.; Timmer-Bosscha, H.; Postma, D.S. ATP-binding cassette (ABC) transporters in normal and pathological lung. Respir. Res. 2005, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Xu, F.-F.; Xiang, C.-P.; Jia, R.; Yan, C.-H.; Ma, S.-Q.; Wang, N.; Wang, A.-J.; Fan, P. Effect of sodium butyrate on ABC transporters in lung cancer A549 and colorectal cancer HCT116 cells. Oncol. Lett. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Setinek, U.; Hollaus, P.; Zidek, T.; Steiner, E.; Elbling, L.; Cantonati, H.; Attems, J.; Gsur, A.; Micksche, M. Multidrug resistance markers P-glycoprotein, multidrug resistance protein 1, and lung resistance protein in non-small cell lung cancer: Prognostic implications. J. Cancer Res. Clin. Oncol. 2005, 131, 355–363. [Google Scholar] [CrossRef]
- Cheng, Q.; Liao, M.; Hu, H.; Li, H.; Wu, L. Asiatic acid (AA) sensitizes multidrug-resistant human lung adenocarcinoma A549/DDP cells to cisplatin (DDP) via downregulation of P-glycoprotein (MDR1) and its targets. Cell. Physiol. Biochem. 2018, 47, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Krystal, G.; Birrer, M.; Way, J.; Nau, M.; Sausville, E.; Thompson, C.; Minna, J.; Battey, J. Multiple mechanisms for transcriptional regulation of the myc gene family in small-cell lung cancer. Mol. Cell. Biol. 1988, 8, 3373–3381. [Google Scholar] [PubMed]
- Johnson, B.E.; Russell, E.; Simmons, A.M.; Phelps, R.; Steinberg, S.M.; Ihde, D.C.; Gazdar, A.F. MYC family DNA amplification in 126 tumor cell lines from patients with small cell lung cancer. J. Cell. Biochem. 1996, 63, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, I.I.; Gazdar, A.F.; Minna, J.D. Molecular genetics of small cell lung carcinoma. In Seminars in Oncology; WB Saunders: Philadelphia, PA, USA, 2001; pp. 3–13. [Google Scholar]
- Sekido, Y.; Fong, K.M.; Minna, J.D. Progress in understanding the molecular pathogenesis of human lung cancer. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 1998, 1378, F21–F59. [Google Scholar] [CrossRef]
- Claudio, P.P.; Caputi, M.; Giordano, A. The RB2/p130 gene: The latest weapon in the war against lung cancer? Clin. Cancer Res. 2000, 6, 754–764. [Google Scholar] [PubMed]
- Nowell, C.S.; Radtke, F. Notch as a tumour suppressor. Nat. Rev. Cancer 2017, 17, 145–159. [Google Scholar] [CrossRef]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef]
- Krencz, I.; Sztankovics, D.; Danko, T.; Sebestyen, A.; Khoor, A. Progression and metastasis of small cell lung carcinoma: The role of the PI3K/Akt/mTOR pathway and metabolic alterations. Cancer Metastasis Rev. 2021, 40, 1141–1157. [Google Scholar] [CrossRef]
- Krystal, G.W.; Sulanke, G.; Litz, J. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol. Cancer Ther. 2002, 1, 913–922. [Google Scholar] [PubMed]
- Brambilla, E.; Negoescu, A.; Gazzeri, S.; Lantuejoul, S.; Moro, D.; Brambilla, C.; Coll, J.-L. Apoptosis-related factors p53, Bcl2, and Bax in neuroendocrine lung tumors. Am. J. Pathol. 1996, 149, 1941. [Google Scholar] [PubMed]



| Mechanism | Pentacyclic Triterpenoids | Target Pathway | References |
|---|---|---|---|
| Induction of Apoptosis | Lupeol | Bax↑, Bcl-2↓ | [63,64,65,66] |
| Betulinic acid | ROS↑, Caspase activation↑ | [67,68] | |
| Betulin | ROS↑, Bax/Bcl-2 ratio | [68,69,70] | |
| Oleanolic acid | Caspase activation↑, Bax↑, Survivin↓ | [71] | |
| Ursolic acid | Release of cytochrome C, Caspase activation↑ | [72,73,74] | |
| Glycyrrhetinic acid | MAPK/STAT3 pathway↓, Bcl-2↓ | [75,76] | |
| Glycyrrhizin | TxAS↓ | [77] | |
| Asiatic acid | p53↑, PI3K pathway↓ | [78,79] | |
| Inhibition of Proliferation | Betulinic acid | CDKs↓, p53↑ | [68,80,81] |
| Betulin | p27,p21↑, Cyclin-B1,-D,-E↓, AMPK pathway↑ | [68,82] | |
| Oleanolic acid | CDK inhibitors↑, Cyclin-D1↓, ROS↑ | [83,84] | |
| Ursolic acid | Cyclin-D1↓, CDK4↓, AKT/mTOR pathway↓ | [85,86,87] | |
| Glycyrrhizin | p27, p21, p18, p16↑ Cyclin-D1,-D3,E2↓, CDK4,6,2↓, HGMB1↓ | [88,89,90] | |
| Asiatic acid | Cyclin-D1, CDK2↓, ROS↑, PI3K/AKT pathway↓ | [78,91] | |
| Inhibition of Angiogenesis | Oleanolic acid | VEGF↓, PI3K/AKT pathway↓ | [71] |
| Ursolic acid | VEGF↓, HIF-1α↓ | [74,86,92] | |
| Suppression of Metastasis | Betulinic acid | E-cadherin ubiquitination↓, F-actin polymerization↓ | [67,93] |
| Betulin | MMP-2,-9↓, Wnt/β-catenin↓ | [68,69] | |
| Ursolic acid | MMP-2,-9↓ | [74,86,94] | |
| Glycyrrhizin | MMP-2,-9↓, NF-κB pathway↓ | [95] | |
| Asiatic acid | E-cadherin↑, Snail↓, N-cadherin↓, Vimentin↓, β-catenin↓ | [96] | |
| Modulation of Inflammatory Pathway | Oleanolic acid | NK-κB pathway↓, MAPK pathway↓ | [97] |
| Ursolic acid | TNF-α↓, IL-1β↓, IL-6↓, COX-2↓, iNOS↓ | [98] | |
| Glycyrrhizin, Glycyrrhetinic acid | NK-κB pathway↓, TNF-α↓, IL-1β↓, IL-6↓ | [99] | |
| Asiatic acid | NK-κB pathway↓, TNF-α↓, IL-1β↓, IL-6↓ | [100] | |
| Immune Modulation | Betulinic acid | T cell activation | [101,102] |
| Betulin | NK cell activation, TGF-β1↓, PGE2↓ | [103] | |
| Ursolic acid | ADCC↑, ACC↑, IL-2↑, NK cell activation | [104] | |
| Asiatic acid | TGF-β1/Smad signaling↓, NK cell activation | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-S.; Kwon, R.J.; Lee, H.S.; Chung, J.H.; Kim, Y.S.; Jeong, H.-S.; Park, S.-J.; Lee, S.Y.; Kim, T.; Yoon, S.H. The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential. Pharmaceutics 2025, 17, 22. https://doi.org/10.3390/pharmaceutics17010022
Lee Y-S, Kwon RJ, Lee HS, Chung JH, Kim YS, Jeong H-S, Park S-J, Lee SY, Kim T, Yoon SH. The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential. Pharmaceutics. 2025; 17(1):22. https://doi.org/10.3390/pharmaceutics17010022
Chicago/Turabian StyleLee, Young-Shin, Ryuk Jun Kwon, Hye Sun Lee, Jae Heun Chung, Yun Seong Kim, Han-Sol Jeong, Su-Jung Park, Seung Yeon Lee, Taehwa Kim, and Seong Hoon Yoon. 2025. "The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential" Pharmaceutics 17, no. 1: 22. https://doi.org/10.3390/pharmaceutics17010022
APA StyleLee, Y.-S., Kwon, R. J., Lee, H. S., Chung, J. H., Kim, Y. S., Jeong, H.-S., Park, S.-J., Lee, S. Y., Kim, T., & Yoon, S. H. (2025). The Role of Pentacyclic Triterpenoids in Non-Small Cell Lung Cancer: The Mechanisms of Action and Therapeutic Potential. Pharmaceutics, 17(1), 22. https://doi.org/10.3390/pharmaceutics17010022









