Abstract
Coumarins are natural organic compounds widely found in plants that show promising anticancer properties. This article reviews the current research on the mechanisms of action of coumarins in cancer therapy, including the induction of apoptosis, inhibition of tumor cell proliferation, modulation of oxidative stress, and inhibition of angiogenesis and metastasis. Examples of coumarins with demonstrated anticancer activity, such as scopoletin, umbeliferon, esculetin and their synthetic derivatives, are also presented. The results of preclinical studies, the potential use of coumarins as stand-alone drugs and their role in combination therapy with chemotherapy are discussed. In addition, challenges related to bioavailability, safety and potential interactions with other drugs are highlighted. This review concludes by pointing out future research directions, such as the design of new coumarin analogs and the use of nanotechnology to enhance their efficacy in cancer treatment.
1. Introduction
Coumarins are a class of organic chemical compounds belonging to the benzopyrone lactone group, which are characterized by the presence of a 2H-1-benzopyran-2-one skeleton [1,2]. Their structure allows for constructing a wide range of derivatives that differ in their pharmacological activity (Figure 1).
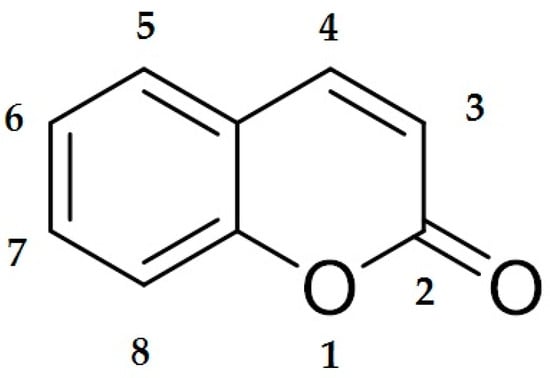
Figure 1.
Chemical structure of coumarin. The illustration shows the basic skeleton of coumarin, consisting of a benzo-α-pyrone system in which the benzene ring is fused to the lactone ring of the α-pyrone. The numbering of the atoms in the molecule follows the convention used in organic chemistry. This structure is the basis for numerous coumarin derivatives with a wide range of biological and therapeutic properties.
Based on the chemical structure, coumarins can be divided into several groups, such as simple coumarins (e.g., umbeliferon, scopoletin, esculetin), furanocoumarins (e.g., psoralen, angelicin), pyranocoumarins (e.g., xanthyletin) and pyrone-substituted coumarins (e.g., dicoumarol). The scheme below shows the most important classes of coumarins (Figure 2).
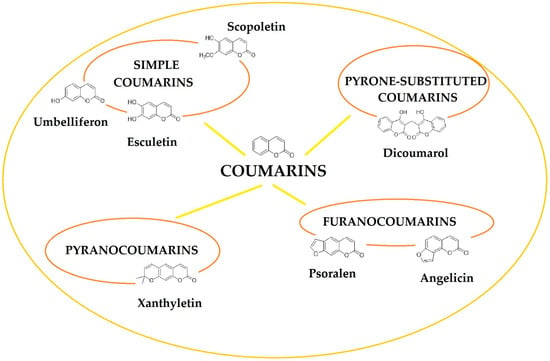
Figure 2.
General classification of coumarins found in foods and plants.
Each of these groups has specific biological properties that can be exploited in medicine and pharmacology [3]. Coumarins have diverse properties and can function as antioxidants, antibacterial agents, anti-inflammatory agents and anticancer agents. It is this latter feature that is of growing interest to scientists investigating the potential applications of coumarins in oncology [4,5,6].
Coumarins occur naturally in plants of the Apiaceae (celery), Fabaceae (legumes), Rutaceae (rutaceae), and Asteraceae (daisy) families [7,8]. These compounds are synthesized by plants as secondary metabolites that are thought to act as protective agents against insects, fungi, and UV radiation. Natural sources of coumarins include horse chestnut (Aesculus hippocastanum), fennel (Foeniculum vulgare), angelica (Angelica archangelica), citrus fruits (Citrus spp.), chamomile (Matricaria chamomilla), and soybean (Glycine max) [9]. Some coumarins (e.g., psoralen and angelicin) form reactive intermediates upon exposure to UV radiation, which allows them to be used in PUVA photochemotherapy (psoralen + UVA), which is used in the treatment of psoriasis and some skin cancers [10].
The first reports of coumarins date back to the 19th century, when Vogel first isolated coumarin from tonka beans (Dipteryx odorata) in 1820, and in 1868, William Perkin synthesized it using the Perkin reaction [11]. In the 20th century, interest in coumarins increased after the discovery of their antithrombotic properties, which led to the development of warfarin, used as an anticoagulant drug [12]. In the following decades, research on coumarins began to focus on their potential use in anticancer therapy. Some natural and synthetic coumarins were found to have the ability to induce apoptosis, inhibit cancer cell proliferation, and inhibit angiogenesis [13,14,15]. In vitro and in vivo studies have confirmed the cytotoxic effects of coumarins, such as esculin, esculetin, scopoletin and umbeliferon, on different cancer cell lines [16,17,18,19]. Synthetic coumarins show even greater selectivity toward cancer cells, suggesting their potential use as modern anticancer drugs [3].
The aim of this article is to review the current research on the use of coumarins in anticancer therapy. The mechanisms of action of these compounds will be discussed, including their effect on apoptosis, cancer cell proliferation, oxidative stress and angiogenesis.
Preclinical and clinical studies will also be analyzed, as well as challenges related to the bioavailability and safety of coumarins. This article will also present future directions of research on new coumarins, especially in the context of the use of nanotechnology to improve their therapeutic efficacy. Due to the promising results of previous studies, coumarins may become a key element of modern oncological therapies in the future. Further research into them may lead to the development of more effective and selective anticancer drugs.
2. Mechanisms of Anticancer Action of Coumarins
Coumarin compounds exhibit multifaceted anticancer activity, including induction of cancer cell apoptosis, inhibition of proliferation and cell cycle, modulation of oxidative stress and antioxidant activity, and inhibition of angiogenesis and metastasis, as well as an influence on the immune system (Figure 3).
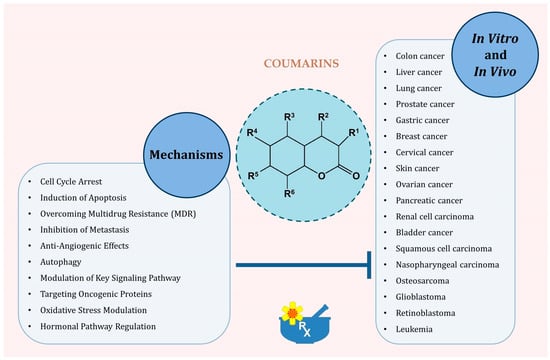
Figure 3.
Principal molecular and cellular mechanisms through which coumarins exert antitumor effects.
2.1. Induction of Apoptosis of Cancer Cells
Coumarins have shown promising anticancer properties, mainly due to their ability to induce apoptosis in cancer cells. Apoptosis, or programmed cell death, is a key mechanism that maintains homeostasis in the body and eliminates damaged or abnormal cells. In the context of cancer therapy, stimulating apoptosis in cancer cells is a promising therapeutic strategy.
Coumarins can initiate apoptosis by destabilizing the mitochondrial membrane, leading to the release of cytochrome c into the cytoplasm, which activates caspases responsible for cell degradation. Studies have shown that osthole, a natural furanocoumarin, induces apoptosis in cancer cells by activating caspases and modulating Bcl-2 and Bax proteins, which regulate mitochondrial membrane permeability [20,21,22]. Some coumarins (e.g., psoralidin) can interact with death receptors on the surface of cancer cells, such as TRAIL-R1/DR4 and TRAIL-R2/DR5. Activation of these receptors leads to a signaling cascade that results in apoptosis [23]. TRAIL (TNF-related apoptosis-inducing ligand) is a protein that induces apoptosis in cancer cells, with low toxicity to normal cells [24]. Coumarins can affect the activity of proteins such as p53, known as the “guardian of the genome”. Activation of p53 leads to cell cycle arrest and induction of apoptosis in cells with damaged DNA. Mutations in the p53 gene are frequently observed in cancers, and modulation of its activity by coumarins may restore the ability of cells to undergo programmed death [25,26].
Cancer cells often show excessive activity of pro-survival signaling pathways such as PI3K/Akt/mTOR [27]. Coumarins can inhibit these pathways, leading to reduced proliferation and induction of apoptosis. Osthole, for example, inhibits cancer cell proliferation by arresting the cell cycle and inducing apoptosis, which may be related to modulation of the PI3K/Akt/mTOR pathway [28].
Coumarins exhibit multifaceted anticancer activity by inducing apoptosis in cancer cells. Their ability to modulate key signaling pathways and proteins involved in cell survival processes makes them promising candidates in anticancer therapy. Further studies on the mechanisms of their action may contribute to the development of new strategies for cancer treatment.
2.2. Inhibition of Cell Proliferation and Cycle
One of the key mechanisms of action is the inhibition of cancer cell proliferation and modulation of the cell cycle. Cancer cell proliferation is a process of uncontrolled cell division leading to the development and progression of cancer. Studies have shown that some coumarins can effectively inhibit this process. For example, esculetin (6,7-dihydroxycoumarin) inhibits cell growth and progression by inducing G1 arrest in human HL-60 leukemia cells. This mechanism involves an increase in the unphosphorylated retinoblastoma protein (pRb) and a decrease in the CDK4 levels, as well as an upregulation of p27 and a downregulation of cyclin D1. The results suggest that by inhibiting pRb phosphorylation, esculetin may arrest the cell cycle in G1, leading to the inhibition of HL-60 leukemia cell growth [29]. In another study, isoimperatorin (ISOIM), a natural furanocoumarin, showed the ability to inhibit the proliferation of gastric cancer cells by inducing cell cycle arrest in the G2/M phase. Additionally, ISOIM induced apoptosis by increasing Bax expression and decreasing Bcl-2 expression, leading to a decreased Bcl-2/Bax ratio compared with control cells [30].
The cell cycle consists of a series of ordered steps leading to cell division. In cancer cells, the regulation of this cycle is often disrupted, which promotes their uncontrolled proliferation. Coumarins can intervene in this process, leading to cell cycle arrest at specific phases. Studies have shown that 7-hydroxycoumarin has a more potent cytostatic effect than coumarin in the human adenocarcinoma cell line A427, which has a positive pRb and homozygous deletion in the p16INK4a gene. Inhibition of the cell cycle at the G1/S transition is consistent with the cytostatic effect of 7-hydroxycoumarin and does not cause changes in the level of cyclin D1 mRNA after transcription [31].
In summary, coumarins show potential as anticancer compounds by inhibiting cancer cell proliferation and modulating the cell cycle. These mechanisms include induction of cell cycle arrest at specific phases and regulation of apoptosis-related proteins, which limits the ability of cancer cells to divide uncontrolled. Further studies on these compounds may contribute to the development of new therapeutic strategies for cancer treatment.
2.3. Modulation of Oxidative Stress and Antioxidant Effects
A pivotal mechanism underlying the anticancer effects of coumarin is the modulation of oxidative stress due to their inherent antioxidant activity. Oxidative stress arises from an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to neutralize them. Elevated ROS levels can inflict damage to DNA, proteins, and lipids, thereby promoting carcinogenesis. Consequently, modulating oxidative stress is a strategic approach in cancer prevention and therapy.
Coumarins, which are heterocyclic compounds, have been shown to have a number of beneficial health effects, including reducing the risk of cancer, diabetes, and cardiovascular and brain diseases. These properties are attributed to their ability to neutralize free radicals through antioxidant activity [32]. Studies have shown that both natural and synthetic coumarin derivatives can significantly affect the functioning of various mammalian cellular systems. Their development as potential antioxidant agents has attracted the attention of scientists due to their unique mechanisms of action [33]. Interestingly, even non-phenolic coumarin derivatives, such as 7-dialkyl-aminocoumarins, have significant antioxidant properties. This mechanism is associated with the presence of trace amounts of reactive compounds such as hydroxycinnamic acid, which can neutralize free radicals. It is also worth noting that structural modifications of the coumarin molecule can affect its antioxidant activity. Changes in the C-3 and C-4 positions can shift the reactivity of the compounds toward different types of free radicals, suggesting the possibility of designing more effective antioxidants based on the coumarin structure [34].
Numerous studies have demonstrated that coumarins possess significant antioxidant capabilities. These compounds can scavenge ROS and inhibit oxidative processes, thereby protecting cells from oxidative damage. For instance, certain coumarin derivatives have been shown to suppress oxidative stress through their ability to scavenge ROS and inhibit neutrophil-dependent superoxide anion formation. Additionally, coumarins can modulate key signaling pathways associated with oxidative stress responses. The Keap1/Nrf2/ARE pathway plays a crucial role in cellular defense against oxidative stress by regulating the expression of antioxidant proteins. Coumarins have been identified as modulators of this pathway, enhancing the transcription of genes involved in antioxidant defense [35].
Interestingly, especially at higher concentrations, coumarins can exhibit pro-oxidant properties, leading to increased ROS production. This controlled elevation in ROS can trigger apoptosis in cancer cells. For example, 4-phenylcoumarin has been reported to induce ROS-dependent cell death in A549 lung cancer cells, highlighting its potential as a therapeutic agent [36].
The dual ability of coumarins to act as antioxidants and, under specific circumstances, as pro-oxidants underscores their potential in cancer therapy. By modulating oxidative stress, coumarins can protect normal cells from damage and selectively induce death in cancer cells. Further research into these mechanisms may pave the way for the development of coumarin-based anticancer therapeutics.
2.4. Inhibition of Angiogenesis and Metastasis
Coumarins, a class of naturally occurring benzopyrone compounds, have demonstrated significant anticancer properties, notably through the inhibition of angiogenesis and metastasis. These processes are critical for tumor growth and dissemination, and targeting them is a promising therapeutic strategy [37].
Angiogenesis, the formation of new blood vessels from existing vasculature, is essential for tumor progression. Coumarins have been shown to inhibit angiogenesis through various mechanisms.
Modulation of VEGF signaling: Vascular endothelial growth factor (VEGF) is a primary mediator of angiogenesis. Coumarins can suppress VEGF expression and its receptor activity, thereby hindering endothelial cell proliferation and new vessel formation. For instance, studies have demonstrated that coumarin derivatives effectively reduce VEGF-induced angiogenesis in cancer models [14,38].
Suppression of endothelial cell functions: Coumarins directly affect endothelial cells by inhibiting their proliferation, migration, and tube formation, which are vital steps in angiogenesis. Research indicates that certain coumarin compounds disrupt endothelial cell functions, leading to impaired angiogenic processes [39].
Metastasis, the spread of cancer cells to distant organs, is a leading cause of cancer-related mortality. Coumarins have been found to impede metastasis through several pathways.
Inhibition of matrix metalloproteinases (MMPs): MMPs degrade extracellular matrix components, facilitating cancer cell invasion. Coumarins have been shown to inhibit metalloproteinase-2 (MMP-2) and metalloproteinase-9 (MMP-9) activity, thereby reducing tumor cell migration and invasion [40].
Suppression of epithelial–mesenchymal transition (EMT): EMT is a process where epithelial cells acquire mesenchymal properties, enhancing migratory and invasive capabilities. Coumarins can inhibit EMT by modulating key signaling pathways, thus reducing the metastatic potential [41,42,43].
The ability of coumarins to inhibit angiogenesis and metastasis underscores their potential as therapeutic agents in cancer treatment. By targeting multiple pathways involved in tumor vascularization and dissemination, coumarins offer a multifaceted approach to impeding cancer progression.
2.5. Impact on the Immune System
Coumarins have demonstrated significant immunomodulatory properties, impacting both innate and adaptive immune responses.
Research has shown that coumarin derivatives can enhance T-cell proliferation and activity. For instance, a study Berkarda et al. observed increased lymphocyte responsiveness to phytohemagglutinin (PHA) in both normal individuals and cancer patients treated with coumarin derivatives [44]. Coumarins influence the production of key cytokines involved in inflammatory responses. A systematic review highlighted that coumarins act on immune cells and cytokines, playing a role in the treatment of autoimmune diseases by regulating signaling pathways such as NF-κB and Keap1/Nrf2. The NF-κB pathway is pivotal in regulating immune responses and inflammation. Coumarins have been found to inhibit NF-κB activation, leading to decreased expression of inflammatory mediators. For example, studies have shown that coumarins can modulate this pathway, thereby exhibiting anti-inflammatory effects. Keap1/Nrf2 Pathway: The Keap1/Nrf2 pathway plays a crucial role in the cellular defense against oxidative stress. Coumarins can activate the Nrf2 pathway, enhancing the expression of antioxidant proteins and contributing to the modulation of immune responses. This mechanism has been highlighted in studies exploring the therapeutic potential of coumarins in autoimmune diseases [35,45].
Due to their immunomodulatory properties, coumarins have been explored as potential therapeutic agents in autoimmune diseases. A comprehensive review discussed the therapeutic effects of natural coumarins in autoimmune diseases, emphasizing their role in modulating immune responses [45].
The therapeutic potential of coumarins in autoimmune diseases has been highlighted in various studies, suggesting their role in modulating immune responses and providing a basis for the development of new treatments. For instance, a study demonstrated that coumarin derivatives ameliorate intestinal inflammation and modulate the gut microbiome, highlighting their potential in treating autoimmune conditions [46].
Coumarins exert significant effects on the immune system by modulating immune cell functions, cytokine production, and key signaling pathways. These properties underscore their potential as therapeutic agents in managing immune-related disorders, including autoimmune diseases and inflammatory conditions.
3. Coumarins with Proven Anticancer Effects
3.1. Scopoletin and Its Biological Activity
Scopoletin (Figure 4A), a member of the coumarins group, is a natural chemical compound found in many plant species. Numerous studies indicate its broad spectrum of biological activity, including anti-inflammatory, antioxidant and antimicrobial effects [47].
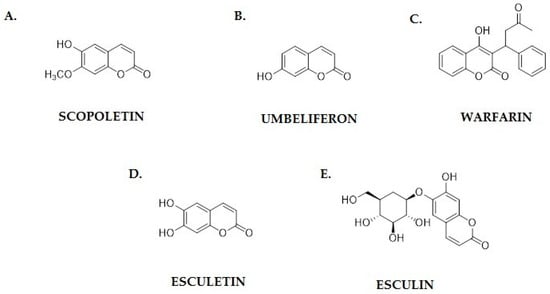
Figure 4.
Chemical structures of coumarins with proven anticancer activity: (A) scopoletin; (B) umbeliferon; (C) warfarin; (D) esculetin; and (E) esculin.
In recent years, there has been growing interest in its anticancer potential, especially in the context of various types of cancers, such as cervical cancer, gynecological cancers and other types of solid tumors. One of the key mechanisms of scopoletin’s anticancer action is the induction of apoptosis in cancer cells. Studies conducted by Karatoprak et al. have shown that scopoletin, as a representative of coumarins, can act as a multi-target therapeutic anticancer agent by activating apoptotic pathways in gynecological cancer cells. The results of in vitro studies on cervical cancer cell lines (HeLa) suggest that this compound leads to DNA fragmentation and increases caspase activity [48,49]. Scopoletin also has antioxidant activity, which may play an important role in inhibiting the growth of cancer cells. Ajiboye et al. analyzed plant extracts containing scopoletin and showed that this substance can reduce oxidative stress in cells, which leads to a reduction in cancer proliferation. An increase in the level of reactive oxygen species (ROS) in cancer cells can lead to their death, and scopoletin modulates this process [47]. Studies by Meng et al. showed that scopoletin affects the biosynthetic pathways of coumarins and the mechanisms regulating the proliferation and apoptosis of cancer cells. Their experiments, conducted on cell cultures, showed that the presence of scopoletin leads to the inhibition of the expression of genes responsible for the development of cancer, such as Bcl-2 and c-Myc [50].
In addition to its direct effect on cancer cells, scopoletin may also affect the tumor microenvironment. The work of Elgabry et al. suggests that this compound may modify the activity of the immune system, strengthening the body’s immune response to cancer cells [51]. In turn, the review by Sun and Shahrajabian indicated a synergistic effect of scopoletin with other plant-derived compounds that support its therapeutic properties. Due to its properties, scopoletin is considered a potential component of anticancer drugs. It can be used as a stand-alone active substance or in combination with other chemotherapeutic drugs to increase the effectiveness of therapy and reduce the side effects of traditional chemotherapy [52]. The work suggests that its combination with other coumarins may additionally enhance the therapeutic effect.
Scopoletin exerts strong anticancer properties by inducing apoptosis, inhibiting proliferation, modulating oxidative stress, and influencing the tumor microenvironment. Its potential use in anticancer therapy requires further clinical trials to confirm its efficacy and safety. The results of the studies to date are promising and indicate the possibility of using scopoletin as an innovative component of cancer therapy.
3.2. Umbeliferon—Mechanisms of Anticancer Action
Umbeliferon (7-hydroxycoumarin; Figure 4B) is a natural phenolic compound belonging to the coumarins group, which exhibits a wide range of biological activities, including anti-inflammatory, antioxidant and antimicrobial activities. In recent years, there has been growing interest in its anticancer potential, resulting from its ability to modulate various signaling pathways important for the proliferation, apoptosis and migration of cancer cells [53]. One of the key mechanisms of action of umbeliferon is the ability to induce apoptosis in cancer cells. Studies by Albratty and Makeen have shown that umbeliferon can reduce the expression of the Bcl-2 protein, which plays a key role in inhibiting apoptosis, while increasing the expression of proapoptotic Bax. As a result, caspases are activated and cancer cells die, which suggests the therapeutic potential of this compound in the treatment of breast cancer [54]. Similar results were obtained by Muthu et al. when examining the effect of umbeliferon on colon cancer cells. Their experiments showed that this compound activates the caspase cascade and increases the cytochrome c levels in the cytoplasm, leading to apoptosis [55].
The mechanism of action of umbeliferon also includes modulation of oxidative stress in cancer cells. Yu et al. demonstrated that in liver cancer, umbeliferon increases the reactive oxygen species (ROS) levels, which causes DNA damage and activation of the p53 pathway. This leads to cell cycle arrest, and ultimately, to cancer cell death [56].
Furthermore, it has been shown that umbeliferon can act as both an antioxidant and a pro-oxidant depending on the biological context. In healthy cells, it counteracts oxidative stress, while in cancer cells, it leads to increased ROS production and apoptosis [57].
The growth and migration of cancer cells are key processes leading to the development of malignant tumors. Ma et al. showed that umbeliferon inhibits glioma cell proliferation and metastasis by regulating the cadherin/β-catenin complex. Reduced activity of this complex leads to decreased intercellular adhesion and inhibition of the invasive properties of cancer cells [58]. Similar effects were reported by Lim et al. in the context of skin disease. Their studies showed that umbeliferon inhibits NF-κB activity, which results in reduced expression of pro-inflammatory factors and reduced ability of the tumor to evade the immune system [59].
Umbeliferon has the ability to affect many signaling pathways crucial to cancer cell survival. It has been proven that this compound blocks the PI3K/Akt/mTOR pathway, which is overactivated in many types of cancer. Inhibition of this pathway leads to cell cycle arrest in the G1 phase and reduced cancer cell survival [53,60]. In turn, An et al. showed that umbeliferon can also affect the MAPK/ERK pathway, which plays an important role in regulating cell proliferation and differentiation. Their studies suggest that umbeliferon may be a promising compound for targeted therapy [61].
Due to its multidirectional anticancer activity, umbeliferon is considered as a potential component of combination therapies [62]. It can be used in combination with other chemotherapeutic drugs to increase their efficacy and reduce cancer’s resistance to treatment. Moreover, its natural origin and relatively low toxicity make it an attractive candidate for clinical trials.
3.3. Warfarin and Its Role in Inhibiting Angiogenesis
Angiogenesis, the process of new blood vessel formation, is crucial for various physiological and pathological processes, including wound healing, embryogenesis, and cancer progression. The regulation of angiogenesis is mediated by a complex interplay of growth factors, signaling pathways, and extracellular matrix remodeling [63]. Targeting angiogenesis has become a prominent strategy in cancer therapy and the treatment of diseases characterized by excessive vascularization, such as diabetic retinopathy [64].
Warfarin (Figure 4C), a widely used anticoagulant, has recently been recognized for its potential antiangiogenic properties. It functions primarily as a vitamin K antagonist, interfering with the γ-carboxylation of coagulation proteins. However, research suggests that its effects extend beyond anticoagulation, influencing angiogenesis by modulating vascular endothelial growth factor (VEGF) signaling, endothelial cell migration, and tumor microenvironment interactions [65].
VEGF is a key regulator of angiogenesis, promoting endothelial cell proliferation and new vessel formation. Warfarin has been shown to interfere with VEGF-driven angiogenesis by disrupting Gla-domain protein activation, which is essential for endothelial function [66,67]. In vitro studies demonstrate that warfarin inhibits VEGF-induced endothelial cell migration and tube formation, leading to the suppression of neovascularization [68]. Additionally, warfarin modulates the expression of VEGF receptors, particularly VEGFR-2, reducing its downstream signaling and limiting the angiogenic response [69]. These findings suggest that warfarin’s inhibitory effects on angiogenesis are mediated through direct interaction with key proangiogenic pathways.
Endothelial cell migration and tube formation are essential steps in angiogenesis. Studies indicate that warfarin suppresses endothelial proliferation by interfering with protease-activated receptor 1 (PAR-1) signaling, which plays a vital role in vascular remodeling [70]. Experimental models reveal that warfarin-treated endothelial cells exhibit reduced motility and an impaired ability to form capillary-like structures, further supporting its antiangiogenic potential.
Furthermore, warfarin has been found to disrupt hypoxia-induced angiogenesis, a key mechanism in tumor vascularization. Hypoxic conditions stimulate the expression of hypoxia-inducible factor-1α (HIF-1α), which in turn upregulates VEGF production. Warfarin counteracts this process by downregulating HIF-1α activity, thereby attenuating the hypoxia-driven angiogenic response [71].
Tumor growth and metastasis depend on the formation of new blood vessels to supply oxygen and nutrients. Warfarin has been shown to inhibit tumor-associated angiogenesis by targeting multiple signaling pathways involved in endothelial activation and vascular remodeling [63]. Studies on cancer models reveal that warfarin, in combination with chemotherapeutic agents, enhances treatment efficacy by reducing the vascular density within tumors and limiting the supply of essential nutrients for tumor progression. Moreover, warfarin’s ability to modulate the tumor microenvironment suggests its potential as an adjuvant therapy in cancer treatment [72].
The antiangiogenic properties of warfarin highlight its potential for repurposing as an adjunctive therapy in cancer treatment. Despite promising preclinical findings, clinical studies are needed to determine the optimal dosing and minimize the potential side effects, particularly the risk of hemorrhage associated with long-term anticoagulant use.
3.4. Esculetin and Esculin in Cancer Models
Esculetin and esculin (Figure 4D,E) are naturally occurring coumarin derivatives that have attracted significant attention due to their anticancer properties. These compounds exhibit a wide range of biological activities, including antioxidant, anti-inflammatory, and antiproliferative effects, making them promising candidates for cancer treatment [15,73]. Studies indicate that esculetin, the aglycone form of esculin, plays a crucial role in modulating key cellular pathways involved in tumor progression, apoptosis, and metastasis [74].
One of the primary anticancer mechanisms of esculetin and esculin is their ability to induce apoptosis in cancer cells. Studies have demonstrated that esculetin triggers apoptosis through the activation of caspase-dependent pathways and mitochondrial dysfunction, leading to the release of cytochrome c and subsequent cell death [15,16]. Additionally, esculetin has been shown to cause G1 and G2/M cell cycle arrest, preventing uncontrolled proliferation of cancer cells [73]. Tumor progression relies heavily on angiogenesis, the formation of new blood vessels, and metastasis, the spread of cancer cells to distant organs. Esculetin exhibits strong antiangiogenic and antimetastatic properties by downregulating vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs), which are critical regulators of cancer cell invasion and migration [35]. Furthermore, in melanoma models, esculetin has demonstrated synergistic effects when combined with conventional chemotherapeutics, enhancing their efficacy in reducing the metastatic potential [75]. Oxidative stress plays a crucial role in carcinogenesis by causing DNA damage, promoting tumor growth, and enhancing drug resistance. Esculin and esculetin exhibit potent antioxidant properties by scavenging reactive oxygen species (ROS) and modulating nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, which regulates cellular antioxidant defense mechanisms [35]. Moreover, these compounds have been found to suppress inflammatory pathways by inhibiting NF-κB activation, which is implicated in tumor initiation and progression [16].
In breast cancer models, esculetin has been found to inhibit the proliferation of estrogen receptor-positive by modulating estrogen receptor signaling and inducing autophagy-related cell death [76]. Furthermore, studies suggest that combining esculetin with standard chemotherapeutic agents enhances treatment outcomes by reducing drug resistance mechanisms [73]. Colorectal cancer (CRC) is one of the most common malignancies worldwide. Esculin and esculetin have shown promising anti-CRC activity by targeting key oncogenic pathways such as Wnt/β-catenin and PI3K/Akt/mTOR signaling [77,78]. Studies also report that esculetin sensitizes CRC cells to radiotherapy and chemotherapy, thereby improving treatment efficacy and reducing tumor recurrence rates [79].
In melanoma models, esculetin has been investigated for its ability to suppress tumor growth and enhance the effects of existing anti-melanoma drugs. Research suggests that esculetin exerts its effects by modulating oxidative stress responses and inhibiting melanogenesis-related proteins. Isobolographic analysis has revealed that esculetin displays synergistic interactions with doxorubicin and cisplatin, leading to enhanced cytotoxic effects in melanoma cells [75].
Gastrointestinal cancers, including gastric and pancreatic cancer, are highly aggressive malignancies with a poor prognosis. Studies indicate that esculetin inhibits tumor growth by downregulating pro-inflammatory cytokines, which contribute to the inflammatory tumor microenvironment. Additionally, esculetin has been shown to target ATP-binding cassette (ABC) transporters, which are responsible for multidrug resistance in gastrointestinal tumors [80].
Despite promising preclinical data, the clinical application of esculetin and esculin remains limited due to challenges related to bioavailability and pharmacokinetics. Studies are currently underway to develop novel drug delivery systems, such as nanoformulations and encapsulation techniques, to improve their stability and therapeutic efficacy [16]. Moreover, ongoing clinical trials are investigating the potential of esculetin as an adjuvant therapy in combination with conventional anticancer agents [81].
Future research should focus on elucidating the molecular targets of esculetin and esculin in different cancer subtypes, optimizing their pharmacological profiles, and conducting large-scale clinical trials to establish their safety and efficacy in human patients [82].
Esculetin and esculin have emerged as promising natural compounds with significant anticancer potential. Their ability to induce apoptosis, inhibit angiogenesis, and modulate oxidative stress makes them attractive candidates for cancer therapy. While current studies provide strong preclinical evidence supporting their efficacy, further research is needed to overcome the pharmacokinetic limitations and translate these findings into clinical applications. The development of novel drug formulations and combination strategies could pave the way for integrating esculetin and esculin into standard cancer treatments.
3.5. New Synthetic Coumarins with Therapeutic Potential
In recent years, advancements in synthetic chemistry have led to the development of novel coumarin derivatives with enhanced therapeutic potential [83]. These synthetic coumarins have demonstrated improved bioavailability, specificity, and efficacy against various diseases, particularly in cancer therapy [39].
One of the most significant advancements in synthetic coumarins is the introduction of four-substituted derivatives, which have shown promising anticancer properties. These compounds exhibit potent cytotoxic effects against cancer cells by targeting specific molecular pathways, such as the inhibition of topoisomerases and modulation of apoptosis-related proteins [84]. Sulfonamide-modified coumarins have gained attention due to their ability to inhibit key enzymes involved in cancer progression. Recent studies have shown that these derivatives act as selective enzyme inhibitors, effectively suppressing tumor growth while minimizing side effects [85]. Hybridization of coumarins with other pharmacophores has led to the development of compounds with enhanced selectivity and potency. Recent research highlights the potential of hybrid coumarins in photodynamic therapy, where they serve as mitochondrial-targeted photosensitizers in anticancer treatment [86].
Synthetic coumarins exert their anticancer effects through multiple mechanisms, with apoptosis induction being a primary mode of action. Many newly developed coumarin derivatives activate intrinsic and extrinsic apoptotic pathways by upregulating pro-apoptotic proteins and downregulating anti-apoptotic proteins [87]. Coumarin-based derivatives have been shown to halt cancer cell proliferation by inducing cell cycle arrest at different phases. Studies indicate that specific coumarins can effectively inhibit cyclin-dependent kinases (CDKs), leading to the accumulation of cells in the G1 or G2/M phase, thereby preventing their progression into mitosis [15,88].
A recent breakthrough in coumarin-based therapy is their application in photodynamic therapy (PDT). COUPY coumarins (Figure 5), for instance, have been engineered to selectively accumulate in mitochondria, enhancing their photodynamic effects and selectively eliminating cancer cells [86]. This targeted approach minimizes the damage to healthy tissues, making PDT a promising strategy in modern oncology.
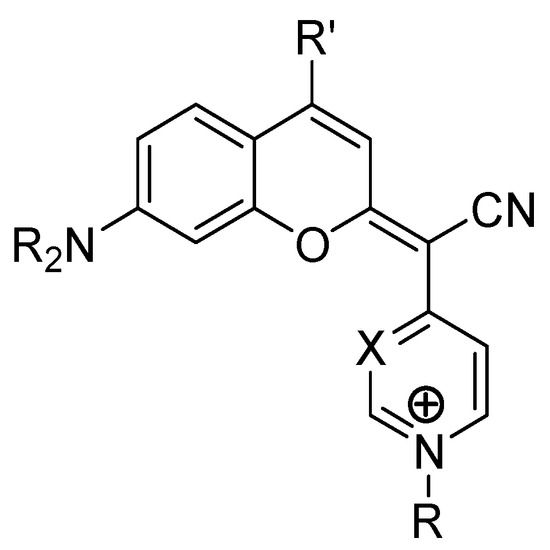
Figure 5.
Chemical structure of coumarin-based COUPY derivatives.
Breast cancer remains one of the most studied malignancies for coumarin-based therapies. Synthetic coumarins have demonstrated potent effects in hormone-sensitive and triple-negative breast cancer models by inhibiting estrogen receptor signaling and inducing autophagic cell death [89]. Lung cancer is another area where synthetic coumarins show great promise. Research indicates that modified coumarin derivatives can effectively suppress key oncogenic pathways, such as PI3K/Akt and MAPK, leading to significant tumor growth inhibition [90]. Colorectal cancer (CRC) is among the leading causes of cancer-related mortality worldwide. Novel coumarins have shown efficacy in CRC models by downregulating β-catenin signaling and inhibiting multidrug resistance proteins [91].
4-Hydroxycoumarin (4-HC) and its derivatives have attracted increasing attention in recent years due to their promising anticancer activities. Structurally, 4-HC serves as a core scaffold for the synthesis of numerous biologically active molecules capable of interacting with key cellular targets involved in tumor progression. Recent research by Dimić et al. (2022) demonstrated that novel derivatives of 4-hydroxycoumarin conjugated with neurotransmitters such as dopamine and octopamine exhibit significant selective cytotoxicity toward colorectal carcinoma (HCT-116) and cervical adenocarcinoma (HeLa) cells, while maintaining low toxicity toward healthy fibroblast cells (MRC-5). The proposed mechanisms underlying the observed anticancer effects include the induction of apoptosis, inhibition of carbonic anhydrase IX (hCA-IX)—a marker of tumor hypoxia—and modulation of oxidative stress through altered reactive oxygen species (ROS) production. Molecular docking and dynamics studies confirmed the strong interactions of these derivatives with hCA-IX, supporting their potential as selective anticancer agents [92]. Additionally, Avdović et al. (2021) reported the synthesis and biological evaluation of new 4-hydroxycoumarin-based ligands and their palladium(II) complexes. The study revealed that these complexes possess enhanced cytotoxic properties against several carcinoma cell lines, including colorectal (HCT116), melanoma (A375), and pancreatic carcinoma (MIA PaCa-2) cells. Notably, the cytotoxicity was primarily mediated through apoptosis induction. The palladium(II) complexes exhibited higher inhibitory activity compared to their parent ligands, suggesting that metal complexation significantly improves the anticancer potential of 4-hydroxycoumarin scaffolds [93].
Both studies emphasized that modifications to the basic 4-HC structure, such as the attachment of neurotransmitter fragments or complexation with metal ions, can optimize pharmacological profiles, enhance selectivity, and reduce toxicity toward healthy tissues. Importantly, ecotoxicological assessments indicated that these new derivatives have a low environmental impact, further supporting their potential in clinical applications.
Despite the promising therapeutic potential of synthetic coumarins, several challenges remain in their clinical translation. Issues such as poor solubility, rapid metabolism, and potential toxicity must be addressed through advanced drug formulation techniques, such as nanoparticle-based delivery systems [94]. Moreover, further research is needed to explore their combinatorial effects with standard chemotherapeutics to enhance their efficacy while reducing their side effects [95].
The development of synthetic coumarins represents a significant advancement in medicinal chemistry, particularly in cancer therapy. These novel compounds exhibit potent anticancer properties through multiple mechanisms, including apoptosis induction, cell cycle arrest, and targeted photodynamic therapy. While challenges in terms of bioavailability and toxicity remain, continued research and technological innovations hold the potential to establish synthetic coumarins as viable therapeutic agents in modern oncology.
4. Research Models and Results of Preclinical Studies
Coumarins, a prominent class of naturally occurring and synthetically modified benzopyrones, have gained considerable attention in anticancer research due to their potent bioactivities. Preclinical studies, encompassing in vitro, in vivo, and pharmacokinetic analyses, play a critical role in evaluating the efficacy, mechanisms of action, and potential clinical applications of these compounds. This section examines recent advancements in coumarin-based preclinical research models, providing an in-depth overview of their impact on cancer cell lines, animal models, and pharmacokinetic properties.
4.1. In Vitro Studies—The Effect of Coumarins on Various Cancer Cell Lines
Coumarin derivatives exhibit significant cytotoxic and pro-apoptotic effects across various cancer cell lines. Recent research has shown that 0-prenylated coumarin derivatives induce cell death in human cervical cancer cells by activating caspase-dependent apoptosis [96]. Similar effects have been observed in melanoma, where daphnetin, a hydroxylated coumarin, enhances chemosensitivity and potentiates the activity of standard chemotherapy agents [97]. Moreover, novel coumarin-6-sulfonamides have been identified as potent apoptotic antiproliferative agents. These derivatives demonstrate strong inhibition of cell viability in colorectal and breast cancer cell lines by downregulating Bcl-2 and upregulating Bax expression [98].
Beyond direct cytotoxicity, coumarins also exhibit antiangiogenic properties by suppressing vascular endothelial growth factor (VEGF) signaling. Studies show that synthetic coumarins can inhibit tumor angiogenesis and block endothelial cell migration, reducing the metastatic potential [14,38]. In hepatocellular carcinoma models, benzylsulfone–coumarin derivatives effectively impair metastatic processes by inhibiting matrix metalloproteinases (MMPs) and disrupting tumor cell adhesion [99].
Recent work highlights coumarin-based organoselenium compounds, which act as targeted myeloprotective agents while synergizing with chemotherapeutic drugs like carboplatin [100]. The development of hybrid coumarin molecules incorporating sulfonamides and alkyl chains has further improved drug specificity and reduced off-target effects [101].
Numerous in vitro studies have shown that coumarins have a significant effect on the proliferation and survival of various cancer cell lines. Particularly promising results have been obtained in the case of hematological cancer cells (e.g., leukemia), melanoma, breast cancer and colon cancer, where coumarins have shown cytotoxic activity, often synergistic with conventional anticancer drugs (Table 1).

Table 1.
Anticancer efficacy of selected natural coumarin derivatives in vitro.
4.2. In Vivo Studies—Use in Animal Models
The transition from cell-based assays to animal models is a crucial step in preclinical validation. Studies in zebrafish xenografts have demonstrated that umbeliprenin, a naturally occurring sesquiterpene coumarin, significantly reduces the tumor burden and inhibits cell proliferation [118]. In murine breast cancer models, 7-isopentenyloxycoumarin has been found to exhibit potent antiangiogenic activity by modulating CCL2 chemokine signaling, leading to decreased tumor vascularization and growth [119]. Similarly, OT48, a synthetic coumarin derivative, was shown to inhibit tumor progression in spheroid-based models and 3D organoid systems (Figure 6) [120].
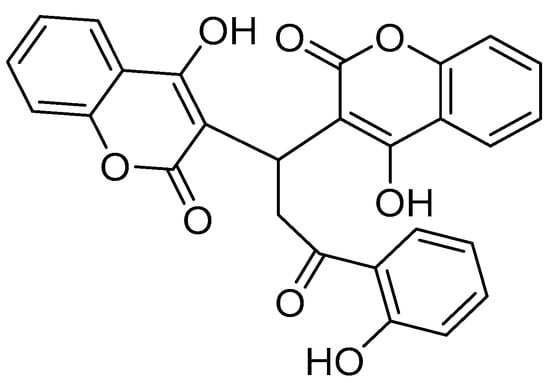
Figure 6.
Chemical structure of the synthetic coumarin derivative OT48 {3,3′-[3-(2-hydroxyphenyl)-3-oxopropane-1,1-diyl]bis(4-hydroxy)-2H-chromen-2-one}.
Preclinical studies suggest that certain coumarin derivatives enhance immune responses by modulating macrophage polarization and T-cell activation [121]. These findings align with nanoparticle-based coumarin formulations, which improve drug bioavailability while boosting immune-mediated tumor suppression [122]. Moreover, pre-treatment with hydroxylated coumarins has been shown to increase the efficacy of platinum-based chemotherapy in lung and ovarian cancer models, suggesting a role in chemosensitization [123].
The antitumor efficacy of selected natural coumarin derivatives has also been confirmed in in vivo models. Studies conducted on laboratory animals, most often mice with implanted human tumors (xenografts), have shown that some compounds from this group significantly inhibit tumor growth, reduce angiogenesis and induce apoptosis of tumor cells. The therapeutic effects are often associated with the modulation of key signaling pathways (e.g., PI3K/Akt, MAPK, NF-κB), which confirms their potential in targeted therapy (Table 2).

Table 2.
Anticancer efficacy of selected natural coumarin derivatives in vivo.
4.3. Review of Pharmacokinetic and Bioavailability Studies
Despite their promising anticancer efficacy, many coumarins suffer from low solubility and bioavailability. Studies indicate that lipophilic coumarins demonstrate enhanced oral absorption, whereas highly hydroxylated derivatives often undergo rapid hepatic metabolism via cytochrome P450 enzymes [15]. Recent pharmacokinetic evaluations of coumarin–palladium(II) complexes show improved plasma stability and prolonged circulation time, suggesting enhanced drug retention and efficacy [133].
To overcome the pharmacokinetic limitations, researchers have explored nanocarrier-based delivery methods. Studies show that polymeric nanoparticles and liposomal encapsulation significantly enhance the drug half-life and tumor accumulation [123]. A nanoformulated coumarin hybrid system, developed for pancreatic cancer, demonstrated a three-fold increase in bioavailability and targeted accumulation in tumor tissues, highlighting the potential of nanomedicine-based interventions [122]. Understanding the structure–activity relationships (SARs) of coumarins is crucial to optimizing their pharmacological profiles. A recent QSAR analysis by Sabt et al. showed that electron-donating substitutions at the 7-position significantly improve antiproliferative activity, while halogenated coumarins exhibit superior enzyme inhibition [98].
Additionally, computational docking studies have provided valuable insights into coumarins’ interactions with oncogenic targets, further guiding drug design and optimization [134].
The preclinical evaluation of coumarins has provided strong evidence supporting their anticancer potential. In vitro studies confirm their cytotoxicity, in vivo models validate their efficacy in animal systems, and pharmacokinetic studies reveal essential modifications needed to optimize their clinical translation. However, challenges such as bioavailability limitations, off-target effects, and metabolic instability remain key barriers. Future research should focus on improving drug delivery, enhancing combinatorial approaches, and bridging the gap between preclinical and clinical studies to fully exploit coumarins’ therapeutic potential in oncology.
5. Potential Use of Coumarins in Cancer Therapy
5.1. Possibilities for Use as Stand-Alone Anticancer Drugs
Coumarins exert direct cytotoxic effects on various cancer cell lines by inducing apoptosis and cell cycle arrest. Several hydroxylated and prenylated derivatives have demonstrated potent antiproliferative activity through the activation of caspases and the mitochondrial apoptotic pathway [87]. For example, daphnetin and esculetin have shown strong activity against melanoma and colorectal cancer by modulating the Bcl-2/Bax ratio and promoting caspase-9 activation [97,135].
Angiogenesis is a key target in cancer therapy, and several coumarin derivatives have exhibited antiangiogenic properties by inhibiting vascular endothelial growth factor (VEGF) signaling [14]. For instance, benzylsulfone–coumarins have been shown to disrupt endothelial tube formation, thereby preventing tumor vascularization and metastasis [99].
Photodynamic therapy (PDT) has emerged as a promising non-invasive cancer treatment approach, utilizing photosensitizing agents that, upon activation by light of a specific wavelength, generate reactive oxygen species (ROS) to induce tumor cell death. Recent advancements in synthetic and naturally derived coumarin-based photosensitizers have demonstrated significant potential in improving the efficacy and selectivity of PDT for various malignancies [86,136]. Coumarins possess intrinsic fluorescence properties that allow their use as light-activated therapeutic agents in PDT. The anticancer activity of coumarin-based photosensitizers relies on three main mechanisms: generation of singlet oxygen (1O₂) and ROS, mitochondrial targeting and apoptosis induction and inhibition of tumor angiogenesis.
A study by Ortega-Forte et al. demonstrated that COUPY–coumarin derivatives selectively accumulate in the mitochondria of HeLa cells. Upon light activation, these compounds induced severe mitochondrial dysfunction, leading to rapid ROS production and cell death. In xenograft models, COUPY–coumarins significantly inhibited tumor progression and reduced recurrence rates (Figure 5) [86].
Recent advancements in PDT include coumarin–porphyrin conjugates, which exhibit enhanced tumor selectivity due to their two-photon absorption properties. Wu et al. reported that these hybrid molecules demonstrated about 50% higher ROS generation efficiency compared to conventional porphyrin-based photosensitizers [137]. Studies on furanocoumarins, such as psoralen, show that these naturally occurring compounds possess dual anticancer effects, acting as both chemotherapeutic agents and photosensitizers. In cancer cells, psoralen-based PDT can lead to tumor regression, suggesting a synergistic interaction between photodynamic and cytotoxic effects [14,138].
One of the primary limitations of PDT is the hydrophobic nature of most photosensitizers, which affects their solubility and bioavailability. To overcome this problem, a method for nano-encapsulation of coumarins was developed, which showed improved bioavailability and targeted accumulation in the tumor [139].
A novel strategy involves the design of coumarin hybrids that act as both photosensitizers and chemotherapeutic agents. Ahmadi and Haddadi-Asl synthesized a doxorubicin–coumarin conjugate, which demonstrated synergistic cytotoxicity in cancer cells models when exposed to visible light irradiation [140].
5.2. Coumarins as Adjuvants in Combination Therapy with Chemotherapy
Coumarins have demonstrated chemosensitizing effects when used in combination with standard chemotherapeutic agents. For example, pre-treatment with hydroxylated coumarins has been found to enhance the efficacy of platinum-based drugs in lung and ovarian cancer models, increasing tumor cell sensitivity to cisplatin and carboplatin [79].
One of the most significant challenges in chemotherapy is multidrug resistance (MDR). Several coumarins act as P-glycoprotein inhibitors, effectively blocking drug efflux pumps and enhancing intracellular drug retention [137,141,142]. In vitro studies have shown that sesquiterpene coumarins (SCs) can be used as chemosensitizers. Kasaian et al. isolated and purified 14 SCs from the roots of four Ferula species. The purified SCs were evaluated for their multidrug resistance (MDR) reversal properties in A2780/RCIS cells (cisplatin-resistant derivatives of the human ovarian cancer cell line A2780P). Among the tested compounds, mogoltacin, mogoltadone, farnesiferol A, farnesiferol B, farnesiferol C, lehmferin, conferdione, and samarcandine showed significant MDR reversal effects. The combination of nontoxic concentrations of SCs (20 μM) with cisplatin significantly enhanced the cytotoxicity of cisplatin on A2780/RCIS cells. The finding showed that conferdione and samarcandine had the strongest inhibitory effect on the efflux of multidrug resistance-associated pump protein 2, and thus these compounds could be considered prime scaffolds for further structural modifications [143].
5.3. Pharmaceutical Formulations—Possibilities for Improving Efficacy and Bioavailability
Despite their promising anticancer properties, many coumarins suffer from poor solubility, rapid metabolism, and low bioavailability. Hydroxylated derivatives are often rapidly metabolized by hepatic enzymes, limiting their systemic availability [15].
Recent advances in nanomedicine have led to the development of nanoformulations of coumarins, which significantly enhance the drug half-life and tumor accumulation. Liposomal and polymeric nanoparticles loaded with coumarin derivatives have exhibited improved plasma retention times and increased tumor specificity [144]. A notable example is coumarin-loaded micelles, which increase water solubility and improve intracellular drug uptake, thereby enhancing anticancer efficacy [145]. Hybrid drug formulations combining coumarins with conventional chemotherapeutics have also been explored to improve treatment outcomes. Coumarin–steroid conjugates have been found to exhibit dual anti-inflammatory and anticancer effects, making them suitable for hormone-sensitive tumors such as prostate and breast cancers [82].
Coumarins exhibit remarkable anticancer potential and can be utilized as stand-alone therapeutic agents, adjuvants in chemotherapy, and components of novel pharmaceutical formulations. Their ability to target multiple pathways, enhance drug efficacy, and modulate drug resistance highlights their clinical relevance in oncology. However, challenges related to bioavailability and metabolism must be addressed through nanotechnology and drug formulation advancements. Future research should focus on optimizing coumarin derivatives, identifying novel molecular targets, and conducting large-scale clinical trials to fully establish their therapeutic utility in cancer treatment.
6. Safety and Limitations of Coumarins in Cancer Therapy
6.1. Potential Toxicity and Side Effects
Coumarins, despite their promising anticancer properties, exhibit potential toxicity risks that must be carefully evaluated before clinical applications. Their adverse effects are mainly associated with hepatotoxicity, nephrotoxicity, and hematological disturbances [15].
One of the most documented safety concerns regarding coumarins is their potential to cause hepatic damage, particularly in high doses or after prolonged use [87]. Some naturally occurring coumarins, such as psoralen and umbeliferon, have been linked to elevated liver enzymes, fatty liver disease, and hepatocellular necrosis Animal studies suggest that chronic exposure can lead to oxidative stress-mediated hepatic injury, limiting their long-term use in therapy [146].
Studies on zebrafish models have shown that some coumarins disrupt kidney function by interfering with renal transporters, leading to electrolyte imbalances and nephrotoxicity [121]. Moreover, some derivatives have demonstrated hematological toxicity, such as leukopenia and thrombocytopenia, raising concerns regarding their safe therapeutic window in oncology [147].
Coumarins like furanocoumarins exhibit phototoxic effects, making patients susceptible to UV-induced skin damage. This reaction is particularly problematic for individuals undergoing photodynamic therapy (PDT), as excessive exposure can trigger erythema, hyperpigmentation, and DNA damage [148].
6.2. Interactions with Other Anticancer Drugs
While coumarins have shown synergistic effects with conventional chemotherapy, they can also interfere with drug metabolism, posing potential risks.
Coumarins have been reported to enhance or inhibit the efficacy of chemotherapy drugs by modulating drug transporters such as P-glycoprotein (P-gp) [8,149]. For instance, warfarin-like coumarins can inhibit CYP450 enzymes, leading to altered drug metabolism and enhanced toxicity [150]. Additionally, combining coumarins with platinum-based chemotherapy has been found to increase nephrotoxicity, necessitating careful dose adjustments [137].
Some coumarin derivatives act as sensitizers in relation to targeted therapy by inhibiting angiogenesis and tumor progression [49]. Curcumin, for example, enhances sorafenib activity in hepatocellular carcinoma models by modulating VEGF and MAPK signaling pathways [151]. However, in some cases, these interactions increase the risk of endothelial dysfunction and bleeding complications [79].
Coumarins can also influence immune checkpoint inhibitors by altering cytokine production and T-cell responses. While this may boost anticancer immune responses, it also raises concerns regarding immune-related adverse effects such as autoimmune hepatitis and colitis [45,79].
6.3. Limitations of Use in Patients with Comorbidities
The potential clinical utility of coumarins in cancer therapy is further limited by pre-existing health conditions, which can exacerbate adverse effects and complicate treatment regimens.
Coumarins, particularly anticoagulant derivatives, have been associated with bleeding risks and thrombocytopenia, making them unsuitable for patients with cardiovascular diseases [152]. Their warfarin-like effects can lead to hemorrhagic complications, especially in patients undergoing chemotherapy with agents like 5-fluorouracil or gemcitabine [153,154].
Patients with pre-existing liver disease may experience enhanced hepatotoxicity, limiting the safe use of esculetin- and psoralen-based coumarins [155]. Similarly, renal impairment patients may be at greater risk of coumarin-induced nephropathy, particularly when combined with nephrotoxic agents such as cisplatin or methotrexate [156].
Diabetic patients may also be at risk, as some coumarins modulate glucose metabolism, potentially leading to hypoglycemia when co-administered with metformin or insulin [157]. Moreover, due to their extensive hepatic metabolism, coumarins require careful monitoring in patients with obesity or metabolic syndrome [158].
While coumarins present exciting opportunities in anticancer therapy, their safety limitations must be critically addressed before widespread clinical adoption. The key concerns include hepatotoxicity, nephrotoxicity, and drug interactions, which pose risks in patients with comorbidities. Strategies such as dose optimization, prodrug formulations, and combination therapy adjustments are necessary to mitigate toxicity and improve their therapeutic index. Future research should focus on large-scale clinical trials to fully assess the long-term safety profiles and develop novel coumarin derivatives with reduced toxicity and enhanced selectivity.
7. Research Perspectives and Future Development Directions
Coumarins, due to their structural diversity and pharmacological activity, are promising candidates for new anticancer drugs. Research on their synthetic derivatives and their use in targeted therapies, as well as technologies for improving their bioavailability, opens up new perspectives in cancer treatment (Table 3).

Table 3.
Promising anticancer efficacy of selected coumarin derivatives in vivo.
7.1. New Synthetic Coumarin Derivatives—Directions for Drug Design
New synthetic coumarins are designed to have enhanced anticancer activity by modifying the benzopyrone ring and adding functional groups that increase the potency of their interactions with target proteins [15]. For example, halogenated coumarins show stronger inhibition of tyrosine kinases, which are key to tumor growth [14], pyrazole-conjugated coumarins show high efficacy in inhibiting melanoma cell proliferation [162], and coumarin hybrids containing sulfonamide groups are more selective for estrogen receptors in breast cancer [163].
Synthetic coumarins act in multiple ways, including by inhibiting angiogenesis caused by blocking the VEGF and HIF-1α pathways [14], by inducing oxidative stress and activating apoptotic pathways [35], and by influencing epigenetics—studies indicate that coumarins can inhibit histone acetylation, which leads to the suppression of oncogene expression [164].
7.2. Application of Nanotechnology to Improve the Efficacy of Coumarins
One of the main limitations of coumarins is their low bioavailability and rapid hepatic metabolism. The use of nanotechnology allows for the improvement of their stability and targeted delivery to cancer cells [165]. Modern nanotechnological strategies for coumarins are liposomal delivery systems—they improve the half-life and penetration of solid tumors [166]—and polymeric nanoparticles—the biophysical characteristics of the nanoformulation, combined with its selective absorption by cancer cells, low in vivo toxicity profile and effective action on tumors, prove the versatility and potential of this nanoparticle formulation in drug delivery applications [167]. Coumarins conjugated with gold nanoparticles show increased absorption by cancer cells and a photosensitizing effect in photodynamic therapy [168].
7.3. Potential of Coumarins in Personalized Therapy
Personalized anticancer therapy is based on adapting the treatment to the genetic profile of the patient, which allows for greater efficacy and fewer side effects. Coumarins show potential in this field, especially due to their ability to interact with key signaling pathways and influence the expression of cancer genes [79].
Studies indicate that some coumarins can act as HDAC (histone deacetylase) inhibitors and modulators of DNA methylation, which allows for the regulation of the expression of genes associated with the development of cancer [169]. Coumarins in combination with immune checkpoint inhibitors (e.g., anti-PD1) can increase the effectiveness of immunotherapy [170].
Pharmacogenetics of coumarins—studies on their metabolism can help in selecting the optimal dose for patients by estimating the therapeutic dose of coumarin, while therapy based on pharmacogenetics can improve the safety and efficacy of coumarin therapy [171].
In the coming years, large-scale clinical trials will be crucial to confirm the therapeutic viability of coumarins in human patients. Their potential application in combination therapies, immunotherapy, and precision medicine requires further validation through comprehensive clinical research. If these challenges can be addressed, coumarins may soon become an integral component of modern oncological treatments, offering novel therapeutic strategies for drug-resistant and hard-to-treat cancers.
8. Conclusions
Coumarins have emerged as a promising class of compounds in anticancer therapy, demonstrating diverse mechanisms of action, including apoptosis induction, inhibition of tumor proliferation, modulation of oxidative stress, and suppression of angiogenesis and metastasis. Their ability to interfere with multiple signaling pathways, such as the PI3K/Akt/mTOR, Wnt/β-catenin, and NF-κB pathways, underpins their therapeutic potential. Additionally, synthetic coumarin derivatives have shown improved selectivity and efficacy, opening up avenues for their development as next-generation anticancer drugs.
Despite these promising results, several challenges hinder the clinical translation of coumarins. Bioavailability issues, such as poor solubility and rapid hepatic metabolism, limit their systemic exposure and therapeutic efficacy. Advances in nanotechnology, including liposomal formulations, polymeric nanoparticles, and targeted drug delivery systems, are crucial to overcoming these limitations and ensuring optimal drug concentrations at tumor sites. Additionally, coumarins’ interactions with chemotherapeutics and their potential role as chemosensitizers provide opportunities for combination therapy, enhancing treatment efficacy while reducing drug resistance.
Safety concerns, including hepatotoxicity, nephrotoxicity, and interactions with other anticancer drugs, necessitate further investigation. Careful dose optimization and pharmacogenetic studies may help minimize these risks, ensuring patient safety and treatment success. Furthermore, the individualized application of coumarins in precision medicine, tailored to tumor-specific genetic and epigenetic profiles, represents a significant step toward personalized cancer therapy.
Looking ahead, future research should focus on the rational design of new coumarin derivatives, advanced formulation strategies, and large-scale clinical trials to validate their efficacy and safety in human patients. Expanding our understanding of coumarin-based pharmacology through molecular modeling, biomarker identification, and drug repurposing approaches will be key to unlocking their full potential in modern oncology.
In summary, coumarins represent a versatile and promising group of anticancer agents, with broad mechanistic capabilities and clinical relevance. While challenges remain, ongoing research efforts in synthetic drug design, nanomedicine, and personalized medicine will play a crucial role in harnessing their full therapeutic potential, paving the way for their integration into next-generation cancer treatment regimens.
Author Contributions
T.P.K. Conceptualization ideas, data curation, investigation, project administration, supervision, visualization, writing—original draft, writing—review and editing. A.M.-K.: Conceptualization ideas, data curation, investigation, resources, writing—original draft. D.A.: Conceptualization ideas, methodology, project administration, supervision, visualization, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.L.; Zhang, Z.W.; Ravindar, L.; Rakesh, K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020, 207, 112832. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, T.; Podgórski, R.; Stompor, M. Natural and Synthetic Coumarins and their Pharmacological Activity. Eur. J. Clin. Exp. Med. 2017, 15, 169–175. [Google Scholar] [CrossRef]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A Review on Pharmacological Properties of Coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Swamy, M.K. Coumarins: An Important Phytochemical with Therapeutic Potential. In Plant-derived Bioactives; Pal, D., Saha, S., Eds.; Springer Singapore: Singapore, 2020; Volume 2, pp. 205–222. [Google Scholar]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell Longev. 2021, 23, 6492346. [Google Scholar] [CrossRef]
- Lončar, M.; Jakovljević, M.; Šubarić, D.; Pavlić, M.; Buzjak Služek, V.; Cindrić, I.; Molnar, M. Coumarins in Food and Methods of Their Determination. Foods 2020, 9, 645. [Google Scholar] [CrossRef]
- Zheng, D.H.; Dou, L.P.; Wei, Y.X.; Du, G.S.; Zou, Y.P.; Song, J.Y.; Zhu, Z.D.; Cai, M.; Qian, Y.Y.; Shi, B.Y. Uptake of donor lymphocytes treated with 8-methoxypsoralen and ultraviolet A light by recipient dendritic cells induces CD4+CD25+Foxp3+ regulatory T cells and down-regulates cardiac allograft rejection. Biochem. Biophys. Res. Commun. 2010, 395, 540–546. [Google Scholar] [CrossRef]
- Toma, A.C.; Stegmüller, S.; Richling, E. Coumarin contents of tonka (Dipteryx odorata) products. Eur. Food Res. Technol. 2025, 251, 513–517. [Google Scholar] [CrossRef]
- Link, K.P. The discovery of dicumarol and its sequels. Circulation 1959, 19, 97–107. [Google Scholar] [CrossRef]
- Kostova, I. Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anticancer. Agents. 2005, 5, 29–46. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Smeriglio, A.; Trombetta, D.; Croley, C.R.; Bhattacharyya, P.; Sobarzo-Sánchez, E.; Farzaei, M.H.; Bishayee, A. Antiangiogenic Effects of Coumarins against Cancer: From Chemistry to Medicine. Molecules 2019, 24, 4278. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Cai, T.; Cai, B. Pharmacological activities of esculin and esculetin: A review. Medicine 2023, 102, e35306. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, A.; Balewski, Ł.; Lahutta, M.; Kokoszka, J. Umbelliferone and Its Synthetic Derivatives as Suitable Molecules for the Development of Agents with Biological Activities: A Review of Their Pharmacological and Therapeutic Potential. Pharmaceuticals 2023, 16, 1732. [Google Scholar] [CrossRef] [PubMed]
- Adhyaksa, I.N.M.P.; Silawarti, P.A.K.; Putra, M.F.A.; Laksmiani, N.P.L. Exploring the anticancer potential of scopoletin against HER-2 positive breast cancer: An in silico molecular docking study. Pharm. Rep. 2024, 3, 63. [Google Scholar] [CrossRef]
- Radha, G.V.; Sadhana, B.; Trideva Sastri, K.; Ganapaty, S. Bioactive umbelliferone and its derivatives: An update. J. Pharmacogn. Phytochem. 2019, 8, 59–66. [Google Scholar]
- Ding, Y.; Lu, X.; Hu, X.; Ma, J.; Ding, H. Osthole inhibits proliferation and induces apoptosis in human osteosarcoma cells. Int. J. Clin. Pharmacol. Ther. 2014, 52, 112–117. [Google Scholar] [CrossRef]
- Zhu, X.; Song, X.; Xie, K.; Zhang, X.; He, W.; Liu, F. Osthole induces apoptosis and suppresses proliferation via the PI3K/Akt pathway in intrahepatic cholangiocarcinoma. Int. J. Mol. Med. 2017, 40, 1143–1151. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, X.J.; Jin, M.Z.; Cao, Z.W.; Ren, Y.Y.; Gu, Z.W. Osthole induces cell cycle arrest and apoptosis in head and neck squamous cell carcinoma by suppressing the PI3K/AKT signaling pathway. Chem. Biol. Interact. 2020, 316, 108934. [Google Scholar] [CrossRef]
- Bronikowska, J.; Szliszka, E.; Jaworska, D.; Czuba, Z.P.; Krol, W. The coumarin psoralidin enhances anticancer effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Molecules 2012, 17, 6449–6464. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Perween, A.; Momal, U.; Imran, M.; Ul Hassan, M.H.; Naeem, H.; Mujtaba, A.; Hussain, M.; Alsagaby, S.A.; Al Abdulmonem, W.; et al. Recent Perspectives on Anticancer Potential of Coumarin Against Different Human Malignancies: An Updated Review. Food Sci. Nutr. 2024, 13, e4696. [Google Scholar] [CrossRef]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. p53 signaling in cancer progression and therapy. Cancer Cell Int. 2021, 21, 703. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Sun, Z.; Lu, Y.; Li, Y.Z.; Xu, H.R.; Zeng, C.Q. Osthole inhibits proliferation and induces apoptosis in BV-2 microglia cells in kainic acid-induced epilepsy via modulating PI3K/AKt/mTOR signalling way. Pharm. Biol. 2019, 57, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.J.; Hsieh, Y.J.; Chu, C.Y.; Lin, Y.L.; Tseng, T.H. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002, 183, 163–168. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, H.R.; Ren, Y.J.; Fang, F.X.; Tian, H.T.; Gao, Z.J.; Song, W.; Huang, S.M.; Zhao, A.F. Effects of isoimperatorin on proliferation and apoptosis of human gastric carcinoma cells. Oncol. Lett. 2018, 15, 7993–7998. [Google Scholar] [CrossRef]
- Jiménez-Orozco, F.A.; López-González, J.S.; Nieto-Rodriguez, A.; Velasco-Velázquez, M.A.; Molina-Guarneros, J.A.; Mendoza-Patiño, N.; García-Mondragón, M.J.; Elizalde-Galvan, P.; León-Cedeño, F.; Mandoki, J.J. Decrease of cyclin D1 in the human lung adenocarcinoma cell line A-427 by 7-hydroxycoumarin. Lung Cancer 2001, 34, 185–194. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; Mohamad, A.B. Antioxidant Activity of Coumarins. Sys. Rev. Pharm. 2017, 8, 24–30. [Google Scholar] [CrossRef]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef]
- Zúñiga-Núñez, D.; Barrias, P.; Cárdenas-Jirón, G.; Ureta-Zanartu, M.S.; Lopez-Alarcón, C.; Vieyra, F.E.; Borsarelli, C.D.; Alarcon, E.I.; Aspée, A. Atypical antioxidant activity of non-phenolic amino-coumarins. RSC Adv. 2018, 8, 1927–1933. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell. Longev. 2020, 1675957. [Google Scholar] [CrossRef]
- Musa, M.A.; Kolawole, Q. 7,8-Diacetoxy-3-(4-methylsulfonylphenyl)-4-phenylcoumarin Induces ROS-dependent Cell Death in the A549 Human Lung Cancer Cell Line. Anticancer. Res. 2023, 43, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Önder, A. Anticancer activity of natural coumarins for biological targets. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 85–109. [Google Scholar]
- Park, S.L.; Won, S.Y.; Song, J.H.; Lee, S.Y.; Kim, W.J.; Moon, S.K. Esculetin Inhibits VEGF-Induced Angiogenesis Both In Vitro and In Vivo. Am. J. Chin. Med. 2016, 44, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Aqib, M.; Khatoon, S.; Ali, M.; Sajid, S.; Assiri, M.A.; Ahamad, S.; Saquib, M.; Hussain, M.K. Exploring the anticancer potential and mechanisms of action of natural coumarins and isocoumarins. Eur. J. Med. Chem. 2025, 282, 117088. [Google Scholar] [CrossRef]
- Hosseini, F.; Ahmadi, A.; Hassanzade, H.; Gharedaghi, S.; Rassouli, F.B.; Jamialahmadi, K. Inhibition of melanoma cell migration and invasion by natural coumarin auraptene through regulating EMT markers and reducing MMP-2 and MMP-9 activity. Eur. J. Pharmacol. 2024, 971, 176517. [Google Scholar] [CrossRef]
- Lee, C.H. Reversal of Epithelial-Mesenchymal Transition by Natural Anti-Inflammatory and Pro-Resolving Lipids. Cancers 2019, 11, 1841. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Garg, C.; Bhat, A.H.; Kaur, S. Phytochemicals in Cancer Therapy: Modulating Cell Cycle, Angiogenesis, and Epithelial-Mesenchymal Transition. Rev. Bras. Farmacogn. 2025, 35, 255–274. [Google Scholar] [CrossRef]
- Berkarda, B.; Bouffard-Eyüboğlu, H.; Derman, U. The effect of coumarin derivatives on the immunological system of man. Agents Actions 1983, 13, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.Q.; Li, Y.B. Therapeutic potential of natural coumarins in autoimmune diseases with underlying mechanisms. Front. Immunol. 2024, 15, 1432846. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Park, Y.J.; Gu, B.H.; Han, G.; Ji, W.; Hwang, S.M.; Kim, M. Coumarin derivatives ameliorate the intestinal inflammation and pathogenic gut microbiome changes in the model of infectious colitis through antibacterial activity. Front. Cell Infect. Microbiol. 2024, 14, 1362773. [Google Scholar] [CrossRef]
- Ajiboye, C.O.; Moronkola, D.O.; Khalid, A.; Adesomoju, A.A.; Bhayye, S.S.; Bharitkar, Y.P.; Yadav, G.; Verma, M.K. Isolation, characterization, HPLC quantification, in-vitro and in-silico evaluations of coumarins and coumarolignans from Blighia unijugata stem with their chemotaxonomic significance. Biochem. Syst. Ecol. 2025, 120, 104950. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, L.; Sun, X.; Zeng, F.; Pan, Q.; Xue, M. Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion and PI3K/AKT signalling pathway. J. Buon. 2019, 24, 997–1002. [Google Scholar]
- Şeker Karatoprak, G.; Dumlupınar, B.; Celep, E.; Kurt Celep, I.; Küpeli Akkol, E.; Sobarzo-Sánchez, E. A comprehensive review on the potential of coumarin and related derivatives as multi-target therapeutic agents in the management of gynecological cancers. Front. Pharmacol. 2024, 15, 1423480. [Google Scholar] [CrossRef]
- Meng, T.; Qin, Q.P.; Wang, Z.R.; Peng, L.T.; Zou, H.H.; Gan, Z.Y.; Tan, M.X.; Wang, K.; Liang, F.P. Synthesis and biological evaluation of substituted 3-(2’-benzimidazolyl)coumarin platinum(II) complexes as new telomerase inhibitors. J. Inorg. Biochem. 2018, 189, 143–150. [Google Scholar] [CrossRef]
- Elgabry, R.M.; Hassan, M.; Fawzy, G.A.; Meselhy, K.M.; Mohamed, O.G.; Al-Taweel, A.M.; Sedeek, M.S. A Comparative Analysis of Polysaccharides and Ethanolic Extracts from Two Egyptian Sweet Potato Cultivars, Abees and A 195: Chemical Characterization and Immunostimulant Activities. Metabolites 2024, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. B-FOPCU 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Albratty, M.; Makeen, H.A. An Umbelliferone-induced Caspase-Mediated Apoptosis in MDA-MB-231 Breast Cancer Cells. Indian J. Pharm. Educ. Res. 2024, 58, 890–898. [Google Scholar] [CrossRef]
- Muthu, R.; Selvaraj, N.; Vaiyapuri, M. Anti-inflammatory and proapoptotic effects of umbelliferone in colon carcinogenesis. Hum. Exp. Toxicol. 2016, 35, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Hu, D.H.; Zhang, J.J. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 3869–3873. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Sindhu, G. Umbelliferone arrest cell cycle at G0/G1 phase and induces apoptosis in human oral carcinoma (KB) cells possibly via oxidative DNA damage. Biomed. Pharmacother. 2017, 92, 661–671. [Google Scholar] [CrossRef]
- Ma, W.; Shi, H.; Dong, X.; Shi, Y.; Zhang, L.; Jiang, B. Umbelliferone inhibits proliferation and metastasis via modulating cadherin/β-catenin complex-aided cell-cell adhesion in glioblastoma cells. Adv. Clin. Exp. Med. 2025. [Google Scholar] [CrossRef]
- Lim, J.Y.; Lee, J.H.; Lee, D.H.; Lee, J.H.; Kim, D.K. Umbelliferone reduces the expression of inflammatory chemokines in HaCaT cells and DNCB/DFE-induced atopic dermatitis symptoms in mice. Int. Immunopharmacol. 2019, 75, 105830. [Google Scholar] [CrossRef]
- Sumorek-Wiadro, J.; Zając, A.; Langner, E.; Skalicka-Woźniak, K.; Maciejczyk, A.; Rzeski, W.; Jakubowicz-Gil, J. Antiglioma Potential of Coumarins Combined with Sorafenib. Molecules 2020, 25, 5192. [Google Scholar] [CrossRef]
- An, G.; Park, S.; Lee, M.; Lim, W.; Song, G. Antiproliferative Effect of 4-Methylumbelliferone in Epithelial Ovarian Cancer Cells Is Mediated by Disruption of Intracellular Homeostasis and Regulation of PI3K/AKT and MAPK Signaling. Pharmaceutics 2020, 12, 640. [Google Scholar] [CrossRef]
- Tamtürk, E.; Yalcin, S.; Ercan, F.; Tunçbilek, A. In vivo, In vitro, and In silico Studies of Umbelliferone and Irinotecan on MDA-MB-231 Breast Cancer Cell Line and Drosophila melanogaster Larvae. Anticancer. Agents Med. Chem. 2024, 25, 499–516. [Google Scholar] [CrossRef]
- Bobek, V.; Kovarík, J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed. Pharmacother. 2004, 58, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.S.; Cavallerano, J.D.; Sun, J.K.; Aiello, L.M.; Aiello, L.P. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat. Rev. Endocrinol. 2010, 6, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Battinelli, E.M.; Markens, B.A.; Kulenthirarajan, R.A.; Machlus, K.R.; Flaumenhaft, R.; Italiano, J.E., Jr. Anticoagulation inhibits tumor cell-mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood 2014, 123, 101–112. [Google Scholar] [CrossRef]
- Demer, L.L.; Boström, K.I. Conflicting forces of warfarin and matrix gla protein in the artery wall. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 9–10. [Google Scholar] [CrossRef]
- Florek, K.; Mendyka, D.; Gomułka, K. Vascular Endothelial Growth Factor (VEGF) and Its Role in the Cardiovascular System. Biomedicines 2024, 12, 1055. [Google Scholar] [CrossRef]
- Benndorf, R.A.; Schwedhelm, E.; Gnann, A.; Taheri, R.; Kom, G.; Didié, M.; Steenpass, A.; Ergün, S.; Böger, R.H. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A(2) receptor: A potential link between oxidative stress and impaired angiogenesis. Circ. Res. 2008, 103, 1037–1046. [Google Scholar]
- Marques, C.S.; Brandão, P.; Burke, A.J. Targeting Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2): Latest Insights on Synthetic Strategies. Molecules 2024, 29, 5341. [Google Scholar] [CrossRef]
- Habibi, A.; Ruf, W.; Schurgers, L. Protease-activated receptors in vascular smooth muscle cells: A bridge between thrombo-inflammation and vascular remodelling. Cell Commun. Signal 2025, 23, 57. [Google Scholar] [CrossRef]
- Yan, L.I.; Jing-Ping, G.E.; Ke, M.A.; Yuan-Yuan, Y.I.N.; Juan, H.E.; Jian-Ping, G.U. The combination of EGCG with warfarin reduces deep vein thrombosis in rabbits through modulating HIF-1α and VEGF via the PI3K/AKT and ERK1/2 signaling pathways. Chin. J. Nat. Med. 2022, 20, 679–690. [Google Scholar]
- Wang, J.; Zhu, C. Anticoagulation in combination with antiangiogenesis and chemotherapy for cancer patients: Evidence and hypothesis. Onco Targets Ther. 2016, 9, 4737–4746. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef] [PubMed]
- Rezoan Hossain, M.; Zahra Shova, F.T.; Akter, M.; Shuvo, S.; Ahmed, N.; Akter, A.; Haque, M.; Salma, U.; Roman Mogal, M.; Saha, H.R.; et al. Esculetin unveiled: Decoding its anti-tumor potential through molecular mechanisms-A comprehensive review. Cancer Rep. 2024, 7, e1948. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Góralczyk, A.; Łuszczki, J.J. Synergy, Additivity and Antagonism between Esculetin and Six Commonly Used Chemotherapeutics in Various Malignant Melanoma Cell Lines—An Isobolographic Analysis. Molecules 2023, 28, 3889. [Google Scholar] [CrossRef]
- Chang, H.T.; Chou, C.T.; Lin, Y.S.; Shieh, P.; Kuo, D.H.; Jan, C.R.; Liang, W.Z. Esculetin, a natural coumarin compound, evokes Ca(2+) movement and activation of Ca(2+)-associated mitochondrial apoptotic pathways that involved cell cycle arrest in ZR-75-1 human breast cancer cells. Tumour Biol. 2016, 37, 4665–4678. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, T.G.; Chen, H.; Jung, S.K.; Lee, H.J.; Lee, M.H.; Kim, D.J.; Shin, A.; Lee, K.W.; Bode, A.M.; et al. Esculetin suppresses proliferation of human colon cancer cells by directly targeting β-catenin. Cancer Prev. Res. (Phila) 2013, 6, 1356–1364. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Huo, X. Inhibitory effects of aesculetin on the proliferation of colon cancer cells by the Wnt/β-catenin signaling pathway. Oncol. Lett. 2018, 15, 7118–7122. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Banikazemi, Z.; Mirazimi, S.M.; Dashti, F.; Mazandaranian, M.R.; Akbari, M.; Morshedi, K.; Aslanbeigi, F.; Rashidian, A.; Chamanara, M.; Hamblin, M.R.; et al. Coumarins and Gastrointestinal Cancer: A New Therapeutic Option? Front. Oncol. 2021, 11, 752784. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sheng, Y.; Guo, F.; Wu, J.; Huang, Y.; Yang, X.; Wang, M.; Zhang, S.; Li, P. Therapeutic potential of esculetin in various cancer types. Oncol. Lett. 2024, 28, 305. [Google Scholar] [CrossRef]
- Pinto, D.C.G.A.; Silva, A.M.S. Anticancer Natural Coumarins as Lead Compounds for the Discovery of New Drugs. Curr. Top. Med. Chem. 2017, 17, 3190–3198. [Google Scholar] [CrossRef]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Vyas, V.K.; Bhatt, S.; Ghate, M.D. Therapeutic potential of 4-substituted coumarins: A conspectus. Eur. J. Med. Chem. Rep. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Rubab, L.; Afroz, S.; Ahmad, S.; Hussain, S.; Nawaz, I.; Irfan, A.; Batool, F.; Kotwica-Mojzych, K.; Mojzych, M. An Update on Synthesis of Coumarin Sulfonamides as Enzyme Inhibitors and Anticancer Agents. Molecules 2022, 27, 1604. [Google Scholar] [CrossRef]
- Ortega-Forte, E.; Rovira, A.; Gandioso, A.; Bonelli, J.; Bosch, M.; Ruiz, J.; Marchán, V. COUPY Coumarins as Novel Mitochondria-Targeted Photodynamic Therapy Anticancer Agents. J. Med. Chem. 2021, 64, 17209–17220. [Google Scholar] [CrossRef] [PubMed]
- Rawat, A.; Reddy, A.V.B. Recent advances on anticancer activity of coumarin derivatives. Eur. J. Med. Chem. Rep. 2022, 5, 100038. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Song, F.; Huo, X.; Guo, Z. Anti-breast Cancer Potential of Natural and Synthetic Coumarin Derivatives. Curr. Top. Med. Chem. 2021, 21, 1692–1709. [Google Scholar] [CrossRef] [PubMed]
- Shaik, B.B.; Katari, N.K.; Seboletswe, P.; Gundla, R.; Kushwaha, N.D.; Kumar, V.; Singh, P.; Karpoormath, R.; Bala, M.D. Recent Literature Review on Coumarin Hybrids as Potential Anticancer Agents. Anticancer. Agents Med. Chem. 2023, 23, 142–163. [Google Scholar]
- Yadav, A.K.; Maharjan Shrestha, R.; Yadav, P.N. Anticancer mechanism of coumarin-based derivatives. Eur. J. Med. Chem. 2024, 267, 116179. [Google Scholar] [CrossRef]
- Dimić, D.S.; Kaluđerović, G.N.; Avdović, E.H.; Milenković, D.A.; Živanović, M.N.; Potočňák, I.; Samoľová, E.; Dimitrijević, M.S.; Saso, L.; Marković, Z.S.; et al. Synthesis, Crystallographic, Quantum Chemical, Antitumor, and Molecular Docking/Dynamic Studies of 4-Hydroxycoumarin-Neurotransmitter Derivatives. Int. J. Mol. Sci. 2022, 23, 1001. [Google Scholar] [CrossRef]
- Avdović, E.H.; Petrović, I.P.; Stevanović, M.J.; Saso, L.; Dimitrić Marković, J.M.; Filipović, N.D.; Živić, M.Ž.; Cvetić Antić, T.N.; Žižić, M.V.; Todorović, N.V.; et al. Synthesis and Biological Screening of New 4-Hydroxycoumarin Derivatives and Their Palladium(II) Complexes. Oxid. Med. Cell Longev. 2021, 28, 8849568. [Google Scholar] [CrossRef] [PubMed]
- Pyrczak-Felczykowska, A.; Herman-Antosiewicz, A. Modification in Structures of Active Compounds in Anticancer Mitochondria-Targeted Therapy. Int. J. Mol. Sci. 2025, 26, 1376. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Singh, K.; Ved, A.; Hasan, S.M.; Mujeeb, S. Therapeutic Journey and Recent Advances in the Synthesis of Coumarin Derivatives. Mini Rev. Med. Chem. 2022, 22, 1314–1330. [Google Scholar] [CrossRef]
- Maleki, E.H.; Bahrami, A.R.; Sadeghian, H.; Matin, M.M. Discovering the structure-activity relationships of different 0-prenylated coumarin derivatives as effective anticancer agents in human cervical cancer cells. Toxicol. In Vitro 2020, 63, 104745. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska-Łuczka, P.; Góralczyk, A.; Łuszczki, J.J. Daphnetin, a Coumarin with Anticancer Potential against Human Melanoma: In Vitro Study of Its Effective Combination with Selected Cytostatic Drugs. Cells 2023, 12, 1593. [Google Scholar] [CrossRef]
- Sabt, A.; Abdelhafez, O.M.; El-Haggar, R.S.; Madkour, H.M.F.; Eldehna, W.M.; El-Khrisy, E.E.A.M.; Abdel-Rahman, M.A.; Rashed, L.A. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: Synthesis, in vitro biological evaluation, and QSAR studies. J. Enzyme Inhib. Med. Chem. 2018, 33, 1095–1107. [Google Scholar] [CrossRef]
- Wang, T.; Peng, T.; Wen, X.; Wang, G.; Sun, Y.; Liu, S.; Zhang, S.; Wang, L. Design, Synthesis and Preliminary Biological Evaluation of Benzylsulfone Coumarin Derivatives as Anti-Cancer Agents. Molecules 2019, 24, 4034. [Google Scholar] [CrossRef]
- Patra, A.R.; Roy, S.S.; Basu, A.; Bhuniya, A.; Bhattacharjee, A.; Hajra, S.; Hossain Sk, U.; Baral, R.; Bhattacharya, S. Design and synthesis of coumarin-based organoselenium as a new hit for myeloprotection and synergistic therapeutic efficacy in adjuvant therapy. Sci. Rep. 2018, 8, 2194. [Google Scholar] [CrossRef]
- Bhat, A.A.; Kaur, G.; Tandon, N.; Tandon, R.; Singh, I. Current advancements in synthesis, anticancer activity, and structure–activity relationship (SAR) of coumarin derivatives. Inorg. Chem. Commun. 2024, 167, 112605. [Google Scholar] [CrossRef]
- Luo, K.; Sun, J.; Chan, J.Y.-W.; Yang, L.; Wu, S.; Fung, K.P.; Liu, F. Anticancer effects of imperatorin isolated from Angelica dahurica: Induction of apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Chemotherapy 2011, 57, 449–459. [Google Scholar] [CrossRef]
- Tong, K.; Xin, C.; Chen, W. Isoimperatorin induces apoptosis of the SGC-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol. Lett. 2017, 13, 518–524. [Google Scholar] [CrossRef]
- Adams, M.; Efferth, T.; Bauer, R. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards CCRF-CEM leukaemia cells and multi-drug resistant CEM/ADR5000 cells, from Artemisia argyi. Planta Med. 2006, 72, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Si, J.; Li, G.; Cao, L. Umbelliprenin isolated from Ferula sinkiangensis inhibits tumor growth and migration through the disturbance of Wnt signaling pathway in gastric cancer. PLoS ONE 2019, 14, e0207169. [Google Scholar] [CrossRef]
- Bartnik, M.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Kania, A.K.; Zdzisińska, B. Quantitative analysis of isopimpinellin from Ammi majus L. fruits and evaluation of its biological effect on selected human tumor cells. Molecules 2024, 29, 2874. [Google Scholar] [CrossRef]
- Charmforoshan, E.; Karimi, E.; Oskoueian, E.; Es-Haghi, A.; Iranshahi, M. Inhibition of human breast cancer cells (MCF-7 cell line) growth via cell proliferation, migration, and angiogenesis by auraptene of Ferula szowitsiana root extract. J. Food Meas. Char. 2019, 13, 2644–2653. [Google Scholar] [CrossRef]
- Weng, M.; Deng, Z.; Huang, S.; Lin, X.; Xu, N.; Sun, X.; Wu, W.; Lu, J.; Wang, D. Fraxetin inhibits proliferation and induces apoptosis of bladder cancer through the Akt pathway in vitro and in vivo. J. Biochem. Mol. Toxicol. 2024, 38, e23556. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lu, C.; Tang, R.; Pan, Y.; Bao, S.; Qiu, Y.; Xie, M. Ellagic acid inhibits the proliferation of human pancreatic carcinoma PANC-1 cells in vitro and in vivo. Oncotarget 2017, 8, 12301–12310. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Tan, Y.; Liu, B.; Liu, C. Ellagic acid inhibits human glioblastoma growth in vitro and in vivo. Oncol. Rep. 2017, 37, 1084–1092. [Google Scholar] [CrossRef]
- Saputri, R.D.; Tjahjandarie, T.S.; Tanjung, M. Two novel coumarins bearing an acetophenone derivative from the leaves of Melicope Quercifolia. Nat. Prod. Res. 2021, 35, 1256–1261. [Google Scholar] [CrossRef]
- Teh, S.S.; Cheng Lian Ee, G.; Mah, S.H.; Lim, Y.M.; Rahmani, M. Mesua beccariana (Clusiaceae), A source of potential anti-cancer lead compounds in drug discovery. Molecules 2012, 17, 10791–10800. [Google Scholar] [CrossRef]
- Lin, W.; Huang, J.; Liao, X.; Yuan, Z.; Feng, S.; Xie, Y.; Ma, W. Neo-tanshinlactone selectively inhibits the proliferation of estrogen receptor positive breast cancer cells through transcriptional down-regulation of estrogen receptor alpha. Pharmacol. Res. 2016, 111, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bastow, K.F.; Sun, C.M.; Lin, Y.L.; Yu, H.J.; Don, M.J.; Wu, T.S.; Nakamura, S.; Lee, K.H. Antitumor Agents. 239. Isolation, Structure Elucidation, Total Synthesis, and Anti-Breast Cancer Activity of Neo-tanshinlactone from Salvia miltiorrhiza. J. Med. Chem. 2004, 47, 5816–5819. [Google Scholar] [CrossRef]
- Li, W.; Shao, Y.T.; Yin, T.P.; Yan, H.; Shen, B.C.; Li, Y.Y.; Xie, H.D.; Sun, Z.W.; Ma, Y.L. Penisarins A and B, sesquiterpene coumarins isolated from an endophytic Penicillium sp. J. Nat. Prod. 2020, 83, 3471–3475. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Pan, D.; Zhen, Y.; Huang, J.; Wang, J.; Zhang, J.; He, Z. Ferulin C triggers potent PAK1 and p21-mediated anti-tumor effects in breast cancer by inhibiting Tubulin polymerization in vitro and in vivo. Pharmacol. Res. 2020, 152, 104605. [Google Scholar] [CrossRef]
- Ekowati, H.; Astuti, I.; Mustofa, M. Anticancer activity of calanone on HeLa cell line. Indones. J. Chem. 2010, 10, 240–244. [Google Scholar] [CrossRef]
- Shahzadi, I.; Ali, Z.; Baek, S.H.; Mirza, B.; Ahn, K.S. Assessment of the Antitumor Potential of Umbelliprenin, a Naturally Occurring Sesquiterpene Coumarin. Biomedicines 2020, 8, 126. [Google Scholar] [CrossRef]
- da Cruz, R.M.D.; Batista, T.M.; de Sousa, T.K.G.; Mangueira, V.M.; Dos Santos, J.A.F.; de Abrantes, R.A.; Ferreira, R.C.; Leite, F.C.; Brito, M.T.; Batista, L.M.; et al. Coumarin derivative 7-isopentenyloxycoumarin induces in vivo antitumor activity by inhibit angiogenesis via CCL2 chemokine decrease. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1701–1714. [Google Scholar] [CrossRef]
- Lee, J.Y.; Mazumder, A.; Diederich, M. Preclinical Assessment of the Bioactivity of the Anticancer Coumarin OT48 by Spheroids, Colony Formation Assays, and Zebrafish Xenografts. J. Vis. Exp. 2018, 136, 57490. [Google Scholar]
- Lachowicz-Radulska, J.; Widelski, J.; Nowaczyński, F.; Serefko, A.; Sobczyński, J.; Ludwiczuk, A.; Kasica, N.; Szopa, A. Zebrafish as a Suitable Model for Utilizing the Bioactivity of Coumarins and Coumarin-Based Compounds. Int. J. Mol. Sci. 2025, 26, 1444. [Google Scholar] [CrossRef]
- Yerer, M.B.; Dayan, S.; Han, M.I.; Sharma, A.; Tuli, H.S.; Sak, K. Nanoformulations of Coumarins and the Hybrid Molecules of Coumarins with Potential Anticancer Effects. Anticancer. Agents Med. Chem. 2020, 20, 1797–1816. [Google Scholar] [CrossRef]
- Koley, M.; Han, J.; Soloshonok, V.A.; Mojumder, S.; Javahershenas, R.; Makarem, A. Latest developments in coumarin-based anticancer agents: Mechanism of action and structure–activity relationship studies. RSC Med. Chem. 2024, 15, 10–54. [Google Scholar] [CrossRef] [PubMed]
- Kleiner-Hancock, H.E.; Shi, R.; Remeika, A.; Robbins, D.; Prince, M.; Gill, J.N.; Syed, Z.; Adegboyega, P.; Mathis, J.M.; Clifford, J.L. Effects of ATRA combined with citrus and ginger-derived compounds in human SCC xenografts. BMC Cancer 2010, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sunita, P.; Jha, S.; Pattanayak, S.P. Daphnetin inhibits TNF-α and VEGF- induced angiogenesis through inhibition of the IKKs/IκBα/NF-κB, Src/FAK/ ERK1/2 and Akt signalling pathways. Clin. Exp. Pharmacol. Physiol. 2016, 43, 939–950. [Google Scholar] [CrossRef]
- Duan, J.; Zhan, J.; Wang, G.; Zhao, X.; Huang, W.; Zhou, G. The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef]
- Bose, P.; Pattanayak, S. Herniarin, a natural coumarin, inhibits mammary carcinogenesis by modulating liver X receptor-α/β-PI3K-Akt-Maf1 Pathway in sprague-dawley rats. Phcog. Mag. 2019, 15, 510. [Google Scholar]
- Lv, X.; Yang, H.; Zhong, H.; He, L.; Wang, L. Osthole exhibits an antitumor effect in retinoblastoma through inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis. Pharm. Biol. 2022, 60, 417–426. [Google Scholar] [CrossRef]
- Tabana, Y.M.; Hassan, L.E.A.; Ahamed, M.B.K.; Dahham, S.S.; Iqbal, M.A.; Saeed, M.A.A.; Khan, M.S.S.; Sandai, D.; Majid, A.S.A.; Oon, C.E.; et al. Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc. Res. 2016, 107, 17–33. [Google Scholar] [CrossRef]
- Rashidi, M.; Khalilnezhad, A.; Amani, D.; Jamshidi, H.; Muhammadnejad, A.; Bazi, A.; Ziai, S.A. Umbelliprenin shows antitumor, antiangiogenesis, antimetastatic, anti- inflammatory, and immunostimulatory activities in 4T1 tumor-bearing Balb/c mice. J. Cell. Physiol. 2018, 233, 8908–8918. [Google Scholar] [CrossRef]
- Khaghanzadeh, N.; Samiei, A.; Ramezani, M.; Mojtahedi, Z.; Hosseinzadeh, M.; Ghaderi, A. Umbelliprenin induced production of IFN- γ and TNF- α, and reduced IL-10, IL-4, Foxp3 and TGF- β in a mouse model of lung cancer. Immunopharmacol. Immunotoxicol. 2014, 36, 25–32. [Google Scholar] [CrossRef]
- Bhagavatheeswaran, S.; Ramachandran, V.; Shanmugam, S.; Balakrishnan, A. Isopimpinellin extends antiangiogenic effect through overexpression of miR-15b- 5p and downregulating angiogenic stimulators. Mol. Biol. Rep. 2022, 49, 279–291. [Google Scholar] [CrossRef]
- Avdović, E.H.; Antonijević, M.; Simijonović, D.; Roca, S.; Topić, D.V.; Grozdanić, N.; Stanojković, T.; Radojević, I.; Vojinović, R.; Marković, Z. Synthesis and Cytotoxicity Evaluation of Novel Coumarin–Palladium(II) Complexes against Human Cancer Cell Lines. Pharmaceuticals 2023, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Gok, B.; Bicak, B.; Akman, G.; Arvas, B.; Aydogan, F.; Yolacan, C. Computer-aided anticancer drug design: In vitro and in silico studies of new iminocoumarin derivative. J. Mol. Struct. 2021, 1239, 130539. [Google Scholar] [CrossRef]
- Singh, L.; Singh, A.P.; Bhatti, R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021, 73, 1220–1229. [Google Scholar] [CrossRef]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853. [Google Scholar] [CrossRef]
- Hübinger, L.; Runge, R.; Rosenberg, T.; Freudenberg, R.; Kotzerke, J.; Brogsitter, C. Psoralen as a Photosensitizers for Photodynamic Therapy by Means of In Vitro Cherenkov Light. Int. J. Mol. Sci. 2022, 23, 15233. [Google Scholar] [CrossRef]
- Modi, S.K.; Mohapatra, P.; Bhatt, P.; Singh, A.; Parmar, A.S.; Roy, A.; Joshi, V.; Singh, M.S. Targeting tumor microenvironment with photodynamic nanomedicine. Med. Res. Rev. 2025, 45, 66–96. [Google Scholar] [CrossRef]
- Ahmadi, H.; Haddadi-Asl, V. Temperature-, pH-, and light-responsive reversibly cross-linked micelle assemblies for efficient DOX delivery. Polym. Bull. 2025, 1–23. [Google Scholar] [CrossRef]
- Kubrak, T.; Bogucka-Kocka, A.; Komsta, Ł.; Załuski, D.; Bogucki, J.; Galkowski, D.; Kaczmarczyk, R.; Feldo, M.; Cioch, M.; Kocki, J. Modulation of Multidrug Resistance Gene Expression by Coumarin Derivatives in Human Leukemic Cells. Oxid. Med. Cell Longev. 2017, 2017, 5647281. [Google Scholar] [CrossRef]
- Wang, G.; Sun, S.; Wu, B.; Liu, J. Coumarins as Potential Anti-drug Resistant Cancer Agents: A Mini Review. Curr. Top. Med. Chem. 2021, 21, 1725–1736. [Google Scholar] [CrossRef]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Iranshahi, M. Modulation of Multidrug Resistance Protein 2 Efflux in the Cisplatin Resistance Human Ovarian Carcinoma Cells A2780/RCIS by Sesquiterpene Coumarins. Phytother. Res. 2016, 30, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Ajith, S.; Almomani, F.; Elhissi, A.; Husseini, G.A. Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Pitaro, M.; Croce, N.; Gallo, V.; Arienzo, A.; Salvatore, G.; Antonini, G. Coumarin-Induced Hepatotoxicity: A Narrative Review. Molecules 2022, 27, 9063. [Google Scholar] [CrossRef]
- Dana, P.; Pimpha, N.; Chaipuang, A.; Thumrongsiri, N.; Tanyapanyachon, P.; Taweechaipaisankul, A.; Chonniyom, W.; Watcharadulyarat, N.; Sathornsumetee, S.; Saengkrit, N. Inhibiting Metastasis and Improving Chemosensitivity via Chitosan-Coated Selenium Nanoparticles for Brain Cancer Therapy. Nanomaterials 2022, 12, 2606. [Google Scholar] [CrossRef]
- Grosu, C.; Jîjie, A.-R.; Manea, H.C.; Moacă, E.-A.; Iftode, A.; Minda, D.; Chioibaş, R.; Dehelean, C.-A.; Vlad, C.S. New Insights Concerning Phytophotodermatitis Induced by Phototoxic Plants. Life 2024, 14, 1019. [Google Scholar] [CrossRef]
- Tripathi, A.; Misra, K. Inhibition of P-Glycoprotein Mediated Efflux of Paclitaxel by Coumarin Derivatives in Cancer Stem Cells: An In Silico Approach. Comb. Chem. High. Throughput Screen. 2016, 19, 497–506. [Google Scholar] [CrossRef]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Lu, X.; Friedrich, L.J.; Efferth, T. Natural products targeting tumour angiogenesis. Br. J. Pharmacol. 2025, 182, 2094–2136. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef]
- Saif, M.W. Interaction between Gemcitabine and Warfarin Causing Gastrointestinal Bleeding in a Patient with Pancreatic Cancer. J. Appl. Res. 2005, 5, 434–437. [Google Scholar] [PubMed]
- Saif, M.W. An adverse interaction between warfarin and fluoropyrimidines revisited. Clin. Colorectal Cancer 2005, 5, 175–180. [Google Scholar] [CrossRef]
- Heghes, S.C.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Filip, L. Safety Profile of Nutraceuticals Rich in Coumarins: An Update. Front. Pharmacol. 2022, 13, 803338. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Sun, M.; Osborne, G.; Ricker, K.; Tsai, F.C.; Li, K.; Tomar, R.; Phuong, J.; Schmitz, R.; Sandy, M.S. Cancer Hazard Identification Integrating Human Variability: The Case of Coumarin. Int. J. Toxicol. 2019, 38, 501–552. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef]
- Marrero, J.; Alonso, M.; Guerrero, I.; Sánchez, C.; Robles, S. Coumarins and metabolic syndrome: Brief Report. Med. Res. Arch. 2017, 5, 1–20. [Google Scholar]
- Wu, C.; Sun, Z.; Guo, B.; Ye, Y.; Han, X.; Qin, Y.; Liu, S. Osthole inhibits bone metastasis of breast cancer. Oncotarget 2017, 8, 58480–58493. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Z.; Zhao, X.; Wang, G.; Lai, R. Anticarin β inhibits human glioma progression by suppressing cancer stemness via STAT3. Front. Oncol. 2021, 11, 715673. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Meng, P.; Long, C.; Luo, X.; Yang, X.; Wang, Y.; Zhang, Z.; Mwangi, J.; Kamau, P.M.; et al. Anticarin-β shows a promising anti- osteosarcoma effect by specifically inhibiting CCT4 to impair proteostasis. Acta Pharm. Sin. B 2022, 12, 2268–2279. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent Advances in the Development of Pyrazole Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef]
- Singh, A.; Singh, K.; Kaur, K.; Singh, A.; Sharma, A.; Kaur, K.; Kaur, J.; Kaur, G.; Kaur, U.; Kaur, H.; et al. Coumarin as an Elite Scaffold in Anti-Breast Cancer Drug Development: Design Strategies, Mechanistic Insights, and Structure–Activity Relationships. Biomedicines 2024, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhu, W.; Zhao, K.; Yu, J.; Lu, W.; Zhou, R.; Fan, S.; Kong, W.; Yang, F.; Shan, P. Discovery of a novel hybrid coumarin-hydroxamate conjugate targeting the HDAC1-Sp1-FOSL2 signaling axis for breast cancer therapy. Cell Commun. Signal. 2024, 22, 361. [Google Scholar] [CrossRef] [PubMed]
- Karahmet Sher, E.; Alebić, M.; Marković Boras, M.; Boškailo, E.; Karahmet Farhat, E.; Karahmet, A.; Pavlović, B.; Sher, F.; Lekić, L. Nanotechnology in medicine revolutionizing drug delivery for cancer and viral infection treatments. Int. J. Pharm. 2024, 660, 124345. [Google Scholar] [CrossRef]
- Allateef, A.; Shalan, N.; Lafi, Z. Anticancer activity of liposomal formulation co-encapsulated with coumarin and phenyl butyric acid. J. Appl. Pharm. Sci. 2024, 14, 208–215. [Google Scholar] [CrossRef]
- Gregoriou, Y.; Gregoriou, G.; Manoli, A.; Papageorgis, P.; Mc Larney, B.; Vangeli, D.; McColman, S.; Yilmaz, V.; Hsu, H.T.; Skubal, M.; et al. Photophysical and biological assessment of coumarin-6 loaded polymeric nanoparticles as a cancer imaging agent. Sens. Diagn. 2023, 2, 1277–1285. [Google Scholar] [CrossRef]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Advancing Photodynamic Therapy with Nano-Conjugated Hypocrellin: Mechanisms and Clinical Applications. Int. J. Nanomedicine 2024, 19, 11023–11038. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Raj, K.; Nandi, S.S.; Sinha, S.; Mishra, A.; Mondal, A.; Lagoa, R.; Burcher, J.T.; Bishayee, A. Potential of Synthetic and Natural Compounds as Novel Histone Deacetylase Inhibitors for the Treatment of Hematological Malignancies. Cancers 2023, 15, 2808. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Rizzo, A.; Ricci, A.D.; Aksoy, S.; Sahin, K. The Use of Phytochemicals to Improve the Efficacy of Immune Checkpoint Inhibitors: Opportunities and Challenges. Appl. Sci. 2022, 12, 10548. [Google Scholar] [CrossRef]
- Gage, B.F. Pharmacogenetics-based coumarin therapy. Hematology Am. Soc. Hematol. Educ. Program. 2006, 1, 67–473. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).