Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance
Abstract
1. Introduction
2. Methodology
3. Case Studies
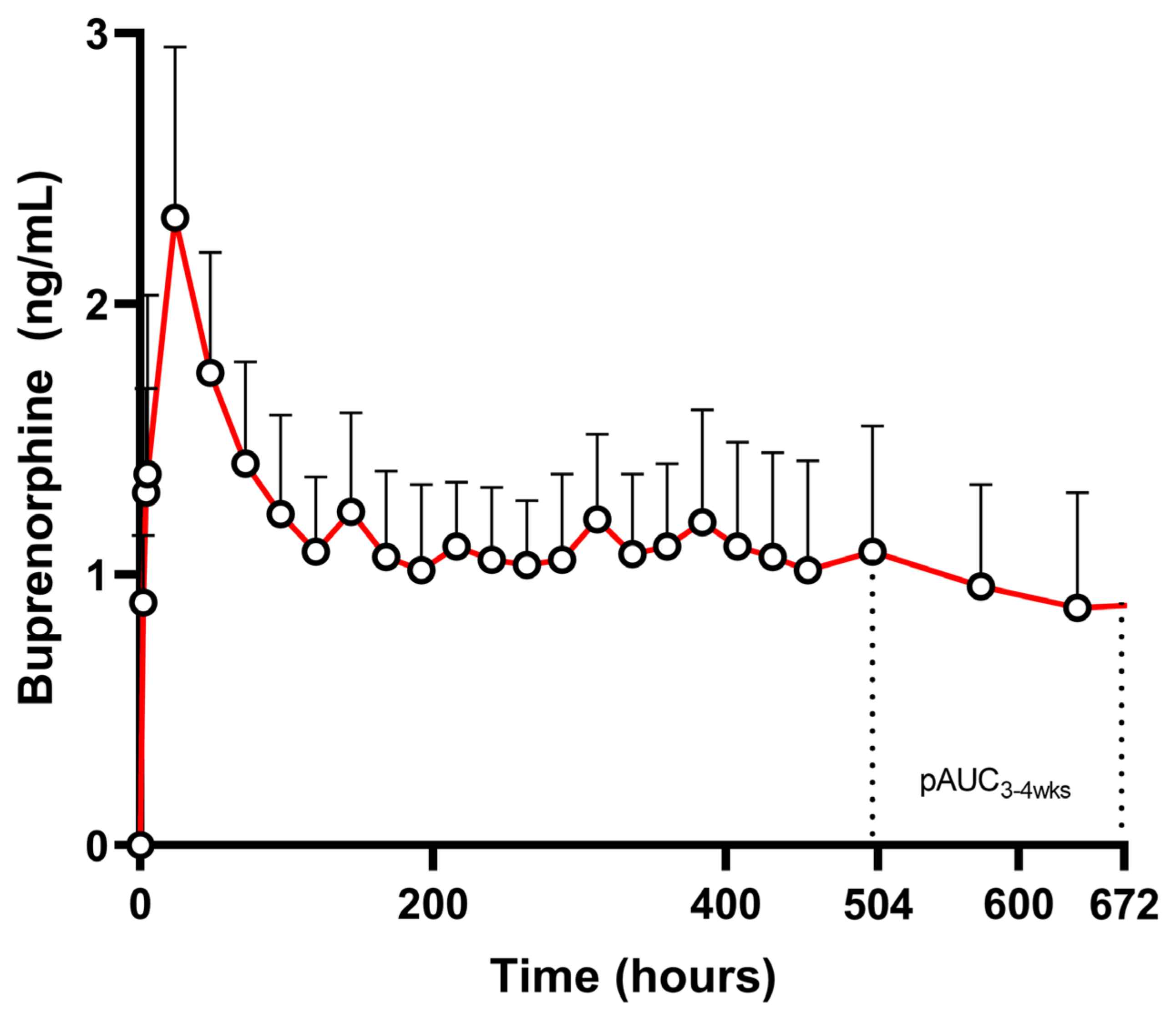
3.1. Case Study: Buprenorphine
3.2. Case Study: Naltrexone
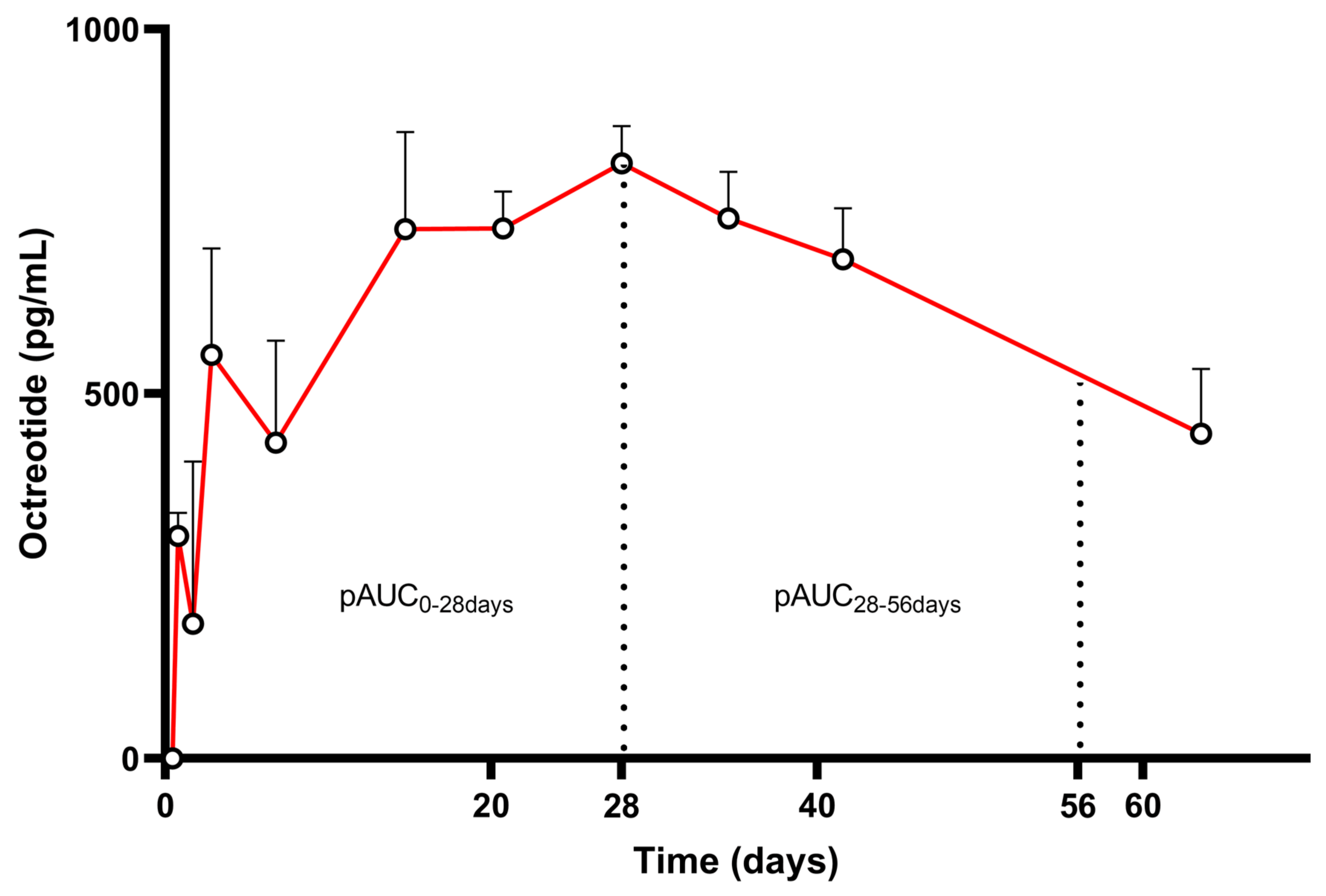
3.3. Case Study: Octreotide
3.4. Case Study: Lanreotide

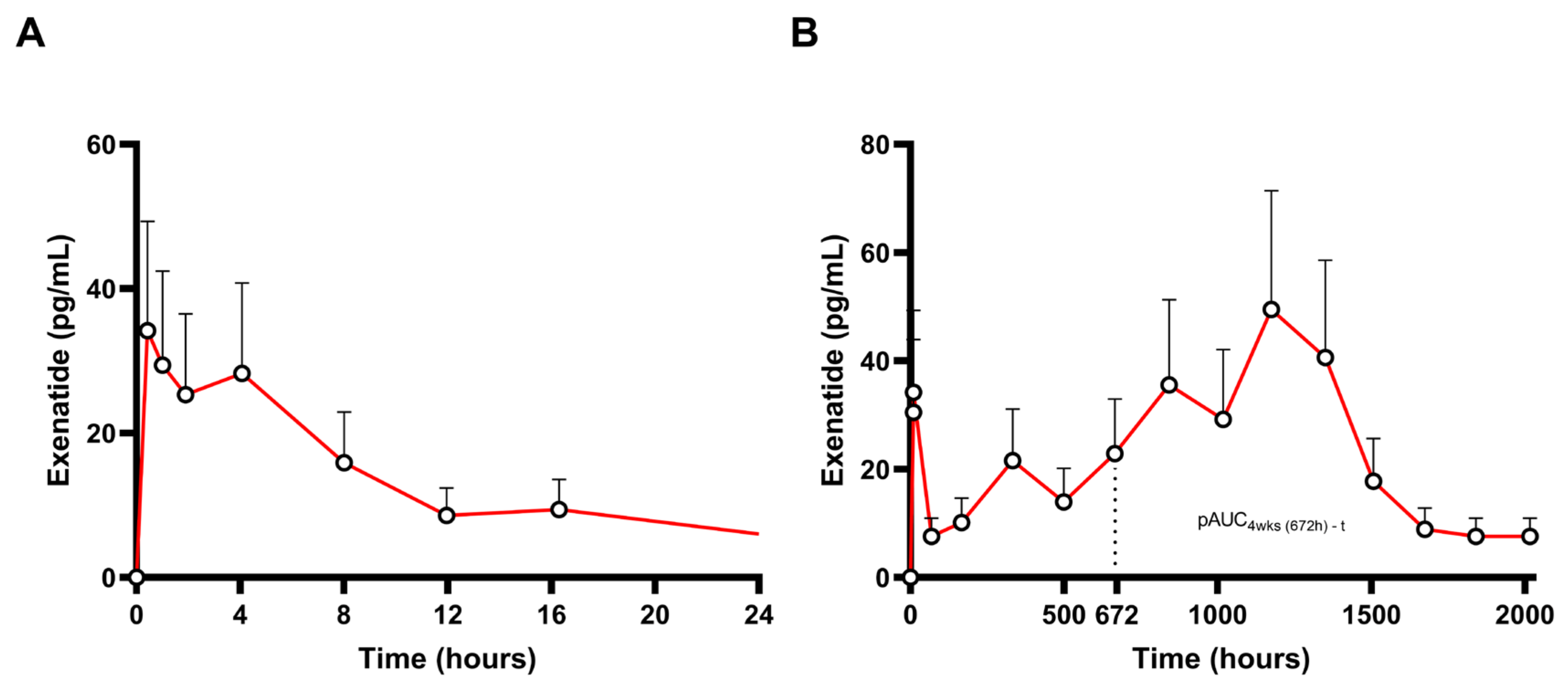
3.5. Case Study: Exenatide
3.6. Case Study: Leuprolide


4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davit, B.; Braddy, A.C.; Conner, D.P.; Yu, L.X. International Guidelines for Bioequivalence of Systemically Available Orally Administered Generic Drug Products: A Survey of Similarities and Differences. AAPS J. 2013, 15, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, M.; Wonnemann, M.; Schug, B.; Toal, C.; Donath, F.; Pontius, A.; Pauli, K.; Brendel, E.; Blume, H. Differences in Bioavailability between 60 Mg of Nifedipine Osmotic Push-Pull Systems after Fasted and Fed Administration. Int. J. Clin. Pharmacol. Ther. 2010, 48, 158–170. [Google Scholar] [CrossRef] [PubMed]
- García-Arieta, A.; Morales-Alcelay, S.; Herranz, M.; De La Torre-Alvarado, J.M.; Blázquez-Pérez, A.; Suárez-Gea, M.L.; Álvarez, C. Investigation on the Need of Multiple Dose Bioequivalence Studies for Prolonged-Release Generic Products. Int. J. Pharm. 2012, 423, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Endrenyi, L.; Tothfalusi, L. Do Regulatory Bioequivalence Requirements Adequately Reflect the Therapeutic Equivalence of Modified-Release Drug Products? J. Pharm. Pharm. Sci. 2010, 13, 107. [Google Scholar] [CrossRef]
- Health Canada. Report C: Report on Bioavailability of Oral Dosage Formulations, not in Modified Release Form, of Drugs Used for Systemic Effects, Having Complicated or Variable Pharmacokinetics; Health Canada; Expert Advisory Committee on Bioavailability, Health Protection Branch: Ottawa, ON, Canada, 1992. [Google Scholar]
- FDA. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations: Guidance for Industry; FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2003. [Google Scholar]
- FDA. Guidance on Zolpidem Extended Release Tablets/Oral. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Zolpidem_ERtab_21774_RC8-09.pdf (accessed on 18 October 2024).
- FDA. Draft Guidance on Methylphenidate Hydrochloride. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_205489.pdf (accessed on 18 October 2024).
- FDA. Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry (Draft Guidance). 2021. Available online: https://www.fda.gov/media/87219/download (accessed on 18 October 2024).
- EMA. Guideline on the Pharmacokinetic and Clinical Evaluation of Modified Release Dosage Forms. 30 Churchill Place, Canary Wharf, London E14 5EU, United Kingdom. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-pharmacokinetic-and-clinical-evaluation-modified-release-dosage-forms_en.pdf (accessed on 18 October 2024).
- Swanson, J.M.; Wigal, S.B.; Wigal, T.; Sonuga-Barke, E.; Greenhill, L.L.; Biederman, J.; Kollins, S.; Nguyen, A.S.; DeCory, H.H.; Hirshe Dirksen, S.J.; et al. A Comparison of Once-Daily Extended-Release Methylphenidate Formulations in Children with Attention-Deficit/Hyperactivity Disorder in the Laboratory School (The Comacs Study). Pediatrics 2004, 113, e206–e216. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Straughn, A.B.; Patrick, K.S. Advances in the Pharmacotherapy of Attention-Deficit-Hyperactivity Disorder: Focus on Methylphenidate Formulations. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 1281–1299. [Google Scholar] [CrossRef]
- Endrenyi, L.; Tothfalusi, L. Metrics for the Evaluation of Bioequivalence of Modified-Release Formulations. AAPS J. 2012, 14, 813–819. [Google Scholar] [CrossRef][Green Version]
- EMA. Methylphenidate, Prolonged-Release Tablet 18 mg, 27 mg, 36 mg and 54 mg and Modified Release Capsule 5 mg, 6 mg, 20 mg, 30 mg, 40 mg, 50 mg and 60 mg Product Specific Bioequivalence Guidance. 2024. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/methylphenidate-prolonged-release-tablet-18-mg-27-mg-36-mg-54-mg-modified-release-capsule-5-mg-10-mg-20-mg-30-mg-40-mg-50-mg-60-mg-product-specific-bioequivalence-guidance_en.pdf (accessed on 18 October 2024).
- Lionberger, R.A.; Raw, A.S.; Kim, S.H.; Zhang, X.; Yu, L.X. Use of partial AUC to demonstrate bioequivalence of zolpidem tartrate extended release formulations. Pharm. Res. 2012, 29, 1110–1120. [Google Scholar] [CrossRef]
- Gagnon, R.C.; Peterson, J.J. Estimation of Confidence Intervals for Area Under the Curve from Destructively Obtained Pharmacokinetic Data. J. Pharmacokinet. Pharmacodyn. 1998, 26, 87–102. [Google Scholar] [CrossRef]
- NSW Ministry of Health. Long-Acting Injectable Buprenorphine (LAIB) for Opioid Dependence Treatment. 2024. Available online: https://www.health.nsw.gov.au/aod/Publications/laib.pdf (accessed on 18 October 2024).
- Albayaty, M.; Linden, M.; Olsson, H.; Johnsson, M.; Strandgården, K.; Tiberg, F. Pharmacokinetic Evaluation of Once-Weekly and Once-Monthly Buprenorphine Subcutaneous Injection Depots (CAM2038) Versus Intravenous and Sublingual Buprenorphine in Healthy Volunteers Under Naltrexone Blockade: An Open-Label Phase 1 Study. Adv. Ther. 2017, 34, 560–575. [Google Scholar] [CrossRef]
- Jones, A.K.; Ngaimisi, E.; Gopalakrishnan, M.; Young, M.A.; Laffont, C.M. Population Pharmacokinetics of a Monthly Buprenorphine Depot Injection for the Treatment of Opioid Use Disorder: A Combined Analysis of Phase II and Phase III Trials. Clin. Pharmacokinet. 2021, 60, 527–540. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Pharmacology and Biopharmaceutics Review Document for SUBLOCADE® (20-9819); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/209819Orig1s000ClinPharmR.pdf (accessed on 3 December 2024).
- Coe, M.A.; Lofwall, M.R.; Walsh, S.L. Buprenorphine Pharmacology Review: Update on Transmucosal and Long-Acting Formulations. J. Addict. Med. 2019, 13, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Hjelmström, P.; Banke Nordbeck, E.; Tiberg, F. Optimal Dose of Buprenorphine in Opioid Use Disorder Treatment: A Review of Pharmacodynamic and Efficacy Data. Drug Dev. Ind. Pharm. 2020, 46, 1–7. [Google Scholar] [CrossRef]
- Kuhlman, J.J.K., Jr.; Levine, B.; Johnson, R.E.; Fudala, P.J.; Cone, E.J. Relationship of Plasm a Buprenorphine and Norbuprenorphine to Withdrawal Symptoms during Dose Induction, Maintenance and Withdrawal from Sublingual Buprenorphine. Addiction 1998, 93, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.; Johanson, C.-E.; Bueller, J.; Chang, Y.; Moody, D.E.; Kilbourn, M.; Koeppe, R.; Zubieta, J.-K. Buprenorphine Duration of Action: Mu-Opioid Receptor Availability and Pharmacokinetic and Behavioral Indices. Biol. Psychiatry 2007, 61, 101–110. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Johanson, C.-E.; Moody, D.E.; Woods, J.H.; Kilbourn, M.R.; Koeppe, R.A.; Schuster, C.R.; Zubieta, J.-K. Effects of Buprenorphine Maintenance Dose on μ-Opioid Receptor Availability, Plasma Concentrations, and Antagonist Blockade in Heroin-Dependent Volunteers. Neuropsychopharmacology 2003, 28, 2000–2009. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Comer, S.D.; Fiellin, D.A. Buprenorphine Maintenance and Mu-Opioid Receptor Availability in the Treatment of Opioid Use Disorder: Implications for Clinical Use and Policy. Drug Alcohol Depend. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Walsh, S.L.; Comer, S.D.; Lofwall, M.R.; Vince, B.; Levy-Cooperman, N.; Kelsh, D.; Coe, M.A.; Jones, J.D.; Nuzzo, P.A.; Tiberg, F.; et al. Effect of Buprenorphine Weekly Depot (CAM2038) and Hydromorphone Blockade in Individuals with Opioid Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 894. [Google Scholar] [CrossRef]
- Fang, L.; Uppoor, R.; Xu, M.; Sharan, S.; Zhu, H.; Tampal, N.; Li, B.; Zhang, L.; Lionberger, R.; Zhao, L. Use of Partial Area Under the Curve in Bioavailability or Bioequivalence Assessments: A Regulatory Perspective. Clin. Pharmacol. Ther. 2021, 110, 880–887. [Google Scholar] [CrossRef]
- FDA. Draft Guidance on Buprenorphine Extended-Release Subcutaneous Solution. 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_209819.pdf (accessed on 18 October 2024).
- Buvidal® 8, 16, 24, 32, 64, 96, 128, 160 mg Prolonged-Release Solution for Injection SmPC; Camurus AB: Lund, Sweden, 2023.
- Vivitrol® 380 mg Extended-Release Injectable Suspension, SmPC; Alkermes, Inc.: Dublin, Ireland, 2022.
- Volpicelli, J.R.; Rhines, K.C.; Rhines, J.S.; Volpicelli, L.A.; Alterman, A.I.; O’Brien, C.P. Naltrexone and alcohol dependence: Role of subject compliance. Arch. Gen. Psychiatry 1997, 54, 737–742. [Google Scholar] [CrossRef]
- Dunbar, J.L.; Turncliff, R.Z.; Dong, Q.; Silverman, B.L.; Ehrich, E.W.; Lasseter, K.C. Single- and Multiple-Dose Pharmacokinetics of Long-acting Injectable Naltrexone. Alcohol. Clin. Exp. Res. 2006, 30, 480–490. [Google Scholar] [CrossRef] [PubMed]
- FDA. Clinical Pharmacology and Biopharmaceutics Review Document for Vivitrol® (21-897); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2006. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021897s000_ClinPharmR.pdf (accessed on 3 December 2024).
- Singh, D.; Saadabadi, A. Naltrexone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Verebey, K.; Volavka, J.; Mule, S.J.; Resnick, R.B. Naltrexone: Disposition, Metabolism, and Effects after Acute and Chronic Dosing. Clin. Pharmacol. Ther. 1976, 20, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.N.; Hollister, L.E.; Kishimoto, A.; Barnett, G. Kinetics of a Naltrexone Sustained-Release Preparation. Clin. Pharmacol. Ther. 1984, 36, 704–708. [Google Scholar] [CrossRef]
- Comer, S.; Collins, E.; Kleber, H.; Nuwayser, E.; Kerrigan, J.; Fischman, M. Depot Naltrexone: Long-Lasting Antagonism of the Effects of Heroin in Humans. Psychopharmacology 2002, 159, 351–360. [Google Scholar] [CrossRef]
- Brewer, C. Serum Naltrexone and 6-beta-naltrexol Levels from Naltrexone Implants Can Block Very Large Amounts of Heroin: A Report of Two Cases. Addict. Biol. 2002, 7, 321–323. [Google Scholar] [CrossRef]
- Lee, M.C.; Wagner, H.N.; Tanada, S.; Frost, J.J.; Bice, A.N.; Dannals, R.F. Duration of occupancy of opiate receptors by naltrexone. J. Nucl. Med. 1988, 29, 1207–1211. [Google Scholar]
- FDA. Draft Guidance on Naltrexone Intramuscular Extended-Release Suspension. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_021897.pdf (accessed on 18 October 2024).
- Croop, R.S. The Safety Profile of Naltrexone in the Treatment of Alcoholism: Results from a Multicenter Usage Study. Arch. Gen. Psychiatry 1997, 54, 1130. [Google Scholar] [CrossRef]
- King, A.C.; Volpicelli, J.R.; Frazer, A.; O’Brien, C.P. Effect of Naltrexone on Subjective Alcohol Response in Subjects at High and Low Risk for Future Alcohol Dependence. Psychopharmacology 1997, 129, 15–22. [Google Scholar] [CrossRef]
- Johnson, B.A. Naltrexone long-acting formulation in the treatment of alcohol dependence. Ther. Clin. Risk Manag. 2007, 3, 741–749. [Google Scholar]
- Lobmaier, P.P.; Kunøe, N.; Gossop, M.; Waal, H.; Lobmaier, P.P.; Kunøe, N.; Gossop, M.; Waal, H. Naltrexone depot formulations for opioid and alcohol dependence: A systematic review. CNS Neurosci. Ther. 2011, 17, 629–636. [Google Scholar] [CrossRef]
- Lamberts, S.W.; Van der Lely, A.J.; de Herder, W.W.; Hofland, L.J. Octreotide. N. Engl. J. Med. 1996, 334, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Astruc, B.; Marbach, P.; Bouterfa, H.; Denot, C.; Safari, M.; Vitaliti, A.; Sheppard, M. Long-Acting Octreotide and Prolonged-Release Lanreotide Formulations Have Different Pharmacokinetic Profiles. J. Clin. Pharmacol. 2005, 45, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Karamouzis, I.; Patelli, I.; Mazziotti, G. Octreotide for Acromegaly Treatment: A Reappraisal. Expert Opin. Pharmacother. 2013, 14, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Lancranjan, I.; Bruns, C.; Grass, P.; Jaquet, P.; Jervell, J.; Kendall-Taylor, P.; Lamberts, S.W.J.; Marbach, P.; Ørskov, H.; Pagani, G.; et al. Sandostatin LAR®: Pharmacokinetics, Pharmacodynamics, Efficacy, and Tolerability in Acromegalic Patients. Metabolism 1995, 44, 18–26. [Google Scholar] [CrossRef]
- FDA. Clinical Pharmacology and Biopharmaceutics Review Document for Santostatin LAR® (21-008); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 1998. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/021008a_clinphrm.pdf (accessed on 3 December 2024).
- Sandostatin® LAR® 10 mg, 20mg or 30mg Powder and Solvent for Suspension for Injection SmPC; Novartis Pharmaceuticals UK Ltd.: London, UK, 2022.
- Kapralos, I.; Dokoumetzidis, A. Population Pharmacokinetic Modelling of the Complex Release Kinetics of Octreotide LAR: Defining Sub-Populations by Cluster Analysis. Pharmaceutics 2021, 13, 1578. [Google Scholar] [CrossRef]
- SOMATULINE® DEPOT 60, 90, 120 mg Injection, for Subcutaneous Use SmPC; Ipsen Pharma Biotech: Paris, France, 2019.
- Valery, C.; Paternostre, M.; Robert, B.; Artzner, F. Biomimetic Organization: Octapeptide Self-Assembly into Nanotubes of Viral Capsid-like Dimension. Proc. Natl. Acad. Sci. USA 2003, 100, 10258–10262. [Google Scholar] [CrossRef]
- Valery, C.; Artzner, F.; Robert, B.; Gulick, T.; Keller, G.; Grabielle-Madelmont, C.; Torres, M.L.; Cherif-Cheikh, R.; Paternostre, M. Self-association process of a peptide in solution: From β-sheet filaments to large embedded nanotubes. Biophys. J. 2004, 86, 2484–2501. [Google Scholar] [CrossRef]
- Pouget, E.; Fay, N.; Dujardin, E.; Jamin, N.; Berthault, P.; Perrin, L.; Pandit, A.; Rose, T.; Valéry, C.; Thomas, D.; et al. Elucidation of the self-assembly pathway of lanreotide octapeptide into β-sheet nanotubes: Role of two stable intermediates. J. Am. Chem. Soc. 2010, 132, 4230–4241. [Google Scholar] [CrossRef]
- Jenkins, P.J.; Akker, S.; Chew, S.L.; Besser, G.M.; Monson, J.P.; Grossman, A.B. Optimal Dosage Interval for Depot Somatostatin Analogue Therapy in Acromegaly Requires Individual Titration. Clin. Endocrinol. 2000, 53, 719–724. [Google Scholar] [CrossRef]
- FDA. Draft Guidance on Injectable Lanreotide Acetate. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Lanreotide%20Acetate_draft_Subcutaneous%20injection_RLD%2022074_RC07-14.pdf (accessed on 18 October 2024).
- EMA. Lanreotide Acetate, Prolonged-Release Solution for 5 Injection in Prefilled Syringe 60, 90 and 120 mg Product6 Specific Bioequivalence Guidance. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/lanreotide-acetate-prolonged-release-solution-injection-prefilled-syringe-60-90-and-120-mg-productspecific-bioequivalence-guidance_en.pdf (accessed on 18 October 2024).
- FDA. Clinical Pharmacology and Biopharmaceutics Review Document for Somatuline Autogel ® (22-074); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2007. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022074s000_ClinPharmR_P1.pdf (accessed on 3 December 2024).
- Bydureon® 2 mg Powder and Solvent for Prolonged-Release Suspension for Injection, SmPC; AstraZeneca AB: Södertalje, Sweden, 2016.
- Fineman, M.; Flanagan, S.; Taylor, K.; Aisporna, M.; Shen, L.Z.; Mace, K.F.; Walsh, B.; Diamant, M.; Cirincione, B.; Kothare, P.; et al. Pharmacokinetics and Pharmacodynamics of Exenatide Extended-Release After Single and Multiple Dosing. Clin. Pharmacokinet. 2011, 50, 65–74. [Google Scholar] [CrossRef]
- FDA. Clinical Pharmacology and Biopharmaceutics Review Document for BYDUREON® (22-200); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022200Orig1s000ClinPharmR.pdf (accessed on 3 December 2024).
- Cervera, A.; Wajcberg, E.; Sriwijitkamol, A.; Fernandez, M.; Zuo, P.; Triplitt, C.; Musi, N.; DeFronzo, R.A.; Cersosimo, E. Mechanism of Action of Exenatide to Reduce Postprandial Hyperglycemia in Type 2 Diabetes. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E846–E852. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; MacConell, L.; Zhuang, D.; Kothare, P.A.; Trautmann, M.; Fineman, M.; Taylor, K. Effects of Once-Weekly Dosing of a Long-Acting Release Formulation of Exenatide on Glucose Control and Body Weight in Subjects with Type 2 Diabetes. Diabetes Care 2007, 30, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.V.; Raskin, P. Exenatide extended-release: A once weekly treatment for patients with type 2 diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 229–239. [Google Scholar]
- FDA. Draft Guidance on Synthetic Exenatide Extended Release Subcutaneous Injection. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_022200.pdf (accessed on 18 October 2024).
- Wang, H.-J.; Hsu, L.-F. The Role of Partial Area under the Curve and Maximum Concentrations in Assessing the Bioequivalence of Long-Acting Injectable Formulation of exenatide—A Sensitivity Analysis. Eur. J. Pharm. Sci. 2024, 195, 106718. [Google Scholar] [CrossRef]
- EMA. Exenatide Powder and Solvent for Prolonged-Release Suspension for Injection, 2 mg, and Powder and Solvent for Prolonged-Release Suspension for Injection in Pre-Filled Pen, 2 mg Product-Specific Bioequivalence Guidance. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/exenatide-powder-and-solvent-prolonged-release-suspension-injection-2-mg-and-powder-and-solvent-prolonged-release-suspension-injection-pre-filled-pen-2-mg-product-specific-bioequivalence-guidance_en.pdf (accessed on 18 October 2024).
- Plosker, G.L.; Brogden, R.N. Leuprorelin: A Review of Its Pharmacology and Therapeutic Use in Prostatic Cancer, Endometriosis and Other Sex Hormone-Related Disorders. Drugs 1994, 48, 930–967. [Google Scholar] [CrossRef]
- Boccardo, F.; Pace, M.; Rubagotti, A.; Guarneri, D.; Decensi, A.; Oneto, F.; Martorana, G.; Giuliani, L.; Selvaggi, F.; Battaglia, M.; et al. Goserelin Acetate with or without Flutamide in the Treatment of Patients with Locally Advanced or Metastatic Prostate Cancer. Eur. J. Cancer 1993, 29, 1088–1093. [Google Scholar] [CrossRef]
- Wilson, A.C.; Vadakkadath Meethal, S.; Bowen, R.L.; Atwood, C.S. Leuprolide Acetate: A Drug of Diverse Clinical Applications. Expert Opin. Investig. Drugs 2007, 16, 1851–1863. [Google Scholar] [CrossRef]
- Fraser, H.M. GnRH Analogues for Contraception. Br. Med. Bull. 1993, 49, 62–72. [Google Scholar] [CrossRef]
- FDA. Draft Guidance on Leuprolide Acetate. 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_019732.pdf (accessed on 18 October 2024).
- Wu, H.M.; Chang, H.M.; Leung, P.C.K. Gonadotropin-Releasing Hormone Analogs: Mechanisms of Action and Clinical Applications in Female Reproduction. Front. Neuroendocrinol. 2021, 60, 100876. [Google Scholar] [CrossRef]
- FDA. Label for Eligard®. 2010. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021343s019,021379s015,021488s016,021731s012lbl.pdf (accessed on 3 December 2024).
- FDA. Approval Package Document for Lupron Depot® (20-517); FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/020517orig1s025s030s032Review.pdf (accessed on 3 December 2024).
- Zhou, H.; Xie, J.; Zhu, X.; Li, X.; Yu, X.; Zhang, Y.; Su, Y.; He, C.; Zhu, M.; Li, X.L.; et al. Pharmacokinetic and Pharmacodynamic Evaluation of the New Prolonged-Release Leuprorelin Acetate Microspheres for Injection Compared with Enantone® in Healthy Chinese Male Volunteers. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1149–1156. [Google Scholar] [CrossRef]
- Lee, L.H.N.; Choi, C.; Gershkovich, P.; Barr, A.M.; Honer, W.G.; Procyshyn, R.M. Proposing the use of partial AUC as an adjunctive measure in establishing bioequivalence between deltoid and gluteal administration of long-acting injectable antipsychotics. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Shah, V.P.; Ganes, D.; Midha, K.K.; Caro, J.; Nambiar, P.; Rocci, M.L.; Thombre, A.G.; Abrahamsson, B.; Conner, D.; et al. Challenges and Opportunities in Establishing Scientific and Regulatory Standards for Assuring Therapeutic Equivalence of Modified Release Products: Workshop Summary Report. AAPS J. 2010, 12, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Guidance document: Conduct and Analysis of Comparative Bioavailability Studies. 2012. Available online: https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/bioavailability-bioequivalence/conduct-analysis-comparative.pdf (accessed on 18 October 2024).
- Endrenyi, L.; Csizmadia, F.; Tothfalusi, L.; Chen, M.L. Metrics comparing simulated early concentration profiles for the determination of bioequivalence. Pharm. Res. 1998, 15, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Macheras, P.; Symillides, M.; Reppas, C. The cutoff time point of the partial area method for assessment of rate of absorption in bioequivalence studies. Pharm. Res. 1994, 11, 831–834. [Google Scholar] [CrossRef]
- Soares, K.C.C.; Chiann, C.; Storpirtis, S. Assessment of the Impact of Partial Area under the Curve in a Bioavailability/Bioequivalence Study on Generic Prolonged-Release Formulations. Eur. J. Pharm. Sci. 2022, 171, 106127. [Google Scholar] [CrossRef]
- Boily, M.; Dussault, C.; Massicotte, J.; Guibord, P.; Lefebvre, M. The Impact of New Partial AUC Parameters for Evaluating the Bioequivalence of Prolonged-Release Formulations. Eur. J. Pharm. Sci. 2015, 66, 70–77. [Google Scholar] [CrossRef]
- Midha, K.K.; Hubbard, J.W.; Rawson, M.J. Retrospective Evaluation of Relative Extent of Absorption by the Use of Partial Areas under Plasma Concentration versus Time Curves in Bioequivalence Studies on Conventional Release Products. Eur. J. Pharm. Sci. 1996, 4, 381–384. [Google Scholar] [CrossRef]
- Patterson, S.; Jones, B. Bioequivalence and Statistics in Clinical Pharmacology; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tylee, A.; Walters, P. Onset of action of antidepressants. BMJ 2007, 334, 911–912. [Google Scholar] [CrossRef]
- Ring, A.; Lang, B.; Kazaroho, C.; Labes, D.; Schall, R.; Schütz, H. Sample size determination in bioequivalence studies using statistical assurance. Br. J. Clin. Pharmacol. 2019, 85, 2369–2377. [Google Scholar] [CrossRef]
- EMA. Guideline on the Investigation of Bioequivalence. 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 18 October 2024).
- EMA. Questions & Answers: Positions on Specific Questions Addressed to the Pharmacokinetics Working Party (PKWP). 2015. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/questions-and-answers-positions-specific-questions-addressed-pharmacokinetics-working-party_en.pdf (accessed on 22 November 2024).
- FDA. Draft Guidance on Draft Guidance on Progesterone. 2011. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Progesterone_caps_19781_RC02-11.pdf (accessed on 22 November 2024).
- Buil-Bruna, N.; Dehez, M.; Manon, A.; Nguyen, T.X.Q.; Trocóniz, I.F. Establishing the quantitative relationship between lanreotide Autogel®, chromogranin A, and progression-free survival in patients with nonfunctioning gastroenteropancreatic neuroendocrine tumors. AAPS J. 2016, 18, 703–712. [Google Scholar] [CrossRef]
- Myrelez 60 mg, 90 mg & 120 mg Solution for Injection in a Prefilled Syringe (Lanreotide acetate); Public Assessment Report, Procedure No. DK/H/3027/001-003/DC; The Danish Medicines Agency: København, Denmark, 2021.
- Benzo, R.M.; Moreno, P.I.; Fox, R.S.; Silvera, C.A.; Walsh, E.A.; Yanez, B.; Penedo, F.J. Comorbidity burden and health-related quality of life in men with advanced prostate cancer. Support. Care Cancer 2023, 31, 496. [Google Scholar] [CrossRef] [PubMed]
- Gren, L.; Broski, K.; Childs, J.; Cordes, J.; Engelhard, D.; Gahagan, B.; Marcus, P. Recruitment methods employed in the prostate, lung, colorectal, and ovarian cancer screening trial. Clin. Trials 2009, 6, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sygna, K.; Johansen, S.; Ruland, C.M. Recruitment challenges in clinical research including cancer patients and caregivers. Trials 2015, 16, 1–9. [Google Scholar] [CrossRef]
- Eckstein, N.; Haas, B. Clinical pharmacology and regulatory consequences of GnRH analogues in prostate cancer. Eur. J. Clin. Pharmacol. 2014, 70, 791–798. [Google Scholar] [CrossRef][Green Version]
- TEVA. October 2024. Available online: https://www.tevapharm.com/news-and-media/latest-news/teva-announces-launch-of-the-first-and-only-generic-version-of-sandostatin-lar-depot-octreotide-acetate/ (accessed on 22 November 2024).
- FDA. Generic Drug User Fee Amendments I (GDUFA I) Commitment Letter. 2012. Available online: https://www.fda.gov/media/82022/download (accessed on 22 November 2024).
- FDA. Generic Drug User Fee Amendments II (GDUFA II) Commitment Letter. 2017. Available online: https://www.fda.gov/media/101052/download?attachment (accessed on 22 November 2024).
- FDA. Generic Drug User Fee Amendments III (GDUFA III) Commitment Letter. 2022. Available online: https://www.fda.gov/media/153631/download?attachment (accessed on 22 November 2024).
- Gajjar, P.; Dickinson, J.; Dickinson, H.; Ruston, L.; Mistry, H.B.; Patterson, C.; Dickinson, P.A. Determining bioequivalence possibilities of long acting injectables through population PK modelling. Eur. J. Pharm. Sci. 2022, 179, 106296. [Google Scholar] [CrossRef]

| FDA [9] | EMA [10] |
|---|---|
| →For “modified-release products in which the different phases of release correspond to a clinical effect” and →For various reasons as described in specific PSGs | →For products with low accumulation along with the traditional PK metrics (Cmax, AUC0-τ) and →For various reasons as described in specific PSGs |
| pAUC | Mean AUC (ng × days/mL) (±SD) | Intersubject Variability (%) |
|---|---|---|
| pAUC0–3d | 15.14 (9.01) | 59.51 |
| pAUC0–14d | 55.92 (20.77) | 37.14 |
| pAUC | Mean AUC (ng × days/mL) (±SD) | Intersubject Variability (%) |
|---|---|---|
| pAUC0–10d | 47.53 (18.84) | 39.64 |
| pAUC10–28d | 71.78 (32.45) | 45.22 |
| pAUC | Mean AUC (ng × days/mL) (±SD) | Intersubject Variability (%) |
|---|---|---|
| pAUC0–7d | 33,458 (17,623) | 52.71 |
| pAUC7–28d | 79,825 (51,176) | 64.11 |
| pAUC28-td | 147,093 (160,237) | 108.90 |
| pAUC | Mean AUC (ng × h/mL) (±SD) | Intersubject Variability (%) |
|---|---|---|
| pAUC24h-t | 273.10 (53.51) | 19.59 |
| pAUC48h-t | 266.20 (50.76) | 19.07 |
| pAUC72h-t | 262.10 (48.07) | 18.34 |
| pAUC5d-t | 254.3 (15.48) | 60.88 |
| pAUC7d-t | 25.88 (13.99) | 62.00 |
| Variability (%) * | Parallel | Crossover |
|---|---|---|
| 20 | 36 | 37 |
| 30 | 78 | 79 |
| 50 | 200 | 201 |
| 80 | 442 | 443 |
| 100 | 619 | 620 |
| FDA | EMA | |||
|---|---|---|---|---|
| Intrasubject Variability (%) | Sample Size | BE Limits | Sample Size | BE Limits |
| 20 | 24 | 80–125% | 18 | 80–125% |
| 30 | 32 | 80–125% | 34 | 80–125% |
| 50 | 24 | If SWR is ≥0.294:
| 28 | 69.84–143.19% |
| 80 | 28 | 50 | 69.84–143.19% | |
| 100 | 40 | 68 | 69.84–143.19% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakiridou, G.; Angelerou, M.-F.-G.; Efentakis, P.; Margaritis, A.; Papanastasiou, A.-M.; Kalantzi, L. Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance. Pharmaceutics 2025, 17, 21. https://doi.org/10.3390/pharmaceutics17010021
Tsakiridou G, Angelerou M-F-G, Efentakis P, Margaritis A, Papanastasiou A-M, Kalantzi L. Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance. Pharmaceutics. 2025; 17(1):21. https://doi.org/10.3390/pharmaceutics17010021
Chicago/Turabian StyleTsakiridou, Georgia, Maria-Faidra-Galini Angelerou, Panagiotis Efentakis, Antonios Margaritis, Antigoni-Maria Papanastasiou, and Lida Kalantzi. 2025. "Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance" Pharmaceutics 17, no. 1: 21. https://doi.org/10.3390/pharmaceutics17010021
APA StyleTsakiridou, G., Angelerou, M.-F.-G., Efentakis, P., Margaritis, A., Papanastasiou, A.-M., & Kalantzi, L. (2025). Partial AUCs in Long-Acting Injectables: Rationale, Challenges, Variability, Usefulness, and Clinical Relevance. Pharmaceutics, 17(1), 21. https://doi.org/10.3390/pharmaceutics17010021




