Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion/Exclusion Criteria
- P: Animal studies: Studies involving rats (Rattus norvegicus) and investigating depression-like behaviour (various animal models of depression). Both adult and juvenile male rats were valid.
- I: Interventions: Only studies investigating the effects of sertraline, citalopram, escitalopram, fluoxetine, and paroxetine on depression-like behaviour of rats were included. The dose of the interventions has not been taken into account.
- C: Control group: Studies with a control group received either a vehicle, CMC, or NaCl and naïve were included.
- O: Outcomes: Only studies using the standard 5 min (300 s) observation of the forced swim test (FST) with the IT factor to measure depression-like behaviour were included.
- Language: Only English-written papers were included.
2.3. Search/Screening/Data Extraction
2.4. Data Availability Statement vs. Reality
2.5. SYRCLE
2.6. Certainty of the Evidence—ARRIVE Instead of GRADE
2.6.1. GRADE
- When conducting a GRADE assessment, it must be assumed that all the studies we analysed were non-randomised (classified as other studies), and therefore, these studies start with a score of 2 out of 4, which can be further upgraded or downgraded.
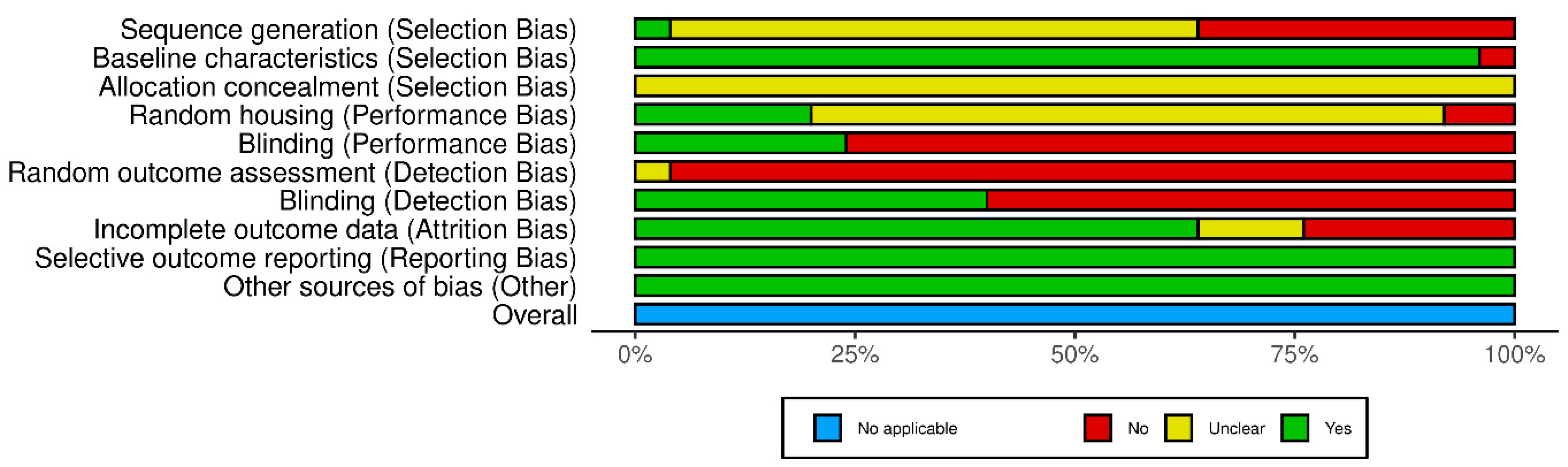
- Based on the SYRCLE analysis, it was determined that most of the analysed studies had a high Risk of Bias in terms of Blinding (Performance Bias), Blinding (Detection Bias), and Random outcome assessment (Detection Bias), which indeed translates to a significant downgrade based on those factors through the GRADE assessment.
- Conducted meta-analyses present high inconsistency due to the high heterogeneity of included studies (Higgins I2), another factor for downgrading these results.
- The overall meta-analysis results were consistent; however, subgroup data for citalopram and a few models (Immobilisation, Maternal Deprivation, Myocardial Infarction, Prenatal Stress, Social Defeat, Thermoregulation) were inconsistent and should be downgraded.
2.6.2. ARRIVE
2.7. Meta-Analysis Methodology
2.8. Sensitivity Analysis
- Various types of ADMs were used in the included studies, leading to inherent heterogeneity that sensitivity analysis could not adequately address.
- Differences in study design include variations in drug dosages, duration of treatment, and experimental conditions.
- Differences in the methodologies used for the forced swimming test across the included studies.
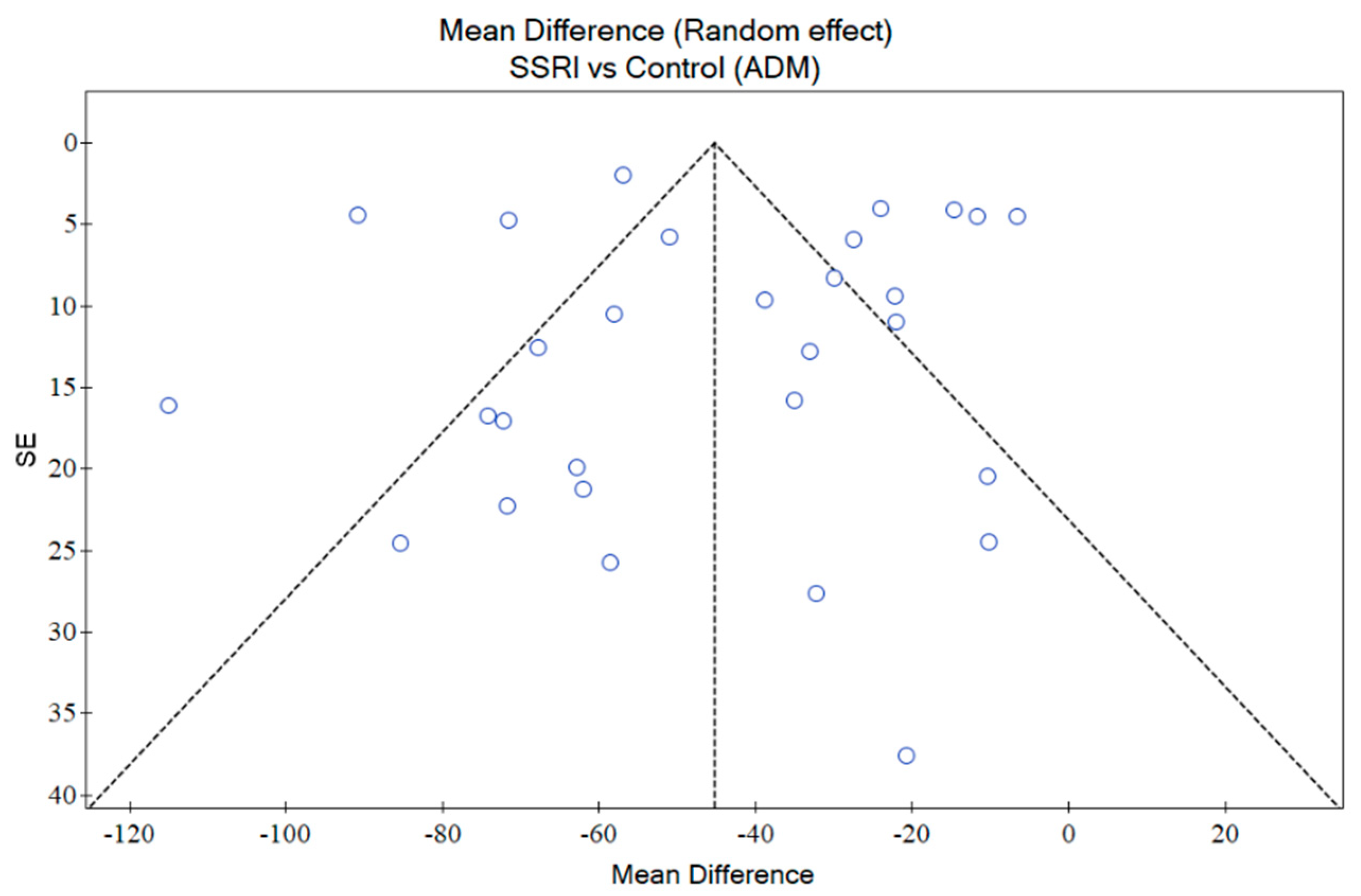
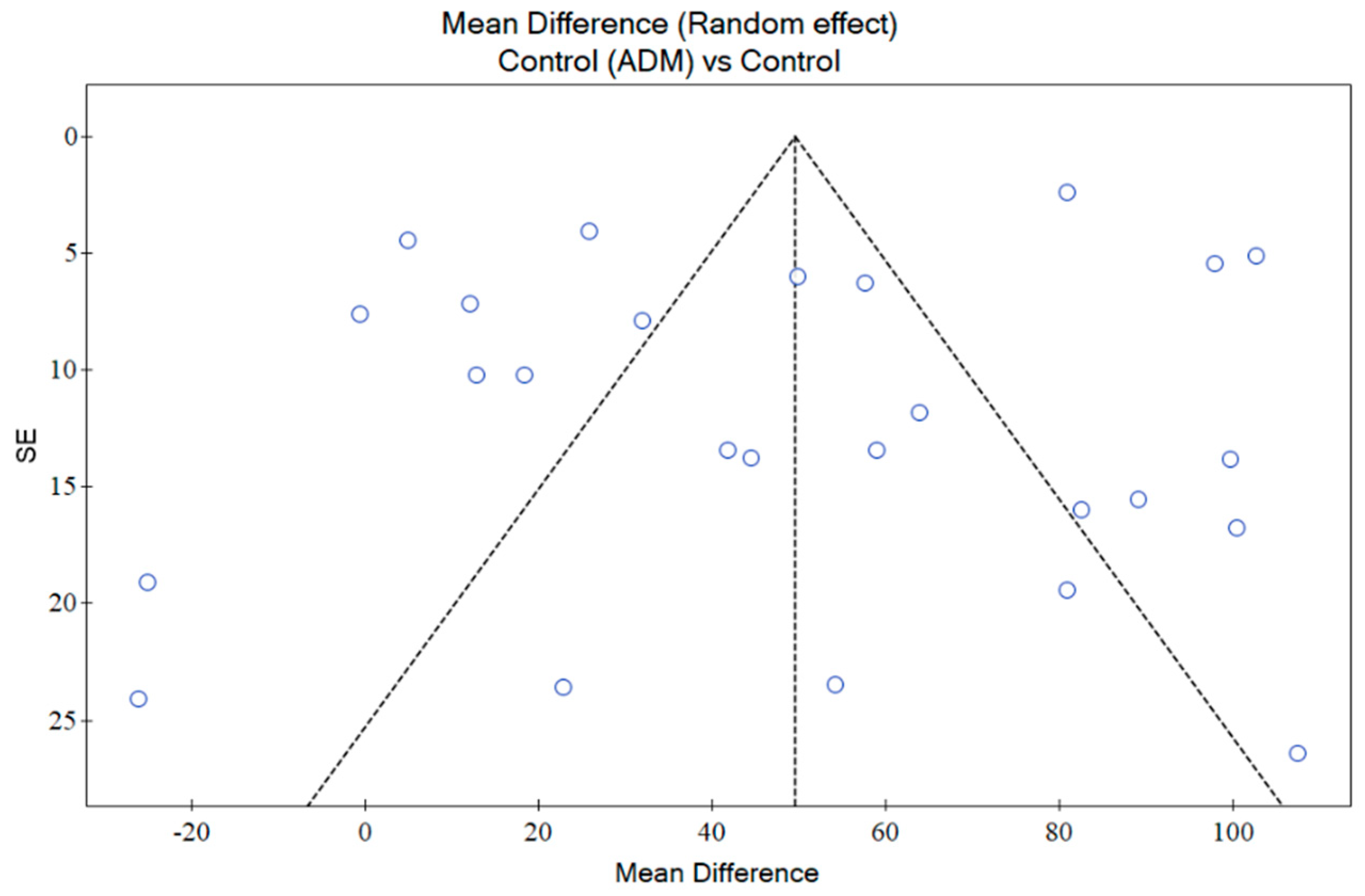
2.9. Publication Bias
3. Results
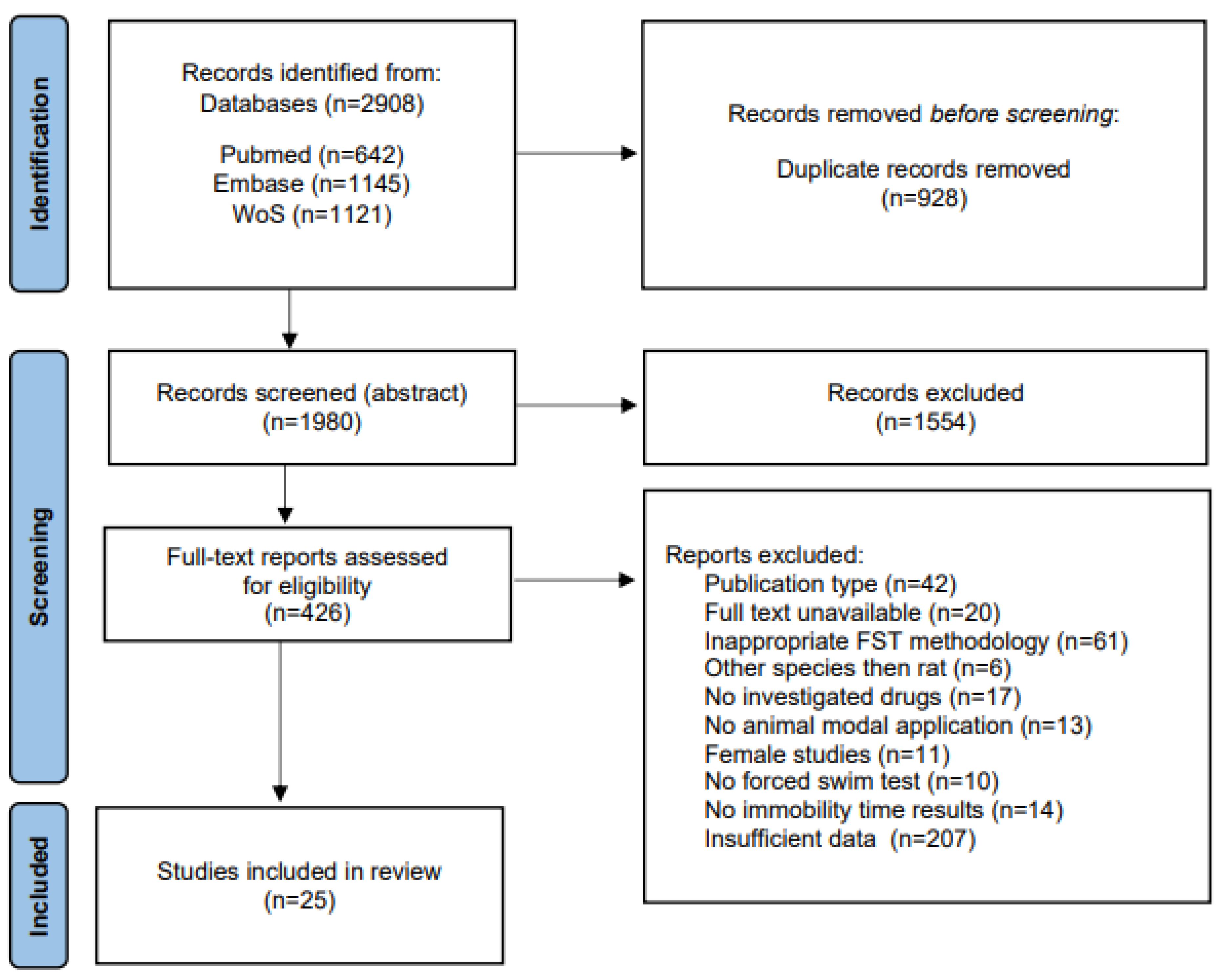
3.1. Literature Search Result
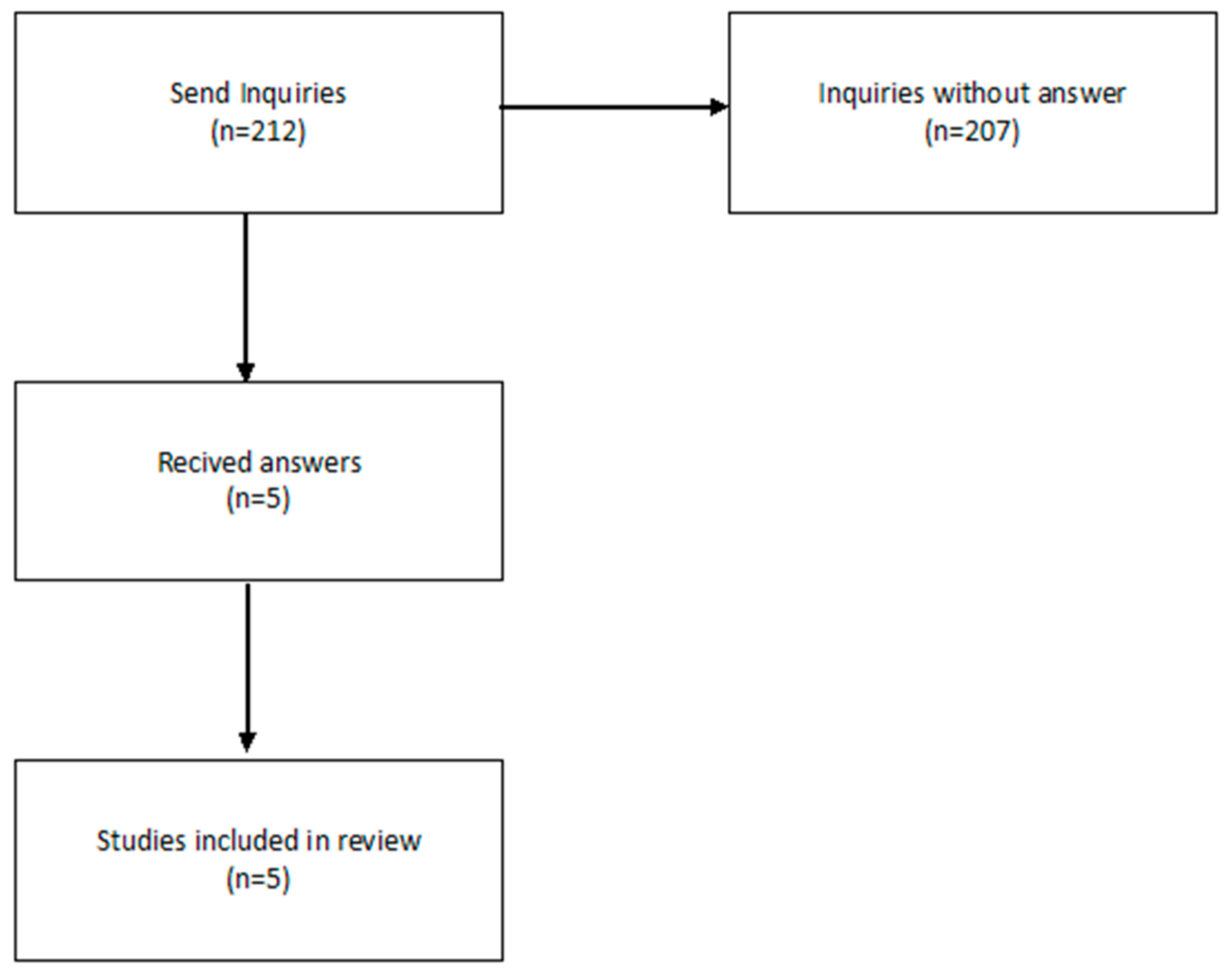
3.2. Data Availability
3.3. Included Studies
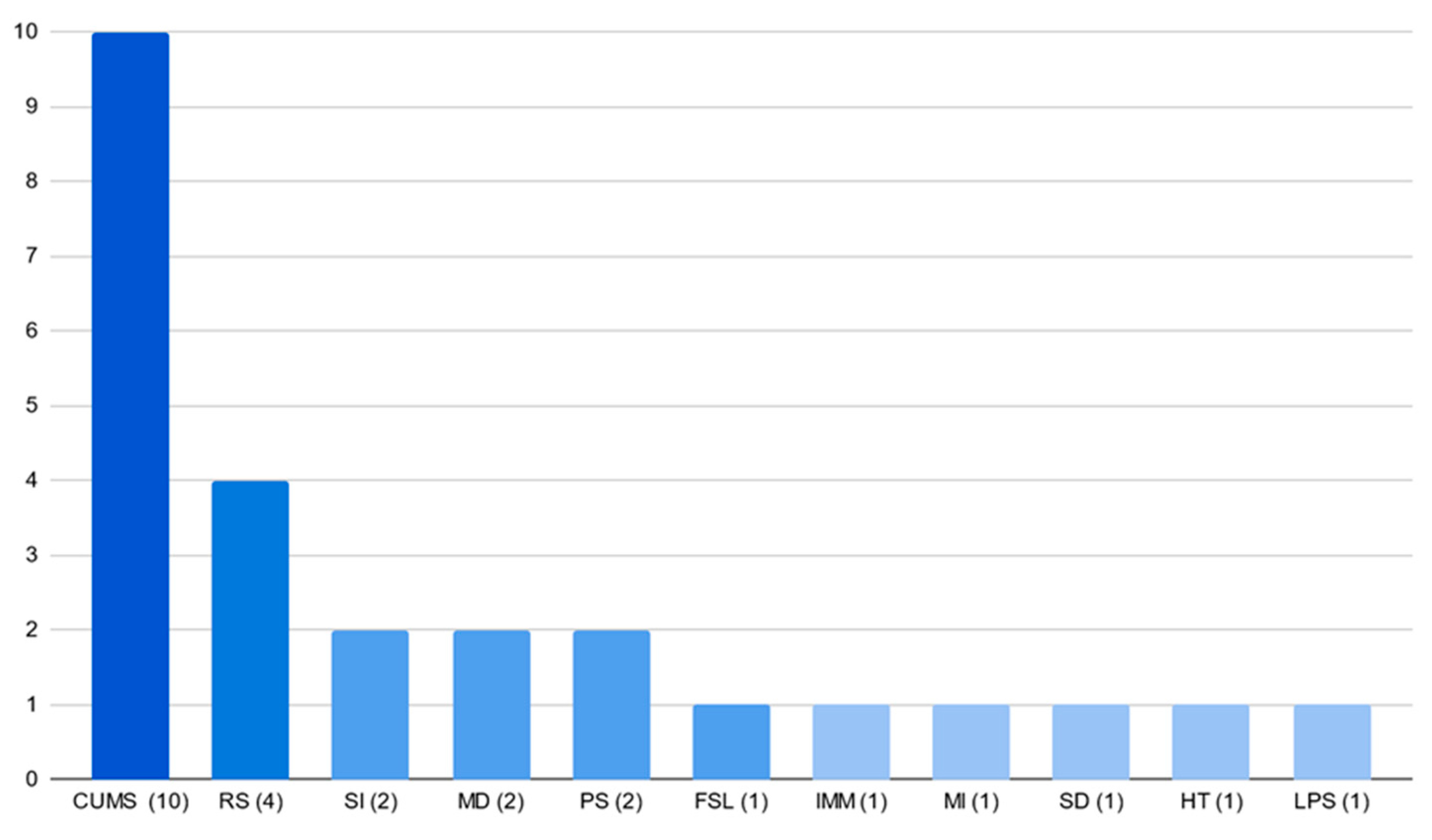
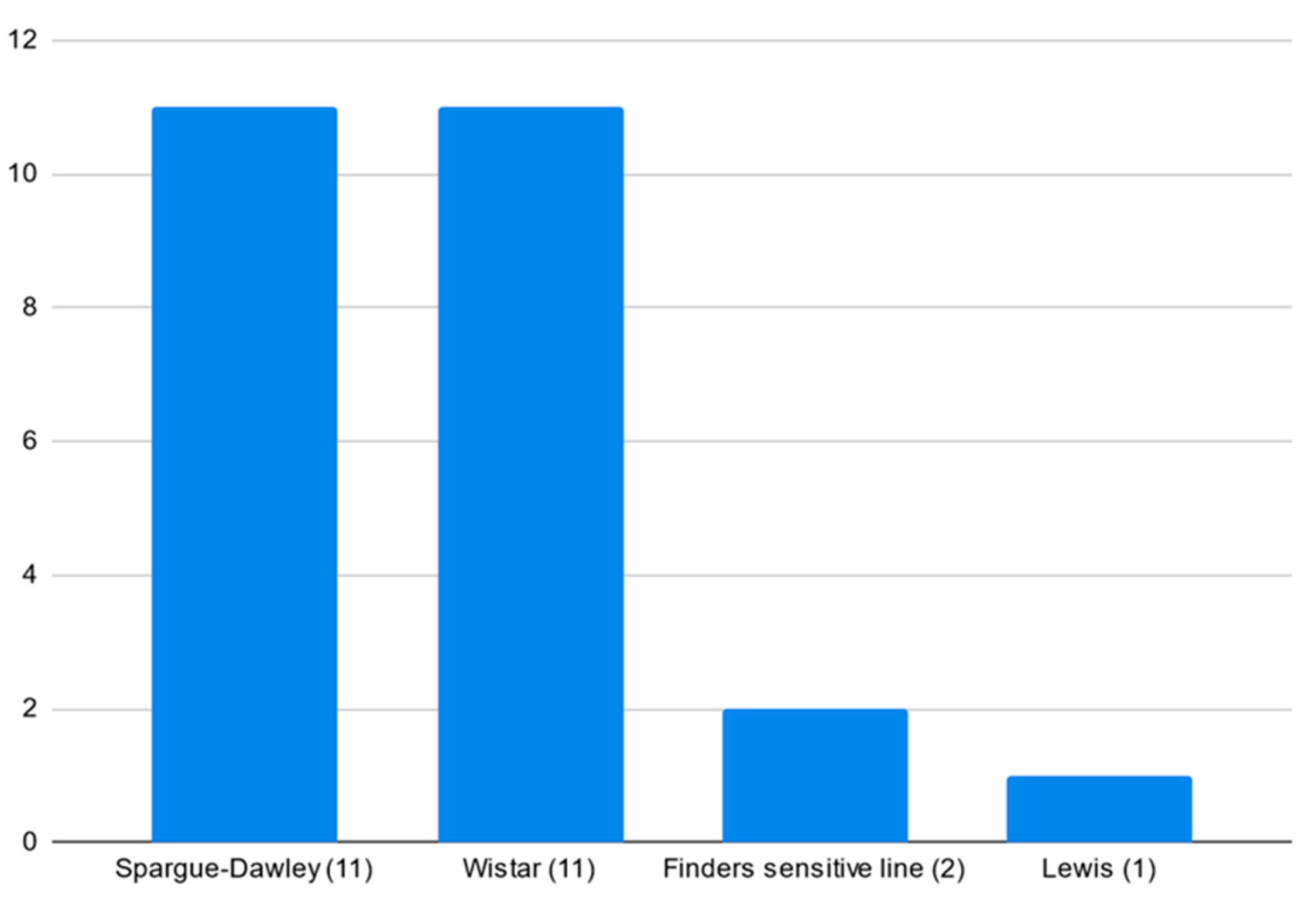
3.4. Models and Strains’ Popularity
3.5. Quality Assessment
ARRIVE Table
3.6. Risk of Bias Assessment
3.7. Receptor Affinity of the Tested Drugs
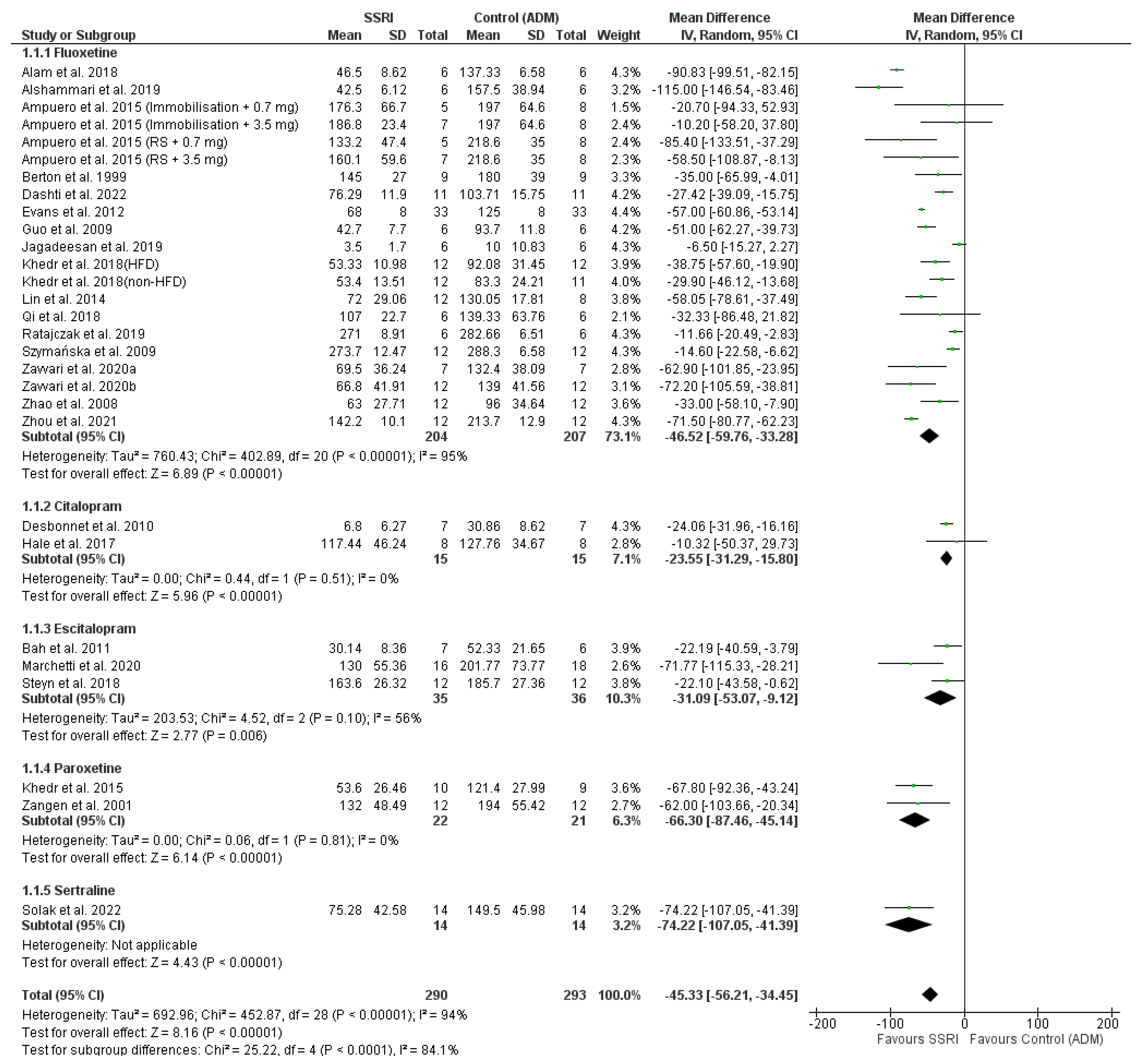
3.8. Meta-Analysis
SSRI Efficacy
- and sertraline (1 study [37], IV-MD −74.22 [−107.05, −41.39] p < 0.00001 (95% CI)).
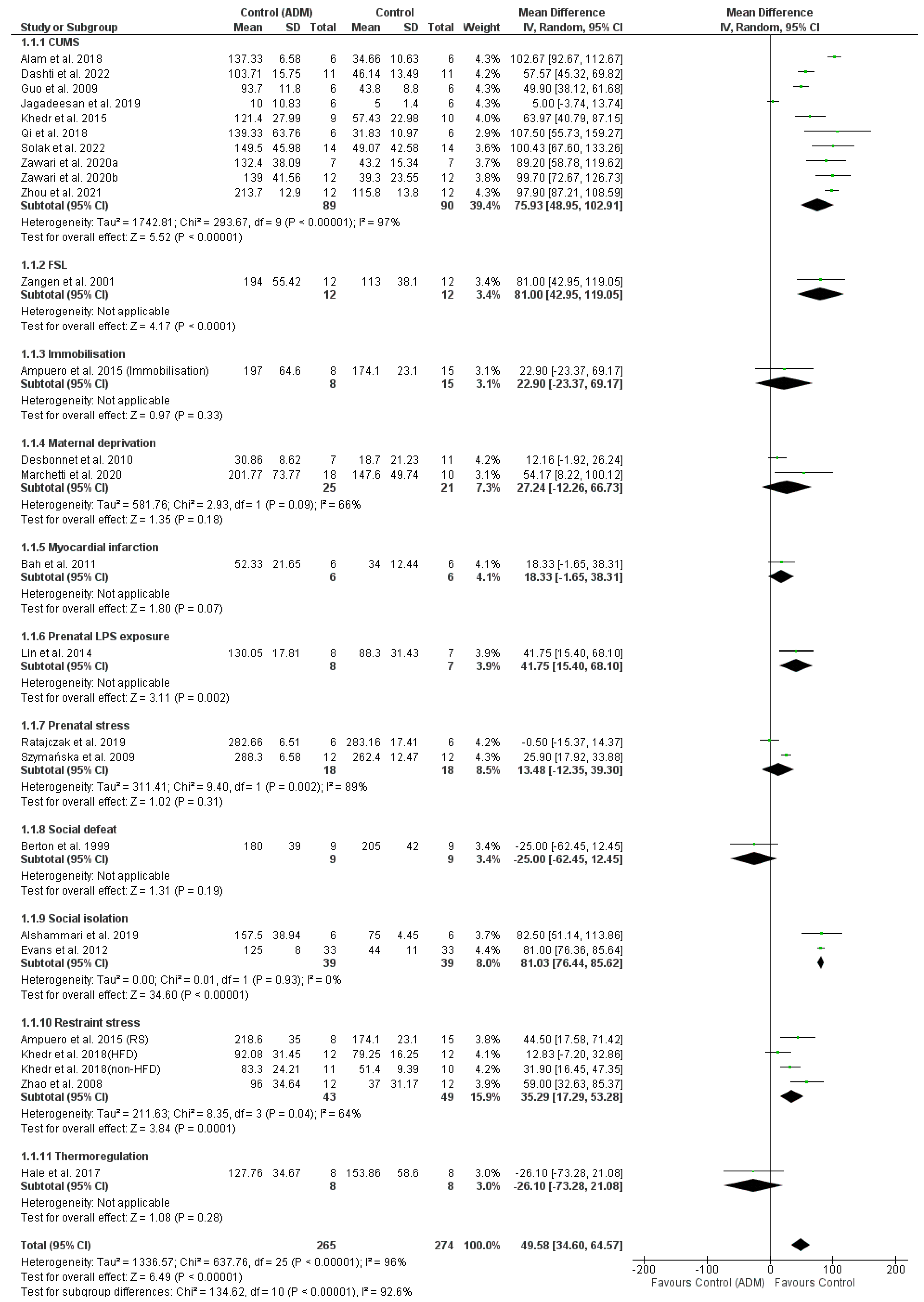
3.9. Animal Depression Model Comparison
3.10. RoB Assessment
4. Discussion
4.1. The Efficacy of SSRIs
4.2. Animal Depression Models
4.3. Animal Strains
4.4. Data Availability
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Depression and Other Common Mental Disorders. Available online: https://www.who.int/publications/i/item/depression-global-health-estimates (accessed on 10 July 2024).
- Neitzke, A.B. An Illness of Power: Gender and the Social Causes of Depression. Cult. Med. Psychiatry 2016, 40, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Gelenberg, A.J.; Marlene Freeman, C.P.; Markowitz, J.C.; Rosenbaum, J.F.; Thase, M.E.; Trivedi, M.H.; Van Rhoads, R.S.; Reus, V.I.; Raymond DePaulo, C.J.; Fawcett, J.A.; et al. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; Work Group on Major Depressive Disorder; American Psychiatric Association: Washington, DC, USA, 2010. [Google Scholar]
- Wang, Q.; Timberlake, M.A.; Prall, K.; Dwivedi, Y. The Recent Progress in Animal Models of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- Becker, M.; Pinhasov, A.; Ornoy, A. Animal Models of Depression: What Can They Teach Us about the Human Disease? Diagnostics 2021, 11, 123. [Google Scholar] [CrossRef]
- Markov, D.D.; Novosadova, E.V. Chronic Unpredictable Mild Stress Model of Depression: Possible Sources of Poor Reproducibility and Latent Variables. Biology 2022, 11, 1621. [Google Scholar] [CrossRef]
- Overstreet, D.H. Modeling Depression in Animal Models. Methods Mol. Biol. 2012, 829, 125–144. [Google Scholar] [CrossRef]
- Petković, A.; Chaudhury, D. Encore: Behavioural Animal Models of Stress, Depression and Mood Disorders. Front. Behav. Neurosci. 2022, 16, 931964. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and Pharmacodynamics: A Comprehensive Analysis of the Absorption, Distribution, Metabolism, and Excretion of Psychiatric Drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Reines, E.H.; Montgomery, S.A. A Comparative Review of Escitalopram, Paroxetine, and Sertraline: Are They All Alike? Int. Clin. Psychopharmacol. 2014, 29, 185–196. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Antidepressants. In Synthesis of Essential Drugs; Elsevier: Amsterdam, The Netherlands, 2006; pp. 103–116. [Google Scholar] [CrossRef]
- Ślifirski, G.; Król, M.; Turło, J. 5-HT Receptors and the Development of New Antidepressants. Int. J. Mol. Sci. 2021, 22, 9015. [Google Scholar] [CrossRef]
- Foster, R.H.; Goa, K.L. Paroxetine: A Review of Its Pharmacology and Therapeutic Potential in the Management of Panic Disorder. CNS Drugs 1997, 8, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Huang, S.Q.; Xiao, T.; Wang, X.P.; Kong, W.; Liu, S.J.; Zhang, Z.; Yang, Y.; Huang, S.S.; Ni, X.J.; et al. Pharmacokinetics of Immediate and Sustained-Release Formulations of Paroxetine: Population Pharmacokinetic Approach to Guide Paroxetine Personalized Therapy in Chinese Psychotic Patients. Front. Pharmacol. 2022, 13, 966622. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- De Lima, M.S. Review: Citalopram Is Effective and Safe for Depression. BMJ Ment. Health 2001, 4, 80. [Google Scholar] [CrossRef]
- Zhong, H.; Haddjeri, N.; Sánchez, C. Escitalopram, an Antidepressant with an Allosteric Effect at the Serotonin Transporter—A Review of Current Understanding of Its Mechanism of Action. Psychopharmacology 2012, 219, 1–13. [Google Scholar] [CrossRef]
- Bræstrup, C.; Sanchez, C. Escitalopram: A Unique Mechanism of Action. Int. J. Psychiatry Clin. Pract. 2004, 8 (Suppl. 1), 11–13. [Google Scholar] [CrossRef] [PubMed]
- CAMARADES Berlin. Preclinical Systematic Reviews & Meta-Analysis Wiki; CAMARADES Berlin: Berlin, Germany, 2024. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Alam, M.; Najmi, A.K.; Ahmad, I.; Ahmad, F.J.; Akhtar, M.J.; Imam, S.S.; Akhtar, M. Formulation and Evaluation of Nano Lipid Formulation Containing CNS Acting Drug: Molecular Docking, in-Vitro Assessment and Bioactivity Detail in Rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 46–57. [Google Scholar] [CrossRef]
- Evans, J.; Sun, Y.; McGregor, A.; Connor, B. Allopregnanolone Regulates Neurogenesis and Depressive/Anxiety-like Behaviour in a Social Isolation Rodent Model of Chronic Stress. Neuropharmacology 2012, 63, 1315–1326. [Google Scholar] [CrossRef]
- Dashti, S.; Nahavandi, A. Neuroprotective Effects of Aripiprazole in Stress-Induced Depressive-like Behavior: Possible Role of CACNA1C. J. Chem. Neuroanat. 2022, 126, 102170. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Zhang, Z.J.; Wang, S.H.; Sui, Y.X.; Sun, Y. Notch1 Signaling, Hippocampal Neurogenesis and Behavioral Responses to Chronic Unpredicted Mild Stress in Adult Ischemic Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Ampuero, E.; Luarte, A.; Santibañez, M.; Varas-Godoy, M.; Toledo, J.; Diaz-Veliz, G.; Cavada, G.; Javier Rubio, F.; Wyneken, U. Two Chronic Stress Models Based on Movement Restriction in Rats Respond Selectively to Antidepressant Drugs: Aldolase C As a Potential Biomarker. Int. J. Neuropsychopharmacol. 2015, 18, pyv038. [Google Scholar] [CrossRef]
- Khedr, S.A.; Elmelgy, A.A.; El-Kharashi, O.A.; Abd-Alkhalek, H.A.; Louka, M.L.; Sallam, H.A.; Aboul-Fotouh, S. Metformin Potentiates Cognitive and Antidepressant Effects of Fluoxetine in Rats Exposed to Chronic Restraint Stress and High Fat Diet: Potential Involvement of Hippocampal c-Jun Repression. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 407–422. [Google Scholar] [CrossRef]
- Bah, T.M.; Benderdour, M.; Kaloustian, S.; Karam, R.; Rousseau, G.; Godbout, R. Escitalopram Reduces Circulating Pro-Inflammatory Cytokines and Improves Depressive Behavior without Affecting Sleep in a Rat Model of Post-Cardiac Infarct Depression. Behav. Brain Res. 2011, 225, 243–251. [Google Scholar] [CrossRef]
- Hale, M.W.; Lukkes, J.L.; Dady, K.F.; Kelly, K.J.; Paul, E.D.; Smith, D.G.; Raison, C.L.; Lowry, C.A. Whole-Body Hyperthermia and a Subthreshold Dose of Citalopram Act Synergistically to Induce Antidepressant-like Behavioral Responses in Adolescent Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 162–168. [Google Scholar] [CrossRef]
- Lin, Y.L.; Wang, S. Prenatal Lipopolysaccharide Exposure Increases Depression-like Behaviors and Reduces Hippocampal Neurogenesis in Adult Rats. Behav. Brain Res. 2014, 259, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Lauria, M.; Caberlotto, L.; Musazzi, L.; Popoli, M.; Mathé, A.A.; Domenici, E.; Carboni, L. Gene Expression Signature of Antidepressant Treatment Response/Non-Response in Flinders Sensitive Line Rats Subjected to Maternal Separation. Eur. Neuropsychopharmacol. 2020, 31, 69–85. [Google Scholar] [CrossRef]
- Khedr, L.H.; Nassar, N.N.; El-Denshary, E.S.; Abdel-Tawab, A.M. Paroxetine Ameliorates Changes in Hippocampal Energy Metabolism in Chronic Mild Stress-Exposed Rats. Neuropsychiatr. Dis. Treat. 2015, 11, 2887–2901. [Google Scholar] [CrossRef]
- Qi, X.; Xu, H.; Wang, L.; Zhang, Z. Comparison of Therapeutic Effects of TREK1 Blockers and Fluoxetine on Chronic Unpredicted Mild Stress Sensitive Rats. ACS Chem. Neurosci. 2018, 9, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Solak, H.; Gormus, Z.I.S.; Koca, R.O.; Gunes, C.E.; Kutlu, S. Does Sertraline Affect Hypothalamic Food Intake Peptides in the Rat Experimental Model of Chronic Mild Stress-Induced Depression? Neurochem. Res. 2022, 47, 1299–1316. [Google Scholar] [CrossRef] [PubMed]
- Zavvari, F.; Nahavandi, A.; Shahbazi, A. Neuroprotective Effects of Cerium Oxide Nanoparticles on Experimental Stress-Induced Depression in Male Rats. J. Chem. Neuroanat. 2020, 106, 101799. [Google Scholar] [CrossRef]
- Zavvari, F.; Nahavandi, A.; Goudarzi, M. Fluoxetine Attenuates Stress-Induced Depressive-like Behavior through Modulation of Hippocampal GAP43 and Neurogenesis in Male Rats. J. Chem. Neuroanat. 2020, 103, 101711. [Google Scholar] [CrossRef]
- Zhou, X.M.; Liu, C.Y.; Liu, Y.Y.; Ma, Q.Y.; Zhao, X.; Jiang, Y.M.; Li, X.J.; Chen, J.X. Xiaoyaosan Alleviates Hippocampal Glutamate-Induced Toxicity in the CUMS Rats via NR2B and PI3K/Akt Signaling Pathway. Front. Pharmacol. 2021, 12, 586788. [Google Scholar] [CrossRef]
- Jagadeesan, S.; Jagadeesan, S.; Chiroma, S.M.; Baharuldin, M.T.H.; Taib, C.N.M.; Amom, Z.; Adenan, M.I.; Moklas, M.A.M. Centella Asiatica Prevents Chronic Unpredictable Mild Stress-Induced Behavioral Changes in Rats. Biomed. Res. Ther. 2019, 6, 3233–3243. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Guo, H.; Zhou, D. Antidepressant-like Effect of Liquiritin from Glycyrrhiza Uralensis in Chronic Variable Stress Induced Depression Model Rats. Behav. Brain Res. 2008, 194, 108–113. [Google Scholar] [CrossRef]
- Alshammari, T.K.; Alghamdi, H.; Green, T.A.; Niazy, A.; Alkahdar, L.; Alrasheed, N.; Alhosaini, K.; Alswayyed, M.; Elango, R.; Laezza, F.; et al. Assessing the Role of Toll-like Receptor in Isolated, Standard and Enriched Housing Conditions. PLoS ONE 2019, 14, e0222818. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Szymańska, M.; Budziszewska, B.; Jaworska-Feil, L.; Basta-Kaim, A.; Kubera, M.; Leśkiewicz, M.; Regulska, M.; Lasoń, W. The Effect of Antidepressant Drugs on the HPA Axis Activity, Glucocorticoid Receptor Level and FKBP51 Concentration in Prenatally Stressed Rats. Psychoneuroendocrinology 2009, 34, 822–832. [Google Scholar] [CrossRef]
- Ratajczak, P.; Kus, K.; Zaprutko, T.; Szczepański, M.; Rusowicz, S.; Nowakowska, E. Antidepressant and Anxiolytic Efficacy of Single, Chronic and Concomitant Use of Vortioxetine, Dapoxetine and Fluoxetine in Prenatally Stressed Rats. Acta Neurobiol. Exp. 2019, 79, 13–24. [Google Scholar] [CrossRef]
- Berton, O.; Durand, M.; Aguerre, S.; Mormède, P.; Chaouloff, F. Behavioral, Neuroendocrine and Serotonergic Consequences of Single Social Defeat and Repeated Fluoxetine Pretreatment in the Lewis Rat Strain. Neuroscience 1999, 92, 327–341. [Google Scholar] [CrossRef]
- Steyn, S.F.; Harvey, B.H.; Brink, C.B. Immediate and Long-Term Antidepressive-like Effects of Pre-Pubertal Escitalopram and Omega-3 Supplementation Combination in Young Adult Stress-Sensitive Rats. Behav. Brain Res. 2018, 351, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Zangen, A.; Nakash, R.; Overstreet, D.H.; Yadid, G. Association between Depressive Behavior and Absence of Serotonin-Dopamine Interaction in the Nucleus Accumbens. Psychopharmacology 2001, 155, 434–439. [Google Scholar] [CrossRef]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults With Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Focus 2018, 16, 420–429. [Google Scholar] [CrossRef]
- Owens, M.J.; Knight, D.L.; Nemeroff, C.B. Second-Generation SSRIs: Human Monoamine Transporter Binding Profile of Escitalopram and R-Fluoxetine. Biol. Psychiatry 2001, 50, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Hansen, R.A.; Morgan, L.C.; Thaler, K.; Lux, L.; van Noord, M.; Mager, U.; Thieda, P.; Gaynes, B.N.; Wilkins, T.; et al. Comparative Benefits and Harms of Second-Generation Antidepressants for Treating Major Depressive Disorder: An Updated Meta-Analysis. Ann. Intern. Med. 2011, 155, 772–785. [Google Scholar] [CrossRef]
- Sawamura, T.; Shimizu, K.; Nibuya, M.; Wakizono, T.; Suzuki, G.; Tsunoda, T.; Takahashi, Y.; Nomura, S. Effect of Paroxetine on a Model of Posttraumatic Stress Disorder in Rats. Neurosci. Lett. 2004, 357, 37–40. [Google Scholar] [CrossRef]
- Qiu, H.M.; Yang, J.X.; Wu, X.H.; Li, N.; Liu, D.; Wang, L.J.; Qin, L.J.; Zhou, Q.X. Antidepressive Effect of Paroxetine in a Rat Model: Upregulating Expression of Serotonin and Norepinephrine Transporter. Neuroreport 2013, 24, 520–525. [Google Scholar] [CrossRef]
- Keller, M.B.; Ryan, N.D.; Strober, M.; Klein, R.G.; Kutcher, S.P.; Birmaher, B.; Hagino, O.R.; Koplewicz, H.; Carlson, G.A.; Clarke, G.N.; et al. Efficacy of Paroxetine in the Treatment of Adolescent Major Depression: A Randomised, Controlled Trial. J. Am. Acad. Child. Adolesc. Psychiatry 2001, 40, 762–772. [Google Scholar] [CrossRef]
- Barbui, C.; Furukawa, T.A.; Cipriani, A. Effectiveness of Paroxetine in the Treatment of Acute Major Depression in Adults: A Systematic Re-Examination of Published and Unpublished Data from Randomised Trials. CMAJ 2008, 178, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sen, S.; Biswas, A.; Barman, T.; Tripathi, S. Impact on Behavioral Changes Due to Chronic Use of Sertraline in Wistar Albino Rats. Indian. J. Pharmacol. 2015, 47, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Okuya, M.; Hatano, M.; Matsuda, Y.; Iwata, N. Antidepressants for the Treatment of Adults with Major Depressive Disorder in the Maintenance Phase: A Systematic Review and Network Meta-Analysis. Mol. Psychiatry 2023, 28, 402–409. [Google Scholar] [CrossRef]
- Kryst, J.; Majcher-Maślanka, I.; Chocyk, A. Effects of Chronic Fluoxetine Treatment on Anxiety- and Depressive-like Behaviors in Adolescent Rodents—Systematic Review and Meta-Analysis. Pharmacol. Rep. 2022, 74, 920. [Google Scholar] [CrossRef] [PubMed]
- Bahji, A.; Ermacora, D.; Stephenson, C.; Hawken, E.R.; Vazquez, G. Comparative Efficacy and Tolerability of Pharmacological Treatments for the Treatment of Acute Bipolar Depression: A Systematic Review and Network Meta-Analysis. J. Affect. Disord. 2020, 269, 154–184. [Google Scholar] [CrossRef]
- Overstreet, D.H.; Keeney, A.; Hogg, S. Antidepressant Effects of Citalopram and CRF Receptor Antagonist CP-154,526 in a Rat Model of Depression. Eur. J. Pharmacol. 2004, 492, 195–201. [Google Scholar] [CrossRef]
- Cui, M.; Huang, C.Y.; Wang, F. Efficacy and Safety of Citalopram for the Treatment of Poststroke Depression: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 2905–2918. [Google Scholar] [CrossRef]
- Hsu, J.W.; Su, T.P.; Huang, C.Y.; Chen, Y.S.; Chou, Y.H. Faster Onset of Antidepressant Effects of Citalopram Compared with Sertraline in Drug-Naïve First-Episode Major Depressive Disorder in a Chinese Population: A 6-Week Double-Blind, Randomised Comparative Study. J. Clin. Psychopharmacol. 2011, 31, 577–581. [Google Scholar] [CrossRef]
- Seo, M.K.; Lee, J.G.; Park, S.W. Effects of Escitalopram and Ibuprofen on a Depression-like Phenotype Induced by Chronic Stress in Rats. Neurosci. Lett. 2019, 696, 168–173. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Andersen, H.F.; Lam, R.W. Efficacy of Escitalopram in the Treatment of Major Depressive Disorder Compared with Conventional Selective Serotonin Reuptake Inhibitors and Venlafaxine XR: A Meta-Analysis. J. Psychiatry Neurosci. 2006, 31, 122. [Google Scholar]
- Antoniuk, S.; Bijata, M.; Ponimaskin, E.; Wlodarczyk, J. Chronic Unpredictable Mild Stress for Modeling Depression in Rodents: Meta-Analysis of Model Reliability. Neurosci. Biobehav. Rev. 2019, 99, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Pollak, D.D.; Rey, C.E.; Monje, F.J. Rodent Models in Depression Research: Classical Strategies and New Directions. Ann. Med. 2010, 42, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Serretti, A. Anhedonia and Depressive Disorders. Clin. Psychopharmacol. Neurosci. 2023, 21, 401–409. [Google Scholar] [CrossRef]
- Wang, S.S.; Yan, X.B.; Hofman, M.A.; Swaab, D.F.; Zhou, J.N. Increased Expression Level of Corticotropin-Releasing Hormone in the Amygdala and in the Hypothalamus in Rats Exposed to Chronic Unpredictable Mild Stress. Neurosci. Bull. 2010, 26, 297. [Google Scholar] [CrossRef]
- Reul, J.M.H.M.; Holsboer, F. On the Role of Corticotropin-Releasing Hormone Receptors in Anxiety and Depression. Dialogues Clin. Neurosci. 2002, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Grippo, A.J.; Francis, J.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Neuroendocrine and Cytokine Profile of Chronic Mild Stress-Induced Anhedonia. Physiol. Behav. 2005, 84, 697–706. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.L.; Bonilla, H.J.; Escobar Villanueva, M.d.C.; Brianza, M.P.; Vázquez, G.P.; Alarcón, F.J.A. Chronic Unpredictable Mild Stress Generates Oxidative Stress and Systemic Inflammation in Rats. Physiol. Behav. 2016, 161, 15–23. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, Y.; Yuan, X. Validity of Chronic Restraint Stress for Modeling Anhedonic-like Behavior in Rodents: A Systematic Review and Meta-Analysis. J. Int. Med. Res. 2022, 50, 03000605221075816. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Kim, S.H.; Kwon, S.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.H. Chronic Administration of Baicalein Decreases Depression-Like Behavior Induced by Repeated Restraint Stress in Rats. Korean J. Physiol. Pharmacol. 2013, 17, 393. [Google Scholar] [CrossRef]
- Bernal-Morales, B.; Contreras, C.M.; Cueto-Escobedo, J. Acute Restraint Stress Produces Behavioral Despair in Weanling Rats in the Forced Swim Test. Behav. Process. 2009, 82, 219–222. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic Restraint Stress Causes Anxiety- and Depression-like Behaviors, Downregulates Glucocorticoid Receptor Expression, and Attenuates Glutamate Release Induced by Brain-Derived Neurotrophic Factor in the Prefrontal Cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Eiden, L.E. Activation of the HPA Axis and Depression of Feeding Behavior Induced by Restraint Stress Are Separately Regulated by PACAPergic Neurotransmission in the Mouse. Stress 2016, 19, 374. [Google Scholar] [CrossRef]
- Wegener, G.; Mathe, A.A.; Neumann, I.D. Selectively Bred Rodents as Models of Depression and Anxiety. Curr. Top. Behav. Neurosci. 2012, 12, 139–187. [Google Scholar] [CrossRef] [PubMed]
- Dulawa, S.C.; Janowsky, D.S. Cholinergic Regulation of Mood: From Basic and Clinical Studies to Emerging Therapeutics. Mol. Psychiatry 2019, 24, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.H.; Friedman, E.; Mathé, A.A.; Yadid, G. The Flinders Sensitive Line Rat: A Selectively Bred Putative Animal Model of Depression. Neurosci. Biobehav. Rev. 2005, 29, 739–759. [Google Scholar] [CrossRef]
- Elfving, B.; Plougmann, P.H.; Müller, H.K.; Mathé, A.A.; Rosenberg, R.; Wegener, G. Inverse Correlation of Brain and Blood BDNF Levels in a Genetic Rat Model of Depression. Int. J. Neuropsychopharmacol. 2010, 13, 563–572. [Google Scholar] [CrossRef]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Eskelund, A.; Budac, D.P.; Sanchez, C.; Elfving, B.; Wegener, G. Female Flinders Sensitive Line Rats Show Estrous Cycle-Independent Depression-like Behavior and Altered Tryptophan Metabolism. Neuroscience 2016, 329, 337–348. [Google Scholar] [CrossRef]
- Begni, V.; Sanson, A.; Pfeiffer, N.; Brandwein, C.; Inta, D.; Talbot, S.R.; Riva, M.A.; Gass, P.; Mallien, A.S. Social Isolation in Rats: Effects on Animal Welfare and Molecular Markers for Neuroplasticity. PLoS ONE 2020, 15, e0240439. [Google Scholar] [CrossRef]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Radojcic, M.B. Effects of Chronic Social Isolation on Wistar Rat Behavior and Brain Plasticity Markers. Neuropsychobiology 2012, 66, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Brenes, J.C.; Fornaguera, J.; Sequeira-Cordero, A. Environmental Enrichment and Physical Exercise Attenuate the Depressive-Like Effects Induced by Social Isolation Stress in Rats. Front. Pharmacol. 2020, 11, 804. [Google Scholar] [CrossRef]
- Zanier-Gomes, P.H.; de Abreu Silva, T.E.; Zanetti, G.C.; Benati, É.R.; Pinheiro, N.M.; Murta, B.M.T.; Crema, V.O. Depressive Behavior Induced by Social Isolation of Predisposed Female Rats. Physiol. Behav. 2015, 151, 292–297. [Google Scholar] [CrossRef]
- Ieraci, A.; Mallei, A.; Popoli, M. Social Isolation Stress Induces Anxious-Depressive-Like Behavior and Alterations of Neuroplasticity-Related Genes in Adult Male Mice. Neural Plast. 2016, 2016, 6212983. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-Induced Depression-like Model in Mice: Meta-Analysis and Systematic Evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef] [PubMed]
- Biesmans, S.; Matthews, L.J.R.; Bouwknecht, J.A.; De Haes, P.; Hellings, N.; Meert, T.F.; Nuydens, R.; Ver Donck, L. Systematic Analysis of the Cytokine and Anhedonia Response to Peripheral Lipopolysaccharide Administration in Rats. BioMed Res. Int. 2016, 2016, 9085273. [Google Scholar] [CrossRef] [PubMed]
- Custódio, C.S.; Mello, B.S.F.; Filho, A.J.M.C.; de Carvalho Lima, C.N.; Cordeiro, R.C.; Miyajima, F.; Réus, G.Z.; Vasconcelos, S.M.M.; Barichello, T.; Quevedo, J.; et al. Neonatal Immune Challenge with Lipopolysaccharide Triggers Long-Lasting Sex- and Age-Related Behavioral and Immune/Neurotrophic Alterations in Mice: Relevance to Autism Spectrum Disorders. Mol. Neurobiol. 2018, 55, 3775–3788. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lin, S.Y.; Wang, S. Prenatal Lipopolysaccharide Exposure Increases Anxiety-like Behaviors and Enhances Stress-Induced Corticosterone Responses in Adult Rats. Brain Behav. Immun. 2012, 26, 459–468. [Google Scholar] [CrossRef]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036. [Google Scholar] [CrossRef]
- Matsubara, T.; Funato, H.; Kobayashi, A.; Nobumoto, M.; Watanabe, Y. Reduced Glucocorticoid Receptor Alpha Expression in Mood Disorder Patients and First-Degree Relatives. Biol. Psychiatry 2006, 59, 689–695. [Google Scholar] [CrossRef]
- Armario, A.; Belda, X.; Gagliano, H.; Fuentes, S.; Molina, P.; Serrano, S.; Nadal, R. Differential Hypothalamic-Pituitary-Adrenal Response to Stress among Rat Strains: Methodological Considerations and Relevance for Neuropsychiatric Research. Curr. Neuropharmacol. 2023, 21, 1906. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.; Roy, N.; Dalziel, J.; Butts, C.; Coad, J.; Young, W.; Parkar, S.G.; Hedderley, D.; Dinnan, H.; Martell, S.; et al. Anxiety-like Behavior in Female Sprague Dawley Rats Associated with Cecal Clostridiales. Microorganisms 2023, 11, 1773. [Google Scholar] [CrossRef] [PubMed]
- Germolec, D.; Kono, D.H.; Pfau, J.C.; Pollard, K.M. Animal Models Used to Examine the Role of the Environment in the Development of Autoimmune Disease: Findings from an NIEHS Expert Panel Workshop. J. Autoimmun. 2012, 39, 285. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-Analysis of Individual Participant Data: Rationale, Conduct, and Reporting. BMJ 2010, 340, 521–525. [Google Scholar] [CrossRef] [PubMed]
| Authors and Year | Arrive Item | Total | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |||
| Alam et al. (2018) [25] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 24 | |
| Alshammari et al. (2019) [43] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 0 | 1 | 1 | 0 | 2 | 2 | 29 | |
| Ampuero et al. (2015) [29] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 28 | |
| Bah et al. (2011) [31] | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 25 | |
| Berton et al. (1999) [47] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 26 | |
| Dashti et al. (2022) [27] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 0 | 2 | 2 | 30 | |
| Desbonet et al. (2010) [44] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 0 | 29 | |
| Evans et al. (2012) [26] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 29 | |
| Guo et al. (2009) [28] | 2 | 1 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 26 | |
| Hale et al. (2017) [32] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 0 | 2 | 28 | |
| Jagadeesan et al. (2019) [41] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 31 | |
| Khedr et al. (2015) [35] | 2 | 1 | 1 | 0 | 0 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 25 | |
| Khedr et al. (2018) [30] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 24 | |
| Lin et al. (2014) [33] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 25 | |
| Marchetti et al. (2020) [34] | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 2 | 0 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 25 | |
| Qi et al. (2018) [36] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 26 | |
| Ratajczak et al. (2019) [46] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 25 | |
| Solak et al. (2022) [37] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 0 | 2 | 2 | 31 | |
| Steyn et al. (2018) [48] | 1 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 27 | |
| Szymańska et al. (2009) [45] | 2 | 1 | 1 | 1 | 0 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 26 | |
| Zavvari et al. (2020) [39] | 2 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 26 | |
| Zaavari et al. (2020) [38] | 2 | 1 | 1 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 26 | |
| Zangen et al. (2001) [49] | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 20 | |
| Zhao et al. (2008) [42] | 2 | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 25 | |
| Zhou et al. (2021) [40] | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 2 | 2 | 29 | |
| Category score | 48 | 23 | 25 | 2 | 13 | 50 | 45 | 50 | 50 | 41 | 29 | 47 | 47 | 39 | 37 | 12 | 25 | 26 | 0 | 19 | 37 | 665 | |
| Maximum score | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 1050 | |
| Ratio | 0.96 | 0.46 | 0.50 | 0.04 | 0.26 | 1.00 | 0.90 | 1.00 | 1.00 | 0.82 | 0.58 | 0.94 | 0.94 | 0.78 | 0.74 | 0.24 | 0.50 | 0.52 | 0.00 | 0.38 | 0.74 | 0.63 | |
| Drug | Affinity | |||||
|---|---|---|---|---|---|---|
| SERT | DA | 5-HT | H | M | Others | |
| Fluoxetine | ||||||
| Prozac | H2 | α2, α3, β4 | ||||
| Fluoxetine hydrochloride | SERT | 5-HT2C | ||||
| Eli Lilly | ||||||
| Paroxetine | ||||||
| Seroxat | 5-HT1 | α1, α2, β | ||||
| Paroxetine hydrochloride | SERT | D2 | 5-HT2 | H1 | ||
| GlaxoSmithKline (GSK) | 5-HT3 | |||||
| Sertraline | ||||||
| Zoloft | σ1 | |||||
| Sertraline hydrochloride | SERT | |||||
| Pfizer | ||||||
| Citalopram | ||||||
| Cipramil | ||||||
| Citalopram hydrobromide | SERT | |||||
| Lundbeck | H1 | |||||
| Escitalopram | ||||||
| Lexapro | 5-HT1A | M1 | ||||
| Escitalopram oxalate | SERT | D2 | 5-HT2A | H1 | ||
| Lundbeck | 5-HT2C | α2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, P.; Martyński, J.; Zięba, J.K.; Świło, K.; Kopciuch, D.; Paczkowska, A.; Zaprutko, T.; Kus, K. Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis. Pharmaceutics 2024, 16, 1144. https://doi.org/10.3390/pharmaceutics16091144
Ratajczak P, Martyński J, Zięba JK, Świło K, Kopciuch D, Paczkowska A, Zaprutko T, Kus K. Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis. Pharmaceutics. 2024; 16(9):1144. https://doi.org/10.3390/pharmaceutics16091144
Chicago/Turabian StyleRatajczak, Piotr, Jakub Martyński, Jan Kazimierz Zięba, Katarzyna Świło, Dorota Kopciuch, Anna Paczkowska, Tomasz Zaprutko, and Krzysztof Kus. 2024. "Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis" Pharmaceutics 16, no. 9: 1144. https://doi.org/10.3390/pharmaceutics16091144
APA StyleRatajczak, P., Martyński, J., Zięba, J. K., Świło, K., Kopciuch, D., Paczkowska, A., Zaprutko, T., & Kus, K. (2024). Comparative Efficacy of Animal Depression Models and Antidepressant Treatment: A Systematic Review and Meta-Analysis. Pharmaceutics, 16(9), 1144. https://doi.org/10.3390/pharmaceutics16091144







