Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy
Abstract
1. Introduction
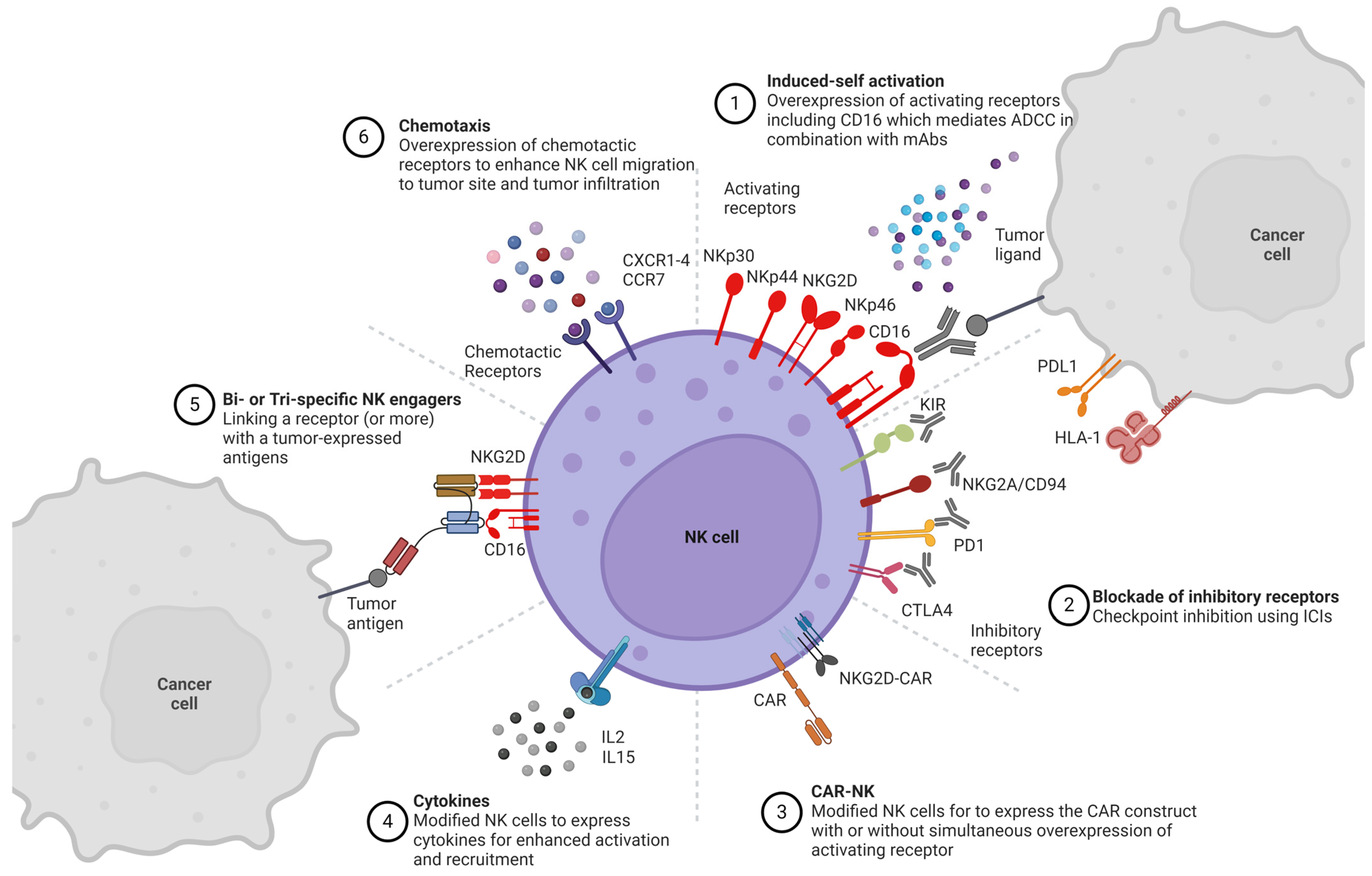
2. NK Cell Receptor Engineering
2.1. Activating NK Cell Receptors
2.1.1. CD16
2.1.2. NKG2D
2.1.3. NKG2C
2.1.4. NKp46
2.1.5. NKp44
2.1.6. NKp30
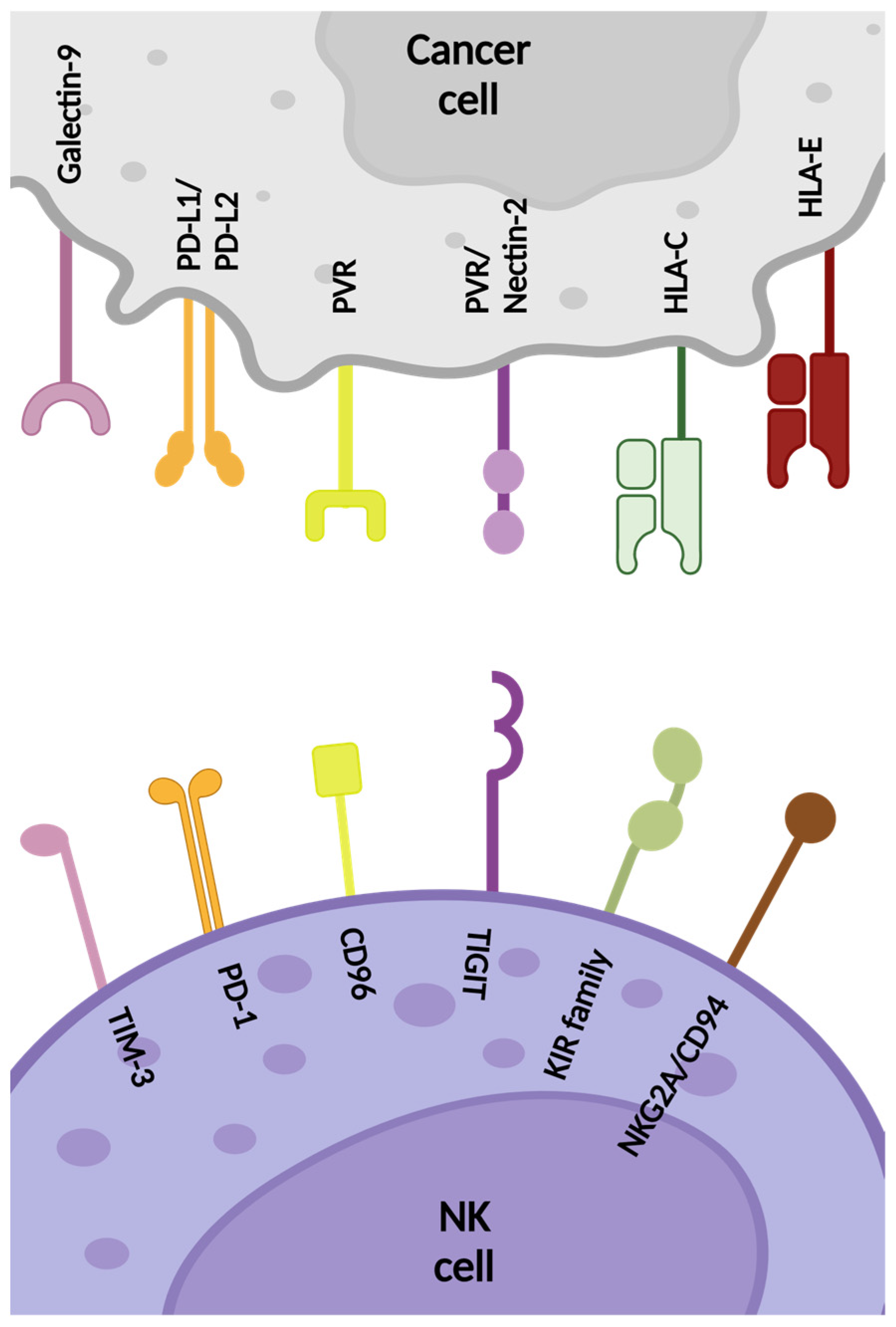
2.2. Inhibitory NK Cell Receptors
2.2.1. HLA-Specific Inhibitory Receptors
KIR
NKG2A/CD94
2.2.2. Non-HLA-Specific Inhibitory Receptors
TIGIT
CD96
CD112R
CTLA-4
PD-1
TIM-3
2.3. Chemotactic Receptors
2.3.1. CXCR4
2.3.2. CCR7
2.3.3. CXCR3
2.3.4. CXCR1-CXCR2
2.4. Cytokine Manipulation
| NK Cell Receptor | Type of Engineering/Antibody | Method of Engineering | Target | NK Cell Source | Key Outcomes | Year | Ref. |
|---|---|---|---|---|---|---|---|
| Activating Receptors | |||||||
| CD16 | Overexpression of hnCD16 and IL-2 | DNA electroporation | Head and neck, breast, lung, colorectal, and pancreatic cancer | NK-92 | In vitro lysis of many tumor cell lines, including lung, breast, head and neck, and colon | 2016 | [62] |
| CD16 | Knockout of ADAM17 and PD-1 | CRISPR/Cas9 electroporation | B-cell lymphoma (Raji cell line) | PB-NK | In vitro enhanced ADCC with rituximab, and elevated IFN-γ levels | 2020 | [63] |
| CD16 | Overexpression of hnCD16 | Lentiviral vectors | B-cell lymphoma, ovarian cancer | iPSC-NK | In vivo enhancement of ADCC with anti-CD20 and anti-HER2 mAbs | 2020 | [60] |
| CD16 | Overexpression of hnCD16 | Retroviral vectors | B-cell leukemia and lymphoma (cell lines and primary patient material) | UCB-NK or PB-NK | Successful expansion and sorting of CD16+ cells and in vitro enhancement of ADCC with rituximab or elotuzumab | 2024 | [64] |
| CD16 CD38 | Overexpression of CD16 and knockout of CD38 | CRISPR/Cas9 and mRNA Electroporation | Myeloma (MM.1S, NCI-H929, and EJM cell lines) | iPSC-NK | In vitro and in vivo enhanced ADCC with daratumumab reaching high lysis levels of NCI-H929 myeloma cells and reduction of tumor burden in MM.1S xenograft mouse | 2022 | [65] |
| CD16 | Overexpression of CD16 in combination with CAR construct | Lentiviral vectors | Triple-negative breast cancer | NK92MI | In vitro enhancement of ADCC with L-ICON immunoconjugate agent and in vivo reduction in tumor growth in cell line- and patient-derived xenograft mouse models | 2020 | [163] |
| CD16 | Bispecific single domain antibody (VHH) to engage CD16 and EGFR | - | Colorectal cancer (cell lines) | PB-NK | In vitro and ex vivo enhanced activation of NK cells and lysis of EGFR-expressing tumor cell lines | 2021 | [57] |
| CD16 | BiKE for CD16 and CD33 engagement | - | Leukemia and B-cell lymphoma (HL60 and Raji cell lines) | PB-NK | In vitro activation of NK cells with CD16xCD33 BiKE and ADAM17 inhibition against refractory CD33+ AML cells | 2013 | [70] |
| CD16 | 161533 TriKE for CD16 and CD33 engagement and IL-15 production | - | Myelodysplastic syndrome (MDS) | PB-NK | In vitro enhancement of NK cell proliferation and targeting of primary MDS blasts | 2018 | [71] |
| CD16 | AFM13 bispecific antibody for CD16 and CD30 engagement | - | T-cell lymphoma (Karpas 299 and HuT-78 cell lines and Karpas-NSG mice) | PB-NK or CB-NK | In vitro and in vivo enhancement of NK cell cytotoxicity and cytokine production against CD30+ lymphoma targets | 2021 | [74] |
| NKG2D | Overexpression of NKG2D and IL-21 | DNA delivery via chitosan-based nanoparticles | CT-26-induced solid tumors in Balb/c mice | - | In vivo transfection of tumor cells increased stimulation and migration of NK cells to tumor sites, slowed tumor growth and prolonged the life spam of tumor-expressing mice | 2017 | [80] |
| NKG2D | Overexpression of miR-486-5p | Lipofection (HiPerfect Transfection Reagent) | Hepatocellular carcinoma (Huh7 cell line) | PB-NK | In vitro activation of NK cells, induction of NKG2D levels, and enhanced lysis of Huh7 cells | 2016 | [82] |
| NKG2D | Overexpression of NKG2D | Lentiviral vectors | Human sarcoma explants and tumor cell lines | NK-92 | In vitro activation of NK-92 cells and enhanced degranulation towards sarcoma explants and tumor cell lines | 2020 | [83] |
| NKG2D | Overexpression of NKG2D in combination with CAR construct | Retroviral vectors | Myeloid-derived suppressor cells (MDSCs) | PB-NK | In vitro and in vivo increased cytotoxicity against MDSCs, pro-inflammatory cytokine and chemokine production, and tumor infiltration. Exogenous NKG2D was not susceptible to TME compared to endogenous NKG2D | 2021 | [164] |
| NKG2D | BiKE for NKG2D and CS1 engagement | - | MM | PB-NK | In vivo activation of NK cells and improved tumor clearance when tested in a xenograft NOD-SCID (NSG) mouse model | 2018 | [86] |
| NKG2D | BiKE for NKG2D and HER2 engagement | - | Breast ductal carcinoma (BT-474 cell line) | Human NK (source not specified) | In vitro enhancement of unstimulated NK-cell-mediated killing of BT-474 cells but did not promote the secretion of pro-inflammatory cytokines | 2020 | [88] |
| NKG2C | Overexpression of NKG2C and TriKE for NKG2C and CD33 engagement and IL-15 production | Feeder cells | AML (cell lines and blasts) | iPSC-NK | In vitro enhancement of NK cell proliferation, degranulation, and IFN-γ production against AML cell lines and primary AML blasts | 2021 | [94] |
| NKp46 | FLEX-NK engager for NKp46, CD38, and Glypican-3 (GPC3) engagement | - | Hepatocellular carcinoma (cell lines and spheroids) | PB-NK | In vitro enhancement of antibody-dependent cellular phagocytosis and complement-dependent cytotoxicity of NK cells towards GPC3-expressing tumors | 2023 | [96] |
| NKp44 | mAb 14-25-9 for NKp44 and PCNA binding inhibition | - | MM | NK92-44-1 (transduced NK-92 cells to express NKp44) | In vitro enhancement of degranulation and IFN-γ production of NK92-44-1 cells against MM primary cells | 2022 | [98] |
| NKp30 | B7-H6 engagers for NKp30 and EGFR engagement | - | Epidermoid carcinoma and non-small cell lung carcinoma (A431 and A549 cell lines) | PB-NK | In vitro enhancement of IFN-γ and TNF-α production, and cell-mediated cytotoxicity of NK cells against EGFR-expressing tumor cell lines | 2021 | [44] |
| NKp30 | Engagers for NKp30 and EGFR engagement | - | Epidermoid carcinoma and non-small cell lung carcinoma, colorectal and colon cancer (A431, A549, SW-480, HCT116, and H2030 cell lines) | MNC-NK | In vitro enhancement of NK cell activation and cell-mediated killing of NK cells against EGFR-expressing cell lines | 2022 | [99] |
| Inhibitory receptors | |||||||
| KIR | IPH2101 mAb to block KIR and HLA-I engagement | - | MM (U266 and K562 cell lines) | PB-NK | In vitro enhancement of NK cell survival and activity against AML cell lines | 2011 | [104] |
| KIR | Overexpression of KIR2DL1 in combination with a CAR construct (iCARs) | Retroviral vectors | B-cell lymphoma (Raji cell line) | CB-NK | In vitro and in vivo improvement of antitumor-activity when iCARs were administrated in Raji tumor-bearing NSG mice. iCARs prevent trogocytosis-induced self-recognition and fratricide, maintaining tumor recognition and cytotoxicity | 2022 | [165] |
| NKG2A | shRNA knockout of NKG2A | Lentiviral vectors | B lymphoblastoid cells | PB-NK | In vitro induction of target cell lysis by 40% compared to NKG2A-expressing NK cells | 2008 | [110] |
| NKG2A | Blockade of NKG2A | Protein expression blockers with retroviral vectors | HLA-E tumors | PB-NK | In vivo enhancement of NK cell cytotoxicity against HLA-E tumor-expressing immunodeficient mice | 2019 | [111] |
| NKG2A | Knockout of NKG2A | CRISPR/Cas9 nucleofection | MM (primary cells) | PB-NK | In vitro enhancement of cytolytic activity of NKG2A–KO NK cells with no significant difference in cytokine production comparing with NKG2A-expressing NK cells | 2022 | [112] |
| NKG2A | Knock out of NKG2A | CRISPR/Cas9 Lentiviral vectors | Metastatic breast cancer | PB-NK | In vivo delay of tumor progression and enhancement of survival in an HLA-E+ metastatic breast cancer xenogeneic mouse model | 2023 | [113] |
| NKG2A | Monalizumab for blockade of NKG2A and CD94 engagement | - | Chronic lymphocytic leukemia (K562 cell line) | PB-NK | In vitro restoration of NK cell activity against HLA-E-expressing targets, without impacting ADCC | 2016 | [115] |
| NKG2A | Knockout of NKG2A in combination with CAR construct | CRISPR/Cas9 Lentiviral vectors | AML | PB-NK | In vivo complete elimination of AML and AML-initiating cells in an AML-xenografted mouse model | 2022 | [166] |
| TIGIT | 13G6 mAb for TIGIT blockade | - | Colon cancer | - | In vivo restoration of NK cell activity and cytokine production, and prolonged CT26 tumor-bearing mice survival | 2018 | [116] |
| TIGIT | Anti-mouse TIGIT (4B1) and aCD266 mAb for the blockade of TIGIT and CD266 | - | Melanoma | - | In vitro and in vivo enhancement of NK-cell mediated cytotoxicity and decrease in tumor metastasis in mouse melanoma models | 2020 | [123] |
| TIGIT | Knockout of TIGIT | CRISPR/Cas9 electroporation | Pediatric and lung cancer (cell lines and spheroids) | PB-NK | In vitro enhancement of NK-cell cytotoxicity against all tumor cell lines and spheroids tested except CHLA90 that expresses less DNAM-1 and H1975 that is generally susceptible to NK-cell-mediated killing | 2023 | [125] |
| TIGIT | CD155 and CD73 targeting | SynNorch Lentiviral vectors | Glioblastoma | iPSC-NK | In vivo decrease in tumor growth by 40% with iNK cells, enhancement of NKp46 and granzyme B in a xenograft glioblastoma mouse model and isolated brain samples | 2024 | [126] |
| CD96 | anti-CD96 mAb for CD96 blockade | - | Lung cancer | - | In vivo enhancement of survival of mice bearing B16F10 or RM-1 lung metastases | 2016 | [127] |
| CD112R | anti-CD112R mAb for CD112R blockade | - | Colon adenocarcinoma | - | In vivo restoration of NK cell and T-cell function, and prolongation of survival of MC38 tumor-bearing mice | 2021 | [131] |
| CTLA-4 | Ipilimumab for CTLA-4 blockade | - | Melanoma | PB-NK | In vivo inhibition of tumor after treatment of chimeric murine xenograft model with allogeneic NK cells and Ipilimumab | 2013 | [133] |
| PD-1 | Anti-PD-1 and anti-PD-L1 for blockage of PD-1 and PD-L1 | - | HLA-negative cancers | - | In vivo enhancement of NK cell response towards HLA-negative tumor-bearing mice | 2018 | [136] |
| TIM-3 | Knockout of TIM-3 | CRISPR/Cas9 electroporation | Glioblastoma (cell lines) | PB-NK | In vitro enhancement of NK-cell-mediated growth inhibition of GBM cells | 2021 | [142] |
| Chemotactic receptors | |||||||
| CXCR4 | Overexpression of CXCR4 | mRNA electroporation | - | PB-NK | In vitro enhanced chemotaxis towards SDF-1a, and in vivo increased bone marrow homing in NSG mice | 2019 | [53] |
| CXCR4 | Knockout of CXCR4 in myeloid cells | Knockout mice | Melanoma | - | In vivo reduction in tumor growth and of FasL-expressing myeloid cells, and enhancement of Fas-expressing NK cells | 2018 | [54] |
| CXCR4 | Overexpression of CXCR4 in combination with CAR construct | Lentiviral vectors | Leukemia (NALM-6 cell line) | PB-NK | In vitro enhancement of migration towards SDF-1a, and CD16+ tumor eradication while retaining functional activity | 2020 | [146] |
| CCR7 | Overexpression of CCR7 | Feeder cells trogocytosis | - | PB-NK | In vitro migration of NK cells towards CCL19 and CCL21 and in vivo enhancement of lymph node homing in athymic nude mice | 2012 | [148] |
| CCR7 | Overexpression of CCR7and CD16 | mRNA electroporation | Chronic myelogenous leukemia and melanoma (K562 and MM.1S cell lines) | PB-NK | In vitro promotion of migration towards CCL19 and enhancement of CD16-mediated ADCC with rituximab | 2016 | [149] |
| CCR7 | Overexpression of CCR7 and CXCR4 | Lentiviral vectors | Colon cancer | NK-92 | In vitro and in vivo promotion of migration to colon cells and increased survival of HT-29 tumor-bearing SCID mice | 2020 | [55] |
| CXCR3 | Overexpression of CXCR3 | Feeder cells | Renal cell carcinoma (cell lines) and melanoma (526MEL tumor-expressing mice) | PB-NK | In vitro and in vivo NK cell infiltration to CXCL10-expressing solid tumors, reduction in tumors, and increased survival of CXCL10-positive melanoma xenograft mice | 2014 | [151] |
| CXCR2 | Overexpression of CXCR2 | Retroviral vectors | Renal cell carcinoma (cell lines) | PB-NK | In vitro promotion of NK cell migration to tumor sites with no significant difference in degranulation against K562 cells compared to CXCR2- NK cells | 2017 | [152] |
| CXCR2 | Overexpression of CXCR2 and IL-2 | CRISPR/Cas9 | Colon cancer | NK-92 | In vitro enhancement of NK cell infiltration to tumor sites and in vivo prolongation of survival and reduction in colon tumor growth in tumor-bearing mice | 2021 | [154] |
| CXCR1 | Overexpression of CXCR1 in combination with CAR construct | mRNA electroporation | Peritoneal ovarian cancer | PB-NK | In vitro enhancement of NK-cell migration and in vivo infiltration to tumor sites and tumor shrinking in a intraperitoneal xenograft NSG mouse model | 2020 | [155] |
| NK Cell Receptor | Product/Study | Malignancy | NK Cell Source | Sponsor | Location | Status | Clinical Phase | Year | Key Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| Activating Receptors | ||||||||||
| CD16 | AFM13 | Hodgkin Lymphoma | Intravenous infusion | Affimed GmbH | Houston TX USA, Würzburg GER | Completed | I | 2010 | In total, 3 of 26 patients achieved partial remission (11.5%) and 13 patients achieved stable disease (50%), with an overall disease control rate of 61.5% | NCT01221571 |
| CD16 | AFM24, SNK01 | Squamous Cell Carcinoma of Head and Neck, Non-Small-Cell Lung Carcinoma, Colorectal Neoplasms, Advanced Solid Tumor, Refractory Tumor, and Metastatic Tumor | Autologous SNK01 | NKGen Biotech, Inc. | Los Angeles CA, Chicago IL USA | Terminated | I/II | 2021 | Patients had a manageable safety profile, and SNK01 monotherapy has also shown to be well-tolerated in patients with rapidly progressive solid tumors | NCT05099549 |
| CD16 | ha-NK, Avelumab, N-803 | Merkel Cell Carcinoma | NK-92 | ImmunityBio, Inc. | San Francisco CA, Miami FL, Saint Louis MO USA | Terminated | II | 2020 | Did not meet recruitment goal | NCT03853317 |
| CD16 | haNK, Avelumab, Bevacizumab | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I/II | 2017 | - | NCT03329248 |
| Terminated | 2017 | The study was terminated early due to low enrollment. Safety data showed a 2/4 (50%) all-cause mortality. | NCT03387098 | |||||||
| Active, not recruiting | 2018 | - | NCT03586869 | |||||||
| CD16 | haNK, Avelumab, Bevacizumab | Squamous Cell Carcinoma | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Terminated | I/II | 2018 | The study was terminated early due to low enrollment. Safety data showed a 4/4 (50%) all-cause mortality. | NCT03387111 |
| CD16 | FT516 (hnCD16), CD20 Ab/PD-L1 Ab | B-cell Lymphoma and Acute Myeloid Leukemia/Solid Tumors | iPSCs | Fate Therapeutics | Phoenix AZ, San Diego CA, Minneapolis MN USA | Terminated | I | 2019 | The study was terminated by the Sponsor | NCT04023071 |
| 2020 | NCT04551885 | |||||||||
| CD16 | FT516 (hnCD16), Enoblituzumab, IL-2 | Ovarian, Fallopian Tube, and Primary Peritoneal Cancer | iPSCs | Masonic Cancer Center, University of Minnesota | Minneapolis MN USA | Completed | I | 2021 | - | NCT04630769 |
| CD16, CD38 | FT538 (hnCD16, CD38KO), IL15RF, Daratumumab | Multiple Myeloma and Acute Myeloid Leukemia/Solid Tumors | iPSCs | Fate Therapeutics | Denver CO, Minneapolis MN, Saint Louis MO, Hackensack NJ, Nashville TN USA | Terminated | I | 2020 | Interim Outcomes: Administration of FT538 cells in combination with daratumumab was safe and well tolerated without indication of CRS, neurotoxicity, or GvHD | NCT04614636 |
| 2021 | This study was terminated by the Sponsor | NCT05069935 | ||||||||
| CD16, CD38 | FT538 (hnCD16, CD38KO), Daratumumab, Fludarabine, Cyclophosphamide | Acute Myeloid Leukemia | iPSCs | Masonic Cancer Center, University of Minnesota | Minneapolis MN USA | Active, not recruiting | I | 2021 | FT538 combined with daratumumab has been well-tolerated in a heavily pre-treated patient group, showing expected toxicities and some signs of efficacy | NCT04714372 |
| NKG2D | NAKIP-AML, Talazoparib | Acute Myeloid Leukemia | Haploidentical human allogeneic NK cells | German Cancer Research Center | - | Not yet recruiting | I/II | 2024 | - | NCT05319249 |
| NKG2C and PD-1 | Dasatinib | Chronic Myeloid Leukemia | CMV-activated NKG2C+NK | Nanfang Hospital of Southern Medical University | Guangzhou CHI | Unknown | Obs. | 2021 | - | NCT04991532 |
| NY-ESO-1 TCR/IL-15 | NY-ESO-1 TCR/IL-15 NK cells | Multiple Myeloma | CB-NK | M.D. Anderson Cancer Center | Houston TX USA | Recruiting | I/II | 2023 | - | NCT06066359 |
| KIR | IPH2101 | Multiple Myeloma, Myeloma, and Smoldering Multiple Myeloma | Intravenous infusion | National Cancer Institute (NCI) | Bethesda MD USA | Terminated | II | 2010 | Lack of patients meeting the defined primary objectives | NCT01248455 |
| Inhibitory Receptors | ||||||||||
| CTLA4 | Ipilimumab, Cetuximab, CIML NK cells, N-803 | Squamous Cell Carcinoma of the Head and Neck, Recurrent Head and Neck Squamous Cell Carcinoma | CIML NK | Dana-Farber Cancer Institute | Boston MA USA | Recruiting | I | 2020 | Initial Outcomes: Allogeneic CIML NK cells combined with N-803 may promote tumor regression in patients with advanced head-and-neck cancer | NCT04290546 |
| PD-1 | SMT-NK Pembrolizumab | Biliary Tract Cancer | Allogeneic SMT-NK | SMT bio Co., Ltd. | Seoul KOR | Completed | I/II | 2019 | In phase 2a, 126 adverse events (AEs) were observed in 29 patients (85.3%). Severe AEs occurred in 16 patients (47.1%), but no dose-limiting toxicities were reported. The overall response rate (ORR) was 17.4% in the full-analysis set and 50.0% in the per-protocol set | NCT03937895 |
| PD-1 | NK cells Sintilimab | Non-small Cell Lung Cancer | Autologous PBMCs | The First Hospital of Jilin University | Changchun CHI | Completed | II | 2019 | Autologous NK cells combined with sintilimab demonstrated promising antitumor activity and had an acceptable safety profile in advanced NSCLC patients. No unexpected AE were observed | NCT03958097 |
| PD-1 | Pembrolizumab, DC-NK cells | Solid Tumors | Intravenous infusion | Allife Medical Science and Technology Co., Ltd. | - | Unknown | I | 2019 | - | NCT03815084 |
| PD-1 | NK and DC cells, Pembrolizumab, Nivolumab, Sintilimab, Toripalimab, Camrelizumab, Tislelizumab | Digestive Carcinoma, Gastrointestinal Tumors | Autologous NK cells | China Medical University | - | Not yet recruiting | II | 2022 | - | NCT05461235 |
| PD-1 | COH06 Azetolizumab | Several types of Non-Small cell Lung carcinoma | CB-NK | City of Hope Medical Center | Duarte CA USA | Active, not recruiting | I | 2022 | - | NCT05334329 |
| PD-1 | D-CIK cells, Axitinib | Renal Metastatic Cancer | PBMCs | Sun Yat-sen University | Guangzhou CHI | Unknown | II | 2018 | - | NCT03736330 |
| PD-1 | CCICC-002b, CIK cells, Sintilimab | Non-small cell lung cancer | Autologous CIK cells | Tianjin Medical University Cancer Institute and Hospital | Tianjin CHI | Unknown | II | 2021 | - | NCT04836728 |
| PD-1 | D-CIK, anti-PD-1 | Refractory Solid Tumors | PBMCs | Sun Yat-sen University | Guangzhou CHI | Unknown | I/II | 2016 | This study indicated enhanced antitumor immunity following combination treatment, particularly in patients with significant long-term survival benefits. In contrast, those with minimal survival benefit exhibited a higher proportion of peripheral CD8+TIM3+ T cells and a lower serum-level immunostimulatory cytokine profile | NCT02886897 |
| PD-1 | D-CIK and Pembrolizumab | Lung cancer neoplasms | Autologous PBMCs | Capital Medical University | Beijing CHI | Completed | I/II | 2016 | - | NCT03360630 |
| PD-1 | Anti-PD-1 P-GEMOX | High-risk Extranodal NK/T-cell lymphoma | Intravenous infusion | Cancer Institute and Hospital, Chinese Academy of Medical Sciences | Beijing CHI | Recruiting | II | 2021 | - | NCT05254899 |
| PD-1 | Pembrolizumab | NK/T cell lymphoma | Intravenous infusion | The University of Hong Kong | Hong Kong HKG | Unknown | II | 2016 | - | NCT03021057 |
| PD-1 | Merck NK-IIT Pembrolizumab | Melanoma | Intravenous infusion | Nina Bhardwaj | New York NY USA | Terminated | II | 2017 | Did not meet recruitment goal | NCT03241927 |
| PD-1 | SHR-1210 CIK cells | Renal Cell Carcinoma | Autologous CIK cells | Tianjin Medical University Cancer Institute and Hospital | Tianjin CHI | Unknown | II | 2019 | - | NCT03987698 |
| PD-1 | Toripalimab | Extranodal NK/T-cell lymphoma | Intravenous infusion | Beijing Tongren Hospital | - | Unknown | II | 2020 | - | NCT04338282 |
| PD-1 | Anti-PD-1 Chidamide Lenalidomide Etoposide | Relapsed or refractory NK/T-cell lymphoma | Intravenous infusion | Mingzhi Zhang | Zhengzhou CHI | Unknown | IV | 2019 | The 12-month progression-free survival (PFS) rate was 86.8%. All 19 patients experienced treatment-related adverse events (TRAEs), with 4 patients (21.1%) reporting immune-related AEs, including grade 1 hypothyroidism | NCT04038411 |
| PD-1 | Anti-PD-1 Pegaspargase | Extranodal NK/T-cell lymphoma | Intravenous infusion | Ruijin Hospital | Shanghai CHI | Unknown | II | 2019 | The combination of pegaspargase and sintilimab is effective and safe for treating advanced-stage NKTCL, with potential benefits in targeting fatty acid metabolism and CTLA-4 to overcome treatment resistance | NCT04096690 |
| PD-1 | SHR1210 Apatinib | NK/T-cell lymphoma | Intravenous infusion | Peking University | - | Unknown | II | 2018 | The overall response rate (ORR) was 30%, with 10% of patients achieving a complete response. The median progression-free survival (PFS) was 5.6 months, and the median overall survival was 16.7 months | NCT03701022 |
| PD-1 | Toripalimab Chemoradiotherapy | NK/T-cell lymphoma | Intravenous infusion | Sun Yat-sen University | Guangzhou CHI | Recruiting | III | 2020 | A total of 714 NKTCL patients were included. The median overall survival (OS) was 36 months, and cancer-specific survival (CSS) was 57 months | NCT04365036 |
| PD-1 | CAR2BRAIN, NK-92/5.28.z Ezabenlimab | Glioblastoma | NK-92 | Johann Wolfgang Goethe University Hospital | Frankfurt, Mannheim, Mainz GER | Active, not recruiting | I | 2017 | Study Objectives: Assessing for safety and tolerability to establish the maximum tolerated dose | NCT03383978 |
| PD-L1 | QUILT-3.060 NANT haNK | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I/II | 2017 | Initial Outcomes: Beneficial to patients with NSCLC | NCT03329248 |
| PD-L1 | QUILT-3.064, PD-L1 t-haNK | Advanced or metastatic solid tumors | NK-92 | ImmunityBio, Inc. | El Segundo CA USA | Active, not recruiting | I | 2019 | - | NCT04050709 |
| PD-L1 | Sacituzumab, PD-L1 t-haNK, N-803 | Advanced Triple Negative Breast Cancer | NK-92 | ImmunityBio, Inc. | Newport Beach CA USA | Terminated | I/II | 2021 | Low enrollment | NCT04927884 |
| PD-L1 | PD-L1 t-haNK, N-803, Aldoxorubicin | Pancreatic Cancer | NK-92 | ImmunityBio, Inc. | El Segundo CA, Newport beach CA, East Brunswick NJ USA | Active, not recruiting | II | 2020 | - | NCT04390399 |
| PD-L1 | QUILT-3.063 Avelumab haNK | Merkel Cell Carcinoma | NK-92 | ImmunityBio, Inc. | San Francisco CA, Miami FL, Saint Louis MO USA | Terminated | II | 2020 | Did not meet recruitment goal | NCT03853317 |
| PD-1/PD-L1 | QUILT-3.055, Anti-PD-1, Anti-PD-L1, PD-L1 t-haNK | Cancers previously treated with PD-1/PD-L1 Immune Checkpoint Inhibitors | NK-92 | ImmunityBio, Inc. | Anchorage AK, Hot Springs AR, El Segundo CA USA | Active, not recruiting | II | 2018 | Initial Outcomes: N803 shows low toxicity and promising efficacy in halting progression and inducing durable stable disease in patients who had previously progressed on various tumor types and CPI regimens | NCT03228667 |
| TGF-β/NR3C1 | CB-NK-TGF-betaR2-/NR3C1- | Glioblastoma | CB-NK | M.D. Anderson Cancer Center | Houston TX USA | Recruiting | I | 2023 | - | NCT04991870 |
| Chemotactic Receptors | ||||||||||
| CXCR4 | Revolution CXCR4 antagonists in combination with Nivolumab | Metastatic Renal Cell Carcinoma | PBMCs | National Cancer Institute, Naples | Naples ITA | Recruiting | I | 2016 | Baseline NK activity and early detection of CXCR4-dependent reversal of Treg suppressive function at two weeks are significant indicators of response in mRCC patients treated with Nivolumab | NCT03891485 |
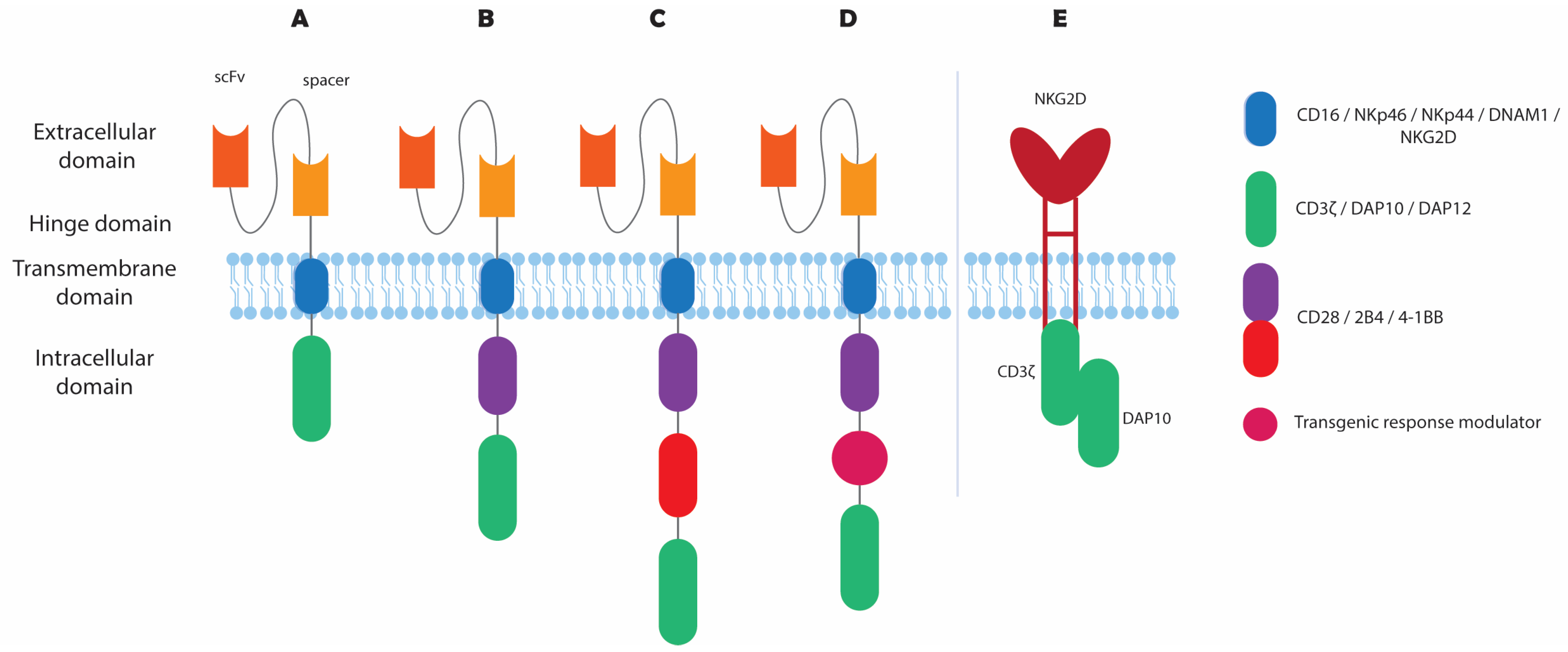
2.5. Combining CAR–NK with NK Receptor Engineering
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Robbins, G.M.; Wang, M.; Pomeroy, E.J.; Moriarity, B.S. Nonviral Genome Engineering of Natural Killer Cells. Stem Cell Res. Ther. 2021, 12, 350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, Y.; Xiao, W.; Tian, Z. Chimeric Antigen Receptor- and Natural Killer Cell Receptor-Engineered Innate Killer Cells in Cancer Immunotherapy. Cell. Mol. Immunol. 2021, 18, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on Γδ T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef]
- Wolf, B.; Zimmermann, S.; Arber, C.; Irving, M.; Trueb, L.; Coukos, G. Safety and Tolerability of Adoptive Cell Therapy in Cancer. Drug Saf. 2019, 42, 315–334. [Google Scholar] [CrossRef]
- Daher, M.; Rezvani, K. Outlook for New Car-Based Therapies with a Focus on Car Nk Cells: What Lies beyond Car-Engineered t Cells in the Race against Cancer. Cancer Discov. 2021, 11, 45–58. [Google Scholar] [CrossRef]
- Wendel, P.; Reindl, L.M.; Bexte, T.; Künnemeyer, L.; Särchen, V.; Albinger, N.; Mackensen, A.; Rettinger, E.; Bopp, T.; Ullrich, E. Arming Immune Cells for Battle: A Brief Journey through the Advancements of t and Nk Cell Immunotherapy. Cancers 2021, 13, 1481. [Google Scholar] [CrossRef] [PubMed]
- Reindl, L.M.; Albinger, N.; Bexte, T.; Müller, S.; Hartmann, J.; Ullrich, E. Immunotherapy with NK Cells: Recent Developments in Gene Modification Open up New Avenues. Oncoimmunology 2020, 9, 1777651. [Google Scholar] [CrossRef]
- Lin, C.Y.; Gobius, I.; Souza-Fonseca-Guimaraes, F. Natural Killer Cell Engineering—A New Hope for Cancer Immunotherapy. Semin. Hematol. 2020, 57, 194–200. [Google Scholar] [CrossRef]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Marco, V. NK Cells and Cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef]
- Rezvani, K.; Rouce, R.H. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front. Immunol. 2015, 6, 578. [Google Scholar] [CrossRef] [PubMed]
- Mantesso, S.; Geerts, D.; Spanholtz, J.; Kučerová, L. Genetic Engineering of Natural Killer Cells for Enhanced Antitumor Function. Front. Immunol. 2020, 11, 607131. [Google Scholar] [CrossRef] [PubMed]
- Michel, T.; Ollert, M.; Zimmer, J. A Hot Topic: Cancer Immunotherapy and Natural Killer Cells. Int. J. Mol. Sci. 2022, 23, 797. [Google Scholar] [CrossRef]
- Müller, S.; Bexte, T.; Gebel, V.; Kalensee, F.; Stolzenberg, E.; Hartmann, J.; Koehl, U.; Schambach, A.; Wels, W.S.; Modlich, U.; et al. High Cytotoxic Efficiency of Lentivirally and Alpharetrovirally Engineered CD19-Specific Chimeric Antigen Receptor Natural Killer Cells Against Acute Lymphoblastic Leukemia. Front. Immunol. 2020, 10, 3123. [Google Scholar] [CrossRef]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]
- Paolini, R.; Bernardini, G.; Molfetta, R.; Santoni, A. NK Cells and Interferons. Cytokine Growth Factor Rev. 2015, 26, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Maddineni, S.; Silberstein, J.L.; Sunwoo, J.B. Emerging NK Cell Therapies for Cancer and the Promise of next Generation Engineering of IPSC-Derived NK Cells. J. Immunother. Cancer 2022, 10, e004693. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Sivori, S.; Della Chiesa, M.; Carlomagno, S.; Quatrini, L.; Munari, E.; Vacca, P.; Tumino, N.; Mariotti, F.R.; Mingari, M.C.; Pende, D.; et al. Inhibitory Receptors and Checkpoints in Human NK Cells, Implications for the Immunotherapy of Cancer. Front. Immunol. 2020, 11, 2156. [Google Scholar] [CrossRef]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK Cell-Based Cancer Immunotherapy: From Basic Biology to Clinical Development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef]
- Shankar, K.; Capitini, C.M.; Saha, K. Genome Engineering of Induced Pluripotent Stem Cells to Manufacture Natural Killer Cell Therapies. Stem Cell Res. Ther. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural Killer Cells in Antitumour Adoptive Cell Immunotherapy. Nat. Rev. Cancer 2022, 22, 557–575. [Google Scholar] [CrossRef]
- Karagiannis, P.; Kim, S.I. IPSC-Derived Natural Killer Cells for Cancer Immunotherapy. Mol. Cells 2021, 44, 541–548. [Google Scholar] [CrossRef]
- Kang, S.; Gao, X.; Zhang, L.; Yang, E.; Li, Y.; Yu, L. The Advances and Challenges of Nk Cell-Based Cancer Immunotherapy. Curr. Oncol. 2021, 28, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Phung, S.K.; Miller, J.S.; Felices, M. Bi-Specific and Tri-Specific NK Cell Engagers: The New Avenue of Targeted NK Cell Immunotherapy. Mol. Diagn. Ther. 2021, 25, 577–592. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune Checkpoint Molecules in Natural Killer Cells as Potential Targets for Cancer Immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lam, K.P.; Xu, S. Natural Killer Cell Engagers (NKCEs): A New Frontier in Cancer Immunotherapy. Front. Immunol. 2023, 14, 1207276. [Google Scholar] [CrossRef]
- El-Mayta, R.; Zhang, Z.; Hamilton, A.G.; Mitchell, M.J. Delivery Technologies to Engineer Natural Killer Cells for Cancer Immunotherapy. Cancer Gene Ther. 2021, 28, 947–959. [Google Scholar] [CrossRef]
- Carlsten, M.; Childs, R.W. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front. Immunol. 2015, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Loo, J.; Sicher, I.; Goff, A.; Kim, O.; Clary, N.; Alexeev, A.; Sulchek, T.; Zamarayeva, A.; Han, S.; Calero-Garcia, M. Microfluidic Transfection of MRNA into Human Primary Lymphocytes and Hematopoietic Stem and Progenitor Cells Using Ultra-Fast Physical Deformations. Sci. Rep. 2021, 11, 21407. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric Antigen Receptor-Engineered Natural Killer Cells for Cancer Immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Habibi, Z.; Hosseini, N.; Abdoli, S.; Rezaei, N. Preclinical Studies of Chimeric Antigen Receptor-Modified Natural Killer Cells in Cancer Immunotherapy: A Review. Expert Opin. Biol. Ther. 2022, 22, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nakade, T.; Sato, Y.; Harashima, H. Delivering MRNA to a Human NK Cell Line, NK-92 Cells, by Lipid Nanoparticles. Int. J. Pharm. 2023, 636, 122810. [Google Scholar] [CrossRef]
- Douka, S.; Brandenburg, L.E.; Casadidio, C.; Walther, J.; Garcia, B.B.M.; Spanholtz, J.; Raimo, M.; Hennink, W.E.; Mastrobattista, E.; Caiazzo, M. Lipid Nanoparticle-Mediated Messenger RNA Delivery for Ex Vivo Engineering of Natural Killer Cells. J. Control. Release 2023, 361, 455–469. [Google Scholar] [CrossRef]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Synergy among Receptors on Resting NK Cells for the Activation of Natural Cytotoxicity and Cytokine Secretion. Blood 2006, 107, 159–166. [Google Scholar] [CrossRef]
- Haroun-Izquierdo, A.; Vincenti, M.; Netskar, H.; Van Ooijen, H.; Zhang, B.; Bendzick, L.; Kanaya, M.; Momayyezi, P.; Li, S.; Wiiger, M.T.; et al. Adaptive Single-KIR + NKG2C + NK Cells Expanded from Select Superdonors Show Potent Missing-Self Reactivity and Efficiently Control HLA-Mismatched Acute Myeloid Leukemia. J. Immunother. Cancer 2022, 10, 168. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK Cells and ILCs in Tumor Immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Ho, J.W.; Hershkovitz, O.; Peiris, M.; Zilka, A.; Bar-Ilan, A.; Nal, B.; Chu, K.; Kudelko, M.; Kam, Y.W.; Achdout, H.; et al. H5-Type Influenza Virus Hemagglutinin Is Functionally Recognized by the Natural Killer-Activating Receptor NKp44. J. Virol. 2008, 82, 2028–2032. [Google Scholar] [CrossRef]
- Gaggero, S.; Bruschi, M.; Petretto, A.; Parodi, M.; Del Zotto, G.; Lavarello, C.; Prato, C.; Santucci, L.; Barbuto, A.; Bottino, C.; et al. Nidogen-1 Is a Novel Extracellular Ligand for the NKp44 Activating Receptor. Oncoimmunology 2018, 7, e1470730. [Google Scholar] [CrossRef] [PubMed]
- Rosental, B.; Brusilovsky, M.; Hadad, U.; Oz, D.; Appel, M.Y.; Afergan, F.; Yossef, R.; Rosenberg, L.A.; Aharoni, A.; Cerwenka, A.; et al. Proliferating Cell Nuclear Antigen Is a Novel Inhibitory Ligand for the Natural Cytotoxicity Receptor NKp44. J. Immunol. 2011, 187, 5693–5702. [Google Scholar] [CrossRef] [PubMed]
- Baychelier, F.; Sennepin, A.; Ermonval, M.; Dorgham, K.; Debre, P.; Vieillard, V. Identification of a Cellular Ligand for the Natural Cytotoxicity Receptor NKp44. Blood 2013, 122, 2935–2942. [Google Scholar] [CrossRef]
- Pekar, L.; Klausz, K.; Busch, M.; Valldorf, B.; Kolmar, H.; Wesch, D.; Oberg, H.-H.; Krohn, S.; Boje, A.S.; Gehlert, C.L.; et al. Affinity Maturation of B7-H6 Translates into Enhanced NK Cell–Mediated Tumor Cell Lysis and Improved Proinflammatory Cytokine Release of Bispecific Immunoligands via NKp30 Engagement. J. Immunol. 2021, 206, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.J.; Lee, K.M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef]
- Stanietsky, N.; Rovis, T.L.; Glasner, A.; Seidel, E.; Tsukerman, P.; Yamin, R.; Enk, J.; Jonjic, S.; Mandelboim, O. Mouse TIGIT Inhibits NK-Cell Cytotoxicity upon Interaction with PVR. Eur. J. Immunol. 2013, 43, 2138–2150. [Google Scholar] [CrossRef]
- Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of Nk Cells for Cancer Immunotherapy. Cells 2021, 10, 1058. [Google Scholar] [CrossRef]
- Xu, J.; Fu, H.; Yang, Y.; Yu, H.; Ai, X.; Lei, Y.; Bao, W.; Tang, Y. Modulation of CXCR1 and CXCR3 Expression on NK Cells via Tim-3 in a Murine Model of Primary Biliary Cholangitis. Mol. Immunol. 2021, 135, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Lacal, P.M.; Martire, M.; Navarra, P.; Graziani, G. Clinical Experience with CTLA-4 Blockade for Cancer Immunotherapy: From the Monospecific Monoclonal Antibody Ipilimumab to Probodies and Bispecific Molecules Targeting the Tumor Microenvironment. Pharmacol. Res. 2022, 175, 105997. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y. Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front. Immunol. 2020, 11, 1295. [Google Scholar] [CrossRef]
- Teijeira, Á.; Garasa, S.; Gato, M.; Alfaro, C.; Migueliz, I.; Cirella, A.; de Andrea, C.; Ochoa, M.C.; Otano, I.; Etxeberria, I.; et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps That Interfere with Immune Cytotoxicity. Immunity 2020, 52, 856–871.e8. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.A.; Wang, S.; Agarwal, V.; Marcisak, E.F.; Zuo, A.; Jablonski, S.A.; Loth, M.; Fertig, E.J.; Macdougall, J.; Zhukovsky, E.; et al. DPP Inhibition Alters the CXCR3 Axis and Enhances NK and CD8+ T Cell Infiltration to Improve Anti-PD1 Efficacy in Murine Models of Pancreatic Ductal Adenocarcinoma. J. Immunother. Cancer 2021, 9, e002837. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following MRNA Transfection with Gain-of-Function Variant CXCR4R334X. Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kumar, A.; Vilgelm, A.E.; Chen, S.; Gregory, D.; Novitskiy, S.V.; Joyce, S.; Richmond, A. Loss of CXCR4 in Myeloid Cells Enhances Antitumor Immunity and Reduces Melanoma Growth through NK Cell and FASL Mechanisms. Cancer Immunol. Res. 2019, 6, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, C.; Wang, C.; Zhang, S.; Li, Z.; Zhu, Y.; Li, D.; Gao, L.; Ge, Z.; Su, M.; et al. Overexpressed CXCR4 and CCR7 on the Surface of NK92 Cell Have Improved Migration and Anti-Tumor Activity in Human Colon Tumor Model. Anticancer Drugs 2020, 31, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Siminska, D.; Kojder, K.; Grochans, S.; Gutowska, I. Fractalkine/CX3CL1 in Neoplastic Processes. Int. J. Mol. Sci. 2020, 21, 3723. [Google Scholar] [CrossRef]
- Toffoli, E.C.; Sheikhi, A.; Lameris, R.; King, L.A.; van Vliet, A.; Walcheck, B.; Verheul, H.M.W.; Spanholtz, J.; Tuynman, J.; de Gruijl, T.D.; et al. Enhancement of Nk Cell Antitumor Effector Functions Using a Bispecific Single Domain Antibody Targeting Cd16 and the Epidermal Growth Factor Receptor. Cancers 2021, 13, 5446. [Google Scholar] [CrossRef]
- Dixon, K.J.; Wu, J.; Walcheck, B. Engineering Anti-Tumor Monoclonal Antibodies and Fc Receptors to Enhance ADCC by Human NK Cells. Cancers 2021, 13, 312. [Google Scholar] [CrossRef]
- Dexiu, C.; Xianying, L.; Yingchun, H.; Jiafu, L. Advances in CD247. Scand. J. Immunol. 2022, 96, e13170. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.H.; Bjordahl, R.; Gaidarova, S.; Rogers, P.; Lee, T.T.; Abujarour, R.; Bonello, G.B.; Wu, J.; Tsai, P.F.; et al. Pluripotent Stem Cell-Derived NK Cells with High-Affinity Noncleavable CD16a Mediate Improved Antitumor Activity. Blood 2020, 135, 399–410. [Google Scholar] [CrossRef]
- Srpan, K.; Ambrose, A.; Karampatzakis, A.; Saeed, M.; Cartwright, A.N.R.; Guldevall, K.; De Matos, G.D.S.C.; Önfelt, B.; Davis, D.M. Shedding of CD16 Disassembles the NK Cell Immune Synapse and Boosts Serial Engagement of Target Cells. J. Cell Biol. 2018, 217, 3267–3283. [Google Scholar] [CrossRef]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Morillon, Y.M.; Greiner, J.W.; Padget, M.R.; Tritsch, S.R.; Tsang, K.Y.; Campbell, K.S.; et al. An NK Cell Line (HaNK) Expressing High Levels of Granzyme and Engineered to Express the High Affinity CD16 Allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, E.J.; Hunzeker, J.T.; Kluesner, M.G.; Lahr, W.S.; Smeester, B.A.; Crosby, M.R.; Lonetree, C.L.; Yamamoto, K.; Bendzick, L.; Miller, J.S.; et al. A Genetically Engineered Primary Human Natural Killer Cell Platform for Cancer Immunotherapy. Mol. Ther. 2020, 28, 52–63. [Google Scholar] [CrossRef]
- van Hauten, P.M.M.; Hooijmaijers, L.; Vidal-Manrique, M.; van der Waart, A.B.; Hobo, W.; Wu, J.; Blijlevens, N.M.A.; Jansen, J.H.; Walcheck, B.; Schaap, N.P.M.; et al. Engineering of CD34+ Progenitor-Derived Natural Killer Cells with Higher-Affinity CD16a for Enhanced Antibody-Dependent Cellular Cytotoxicity. Cytotherapy 2024, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.A.; Levy, E.R.; Reger, R.; Barisic, S.; Chen, L.; Cherkasova, E.; Chakraborty, M.; Allan, D.S.J.; Childs, R. High-Affinity CD16 Integration into a CRISPR/Cas9-Edited CD38 Locus Augments CD38-Directed Antitumor Activity of Primary Human Natural Killer Cells. J. Immunother. Cancer 2022, 10, e003804. [Google Scholar] [CrossRef] [PubMed]
- Piedra-Quintero, Z.L.; Wilson, Z.; Nava, P.; Guerau-de-Arellano, M. CD38: An Immunomodulatory Molecule in Inflammation and Autoimmunity. Front. Immunol. 2020, 11, 597959. [Google Scholar] [CrossRef] [PubMed]
- Juckett, M.; Mailankody, S.; Eghtedar, A.; Bickers, C.; Zong, X.; Wong, L.; Ly, T.; Byon, J.; Cooley, S.; Valamehr, B.; et al. A Phase I Study of FT538, an Off-the-Shelf, Multiplexed-Engineered, IPSC-Derived NK Cell Therapy in Combination with Daratumumab in Relapsed/Refractory Multiple Myeloma. Blood 2022, 140, 10327–10328. [Google Scholar] [CrossRef]
- Maakaron, J.E.; Seichter, C.; Wangen, R.; Hoeschen, A.; Kolaseri Krishnadas, D.; Kent, K.; Shanley, R.; O’Leary, D.; El Jurdi, N.H.; Holtan, S.G.; et al. Phase I Study of FT538 + Daratumumab for Treatment of r/r AML. Blood 2023, 142, 4842. [Google Scholar] [CrossRef]
- Shin, H.G.; Yang, H.R.; Yoon, A.; Lee, S. Bispecific Antibody-Based Immune-Cell Engagers and Their Emerging Therapeutic Targets in Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 5686. [Google Scholar] [CrossRef]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting Natural Killer Cells to Acute Myeloid Leukemia in Vitro with a CD16×33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef]
- Sarhan, D.; Brandt, L.; Felices, M.; Guldevall, K.; Lenvik, T.; Hinderlie, P.; Curtsinger, J.; Warlick, E.; Spellman, S.R.; Blazar, B.R.; et al. 161533 TriKE Stimulates NK-Cell Function to Overcome Myeloid-Derived Suppressor Cells in MDS. Blood Adv. 2018, 2, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of Natural Killer Cells in Immunity to Cancer, and Applications to Immunotherapy. Nat. Rev. Immunol. 2022, 3, 90–105. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158. [Google Scholar] [CrossRef]
- Kerbauy, L.N.; Marin, N.D.; Kaplan, M.; Banerjee, P.P.; Berrien-Elliott, M.M.; Becker-Hapak, M.; Basar, R.; Foster, M.; Melo, L.G.; Neal, C.C.; et al. Combining AFM13, a Bispecific CD30/CD16 Antibody, with Cytokine-Activated Blood and Cord Blood-Derived NK Cells Facilitates CAR-like Responses Against CD30+ Malignancies. Clin. Cancer Res. 2021, 27, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Rothe, A.; Sasse, S.; Topp, M.S.; Eichenauer, D.A.; Hummel, H.; Reiners, K.S.; Dietlein, M.; Kuhnert, G.; Kessler, J.; Buerkle, C.; et al. A Phase 1 Study of the Bispecific Anti-CD30/CD16A Antibody Construct AFM13 in Patients with Relapsed or Refractory Hodgkin Lymphoma. Blood 2015, 125, 4024–4031. [Google Scholar] [CrossRef] [PubMed]
- Siemaszko, J.; Marzec-przyszlak, A.; Bogunia-kubik, K. Nkg2d Natural Killer Cell Receptor—A Short Description and Potential Clinical Applications. Cells 2021, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Ferrari de Andrade, L. NKG2D and MICA/B Shedding: A ‘Tag Game’ between NK Cells and Malignant Cells. Clin. Transl. Immunol. 2020, 9, e1230. [Google Scholar] [CrossRef]
- Afolabi, L.O.; Adeshakin, A.O.; Sani, M.M.; Bi, J.; Wan, X. Genetic Reprogramming for NK Cell Cancer Immunotherapy with CRISPR/Cas9. Immunology 2019, 158, 63–69. [Google Scholar] [CrossRef]
- Sekiba, K.; Yamagami, M.; Otsuka, M.; Suzuki, T.; Kishikawa, T.; Ishibashi, R.; Ohno, M.; Sato, M.; Koike, K. Transcriptional Activation of the MICA Gene with an Engineered CRISPR-Cas9 System. Biochem. Biophys. Res. Commun. 2017, 486, 521–525. [Google Scholar] [CrossRef]
- Tan, L.; Han, S.; Ding, S.; Xiao, W.; Ding, Y.; Qian, L.; Wang, C.; Gong, W. Chitosan Nanoparticle-Based Delivery of Fused NKG2D-IL-21 Gene Suppresses Colon Cancer Growth in Mice. Int. J. Nanomed. 2017, 12, 3095–3107. [Google Scholar] [CrossRef]
- Raza, A.; Rossi, G.R.; Janjua, T.I.; Souza-Fonseca-Guimaraes, F.; Popat, A. Nanobiomaterials to Modulate Natural Killer Cell Responses for Effective Cancer Immunotherapy. Trends Biotechnol. 2023, 41, 77–92. [Google Scholar] [CrossRef]
- Youness, R.A.; Rahmoon, M.A.; Assal, R.A.; Gomaa, A.I.; Hamza, M.T.; Waked, I.; El Tayebi, H.M.; Abdelaziz, A.I. Contradicting Interplay between Insulin-like Growth Factor-1 and MiR-486-5p in Primary NK Cells and Hepatoma Cell Lines with a Contemporary Inhibitory Impact on HCC Tumor Progression. Growth Factors 2016, 34, 128–140. [Google Scholar] [CrossRef]
- Sayitoglu, E.C.; Georgoudaki, A.M.; Chrobok, M.; Ozkazanc, D.; Josey, B.J.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.M.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Paczulla, A.M.; Rothfelder, K.; Raffel, S.; Konantz, M.; Steinbacher, J.; Wang, H.; Ndoh Mbarga, M.; Schaefer, T.; Dörfel, D.; Falcone, M.; et al. Absence of NKG2D Ligands Defines Human Acute Myeloid Leukaemia Stem Cells and Mediates Their Immune Evasion. Blood 2018, 132, 769. [Google Scholar] [CrossRef]
- Jelenčić, V.; Šestan, M.; Kavazović, I.; Lenartić, M.; Marinović, S.; Holmes, T.D.; Prchal-Murphy, M.; Lisnić, B.; Sexl, V.; Bryceson, Y.T.; et al. NK Cell Receptor NKG2D Sets Activation Threshold for the NCR1 Receptor Early in NK Cell Development. Nat. Immunol. 2018, 19, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Kang, S.; Youssef, Y.; Glankler, E.N.; Barrett, E.R.; Carter, A.M.; Ahmed, E.H.; Prasad, A.; Chen, L.; Zhang, J.; et al. A CS1-NKG2D Bispecific Antibody Collectivel Activates Cytolytic Immune Cells against Multiple Myeloma. Cancer Immunol. Res. 2018, 6, 776–787. [Google Scholar] [CrossRef]
- Demaria, O.; Gauthier, L.; Debroas, G.; Vivier, E. Natural Killer Cell Engagers in Cancer Immunotherapy: Next Generation of Immuno-Oncology Treatments. Eur. J. Immunol. 2021, 51, 1934–1942. [Google Scholar] [CrossRef]
- Raynaud, A.; Desrumeaux, K.; Vidard, L.; Termine, E.; Baty, D.; Chames, P.; Vigne, E.; Kerfelec, B. Anti-NKG2D Single Domain-Based Antibodies for the Modulation of Anti-Tumor Immune Response. Oncoimmunology 2021, 10, e1854529. [Google Scholar] [CrossRef] [PubMed]
- Gumá, M.; Budt, M.; Sáez, A.; Brckalo, T.; Hengel, H.; Angulo, A.; López-Botet, M. Expansion of CD94/NKG2C+ NK Cells in Response to Human Cytomegalovirus-Infected Fibroblasts. Blood 2006, 107, 3624–3631. [Google Scholar] [CrossRef]
- Vacca, P.; Pietra, G.; Tumino, N.; Munari, E.; Mingari, M.C.; Moretta, L. Exploiting Human NK Cells in Tumor Therapy. Front. Immunol. 2020, 10, 3013. [Google Scholar] [CrossRef]
- Capuano, C.; Pighi, C.; Battella, S.; De Federicis, D.; Galandrini, R.; Palmieri, G. Harnessing Cd16-Mediated Nk Cell Functions to Enhance Therapeutic Efficacy of Tumor-Targeting Mabs. Cancers 2021, 13, 2500. [Google Scholar] [CrossRef]
- Cichocki, F.; Cooley, S.; Davis, Z.; Defor, T.E.; Schlums, H.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J.; Verneris, R.; et al. CD56dimCD57+ NKG2C+ NK Cell Expansion Is Associated with Reduced Leukemia Relapse after Reduced Intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Murad, S.; Michen, S.; Becker, A.; Füssel, M.; Schackert, G.; Tonn, T.; Momburg, F.; Temme, A. NKG2C+ NK Cells for Immunotherapy of Glioblastoma Multiforme. Int. J. Mol. Sci. 2022, 23, 5857. [Google Scholar] [CrossRef]
- Chiu, E.; Felices, M.; Cichocki, F.; Davis, Z.; Wang, H.; Tuninga, K.; Vallera, D.A.; Lee, T.; Bjordahl, R.; Malmberg, K.J.; et al. Anti-NKG2C/IL-15/Anti-CD33 Killer Engager Directs Primary and IPSC-Derived NKG2C+ NK Cells to Target Myeloid Leukemia. Mol. Ther. 2021, 29, 3410–3421. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e16. [Google Scholar] [CrossRef] [PubMed]
- Arulanandam, A.; Lin, L.; Chang, H.M.; Cerutti, M.; Choblet, S.; Gao, P.; Rath, A.; Bensussan, A.; Kadouche, J.; Teper, D.; et al. Derivation and Preclinical Characterization of CYT-303, a Novel NKp46-NK Cell Engager Targeting GPC3. Cells 2023, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Barrow, A.D.; Edeling, M.A.; Trifonov, V.; Luo, J.; Goyal, P.; Bohl, B.; Bando, J.K.; Kim, A.H.; Walker, J.; Andahazy, M.; et al. Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172, 534–548.e19. [Google Scholar] [CrossRef]
- Iraqi, M.; Edri, A.; Greenshpan, Y.; Goldstein, O.; Ofir, N.; Bolel, P.; Ahmad, M.A.; Zektser, M.; Campbell, K.S.; Rouvio, O.; et al. Blocking the PCNA/NKp44 Checkpoint to Stimulate NK Cell Responses to Multiple Myeloma. Int. J. Mol. Sci. 2022, 23, 4717. [Google Scholar] [CrossRef]
- Klausz, K.; Pekar, L.; Boje, A.S.; Gehlert, C.L.; Krohn, S.; Gupta, T.; Xiao, Y.; Krah, S.; Zaynagetdinov, R.; Lipinski, B.; et al. Multifunctional NK Cell–Engaging Antibodies Targeting EGFR and NKp30 Elicit Efficient Tumor Cell Killing and Proinflammatory Cytokine Release. J. Immunol. 2022, 209, 1724–1735. [Google Scholar] [CrossRef]
- Rajalingam, R. The Impact of HLA Class I-Specific Killer Cell Immunoglobulin-like Receptors on Antibody-Dependent Natural Killer Cell-Mediated Cytotoxicity and Organ Allograft Rejection. Front. Immunol. 2016, 7, 585. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Dysfunction and Checkpoint Immunotherapy. Front. Immunol. 2019, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Arooj, S.; Wang, H. NK Cell-Based Immune Checkpoint Inhibition. Front. Immunol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gao, F.; Yan, M.; Zhao, S.; Yan, Z.; Shi, B.; Liu, Y. Natural Killer Cells: A Promising Immunotherapy for Cancer. J. Transl. Med. 2022, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M.; Bakan, C.E.; Zhang, S.; Collins, S.M.; Liang, J.; Srivastava, S.; Hofmeister, C.C.; Efebera, Y.; Andre, P.; Romagne, F.; et al. IPH2101, a Novel Anti-Inhibitory KIR Antibody, and Lenalidomide Combine to Enhance the Natural Killer Cell versus Multiple Myeloma Effect. Blood 2011, 118, 6387–6391. [Google Scholar] [CrossRef]
- Alfarra, H.; Weir, J.; Grieve, S.; Reiman, T. Targeting NK Cell Inhibitory Receptors for Precision Multiple Myeloma Immunotherapy. Front. Immunol. 2020, 11, 575609. [Google Scholar] [CrossRef]
- Navin, I.; Lam, M.T.; Parihar, R. Design and Implementation of NK Cell-Based Immunotherapy to Overcome the Solid Tumor Microenvironment. Cancers 2020, 12, 3871. [Google Scholar] [CrossRef]
- Debska-Zielkowska, J.; Moszkowska, G.; Zielinski, M.; Zielinska, H.; Dukat-Mazurek, A.; Piotr, T.; Stefanska, K. KIR Receptors as Key Regulators of NK Cells Activity in Health and Disease. Cells 2021, 10, 1777. [Google Scholar] [CrossRef]
- Wei, Y.; Ren, X.; Galbo, P.M.; Moerdler, S.; Wang, H.; Alejandro Sica, R.; Etemad-Gilbertson, B.; Shi, L.; Zhu, L.; Tang, X.; et al. KIR3DL3-HHLA2 Is a Human Immunosuppressive Pathway and a Therapeutic Target. Sci. Immunol. 2021, 6, 9792. [Google Scholar] [CrossRef]
- Forslund, E.; Sohlberg, E.; Enqvist, M.; Olofsson, P.E.; Malmberg, K.-J.; Önfelt, B. Microchip-Based Single-Cell Imaging Reveals That CD56dimCD57-KIR-NKG2A+ NK Cells Have More Dynamic Migration Associated with Increased Target Cell Conjugation and Probability of Killing Compared to CD56dimCD57-KIR-NKG2A- NK Cells. J. Immunol. 2015, 195, 3374–3381. [Google Scholar] [CrossRef]
- Figueiredo, C.; Seltsam, A.; Blasczyk, R. Permanent Silencing of NKG2A Expression for Cell-Based Therapeutics. J. Mol. Med. 2009, 87, 199–210. [Google Scholar] [CrossRef]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking Expression of Inhibitory Receptor NKG2A Overcomes Tumor Resistance to NK Cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef]
- Bexte, T.; Alzubi, J.; Reindl, L.M.; Wendel, P.; Schubert, R.; Salzmann-Manrique, E.; von Metzler, I.; Cathomen, T.; Ullrich, E. CRISPR-Cas9 Based Gene Editing of the Immune Checkpoint NKG2A Enhances NK Cell Mediated Cytotoxicity against Multiple Myeloma. Oncoimmunology 2022, 11, e2081415. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, A.; Guipouy, D.; Lemieux, W.; Harvey, M.; Bordeleau, L.J.; Guay, D.; Roméro, H.; Li, Y.; Dion, R.; Béland, K.; et al. KLRC1 Knockout Overcomes HLA-E-Mediated Inhibition and Improves NK Cell Antitumor Activity against Solid Tumors. Front. Immunol. 2023, 14, 1231916. [Google Scholar] [CrossRef]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A MAb Is a Checkpoint Inhibitor That Promotes Anti-Tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, E.M.; Mele, J.M.; Cheney, C.; Timmerman, E.A.; Fiazuddin, F.; Strattan, E.J.; Mo, X.; Byrd, J.C.; Muthusamy, N.; Awan, F.T. Therapeutic CD94/NKG2A Blockade Improves Natural Killer Cell Dysfunction in Chronic Lymphocytic Leukemia. Oncoimmunology 2016, 5, e1226720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Blake, S.J.; Dougall, W.C.; Miles, J.J.; Teng, M.W.L.; Smyth, M.J. Molecular Pathways: Targeting CD96 and TIGIT for Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 5183–5188. [Google Scholar] [CrossRef]
- Yeo, J.; Ko, M.; Lee, D.H.; Park, Y.; Jin, H.S. Tigit/Cd226 Axis Regulates Anti-Tumor Immunity. Pharmaceuticals 2021, 14, 200. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-Inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef]
- Rousseau, A.; Parisi, C.; Barlesi, F. Anti-TIGIT Therapies for Solid Tumors: A Systematic Review. ESMO Open 2023, 8, 101184. [Google Scholar] [CrossRef]
- Jin, H.; Park, Y. Hitting the Complexity of the TIGIT-CD96-CD112R-CD226 Axis for next-Generation Cancer Immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef]
- Chiang, E.Y.; Mellman, I. TIGIT-CD226-PVR Axis: Advancing Immune Checkpoint Blockade for Cancer Immunotherapy. J. Immunother. Cancer 2022, 10, e004711. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Ka, M.; Pagliano, O.; Menna, C.; Ding, Q.; DeBlasio, R.; Sanders, C.; Hou, J.; Li, X.Y.; Ferrone, S.; et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-Mediated NK-Cell Dysfunction in Melanoma. Clin. Cancer Res. 2020, 26, 5520–5533. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Peppelenbosch, M.P.; Sprengers, D.; Kwekkeboom, J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021, 12, 699895. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.F.; Campbell, A.R.; Croom-Perez, T.J.; Oyer, J.L.; Dieffenthaller, T.A.; Robles-Carrillo, L.D.; Cash, C.A.; Eloriaga, J.E.; Kumar, S.; Andersen, B.W.; et al. Knockout of the Inhibitory Receptor TIGIT Enhances the Antitumor Response of Ex Vivo Expanded NK Cells and Prevents Fratricide with Therapeutic Fc-Active TIGIT Antibodies. J. Immunother. Cancer 2023, 11, e007502. [Google Scholar] [CrossRef] [PubMed]
- Lupo, K.B.; Yao, X.; Borde, S.; Wang, J.; Torregrosa-Allen, S.; Elzey, B.D.; Utturkar, S.; Lanman, N.A.; McIntosh, M.; Matosevic, S. SynNotch-Programmed IPSC-Derived NK Cells Usurp TIGIT and CD73 Activities for Glioblastoma Therapy. Nat. Commun. 2024, 15, 1909. [Google Scholar] [CrossRef]
- Blake, S.J.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.C.R.; Mittal, D.; Aguilera, A.R.; Miles, J.J.; Lutzky, V.P.; de Andrade, L.F.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Liu, F.; Liu, Z.; Chen, F. CD96 Correlates with Immune Infiltration and Impacts Patient Prognosis: A Pan-Cancer Analysis. Front. Oncol. 2021, 11, 634617. [Google Scholar] [CrossRef]
- Zeng, T.; Cao, Y.; Jin, T.; Tian, Y.; Dai, C.; Xu, F. The CD112R/CD112 Axis: A Breakthrough in Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 285. [Google Scholar] [CrossRef]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.D.; Edil, B.H.; Zhu, Y. Blockade of CD112R and TIGIT Signaling Sensitizes Human Natural Killer Cell Functions. Cancer Immunol. Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Cao, G.; Zheng, X.; Sun, C.; Wei, H.; Tian, Z.; Xiao, W.; Sun, R.; Sun, H. Blockade of Checkpoint Receptor PVRIG Unleashes Anti-Tumor Immunity of NK Cells in Murine and Human Solid Tumors. J. Hematol. Oncol. 2021, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhong, C.; Lang, Q.; Liang, Z.; Zhang, Y.; Zhao, X.; Yu, Y.; Zhang, H.; Xu, F.; Tian, Y. Poliovirus Receptor (PVR)-like Protein Cosignaling Network: New Opportunities for Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 267. [Google Scholar] [CrossRef]
- Laurent, S.; Queirolo, P.; Boero, S.; Salvi, S.; Piccioli, P.; Boccardo, S.; Minghelli, S.; Morabito, A.; Fontana, V.; Pietra, G.; et al. The Engagement of CTLA-4 on Primary Melanoma Cell Lines Induces Antibody-Dependent Cellular Cytotoxicity and TNF-α Production. J. Transl. Med. 2013, 11, 108. [Google Scholar] [CrossRef]
- Gemelli, M.; Noonan, D.M.; Carlini, V.; Pelosi, G.; Barberis, M.; Ricotta, R.; Albini, A. Overcoming Resistance to Checkpoint Inhibitors: Natural Killer Cells in Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 886440. [Google Scholar] [CrossRef] [PubMed]
- Hasim, M.S.; Marotel, M.; Hodgins, J.J.; Vulpis, E.; Makinson, O.J.; Asif, S.; Shih, H.Y.; Scheer, A.K.; MacMillan, O.; Alonso, F.G.; et al. When Killers Become Thieves: Trogocytosed PD-1 Inhibits NK Cells in Cancer. Sci. Adv. 2022, 8, eabj3286. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK Cells to Immunotherapy Mediated by PD-1/PD-L1 Blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Makowska, A.; Braunschweig, T.; Denecke, B.; Shen, L.; Baloche, V.; Busson, P.; Kontny, U. Interferon β and Anti-PD-1/PD-L1 Checkpoint Blockade Cooperate in NK Cell-Mediated Killing of Nasopharyngeal Carcinoma Cells. Transl. Oncol. 2019, 12, 1237–1256. [Google Scholar] [CrossRef]
- Makowska, A.; Meier, S.; Shen, L.; Busson, P.; Baloche, V.; Kontny, U. Anti-PD-1 Antibody Increases NK Cell Cytotoxicity towards Nasopharyngeal Carcinoma Cells in the Context of Chemotherapy-Induced Upregulation of PD-1 and PD-L1. Cancer Immunol. Immunother. 2021, 70, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Chen, N.; Chen, X.; Niu, C.; Liu, Z.; Ma, K.; Wang, N.; Yang, L.; Zhao, Y.; Song, W.; et al. Sintilimab plus Autologous NK Cells as Second-Line Treatment for Advanced Non-Small-Cell Lung Cancer Previous Treated with Platinum-Containing Chemotherapy. Front. Immunol. 2022, 13, 1074906. [Google Scholar] [CrossRef]
- Armand, P.; Lesokhin, A.; Borrello, I.; Timmerman, J.; Gutierrez, M.; Zhu, L.; Popa McKiver, M.; Ansell, S.M. A Phase 1b Study of Dual PD-1 and CTLA-4 or KIR Blockade in Patients with Relapsed/Refractory Lymphoid Malignancies. Leukemia 2021, 35, 777–786. [Google Scholar] [CrossRef]
- Ohs, I.; Ducimetière, L.; Marinho, J.; Kulig, P.; Becher, B.; Tugues, S. Restoration of Natural Killer Cell Antimetastatic Activity by IL12 and Checkpoint Blockade. Cancer Res. 2017, 77, 7059–7071. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Nakazawa, T.; Matsuda, R.; Nishimura, F.; Nakamura, M.; Yamada, S.; Nakagawa, I.; Park, Y.S.; Tsujimura, T.; Nakase, H. CRISPR-Cas9–Mediated TIM3 Knockout in Human Natural Killer Cells Enhances Growth Inhibitory Effects on Human Glioma Cells. Int. J. Mol. Sci. 2021, 22, 3489. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, Y.; Tan, L.; Yu, W.; Chen, D.; Lu, C.; He, J.; Wu, G.; Liu, X.; Zhang, Y. Increased Tim-3 Expression in Peripheral NK Cells Predicts a Poorer Prognosis and Tim-3 Blockade Improves NK Cell-Mediated Cytotoxicity in Human Lung Adenocarcinoma. Int. Immunopharmacol. 2015, 29, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Susek, K.H.; Karvouni, M.; Alici, E.; Lundqvist, A. The Role of CXC Chemokine Receptors 1–4 on Immune Cells in the Tumor Microenvironment. Front. Immunol. 2018, 9, 2159. [Google Scholar] [CrossRef]
- Müller, N.; Michen, S.; Tietze, S.; Töpfer, K.; Schulte, A.; Lamszus, K.; Schmitz, M.; Schackert, G.; Pastan, I.; Temme, A. Engineering NK Cells Modified with an EGFRvIII-Specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1α-Secreting Glioblastoma. J. Immunother. 2015, 38, 197–210. [Google Scholar] [CrossRef]
- Jamali, A.; Hadjati, J.; Madjd, Z.; Mirzaei, H.R.; Thalheimer, F.B.; Agarwal, S.; Bonig, H.; Ullrich, E.; Hartmann, J. Highly Efficient Generation of Transgenically Augmented CAR NK Cells Overexpressing CXCR4. Front. Immunol. 2020, 11, 2028. [Google Scholar] [CrossRef]
- Levy, E.R.; Carlsten, M.; Childs, R.W. MRNA Transfection to Improve NK Cell Homing to Tumors. Methods Mol. Biol. 2016, 1441, 231–240. [Google Scholar] [CrossRef]
- Somanchi, S.S.; Somanchi, A.; Cooper, L.J.N.; Lee, D.A. Engineering Lymph Node Homing of Ex Vivo-Expanded Human Natural Killer Cells via Trogocytosis of the Chemokine Receptor CCR7. Blood 2012, 119, 5164–5172. [Google Scholar] [CrossRef]
- Carlsten, M.; Levy, E.; Karambelkar, A.; Li, L.; Reger, R.; Berg, M.; Peshwa, M.V.; Childs, R.W. Efficient MRNA-Based Genetic Engineering of Human NK Cells with High-Affinity CD16 and CCR7 Augments Rituximab-Induced ADCC against Lymphoma and Targets NK Cell Migration toward the Lymph Node-Associated Chemokine CCL19. Front. Immunol. 2016, 7, 105. [Google Scholar] [CrossRef]
- Bonanni, V.; Antonangeli, F.; Santoni, A.; Bernardini, G. Targeting of CXCR3 Improves Anti-Myeloma Efficacy of Adoptively Transferred Activated Natural Killer Cells. J. Immunother. Cancer 2019, 7, 290. [Google Scholar] [CrossRef]
- Wennerberg, E.; Kremer, V.; Childs, R.; Lundqvist, A. CXCL10-Induced Migration of Adoptively Transferred Human Natural Killer Cells toward Solid Tumors Causes Regression of Tumor Growth in Vivo. Cancer Immunol. Immunother. 2015, 64, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kremer, V.; Ligtenberg, M.; Zendehdel, R.; Seitz, C.; Duivenvoorden, A.; Wennerberg, E.; Colón, E.; Scherman-Plogell, A.H.; Lundqvist, A. Genetic Engineering of Human NK Cells to Express CXCR2 Improves Migration to Renal Cell Carcinoma. J. Immunother. Cancer 2017, 5, 73. [Google Scholar] [CrossRef]
- Groth, C.; Arpinati, L.; Shaul, M.E.; Winkler, N.; Diester, K.; Gengenbacher, N.; Weber, R.; Arkhypov, I.; Lasser, S.; Petrova, V.; et al. Blocking Migration of Polymorphonuclear Myeloid-Derived Suppressor Cells Inhibits Mouse Melanoma Progression. Cancers 2021, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yang, L.; Zhang, S.; Ge, Z.; Su, M.; Shi, Y.; Wang, X.; Huang, C. Engineering NK-92 Cell by Upregulating CXCR2 and IL-2 Via CRISPR-Cas9 Improves Its Antitumor Effects as Cellular Immunotherapy for Human Colon Cancer. J. Interferon Cytokine Res. 2021, 41, 450–460. [Google Scholar] [CrossRef]
- Ng, Y.Y.; Tay, J.C.K.; Wang, S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther.—Oncolytics 2020, 16, 75–85. [Google Scholar] [CrossRef]
- Mylod, E.; Melo, A.M.; Donlon, N.E.; Davern, M.; Bhardwaj, A.; Reynolds, J.V.; Lysaght, J.; Conroy, M.J. Fractalkine Elicits Chemotactic, Phenotypic, and Functional Effects on CX3CR1+CD27− NK Cells in Obesity-Associated Cancer. J. Immunol. 2021, 207, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.V.; Alici, E.; Aints, A.; Christensson, B.; Ljunggren, H.G.; Dilber, M.S. Targeting IL-2 to the Endoplasmic Reticulum Confines Autocrine Growth Stimulation to NK-92 Cells. Exp. Hematol. 2005, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Matosevic, S. Viral and Nonviral Engineering of Natural Killer Cells as Emerging Adoptive Cancer Immunotherapies. J. Immunol. Res. 2018, 2018, 4054815. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord Blood NK Cells Engineered to Express IL-15 and a CD19-Targeted CAR Show Long-Term Persistence and Potent Antitumor Activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef]
- Delconte, R.B.; Kolesnik, T.B.; Dagley, L.F.; Rautela, J.; Shi, W.; Putz, E.M.; Stannard, K.; Zhang, J.G.; Teh, C.; Firth, M.; et al. CIS Is a Potent Checkpoint in NK Cell-Mediated Tumor Immunity. Nat. Immunol. 2016, 17, 816–824. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Zhou, K.; Brown, J.; Tsao, T.; Cen, X.; Husman, T.; Bajpai, A.; Dunn, Z.S.; Yang, L. Engineering-Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers 2022, 14, 2266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Blum, R.H.; Bernareggi, D.; Ask, E.H.; Wu, Z.; Hoel, H.J.; Meng, Z.; Wu, C.; Guan, K.L.; Malmberg, K.J.; et al. Metabolic Reprograming via Deletion of CISH in Human IPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-Tumor Activity. Cell Stem Cell 2020, 27, 224–237.e6. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z. Tissue Factor as a New Target for CAR-NK Cell Immunotherapy of Triple-Negative Breast Cancer. Sci. Rep. 2020, 10, 2815. [Google Scholar] [CrossRef]
- Parihar, R.; Rivas, C.; Huynh, M.; Omer, B.; Lapteva, N.; Metelitsa, L.S.; Gottschalk, S.M.; Rooney, C.M. NK Cells Expressing a Chimeric Activating Receptor Eliminate MDSCs and Rescue Impaired CAR-T Cell Activity against Solid Tumors. Cancer Immunol. Res. 2019, 7, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Uprety, N.; Fathi, M.; Rezvan, A.; et al. KIR-Based Inhibitory CARs Overcome CAR-NK Cell Trogocytosis-Mediated Fratricide and Tumor Escape. Nat. Med. 2022, 28, 2133–2144. [Google Scholar] [CrossRef]
- Albinger, N.; Bexte, T.; Buchinger, L.; Wendel, P.; Al-Ajami, A.; Gessner, A.; Särchen, V.; Alzubi, J.; Mertlitz, S.; Penack, O.; et al. CRISPR/Cas9 Gene Editing of Immune Checkpoint Receptor NKG2A Improves the Efficacy of Primary CD33-CAR-NK Cells Against AML. Blood 2022, 140, 4558–4559. [Google Scholar] [CrossRef]
- Pan, K.; Farrukh, H.; Chittepu, V.C.S.R.; Xu, H.; Pan, C.; Zhu, Z. CAR Race to Cancer Immunotherapy: From CAR T, CAR NK to CAR Macrophage Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 119. [Google Scholar] [CrossRef]
- Li, H.; Song, W.; Li, Z.; Zhang, M. Preclinical and Clinical Studies of CAR-NK-Cell Therapies for Malignancies. Front. Immunol. 2022, 13, 992232. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Feng, X.; Han, Z. CAR-NK Cells for Cancer Immunotherapy: From Bench to Bedside. Biomark. Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.J.; Wang, J.; Bos, G.M.J.; Germeraad, W.T.V. Chimeric Antigen Receptor Natural Killer (CAR-NK) Cell Design and Engineering for Cancer Therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef]
- Marin, D.; Li, Y.; Basar, R.; Rafei, H.; Daher, M.; Dou, J.; Mohanty, V.; Dede, M.; Nieto, Y.; Uprety, N.; et al. Safety, Efficacy and Determinants of Response of Allogeneic CD19-Specific CAR-NK Cells in CD19+ B Cell Tumors: A Phase 1/2 Trial. Nat. Med. 2024, 30, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, J.; Wu, C. CAR-NK for Tumor Immunotherapy: Clinical Transformation and Future Prospects. Cancer Lett. 2020, 472, 175–180. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.; Li, Z.; Hu, Y.; Wang, H. From CAR-T Cells to CAR-NK Cells: A Developing Immunotherapy Method for Hematological Malignancies. Front. Oncol. 2021, 11, 720501. [Google Scholar] [CrossRef]
- Chan, L.Y.; Dass, S.A.; Tye, G.J.; Imran, S.A.M.; Wan Kamarul Zaman, W.S.; Nordin, F. CAR-T Cells/-NK Cells in Cancer Immunotherapy and the Potential of MSC to Enhance Its Efficacy: A Review. Biomedicines 2022, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, H.; Lian, K.; Zhang, D.; Hu, P.; He, Z.; Zhang, Z.; Wang, Y. CAR-NK Cells in Combination Therapy against Cancer: A Potential Paradigm. Heliyon 2024, 10, e27196. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.H.; Walter, R.B. Chimeric Antigen Receptor (CAR)-Modified Immune Effector Cell Therapy for Acute Myeloid Leukemia (AML). Cancers 2020, 12, 3617. [Google Scholar] [CrossRef] [PubMed]
- Khawar, M.B.; Sun, H. CAR-NK Cells: From Natural Basis to Design for Kill. Front. Immunol. 2021, 12, 707542. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK Cells: A Promising Cellular Immunotherapy for Cancer. EBioMedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Lu, T.; Ma, R.; Dong, W.; Teng, K.Y.; Kollath, D.S.; Li, Z.; Yi, J.; Bustillos, C.; Ma, S.; Tian, L.; et al. Off-the-Shelf CAR Natural Killer Cells Secreting IL-15 Target Spike in Treating COVID-19. Nat. Commun. 2022, 13, 2576. [Google Scholar] [CrossRef]
- Li, L.; Mohanty, V.; Dou, J.; Huang, Y.; Banerjee, P.P.; Miao, Q.; Lohr, J.G.; Vijaykumar, T.; Frede, J.; Knoechel, B.; et al. Loss of Metabolic Fitness Drives Tumor Resistance after CAR-NK Cell Therapy and Can Be Overcome by Cytokine Engineering. Sci. Adv. 2023, 9, eadd6997. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, R.N.; Eitler, J.; de Azevedo, J.T.C.; Tirapelle, M.C.; Fantacini, D.M.C.; de Souza, L.E.B.; Swiech, K.; Covas, D.T.; Calado, R.T.; Montero, P.O.; et al. Engineering NK-CAR.19 Cells with the IL-15/IL-15Rα Complex Improved Proliferation and Anti-Tumor Effect in Vivo. Front. Immunol. 2023, 14, 1226518. [Google Scholar] [CrossRef]
- Frazao, A.; Rethacker, L.; Messaoudene, M.; Avril, M.F.; Toubert, A.; Dulphy, N.; Caignard, A. NKG2D/NKG2-Ligand Pathway Offers New Opportunities in Cancer Treatment. Front. Immunol. 2019, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Wrona, E.; Borowiec, M.; Potemski, P. CAR-NK Cells in the Treatment of Solid Tumors. Int. J. Mol. Sci. 2021, 22, 5899. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Chuvin, N.; Valladeau-Guilemond, J.; Depil, S. Development of NK Cell-Based Cancer Immunotherapies through Receptor Engineering. Cell. Mol. Immunol. 2024, 21, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.R.; Felices, M.; Miller, J.S. Challenges to the Broad Application of Allogeneic Natural Killer Cell Immunotherapy of Cancer. Stem Cell Res. Ther. 2022, 13, 165. [Google Scholar] [CrossRef]
- Heuser, M.; Tschan-Plessl, A.; Thol, F.; Schwarzer, A.; Kloos, A.; Kattre, N.; Passweg, J.; Pinkernell, K.; Ganser, A. Allogeneic, CD34 +, Umbilical Cordblood-Derived NK Cell Adoptive Immunotherapy for the Treatment of Acute Myeloid Leukemia Patients with Measurable Residual Disease. Blood 2021, 138, 1745–1746. [Google Scholar] [CrossRef]
- Choi, I.; Yoon, S.R.; Park, S.Y.; Kim, H.; Jung, S.J.; Jang, Y.J.; Kang, M.; Yeom, Y.; Lee, J.L.; Kim, D.Y.; et al. Donor-Derived Natural Killer Cells Infused after Human Leukocyte Antigen-Haploidentical Hematopoietic Cell Transplantation: A Dose-Escalation Study. Biol. Blood Marrow Transplant. 2014, 20, 696–704. [Google Scholar] [CrossRef]
- Bachanova, V.; Maakaron, J.E.; Cichocki, F.; McKenna, D.H.; Cao, Q.; DeFor, T.E.; Janakiram, M.; Wangen, R.; Cayci, Z.; Grzywacz, B.; et al. Gda-201, a Novel Metabolically Enhanced Allogeneic Natural Killer (NK) Cell Product Yields High Remission Rates in Patients with Relapsed/Refractory Non-Hodgkin Lymphoma (NHL): 2-Year Survival and Correlation with Cytokine IL7. Blood 2021, 138, 3854. [Google Scholar] [CrossRef]
- Lian, G.Y.; Mak, T.S.K.; Yu, X.Q.; Lan, H.Y. Challenges and Recent Advances in NK Cell-Targeted Immunotherapies in Solid Tumors. Int. J. Mol. Sci. 2022, 23, 164. [Google Scholar] [CrossRef]
- Tong, L.; Jiménez-Cortegana, C.; Tay, A.H.M.; Wickström, S.; Galluzzi, L.; Lundqvist, A. NK Cells and Solid Tumors: Therapeutic Potential and Persisting Obstacles. Mol. Cancer 2022, 21, 206. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.V.; Kim, H.; Bjordahl, R.; Davis, Z.B.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H.; et al. Harnessing Features of Adaptive NK Cells to Generate IPSC-Derived NK Cells for Enhanced Immunotherapy. Cell Stem Cell 2021, 28, 2062–2075.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Ji, Y.; Gao, Y.; Zhang, M.; Liu, Y.; Zhu, W.; Lu, P. Cytokine Release Syndrome after Modified CAR-NK Therapy in an Advanced Non-Small Cell Lung Cancer Patient: A Case Report. Cell Transplant. 2022, 31, 9636897221094244. [Google Scholar] [CrossRef] [PubMed]
- Delehedde, C.; Ciganek, I.; Bernard, P.L.; Laroui, N.; Da Silva, C.C.; Gonçalves, C.; Nunes, J.; Bennaceur-Griscelli, A.L.; Imeri, J.; Huyghe, M.; et al. Enhancing Natural Killer Cells Proliferation and Cytotoxicity Using Imidazole-Based Lipid Nanoparticles Encapsulating Interleukin-2 MRNA. Mol. Ther.—Nucleic Acids 2024, 35, 102263. [Google Scholar] [CrossRef]
- Wang, L.; Wu, L.; Zhu, Z.; Zhang, Q.; Li, W.; Gonzalez, G.M.; Wang, Y.; Rana, T.M. Role of PCIF1-mediated 5′-cap N6-methyladeonsine MRNA Methylation in Colorectal Cancer and Anti-PD-1 Immunotherapy. EMBO J. 2023, 42, e111673. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Li, J.; Su, F.; Kuo, J.C.T.; Hu, Y.; Zhao, X.; Lee, R.J. Antitumor Activity of Anti-MiR-21 Delivered through Lipid Nanoparticles. Adv. Healthc. Mater. 2023, 12, 2202412. [Google Scholar] [CrossRef]
- Huang, C.; Duan, X.; Wang, J.; Tian, Q.; Ren, Y.; Chen, K.; Zhang, Z.; Li, Y.; Feng, Y.; Zhong, K.; et al. Lipid Nanoparticle Delivery System for MRNA Encoding B7H3-Redirected Bispecific Antibody Displays Potent Antitumor Effects on Malignant Tumors. Adv. Sci. 2023, 10, 2205532. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, F.; Yin, Y.; Zhang, Q.; Jin, H.; Wu, Y.; Pang, L.; Li, J.; Gao, J. Immunotherapy of Tumor RNA-Loaded Lipid Nanoparticles against Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 1553–1564. [Google Scholar] [CrossRef]

| Receptor/Molecule | Ligand/Mode of Action | References |
|---|---|---|
| Activating Receptors | ||
| CD16 (FcγRIII) | IgG-ADCC | [25] |
| NKG2D | MHC-I, MICA, MICB, ULBPs | [16,37] |
| NKG2C | HLA-E | [38] |
| NKp46 (NCR1, CD335) | Viral hemagglutinins | [39] |
| NKp44 (NCR2, CD336) | Viral hemagglutinins, Nidogen-1, PCNA, 21spe MML5 | [39,40,41,42,43] |
| NKp30 (NCR3, CD337) | B7–H6, BAT3, pp65 | [39,44] |
| Inhibiting Receptors | ||
| KIR family | HLA-A,B,C | [11,24] |
| NKG2A/CD94 | HLA-E | [45] |
| TIGIT | PVR (CD155), Nectin-2 (CD112) | [13,39,46] |
| TIM3 | Galectin-9 | [27,47,48] |
| PD-1 | PD–L1, PD–L2 | [28,39] |
| CTLA-4 | B7-1 (CD80), B7-2 (CD86) | [49] |
| CD96 | PVR (CD155) | [39,50] |
| Chemotactic Receptors | ||
| CXCR1 | CXCL6, CXCL8 | [51] |
| CXCR2 | CXCL1–CXCL7 | [6,51] |
| CXCR3 | CXCL9, CXCL10, CXCL11 | [23,52] |
| CXCR4 | CXCL12 (or SDF-1a) | [53,54] |
| CCR7 | CCL19, CCL21 | [55] |
| CX3CR1 | CX3CL1 | [56] |
| NK Cell Receptor | Product Name | Malignancy | NK Cell Source | Sponsor | Location | Status | Clinical Phase | Year | Key Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| NKG2DL | NKG2D-CAR–NK92 cells | Relapsed/Refractory Solid Tumors | NK-92 | Xinxiang medical university | Xinxiang CHI | Recruiting | I | 2023 | - | NCT05528341 |
| NKG2DL | NKG2D CAR–NK Cell Therapy | Relapsed or Refractory Acute Myeloid Leukemia | Intravenous infusion | Hangzhou Cheetah Cell Therapeutics Co., Ltd. | Sanhe CHI | Terminated | Unknown | 2021 | - | NCT05247957 |
| NKG2DL | CAR–NK cells targeting NKG2D ligands | Metastatic Solid Tumors | PBMCs | The Third Affiliated Hospital of Guangzhou Medical University | Guangzhou CHI | Unknown | I | 2018 | In total, 2 out of 3 patients showed reduced ascites and fewer tumor cells. 1 out of 3 patients experienced rapid tumor regression and complete metabolic liver response. | NCT03415100 |
| NKG2DL | NKG2D CAR–NK | Refractory Metastatic Colorectal Cancer | - | Zhejiang University | Hangzhou CHI | Recruiting | I | 2021 | - | NCT05213195 |
| NKG2DL | NKX101 | Relapsed/Refractory AML and MDS | PB-NK | Nkarta Inc. | Denver CO, Jacksonville FL, Atlanta GA USA | Active, not recruiting | I | 2020 | Initial Outcomes: NKX101 shows encouraging early responses in relapsed/refractory AML, even in high-risk cases. The toxicity profile matches expectations, with no CRS, ICANS, or treatment-related deaths. | NCT04623944 |
| NKG2DL | NKG2D CAR–NK | AML | Unknown | Zhejiang University | Hangzhou CHI | Recruiting | Unknown | 2023 | - | NCT05734898 |
| NKG2DL | NKG2D CAR–NK | Ovarian Cancer | Unknown | Hangzhou Cheetah Cell Therapeutics Co., Ltd. | Hangzhou CHI | Recruiting | NA | 2023 | - | NCT05776355 |
| NKG2DL and PD-L1 | PD-1/NKG2D CAR–NK | Non-small Cell Lung Carcinoma | NK-92 | Xinxiang medical university | Xinxiang CHI | Completed | I | 2018 | A previously unreported instance of cytokine release syndrome (CRS) occurred, marking the first known case during CAR–NK therapy | NCT03656705 |
| PD-L1 | PD-L1 CAR–NK, Pembrolizumab, N-803 | Gastroesophageal Junction (GEJ) Cancers, Advanced HNSCC | t-haNK | National Cancer Institute (NCI) | Bethesda MD USA | Recruiting | II | 2021 | - | NCT04847466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douka, S.; Papamoschou, V.; Raimo, M.; Mastrobattista, E.; Caiazzo, M. Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy. Pharmaceutics 2024, 16, 1143. https://doi.org/10.3390/pharmaceutics16091143
Douka S, Papamoschou V, Raimo M, Mastrobattista E, Caiazzo M. Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy. Pharmaceutics. 2024; 16(9):1143. https://doi.org/10.3390/pharmaceutics16091143
Chicago/Turabian StyleDouka, Stefania, Vasilis Papamoschou, Monica Raimo, Enrico Mastrobattista, and Massimiliano Caiazzo. 2024. "Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy" Pharmaceutics 16, no. 9: 1143. https://doi.org/10.3390/pharmaceutics16091143
APA StyleDouka, S., Papamoschou, V., Raimo, M., Mastrobattista, E., & Caiazzo, M. (2024). Harnessing the Power of NK Cell Receptor Engineering as a New Prospect in Cancer Immunotherapy. Pharmaceutics, 16(9), 1143. https://doi.org/10.3390/pharmaceutics16091143







