Microporation-Mediated Transdermal Delivery of In Situ Gel Incorporating Etodolac-Loaded PLGA Nanoparticles for Management of Rheumatoid Arthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
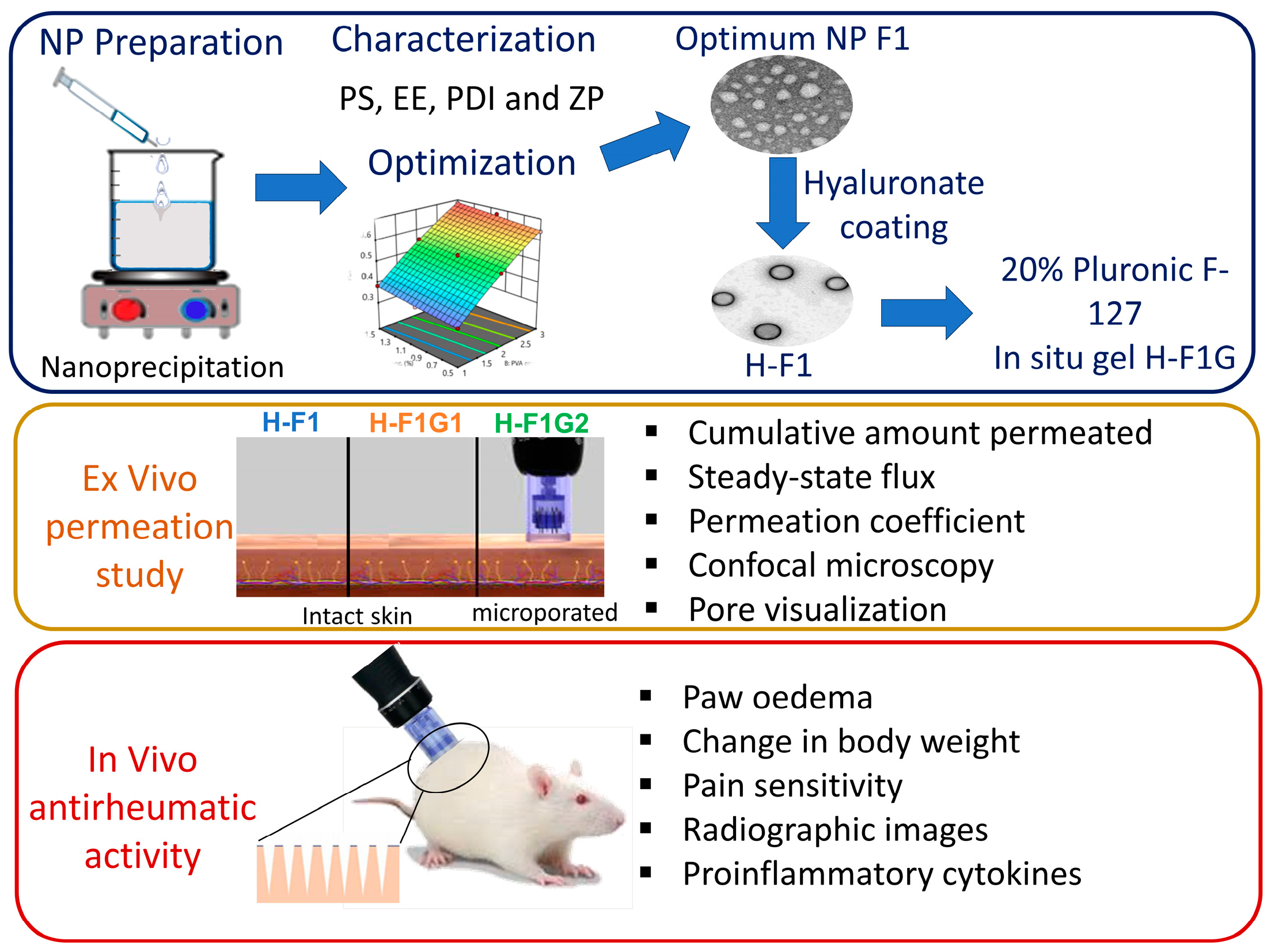
2.3. Preparation of ETD-Loaded PLGA NPs
2.4. Experimental Design
2.5. Characterization of ETD-Loaded Polymeric NPs
2.5.1. Determination of EE
2.5.2. Determination of PS, PDI, and Zeta Potential (ZP)
2.6. Optimization of ETD-Loaded NPs
2.7. Determination of Drug Loading Capacity (DL) of the Optimum Formulation F1
2.8. Preparation of Hyaluronate-Coated ETD-Loaded NPs
2.9. Transmission Electron Microscopy (TEM)
2.10. Preparation of ETD-Loaded NP In Situ Hydrogel
2.11. Evaluation of ETD-Loaded NP In Situ Hydrogel
2.11.1. Evaluation of Gelation Time
2.11.2. Evaluation of the Lowest Gelation Temperature
2.11.3. Determination of Drug Loading
2.12. Ex Vivo Skin Permeation Study
2.12.1. Skin Preparation
2.12.2. Induction of Microporation
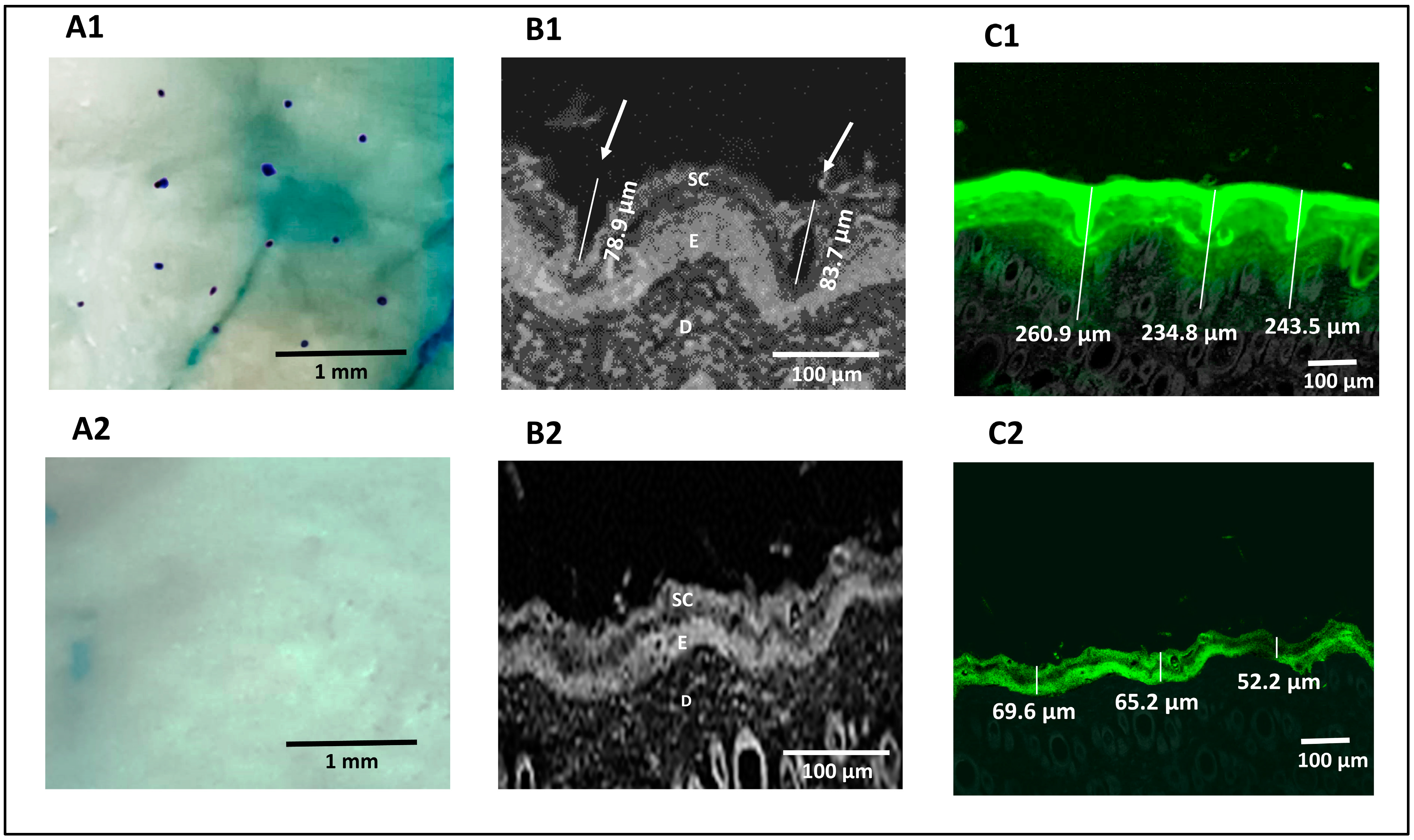
2.12.3. Visualization of Pores
2.12.4. Confocal Microscopy
2.12.5. Ex Vivo ETD Skin Permeation
2.13. In Vivo Antirheumatic Activity
2.13.1. Induction of Arthritis and Experimental Design
2.13.2. Measuring Paw Oedema
2.13.3. Measuring the Change in Body Weight
2.13.4. Behavioral Assessment of Pain Sensitivity
2.13.5. Assessment of Radiographic Images
2.13.6. Assessment of Pro-Inflammatory Cytokines
3. Results and Discussion
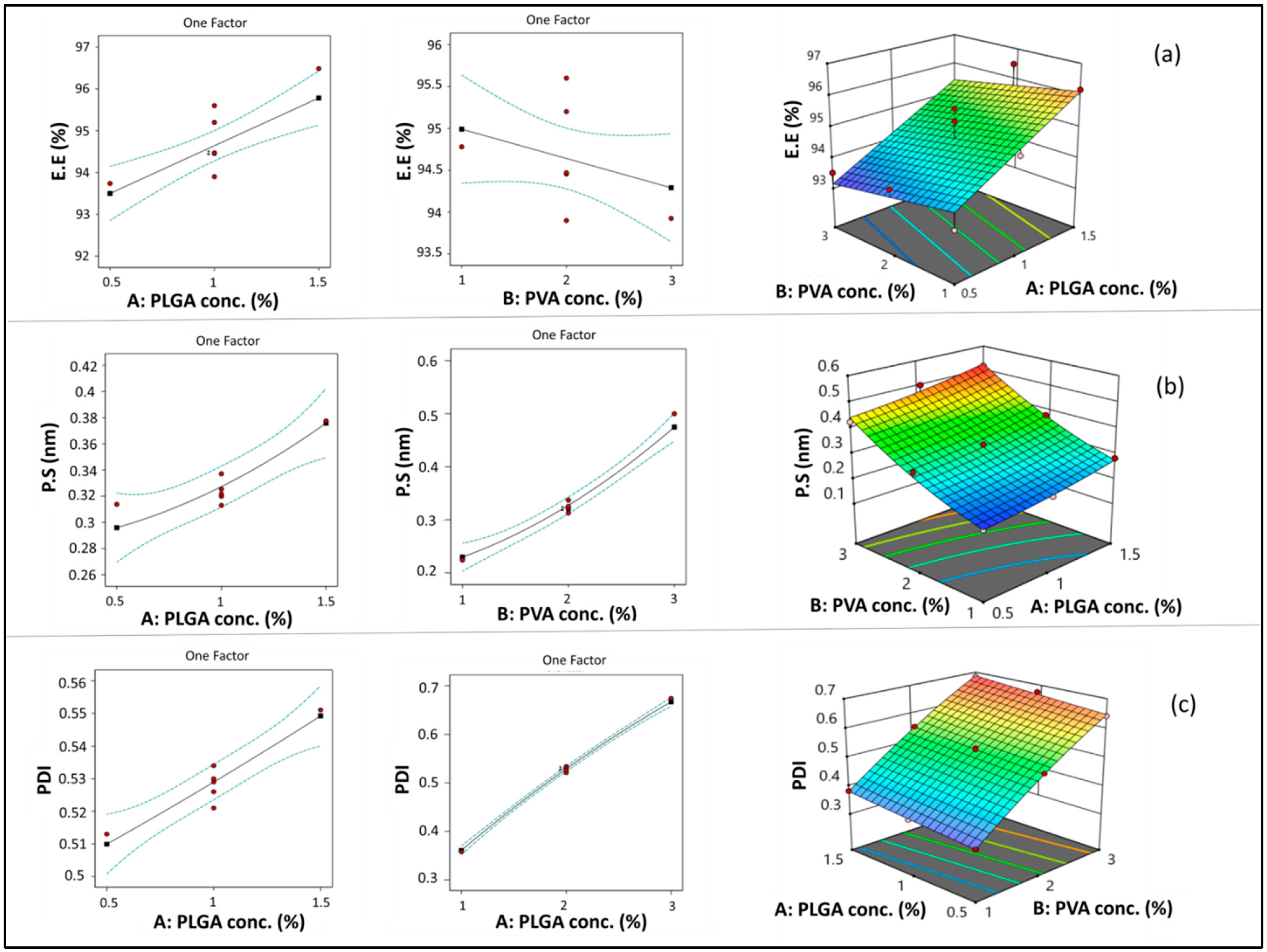
3.1. Statistical Output of the Central Composite Face-Centered Design and Diagnostic Analysis
3.1.1. Model Analysis of EE
3.1.2. Model Analysis of PS
3.1.3. Model Analysis of PDI
3.2. Optimization of ETD-Loaded NPs
3.3. Determination of DL
3.4. Preparation of Hyaluronate-Coated NPs
3.5. Transmission Electron Microscopy
3.6. Evaluation of ETD-Loaded NP In Situ Hydrogel
3.6.1. Evaluation Gelation Time
3.6.2. Evaluation of the Lowest Gelation Temperature
3.6.3. Determination of DL of the In Situ Gel Formulation (H-F1G)
3.7. Ex Vivo Permeation Study
3.7.1. Visualization of Pores
3.7.2. Confocal Microscopy
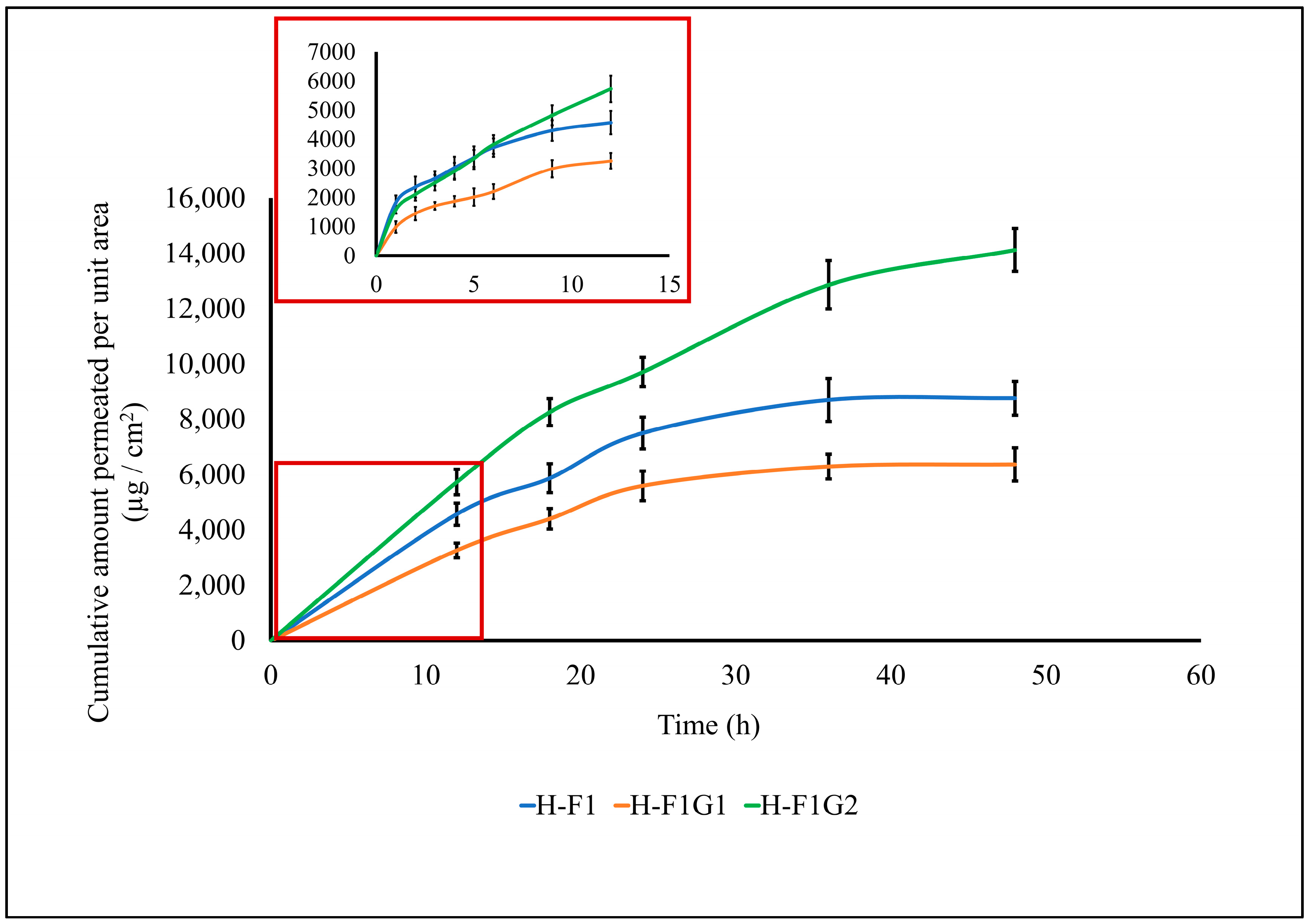
3.7.3. Ex Vivo ETD Skin Permeation
3.8. In Vivo Antirheumatic Activity
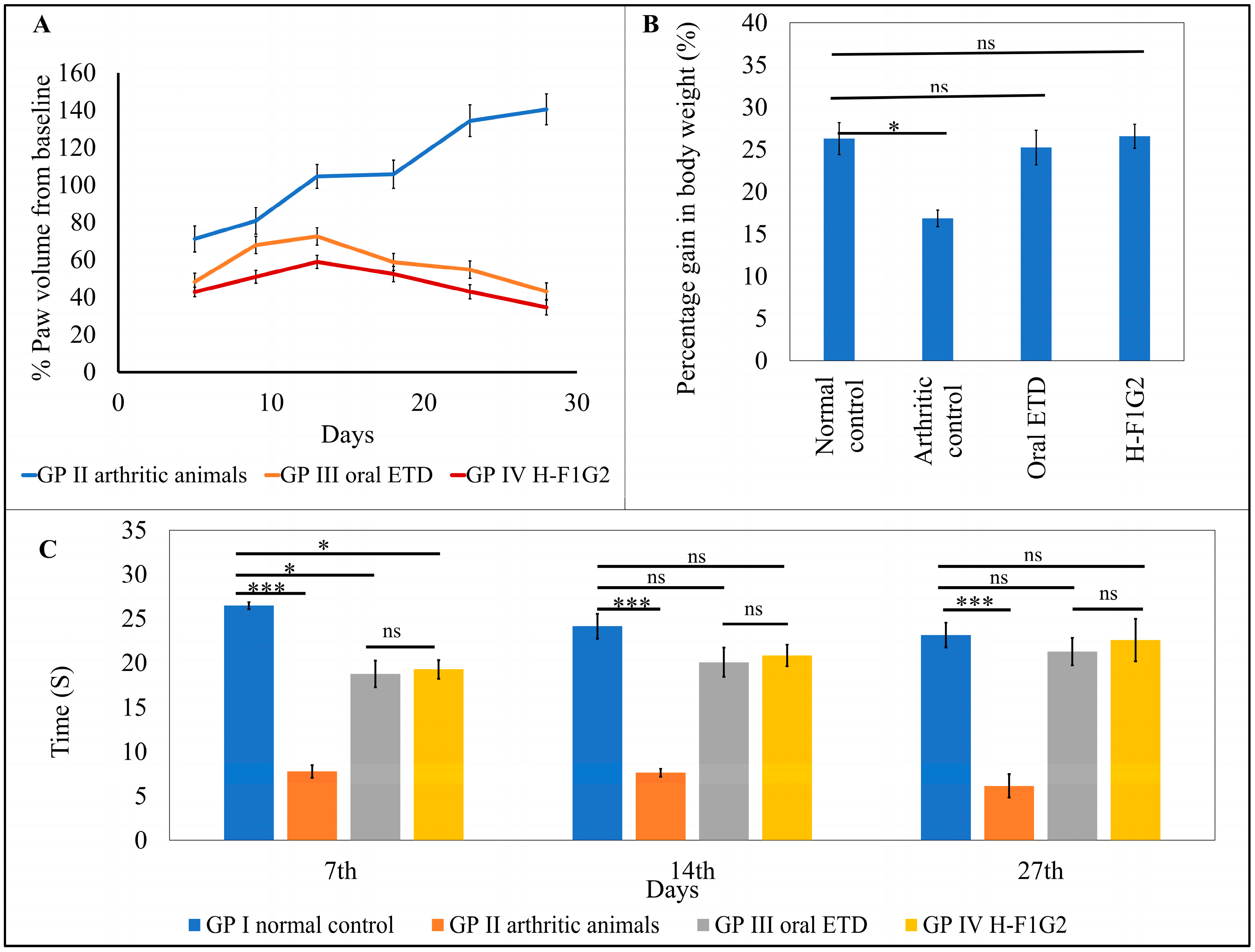
3.8.1. Measuring Paw Oedema
3.8.2. Measuring the Change in Body Weight
3.8.3. Behavioral Assessment of Pain Sensitivity
3.8.4. Assessment of Radiographic Images
3.8.5. Assessment of Pro-Inflammatory Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| NSAIDS | Nonsteroidal anti-inflammatory medications |
| ETD | Etodolac |
| COX | Cyclo-oxygenase |
| NPs | Polymeric nanoparticle drug delivery system |
| SC | Stratum corneum |
| MN | Microneedle |
| CFAs | Complete Freund’s adjuvants |
| PVA | Polyvinyl alcohol |
| EE | Entrapment efficiency |
| PS | Particle size |
| PDI | Polydispersity index |
| ZP | Zeta potential |
| DL | Loading capacity |
| TEM | Transmission electron microscopy |
| PBS | Phosphate-buffered saline |
| TNF-α | Tumor necrosis factor alpha |
| IL-6 | Interleukin 6 |
| IL-1 β | Interleukin 1-beta. |
References
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Anita, C.; Munira, M.; Mural, Q.; Shaily, L. Topical nanocarriers for management of Rheumatoid Arthritis: A review. Biomed. Pharmacother. 2021, 141, 111880. [Google Scholar] [CrossRef] [PubMed]
- Pirmardvand Chegini, S.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mousavi, M.J.; Jamalzehi, S.; Alimohammadi, R.; Bezvan, M.H.; Mohammadi, H.; Aslani, S. Strategies toward rheumatoid arthritis therapy; the old and the new. J. Cell Physiol. 2019, 234, 10018–10031. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xia, W.; Wang, S.; Dai, G.; Jiao, W.; Guo, N.; Li, H.; Zhang, G. Etodolac improves collagen induced rheumatoid arthritis in rats by inhibiting synovial inflammation, fibrosis and hyperplasia. Mol. Biomed. 2021, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Wairkar, S. Dietary polyphenols for management of rheumatoid arthritis: Pharmacotherapy and novel delivery systems. Phytother. Res. 2022, 36, 2324–2341. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Nakamura, H.; Lam, V.; Hofs, E.; Cederberg, R.; Cait, J.; Hughes, M.R.; Lee, L.; Jia, W.; Adomat, H.H.; et al. DMSO Represses Inflammatory Cytokine Production from Human Blood Cells and Reduces Autoimmune Arthritis. PLoS ONE 2016, 11, e0152538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C. Acupuncture and Chronic Musculoskeletal Pain. Curr. Rheumatol. Rep. 2020, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E.; Bernatsky, S.; Lévesque, J.F.; Van, M.T.; Houde, M.; April, K.T. Access and perceived need for physical and occupational therapy in chronic arthritis. Disabil. Rehabil. 2010, 32, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Chehade, L.; Jaafar, Z.A.; El Masri, D.; Zmerly, H.; Kreidieh, D.; Tannir, H.; Itani, L.; El Ghoch, M. Lifestyle Modification in Rheumatoid Arthritis: Dietary and Physical Activity Recommendations Based on Evidence. Curr. Rheumatol. Rev. 2019, 15, 209–214. [Google Scholar] [CrossRef]
- Jones, R.A. Etodolac: An overview of a selective COX-2 inhibitor. Inflammopharmacology 1999, 7, 269–275. [Google Scholar] [CrossRef]
- Hasegawa, K.; Torii, Y.; Ishii, R.; Oe, S.; Kato, R.; Udagawa, Y. Effects of a selective COX-2 inhibitor in patients with uterine endometrial cancers. Arch. Gynecol. Obstet. 2011, 284, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, T.; Katsumata, K.; Tsuchida, A.; Wada, T.; Mori, Y.; Hisada, M.; Kawakita, H.; Aoki, T. Etodolac, a selective cyclooxygenase-2 inhibitor, inhibits liver metastasis of colorectal cancer cells via the suppression of MMP-9 activity. Int. J. Mol. Med. 2006, 17, 357–362. [Google Scholar] [CrossRef][Green Version]
- Reynolds, J.E.F. Martindale: The Extra Pharmacopoeia; Deutscher Apotheker Vlg: Gerlingen, Germany, 1996. [Google Scholar]
- Shilakari Asthana, G.; Asthana, A.; Singh, D.; Sharma, P.K. Etodolac containing topical niosomal gel: Formulation development and evaluation. J. Drug Deliv. 2016, 2016, 9324567. [Google Scholar] [CrossRef]
- Yuan, F.; Quan, L.D.; Cui, L.; Goldring, S.R.; Wang, D. Development of macromolecular prodrug for rheumatoid arthritis. Adv. Drug Deliv. Rev. 2012, 64, 1205–1219. [Google Scholar] [CrossRef]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: Ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Mahajan, A.; Thakur, K.; Kaur, G.; Goni, V.G.; Kumar, M.V.; Barnwal, R.P.; Singh, G.; Singh, B.; Katare, O.P. Exploring the therapeutic potential of sodium deoxycholate tailored deformable-emulsomes of etodolac for effective management of arthritis. Sci. Rep. 2023, 13, 21681. [Google Scholar] [CrossRef]
- Özdemir, S.; Üner, B.; Karaküçük, A.; Çelik, B.; Sümer, E.; Taş, Ç. Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization. Pharmaceutics 2023, 15, 2510. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska-Kośnik, A.; Szymańska, E.; Czarnomysy, R.; Jacyna, J.; Markuszewski, M.; Basa, A.; Winnicka, K. Nanostructured Lipid Carriers Engineered as Topical Delivery of Etodolac: Optimization and Cytotoxicity Studies. Materials 2021, 14, 596. [Google Scholar] [CrossRef]
- Shaji, J.; Lal, M. Nanocarriers for targeting in inflammation. Asian J. Pharm. Clin. Res. 2013, 6, 3–12. [Google Scholar]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Banga, A.K. Microporation applications for enhancing drug delivery. Expert. Opin. Drug Deliv. 2009, 6, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Vandervoort, J.; Ludwig, A. Microneedles for transdermal drug delivery: A minireview. Front. Biosci. A J. Virtual Libr. 2008, 13, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Alberti, I.; Lapteva, M.; Kalia, Y.N. Next generation intra- and transdermal therapeutic systems: Using non- and minimally-invasive technologies to increase drug delivery into and across the skin. Eur. J. Pharm. Sci. 2013, 50, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Khurana, R.K.; Jain, A.; Kaur, R.; Kumar, R. Chapter 15—Microporation and Nanoporation for Effective Delivery of Drugs and Genes. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 485–514. [Google Scholar]
- Sivaraman, A.; Banga, A.K. Novel in situ forming hydrogel microneedles for transdermal drug delivery. Drug Deliv. Transl. Res. 2017, 7, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Habib, B.A.; Sayed, S.; Elsayed, G.M. Enhanced transdermal delivery of ondansetron using nanovesicular systems: Fabrication, characterization, optimization and ex-vivo permeation study-Box-Cox transformation practical example. Eur. J. Pharm. Sci. 2018, 115, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.; Engelhardt, B.; Alyautdin, R.; Von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 2004, 20, 409–416. [Google Scholar] [CrossRef]
- Mondalek, F.G.; Ashley, R.A.; Roth, C.C.; Kibar, Y.; Shakir, N.; Ihnat, M.A.; Fung, K.-M.; Grady, B.P.; Kropp, B.P.; Lin, H.-K. Enhanced angiogenesis of modified porcine small intestinal submucosa with hyaluronic acid-poly(lactide-co-glycolide) nanoparticles: From fabrication to preclinical validation. J. Biomed. Mater. Res. A 2010, 94, 712–719. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, J.-H.; Meng, M.; Cui, N.; Dai, C.-Y.; Jia, Q.; Lee, E.-S.; Jiang, H.-B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials 2021, 14, 4522. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan/β-glycerophosphate in situ gelling mucoadhesive systems for intravesical delivery of mitomycin-C. Int. J. Pharm. X 2019, 1, 100007. [Google Scholar] [CrossRef] [PubMed]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. App. 2016, 8, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.A.; Chen, G.; Domínguez-Robles, J.; Thakur, R.R.S.; Hamdan, I.M.N.; Vora, L.; Larrañeta, E.; McElnay, J.C.; Rooney, M.; Donnelly, R.F. Development and characterisation of novel poly (vinyl alcohol)/poly (vinyl pyrrolidone)-based hydrogel-forming microneedle arrays for enhanced and sustained transdermal delivery of methotrexate. Int. J. Pharm. 2020, 586, 119580. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.A.; Garimella, H.T.; German, C.L.; Banga, A.K. Microneedle Mediated Iontophoretic Delivery of Tofacitinib Citrate. Pharm. Res. 2023, 40, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Banga, A.K. Fabrication, characterization and application of sugar microneedles for transdermal drug delivery. Ther. Deliv. 2017, 8, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Minhas, M.U.; Tekko, I.A.; Donnelly, R.F.; Thakur, R.R.S. Evaluation of microneedles-assisted in situ depot forming poloxamer gels for sustained transdermal drug delivery. Drug Deliv. Transl. Res. 2019, 9, 764–782. [Google Scholar] [CrossRef]
- Morsi, N.; Ghorab, D.; Refai, H.; Teba, H. Ketoroloac tromethamine loaded nanodispersion incorporated into thermosensitive in situ gel for prolonged ocular delivery. Int. J. Pharm. 2016, 506, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nageeb El-Helaly, S.; Abd-Elrasheed, E.; Salim, S.A.; Fahmy, R.H.; Salah, S.; EL-Ashmoony, M.M. Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 474. [Google Scholar] [CrossRef]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In-vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555–6574. [Google Scholar] [CrossRef]
- Teba, H.E.; Khalil, I.A.; El Sorogy, H.M. Novel cubosome based system for ocular delivery of acetazolamide. Drug Deliv. 2021, 28, 2177–2186. [Google Scholar] [CrossRef]
- Hegde, R.R.; Bhattacharya, S.S.; Verma, A.; Ghosh, A. Physicochemical and pharmacological investigation of water/oil microemulsion of non-selective beta blocker for treatment of glaucoma. Curr. Eye Res. 2014, 39, 155–163. [Google Scholar] [CrossRef]
- Elshall, A.A.; Ghoneim, A.M.; Abdel-Mageed, H.M.; Osman, R.; Shaker, D.S. Ex vivo permeation parameters and skin deposition of melatonin-loaded microemulsion for treatment of alopecia. Future J. Pharm. Sci. 2022, 8, 28. [Google Scholar] [CrossRef]
- Helyes, Z.; Pintér, E.; Németh, J.; Kéri, G.; Thán, M.; Oroszi, G.; Horváth, A.; Szolcsányi, J. Anti-inflammatory effect of synthetic somatostatin analogues in the rat. Br. J. Pharmacol. 2001, 134, 1571–1579. [Google Scholar] [CrossRef]
- Inoue, N.; Ito, S.; Tajima, K.; Nogawa, M.; Takahashi, Y.; Sasagawa, T.; Nakamura, A.; Kyoi, T. Etodolac attenuates mechanical allodynia in a mouse model of neuropathic pain. J. Pharmacol. Sci. 2009, 109, 600–605. [Google Scholar] [CrossRef]
- Price, D.D.; Mao, J.; Lu, J.; Caruso, F.S.; Frenk, H.; Mayer, D.J. Effects of the combined oral administration of NSAIDs and dextromethorphan on behavioral symptoms indicative of arthritic pain in rats. Pain 1996, 68, 119–127. [Google Scholar] [CrossRef]
- Madhavi, N.; Sudhakar, B.; Reddy, K.; Ratna, J.V. Design by optimization and comparative evaluation of vesicular gels of etodolac for transdermal delivery. Drug Dev. Ind. Pharm. 2019, 45, 611–628. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Saksena, A.K.; Pal, R.; Jaiswal, R.; Kumar, R. Effect of Boswellia Serrata extract on acute inflammatory parameters and tumor necrosis factor-α in complete freund’s adjuvant-induced animal model of rheumatoid arthritis. Int. J. Appl. Basic Med. Res. 2019, 9, 100–106. [Google Scholar] [CrossRef]
- Swathi, K.P.; Jayaram, S.; Sugumar, D.; Rymbai, E. Evaluation of anti-inflammatory and anti-arthritic property of ethanolic extract of Clitoria ternatea. Chin. Herb. Med. 2021, 13, 243–249. [Google Scholar] [CrossRef]
- Laste, G.; Ripoll Rozisky, J.; de Macedo, I.C.; Souza Dos Santos, V.; Custódio de Souza, I.C.; Caumo, W.; Torres, I.L.S. Spinal cord brain-derived neurotrophic factor levels increase after dexamethasone treatment in male rats with chronic inflammation. Neuroimmunomodulation 2013, 20, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.M.; Goicoechea, C.; Avellanal, M.; Traseira, S.; Martín, M.I.; Sánchez-Robles, E.M. Comparison of the antinociceptive profiles of morphine and oxycodone in two models of inflammatory and osteoarthritic pain in rat. Eur. J. Pharmacol. 2019, 854, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Implementing central composite design for developing transdermal diacerein-loaded niosomes: Ex vivo permeation and in vivo deposition. Curr. Drug Deliv. 2018, 15, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Al-mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Derman, S. Caffeic acid phenethyl ester loaded PLGA nanoparticles: Effect of various process parameters on reaction yield, encapsulation efficiency, and particle size. J. Nanomater. 2015, 2015, 341848. [Google Scholar] [CrossRef]
- Halayqa, M.; Domańska, U. PLGA biodegradable nanoparticles containing perphenazine or chlorpromazine hydrochloride: Effect of formulation and release. Int. J. Mol. Sci. 2014, 15, 23909–23923. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A.; Badr-Eldin, S.M. Biodegradable self-assembled nanoparticles of PEG-PLGA amphiphilic diblock copolymer as a promising stealth system for augmented vinpocetine brain delivery. Int. J. Pharm. 2020, 588, 119778. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Andresen, T.L. Factors controlling nanoparticle pharmacokinetics: An integrated analysis and perspective. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 481–503. [Google Scholar] [CrossRef]
- Noori Koopaei, M.; Khoshayand, M.R.; Mostafavi, S.H.; Amini, M.; Khorramizadeh, M.R.; Jeddi Tehrani, M.; Atyabi, F.; Dinarvand, R. Docetaxel loaded PEG-PLGA nanoparticles: Optimized drug loading, in-vitro cytotoxicity and in-vivo antitumor effect. Iran. J. Pharm. Res. 2014, 13, e125503. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Formulation and optimization of temozolomide nanoparticles by 3 factor 2 level factorial design. Biomatter 2013, 3, e25102. [Google Scholar] [CrossRef]
- Fodor-Kardos, A.; Kiss, Á.F.; Monostory, K.; Feczkó, T. Sustained in vitro interferon-beta release and in vivo toxicity of PLGA and PEG-PLGA nanoparticles. RSC Adv. 2020, 10, 15893–15900. [Google Scholar] [CrossRef]
- Zweers, M.L.; Grijpma, D.W.; Engbers, G.H.; Feijen, J. The preparation of monodisperse biodegradable polyester nanoparticles with a controlled size. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 559–566. [Google Scholar] [CrossRef]
- Aithal, B.K.; Sunil Kumar, M.R.; Rao, B.N.; Upadhya, R.; Prabhu, V.; Shavi, G.; Arumugam, K.; Sajankila, S.P.; Udupa, N.; Satyamoorthy, K.; et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J. Pharm. Sci. 2011, 100, 3517–3528. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Kumari, M.; Pahuja, R.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Hyaluronic acid-grafted PLGA nanoparticles for the sustained delivery of berberine chloride for an efficient suppression of Ehrlich ascites tumors. Drug Deliv. Transl. Res. 2018, 8, 565–579. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, J.; Chen, W.; Jiang, H.; Zhang, Z.; Sun, X. Targeted delivery of low-dose dexamethasone using PCL-PEG micelles for effective treatment of rheumatoid arthritis. J. Control Release 2016, 230, 64–72. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Wang, Q.; Zhou, M.; Li, M.; Gong, T.; Zhang, Z.; Sun, X. pH-sensitive polymeric micelles for targeted delivery to inflamed joints. J. Control Release 2017, 246, 133–141. [Google Scholar] [CrossRef]
- Tuan-Mahmood, T.M.; McCrudden, M.T.; Torrisi, B.M.; McAlister, E.; Garland, M.J.; Singh, T.R.; Donnelly, R.F. Microneedles for intradermal and transdermal drug delivery. Eur. J. Pharm. Sci. 2013, 50, 623–637. [Google Scholar] [CrossRef]
- Talbot, S.R.; Biernot, S.; Bleich, A.; van Dijk, R.M.; Ernst, L.; Häger, C.; Helgers, S.O.A.; Koegel, B.; Koska, I.; Kuhla, A.; et al. Defining body-weight reduction as a humane endpoint: A critical appraisal. Lab. Anim. 2020, 54, 99–110. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431–1445. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]



| Independent Variables | Level | Optimized Levels | ||||||
|---|---|---|---|---|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | ||||||
| PLGA (%w/w) (A) | 0.5 | 1 | 1.5 | 1.5 | ||||
| PVA (%w/w) (B) | 1 | 2 | 3 | 1 | ||||
| Dependent Variables | Desirability Constraints | Model | Model Summary Statistics | |||||
| R2 | Adjusted R2 | Predicted R2 | Adequate Precision | p-Value | Significant Factors | |||
| EE (%) (Y1) | Maximize | Linear | 0.712 | 0.6547 | 0.5025 | 10.5631 | 0.002 | A |
| PS (nm) (Y2) | Minimize | Quadratic | 0.983 | 0.9709 | 0.8712 | 30.0642 | <0.0001 | A, B, B2 |
| PDI (Y3) | Minimize | Quadratic | 0.998 | 0.9974 | 0.9913 | 91.6582 | <0.0001 | A, B, B2 |
| Formula No. | Space Type | PLGA Conc. (A) (%w/w) | PVA Conc. (B) (%w/w) | Y1 EE (%) | Y2 PS (nm) | Y3 PDI |
|---|---|---|---|---|---|---|
| F1 | Factorial | 1.5 | 1 | 96.19 ± 2.31 | 282.3 ± 0.62 | 0.383 ± 0.04 |
| F2 | Factorial | 0.5 | 3 | 93.52 ± 1.52 | 424.1 ± 4.54 | 0.643 ± 0.03 |
| F3 | Factorial | 0.5 | 1 | 93.32 ± 1.22 | 199.9 ± 1.01 | 0.343 ± 0.02 |
| F4 | Center | 1 | 2 | 94.46 ± 2.14 | 321.6 ± 2.84 | 0.529 ± 0.05 |
| F5 | Center | 1 | 2 | 95.2 ± 1.85 | 330.4 ± 2.32 | 0.526 ± 0.02 |
| F6 | Axial | 1 | 3 | 93.93 ± 0.91 | 499.9 ± 5.66 | 0.674 ± 0.03 |
| F7 | Center | 1 | 2 | 93.9 ± 1.79 | 337.1 ± 2.11 | 0.53 ± 0.03 |
| F8 | Axial | 1.5 | 2 | 96.49 ± 2.33 | 377.5 ± 1.56 | 0.551 ± 0.04 |
| F9 | Center | 1 | 2 | 95.6 ± 3.24 | 319.9 ± 3.96 | 0.521 ± 0.05 |
| F10 | Axial | 0.5 | 2 | 93.74 ± 1.54 | 313.8 ± 3.12 | 0.513 ± 0.01 |
| F11 | Factorial | 1.5 | 3 | 94.75 ± 2.47 | 517.5 ± 2.25 | 0.683 ± 0.03 |
| F12 | Center | 1 | 2 | 94.47 ± 1.65 | 325.4 ± 5.47 | 0.534 ± 0.02 |
| F13 | Axial | 1 | 1 | 94.78 ± 0.79 | 224.1 ± 1.32 | 0.358 ± 0.01 |
| NP | PS (nm) | PDI | ZP (mV) | DL (mg·mg−1) |
|---|---|---|---|---|
| Uncoated NPs (F1) | 282.3 ± 0.62 | 0.383 ± 0.04 | −6.44 ± 1.69 | 0.138 ± 0.0061 |
| 0.1% H-F1 | 287.4 ± 4.2 | 0.267 ± 0.02 | −23.7 ± 3.77 | 0.135 ± 0.0057 |
| 0.2% H-F1 | 300.5 ± 5.77 | 0.591 ± 0.04 | −27.8 ± 2.87 | 0.131 ± 0.0086 |
| 0.4% H-F1 | 439 ± 5.42 | 0.670 ± 0.05 | −32.5 ± 5.12 | 0.102 ± 0.0092 |
| Permeation Parameter | H-F1 | H-F1G1 | H-F1G2 |
|---|---|---|---|
| Cumulative amount of ETD permeated per unit area after 48 h (µg/cm2) | 8764.97 ± 611.8 | 6368.79 ± 599.4 | 14,124.84 ± 777.9 |
| Steady-state flux Jss (µg/cm2·h) | 172.42 ± 29.75 | 124.79 ± 13.89 | 288.47 ± 7.25 |
| Permeation coefficient Kp (cm/h) | 1.79 × 10−2 ± 3.13 × 10−3 | 1.3 × 10−2 ± 1.44 × 10−3 | 3 × 10−2 ± 7.54 × 10−4 |
| Days | Average Percentage Change in Paw Volume | Percentage Inhibition in Paw Volume | |||
|---|---|---|---|---|---|
| GP II Arthritic Animals | GP III Oral ETD | GP IV H-F1G2 | GP III Oral ETD | GP IV H-F1G2 | |
| 5 | 71.17 ± 7.02 | 48.22 ± 3.35 | 42.83 ± 2.59 | 32.2 | 39.8 |
| 9 | 80.86 ± 7.08 | 67.93 ± 4.62 | 50.87 ± 3.38 | 16 | 37.1 |
| 13 | 104.62 ± 6.46 | 72.53 ± 6.35 | 58.92 ± 3.48 | 30.7 | 43.7 |
| 18 | 105.73 ± 7.59 | 58.78 ± 4.31 | 52.38 ± 4.14 | 44.4 | 50.5 |
| 23 | 134.36 ± 8.33 | 54.74 ± 2.30 | 42.96 ± 3.85 | 59.3 | 68 |
| 28 | 140.41 ± 8.45 | 43.04 ± 3.69 | 34.61 ± 4.16 | 69.3 | 75.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Sorogy, H.M.; Fayez, S.M.; Khalil, I.A.; Abdel Jaleel, G.A.; Fayez, A.M.; Eliwa, H.A.; Teba, H.E. Microporation-Mediated Transdermal Delivery of In Situ Gel Incorporating Etodolac-Loaded PLGA Nanoparticles for Management of Rheumatoid Arthritis. Pharmaceutics 2024, 16, 844. https://doi.org/10.3390/pharmaceutics16070844
El Sorogy HM, Fayez SM, Khalil IA, Abdel Jaleel GA, Fayez AM, Eliwa HA, Teba HE. Microporation-Mediated Transdermal Delivery of In Situ Gel Incorporating Etodolac-Loaded PLGA Nanoparticles for Management of Rheumatoid Arthritis. Pharmaceutics. 2024; 16(7):844. https://doi.org/10.3390/pharmaceutics16070844
Chicago/Turabian StyleEl Sorogy, Heba M., Sahar M. Fayez, Islam A. Khalil, Gehad A. Abdel Jaleel, Ahmed M. Fayez, Hesham A. Eliwa, and Hoda E. Teba. 2024. "Microporation-Mediated Transdermal Delivery of In Situ Gel Incorporating Etodolac-Loaded PLGA Nanoparticles for Management of Rheumatoid Arthritis" Pharmaceutics 16, no. 7: 844. https://doi.org/10.3390/pharmaceutics16070844
APA StyleEl Sorogy, H. M., Fayez, S. M., Khalil, I. A., Abdel Jaleel, G. A., Fayez, A. M., Eliwa, H. A., & Teba, H. E. (2024). Microporation-Mediated Transdermal Delivery of In Situ Gel Incorporating Etodolac-Loaded PLGA Nanoparticles for Management of Rheumatoid Arthritis. Pharmaceutics, 16(7), 844. https://doi.org/10.3390/pharmaceutics16070844







