Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Particle Preparation Using Microfluidics with Simultaneous Loading of Calcein or Insulin
2.2.2. Particle Size Analysis
2.2.3. Zeta Potential
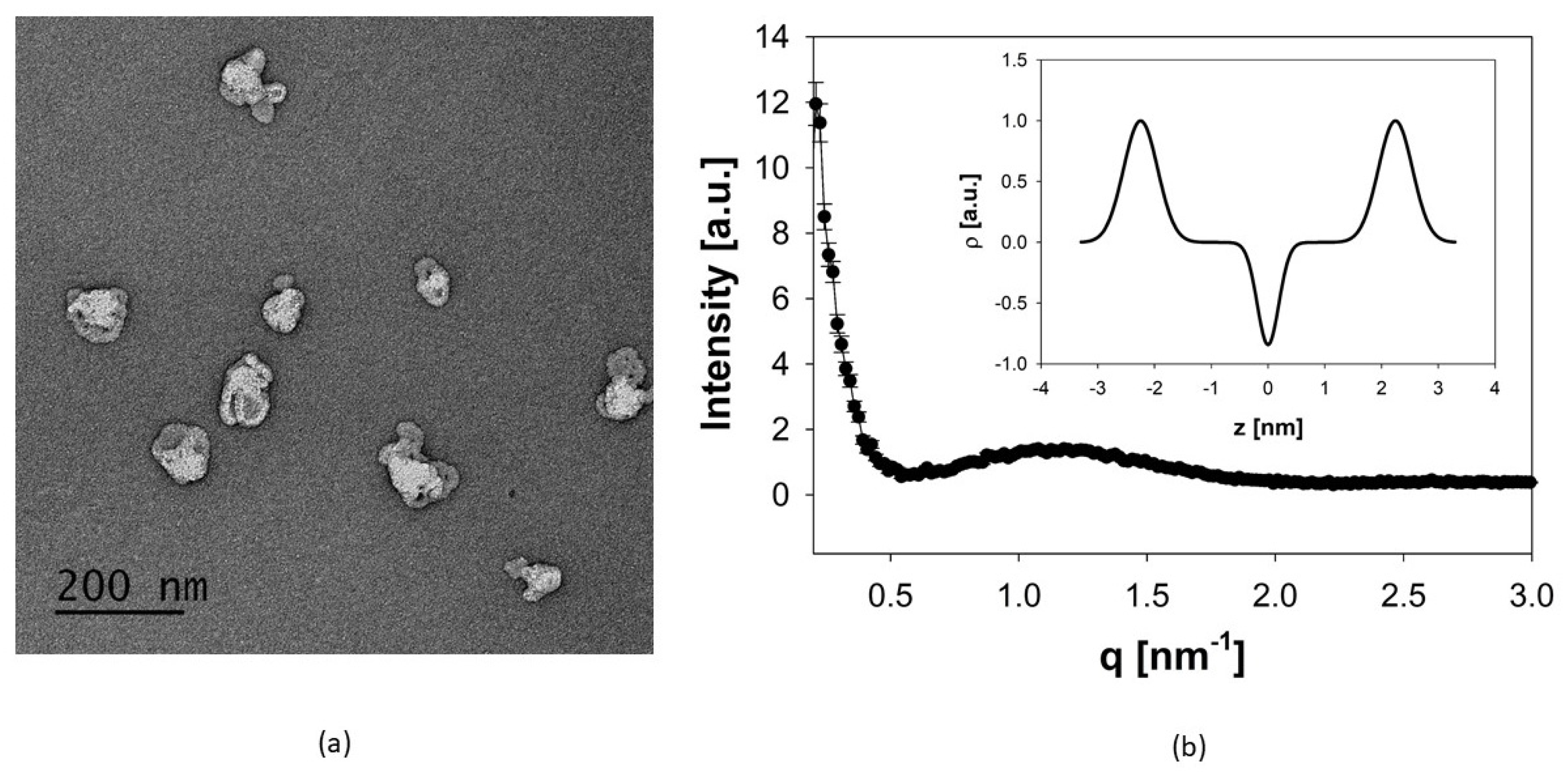
2.2.4. Small-Angle X-ray Scattering (SAXS)
2.2.5. Transmission Electron Microscopy (TEM)
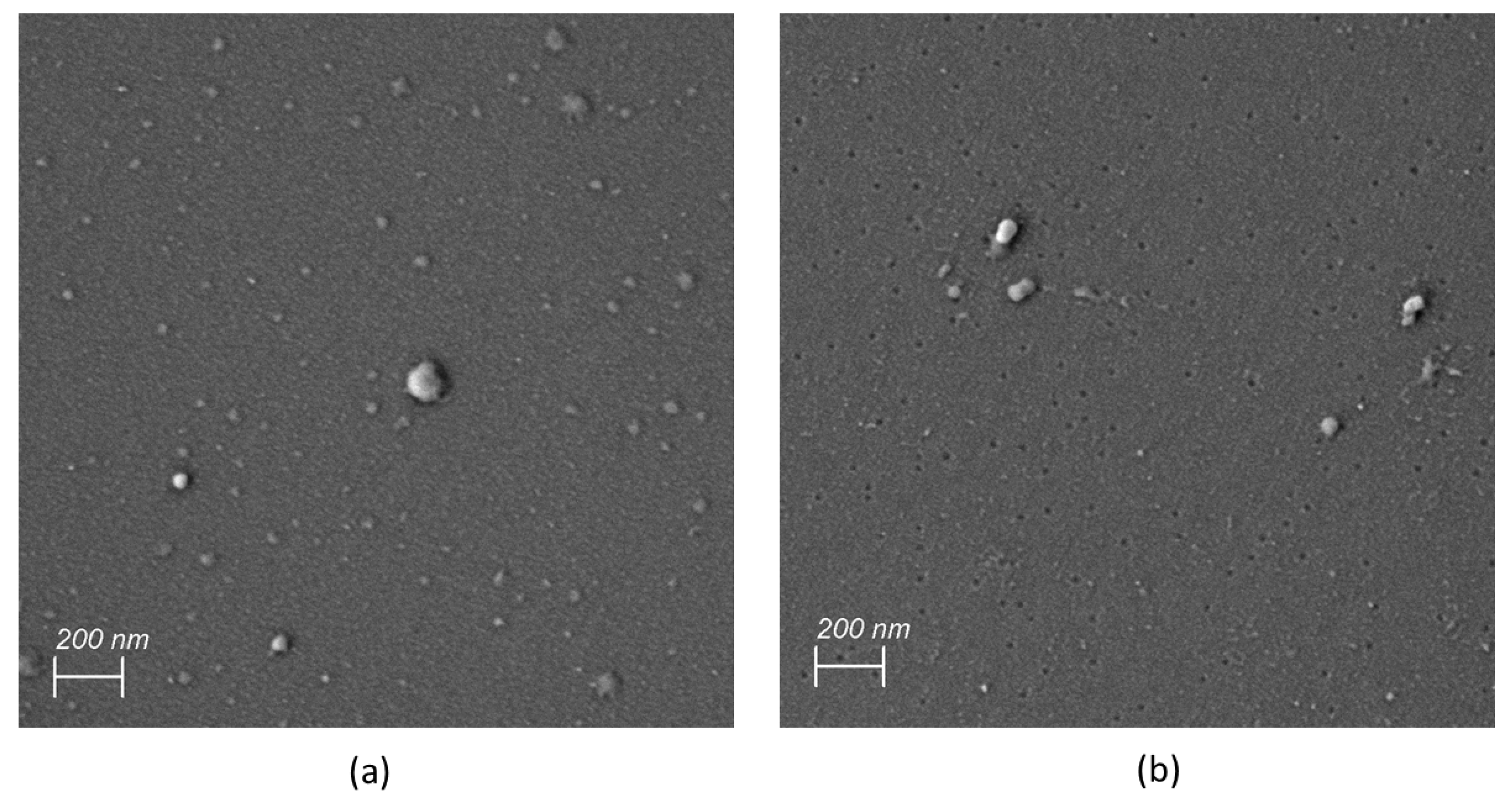
2.2.6. Scanning Electron Microscopy (SEM)
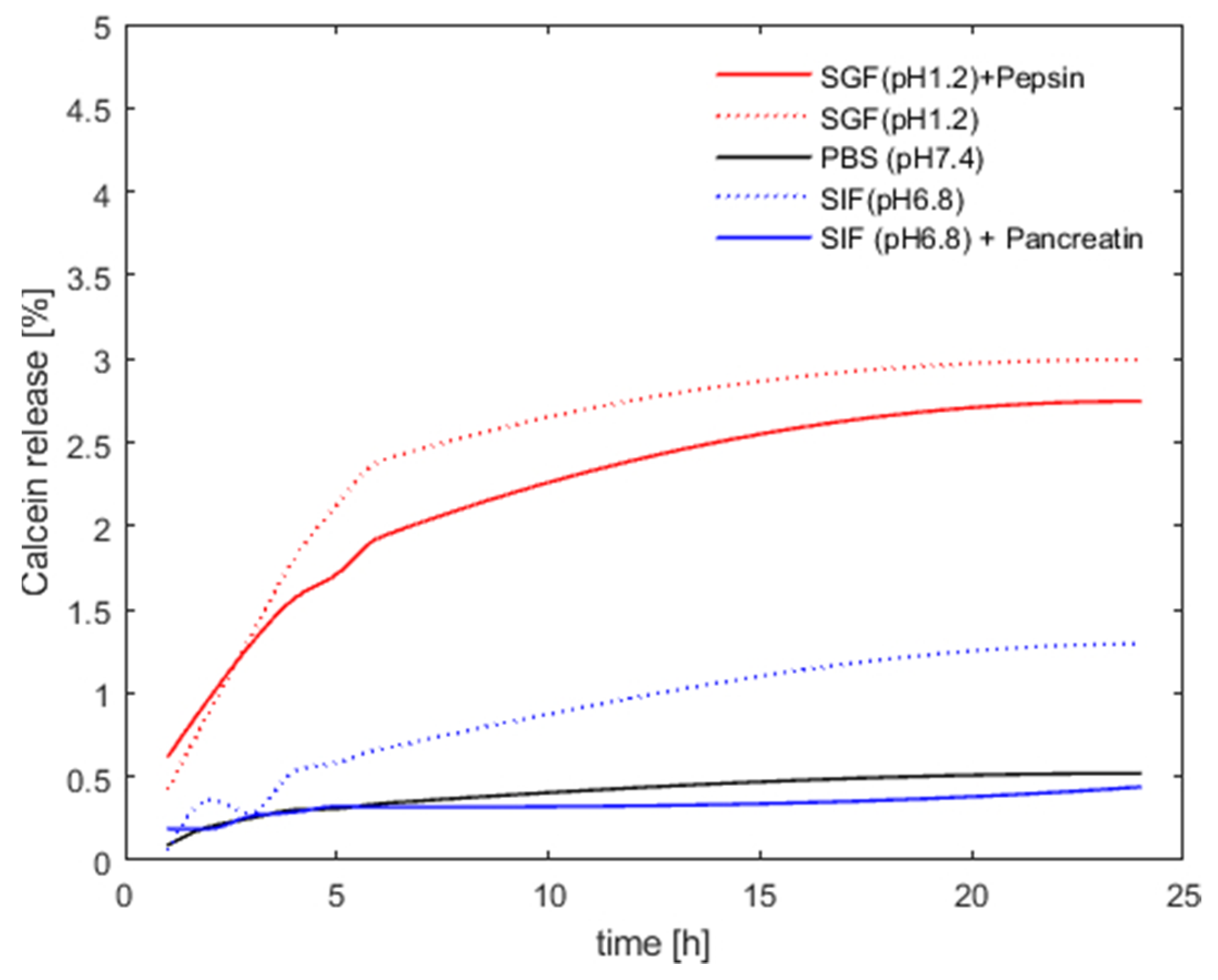
2.2.7. Stability in Simulated Gastric and Intestinal Fluid
2.2.8. In Vitro Uptake Studies
2.2.9. Lyophilization
2.2.10. Spray Drying
2.2.11. Statistical Analysis
3. Results
3.1. Manufacturing and Physicochemical Characterization of Archaeosomes
3.2. Archaeosome Stability in Simulated Intestinal Fluids and Drug Release Behavior
3.3. In Vitro Release and Cellular Uptake Behavior
3.4. Dry Powder Formulations of Archaeosomes
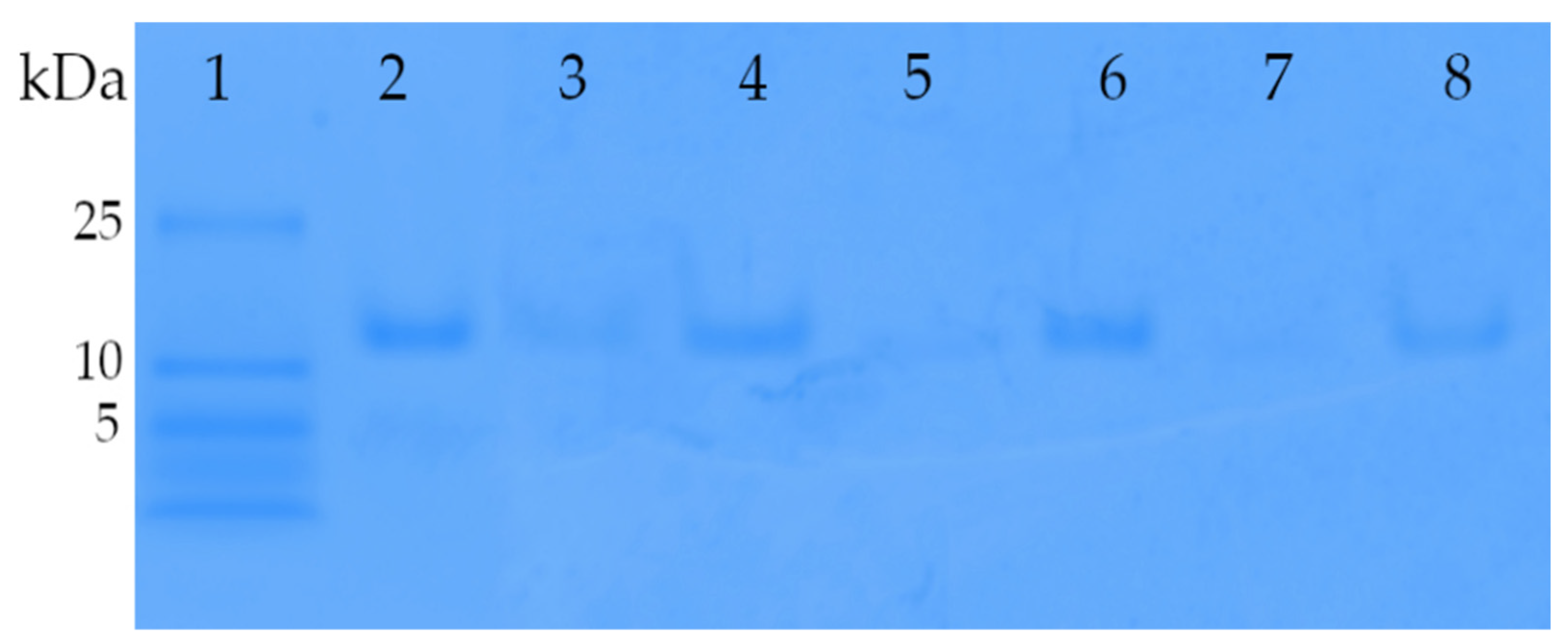
3.5. Insulin Loading for Dry Powder Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Rosa, M.; Gambacorta, A.; Gliozzi, A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 1986, 50, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Charles-Orszag, A.; Lord, S.J.; Mullins, R.D. High-Temperature Live-Cell Imaging of Cytokinesis, Cell Motility, and Cell-Cell Interactions in the Thermoacidophilic Crenarchaeon Sulfolobus acidocaldarius. Front. Microbiol. 2021, 12, 707124. [Google Scholar] [CrossRef]
- Quehenberger, J.; Shen, L.; Albers, S.V.; Siebers, B.; Spadiut, O. Sulfolobus—A Potential Key Organism in Future Biotechnology. Front. Microbiol. 2017, 8, 2474. [Google Scholar] [CrossRef]
- Salvador-Castell, M.; Golub, M.; Erwin, N.; Deme, B.; Brooks, N.J.; Winter, R.; Peters, J.; Oger, P.M. Characterisation of a synthetic Archeal membrane reveals a possible new adaptation route to extreme conditions. Commun. Biol. 2021, 4, 653. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, A.; Barbeau, J.; Lemiègre, L.; Benvegnu, T. Archaeal tetraether bipolar lipids: Structures, functions and applications. Biochimie 2009, 91, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Gulik, A.; Luzzati, V.; De Rosa, M.; Gambacorta, A. Structure and polymorphism of bipolar isopranyl ether lipids from archaebacteria. J. Mol. Biol. 1985, 182, 131–149. [Google Scholar] [CrossRef]
- Chong, P.L.; Ravindra, R.; Khurana, M.; English, V.; Winter, R. Pressure perturbation and differential scanning calorimetric studies of bipolar tetraether liposomes derived from the thermoacidophilic archaeon Sulfolobus acidocaldarius. Biophys. J. 2005, 89, 1841–1849. [Google Scholar] [CrossRef]
- Chong, P.L.; Sulc, M.; Winter, R. Compressibilities and volume fluctuations of archaeal tetraether liposomes. Biophys. J. 2010, 99, 3319–3326. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Nicolaus, B. A New type of cell membrane, in thermophilic archaebacteria, based on bipolar ether lipids. J. Membr. Sci. 1983, 16, 287–294. [Google Scholar] [CrossRef]
- Chong, P.L. Archaebacterial bipolar tetraether lipids: Physico-chemical and membrane properties. Chem. Phys. Lipids 2010, 163, 253–265. [Google Scholar] [CrossRef]
- Jeworrek, C.; Evers, F.; Erlkamp, M.; Grobelny, S.; Tolan, M.; Chong, P.L.; Winter, R. Structure and phase behavior of archaeal lipid monolayers. Langmuir 2011, 27, 13113–13121. [Google Scholar] [CrossRef]
- Kaur, G.; Garg, T.; Rath, G.; Goyal, A.K. Archaeosomes: An excellent carrier for drug and cell delivery. Drug Deliv. 2016, 23, 2497–2512. [Google Scholar] [CrossRef]
- Romero, E.L.; Morilla, M.J. Ether lipids from archaeas in nano-drug delivery and vaccination. Int. J. Pharm. 2023, 634, 122632. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Sun, W.; Xu, Y. Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem. Biophys. Res. Commun. 2010, 394, 412–417. [Google Scholar] [CrossRef]
- Uhl, P.; Helm, F.; Hofhaus, G.; Brings, S.; Kaufman, C.; Leotta, K.; Urban, S.; Haberkorn, U.; Mier, W.; Fricker, G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur. J. Pharm. Biopharm. 2016, 103, 159–166. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Santonocito, D.; Sarpietro, M.G.; Castelli, F.; Lauro, M.R.; Torrisi, C.; Russo, S.; Puglia, C. Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations. Moleculea 2023, 28, 1545. [Google Scholar] [CrossRef]
- Quehenberger, J.; Pittenauer, E.; Allmaier, G.; Spadiut, O. The influence of the specific growth rate on the lipid composition of Sulfolobus acidocaldarius. Extremophiles 2020, 24, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.; Hussain, M.T.; Briuglia, M.L.; Edwards, D.P.; ter Horst, J.H.; Szita, N.; Perrie, Y. Rapid and scale-independent microfluidic manufacture of liposomes entrapping protein incorporating in-line purification and at-line size monitoring. Int. J. Pharm. 2019, 556, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef]
- Perrie, Y.; Kastner, E.; Khadke, S.; Roces, C.B.; Stone, P. Manufacturing Methods for Liposome Adjuvants. Methods Mol. Biol. 2017, 1494, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Kendall, D.A.; MacDonald, R.C. A simple procedure for the determination of the trapped volume of liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 1982, 691, 332–340. [Google Scholar] [CrossRef]
- Gradauer, K.; Vonach, C.; Leitinger, G.; Kolb, D.; Frohlich, E.; Roblegg, E.; Bernkop-Schnurch, A.; Prassl, R. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. Int. J. Nanomed. 2012, 7, 2523–2534. [Google Scholar] [CrossRef]
- Sleigh, S.H. Insulin preparations and analogues: Structure and properties. J. Diabetes Nurs. 1998, 2, 150. [Google Scholar]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality x-ray data. Phys. Rev. E. Stat. Phys. Plasmas. Fluids Relat. Interdiscip. Top. 2000, 62, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Koschuch, R.; Pozo-Navas, B.; Rappolt, M.; Lohner, K.; Laggner, P. Structural analysis of weakly ordered membrane stacks. J. Appl. Crystallogr. 2003, 63, 1378–1388. [Google Scholar] [CrossRef]
- Kornmueller, K.; Lehofer, B.; Leitinger, G.; Amenitsch, H.; Prassl, R. Peptide self-assembly into lamellar phases and the formation of lipid-peptide nanostructures. Nano Res. 2017, 11, 913–928. [Google Scholar] [CrossRef]
- Schimpel, C.; Teubl, B.; Absenger, M.; Meindl, C.; Frohlich, E.; Leitinger, G.; Zimmer, A.; Roblegg, E. Development of an advanced intestinal in vitro triple culture permeability model to study transport of nanoparticles. Mol. Pharm. 2014, 11, 808–818. [Google Scholar] [CrossRef]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Tetyczka, C.; Hartl, S.; Jeitler, R.; Absenger-Novak, M.; Meindl, C.; Froehlich, E.; Riedl, S.; Zweytick, D.; Roblegg, E. Cytokine-Mediated Inflammation in the Oral Cavity and Its Effect on Lipid Nanocarriers. Nanomaterials 2021, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Agbayani, G.; Chandan, V.; Iqbal, U.; Dudani, R.; Qian, H.; Jakubek, Z.; Chan, K.; Harrison, B.; Deschatelets, L.; et al. Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology. Pharmaceutics 2022, 14, 2291. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.-G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef] [PubMed]
- Piunti, C.; Cimetta, E. Microfluidic approaches for producing lipid-based nanoparticles for drug delivery applications. Biophys. Rev. 2023, 4, 031304. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Tarek, M.; Tomšič, M.; Valant, J.; Ulrih, N.P.; Jamnik, A.; Kramar, P.; Miklavčič, D. Structural properties of archaeal lipid bilayers: Small-angle X-ray scattering and molecular dynamics simulation study. Langmuir 2014, 30 28, 8308–8315. [Google Scholar] [CrossRef]
- Balgavý, P.; Dubničková, M.; Kučerka, N.; Kiselev, M.A.; Yaradaikin, S.P.; Uhríková, D. Bilayer thickness and lipid interface area in unilamellar extruded 1,2-diacylphosphatidylcholine liposomes: A small-angle neutron scattering study. Biochim. Biophys. Acta (BBA) Biomembr. 2001, 1512, 40–52. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Salhi, A.; Amara, S.; Mansuelle, P.; Puppo, R.; Lebrun, R.; Gontero, B.; Aloulou, A.; Carrière, F. Characterization of all the lipolytic activities in pancreatin and comparison with porcine and human pancreatic juices. Biochimie 2020, 169, 106–120. [Google Scholar] [CrossRef]
- Patel, G.B.; Sprott, G.D. Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit. Rev. Biotechnol. 1999, 19, 317–357. [Google Scholar] [CrossRef]
- Guldiken, B.; Linke, A.; Capanoglu, E.; Boyacioglu, D.; Kohlus, R.; Weiss, J.; Gibis, M. Formation and characterization of spray dried coated and uncoated liposomes with encapsulated black carrot extract. J. Food Eng. 2019, 246, 42–50. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-Drying Microencapsulation of Polyphenol Bioactives: A Comparative Study Using Different Natural Fibre Polymers as Encapsulants. Food Bioprocess. Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Sedlmayr, V.; Horn, C.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. Archaeosomes facilitate storage and oral delivery of cannabidiol. Int. J. Pharm. 2023, 645, 123434. [Google Scholar] [CrossRef] [PubMed]
- Rastädter, K.; Wurm, D.J.; Spadiut, O.; Quehenberger, J. The Cell Membrane of Sulfolobus spp.-Homeoviscous Adaption and Biotechnological Applications. Int. J. Mol. Sci. 2020, 21, 3935. [Google Scholar] [CrossRef] [PubMed]
- Bramkamp, M. Fluidity is the way to life: Lipid phase separation in bacterial membranes. EMBO J. 2022, 41, e110737. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, P.B.; Genova, J. Archaeosomes: New Generation of Liposomes Based on Archaeal Lipids for Drug Delivery and Biomedical Applications. ACS Omega 2023, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kejžar, J.; Osojnik Črnivec, I.G.; Poklar Ulrih, N. Advances in Physicochemical and Biochemical Characterization of Archaeosomes from Polar Lipids of Aeropyrum pernix K1 and Stability in Biological Systems. ACS Omega 2023, 8, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Mucus as barrier for drug delivery by nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 126–136. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Poelma, F.G.J.; Junginger, H.E.; Tukker, J.J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 1991, 70, 235–240. [Google Scholar] [CrossRef]
- Patel, G.B.; Agnew, B.J.; Deschatelets, L.; Fleming, L.P.; Sprott, G.D. In vitro assessment of archaeosome stability for developing oral delivery systems. Int. J. Pharm. 2000, 194, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sprott, G.D. Structures of archaebacterial membrane lipids. J. Bioenerg. Biomembr. 1992, 24, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.; Hofhaus, G.; Thomas, S.; Cuesta, L.C.; Gropp, F.; Schröder, R.; Hartmann, K.; Fricker, G. Improved Oral Bioavailability of Human Growth Hormone by a Combination of Liposomes Containing Bio-Enhancers and Tetraether Lipids and Omeprazole. J. Pharm. Sci. 2014, 103, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, J.; Thewes, B.; Gropp, F.; Fricker, G. Oral peptide delivery by tetraether lipid liposomes. Int. J. Pharm. 2011, 415, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Morilla, M.J.; Gomez, D.M.; Cabral, P.; Cabrera, M.; Balter, H.; Tesoriero, M.V.; Higa, L.; Roncaglia, D.; Romero, E.L. M cells prefer archaeosomes: An in vitro/in vivo snapshot upon oral gavage in rats. Curr. Drug Deliv. 2011, 8, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, L.; Dicaire, C.J.; Patel, G.B.; Sprott, G.D. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: Comparison to conventional liposomes and alum. Infect. Immun. 2000, 68, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.B.; Zhou, H.; KuoLee, R.; Chen, W. Archaeosomes as adjuvants for combination vaccines. J. Liposome Res. 2004, 14, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J. The Centenary of the Discovery of Insulin: An Update on the Quest for Oral Delivery. Front. Drug Deliv. 2021, 1, 726675. [Google Scholar] [CrossRef]
- Gedawy, A.; Martinez, J.; Al-Salami, H.; Dass, C.R. Oral insulin delivery: Existing barriers and current counter-strategies. J. Pharm. Pharmacol. 2018, 70, 197–213. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef]
- Limenh, L.W. A review on oral novel delivery systems of insulin through the novel delivery system formulations: A review. SAGE Open Med. 2024, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, R.; Roque, L.; Reis, C.P. Oral insulin delivery: Utopia, currently possible or a near reality? Ther. Deliv. 2021, 12, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]



| Organic Solvent | Loading | Mean Particle Size [nm] | PDI |
|---|---|---|---|
| Stability assays | |||
| EtOH | - | 85.4 ± 1.1 | 0.336 ± 0.017 |
| DMSO-iPA (2:1 v/v) | - | 86.3 ± 0.7 | 0.423 ± 0.140 |
| DMSO-iPA (2:1 v/v) | calcein | 111.6 ± 1.3 | 0.070 ± 0.002 |
| Cellular uptake studies | |||
| DMSO-iPA (2:1 v/v) | calcein | 129.6 ± 0.7 | 0.119 ± 0.013 |
| DMSO-iPA (2:1 v/v) | calcein + DOPE-rhodamine | 127.0 ± 0.2 | 0.107 ± 0.006 |
| DMSO-iPA (2:1 v/v) | DOPE-rhodamine | 137.7 ± 0.5 | 0.220 ± 0.050 |
| Powder production | |||
| EtOH | insulin | 97.5 ± 1.7 | 0.285 ± 0.031 |
| EtOH | insulin * | 103.1 ± 1.2 | 0.348 ± 0.079 |
| EtOH | insulin ** | 105.9 ± 2.1 | 0.324 ± 0.064 |
| Inserted | Apical | Basal | Cell Attached | |
|---|---|---|---|---|
| TR [%] | 100 | 72.982 ± 1.672 | 0.063 ± 0.007 | ~27 |
| TR_C [%] | 100 | 70.994 ± 13.036 | 0.065 ± 0.009 | ~29 |
| Inserted | Apical | Basal | Cell Attached | ||
|---|---|---|---|---|---|
| T_C | Calcein [%] | 100 | 96.510 ± 0.382 | 0.418 ± 0.002 | ~3 |
| TR_C | Calcein [%] | 100 | 80.417 ± 0.385 | 0.443 ± 0.002 | ~19 |
| Insulin | T_ins | T_ins SD * | TEL_ins LYO * | |
|---|---|---|---|---|
| Mean particle size [nm] | 97.5 ± 0.5 | 103.1 ± 1.2 | 105.9 ± 1.4 | |
| PDI | 0.285 ± 0.003 | 0.348 ± 0.009 | 0.324 ± 0.004 | |
| Insulin [µg/mL] | 10,261 ± 35 | 3631 ± 13 | 2045 ± 17 | 2120 ± 12 |
| % EE | ~35 | |||
| Overall recovery % | ~20 | ~21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidakovic, I.; Kornmueller, K.; Fiedler, D.; Khinast, J.; Fröhlich, E.; Leitinger, G.; Horn, C.; Quehenberger, J.; Spadiut, O.; Prassl, R. Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations. Pharmaceutics 2024, 16, 694. https://doi.org/10.3390/pharmaceutics16060694
Vidakovic I, Kornmueller K, Fiedler D, Khinast J, Fröhlich E, Leitinger G, Horn C, Quehenberger J, Spadiut O, Prassl R. Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations. Pharmaceutics. 2024; 16(6):694. https://doi.org/10.3390/pharmaceutics16060694
Chicago/Turabian StyleVidakovic, Ivan, Karin Kornmueller, Daniela Fiedler, Johannes Khinast, Eleonore Fröhlich, Gerd Leitinger, Christina Horn, Julian Quehenberger, Oliver Spadiut, and Ruth Prassl. 2024. "Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations" Pharmaceutics 16, no. 6: 694. https://doi.org/10.3390/pharmaceutics16060694
APA StyleVidakovic, I., Kornmueller, K., Fiedler, D., Khinast, J., Fröhlich, E., Leitinger, G., Horn, C., Quehenberger, J., Spadiut, O., & Prassl, R. (2024). Archaeosomes for Oral Drug Delivery: From Continuous Microfluidics Production to Powdered Formulations. Pharmaceutics, 16(6), 694. https://doi.org/10.3390/pharmaceutics16060694










