Daptomycin Liposomes Exhibit Enhanced Activity against Staphylococci Biofilms Compared to Free Drug
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Daptomycin-Loaded Liposomes
2.1.1. Thin Film Method (TFM)
2.1.2. DRV Method
2.1.3. MicroFluidics Mixing (MM)
2.2. Physicochemical Characterization of Dapto-Liposomes
2.2.1. Dapto Encapsulation Efficiency
2.2.2. Liposome Size Distribution, Zeta-Potential and Morphology
2.2.3. Drug Release and Stability Studies
2.3. Antimicrobial Activity (In Vitro)
2.3.1. Bacterial Strains and Growth Conditions
2.3.2. Bacterial Growth Curve Assay
2.3.3. Biofilm Susceptibility Assays (Prevention and Treatment)
2.4. Statistical Analysis
3. Results
3.1. Dapto Liposome Physicochemical Properties
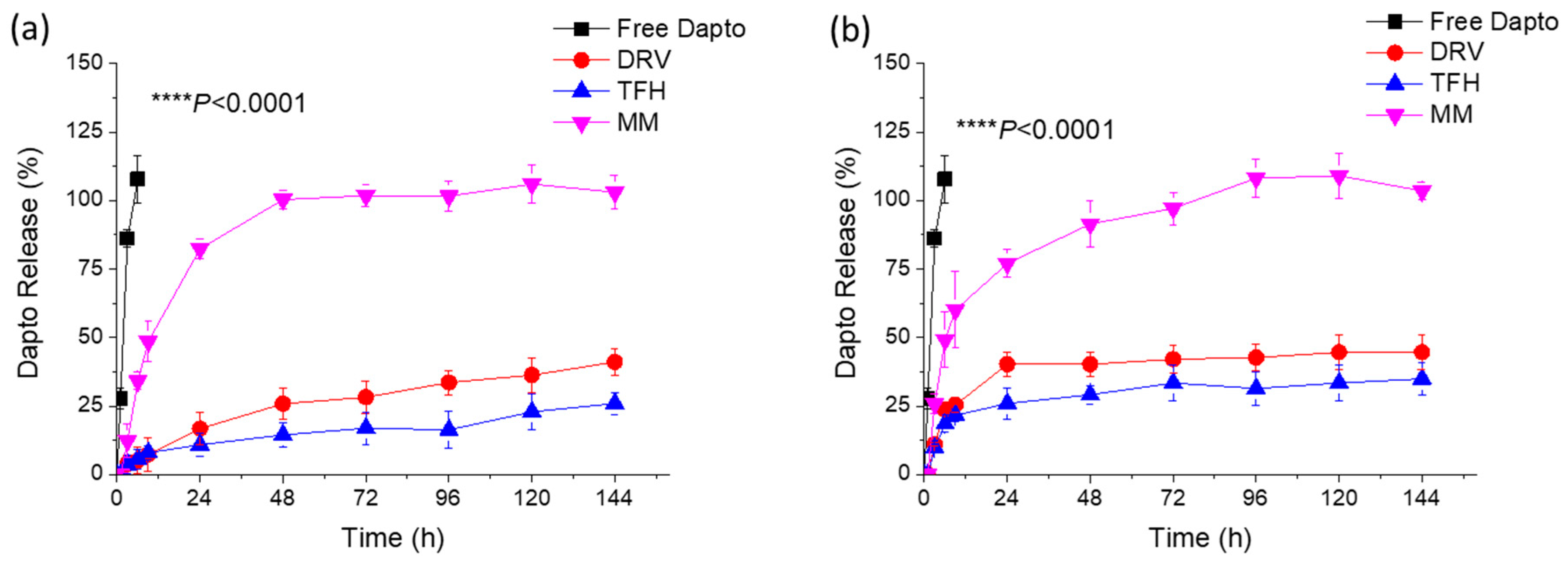
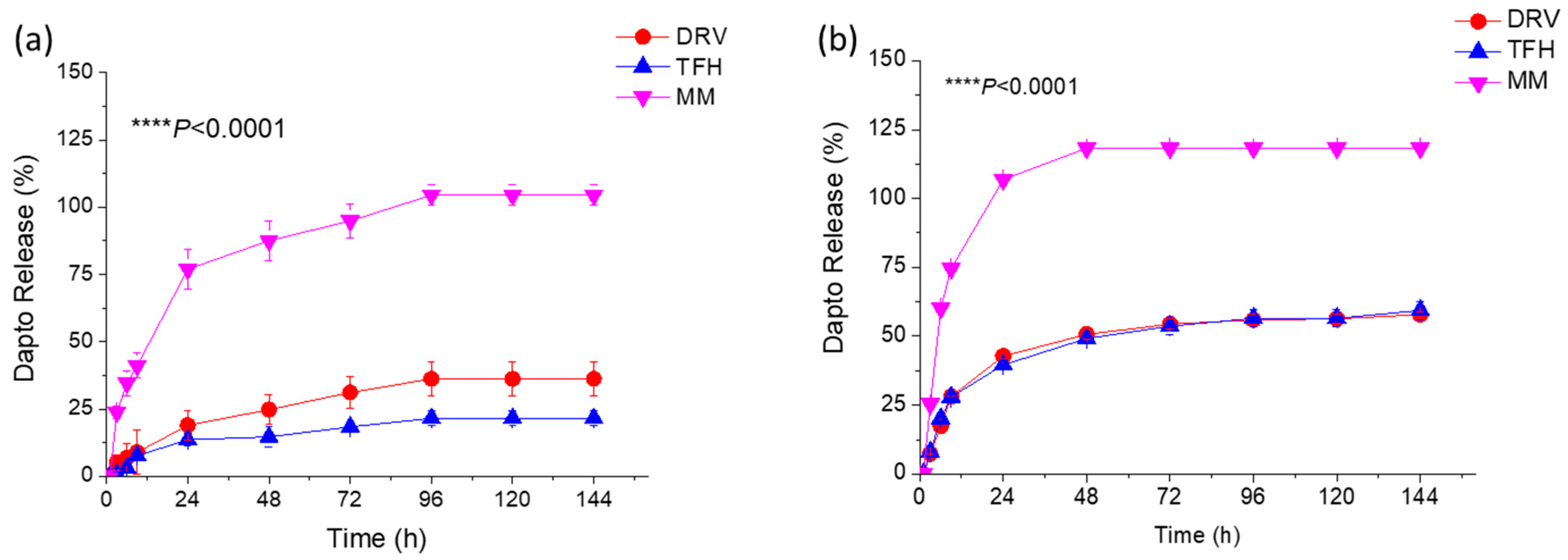
3.2. Liposomal Dapto Release and Chemical Stability Studies
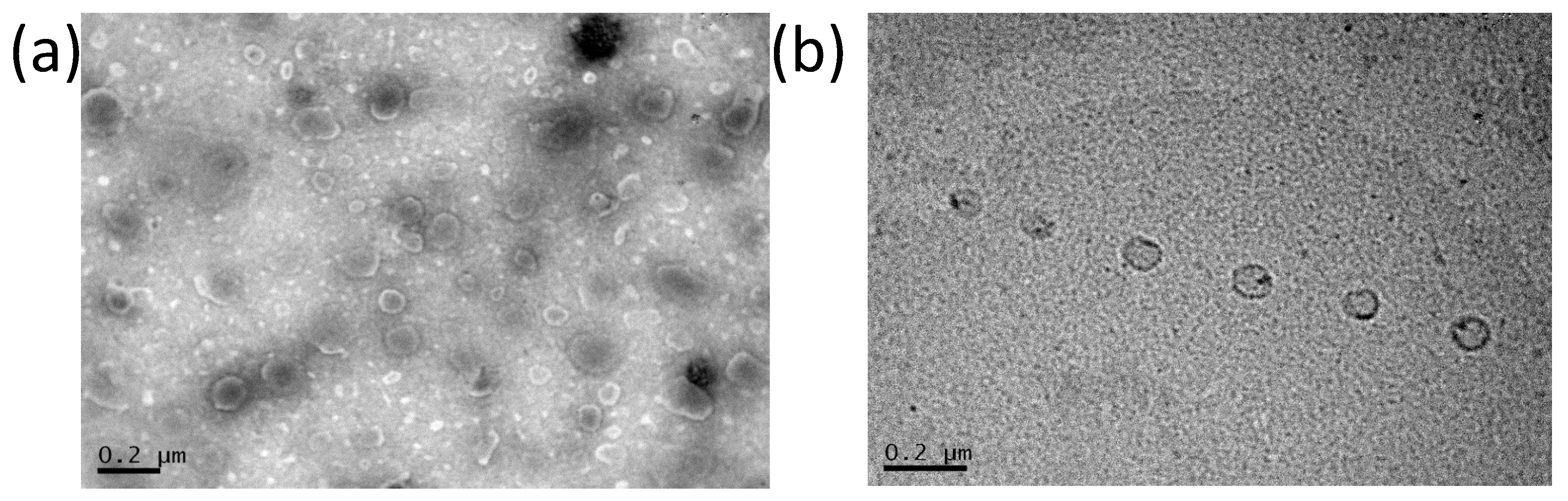
3.3. Transmission Electron Microscopy of Dapto Liposomes
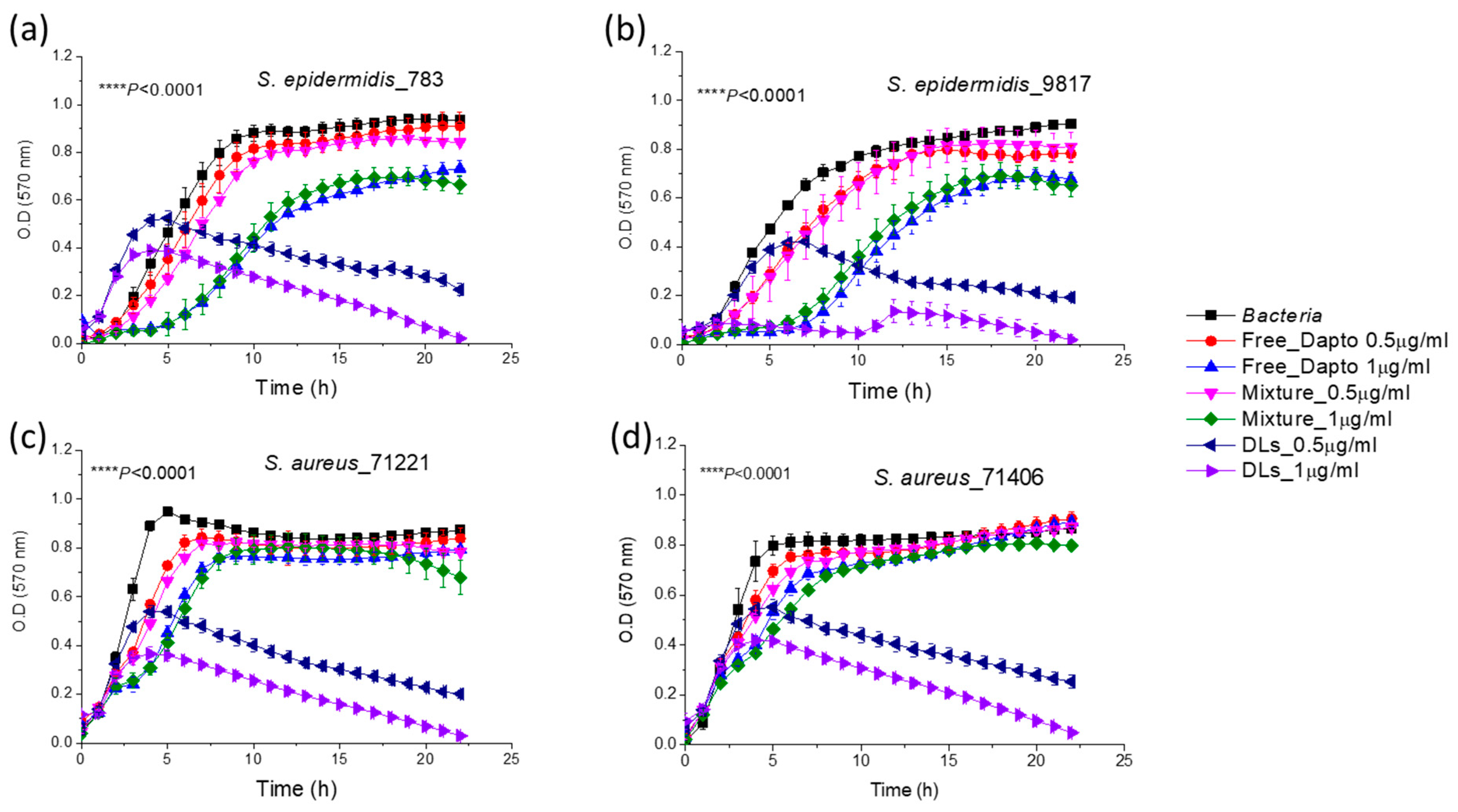
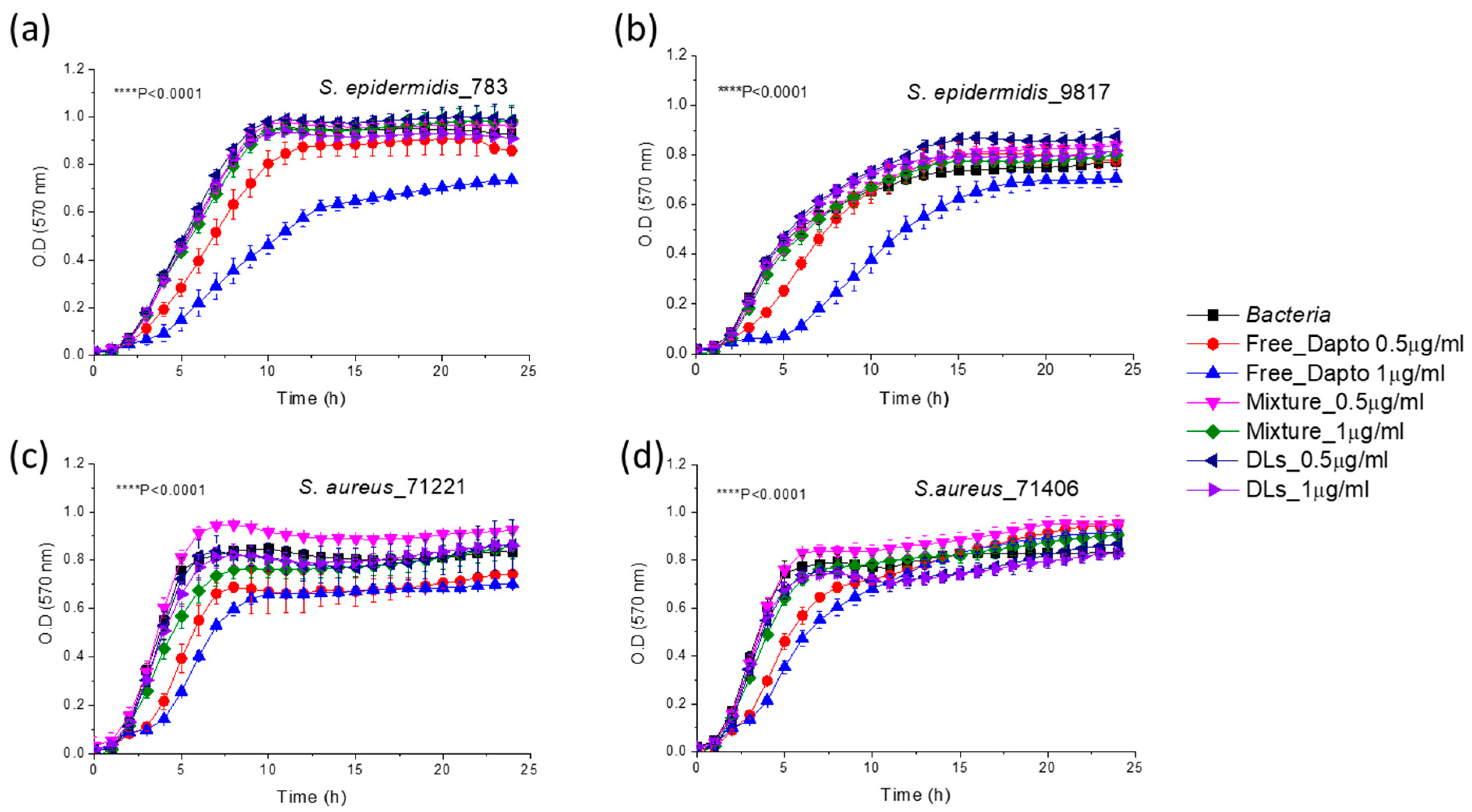
3.4. Inhibition of Planktonic Bacterial Growth by Dapto Liposomes-Effect of Lipid Composition
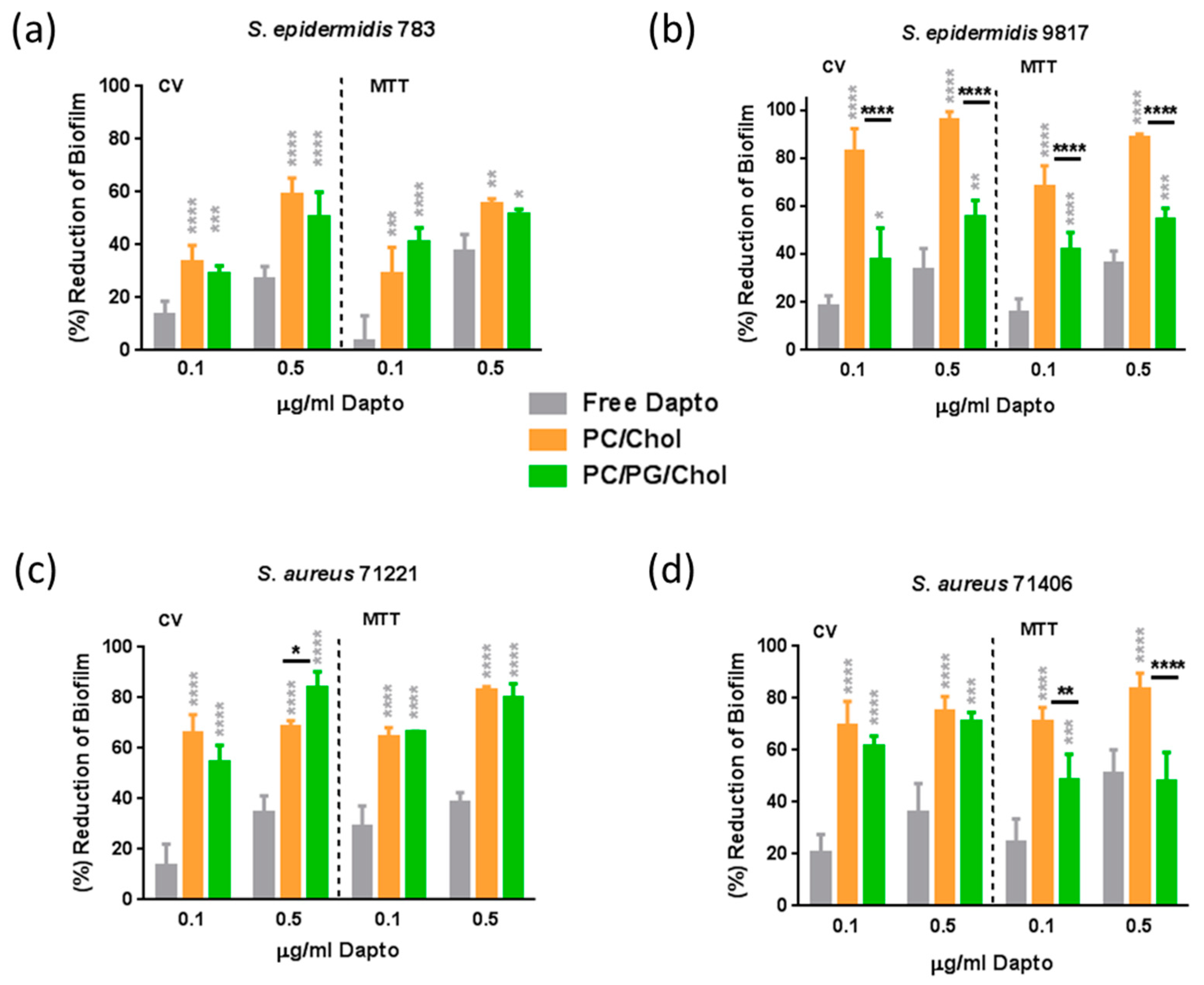
3.5. Antibiofilm Activity of Dapto Liposomes—Effect of Lipid Composition
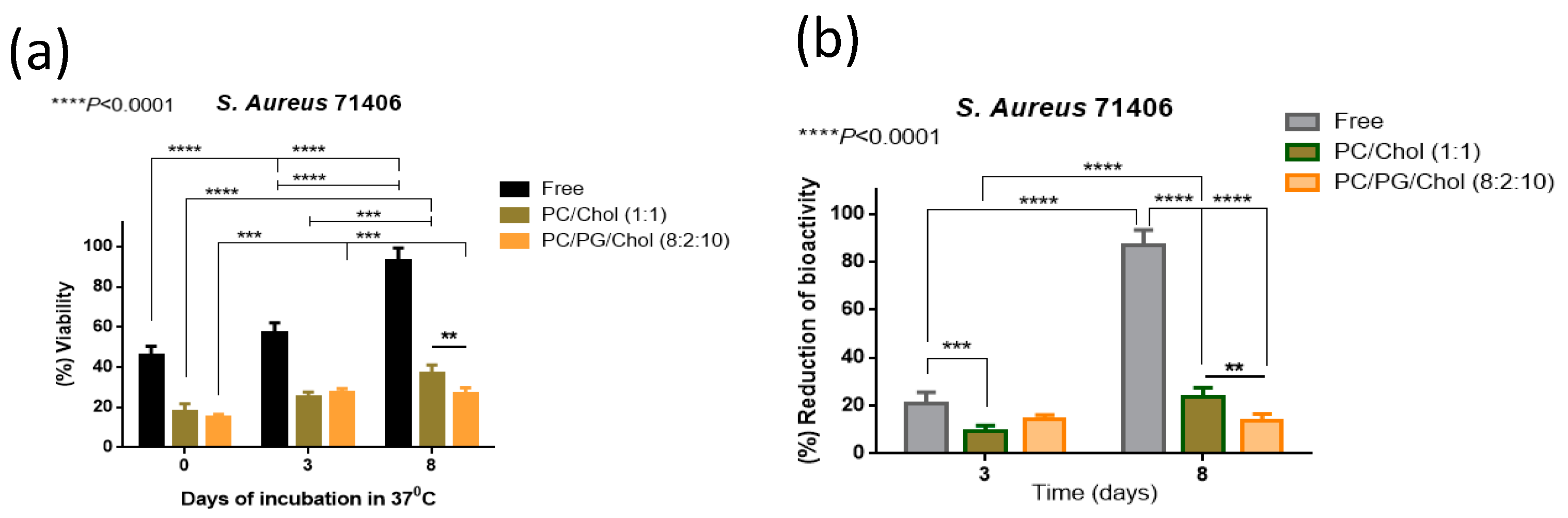
3.6. Preservation of Antibiofilm Activity of Dapto by Liposome Encapsulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, E.Y.; Jiang, W.; Mojica, N.; Tseng, K.K.; McNeill, R.; Cosgrove, S.E.; Perl, T.M. National Costs Associated with Methicillin-Susceptible and Methicillin-Resistant Staphylococcus aureus Hospitalizations in the United States, 2010-2014. Clin. Infect. Dis. 2019, 68, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Sharma, M.; Raut, S.; Gupta, P.; Khatri, N. Healing wounds, defeating biofilms: Lactiplantibacillus plantarum in tackling MRSA infections. Front. Microbiol. 2023, 14, 1284195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of Medical Devices by Staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus aureus. Nat. Rev. Dis. Primer 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Fischer, A.; Yang, S.J.; Bayer, A.S.; Vaezzadeh, A.R.; Herzig, S.; Stenz, L.; Girard, M.; Sakoulas, G.; Scherl, A.; Yeaman, M.R.; et al. Daptomycin resistance mechanisms in clinically derived Staphylococcus aureus strains assessed by a combined transcriptomics and proteomics approach. J. Antimicrob. Chemother. 2011, 66, 1696–1711. [Google Scholar] [CrossRef]
- Lambert, M. IDSA Guidelines on the Treatment of MRSA Infections in Adults and Children. Am. Fam. Physician 2011, 84, 455–463. [Google Scholar]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tzalis, S.; Ioannou, P.; Billiari, E.; Kofteridis, D.P.; Karakonstantis, S. Daptomycin as an option for lock therapy: A systematic literature review. Future Microbiol. 2023, 18, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Pai, L.; Patil, S.; Liu, S.; Wen, F. A growing battlefield in the war against biofilm-induced antimicrobial resistance: Insights from reviews on antibiotic resistance. Front. Cell Infect. Microbiol. 2023, 13, 1327069. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Davison, W.M.; Steenbergen, J.N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 2009, 53, 3505–3507. [Google Scholar] [CrossRef] [PubMed]
- Plota, M.; Sazakli, E.; Giormezis, N.; Gkartziou, F.; Kolonitsiou, F.; Leotsinidis, M.; Antimisiaris, S.G.; Spiliopoulou, I. In Vitro Anti-Biofilm Activity of Bacteriophage K (ATCC 19685-B1) and Daptomycin against Staphylococci. Microorganisms 2021, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Pherez, F.M. Daptomycin resistance and treatment failure following vancomycin for methicillin-resistant Staphylococcus aureus (MRSA) mitral valve acute bacterial endocarditis (ABE). Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Mangili, A.; Bica, I.; Snydman, D.R.; Hamer, D.H. Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 2005, 40, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Santos Ferreira, I.; Kikhney, J.; Kursawe, L.; Kasper, S.; Gonçalves, L.M.D.; Trampuz, A.; Moter, A.; Bettencourt, A.F.; Almeida, A.J. Encapsulation in Polymeric Microparticles Improves Daptomycin Activity Against Mature Staphylococci Biofilms-a Thermal and Imaging Study. AAPS PharmSciTech 2018, 19, 1625–1636. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Santos, S.B.; Melo, L.D.R.; Santos, R.S.; Guimarães, N.; Azevedo, N.F.; Loureiro, J.A.; Pereira, M.C. Fighting Methicillin-Resistant Staphylococcus aureus with Targeted Nanoparticles. Int. J. Mol. Sci. 2023, 24, 9030. [Google Scholar] [CrossRef]
- Arroyo-Urea, E.M.; Lázaro-Díez, M.; Garmendia, J.; Herranz, F.; González-Paredes, A. Lipid-based nanomedicines for the treatment of bacterial respiratory infections: Current state and new perspectives. Nanomedicine 2024, 19, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-da-Silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Arregui, J.R.; Kovvasu, S.P.; Betageri, G.V. Daptomycin proliposomes for oral delivery: Formulation, characterization, and in vivo pharmacokinetics. AAPS PharmSciTech 2018, 19, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.; Huang, X.; Wang, X.; Liao, G.; Chen, Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int. J. Nanomed. 2013, 8, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Su, T.; Zhang, Y.; Huang, X.; Li, J.; Li, C. Liposomal co-delivery of daptomycin and clarithromycin at an optimized ratio for treatment of methicillin-resistant Staphylococcus aureus infection. Drug Deliv. 2015, 22, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Kogkos, G.; Gkartziou, F.; Mourtas, S.; Barlos, K.K.; Klepetsanis, P.; Barlos, K.; Antimisiaris, S.G. Liposomal Entrapment or Chemical Modification of Relaxin2 for Prolongation of Its Stability and Biological Activity. Biomolecules 2022, 12, 1362. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G. Preparation of DRV Liposomes. Methods Mol. Biol. 2023, 2622, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Natsaridis, E.; Gkartziou, F.; Mourtas, S.; Stuart, M.C.A.; Kolonitsiou, F.; Klepetsanis, P.; Spiliopoulou, I.; Antimisiaris, S.G. Moxifloxacin Liposomes: Effect of Liposome Preparation Method on Physicochemical Properties and Antimicrobial Activity against Staphylococcus epidermidis. Pharmaceutics 2022, 14, 370. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Sánchez-Rubio Ferrández, J.; Vázquez Sánchez, R.; Córdoba Díaz, D.; Córdoba Díaz, M.; Lozano Estevan, M.C.; Molina García, T. Stability of daptomycin reconstituted vials and infusion solutions. Eur. J. Hosp. Pharm. 2018, 25, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta potential and membrane permeability in bacteria: A study with cationic agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef]
- Li, C.; Cui, J.; Wang, C.; Zhang, L.; Xiu, X.; Li, Y.; Wei, N.; Li, Y.; Zhang, L. Encapsulation of vinorelbine into cholesterol-polyethylene glycol coated vesicles: Drug loading and pharmacokinetic studies. J. Pharm. Pharmacol. 2011, 63, 376–384. [Google Scholar] [CrossRef]
- Zhang, T.; Muraih, J.K.; Tishbi, N.; Herskowitz, J.; Victor, R.L.; Silverman, J.; Uwumarenogie, S.; Taylor, S.D.; Palmer, M.; Mintzer, E. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J. Biol. Chem. 2014, 289, 11584–11591. [Google Scholar] [CrossRef] [PubMed]
- Matloob, A.H.; Mourtas, S.; Klepetsanis, P.; Antimisiaris, S.G. Increasing the stability of curcumin in serum with liposomes or hybrid drug-in-cyclodextrin-in-liposome systems: A comparative study. Int. J. Pharm. 2014, 476, 108–115. [Google Scholar] [CrossRef]
- Alhariri, M.; Majrashi, M.A.; Bahkali, A.H.; Almajed, F.S.; Azghani, A.O.; Khiyami, M.A.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M.A. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, 12, 6949–6961. [Google Scholar] [CrossRef]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time-kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Gubernator, J.; Dorotkiewicz-Jach, A.; Doroszkiewicz, W.; Kozubek, A. A comparison of the in vitro antimicrobial activity of liposomes containing meropenem and gentamicin. Cell. Mol. Biol. Lett. 2006, 11, 360–375. [Google Scholar] [CrossRef]
- Alhajlan, M.; Alhariri, M.; Omri, A. Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors. Antimicrob. Agents Chemother. 2013, 57, 2694–2704. [Google Scholar] [CrossRef]
- Messiaen, A.S.; Forier, K.; Nelis, H.; Braeckmans, K.; Coenye, T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS ONE 2013, 8, e79220. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.M.; Omri, A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Natsaridis, E.; Mouzoura, P.; Gkartziou, F.; Marazioti, A.; Antimisiaris, S.G. Development of growth factor-incorporating liposomes for integration into scaffolds as a method to improve tissue regeneration. Int. J. Dev. Biol. 2022, 66, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xiong, M.; Bi, Q.; Wang, Y.; Li, C. Self-enhanced targeted delivery of a cell wall- and membrane-active antibiotics, daptomycin, against staphylococcal pneumonia. Acta Pharm. Sin. B 2016, 6, 319–328. [Google Scholar] [CrossRef]
- Rani, N.N.I.M.; Chen, X.Y.; Al-Zubaidi, Z.M.; Azhari, H.; Khaitir, T.M.N.; Ng, P.Y.; Buang, F.; Tan, G.C.; Wong, Y.P.; Said, M.M.; et al. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J. Pharm. Sci. 2022, 17, 102–119. [Google Scholar] [CrossRef]


| Method | EE (%) | Mean Hydr. Diameter (nm) | PDI | ζ-Pot (mV) |
|---|---|---|---|---|
| TFH | 27.9 ± 1.8 | 119.2 ± 6.5 | 0.198 | −8.7 ± 2.3 |
| DRV | 31.7 ± 4.0 | 102.6 ± 5.7 | 0.068 | −8.80 ± 0.17 |
| MM | 8.1 ± 1.0 | 113.8 ± 7.6 | 0.282 | −3.7 ± 1.4 |
| Method | EE (%) | Mean Hydr. Diameter (nm) | PDI | ζ-Pot (mV) |
|---|---|---|---|---|
| TFH | 30.1 ± 6.6 | 98.3 ± 6.3 | 0.065 | −23.2 ± 1.1 |
| DRV | 37.6 ± 7.3 | 103.5 ± 1.77 | 0.046 | −21.9 ± 1.8 |
| MM | 4.2 ± 0.5 | 83.8 ± 4.4 | 0.192 | −20.5 ± 2.3 |
| Lipid Composition | EE (% D/L) | Mean Hydr. Diameter (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|---|
| PC/Chol (2:1) | 25.8 ± 5.8 | 122.2 ± 9.5 | 0.93 | −6.2 ± 2.9 |
| PC/Chol (1:1) | 30.06 ± 5.2 | 119.23 ± 6.48 | 0.198 | −8.8 ± 2.3 |
| PC/Chol/PEG (1:1:0.17) | 3.56 ± 0.009 | 117.1 ± 8.4 | 0.125 | −7.7 ± 0.6 |
| PC/PG/Chol (8:2:5) | 37.2 ± 9 | 127 ± 12 | 0.182 | −21.60 ± 0.75 |
| PC/PG/Chol (8:2:10) | 31.2 ± 5 | 120.3 ± 9.3 | 0.182 | −30.9 ± 1.6 |
| PC/PG/Chol/PEG (8:2:10:1.7) | 3.56 ± 0.21 | 98.6 ± 5.3 | 0.187 | −8.7 ± 1.0 |
| PC/Chol/PEG (1:1:0.17) Post-PEG * | 25.6 ± 2.0 | 106.7 ± 6.3 | 0.159 | −8.93 ± 0.47 |
| PC/PG/Chol/PEG (8:2:10:1.7) Post-PEG * | 32.2 ± 1.5 | 138.7 ± 8.8 | 0.175 | −13.4 ± 3.0 |
| Bacterial Strain | ζ-Potential (mV) |
|---|---|
| S. epidermidis _783 | −28.7 ± 1.30 |
| S. epidermidis _9817 | −20.8 ± 1.48 |
| S. aureus _71221 | −11.7 ± 3.44 |
| S. aureus _71406 | −23.8 ± 1.88 |
| Bacterial Strain | Biofilm Prevention Studies | Biofilm Reduction Studies | ||
|---|---|---|---|---|
| CV | MTT | CV | MTT | |
| S. epidermidis 783 | 1.6 ± 0.03 | 0.9 ± 0.07 | 2.3 ± 0.09 | 1.5 ± 0.04 |
| S. epidermidis 9817 | 0.32 ± 0.04 | 0.3 ± 0.08 | 0.8 ± 0.09 | 0.5 ± 0.09 |
| S. aureus 71406 | 0.6 ± 0.1 | 0.8 ± 0.08 | 2.1 ± 0.1 | 0.8 ± 0.1 |
| S. aureus 71221 | 0.4 ± 0.2 | 0.4 ± 0.08 | 1.7 ± 0.02 | 0.9 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkartziou, F.; Plota, M.; Kypraiou, C.; Gauttam, I.; Kolonitsiou, F.; Klepetsanis, P.; Spiliopoulou, I.; Antimisiaris, S.G. Daptomycin Liposomes Exhibit Enhanced Activity against Staphylococci Biofilms Compared to Free Drug. Pharmaceutics 2024, 16, 459. https://doi.org/10.3390/pharmaceutics16040459
Gkartziou F, Plota M, Kypraiou C, Gauttam I, Kolonitsiou F, Klepetsanis P, Spiliopoulou I, Antimisiaris SG. Daptomycin Liposomes Exhibit Enhanced Activity against Staphylococci Biofilms Compared to Free Drug. Pharmaceutics. 2024; 16(4):459. https://doi.org/10.3390/pharmaceutics16040459
Chicago/Turabian StyleGkartziou, Foteini, Maria Plota, Charikleia Kypraiou, Iti Gauttam, Fevronia Kolonitsiou, Pavlos Klepetsanis, Iris Spiliopoulou, and Sophia G. Antimisiaris. 2024. "Daptomycin Liposomes Exhibit Enhanced Activity against Staphylococci Biofilms Compared to Free Drug" Pharmaceutics 16, no. 4: 459. https://doi.org/10.3390/pharmaceutics16040459
APA StyleGkartziou, F., Plota, M., Kypraiou, C., Gauttam, I., Kolonitsiou, F., Klepetsanis, P., Spiliopoulou, I., & Antimisiaris, S. G. (2024). Daptomycin Liposomes Exhibit Enhanced Activity against Staphylococci Biofilms Compared to Free Drug. Pharmaceutics, 16(4), 459. https://doi.org/10.3390/pharmaceutics16040459







