Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Mutational Profile Analysis
2.3. Cell Growth Rate Estimation
2.4. Cell Viability Assay
2.5. Extracellular Lactate and Glucose Quantification
2.6. Bioenergetic Measurements (Seahorse Assays)
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
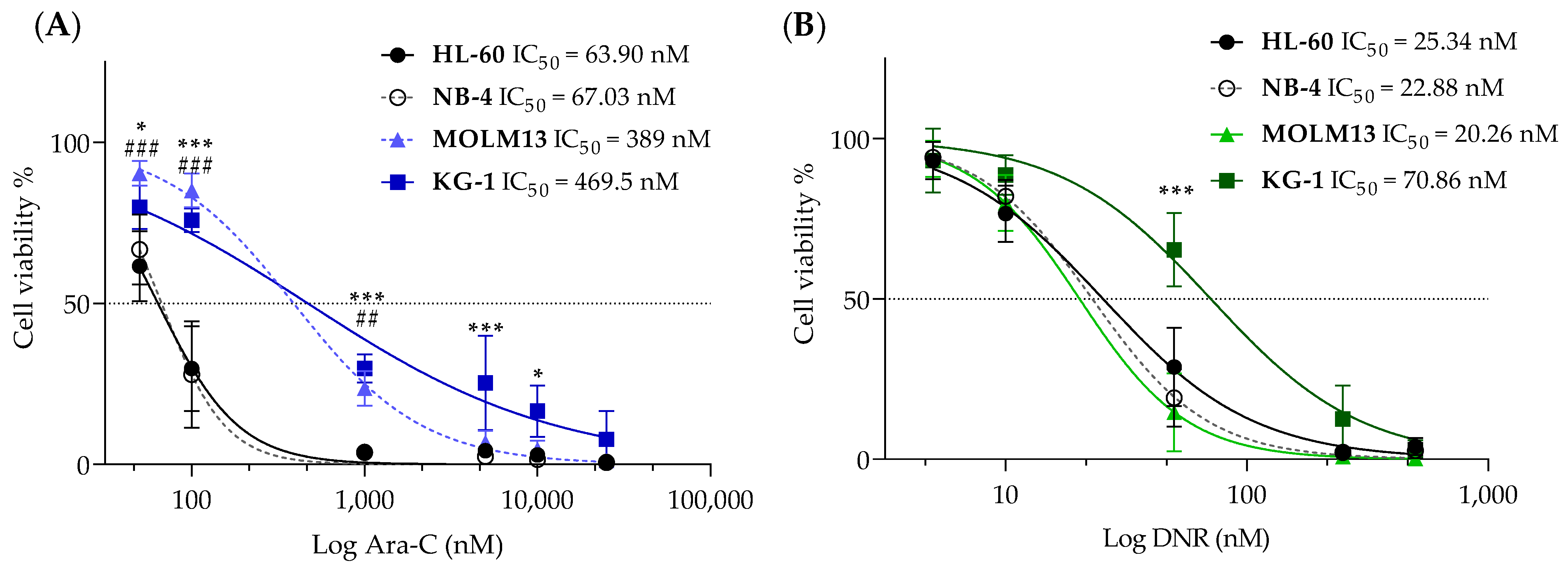
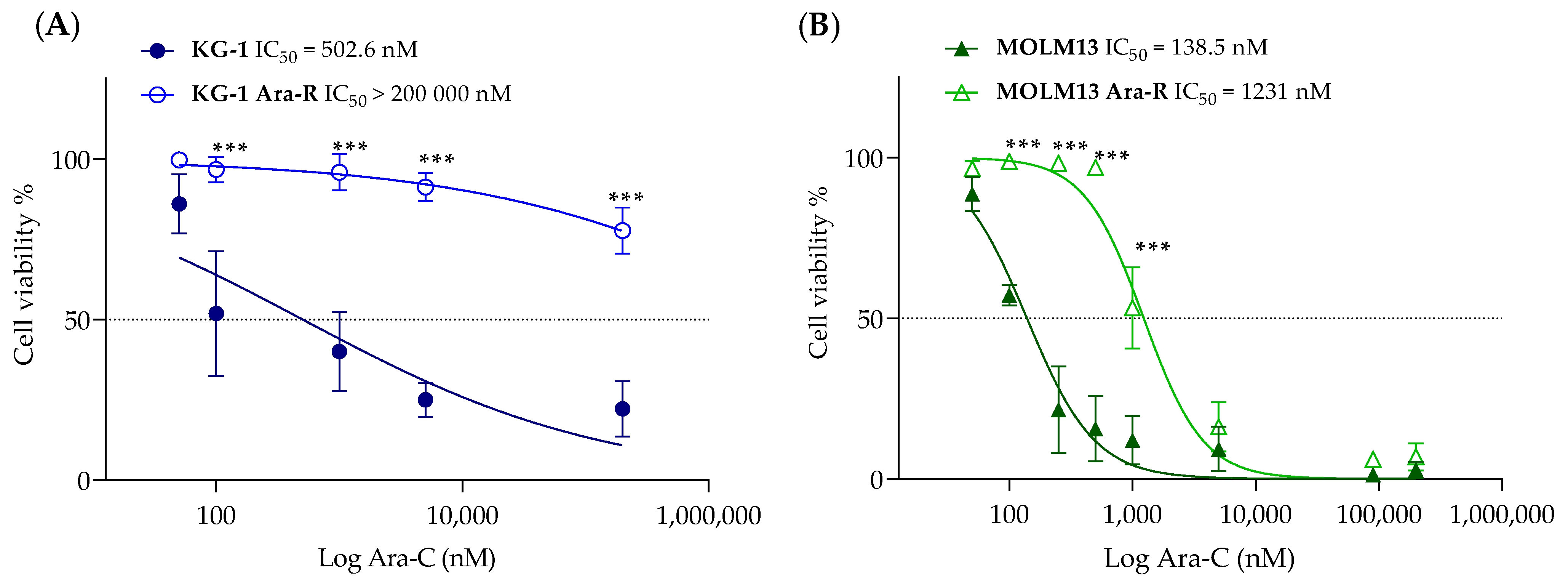
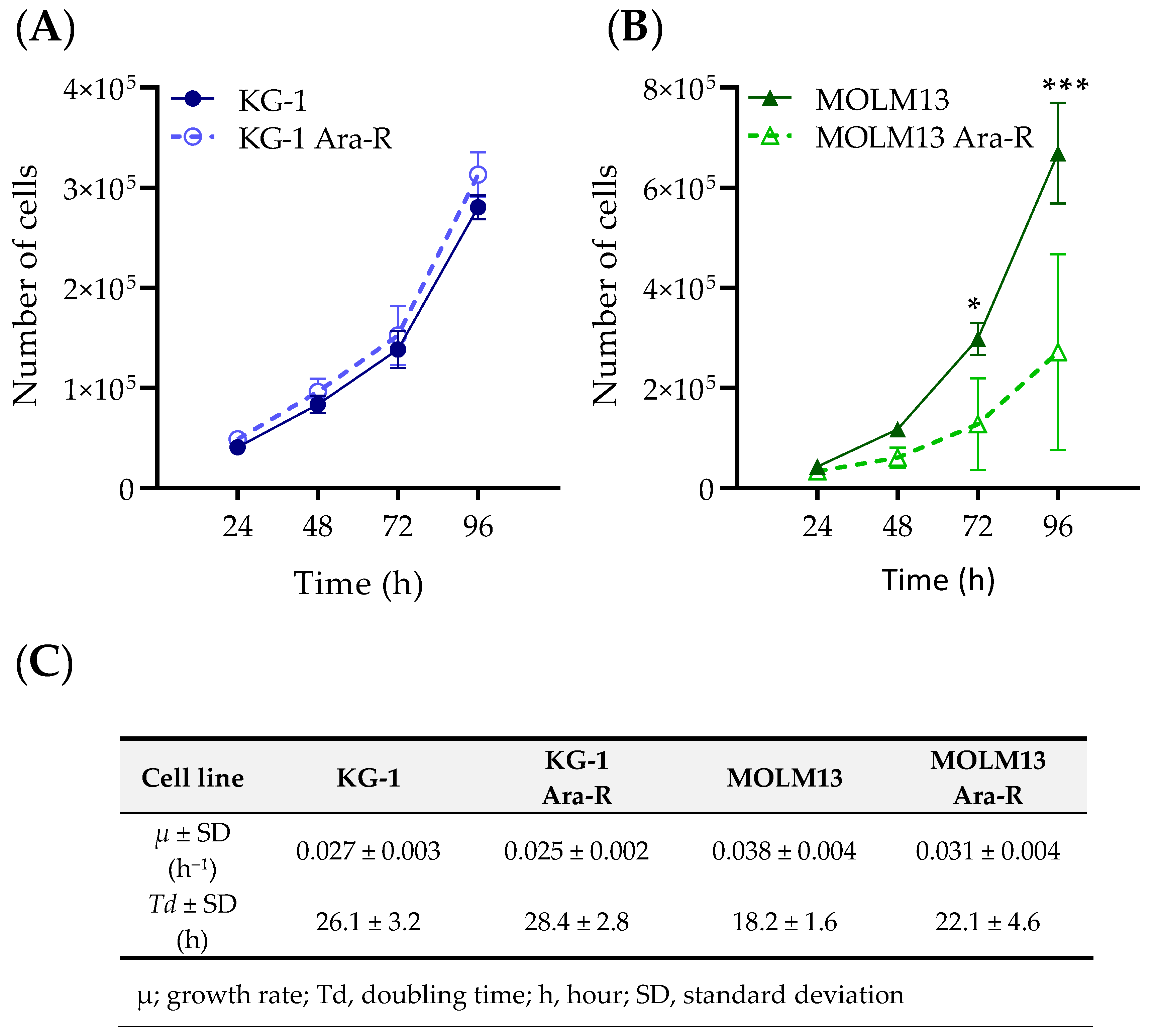
3.1. Development and Characterization of Cytarabine-Resistant KG-1 and MOLM13 Variants
3.2. Characterization of the Glycolytic and Respiratory Profile of Parental and Ara-R AML Cell Lines
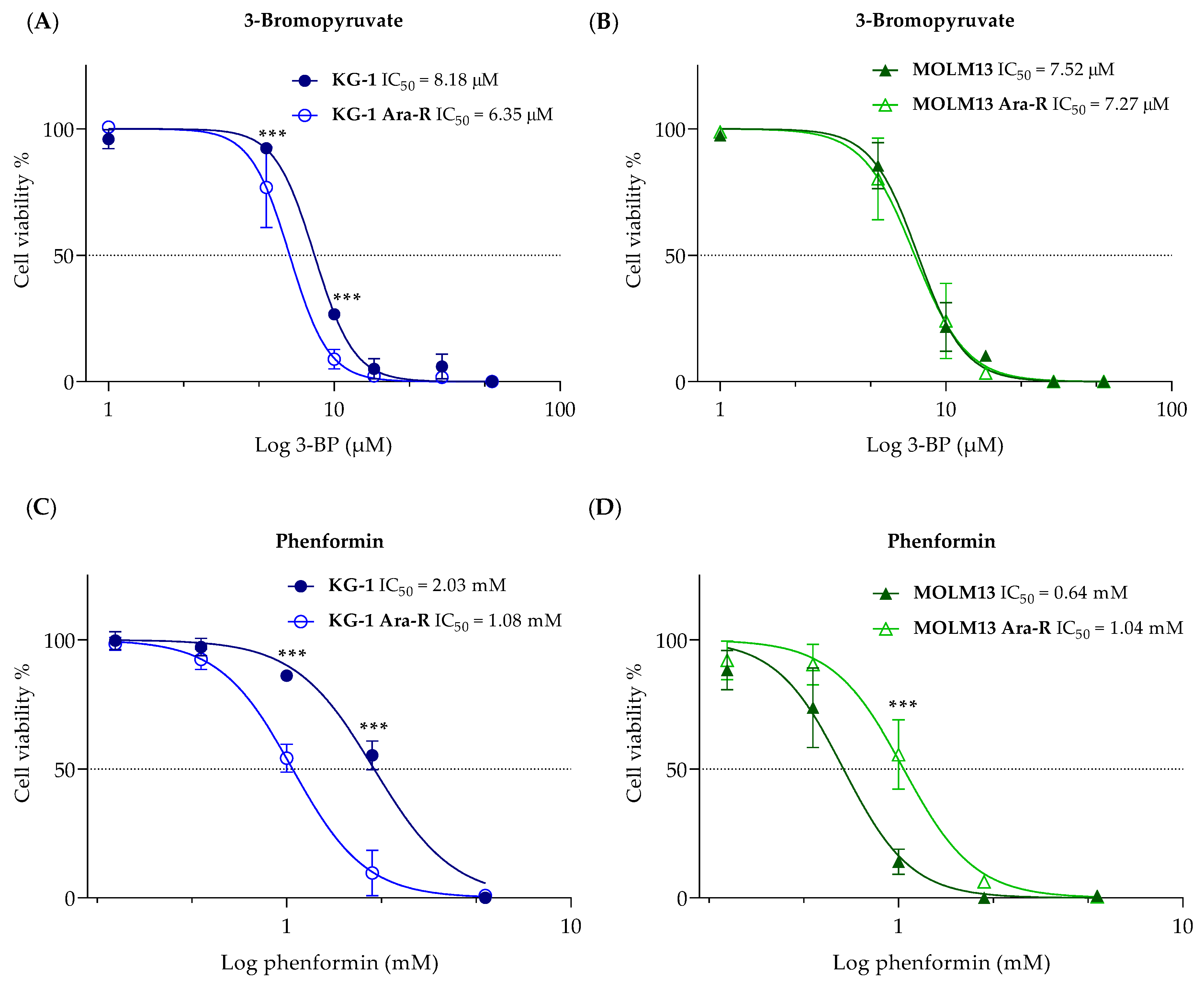
3.3. Effect of Metabolic Inhibitors on Cytarabine-Resistant Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoury, J.D.; Solary, E.; Abla, O.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Hochhaus, A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Ganzel, C.; Sun, Z.; Cripe, L.D.; Fernandez, H.F.; Douer, D.; Rowe, J.M.; Paietta, E.M.; Ketterling, R.; O’Connell, M.J.; Wiernik, P.H.; et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience. Am. J. Hematol. 2018, 93, 1074–1081. [Google Scholar] [CrossRef]
- Cancer.Net. Leukemia-Acute Myeloid-AML. Available online: https://www.cancer.net/cancer-types/leukemia-acute-myeloid-aml/statistics%2C%202023 (accessed on 5 August 2023).
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.A.; Alfayez, M.; Kadia, T.; Ravandi-Kashani, F.; Daver, N. Midostaurin In Acute Myeloid Leukemia: An Evidence-Based Review And Patient Selection. Cancer Manag. Res. 2019, 11, 8817–8828. [Google Scholar] [CrossRef]
- Wouters, B.J. Targeting IDH1 and IDH2 Mutations in Acute Myeloid Leukemia: Emerging Options and Pending Questions. HemaSphere 2021, 5, e583. [Google Scholar] [CrossRef]
- Campos, E.D.V.; Pinto, R. Targeted therapy with a selective BCL-2 inhibitor in older patients with acute myeloid leukemia. Hematol. Transfus. Cell Ther. 2019, 41, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Ganser, A. Treatment of Relapsed Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2020, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Shlush, L.I.; Mitchell, A.; Heisler, L.; Abelson, S.; Ng, S.W.K.; Trotman-Grant, A.; Medeiros, J.J.F.; Rao-Bhatia, A.; Jaciw-Zurakowsky, I.; Marke, R.; et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature 2017, 547, 104–108. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of drug resistance in acute myeloid leukemia. OncoTargets Ther. 2019, 12, 1937–1945. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Devendran, R.; Ramesh, V.; Gnanasekaran, P. CHAPTER 8-Fundamentals of cell metabolism and cancer. In Understanding Cancer; Jain, B., Pandey, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 117–132. ISBN 978-0-323-99883-3. [Google Scholar]
- Grassian, A.R.; Parker, S.J.; Davidson, S.M.; Divakarun, A.S.; Green, C.R.; Zhang, X.; Slocum, K.L.; Pu, M.; Lin, F.; Vickers, C.; et al. IDH1 Mutations Alter Citric Acid Cycle Metabolism and Increase Dependence on Oxidative Mitochondrial Metabolism. Cancer Res. 2016, 74, 3317–3331. [Google Scholar] [CrossRef] [PubMed]
- Raffel, S.; Falcone, M.; Kneisel, N.; Hansson, J.; Wang, W.; Lutz, C.; Bullinger, L.; Poschet, G.; Nonnenmacher, Y.; Barnert, A.; et al. BCAT1 restricts αKG levels in AML stem cells leading to IDHmut-like DNA hypermethylation. Nature 2017, 551, 384–388. [Google Scholar] [CrossRef]
- Poulain, L.; Sujobert, P.; Zylbersztejn, F.; Barreau, S.; Stuani, L.; Lambert, M.; Palama, T.L.; Chesnais, V.; Birsen, R.; Vergez, F.; et al. High mTORC1 activity drives glycolysis addiction and sensitivity to G6PD inhibition in acute myeloid leukemia cells. Leukemia 2017, 31, 2326–2335. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wang, J.-H.; Zhao, A.-H.; Xu, X.; Wang, Y.-H.; Chen, T.-L.; Li, J.-M.; Mi, J.-Q.; Zhu, Y.-M.; Liu, Y.-F.; et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood 2014, 124, 1645–1654. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Ma, L.; Zong, X. Metabolic Symbiosis in Chemoresistance: Refocusing the Role of Aerobic Glycolysis. Front. Oncol. 2020, 10, 5. [Google Scholar] [CrossRef]
- Herst, P.M.; Hesketh, E.L.; Ritchie, D.S.; Berridge, M.V. Glycolytic metabolism confers resistance to combined all-trans retinoic acid and arsenic trioxide-induced apoptosis in HL60ρ0 cells. Leuk. Res. 2008, 32, 327–333. [Google Scholar] [CrossRef]
- Herst, P.M.; Howman, R.A.; Neeson, P.J.; Berridge, M.V.; Ritchie, D.S. The level of glycolytic metabolism in acute myeloid leukemia blasts at diagnosis is prognostic for clinical outcome. J. Leukoc. Biol. 2011, 89, 51–55. [Google Scholar] [CrossRef]
- Song, K.; Li, M.; Xu, X.; Xuan, L.; Huang, G.; Liu, Q. Resistance to chemotherapy is associated with altered glucose metabolism in acute myeloid leukemia. Oncol. Lett. 2016, 12, 334–342. [Google Scholar] [CrossRef]
- Li, M.M.; Datto, M.; Duncavage, E.J.; Kulkarni, S.; Lindeman, N.I.; Roy, S.; Tsimberidou, A.M.; Vnencak-Jones, C.L.; Wolff, D.J.; Younes, A.; et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer. J. Mol. Diagn. 2017, 19, 4–23. [Google Scholar] [CrossRef]
- Horak, P.; Griffith, M.; Danos, A.M.; Pitel, B.A.; Madhavan, S.; Liu, X.; Chow, C.; Williams, H.; Carmody, L.; Barrow-Laing, L.; et al. Standards for the classification of pathogenicity of somatic variants in cancer (oncogenicity): Joint recommendations of Clinical Genome Resource (ClinGen), Cancer Genomics Consortium (CGC), and Variant Interpretation for Cancer Consortium (VICC). Genet. Med. 2022, 24, 986–998. [Google Scholar] [CrossRef]
- Lindström, H.J.G.; Friedman, R. Inferring time-dependent population growth rates in cell cultures undergoing adaptation. BMC Bioinform. 2020, 21, 583. [Google Scholar] [CrossRef]
- Pereira-Nunes, A.; Ferreira, H.; Abreu, S.; Guedes, M.; Neves, N.M.; Baltazar, F.; Granja, S. Combination Therapy with CD147-Targeted Nanopartic.pdf. Adv. Biol. 2023, 7, 2300080. [Google Scholar] [CrossRef] [PubMed]
- Klco, J.M.; Mullighan, C.G. Advances in germline predisposition to acute leukaemias and myeloid neoplasms. Nat. Rev. Cancer 2021, 21, 122–137. [Google Scholar] [CrossRef]
- Kishtagari, A.; Levine, R.L. The Role of Somatic Mutations in Acute Myeloid Leukemia Pathogenesis. Cold Spring Harb. Perspect. Med. 2021, 11, a034975. [Google Scholar] [CrossRef] [PubMed]
- KEGG PATHWAY: Acute Myeloid Leukemia-Homo Sapiens (Human). Available online: https://www.genome.jp/kegg-bin/show_pathway?hsa05221 (accessed on 29 November 2023).
- Wakita, S.; Sakaguchi, M.; Oh, I.; Kako, S.; Toya, T.; Najima, Y.; Doki, N.; Kanda, J.; Kuroda, J.; Mori, S.; et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022, 6, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chu, T.-T.; Han, S.-Y.; Qi, J.-Q.; Tang, Y.-Q.; Qiu, H.-Y.; Fu, C.-C.; Tang, X.-W.; Ruan, C.-G.; Wu, D.-P.; et al. FLT3-ITD and CEBPA Mutations Predict Prognosis in Acute Myelogenous Leukemia Irrespective of Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, E.; Taylor-Weiner, A.N.; Chang, T.Y.; Gelston, L.C.; Wang, Y.-D.; Mazor, T.; Esquivel, E.; Yu, A.; Seepo, S.; Olsen, S.R.; et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat. Genet. 2015, 47, 1326–1333. [Google Scholar] [CrossRef]
- Gui, P.; Bivona, T.G. Stepwise evolution of therapy resistance in AML. Cancer Cell 2021, 39, 904–906. [Google Scholar] [CrossRef]
- Farrar, J.E.; Schuback, H.L.; Ries, R.E.; Wai, D.; Hampton, O.A.; Trevino, L.R.; Alonzo, T.A.; Guidry Auvil, J.M.; Davidsen, T.M.; Gesuwan, P.; et al. Genomic Profiling of Pediatric Acute Myeloid Leukemia Reveals a Changing Mutational Landscape from Disease Diagnosis to Relapse. Cancer Res. 2016, 76, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Mesbahi, Y.; Trahair, T.N.; Lock, R.B.; Connerty, P. Exploring the Metabolic Landscape of AML: From Haematopoietic Stem Cells to Myeloblasts and Leukaemic Stem Cells. Front. Oncol. 2022, 12, 807266. [Google Scholar] [CrossRef] [PubMed]
- Decroocq, J.; Birsen, R.; Montersino, C.; Chaskar, P.; Mano, J.; Poulain, L.; Friedrich, C.; Alary, A.-S.; Guermouche, H.; Sahal, A.; et al. RAS activation induces synthetic lethality of MEK inhibition with mitochondrial oxidative metabolism in acute myeloid leukemia. Leukemia 2022, 36, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.; Birsen, R.; Lauture, L.; Mouche, S.; Angelino, P.; Dehairs, J.; Goupille, L.; Boussaid, I.; Heiblig, M.; Boet, E.; et al. C/EBPα Confers Dependence to Fatty Acid Anabolic Pathways and Vulnerability to Lipid Oxidative Stress–Induced Ferroptosis in FLT3-Mutant Leukemia. Cancer Discov. 2023, 13, 1720–1747. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Jayasena, S. The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites 2023, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Kreitz, J.; Schönfeld, C.; Seibert, M.; Stolp, V.; Alshamleh, I.; Oellerich, T.; Steffen, B.; Schwalbe, H.; Schnütgen, F.; Kurrle, N.; et al. Metabolic Plasticity of Acute Myeloid Leukemia. Cells 2019, 8, 805. [Google Scholar] [CrossRef]
- Tabe, Y.; Konopleva, M.; Andreeff, M. Fatty Acid Metabolism, Bone Marrow Adipocytes, and AML. Front. Oncol. 2020, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Farge, T.; Saland, E.; De Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef]
- Azevedo-Silva, J.; Queirós, O.; Baltazar, F.; Ułaszewski, S.; Goffeau, A.; Ko, Y.H.; Pedersen, P.L.; Preto, A.; Casal, M. The anticancer agent 3-bromopyruvate: A simple but powerful molecule taken from the lab to the bedside. J. Bioenerg. Biomembr. 2016, 48, 349–362. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 553–560. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Liu, Y.; Luo, X.; Yang, C.; Xiao, W.; Li, Q.; Yang, L.; Zhang, X. 3-Bromopyruvic acid regulates glucose metabolism by targeting the c-Myc/TXNIP axis and induces mitochondria-mediated apoptosis in TNBC cells. Exp. Ther. Med. 2022, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jardim-Messeder, D.; Moreira-Pacheco, F. 3-Bromopyruvic Acid Inhibits Tricarboxylic Acid Cycle and Glutaminolysis in HepG2 Cells. Anticancer Res. 2016, 36, 2233–2241. [Google Scholar] [PubMed]
- García Rubiño, M.E.; Carrillo, E.; Ruiz Alcalá, G.; Domínguez-Martín, A.; Marchal, J.A.; Boulaiz, H. Phenformin as an Anticancer Agent: Challenges and Prospects. Int. J. Mol. Sci. 2019, 20, 3316. [Google Scholar] [CrossRef] [PubMed]
- Veiga, S.R.; Ge, X.; Mercer, C.A.; Hernández-Álvarez, M.I.; Thomas, H.E.; Hernandez-Losa, J.; Ramón Y Cajal, S.; Zorzano, A.; Thomas, G.; Kozma, S.C. Phenformin-Induced Mitochondrial Dysfunction Sensitizes Hepatocellular Carcinoma for Dual Inhibition of mTOR. Clin. Cancer Res. 2018, 24, 3767–3780. [Google Scholar] [CrossRef]



| KG-1 | KG-1 Ara-R | COSMIC ID | Clinical Significance @ | Consequence | |
|---|---|---|---|---|---|
| Mutated genes | BCOR (c.4886G>A; p.(Trp1663*)) | BCOR (c.4886G>A; p.(Trp1663*)) | Tier III | Nonsense | |
| DDX41 (c.27+2_27+5dup) | DDX41 (c.27+2_27+5dup) | Tier IV | Frameshift | ||
| FLT3 (c.1669G>A; p.(Val557Ile)) | FLT3 (c.1669G>A; p.(Val557Ile)) | COSM28043 | Tier IV | Missense | |
| NRAS (c.360G>T; p.(Leu120Phe)) | - | Tier III | Missense | ||
| SAMD9 (c.223C>T; p.(Arg75Trp)) | SAMD9 (c.223C>T; p.(Arg75Trp)) | Tier IV | Missense | ||
| SAMD9L (c.1217G>A; p.(Arg406Gln)) | SAMD9L (c.1217G>A; p.(Arg406Gln)) | Tier IV | Missense | ||
| TP53 (c.672+1G>A) | TP53 (c.672+1G>A) | COSM2744696 | Tier II | Splice donor | |
| MOLM13 | MOLM13 Ara-R | COSMIC ID | Clinical Significance @ | Consequence | |
| Mutated genes | ASXL1 (c.1954G>A; p.(Gly652Ser)) | ASXL1 (c.1954G>A; p.(Gly652Ser)) | COSM1716555 | Tier IV | Missense |
| CBL (c.1227_1227+13del) | CBL (c.1227_1227+13del) | Tier IV | Frameshift | ||
| CEBPA (c.584_589dup; p.(His195_Pro196dup)) | CEBPA (c.584_589dup; p.(His195_Pro196dup)) | Tier IV | Frameshift | ||
| CEBPA (c.568T>C; p.(Ser190Pro)) | CEBPA (c.568T>C; p.(Ser190Pro)) | Tier III | Missense | ||
| - | CEBPA (c.566C>A; p.(Pro189His)) | Tier III | Missense | ||
| FLT3 (c.1775_1795dup; p.(Glu598_Tyr599insPheAspPheArgGluTyrGlu) (FLT3-ITD, 21bp) | FLT3 (c.1775_1795dup; p.(Glu598_Tyr599insPheAspPheArgGluTyrGlu) (FLT3-ITD, 21bp) | COSM849 | Tier I | Inframe insertion | |
| SAMD9L (c.866T>C; p.(Phe289Ser)) | SAMD9L (c.866T>C; p.(Phe289Ser))) | COSM3982291 | Tier IV | Missense |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Vieira, J.; Weber, D.D.; Silva, S.; Barbosa-Matos, C.; Granja, S.; Reis, R.M.; Queirós, O.; Ko, Y.H.; Kofler, B.; Casal, M.; et al. Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia. Pharmaceutics 2024, 16, 442. https://doi.org/10.3390/pharmaceutics16040442
Pereira-Vieira J, Weber DD, Silva S, Barbosa-Matos C, Granja S, Reis RM, Queirós O, Ko YH, Kofler B, Casal M, et al. Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia. Pharmaceutics. 2024; 16(4):442. https://doi.org/10.3390/pharmaceutics16040442
Chicago/Turabian StylePereira-Vieira, Joana, Daniela D. Weber, Sâmia Silva, Catarina Barbosa-Matos, Sara Granja, Rui Manuel Reis, Odília Queirós, Young H. Ko, Barbara Kofler, Margarida Casal, and et al. 2024. "Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia" Pharmaceutics 16, no. 4: 442. https://doi.org/10.3390/pharmaceutics16040442
APA StylePereira-Vieira, J., Weber, D. D., Silva, S., Barbosa-Matos, C., Granja, S., Reis, R. M., Queirós, O., Ko, Y. H., Kofler, B., Casal, M., & Baltazar, F. (2024). Glucose Metabolism as a Potential Therapeutic Target in Cytarabine-Resistant Acute Myeloid Leukemia. Pharmaceutics, 16(4), 442. https://doi.org/10.3390/pharmaceutics16040442









