Synthesis and Characterization of ZIF-90 Nanoparticles as Potential Brain Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZIF-90, ZIF-90@Acr, and ZIF-90@Berb Synthesis
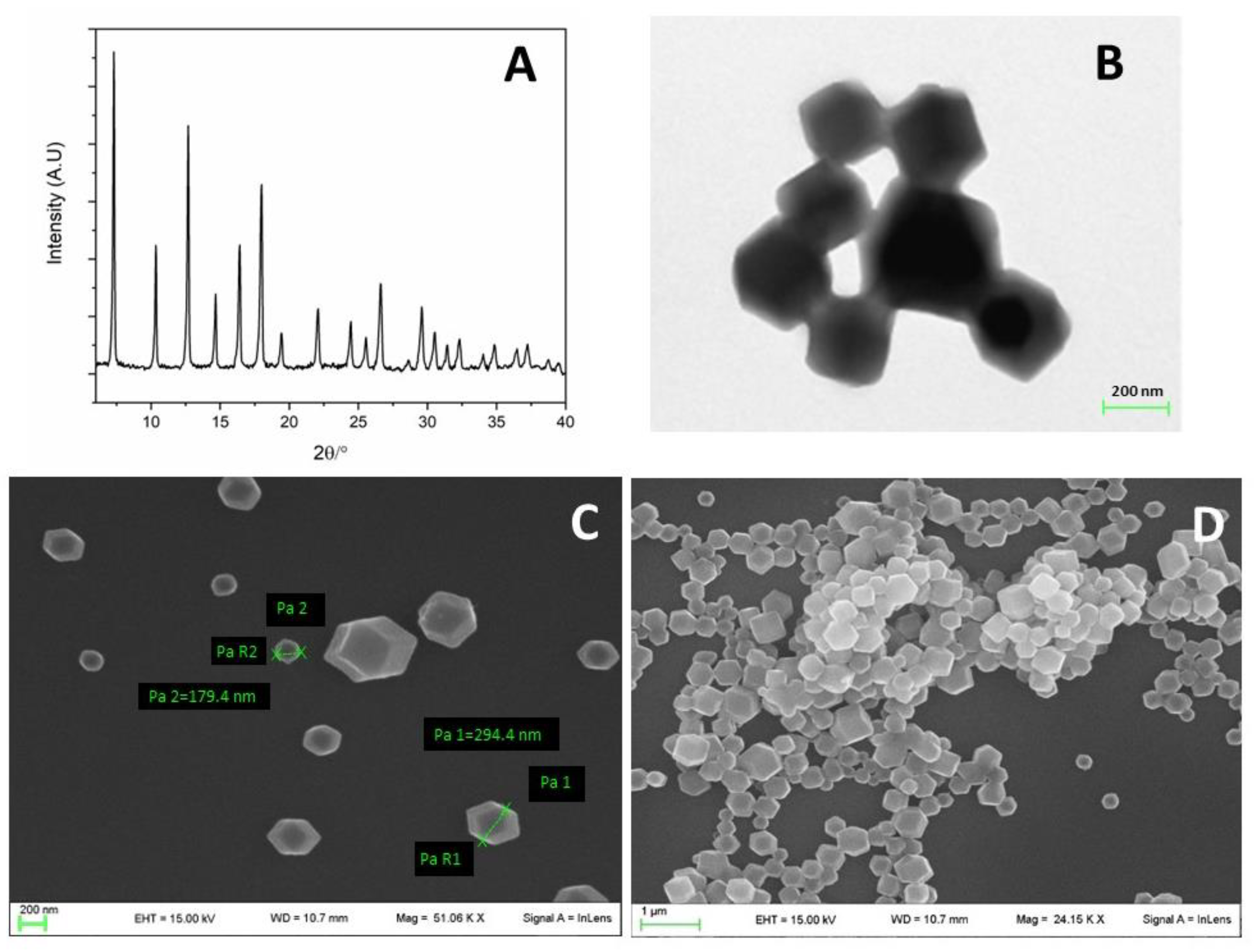
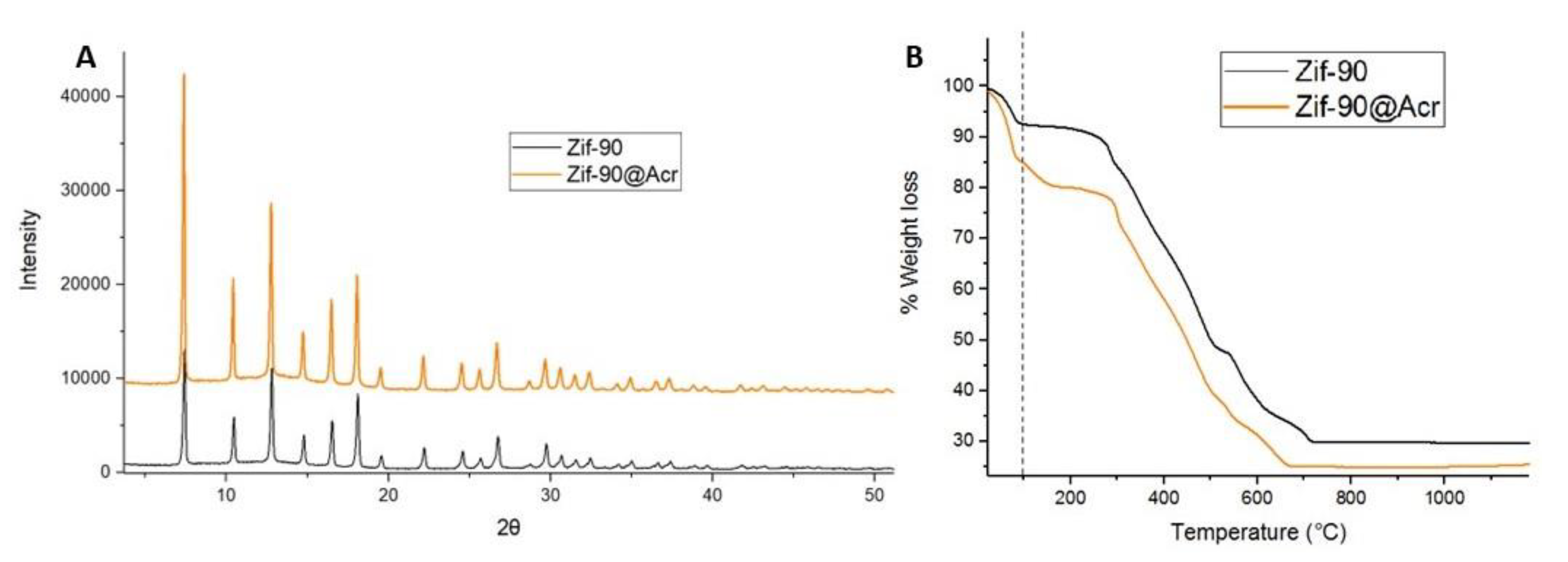
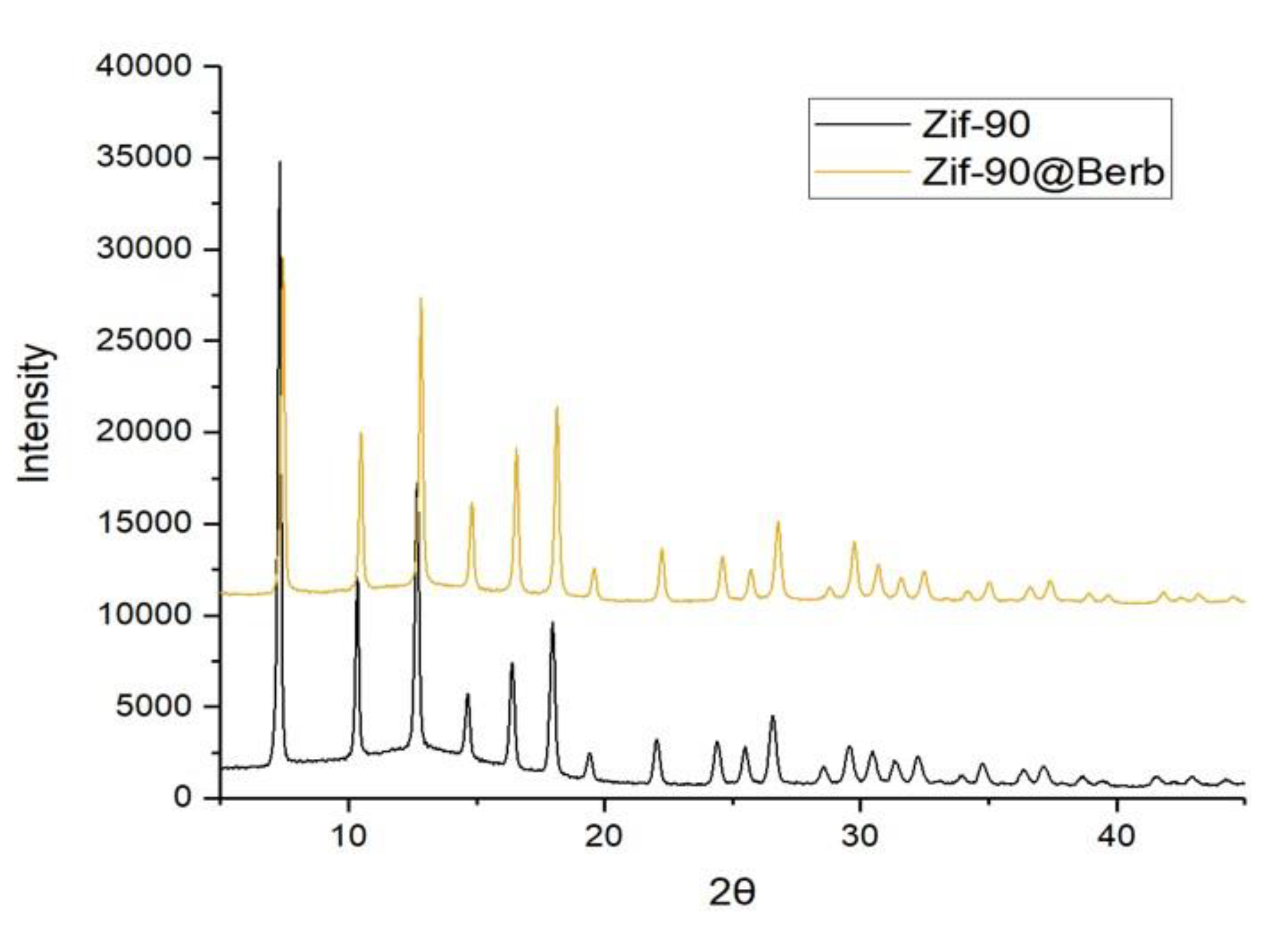
2.3. X-ray Powder Diffraction (XRPD) Analysis
2.4. Electron Microscopy Analysis
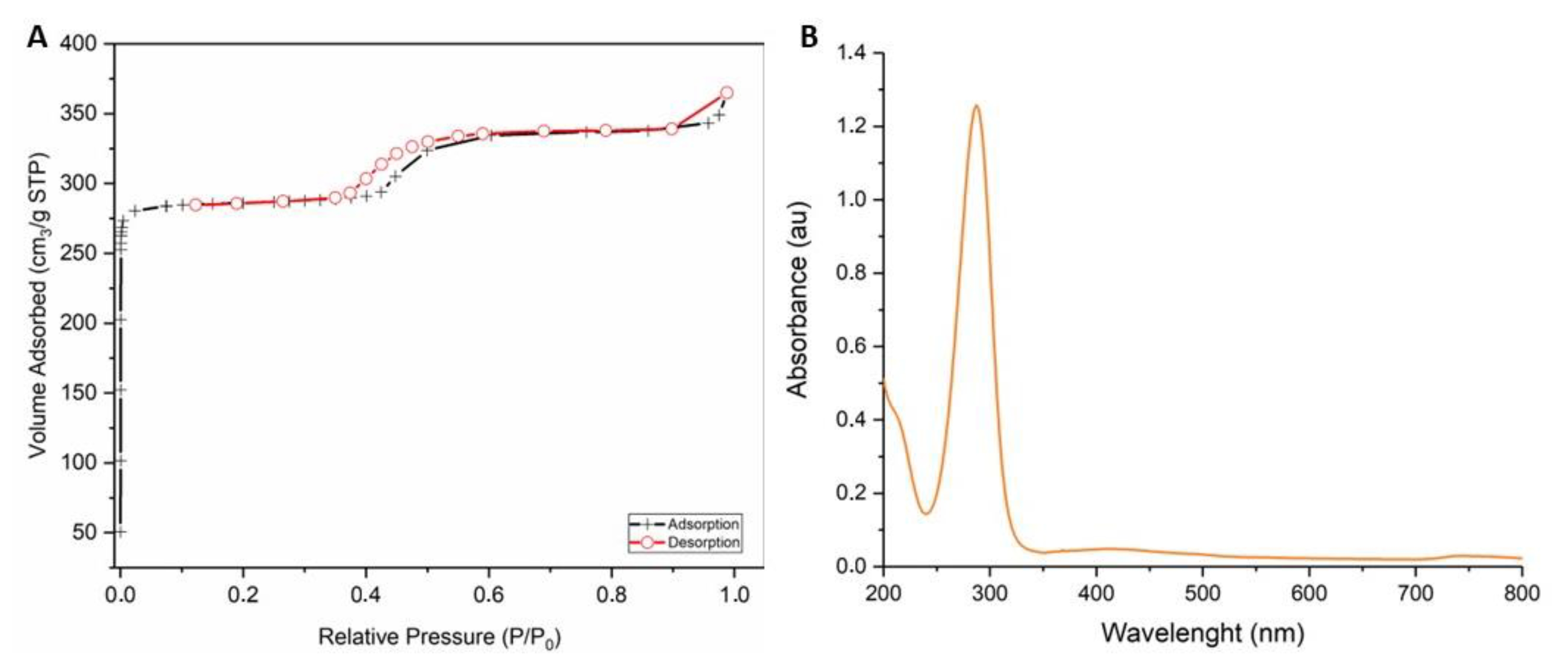
2.5. Gas Sorption Measurements
2.6. UV–Vis Spectroscopy
2.7. Thermogravimetric (TG) Analysis
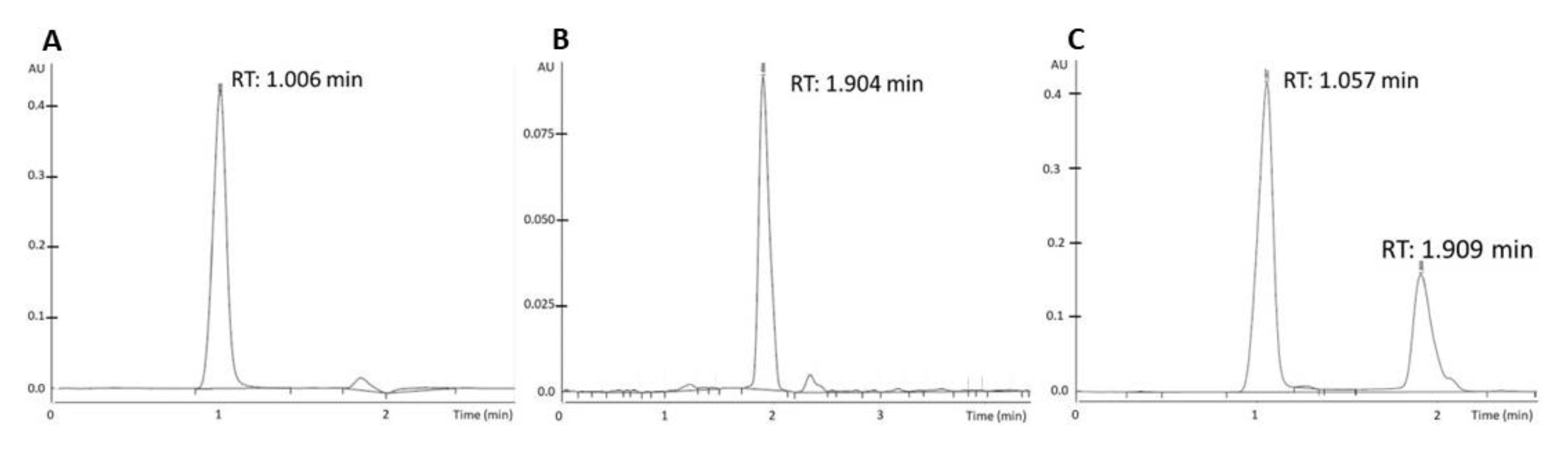
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
2.9. Cell Culture
2.10. Internalization of ZIF-90 Evaluation
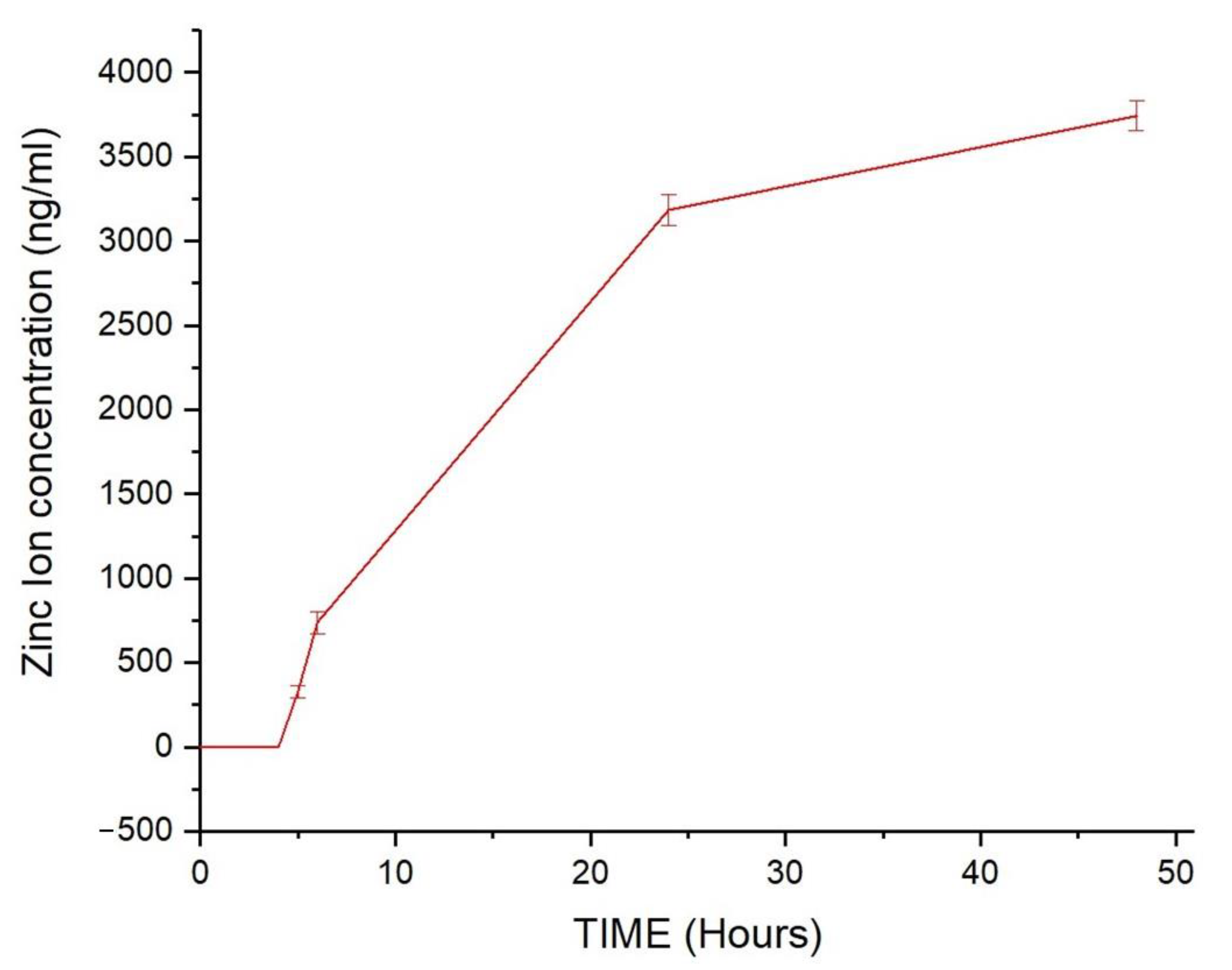
2.11. Stability of ZIF-90 in Simulated Body Fluid (SBF) Evaluation
2.12. Cellular Vitality Assay
2.13. Chick Embryo Chorioallantoic Membrane (CAM) Assay
2.14. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro Oncol. 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Grech, N.; Dalli, T.; Mizzi, S.; Meilak, L.; Calleja, N.; Zrinzo, A. Rising Incidence of Glioblastoma Multiforme in a Well-Defined Population. Cureus 2020, 12, e8195. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Tanzilli, A.; Benincasa, D. Prognostication in Brain Tumors. Handb. Clin. Neurol. 2022, 190, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.; Saini, S.; Bhadra, S.; Kulavi, S.; Bandyopadhyay, J. Precision Medicine Advancements in Glioblastoma: A Systematic Review. Biomedicine 2023, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oberheim Bush, N.A.; Hervey-Jumper, S.L.; Berger, M.S. Management of Glioblastoma, Present and Future. World Neurosurg. 2019, 131, 328–338. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit with Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Liu, Y.; Qu, Y.; Wang, K.; Zhang, Y.; Chang, Y.; Yang, Z.; Wan, J.; Liu, J.; et al. Variants of the Adeno-Associated Virus Serotype 9 with Enhanced Penetration of the Blood–Brain Barrier in Rodents and Primates. Nat. Biomed. Eng. 2022, 6, 1257–1271. [Google Scholar] [CrossRef]
- Marin, B.-M.; Porath, K.A.; Jain, S.; Kim, M.; Conage-Pough, J.E.; Oh, J.-H.; Miller, C.L.; Talele, S.; Kitange, G.J.; Tian, S.; et al. Heterogeneous Delivery across the Blood-Brain Barrier Limits the Efficacy of an EGFR-Targeting Antibody Drug Conjugate in Glioblastoma. Neuro-Oncology 2021, 23, 2042–2053. [Google Scholar] [CrossRef]
- Mi, Z.; Yao, Q.; Qi, Y.; Zheng, J.; Liu, J.; Liu, Z.; Tan, H.; Ma, X.; Zhou, W.; Rong, P. Salmonella-Mediated Blood–brain Barrier Penetration, Tumor Homing and Tumor Microenvironment Regulation for Enhanced Chemo/Bacterial Glioma Therapy. Acta Pharm. Sin. B 2023, 13, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A Comprehensive Profile of Recurrent Glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Zha, S.; Liu, H.; Li, H.; Li, H.; Wong, K.-L.; All, A.H. Functionalized Nanomaterials Capable of Crossing the Blood-Brain Barrier. ACS Nano 2024, 18, 1820–1845. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv. Mater. 2018, 30, e1707365. [Google Scholar] [CrossRef] [PubMed]
- Goetjen, T.A.; Liu, J.; Wu, Y.; Sui, J.; Zhang, X.; Hupp, J.T.; Farha, O.K. Metal-Organic Framework (MOF) Materials as Polymerization Catalysts: A Review and Recent Advances. Chem. Commun. 2020, 56, 10409–10418. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Chen, O.I.-F.; Hanikel, N.; Hossain, M.I.; Flaig, R.W.; Pei, X.; Amin, A.; Doherty, M.D.; Impastato, R.K.; Glover, T.G.; et al. Carbon Dioxide Capture Chemistry of Amino Acid Functionalized Metal-Organic Frameworks in Humid Flue Gas. J. Am. Chem. Soc. 2022, 144, 2387–2396. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-Organic Framework (MOF) Composites as Promising Materials for Energy Storage Applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Zhou, S.; Shekhah, O.; Ramírez, A.; Lyu, P.; Abou-Hamad, E.; Jia, J.; Li, J.; Bhatt, P.M.; Huang, Z.; Jiang, H.; et al. Asymmetric Pore Windows in MOF Membranes for Natural Gas Valorization. Nature 2022, 606, 706–712. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Lei, H.; Wang, M.; Jiao, S. Alternate Storage of Opposite Charges in Multisites for High-Energy-Density Al-MOF Batteries. Adv. Mater. 2022, 34, e2110109. [Google Scholar] [CrossRef]
- Du, Y.; Jie, G.; Jia, H.; Liu, J.; Wu, J.; Fu, Y.; Zhang, F.; Zhu, W.; Fan, M. Visible-Light-Induced Photocatalytic CO2 Reduction over Zirconium Metal Organic Frameworks Modified with Different Functional Groups. J. Environ. Sci. 2023, 132, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.J.; Horcajada, P. Cytotoxicity of Nanoscaled Metal–Organic Frameworks. J. Mater. Chem. B 2013, 2, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, C.S.; Dai, Y.; Yang, Z.; Yu, G.; Liu, Y.; Zhang, M.; Lin, L.; Tang, W.; Zhou, Z.; et al. In Situ Polymerization on Nanoscale Metal-Organic Frameworks for Enhanced Physiological Stability and Stimulus-Responsive Intracellular Drug Delivery. Biomaterials 2019, 218, 119365. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, C.; Monarca, L.; Ragonese, F.; Mecca, C.; Bruscoli, S.; Giovagnoli, S.; Donato, R.; Bereshchenko, O.; Fioretti, B.; Costantino, F. Probing Internalization Effects and Biocompatibility of Ultrasmall Zirconium Metal-Organic Frameworks UiO-66 NP in U251 Glioblastoma Cancer Cells. Nanomaterials 2018, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, A.; Li, C.; Panwar Hazari, P.; Mahajan, S.D.; Aalinkeel, R.; Sharma, R.K.; Swihart, M.T. A Cannabidiol-Loaded Mg-Gallate Metal-Organic Framework-Based Potential Therapeutic for Glioblastomas. J. Mater. Chem. B 2021, 9, 2505–2514. [Google Scholar] [CrossRef]
- Chen, S.; Pang, H.; Sun, J.; Li, K. Research Advances and Applications of ZIF-90 Metal–Organic Framework Nanoparticles in the Biomedical Field. Mater. Chem. Front. 2024. [Google Scholar] [CrossRef]
- Zhu, Q.; Zheng, Y.; Zhang, Z.; Chen, Y. Enzyme Immobilization on Covalent Organic Framework Supports. Nat. Protoc. 2023, 18, 3080–3125. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, Y.; Chen, L.; Wang, S.; Liu, C.; Zhao, H.; Jin, M.; Chang, S.; Quan, X.; Cui, M.; et al. Combined Biomimetic MOF-RVG15 Nanoformulation Efficient Over BBB for Effective Anti-Glioblastoma in Mice Model. Int. J. Nanomed. 2022, 17, 6377–6398. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Q.; Zhang, R.; Li, J.; Lin, Q.; Li, J.; Zhou, X.; Yan, X.; Fan, K. Chiral Metal-Organic Frameworks Incorporating Nanozymes as Neuroinflammation Inhibitors for Managing Parkinson’s Disease. Nat. Commun. 2023, 14, 8137. [Google Scholar] [CrossRef]
- Dionigi, L.; Ragonese, F.; Monarca, L.; Covino, S.; de Luca, A.; Iannitti, R.G.; Bastioli, F.; Moulas, A.N.; Allegretti, M.; Fioretti, B. Focus on the Use of Resveratrol as an Adjuvant in Glioblastoma Therapy. Curr. Pharm. Des. 2020, 26, 2102–2108. [Google Scholar] [CrossRef]
- Bibak, B.; Shakeri, F.; Keshavarzi, Z.; Mollazadeh, H.; Javid, H.; Jalili-Nik, M.; Sathyapalan, T.; Afshari, A.R.; Sahebkar, A. Anticancer Mechanisms of Berberine: A Good Choice for Glioblastoma Multiforme Therapy. Curr. Med. Chem. 2022, 29, 4507–4528. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Recent Advances in Berberine Inspired Anticancer Approaches: From Drug Combination to Novel Formulation Technology and Derivatization. Molecules 2020, 25, 1426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xiao, G.; Cheng, P.; Zeng, J.; Liu, Y. Protective Application of Chinese Herbal Compounds and Formulae in Intestinal Inflammation in Humans and Animals. Molecules 2023, 28, 6811. [Google Scholar] [CrossRef] [PubMed]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, J.; Pan, Y.; Shen, W. Berberine Suppressed the Progression of Human Glioma Cells by Inhibiting the TGF-Β1/SMAD2/3 Signaling Pathway. Integr. Cancer Ther. 2022, 21, 15347354221130303. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.-Q.; Fan, D.-J.; Yang, S.-S.; Lin, X.; Meng, L.-K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Hao, H.-P.; Xie, H.-G.; Lai, L.; Wang, Q.; Liu, C.-X.; Wang, G.-J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Cao, B.; Sun, R.; Tang, Y.; Paletta, J.L.; Wu, X.; Liu, L.; Zha, W.; Zhao, C.; Li, Y.; et al. A Metabolomic and Pharmacokinetic Study on the Mechanism Underlying the Lipid-Lowering Effect of Orally Administered Berberine. Mol. Biosyst. 2015, 11, 463–474. [Google Scholar] [CrossRef]
- Tan, X.-S.; Ma, J.-Y.; Feng, R.; Ma, C.; Chen, W.-J.; Sun, Y.-P.; Fu, J.; Huang, M.; He, C.-Y.; Shou, J.-W.; et al. Tissue Distribution of Berberine and Its Metabolites after Oral Administration in Rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef]
- Spogli, R.; Bastianini, M.; Ragonese, F.; Iannitti, R.G.; Monarca, L.; Bastioli, F.; Nakashidze, I.; Brecchia, G.; Menchetti, L.; Codini, M.; et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients 2018, 10, 1925. [Google Scholar] [CrossRef] [PubMed]
- Iannitti, R.G.; Floridi, A.; Lazzarini, A.; Tantucci, A.; Russo, R.; Ragonese, F.; Monarca, L.; Caglioti, C.; Spogli, R.; Leonardi, L.; et al. Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Represents an Oral Formulation of Resveratrol with Better Gastric Absorption and Bioavailability Respect to Pure Resveratrol. Front. Nutr. 2020, 7, 570047. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-I.; Liu, S.-M.; Lo, W.-S.; Wu, J.-W.; Liu, Y.-H.; Chein, R.-J.; Yang, R.; Wu, K.C.-W.; Hwu, J.R.; Ma, N.; et al. Cytotoxicity of Postmodified Zeolitic Imidazolate Framework-90 (ZIF-90) Nanocrystals: Correlation between Functionality and Toxicity. Chemistry 2016, 22, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Park, C.R. Preparation of Highly Moisture-Resistant Black-Colored Metal Organic Frameworks. Adv. Mater. 2012, 24, 4010–4013. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and Enhanced Hydrostability and Hydrogen Storage Capacity of CNT@MOF-5 Hybrid Composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Yang, T.; Chung, T.-S. Room-Temperature Synthesis of ZIF-90 Nanocrystals and the Derived Nano-Composite Membranes for Hydrogen Separation. J. Mater. Chem. A 2013, 1, 6081–6090. [Google Scholar] [CrossRef]
- Shieh, F.-K.; Wang, S.-C.; Yen, C.-I.; Wu, C.-C.; Dutta, S.; Chou, L.-Y.; Morabito, J.V.; Hu, P.; Hsu, M.-H.; Wu, K.C.-W.; et al. Imparting Functionality to Biocatalysts via Embedding Enzymes into Nanoporous Materials by a de Novo Approach: Size-Selective Sheltering of Catalase in Metal–Organic Framework Microcrystals. J. Am. Chem. Soc. 2015, 137, 4276–4279. [Google Scholar] [CrossRef]
- Usman, M.; Khan, M.Y.; Anjum, T.; Khan, A.L.; Hoque, B.; Helal, A.; Hakeem, A.S.; Al-Maythalony, B.A. Controlled Covalent Functionalization of ZIF-90 for Selective CO2 Capture & Separation. Membranes 2022, 12, 1055. [Google Scholar] [CrossRef]
- Mikhail, R.S.; Brunauer, S.; Bodor, E.E. Investigations of a Complete Pore Structure Analysis: I. Analysis of Micropores. J. Colloid Interface Sci. 1968, 26, 45–53. [Google Scholar] [CrossRef]
- Vandenbulcke, F.; Nouel, D.; Vincent, J.P.; Mazella, J.; Beaudet, A. Ligand-Induced Internalization of Neurotensin in Transfected COS-7 Cells: Differential Intracellular Trafficking of Ligand and Receptor. J. Cell Sci. 2000, 113 Pt 17, 2963–2975. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N. Zeolitic Imidazolate Frameworks (ZIF-8) for Biomedical Applications: A Review. Curr. Med. Chem. 2021, 28, 7023–7075. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.M.; Bhardwaj, G.; Goswami, S.; Tonk, R.K.; Goyal, R.K.; Abu-Izneid, T.; Pottoo, F.H. Management of Glioblastoma Multiforme by Phytochemicals: Applications of Nanoparticle-Based Targeted Drug Delivery System. Curr. Drug Targets 2021, 22, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Xie, T.; Huang, X.; Zhao, X. Berberine Inhibits Angiogenesis in Glioblastoma Xenografts by Targeting the VEGFR2/ERK Pathway. Pharm. Biol. 2018, 56, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Gutkin, A.; Cohen, Z.R.; Peer, D. Harnessing Nanomedicine for Therapeutic Intervention in Glioblastoma. Expert Opin. Drug Deliv. 2016, 13, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Caglioti, C.; Palazzetti, F.; Monarca, L.; Lobello, R.; Ceccarini, M.R.; Iannitti, R.G.; Russo, R.; Ragonese, F.; Pennetta, C.; De Luca, A.; et al. LY294002 Inhibits Intermediate Conductance Calcium-Activated Potassium (KCa3.1) Current in Human Glioblastoma Cells. Front. Physiol. 2021, 12, 790922. [Google Scholar] [CrossRef]
- Fioretti, B.; Castigli, E.; Micheli, M.R.; Bova, R.; Sciaccaluga, M.; Harper, A.; Franciolini, F.; Catacuzzeno, L. Expression and Modulation of the Intermediate- Conductance Ca2+-Activated K+ Channel in Glioblastoma GL-15 Cells. Cell Physiol. Biochem. 2006, 18, 47–56. [Google Scholar] [CrossRef]
- Stransky, N.; Ganser, K.; Quintanilla-Martinez, L.; Gonzalez-Menendez, I.; Naumann, U.; Eckert, F.; Koch, P.; Huber, S.M.; Ruth, P. Efficacy of Combined Tumor Irradiation and KCa3.1-Targeting with TRAM-34 in a Syngeneic Glioma Mouse Model. Sci. Rep. 2023, 13, 20604. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monarca, L.; Ragonese, F.; Sabbatini, P.; Caglioti, C.; Stamegna, M.; Palazzetti, F.; Sportoletti, P.; Costantino, F.; Fioretti, B. Synthesis and Characterization of ZIF-90 Nanoparticles as Potential Brain Cancer Therapy. Pharmaceutics 2024, 16, 414. https://doi.org/10.3390/pharmaceutics16030414
Monarca L, Ragonese F, Sabbatini P, Caglioti C, Stamegna M, Palazzetti F, Sportoletti P, Costantino F, Fioretti B. Synthesis and Characterization of ZIF-90 Nanoparticles as Potential Brain Cancer Therapy. Pharmaceutics. 2024; 16(3):414. https://doi.org/10.3390/pharmaceutics16030414
Chicago/Turabian StyleMonarca, Lorenzo, Francesco Ragonese, Paola Sabbatini, Concetta Caglioti, Matteo Stamegna, Federico Palazzetti, Paolo Sportoletti, Ferdinando Costantino, and Bernard Fioretti. 2024. "Synthesis and Characterization of ZIF-90 Nanoparticles as Potential Brain Cancer Therapy" Pharmaceutics 16, no. 3: 414. https://doi.org/10.3390/pharmaceutics16030414
APA StyleMonarca, L., Ragonese, F., Sabbatini, P., Caglioti, C., Stamegna, M., Palazzetti, F., Sportoletti, P., Costantino, F., & Fioretti, B. (2024). Synthesis and Characterization of ZIF-90 Nanoparticles as Potential Brain Cancer Therapy. Pharmaceutics, 16(3), 414. https://doi.org/10.3390/pharmaceutics16030414






