Pharmacokinetic Interaction of Kratom and Cannabidiol in Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Dosing
2.3. Sample Preparation
2.4. Sample Analysis
2.5. Data Analysis
3. Results
3.1. Formulation Analysis
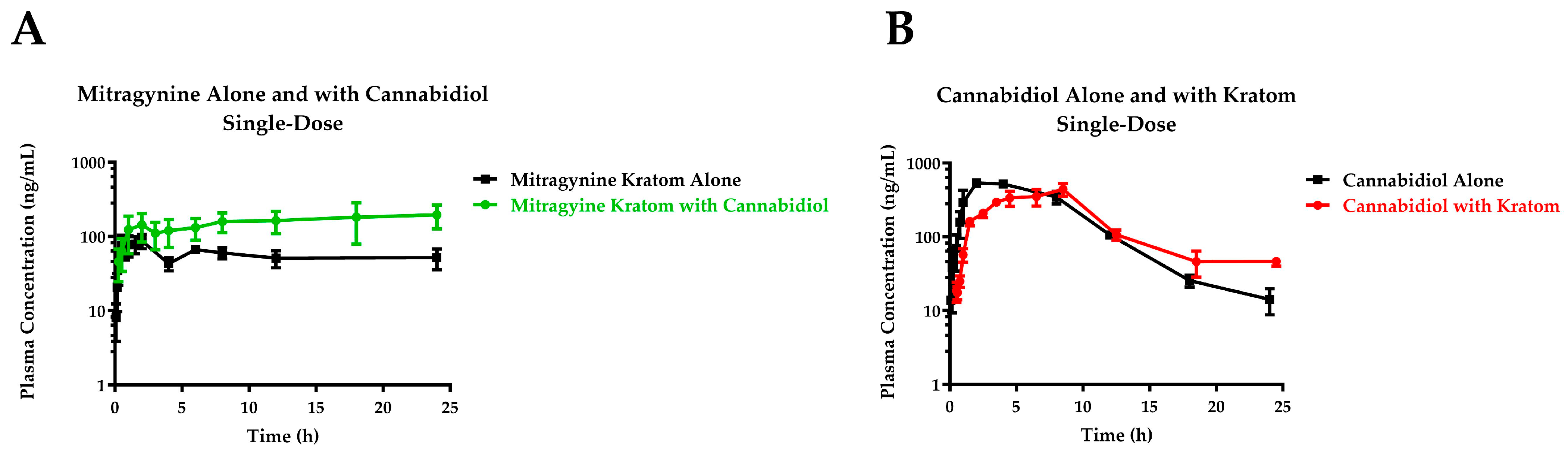
3.2. Single-Dose Pharmacokinetic Study
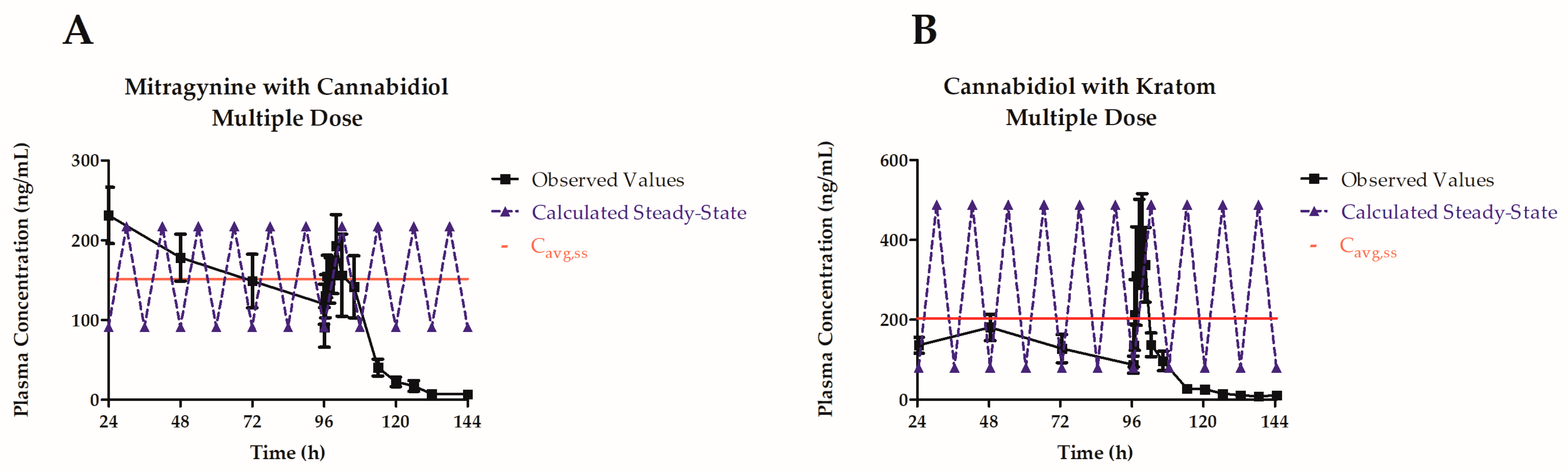
3.3. Multiple-Dose Pharmacokinetic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corroon, J.; Phillips, J.A. A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res. 2018, 3, 152–161. [Google Scholar] [CrossRef]
- Veltri, C.; Grundmann, O. Current Perspectives on The Impact of Kratom Use. Subst. Abus. Rehabil. 2019, 10, 23–31. [Google Scholar] [CrossRef]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The Essential Medicinal Chemistry of Cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Schoedel, K.A.; Szeto, I.; Setnik, B.; Sellers, E.M.; Levy-Cooperman, N.; Mills, C.; Etges, T.; Sommerville, K. Abuse Potential Assessment of Cannabidiol (CBD) in Recreational Polydrug Users: A Randomized, Double-Blind, Controlled Trial. Epilepsy Behav. 2018, 88, 162–171. [Google Scholar] [CrossRef]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Hanapi, N.A.; Chear, N.J.; Azizi, J.; Yusof, S.R. Kratom Alkaloids: Interactions With Enzymes, Receptors, and Cellular Barriers. Front. Pharmacol. 2021, 12, 751656. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, J.; Amioka, E.; Rockhill, K.; Haynes, C.M.; Black, J.C.; Dart, R.C.; Iwanicki, J.L. Prevalence and Description of Kratom (Mitragyna speciosa) Use in The United States: A Cross-Sectional Study. Addiction 2021, 116, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tanna, R.S.; Nguyen, J.T.; Hadi, D.L.; Manwill, P.K.; Flores-Bocanegra, L.; Layton, M.E.; White, J.R.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; et al. Clinical Pharmacokinetic Assessment of Kratom (Mitragyna speciosa), a Botanical Product with Opioid-like Effects, in Healthy Adult Participants. Pharmaceutics 2022, 14, 620. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.A.; Kellogg, J.J.; Wallace, E.D.; Khin, M.; Flores-Bocanegra, L.; Tanna, R.S.; McIntosh, S.; Raja, H.A.; Graf, T.N.; Hemby, S.E.; et al. Chemical Composition and Biological Effects of Kratom (Mitragyna speciosa): In Vitro Studies With Implications for Efficacy and Drug Interactions. Sci. Rep. 2020, 10, 19158. [Google Scholar] [CrossRef]
- Tanna, R.S.; Nguyen, J.T.; Hadi, D.L.; Layton, M.E.; White, J.R.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; Thummel, K.E.; Paine, M.F. Clinical Assessment of the Drug Interaction Potential of the Psychotropic Natural Product Kratom. Clin. Pharmacol. Ther. 2023, 113, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Tanna, R.S.; Tian, D.D.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; Thummel, K.E.; Paine, M.F. Refined Prediction of Pharmacokinetic Kratom-Drug Interactions: Time-Dependent Inhibition Considerations. J. Pharmacol. Exp. Ther. 2021, 376, 64–73. [Google Scholar] [CrossRef]
- Tanna, R.S.; Cech, N.B.; Oberlies, N.H.; Rettie, A.E.; Thummel, K.E.; Paine, M.F. Translating Kratom-Drug Interactions: From Bedside to Bench and Back. Drug Metab. Dispos. 2023, 51, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtelova, G.; Noskova, K.; Turjap, M.; Sulcova, A.; Hanus, L.; Jurica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Madden, K.; Tanco, K.; Bruera, E. Clinically Significant Drug-Drug Interaction Between Methadone and Cannabidiol. Pediatrics 2020, 145, e20193256. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Absalom, N.L.; Abelev, S.V.; Low, I.K.; Doohan, P.T.; Martin, L.J.; Chebib, M.; McGregor, I.S.; Arnold, J.C. Coadministered Cannabidiol and Clobazam: Preclinical Evidence for Both Pharmacodynamic and Pharmacokinetic Interactions. Epilepsia 2019, 60, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug-Drug Interaction Between Clobazam and Cannabidiol in Children WITH Refractory Epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Ampah, S.B.; Liu, Y.; Grayson, L.P.; Szaflarski, J.P.; Program, U.C. Drug-Drug Interactions with Cannabidiol (CBD) Appear to Have No Effect on Treatment Response in an Open-Label Expanded Access Program. Epilepsy Behav. 2019, 98, 201–206. [Google Scholar] [CrossRef]
- Leino, A.D.; Emoto, C.; Fukuda, T.; Privitera, M.; Vinks, A.A.; Alloway, R.R. Evidence of a Clinically Significant Drug-Drug Interaction Between Cannabidiol and Tacrolimus. Am. J. Transplant. 2019, 19, 2944–2948. [Google Scholar] [CrossRef]
- Kamble, S.H.; Sharma, A.; King, T.I.; Berthold, E.C.; Leon, F.; Meyer, P.K.L.; Kanumuri, S.R.R.; McMahon, L.R.; McCurdy, C.R.; Avery, B.A. Exploration of Cytochrome P450 Inhibition Mediated Drug-Drug Interaction Potential of Kratom Alkaloids. Toxicol. Lett. 2020, 319, 148–154. [Google Scholar] [CrossRef]
- Grayson, L.; Vines, B.; Nichol, K.; Szaflarski, J.P.; Program, U.C. An Interaction Between Warfarin and Cannabidiol, A Case Report. Epilepsy Behav. Case Rep. 2018, 9, 10–11. [Google Scholar] [CrossRef]
- Landmark, C.J.; Brandl, U. Pharmacology and Drug Interactions of Cannabinoids. Epileptic Disord. 2020, 22, S16–S22. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef]
- VanLandingham, K.E.; Crockett, J.; Taylor, L.; Morrison, G. A Phase 2, Double-Blind, Placebo-Controlled Trial to Investigate Potential Drug-Drug Interactions Between Cannabidiol and Clobazam. J. Clin. Pharmacol. 2020, 60, 1304–1313. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Gunning, B.; Arenas Cabrera, C.M.; VanLandingham, K.; Crockett, J.; Critchley, D.; Wray, L.; Tayo, B.; Morrison, G.; Toledo, M. A Phase II Randomized Trial to Explore the Potential for Pharmacokinetic Drug-Drug Interactions With Stiripentol or Valproate When Combined with Cannabidiol in Patients with Epilepsy. CNS Drugs 2020, 34, 661–672. [Google Scholar] [CrossRef]
- Brown, J.D.; Winterstein, A.G. Potential Adverse Drug Events and Drug-Drug Interactions with Medical and Consumer Cannabidiol (CBD) Use. J. Clin. Med. 2019, 8, 989. [Google Scholar] [CrossRef]
- Ortiz, Y.T.; Bilbrey, J.A.; Felix, J.S.; Kienegger, E.A.; Mottinelli, M.; Mukhopadhyay, S.; McCurdy, C.R.; McMahon, L.R.; Wilkerson, J.L. Cannabidiol and Mitragynine Exhibit Differential Interactive Effects in the Attenuation of Paclitaxel-Induced Mechanical Allodynia, Acute Antinociception, and Schedule-Controlled Responding in Mice. Pharmacol. Rep. 2023, 75, 937–950. [Google Scholar] [CrossRef]
- Smith, K.E.; Dunn, K.E.; Rogers, J.M.; Grundmann, O.; McCurdy, C.R.; Garcia-Romeu, A.; Schriefer, D.; Swogger, M.T.; Epstein, D.H. Kratom Use as More Than a “Self-Treatment”. Am. J. Drug Alcohol. Abus. 2022, 48, 684–694. [Google Scholar] [CrossRef]
- Smith, K.E.; Dunn, K.E.; Grundmann, O.; Garcia-Romeu, A.; Rogers, J.M.; Swogger, M.T.; Epstein, D.H. Social, Psychological, and Substance use Characteristics of U.S. Adults who Use Kratom: Initial Findings from an Online, Crowdsourced Study. Exp. Clin. Psychopharmacol. 2021, 30, 983. [Google Scholar] [CrossRef]
- Grundmann, O.; Veltri, C.A.; Morcos, D.; Knightes, D.; Smith, K.E.; Singh, D.; Corazza, O.; Cinosi, E.; Martinotti, G.; Walsh, Z.; et al. Exploring the Self-Reported Motivations of Kratom (Mitragyna speciosa Korth.) Use: A Cross-Sectional Investigation. Am. J. Drug Alcohol. Abus. 2022, 48, 433–444. [Google Scholar] [CrossRef]
- Farkas, D.J.; Cooper, Z.D.; Heydari, L.N.; Hughes, A.C.; Rawls, S.M.; Ward, S.J. Kratom Alkaloids, Cannabinoids, and Chronic Pain: Basis of Potential Utility and Role in Therapy. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.; Anyangwe, N.; Carlacci, L.; Casper, S.; Danam, R.P.; Enongene, E.; Erives, G.; Fabricant, D.; Gudi, R.; Hilmas, C.J.; et al. The Safety and Regulation of Natural Products Used as Foods and Food Ingredients. Toxicol. Sci. An Off. J. Soc. Toxicol. 2011, 123, 333–348. [Google Scholar] [CrossRef]
- Rosenkranz, B.; Fasinu, P.; Bouic, P. An Overview of The Evidence and Mechanisms of Herb–Drug Interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef]
- Sharma, A.; Kamble, S.H.; Leon, F.; Chear, N.J.; King, T.I.; Berthold, E.C.; Ramanathan, S.; McCurdy, C.R.; Avery, B.A. Simultaneous Quantification of Ten Key Kratom Alkaloids in Mitragyna speciosa Leaf Extracts and Commercial Products by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. Drug Test. Anal. 2019, 11, 1162–1171. [Google Scholar] [CrossRef]
- Kamble, S.H.; Berthold, E.C.; King, T.I.; Raju Kanumuri, S.R.; Popa, R.; Herting, J.R.; Leon, F.; Sharma, A.; McMahon, L.R.; Avery, B.A.; et al. Pharmacokinetics of Eleven Kratom Alkaloids Following an Oral Dose of Either Traditional or Commercial Kratom Products in Rats. J. Nat. Prod. 2021, 84, 1104–1112. [Google Scholar] [CrossRef]
- Epidiolex (Cannabidiol) Oral Solution. Package Insert. 2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (accessed on 4 February 2024).
- Berthold, E.C.; Kamble, S.H.; Kanumuri, S.R.R.; Kuntz, M.A.; Senetra, A.S.; Chiang, Y.H.; McMahon, L.R.; McCurdy, C.R.; Sharma, A. Comparative Pharmacokinetics of Commercially Available Cannabidiol Isolate, Broad-Spectrum, and Full-Spectrum Products. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 427–435. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Clinical Drug Interaction Studies-Cytochrome P450 Enzyme-and Transporter-Mediated Drug Interactions Guidance for Industry; FAD, 2020. Available online: https://www.fda.gov/media/134581/download (accessed on 4 February 2024).
- Yu, J.; Wang, Y.; Ragueneau-Majlessi, I. Pharmacokinetic Drug-Drug Interactions with Drugs Approved by the US Food and Drug Administration in 2020: Mechanistic Understanding and Clinical Recommendations. Drug Metab. Dispos. 2022, 50, 1–7. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uprety, R.; Daibani, A.E.; Rouzic, V.L.; Hunkele, A.; Appourchaux, K.; Eans, S.O.; Nuthikattu, N.; Jilakara, R.; Thammavong, L.; et al. Kratom Alkaloids as Probes for Opioid Receptor Function: Pharmacological Characterization of Minor Indole and Oxindole Alkaloids from Kratom. ACS Chem. Neurosci. 2021, 12, 2661–2678. [Google Scholar] [CrossRef]
- King, T.I.; Sharma, A.; Kamble, S.H.; Leon, F.; Berthold, E.C.; Popa, R.; Cerlati, O.; Prentice, B.M.; McMahon, L.R.; McCurdy, C.R.; et al. Bioanalytical Method Development and Validation of Corynantheidine, A Kratom Alkaloid, Using UPLC-MS/MS, and its Application to Preclinical Pharmacokinetic Studies. J. Pharm. Biomed. Anal. 2020, 180, 113019. [Google Scholar] [CrossRef]
- Nasrin, S.; Watson, C.J.W.; Perez-Paramo, Y.X.; Lazarus, P. Cannabinoid Metabolites as Inhibitors of Major Hepatic CYP450 Enzymes, with Implications for Cannabis-Drug Interactions. Drug Metab. Dispos. 2021, 49, 1070–1080. [Google Scholar] [CrossRef]
- Kamble, S.H.; Sharma, A.; King, T.I.; Leon, F.; McCurdy, C.R.; Avery, B.A. Metabolite Profiling and Identification of Enzymes Responsible for the Metabolism of Mitragynine, The Major Alkaloid of Mitragyna speciosa (Kratom). Xenobiotica 2019, 49, 1279–1288. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uprety, R.; Slocum, S.T.; Irie, T.; Le Rouzic, V.; Li, X.; Wilson, L.L.; Scouller, B.; Alder, A.F.; Kruegel, A.C.; et al. Oxidative Metabolism as a Modulator of Kratom’s Biological Actions. J. Med. Chem. 2021, 64, 16553–16572. [Google Scholar] [CrossRef]
- Matheson, J.; Bourgault, Z.; Le Foll, B. Sex Differences in the Neuropsychiatric Effects and Pharmacokinetics of Cannabidiol: A Scoping Review. Biomolecules 2022, 12, 1462. [Google Scholar] [CrossRef]
- MacNair, L.; Kulpa, J.; Hill, M.L.; Eglit, G.M.L.; Mosesova, I.; Bonn-Miller, M.O.; Peters, E.N. Sex Differences in the Pharmacokinetics of Cannabidiol and Metabolites Following Oral Administration of a Cannabidiol-Dominant Cannabis Oil in Healthy Adults. Cannabis Cannabinoid Res. 2023. [Google Scholar] [CrossRef]
| Parameter | Kratom Product Alone (n = 4) [35] | Kratom Product Plus Cannabidiol (n = 5) | Fold Change (90% CI Ratio) |
|---|---|---|---|
| Cmax (ng/mL) | 111.9 ± 15.6 | 253.4 ± 68.3 | 2.3 (1.6, 2.7) |
| Tmax (h) | 3.1 ± 1.7 | 17.0 ± 4.6 | 5.4 (24.3, 4.2) |
| AUC0–24 (h·ng/mL) | 1306.8 ± 126.1 | 5354.9 ± 1145.5 | 2.8 (1.6, 3.7) |
| Parameter | Cannabidiol Product Alone (n = 4) | Cannabidiol Plus Kratom Product (n = 5) | Fold Change (90% CI Ratio) |
|---|---|---|---|
| Cmax (ng/mL) | 588.8 ± 38.3 | 490.9 ± 69.2 | 0.8 (0.7, 0.9) |
| Tmax (h) | 3.8 ± 1.5 | 6.3 ± 1.0 | 1.7 (3.8, 1.3) |
| AUC0–24 (h·ng/mL) | 4400.6 ± 319.2 | 4182.6 ± 614.5 | 1.0 (0.8, 1.1) |
| Kratom Product Alone (n = 4) [35] | Kratom Product Plus Cannabidiol (n = 5) | |
|---|---|---|
| Corynantheidine (0.2 mg/kg) | ||
| Cmax (ng/mL) | 3.1 ± 0.5 | 12.9 ± 2.0 |
| Tmax (h) | 3.1 ± 1.7 | 12.6 ± 11.4 |
| AUC0–24 (h·ng/mL) | 30.4 ± 9.1 | 195.9 ± 111.1 |
| Speciociliatine (2.2 mg/kg) | ||
| Cmax (ng/mL) | 23.8 ± 1.4 | 68.1 ± 5.9 |
| Tmax (h) | 3.2 ± 1.6 | 17.0 ± 5.9 |
| AUC0–24 (h·ng/mL) | 222.7 ± 22.2 | 989.7 ± 93.7 |
| Paynantheine (1.8 mg/kg) | ||
| Cmax (ng/mL) | 3.0 ± 1.7 | 19.8 ± 5.8 |
| Tmax (h) | 0.08 ± 0.0 | 16.2 ± 4.9 |
| AUC0–24 (h·ng/mL) | - | 290.0 ± 96.4 |
| 7-hydroxymitragynine (<0.1 mg/kg) | ||
| Cmax (ng/mL) | 4.0 ± 0.6 | 17.5 ± 2.3 |
| Tmax (h) | 3.1 ± 1.7 | 15.0 ± 5.5 |
| AUC0–24 (h·ng/mL) | 41.0 ± 7.6 | 189.2 ± 27.7 |
| Parameter | Mitragynine | Cannabidiol |
|---|---|---|
| Cmin,ss (ng/mL) | 91.1 ± 24.9 | 80.9 ± 23.7 |
| Cavg,ss (ng/mL) | 151.1 ± 34.7 | 203.8 ± 51.3 |
| Cmax,ss (ng/mL) | 217.5 ± 40.0 | 488.6 ± 96.2 |
| Fluctuation (%) | 90.0 ± 11.3 | 219.0 ± 42.7 |
| Accumulation Index | 1.4 ± 0.1 | 1.8 ± 0.0 |
| AUC0–∞ (h·ng/mL) | 2908.0 ± 735.0 | 3519.3 ± 851.1 |
| AUC/τ (h·ng/mL) | 1813.1 ± 416.6 | 2446.0 ± 615.1 |
| Parameter | Single-Dose | Multiple-Dose |
|---|---|---|
| Mitragynine | ||
| AUC0–∞/dose (h·ng/mL/mg/kg) | 578.6 ± 187.9 | 605.8 ± 153.1 |
| V/F (L/kg) | 25.8 ± 7.5 | 28.7 ± 3.7 |
| CL/F (L/h/kg) | 2.3 ± 0.5 | 3.1 ± 0.5 |
| Cannabidiol | ||
| AUC0–∞/dose (h·ng/mL/mg/kg) | 97.8 ± 18.5 | 140.8 ± 34.0 |
| V/F (L/kg) | 164.5 ± 26.3 | 192.3 ± 42.1 |
| CL/F (L/h/kg) | 11.5 ± 1.6 | 13.0 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthold, E.C.; Kamble, S.H.; Kanumuri, S.R.R.; Kuntz, M.A.; Senetra, A.S.; Chiang, Y.-H.; Mukhopadhyay, S.; McCurdy, C.R.; Sharma, A. Pharmacokinetic Interaction of Kratom and Cannabidiol in Male Rats. Pharmaceutics 2024, 16, 318. https://doi.org/10.3390/pharmaceutics16030318
Berthold EC, Kamble SH, Kanumuri SRR, Kuntz MA, Senetra AS, Chiang Y-H, Mukhopadhyay S, McCurdy CR, Sharma A. Pharmacokinetic Interaction of Kratom and Cannabidiol in Male Rats. Pharmaceutics. 2024; 16(3):318. https://doi.org/10.3390/pharmaceutics16030318
Chicago/Turabian StyleBerthold, Erin C., Shyam H. Kamble, Siva Rama Raju Kanumuri, Michelle A. Kuntz, Alexandria S. Senetra, Yi-Hua Chiang, Sushobhan Mukhopadhyay, Christopher R. McCurdy, and Abhisheak Sharma. 2024. "Pharmacokinetic Interaction of Kratom and Cannabidiol in Male Rats" Pharmaceutics 16, no. 3: 318. https://doi.org/10.3390/pharmaceutics16030318
APA StyleBerthold, E. C., Kamble, S. H., Kanumuri, S. R. R., Kuntz, M. A., Senetra, A. S., Chiang, Y.-H., Mukhopadhyay, S., McCurdy, C. R., & Sharma, A. (2024). Pharmacokinetic Interaction of Kratom and Cannabidiol in Male Rats. Pharmaceutics, 16(3), 318. https://doi.org/10.3390/pharmaceutics16030318








