Measuring and Modeling of Melt Viscosity for Drug Polymer Mixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation Method for Rheological Measurements
2.3. Rheology Measurement Method
3. Results and Discussion
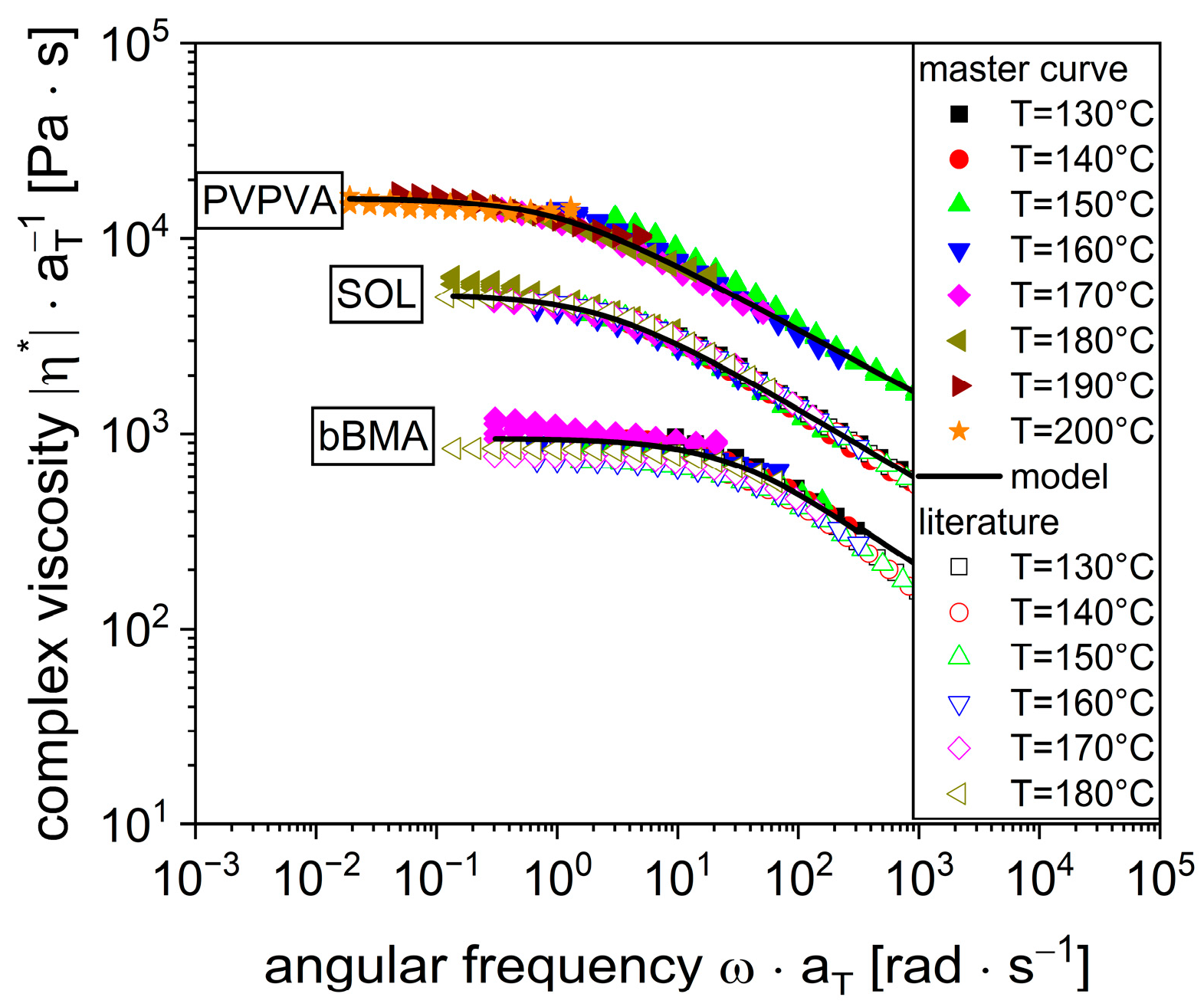
3.1. Viscosity Curves and Modeling for Pure Polymers
| Pure Polymer | bBMA | bBMA [17] | SOL | SOL [17] | PVPVA |
|---|---|---|---|---|---|
| 1011 ± 34 | 772 | 5146 ± 93 | 5063 | 18,296 ± 486 | |
| 26.8 ± 5.5 | 52.0 | 2.5 ± 0.1 | 4.5 | 1.0 ± 0.0 | |
| 0.433 ± 0.018 | 0.543 | 0.363 ± 0.002 | 0.416 | 0.368 ± 0.003 | |
| 122,498 ± 801 | 128,761 | 130,846 ± 344 | 139,821 | 184,119 ± 2442 |
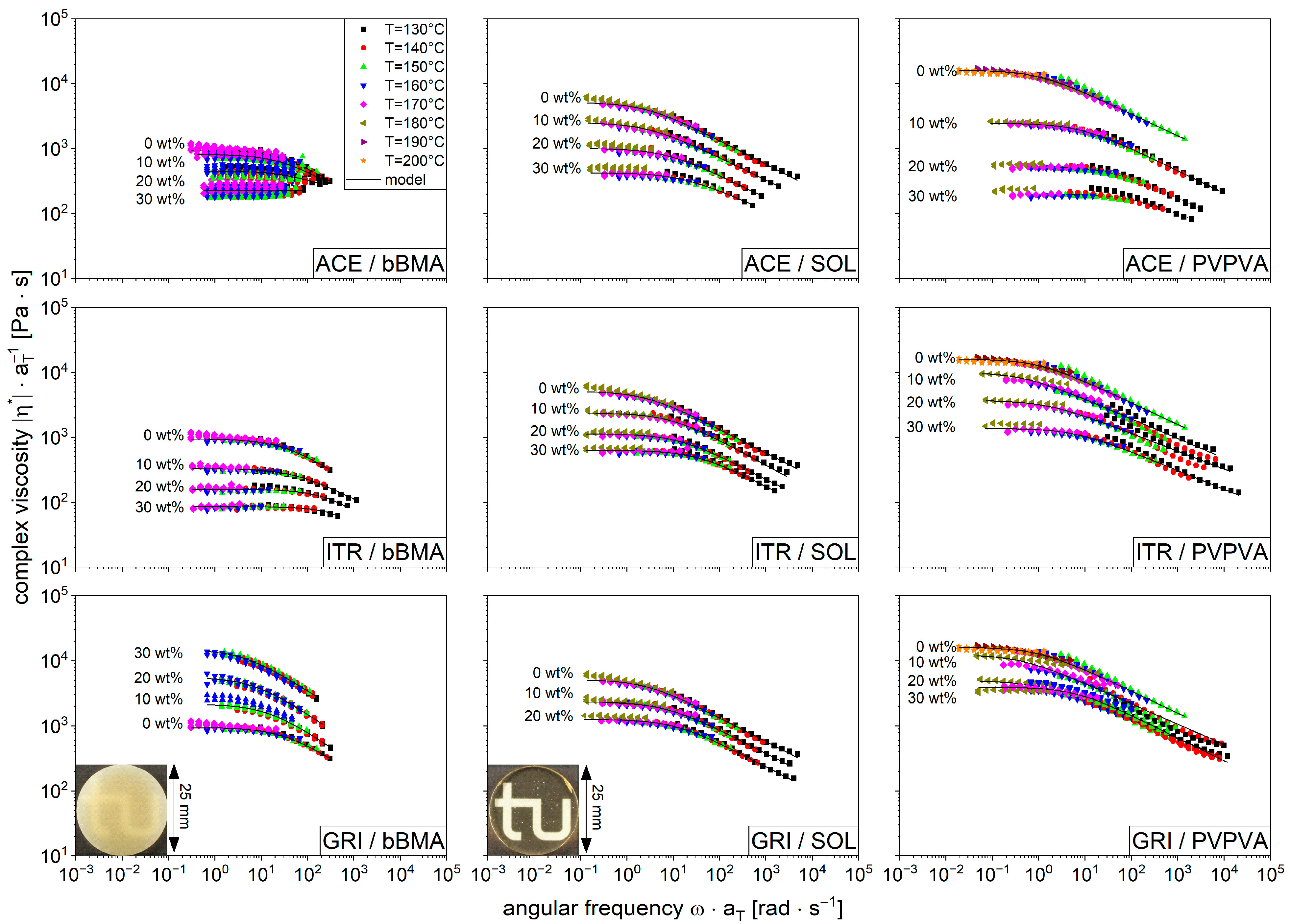
3.2. Viscosity Curves of Drug/Polymer Mixtures
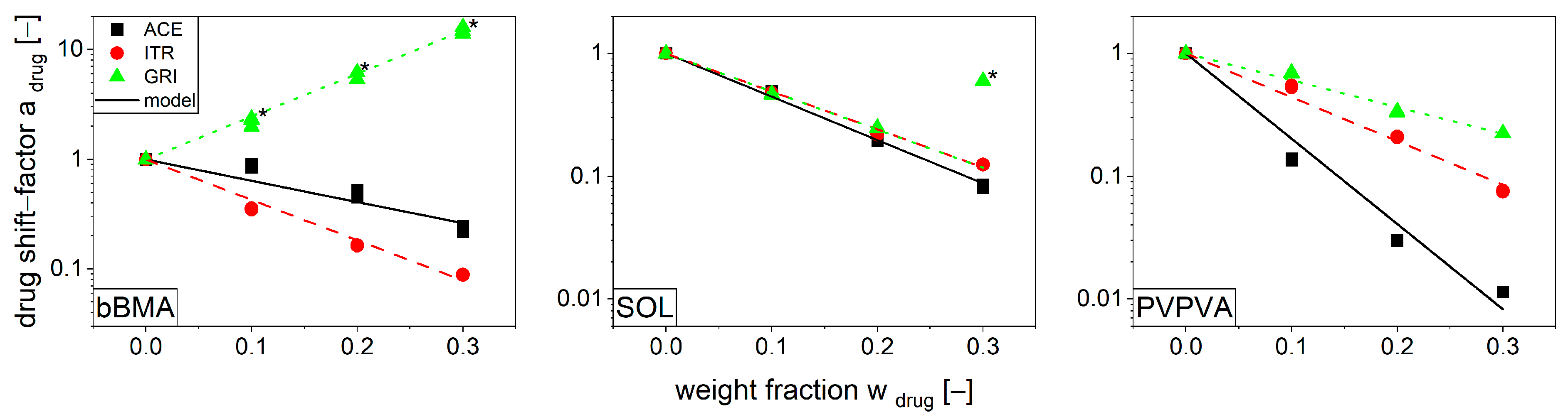
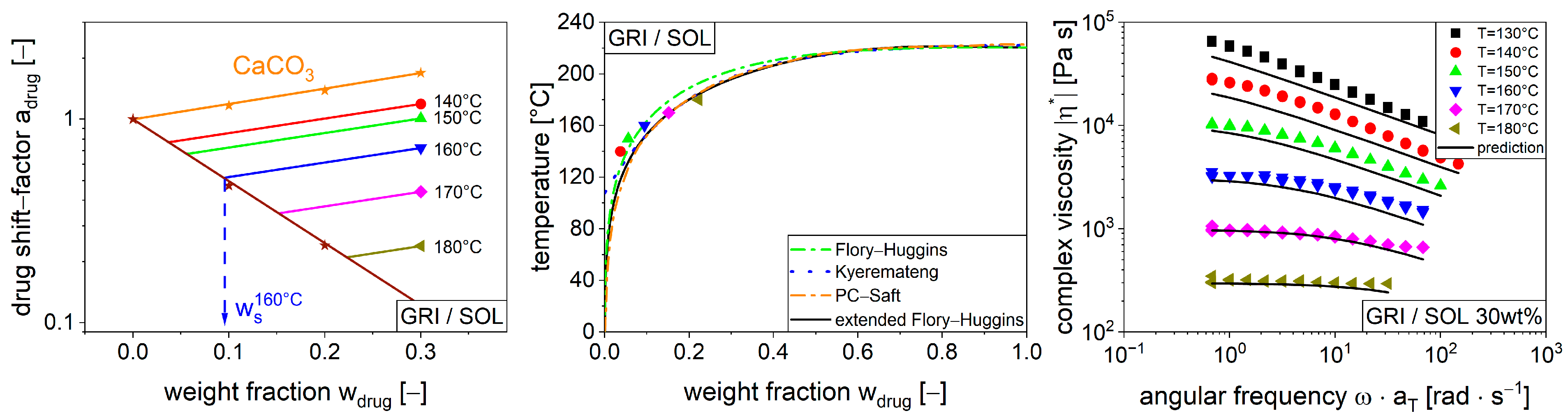
3.3. Modeling Function for Viscosity with Drug Shift Factor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols and Abbreviations
| Latin Symbols | Definition | Unit |
| temperature shift factor | ||
| drug shift factor | ||
| flow index | ||
| Arrhenius activation energy | ||
| ideal gas constant | ||
| drug relation parameter of a plasticizing drug | ||
| drug relation parameter of filler | ||
| temperature | ||
| glass transition temperature | ||
| reference temperature | ||
| weight fraction of the drug | ||
| reference weight fraction of the drug | ||
| weight fraction of the dissolved drug | ||
| Greek Symbols | Definition | Unit |
| shear rate | ||
| critical shear rate | ||
| zero shear rate viscosity | ||
| dynamic viscosity | ||
| complex viscosity | ||
| angular frequency | ||
| Abbreviations | Definition | |
| ACE | acetaminophen | |
| bBMA | basic butylated methacrylate copolymer | |
| CaCO3 | calcium carbonate | |
| GRI | griseofulvin | |
| ITR | itraconazole | |
| PVPVA | polyvinylpyrrolidone/vinyl acetate (copovidone) | |
| SOL | Soluplus | |
| Note: Tradenames and trademarks are used without particular labeling. | ||
Appendix A
| Drug | Content [wt%] | ||||
|---|---|---|---|---|---|
| - | 0 | 1011 ± 34 | 26.8 ± 5.5 | 0.433 ± 0.018 | 122,498 ± 801 |
| ACE | 10 | 806 ± 54 | 26.7 ± 6.0 | 0.400 ± 0.028 | 79,357 ± 1356 |
| ACE | 20 | 451 ± 46 | 30.0 ± 9.8 | 0.162 ± 0.033 | 43,055 ± 1883 |
| ACE | 30 | 179 ± 22 | 77.4 ± 1.2 | 0.117 ± 0.028 | 44,991 ± 2784 |
| ITR | 10 | 343 ± 7 | 72.8 ± 13.4 | 0.437 ± 0.027 | 115,075 ± 480 |
| ITR | 20 | 158 ± 3 | 369.4 ± 98.8 | 0.581 ± 0.098 | 114,195 ± 1198 |
| ITR | 30 | 86 ± 1 | 253.56 ± 49.8 | 0.368 ± 0.044 | 1,113,365 ± 865 |
| GRI | 10 | 2172 ± 159 | 14.8 ± 1.8 | 0.495 ± 0.009 | 110,747 ± 6318 |
| GRI | 20 | 5607 ± 462 | 7.1 ± 1.0 | 0.484 ± 0.015 | 110,022 ± 5735 |
| GRI | 30 | 14,928 ± 1 386 | 4.5 ± 1.1 | 0.467 ± 0.020 | 132,317 ± 6475 |
| Drug | Content [wt%] | ||||
|---|---|---|---|---|---|
| - | 0 | 5146 ± 93 | 2.5 ± 0.1 | 0.363 ± 0.002 | 130,846 ± 344 |
| ACE | 10 | 2484 ± 55 | 3.1 ± 0.2 | 0.349 ± 0.001 | 123,170 ± 405 |
| ACE | 20 | 1003 ± 30 | 7.3 ± 0.7 | 0.351 ± 0.004 | 117,018 ± 420 |
| ACE | 30 | 424 ± 11 | 17.0 ± 2.7 | 0.322 ± 0.009 | 113,743 ± 595 |
| ITR | 10 | 2410 ± 16 | 7.7 ± 0.3 | 0.380 ± 0.004 | 128,483 ± 230 |
| ITR | 20 | 1123 ± 22 | 14.3 ± 1.2 | 0.368 ± 0.003 | 129,862 ± 585 |
| ITR | 30 | 631 ± 6 | 33.8 ± 1.1 | 0.371 ± 0.004 | 131,218 ± 604 |
| GRI | 10 | 2326 ± 39 | 6.8 ± 0.4 | 0.362 ± 0.004 | 131,394 ± 304 |
| GRI | 20 | 1237 ± 11 | 13.0 ± 0.4 | 0.373 ± 0.002 | 138,862 ± 520 |
| GRI | 30 | 2987 ± 40 | 15.4 ± 1.0 | 0.456 ± 0.005 | 181,353 ± 816 |
| CaCO3 | 10 | 5497 ± 15 | 4.0 ± 0.1 | 0.389 ± 0.002 | 159,612 ± 164 |
| CaCO3 | 20 | 6616 ± 12 | 3.9 ± 0.1 | 0.389 ± 0.002 | 162,443 ± 1 004 |
| CaCO3 | 30 | 8191 ± 47 | 3.5 ± 0.1 | 0.391 ± 0.004 | 161,034 ± 525 |
| Drug | Content [wt%] | ||||
|---|---|---|---|---|---|
| - | 0 | 18,296 ± 486 | 1.0 ± 0.0 | 0.368 ± 0.003 | 184,119 ± 2442 |
| ACE | 10 | 2432 ± 44 | 8.4 ± 0.4 | 0.348 ± 0.003 | 161,786 ± 377 |
| ACE | 20 | 534 ± 10 | 37.2 ± 2.8 | 0.333 ± 0.006 | 146,410 ± 656 |
| ACE | 30 | 202 ± 1 | 89.4 ± 0.6 | 0.286 ± 0.001 | 145,155 ± 388 |
| ITR | 10 | 9596 ± 268 | 0.6 ± 0.0 | 0.307 ± 0.002 | 193,820 ± 1706 |
| ITR | 20 | 3697 ± 60 | 1.5 ± 0.1 | 0.275 ± 0.002 | 182,274 ± 458 |
| ITR | 30 | 1333 ± 12 | 6.6 ± 0.7 | 0.278 ± 0.003 | 178,054 ± 984 |
| GRI | 10 | 12,303 ± 96 | 0.4 ± 0.0 | 0.310 ± 0.003 | 217,872 ± 1567 |
| GRI | 20 | 5872 ± 78 | 0.3 ± 0.0 | 0.258 ± 0.002 | 212,188 ± 1318 |
| GRI | 30 | 3965 ± 25 | 6.2 ± 0.7 | 0.351 ± 0.002 | 211,522 ± 1088 |
| Additive/Polymer | bBMA | SOL | PVPVA |
|---|---|---|---|
| ACE | −4.47 ± 0.37 | −8.11 ± 0.11 | −15.98 ± 0.05 |
| ITR | −8.49 ± 0.07 | −7.11 ± 0.05 | −8.19 ± 0.05 |
| GRI | 8.97 ± 0.34 | −7.13 ± 0.09 | −5.02 ± 0.02 |
| CaCO3 | - | 1.51 ± 0.01 | - |
References
- Aho, J.; Boetker, J.P.; Baldursdottir, S.; Rantanen, J. Rheology as a tool for evaluation of melt processability of innovative dosage forms. Int. J. Pharm. 2015, 494, 623–642. [Google Scholar] [CrossRef]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef]
- Repka, M.A.; Battu, S.K.; Upadhye, S.B.; Thumma, S.; Crowley, M.M.; Zhang, F.; Martin, C.; McGinity, J.W. Pharmaceutical applications of hot-melt extrusion: Part II. Drug Dev. Ind. Pharm. 2007, 33, 1043–1057. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Scoutaris, N.; Gong, Y.; Hui, H.-W.; Kumar, S.; Douroumis, D. Investigation on hot melt extrusion and prediction on 3D printability of pharmaceutical grade polymers. Int. J. Pharm. 2021, 604, 120755. [Google Scholar] [CrossRef] [PubMed]
- Duty, C.; Ajinjeru, C.; Kishore, V.; Compton, B.; Hmeidat, N.; Chen, X.; Liu, P.; Hassen, A.A.; Lindahl, J.; Kunc, V. What makes a material printable? A viscoelastic model for extrusion-based 3D printing of polymers. J. Manuf. Process. 2018, 35, 526–537. [Google Scholar] [CrossRef]
- Seo, K.-S.; Bajracharya, R.; Lee, S.H.; Han, H.-K. Pharmaceutical Application of Tablet Film Coating. Pharmaceutics 2020, 12, 853. [Google Scholar] [CrossRef]
- Kapoor, D.; Maheshwari, R.; Verma, K.; Sharma, S.; Ghode, P.; Tekade, R.K. Coating technologies in pharmaceutical product development. In Drug Delivery Systems; Academic Press: Cambridge, MA, USA, 2020; pp. 665–719. [Google Scholar] [CrossRef]
- Bose, S.; Bogner, R.H. Solventless pharmaceutical coating processes: A review. Pharm. Dev. Technol. 2007, 12, 115–131. [Google Scholar] [CrossRef]
- Zecevic, D.E.; Evans, R.C.; Paulsen, K.; Wagner, K.G. From benchtop to pilot scale-experimental study and computational assessment of a hot-melt extrusion scale-up of a solid dispersion of dipyridamole and copovidone. Int. J. Pharm. 2018, 537, 132–139. [Google Scholar] [CrossRef]
- Gupta, S.; Solanki, N.; Serajuddin, A.T.M. Investigation of Thermal and Viscoelastic Properties of Polymers Relevant to Hot Melt Extrusion, IV: Affinisol™ HPMC HME Polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Osswald, T.A.; Rudolph, N. Polymer Rheology: Fundamentals and Applications; Hanser: Munich, Germany, 2015; ISBN 978-1-56990-517-3. [Google Scholar]
- Schröder, T. Rheologie der Kunststoffe: Theorie und Praxis; Hanser: München, Germany, 2018; ISBN 978-3-446-45405-7. [Google Scholar]
- Mezger, T. Das Rheologie Handbuch: Für Anwender von Rotations- und Oszillations-Rheometern; Auflage; Vincentz Network: Hannover, Germany, 2016; ISBN 978-3-74860-012-1. [Google Scholar]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Treffer, D.; Troiss, A.; Khinast, J. A novel tool to standardize rheology testing of molten polymers for pharmaceutical applications. Int. J. Pharm. 2015, 495, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Agassant, J.-F.; Avenas, P.; Carreau, P.J.; Vergnes, B.; Vincent, M. Polymer Processing, 2nd ed.; Hanser: München, Germany; Cincinatti, OH, USA, 2017; ISBN 978-1-56990-606-4. [Google Scholar]
- Osswald, T.A.; Hernández-Ortiz, J.P. Polymer Processing: Modeling and Simulation, 1st ed.; Hanser; Hanser Gardner: München, Germany; Cincinatti, OH, USA, 2006; ISBN 978-3-446-40381-9. [Google Scholar]
- de Miranda, D.A.; Rauber, W.K.; Vaz Júnior, M.; Nogueira, A.L.; Bom, R.P.; Zdanski, P.S.B. Evaluation of the Predictive Capacity of Viscosity Models in Polymer Melt Filling Simulations. J. Mater. Eng. Perform. 2023, 32, 1707–1720. [Google Scholar] [CrossRef]
- Zare, Y.; Park, S.P.; Rhee, K.Y. Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau–Yasuda model. Results Phys. 2019, 13, 102245. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef]
- Kohlgrüber, K. Der Gleichläufige Doppelschneckenextruder: Grundlagen, Technologie, Anwendungen; Auflage; Hanser: München, Germany, 2016; ISBN 978-3-446-43361-8. [Google Scholar]
- Rueda, M.M.; Auscher, M.-C.; Fulchiron, R.; Périé, T.; Martin, G.; Sonntag, P.; Cassagnau, P. Rheology and applications of highly filled polymers: A review of current understanding. Prog. Polym. Sci. 2017, 66, 22–53. [Google Scholar] [CrossRef]
- Chong, J.S.; Christiansen, E.B.; Baer, A.D. Rheology of concentrated suspensions. J. Appl. Polym. Sci. 1971, 15, 2007–2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Fassihi, M.A.; Fassihi, R. Delivery Considerations of Highly Viscous Polymeric Fluids Mimicking Concentrated Biopharmaceuticals: Assessment of Injectability via Measurement of Total Work Done “WT”. AAPS PharmSciTech 2018, 19, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Maru, S.M.; de Matas, M.; Kelly, A.; Paradkar, A. Characterization of thermal and rheological properties of zidovudine, lamivudine and plasticizer blends with ethyl cellulose to assess their suitability for hot melt extrusion. Eur. J. Pharm. Sci. 2011, 44, 471–478. [Google Scholar] [CrossRef]
- Sarode, A.L.; Sandhu, H.; Shah, N.; Malick, W.; Zia, H. Hot melt extrusion (HME) for amorphous solid dispersions: Predictive tools for processing and impact of drug-polymer interactions on supersaturation. Eur. J. Pharm. Sci. 2013, 48, 371–384. [Google Scholar] [CrossRef]
- Chomcharn, N.; Xanthos, M. Properties of aspirin modified enteric polymer prepared by hot-melt mixing. Int. J. Pharm. 2013, 450, 259–267. [Google Scholar] [CrossRef]
- Song, Y.; Mathias, P.M.; Tremblay, D.; Chen, C.-C. Liquid Viscosity Model for Polymer Solutions and Mixtures. Ind. Eng. Chem. Res. 2003, 42, 2415–2422. [Google Scholar] [CrossRef]
- Aho, J.; Edinger, M.; Botker, J.; Baldursdottir, S.; Rantanen, J. Oscillatory Shear Rheology in Examining the Drug-Polymer Interactions Relevant in Hot Melt Extrusion. J. Pharm. Sci. 2016, 105, 160–167. [Google Scholar] [CrossRef]
- Sathigari, S.K.; Radhakrishnan, V.K.; Davis, V.A.; Parsons, D.L.; Babu, R.J. Amorphous-state characterization of efavirenz--polymer hot-melt extrusion systems for dissolution enhancement. J. Pharm. Sci. 2012, 101, 3456–3464. [Google Scholar] [CrossRef]
- van Renterghem, J.; Vervaet, C.; de Beer, T. Rheological Characterization of Molten Polymer-Drug Dispersions as a Predictive Tool for Pharmaceutical Hot-Melt Extrusion Processability. Pharm. Res. 2017, 34, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, E.S.; Üstüner, E.E.; Gryczke, A.; Wagner, K.G. Predicting melt rheology for hot-melt extrusion by means of a simple Tg-measurement. Eur. J. Pharm. Biopharm. 2017, 119, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, E.S.; Gryczke, A.; Wagner, K.G. Validation of Model-Based Melt Viscosity in Hot-Melt Extrusion Numerical Simulation. Pharmaceutics 2018, 10, 132. [Google Scholar] [CrossRef]
- Suwardie, H.; Wang, P.; Todd, D.B.; Panchal, V.; Yang, M.; Gogos, C.G. Rheological study of the mixture of acetaminophen and polyethylene oxide for hot-melt extrusion application. Eur. J. Pharm. Biopharm. 2011, 78, 506–512. [Google Scholar] [CrossRef]
- Yang, M.; Wang, P.; Suwardie, H.; Gogos, C. Determination of acetaminophen’s solubility in poly(ethylene oxide) by rheological, thermal and microscopic methods. Int. J. Pharm. 2011, 403, 83–89. [Google Scholar] [CrossRef]
- Song, S.; Wang, C.; Zhang, B.; Sun, C.C.; Lodge, T.P.; Siegel, R.A. A Rheological Approach for Predicting Physical Stability of Amorphous Solid Dispersions. J. Pharm. Sci. 2023, 112, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.; Gupta, S.S.; Serajuddin, A.T.M. Rheological analysis of itraconazole-polymer mixtures to determine optimal melt extrusion temperature for development of amorphous solid dispersion. Eur. J. Pharm. Sci. 2018, 111, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Klueppelberg, J.; Handge, U.A.; Thommes, M.; Winck, J. Composition Dependency of the Flory–Huggins Interaction Parameter in Drug–Polymer Phase Behavior. Pharmaceutics 2023, 15, 2650. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Suwardie, H.; Wang, P.; Gogos, C.G. Miscibility studies of indomethacin and Eudragit® E PO by thermal, rheological, and spectroscopic analysis. J. Pharm. Sci. 2012, 101, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Parikh, T.; Meena, A.K.; Mahajan, N.; Vitez, I.; Serajuddin, A.T.M. Effect of carbamazepine on viscoelastic properties and hot melt extrudability of Soluplus®. Int. J. Pharm. 2015, 478, 232–239. [Google Scholar] [CrossRef]
- MeltPrep GmbH. Manual for Vacuum Compression Molding System; MeltPrep GmbH: Graz, Austria, 2022. [Google Scholar]
- MathWorks. Fmincon. Available online: https://de.mathworks.com/help/optim/ug/fmincon.html (accessed on 16 February 2023).
- Flügel, K.; Schmidt, K.; Mareczek, L.; Gäbe, M.; Hennig, R.; Thommes, M. Impact of incorporated drugs on material properties of amorphous solid dispersions. Eur. J. Pharm. Biopharm. 2021, 159, 88–98. [Google Scholar] [CrossRef]
- Utracki, L.A. Polymer Blends Handbook; Kluwer: Dordrecht, The Netherlands, 2002; ISBN 1-4020-1111-3. [Google Scholar]
- Bochmann, E.S.; Steffens, K.E.; Gryczke, A.; Wagner, K.G. Numerical simulation of hot-melt extrusion processes for amorphous solid dispersions using model-based melt viscosity. Eur. J. Pharm. Biopharm. 2018, 124, 34–42. [Google Scholar] [CrossRef]
- Wolbert, F.; Fahrig, I.-K.; Gottschalk, T.; Luebbert, C.; Thommes, M.; Sadowski, G. Factors Influencing the Crystallization-Onset Time of Metastable ASDs. Pharmaceutics 2022, 14, 269. [Google Scholar] [CrossRef]
- Huggins, M.L. Thermodynamic properties of solutions of long-chain compounds. Ann. N. Y. Acad. Sci. 1942, 43, 1–32. [Google Scholar] [CrossRef]
- Kyeremateng, S.O.; Pudlas, M.; Woehrle, G.H. A fast and reliable empirical approach for estimating solubility of crystalline drugs in polymers for hot melt extrusion formulations. J. Pharm. Sci. 2014, 103, 2847–2858. [Google Scholar] [CrossRef]
- Six, K.; Berghmans, H.; Leuner, C.; Dressman, J.; van Werde, K.; Mullens, J.; Benoist, L.; Thimon, M.; Meublat, L.; Verreck, G.; et al. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion, Part II. Pharm. Res. 2003, 20, 1047–1054. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimmel, V.; Ercolin, E.; Zimmer, R.; Yörük, M.; Winck, J.; Thommes, M. Measuring and Modeling of Melt Viscosity for Drug Polymer Mixtures. Pharmaceutics 2024, 16, 301. https://doi.org/10.3390/pharmaceutics16030301
Kimmel V, Ercolin E, Zimmer R, Yörük M, Winck J, Thommes M. Measuring and Modeling of Melt Viscosity for Drug Polymer Mixtures. Pharmaceutics. 2024; 16(3):301. https://doi.org/10.3390/pharmaceutics16030301
Chicago/Turabian StyleKimmel, Vincent, Enrico Ercolin, Robin Zimmer, Muhammet Yörük, Judith Winck, and Markus Thommes. 2024. "Measuring and Modeling of Melt Viscosity for Drug Polymer Mixtures" Pharmaceutics 16, no. 3: 301. https://doi.org/10.3390/pharmaceutics16030301
APA StyleKimmel, V., Ercolin, E., Zimmer, R., Yörük, M., Winck, J., & Thommes, M. (2024). Measuring and Modeling of Melt Viscosity for Drug Polymer Mixtures. Pharmaceutics, 16(3), 301. https://doi.org/10.3390/pharmaceutics16030301






