Nanomaterials in the Wound Healing Process: New Insights and Advancements
Abstract
1. Introduction
2. Wound Processes
- Acute wounds: short-term (more prevalent), and sometimes inflicted by radiation, shock (electrical), excessive, or mechanical injury.
3. Wound Healing Process
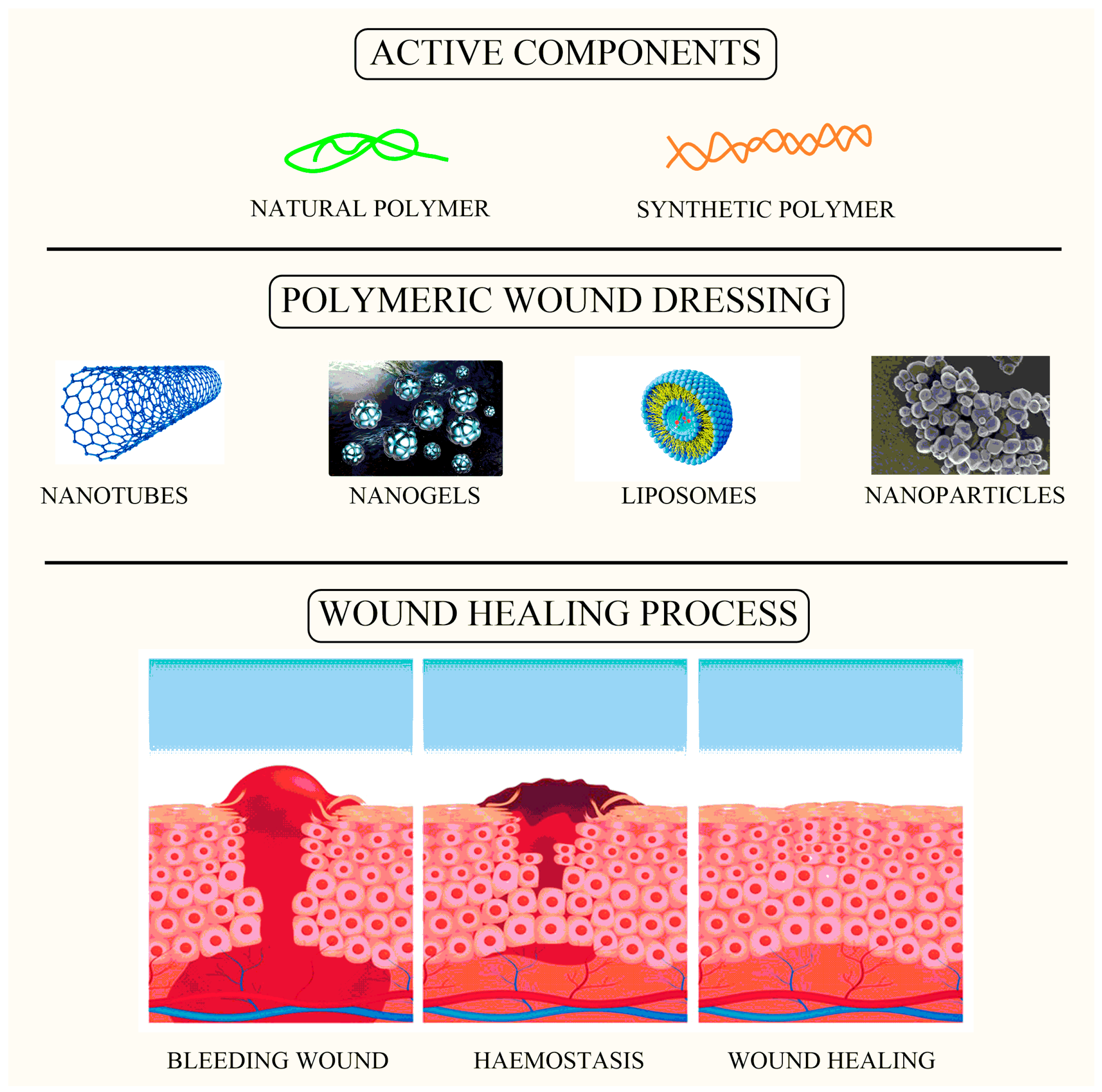
3.1. Hemostasis
3.2. Inflammation
3.3. Proliferation
3.4. Maturation
4. Available Therapeutic Approaches in Wound Healing
5. Applications of Different Nanomaterials in Wound Healing
6. Carbon-Based Nanomaterial
6.1. Carbon Nanotubes
6.2. Single-Walled Nanotube
6.3. Double-Walled Nanotube
6.4. Multi-Walled Nanotube
7. Nanostructured Carriers
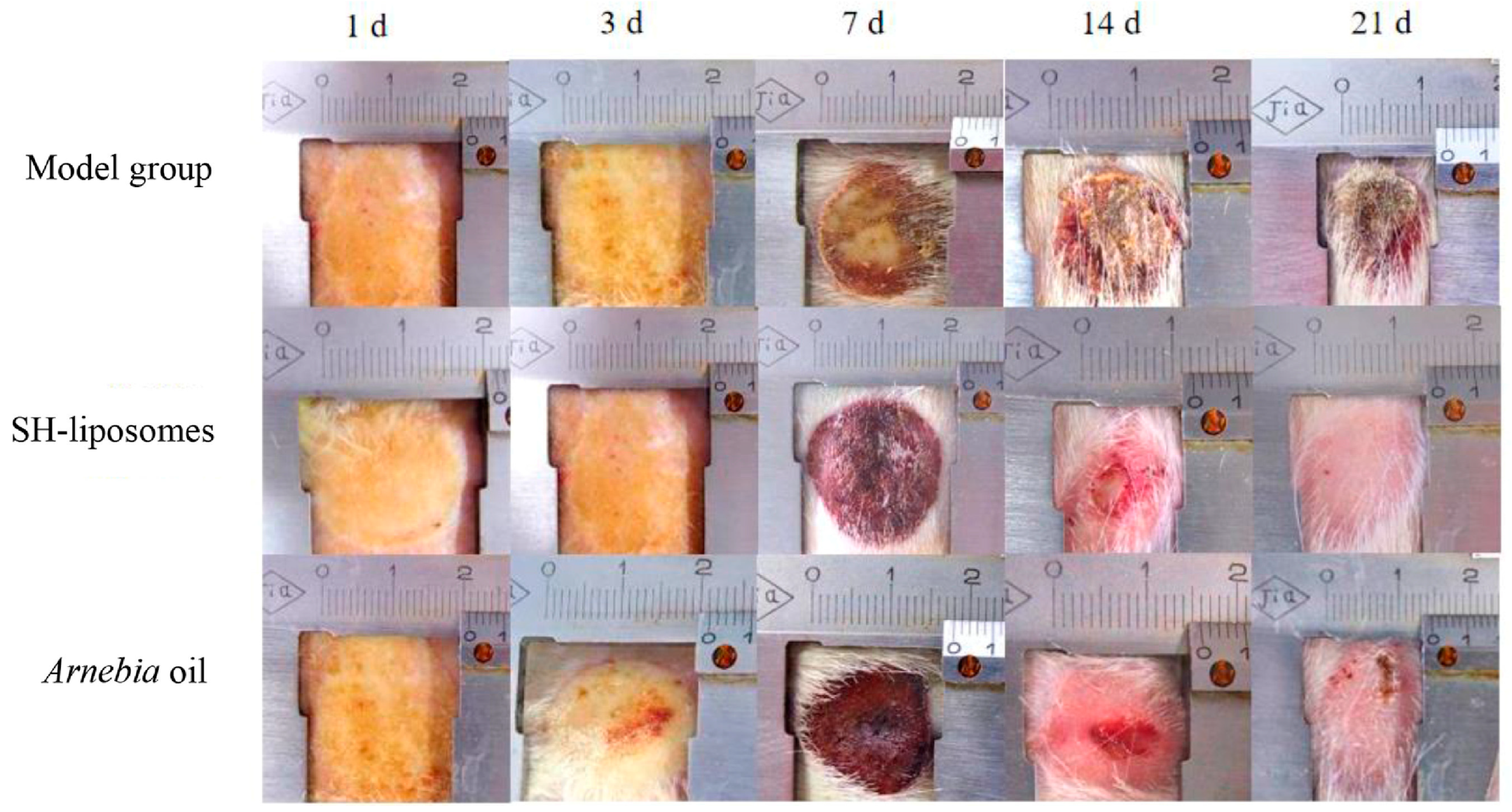
7.1. Liposomal Delivery
7.2. Nanohydrogels
7.3. Metals and Metal Nanoparticles as Antimicrobials
7.4. Silver Nanoparticles
7.5. Zinc Oxide Nanoparticles
7.6. Titanium Oxide Nanoparticles
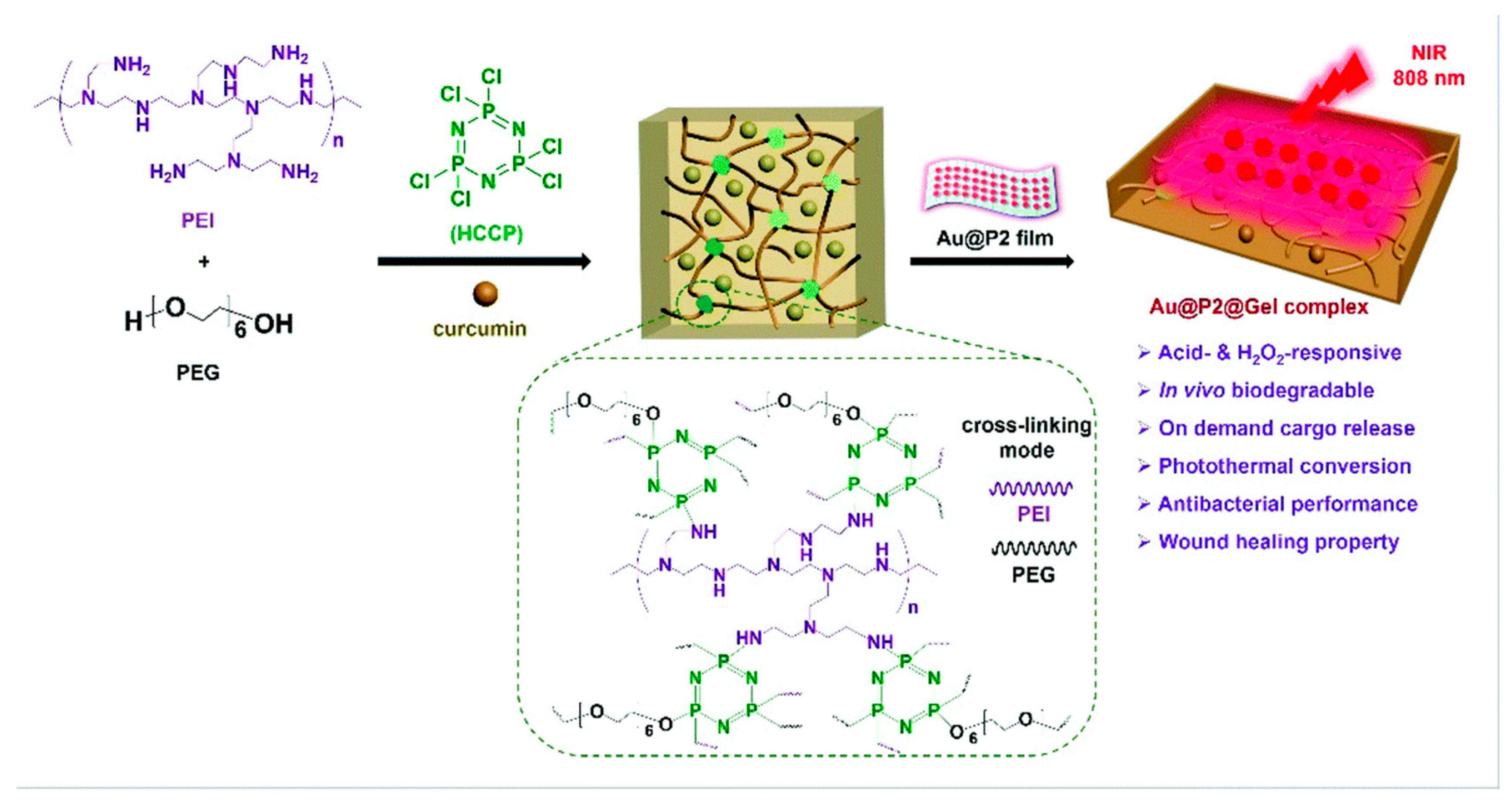
7.7. Gold Nanoparticles
7.8. Nanofibers
8. Metal Nanoparticles with Biomaterials in Wound Healing
8.1. Zero-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
8.2. One-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
8.3. Two-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
8.4. Three-Dimensional Metal-Based Biomaterial Platforms in Wound Repair
9. Metal Nanoparticle Potential Cytotoxicity
9.1. Neurotoxic Effects
9.2. Immunotoxicity
9.3. Genotoxicity
10. Future Challenges
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarei, M.; Lee, G.; Lee, S.G.; Cho, K. Advances in Biodegradable Electronic Skin: Material Progress and Recent Applications in Sensing, Robotics, and Human–Machine Interfaces. Adv. Mater. 2023, 35, e2203193. [Google Scholar] [CrossRef]
- Sharma, A.; Chopra, H.; Singh, I.; Emran, T.B. Physically and Chemically Crosslinked Hydrogels for Wound Healing Applications. Int. J. Surg. 2022, 106, 106915. [Google Scholar] [CrossRef] [PubMed]
- Vinaik, R.; Barayan, D.; Jeschke, M.G. Pathophysiology and Hypermetabolic Response to Burn. In Essential Burn Care for Non-Burn Specialists; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Nosrati, H.; Heydari, M.; Tootiaei, Z.; Ganjbar, S.; Khodaei, M. Delivery of Antibacterial Agents for Wound Healing Applications Using Polysaccharide-Based Scaffolds. J. Drug Deliv. Sci. Technol. 2023, 84, 104516. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Wenthen, R.; Landers, Z.A. Traumatic Injury and Traumatic Brain Injury. In The Practice of Clinical Social Work in Healthcare; Hemphill, M., Nathanson, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 215–239. [Google Scholar] [CrossRef]
- Jan, B.; Jan, R.; Afzal, S.; Ayoub, M.; Masoodi, M.H. Treatment Strategies to Combat Multidrug Resistance (MDR) in Bacteria. In Non-Traditional Approaches to Combat Antimicrobial Drug Resistance; Wani, M.Y., Ahmad, A., Eds.; Springer Nature: Singapore, 2023; pp. 79–100. [Google Scholar] [CrossRef]
- Chopra, H.; Kumar, S.; Singh, I. Strategies and Therapies for Wound Healing: A Review. Curr. Drug Targets 2021, 23, 87–98. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Kharazmi, M.S.; Jafari, S.M. Role of Nanomaterials in Improving the Functionality of Probiotics; Integration of Nanotechnology onto Micro-Structured Platforms. Food Biosci. 2023, 53, 102843. [Google Scholar] [CrossRef]
- Chhabra, J.; Chopra, H.; Pahwa, R.; Raina, N.; Wadhwa, K.; Saini, S.; Negi, P.; Gupta, M.; Singh, I.; Dureja, H.; et al. Potential of Nanoemulsions for Accelerated Wound Healing: Innovative Strategies. Int. J. Surg. 2023, 109, 2365–2377. [Google Scholar] [CrossRef]
- Sharma, R.; Borah, S.J.; Bhawna, N.; Kumar, S.; Gupta, A.; Kumari, V.; Kumar, R.; Dubey, K.K.; Kumar, V. Emerging Trends in Nano-Based Antidiabetic Therapeutics: A Path to Effective Diabetes Management. Mater. Adv. 2023, 4, 3091–3113. [Google Scholar] [CrossRef]
- Roy, M.; Roy, A.; Rustagi, S.; Pandey, N. An Overview of Nanomaterial Applications in Pharmacology. BioMed Res. Int. 2023, 2023, 4838043. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Negi, A.; Bhattacharya, B.; Dey, T.; Mitra, P.; Preetam, S.; Kumar, L.; Kar, S.; Das, S.S.; Iqbal, D.; et al. Nanotheranostics to Target Antibiotic-Resistant Bacteria: Strategies and Applications. OpenNano 2023, 11, 100138. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The Initiation of Oxidative Stress and Therapeutic Strategies in Wound Healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Sung, S.; Steele, L.A.; Risser, G.E.; Spiller, K.L. Biomaterial-Assisted Macrophage Cell Therapy for Regenerative Medicine. Adv. Drug Deliv. Rev. 2023, 199, 114979. [Google Scholar] [CrossRef]
- Muñoz-González, P.U.; Flores-Moreno, J.M.; Quintero-Ortega, I.A.; Mantovani, D.; Mendoza-Novelo, B.; González-García, G. Water-Dispersible Fluorescent Silicon Nanoparticles That Modulate Inflammatory Response in Macrophages. ACS Appl. Nano Mater. 2023, 6, 11187–11197. [Google Scholar] [CrossRef]
- Benoit, A.; Vogin, G.; Duhem, C.; Berchem, G.; Janji, B. Lighting Up the Fire in the Microenvironment of Cold Tumors: A Major Challenge to Improve Cancer Immunotherapy. Cells 2023, 12, 1787. [Google Scholar] [CrossRef]
- Parikh, S.D.; Wang, W.; Nelson, M.T.; Sulentic, C.E.W.; Mukhopadhyay, S.M. Bioinspired Hierarchical Carbon Structures as Potential Scaffolds for Wound Healing and Tissue Regeneration Applications. Nanomaterials 2023, 13, 1791. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.S.; D’Elia, A.M.; Rodell, C.B. Control of the Post-Infarct Immune Microenvironment through Biotherapeutic and Biomaterial-Based Approaches. Drug Deliv. Transl. Res. 2023, 13, 1983–2014. [Google Scholar] [CrossRef] [PubMed]
- Mehryab, F.; Taghizadeh, F.; Goshtasbi, N.; Merati, F.; Rabbani, S.; Haeri, A. Exosomes as Cutting-Edge Therapeutics in Various Biomedical Applications: An Update on Engineering, Delivery, and Preclinical Studies. Biochimie 2023, 213, 139–167. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, H.; Liu, R.; Cui, R. Advances in Immunomodulatory Mechanisms of Mesenchymal Stem Cells-Derived Exosome on Immune Cells in Scar Formation. Int. J. Nanomed. 2023, 18, 3643–3662. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Pang, Q.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Nanomaterials-Based Wound Dressing for Advanced Management of Infected Wound. Antibiotics 2023, 12, 351. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Gao, W.; Li, X.; Long, L.; Hou, X.; Zhao, J.; Li, S.; Yuan, X. Microstructure-United Heterogeneous Sodium Alginate Doped Injectable Hydrogel for Stable Hemostasis in Dynamic Mechanical Environments. Int. J. Biol. Macromol. 2023, 248, 125877. [Google Scholar] [CrossRef]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D.; et al. Metal Nanoparticles in Cancer: From Synthesis and Metabolism to Cellular Interactions. J. Nanostructure Chem. 2023, 13, 321–348. [Google Scholar] [CrossRef]
- Lu, Z.; Li, J.; Chen, B.; Feng, J.; Hu, Q.; Jin, Y.; Fu, Z. Mitochondria Targeted Nanoparticles Potentiate Tumor Chemo-Phototherapy by Toxic Oxidative Stress Mediated Oxeiptosis. Macromol. Biosci. 2023, 23, e2300151. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, S.; Park, H.; Sun, J.G.; Kim, E.C.; Shim, G. Nanoparticles for Lymph Node-Directed Delivery. Pharmaceutics 2023, 15, 565. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, A. Strategies for Cancer Therapy: Targeting Tumor Microenvironment and Nanotechnology. Egypt. Pharm. J. 2023, 22, 165–176. [Google Scholar] [CrossRef]
- Choudhury, H.; Pandey, M.; Saravanan, V.; Mun, A.T.Y.; Bhattamisra, S.K.; Parikh, A.; Garg, S.; Gorain, B. Recent Progress of Targeted Nanocarriers in Diagnostic, Therapeutic, and Theranostic Applications in Colorectal Cancer. Biomater. Adv. 2023, 153, 213556. [Google Scholar] [CrossRef]
- Bao, M.; Wang, K.; Li, J.; Li, Y.; Zhu, H.; Lu, M.; Zhang, Y.; Fan, Q.; Han, L.; Wang, K.; et al. ROS Scavenging and Inflammation-Directed Polydopamine Nanoparticles Regulate Gut Immunity and Flora Therapy in Inflammatory Bowel Disease. Acta Biomater. 2023, 161, 250–264. [Google Scholar] [CrossRef]
- Chen, S.; Xing, Z.; Geng, M.; Zhao, R.; Yang, X.; Zhu, X.; Anderson, J.M.; Zhang, X. Macrophage Fusion Event as One Prerequisite for Inorganic Nanoparticle-Induced Antitumor Response. Sci. Adv. 2023, 9, eadd9871. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, Y.; Huang, H.; Roy, S.; Song, Z.; Guo, B. Recent Advances in Nanomedicines for Imaging and Therapy of Myocardial Ischemia-Reperfusion Injury. J. Control. Release 2023, 353, 563–590. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The Oral Microbiome in the Pathophysiology of Cardiovascular Disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, Z.; Jolly, K.J. Myeloid Cell-Mediated Drug Delivery: From Nanomedicine to Cell Therapy. Adv. Drug Deliv. Rev. 2023, 197, 114827. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Santos, A.I.; Veiga, F.; Figueiras, A. Lignin Nanoparticle–Based Nanocomposite Hydrogels for Biomedical Applications. In Functional Nanocomposite Hydrogels: Synthesis, Characterization, and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Kushwaha, R.; Kumar, S.; Das, A.; Sukriti; Verma, M.L. Silver Nanoparticle-Based Nanocomposite Hydrogels for Biomedical Applications. In Functional Nanocomposite Hydrogels: Synthesis, Characterization, and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Okoye, C.O.; Okeke, E.S.; Ezeorba, T.P.C.; Chukwudozie, K.I.; Chiejina, C.O.; Fomena Temgoua, N.S. Microbial and Bio-Based Preservatives: Recent Advances in Antimicrobial Compounds. In Microbes for Natural Food Additives; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Rahim, G.; Qureshi, R.; Hazrat, A.; Ahmad, B.; Khan, A.A.; Aziz, T.; Alharbi, M.; Alshammari, A. Phytochemical, Antimicrobial, Radical Scavenging and In-Vitro Biological Activities of Teucrium stocksianum Leaves. J. Chil. Chem. Soc. 2023, 68, 5748–5754. [Google Scholar] [CrossRef]
- Gambhir, K.; Tyagi, N.; Verma, Y.K. Next-Generation Bandages to Overcome Oxygen Limitation during Wound Healing/Tissue Repair. In Fiber and Textile Engineering in Drug Delivery Systems; Woodhead Publishing: Sawston, UK, 2023. [Google Scholar] [CrossRef]
- Musyuni, P.; Nagpal, M.; Singh, M.; Goyal, R.K.; Aggarwal, G. Nanotechnology Enabled Solutions to Combat COVID-19: Prevention, Treatment, and Diagnosis. Curr. Pharm. Biotechnol. 2021, 23, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Aydeger, A.; Aysit, N.; Baydas, G.; Cakici, C.; Erim, U.C.; Arpa, M.D.; Ozcicek, I. Design of IKVAV Peptide/Gold Nanoparticle Decorated, Micro/Nano-Channeled PCL/PLGA Film Scaffolds for Neuronal Differentiation and Neurite Outgrowth. Biomater. Adv. 2023, 152, 213472. [Google Scholar] [CrossRef]
- Londhe, S.; Haque, S.; Patra, C.R. Silver and Gold Nanoparticles: Potential Cancer Theranostic Applications, Recent Development, Challenges, and Future Perspectives. In Gold and Silver Nanoparticles: Synthesis and Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Dam, P.; Celik, M.; Ustun, M.; Saha, S.; Saha, C.; Kacar, E.A.; Kugu, S.; Karagulle, E.N.; Tasoglu, S.; Buyukserin, F.; et al. Wound Healing Strategies Based on Nanoparticles Incorporated in Hydrogel Wound Patches. RSC Adv. 2023, 13, 21345–21364. [Google Scholar] [CrossRef]
- Alvandi, H.; Rajati, H.; Naseriyeh, T.; Rahmatabadi, S.S.; Hosseinzadeh, L.; Arkan, E. Incorporation of Aloe vera and Green Synthesized ZnO Nanoparticles into the Chitosan/PVA Nanocomposite Hydrogel for Wound Dressing Application. Polym. Bull. 2023, 2023, 1–16. [Google Scholar] [CrossRef]
- Xiang, Y.; Qi, X.; Cai, E.; Zhang, C.; Wang, J.; Lan, Y.; Deng, H.; Shen, J.; Hu, R. Highly Efficient Bacteria-Infected Diabetic Wound Healing Employing a Melanin-Reinforced Biopolymer Hydrogel. Chem. Eng. J. 2023, 460, 141852. [Google Scholar] [CrossRef]
- Tiwari, N.; Kumar, D.; Priyadarshani, A.; Jain, G.K.; Mittal, G.; Kesharwani, P.; Aggarwal, G. Recent Progress in Polymeric Biomaterials and Their Potential Applications in Skin Regeneration and Wound Care Management. J. Drug Deliv. Sci. Technol. 2023, 82, 104319. [Google Scholar] [CrossRef]
- Latiyan, S.; Kumar, T.S.S.; Doble, M.; Kennedy, J.F. Perspectives of Nanofibrous Wound Dressings Based on Glucans and Galactans-A Review. Int. J. Biol. Macromol. 2023, 244, 125358. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Ma, P.; Wu, H.; Xiao, D.; Zhang, Y.; Sui, X.; Zhang, L.; Dong, A. Functional Carbohydrate-Based Hydrogels for Diabetic Wound Therapy. Carbohydr. Polym. 2023, 312, 120823. [Google Scholar] [CrossRef] [PubMed]
- Rezvani Ghomi, E.; Niazi, M.; Ramakrishna, S. The Evolution of Wound Dressings: From Traditional to Smart Dressings. Polym. Adv. Technol. 2023, 34, 520–530. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Yeo, J.C.C.; Soo, X.Y.D.; Thitsartarn, W.; Liu, S.; Tan, B.H.; Suwardi, A.; Li, Z.; Zhu, Q.; et al. Responsive Hydrogel Dressings for Intelligent Wound Management. BMEMat 2023, 1, e12021. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhang, X.J.; Gao, Y.M.; Song, Y.; Sands, M.X.; Zhou, S.B.; Li, Q.F.; Zhang, J. Photo-Responsive Hydrogel for Contactless Dressing Change to Attenuate Secondary Damage and Promote Diabetic Wound Healing. Adv. Health Mater. 2023, 12, e2202770. [Google Scholar] [CrossRef] [PubMed]
- Huanbutta, K.; Sittikijyothin, W.; Sangnim, T. Development of Topical Natural Based Film Forming System Loaded Propolis from Stingless Bees for Wound Healing Application. J. Pharm. Investig. 2020, 50, 625–634. [Google Scholar] [CrossRef]
- Sangnim, T.; Meeboon, P.; Phongsewalak, P.; Prasongdee, P.; Sriamornsak, P.; Singh, I.; Manmuan, S.; Huanbutta, K. Development and Evaluation of Liquid Plaster Loaded with Chromolaena Odorata Leaf Extract Endowed with Several Beneficial Properties to Wound Healing. Gels 2022, 8, 72. [Google Scholar] [CrossRef]
- Lu, H.; Shao, W.; Gao, B.; Zheng, S.; He, B. Intestine-Inspired Wrinkled MXene Microneedle Dressings for Smart Wound Management. Acta Biomater. 2023, 159, 201–210. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, G.; Wu, X.; Xie, J.; Li, D.; Peng, S.; Tang, D.; Zhou, G. Recent Advances of Three-Dimensional Bioprinting Technology in Hepato-Pancreato-Biliary Cancer Models. Front. Oncol. 2023, 13, 1143600. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Edmonds, M.E.; Serena, T.E. Point-of-Care Fluorescence Imaging Reveals Extent of Bacterial Load in Diabetic Foot Ulcers. Int. Wound J. 2023, 20, 554–566. [Google Scholar] [CrossRef]
- Arshad, R.; Razlansari, M.; Maryam Hosseinikhah, S.; Tiwari Pandey, A.; Ajalli, N.; Ezra Manicum, A.L.; Thorat, N.; Rahdar, A.; Zhu, Y.; Tabish, T.A. Antimicrobial and Anti-Biofilm Activities of Bio-Inspired Nanomaterials for Wound Healing Applications. Drug Discov. Today 2023, 28, 103673. [Google Scholar] [CrossRef]
- Yaşayan, G.; Nejati, O.; Ceylan, A.F.; Karasu, Ç.; Kelicen Ugur, P.; Bal-Öztürk, A.; Zarepour, A.; Zarrabi, A.; Mostafavi, E. Tackling Chronic Wound Healing Using Nanomaterials: Advancements, Challenges, and Future Perspectives. Appl. Mater. Today 2023, 32, 101829. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Krishnan, M.; Bupesh, G.; Chacko, S.; Gawade, O.; Hasan, S.; George, E.; Vijayakumar, T.S.; Sundaram, M.; Sagadevan, S. Prospective Features of Functional 2D Nanomaterial Graphene Oxide in the Wound Healing Process. J. Drug Deliv. Sci. Technol. 2023, 82, 104352. [Google Scholar] [CrossRef]
- Prakashan, D.; Roberts, A.; Gandhi, S. Recent Advancement of Nanotherapeutics in Accelerating Chronic Wound Healing Process for Surgical Wounds and Diabetic Ulcers. Biotechnol. Genet. Eng. Rev. 2023, 2023, 1–29. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, X.; Du, H. Advances in Mesenchymal Stromal Cells and Nanomaterials for Diabetic Wound Healing. Diabetes/Metab. Res. Rev. 2023, 39, e3638. [Google Scholar] [CrossRef]
- Issaka, E. State-of-the-Art of Synthesized Exosomes and NPs-Based Biomimetic Nanoparticles for Wound Rehabilitation: A Review. Biomed. Mater. Devices 2023, 2023, 1–34. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Zhan, R.; Qian, W.; Luo, G. Nanomaterials and Nanomaterials-Based Drug Delivery to Promote Cutaneous Wound Healing. Adv. Drug Deliv. Rev. 2023, 193, 114670. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Goswami, L.; Kim, B.S. Nanomaterial-Based Therapy for Wound Healing. Nanomaterials 2022, 12, 618. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and Behavior of Carbon Nanomaterials When Interfacing Neuronal Cells: How Far Have We Come? Carbon 2019, 143, 430–446. [Google Scholar] [CrossRef]
- Chen, D.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic Applications of Carbon Nanomaterials in Cancer: Focus on Imaging and Cargo Delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, M.; Nasrollahzadeh, M.; Jaleh, B.; Soufi, G.J.; Iravani, S. Carbon-Based Nanomaterials for Targeted Cancer Nanotherapy: Recent Trends and Future Prospects. J. Drug Target. 2021, 29, 716–741. [Google Scholar] [CrossRef]
- Tonelli, F.M.; Goulart, V.A.; Gomes, K.N.; Ladeira, M.S.; Santos, A.K.; Lorençon, E.; Ladeira, L.O.; Resende, R.R. Graphene-Based Nanomaterials: Biological and Medical Applications and Toxicity. Nanomedicine 2015, 10, 2423–2450. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological Interactions of Carbon-Based Nanomaterials: From Coronation to Degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ahmad, M.; Silva, S.R.P. Low Temperature Growth of Carbon Nanotubes—A Review. Carbon 2020, 158, 24–44. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Faraji, S.; Stano, K.L.; Yildiz, O.; Li, A.; Zhu, Y.; Bradford, P.D. Ultralight Anisotropic Foams from Layered Aligned Carbon Nanotube Sheets. Nanoscale 2015, 7, 17038–17047. [Google Scholar] [CrossRef]
- Lee, E.; Salgado, R.A.; Lee, B.; Sumant, A.V.; Rajh, T.; Johnson, C.; Balandin, A.A.; Shevchenko, E.V. Design of Lithium Cobalt Oxide Electrodes with High Thermal Conductivity and Electrochemical Performance Using Carbon Nanotubes and Diamond Particles. Carbon N. Y. 2018, 129, 702–710. [Google Scholar] [CrossRef]
- Marconnet, A.M.; Panzer, M.A.; Goodson, K.E. Thermal Conduction Phenomena in Carbon Nanotubes and Related Nanostructured Materials. Rev. Mod. Phys. 2013, 85, 1295–1326. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Maruyama, S. A Molecular Dynamics Simulation of Heat Conduction of a Finite Length Single-Walled Carbon Nanotube. Microscale Thermophys. Eng. 2003, 7, 41–50. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.K.; Tománek, D. Unusually High Thermal Conductivity of Carbon Nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef]
- Osman, M.A.; Srivastava, D. Temperature Dependence of the Thermal Conductivity of Single-Wall Carbon Nanotubes. Nanotechnology 2001, 12, 21–24. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Randhawa, A.; Lim, K.T. Carbon Nanotubes-Based Hydrogels for Bacterial Eradiation and Wound-Healing Applications. Appl. Sci. 2021, 11, 9550. [Google Scholar] [CrossRef]
- Khalid, A.; Madni, A.; Raza, B.; Islam, M.U.; Hassan, A.; Ahmad, F.; Ali, H.; Khan, T.; Wahid, F. Multiwalled Carbon Nanotubes Functionalized Bacterial Cellulose as an Efficient Healing Material for Diabetic Wounds. Int. J. Biol. Macromol. 2022, 203, 256–267. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Y.; Yu, D.; Li, R.; Luo, W.; Ma, G.; Zhang, C. Isoniazid-Loaded Chitosan/Carbon Nanotubes Microspheres Promote Secondary Wound Healing of Bone Tuberculosis. J. Biomater. Appl. 2019, 33, 989–996. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Abu-Rass, H.; Lutz, S.; Hindawi, R.; Ghannam, L.; Zakarneh, M.; Mousa, A. Enhancement of Wound Healing by Single-Wall/Multi-Wall Carbon Nanotubes Complexed with Chitosan. Int. J. Nanomed. 2018, 13, 7195–7206. [Google Scholar] [CrossRef]
- Kolahdouz, M.; Xu, B.; Nasiri, A.F.; Fathollahzadeh, M.; Manian, M.; Aghababa, H.; Wu, Y.; Radamson, H.H. Carbon-Related Materials: Graphene and Carbon Nanotubes in Semiconductor Applications and Design. Micromachines 2022, 13, 1257. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the Functionalized Carbon Nanotubes as Smart Nanocarriers for Drug Delivery Applications. In Fullerenes, Graphenes and Nanotubes: A Pharmaceutical Approach; William Andrew Publishing: Norwich, NY, USA, 2018. [Google Scholar] [CrossRef]
- Liang, F.; Chen, B. A Review on Biomedical Applications of Single-Walled Carbon Nanotubes. Curr. Med. Chem. 2009, 17, 10–24. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Berger, G.M.; Ticich, T.M. Carbon Nanotube Synthesis in a Flame Using Laser Ablation for in Situ Catalyst Generation. Appl. Phys. A Mater. Sci. Process. 2003, 77, 885–889. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes–Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, e1701213. [Google Scholar] [CrossRef]
- Punbusayakul, N.; Talapatra, S.; Ajayan, P.M.; Surareungchai, W. Label-Free as-Grown Double Wall Carbon Nanotubes Bundles for Salmonella Typhimurium Immunoassay. Chem. Cent. J. 2013, 7, 102. [Google Scholar] [CrossRef]
- Bhatt, A.; Jain, A.; Gurnany, E.; Jain, R.; Modi, A.; Jain, A. Carbon Nanotubes: A Promising Carrier for Drug Delivery and Targeting. In Nanoarchitectonics for Smart Delivery and Drug Targeting; William Andrew Publishing: Norwich, NY, USA, 2016. [Google Scholar] [CrossRef]
- Matta-Domjan, B.; King, A.; Totti, S.; Matta, C.; Dover, G.; Martinez, P.; Zakhidov, A.; La Ragione, R.; Macedo, H.; Jurewicz, I.; et al. Biophysical Interactions between Pancreatic Cancer Cells and Pristine Carbon Nanotube Substrates: Potential Application for Pancreatic Cancer Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [PubMed]
- Kordy, M.G.M.; Abdel-Gabbar, M.; Soliman, H.A.; Aljohani, G.; Binsabt, M.; Ahmed, I.A.; Shaban, M. Phyto-Capped Ag Nanoparticles: Green Synthesis, Characterization, and Catalytic and Antioxidant Activities. Nanomaterials 2022, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Q.; Yang, X.; Wu, X.F.; Fan, Y.B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Balaure, P.C.; Grumezescu, A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part i: Molecular Basis of Biofilm Recalcitrance. Passive Anti-Biofouling Nanocoatings. Nanomaterials 2020, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Ma, C.; Choukourov, A.; Palumbo, F. Plasma Technology in Antimicrobial Surface Engineering. J. Appl. Phys. 2022, 131, 011102. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci. Adv. Mater. Devices 2020, 5, 417–435. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization–The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sittikijyothin, W.; Sangnim, T. Development and Characterization of Bilayer Wound Healing Patch Nanofiber Fabricated by Electrospinning. J. Nano Res. 2019, 59, 46–56. [Google Scholar] [CrossRef]
- Doolan, J.A.; Williams, G.T.; Hilton, K.L.F.; Chaudhari, R.; Fossey, J.S.; Goult, B.T.; Hiscock, J.R. Advancements in Antimicrobial Nanoscale Materials and Self-Assembling Systems. Chem. Soc. Rev. 2022, 51, 8696–8755. [Google Scholar] [CrossRef] [PubMed]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters with Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef] [PubMed]
- Ruseska, I.; Fresacher, K.; Petschacher, C.; Zimmer, A. Use of Protamine in Nanopharmaceuticals—A Review. Nanomaterials 2021, 11, 1508. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Brayden, D.J.; Hill, T.A.; Fairlie, D.P.; Maher, S.; Mrsny, R.J. Systemic Delivery of Peptides by the Oral Route: Formulation and Medicinal Chemistry Approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Eid, H.M.; Ali, A.A.; Ali, A.M.A.; Eissa, E.M.; Hassan, R.M.; El-Ela, F.I.A.; Hassan, A.H. Potential Use of Tailored Citicoline Chitosan-Coated Liposomes for Effective Wound Healing in Diabetic Rat Model. Int. J. Nanomed. 2022, 17, 555–575. [Google Scholar] [CrossRef]
- Cardoso-Daodu, I.M.; Ilomuanya, M.O.; Azubuike, C.P. Development of Curcumin-Loaded Liposomes in Lysine–Collagen Hydrogel for Surgical Wound Healing. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 100. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Panchai, P.; Julin, K.; Basnet, P.; Nystad, M.; Johannessen, M.; Škalko-Basnet, N. Chitosan-Based Delivery System Enhances Antimicrobial Activity of Chlorhexidine. Front. Microbiol. 2022, 13, 1023083. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Xu, D.; Zhang, W.; Zhao, X.; Li, H.; Xu, F.; Yin, L.; Peng, X.; Fu, H.; Chang, L.J.; et al. Preparation of Shikonin Liposome and Evaluation of Its in Vitro Antibacterial and in Vivo Infected Wound Healing Activity. Phytomedicine 2022, 99, 154035. [Google Scholar] [CrossRef] [PubMed]
- Mengoni, T.; Adrian, M.; Pereira, S.; Santos-Carballal, B.; Kaiser, M.; Goycoolea, F.M. A Chitosan-Based Liposome Formulation Enhances the in Vitro Wound Healing Efficacy of Substance P Neuropeptide. Pharmaceutics 2017, 9, 56. [Google Scholar] [CrossRef]
- Ternullo, S.; Werning, L.V.S.; Holsæter, A.M.; Škalko-Basnet, N. Curcumin-in-Deformable Liposomes-in-Chitosan-Hydrogel as a Novel Wound Dressing. Pharmaceutics 2020, 12, 8. [Google Scholar] [CrossRef]
- Liu, Y.; Song, S.; Liu, S.; Zhu, X.; Wang, P. Application of Nanomaterial in Hydrogels Related to Wound Healing. J. Nanomater. 2022, 2022, 4656037. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Divyashri, G.; Badhe, R.V.; Sadanandan, B.; Vijayalakshmi, V.; Kumari, M.; Ashrit, P.; Bijukumar, D.; Mathew, M.T.; Shetty, K.; Raghu, A.V. Applications of Hydrogel-Based Delivery Systems in Wound Care and Treatment: An up-to-Date Review. Polym. Adv. Technol. 2022, 33, 2025–2043. [Google Scholar] [CrossRef]
- Ding, Y.W.; Wang, Z.Y.; Ren, Z.W.; Zhang, X.W.; Wei, D.X. Advances in Modified Hyaluronic Acid-Based Hydrogels for Skin Wound Healing. Biomater. Sci. 2022, 10, 3393–3409. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Hu, D.; He, X.; Ji, X.; Li, T.; Wei, X.; Qian, Z. Mussel-Inspired Hydrogel with Injectable Self-Healing and Antibacterial Properties Promotes Wound Healing in Burn Wound Infection. NPG Asia Mater. 2022, 14, 86. [Google Scholar] [CrossRef]
- Shang, K.; Tao, L.; Jiang, S.; Yan, J.; Hu, S.; Yang, G.; Ma, C.; Cheng, S.; Wang, X.; Yin, J. Highly Flexible Hydrogel Dressing with Efficient Antibacterial, Antioxidative, and Wound Healing Performances. Biomater. Sci. 2022, 10, 1373–1383. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic Resistance in Microbes: History, Mechanisms, Therapeutic Strategies and Future Prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Duan, C.; Yu, M.; Xu, J.; Li, B.Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Pasika, S.R.; Bulusu, R.; Rao, B.V.K.; Kommineni, N.; Bolla, P.K.; Kala, S.G.; Godugu, C. Nanotechnology for Biomedical Applications. In Nanomaterials: Advances and Applications; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Edirisinghe, M. Metal-Based Nanoparticles for Combating Antibiotic Resistance. Appl. Phys. Rev. 2021, 8, 041303. [Google Scholar] [CrossRef]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Crabbe-Mann, M.; Ahmed, J.; Brako, F.; Karademir, B.; Aksu, B.; Sennaroglu, M.; Eroglu, M.S.; Ren, G.; et al. Co-Culture of Keratinocyte-Staphylococcus Aureus on Cu-Ag-Zn/CuO and Cu-Ag-W Nanoparticle Loaded Bacterial Cellulose:PMMA Bandages. Macromol. Mater. Eng. 2019, 304, 1800537. [Google Scholar] [CrossRef]
- Saleh, A.; Akkuş-Daǧdeviren, Z.B.; Haddadzadegan, S.; Wibel, R.; Bernkop-Schnürch, A. Peptide Antibiotic-Polyphosphate Nanoparticles: A Promising Strategy to Overcome the Enzymatic and Mucus Barrier of the Intestine. Biomacromolecules 2023, 24, 2587–2595. [Google Scholar] [CrossRef]
- Singh, C.; Mehata, A.K.; Priya, V.; Malik, A.K.; Setia, A.; Suseela, M.N.L.; Vikas, M.N.L.; Gokul, P.; Samridhi, P.; Singh, S.K.; et al. Bimetallic Au–Ag Nanoparticles: Advanced Nanotechnology for Tackling Antimicrobial Resistance. Molecules 2022, 27, 7059. [Google Scholar] [CrossRef]
- Meng, Y.; Chen, L.; Chen, Y.; Shi, J.; Zhang, Z.; Wang, Y.; Wu, F.; Jiang, X.; Yang, W.; Zhang, L.; et al. Reactive Metal Boride Nanoparticles Trap Lipopolysaccharide and Peptidoglycan for Bacteria-Infected Wound Healing. Nat. Commun. 2022, 13, 7353. [Google Scholar] [CrossRef]
- Soares, C.; Pereira, R.; Fidalgo, F. Metal-Based Nanomaterials and Oxidative Stress in Plants: Current Aspects and Overview. In Phytotoxicity of Nanoparticles; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Bhatt, S.; Upadhyay, T.; Patil, C.; Pai, K.S.R.; Chellappan, D.K.; Dua, K. Role of Oxidative Stress in Pathophysiological Progression of Schizophrenia. Curr. Psychiatry Res. Rev. 2023, 19, 11–27. [Google Scholar] [CrossRef]
- Parmar, S.; Kaur, H.; Singh, J.; Matharu, A.S.; Ramakrishna, S.; Bechelany, M. Recent Advances in Green Synthesis of Ag NPs for Extenuating Antimicrobial Resistance. Nanomaterials 2022, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yi, Y.; Wang, S.; Dou, H.; Fan, Y.; Tian, L.; Zhao, J.; Ren, L. Bio-Inspired Self-Adaptive Nanocomposite Array: From Non-Antibiotic Antibacterial Actions to Cell Proliferation. ACS Nano 2022, 16, 16549–16562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, Q.; Wei, Y.; Meng, W.; Wang, R.; Liu, J.; Nie, Y.; Luo, R.; Wang, Y.; Shen, B. Surface Modification of Titanium Implants by PH-Responsive Coating Designed for Self-Adaptive Antibacterial and Promoted Osseointegration. Chem. Eng. J. 2022, 435, 134802. [Google Scholar] [CrossRef]
- Pemmada, R.; Shrivastava, A.; Dash, M.; Cui, K.; Kumar, P.; Ramakrishna, S.; Zhou, Y.; Thomas, V.; Nanda, H.S. Science-Based Strategies of Antibacterial Coatings with Bactericidal Properties for Biomedical and Healthcare Settings. Curr. Opin. Biomed. Eng. 2023, 25, 100442. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Li, M.; Shakoor, N.; Adeel, M.; Zhou, P.; Guo, M.; Jiang, Y.; Zhao, W.; Lou, B.Z.; et al. Application and Mechanisms of Metal-Based Nanoparticles in the Control of Bacterial and Fungal Crop Diseases. Pest Manag. Sci. 2023, 79, 21–36. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life 2023, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Toczek, J.; Sadłocha, M.; Major, K.; Stojko, R. Benefit of Silver and Gold Nanoparticles in Wound Healing Process after Endometrial Cancer Protocol. Biomedicines 2022, 10, 679. [Google Scholar] [CrossRef]
- Sabarees, G.; Velmurugan, V.; Tamilarasi, G.P.; Alagarsamy, V.; Raja Solomon, V. Recent Advances in Silver Nanoparticles Containing Nanofibers for Chronic Wound Management. Polymers 2022, 14, 3994. [Google Scholar] [CrossRef]
- Banerjee, K.; Madhyastha, R.; Nakajima, Y.; Maruyama, M.; Madhyastha, H. Nanoceutical Adjuvants as Wound Healing Material: Precepts and Prospects. Int. J. Mol. Sci. 2021, 22, 4748. [Google Scholar] [CrossRef]
- Siddique, M.H.; Aslam, B.; Imran, M.; Ashraf, A.; Nadeem, H.; Hayat, S.; Khurshid, M.; Afzal, M.; Malik, I.R.; Shahzad, M.; et al. Effect of Silver Nanoparticles on Biofilm Formation and EPS Production of Multidrug-Resistant Klebsiella Pneumoniae. BioMed Res. Int. 2020, 2020, 6398165. [Google Scholar] [CrossRef]
- Miškovská, A.; Rabochová, M.; Michailidu, J.; Masák, J.; Čejková, A.; Lorinčík, J.; Maťátková, O. Antibiofilm Activity of Silver Nanoparticles Biosynthesized Using Viticultural Waste. PLoS ONE 2022, 17, e0272844. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Tran, N.T.K.; Le, T.Q.; Nguyen, T.T.A.; Nguyen, L.T.M.; Van Tran, T. Passion Fruit Peel Pectin/Chitosan Based Antibacterial Films Incorporated with Biosynthesized Silver Nanoparticles for Wound Healing Application. Alex. Eng. J. 2023, 69, 419–430. [Google Scholar] [CrossRef]
- Rather, A.H.; Khan, R.S.; Wani, T.U.; Rafiq, M.; Jadhav, A.H.; Srinivasappa, P.M.; Abdal-hay, A.; Sultan, P.; Rather, S.U.; Macossay, J.; et al. Polyurethane and Cellulose Acetate Micro-Nanofibers Containing Rosemary Essential Oil, and Decorated with Silver Nanoparticles for Wound Healing Application. Int. J. Biol. Macromol. 2023, 226, 690–705. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.D.; Hassan, H.; Khallaf, R.A. Rosuvastatin Calcium-Based Novel Nanocubic Vesicles Capped with Silver Nanoparticles-Loaded Hydrogel for Wound Healing Management: Optimization Employing Box–Behnken Design: In Vitro and in Vivo Assessment. J. Liposome Res. 2022, 32, 45–61. [Google Scholar] [CrossRef]
- Massey, S.; Iqbal, F.; Rehman, A.U.; Iqbal, M.S.; Iram, F. Preparation, Characterization and Biological Evaluation of Silver Nanoparticles and Drug Loaded Composites for Wound Dressings Formed from Lallemantia Royleana Seeds’ Mucilage. J. Biomater. Sci. Polym. Ed. 2022, 33, 481–498. [Google Scholar] [CrossRef]
- Pino, P.; Bosco, F.; Mollea, C.; Onida, B. Antimicrobial Nano-Zinc Oxide Biocomposites for Wound Healing Applications: A Review. Pharmaceutics 2023, 15, 970. [Google Scholar] [CrossRef]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Rosman, N.F.; Nurfazianawatie, M.Z.; Omar, H.; Malek, N.S.A.; Hajar, N.; Buniyamin, I.; Abdullah, S.; Rusop, M.; Asli, N.A. Fungal Growth Physicochemical Properties Inhibition by Novel Zinc Oxide/Glutinous Tapioca Starch Composite. J. Adv. Res. Appl. Sci. Eng. Technol. 2022, 29, 76–89. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Fromell, K.; Vinogradov, V.V.; Terekhov, A.N.; Pakhomov, A.V.; Nilsson, B.; Ekdahl, K.N.; Vinogradov, V.V.; Kessler, V.G. Dispersion of TiO2 Nanoparticles Improves Burn Wound Healing and Tissue Regeneration through Specific Interaction with Blood Serum Proteins. Sci. Rep. 2017, 7, 15448. [Google Scholar] [CrossRef]
- De Moura, F.B.R.; Ferreira, B.A.; Muniz, E.H.; Santos, R.A.; Gomide, J.A.L.; Justino, A.B.; Silva, A.C.A.; Dantas, N.O.; Ribeiro, D.L.; Araújo, F.A.; et al. TiO2 Nanocrystals and Annona Crassiflora Polyphenols Used Alone or Mixed Impact Differently on Wound Repair. Acad. Bras. Cienc. 2022, 94, e20210230. [Google Scholar] [CrossRef]
- Aleem, A.R.; Shahzadi, L.; Nasir, M.; Hajivand, P.; Alvi, F.; Akhtar, A.; Zehra, M.; Mehmood, A.; Yar, M. Developing Sulfur-Doped Titanium Oxide Nanoparticles Loaded Chitosan/Cellulose-Based Proangiogenic Dressings for Chronic Ulcer and Burn Wounds Healing. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1069–1081. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan Gum Incorporating Titanium Dioxide Nanoparticles Biofilm as Wound Dressing: Physicochemical, Mechanical, Antibacterial Properties and Wound Healing Studies. Mater. Sci. Eng. C 2019, 103, 109770. [Google Scholar] [CrossRef]
- Mahdavi, B.; Paydarfard, S.; Zangeneh, M.M.; Goorani, S.; Seydi, N.; Zangeneh, A. Assessment of Antioxidant, Cytotoxicity, Antibacterial, Antifungal, and Cutaneous Wound Healing Activities of Green Synthesized Manganese Nanoparticles Using Ziziphora Clinopodioides Lam Leaves under in Vitro and in Vivo Condition. Appl. Organomet. Chem. 2020, 34, e5248. [Google Scholar] [CrossRef]
- Chia, C.H.; Lau, K.S.; Chin, S.X.; Rosli, N.H.; Vincent, J.; Chowdhury, M.S. Carbon Nanotubes for Biomedical Applications and Health Care: New Horizons. In Carbon Nanotubes for Biomedical Applications and Healthcare; Apple Academic Press: Burlington, ON, Canada, 2024; pp. 255–331. ISBN 9781003396390. [Google Scholar]
- Nanda, S.S.; Wang, T.; Hossain, M.I.; Yoon, H.Y.; Selvan, S.T.; Kim, K.; Yi, D.K. Gold-Nanorod-Based Scaffolds for Wound-Healing Applications. ACS Appl. Nano Mater. 2022, 5, 8640–8648. [Google Scholar] [CrossRef]
- Naraginti, S.; Kumari, P.L.; Das, R.K.; Sivakumar, A.; Patil, S.H.; Andhalkar, V.V. Amelioration of Excision Wounds by Topical Application of Green Synthesized, Formulated Silver and Gold Nanoparticles in Albino Wistar Rats. Mater. Sci. Eng. C 2016, 62, 293–300. [Google Scholar] [CrossRef]
- Shalaby, M.A.; Anwar, M.M.; Saeed, H. Nanomaterials for Application in Wound Healing: Current State-of-the-Art and Future Perspectives. J. Polym. Res. 2022, 29, 91. [Google Scholar] [CrossRef]
- He, X.; Dai, L.; Ye, L.; Sun, X.; Enoch, O.; Hu, R.; Zan, X.; Lin, F.; Shen, J. A Vehicle-Free Antimicrobial Polymer Hybrid Gold Nanoparticle as Synergistically Therapeutic Platforms for Staphylococcus Aureus Infected Wound Healing. Adv. Sci. 2022, 9, e2105223. [Google Scholar] [CrossRef]
- Batool, Z.; Muhammad, G.; Iqbal, M.M.; Aslam, M.S.; Raza, M.A.; Sajjad, N.; Abdullah, M.; Akhtar, N.; Syed, A.; Elgorban, A.M.; et al. Hydrogel Assisted Synthesis of Gold Nanoparticles with Enhanced Microbicidal and in Vivo Wound Healing Potential. Sci. Rep. 2022, 12, 6575. [Google Scholar] [CrossRef]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Collagen nanofiber containing silver nanoparticles for improved wound-healing applications. J. Drug Target. 2016, 24, 520–529. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Mendes, C.; Thirupathi, A.; Corrêa, M.E.A.B.; Gu, Y.; Silveira, P.C.L. The Use of Metallic Nanoparticles in Wound Healing: New Perspectives. Int. J. Mol. Sci. 2022, 23, 15376. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Li, Q.; Wu, J. Application of Metal-Based Biomaterials in Wound Repair. Eng. Regen. 2021, 2, 137–153. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.W.; Hu, X.K.; et al. Ångstrom-Scale Silver Particle-Embedded Carbomer Gel Promotes Wound Healing by Inhibiting Bacterial Colonization and Inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef]
- Singh, V.; Marimuthu, T.; Makatini, M.M.; Choonara, Y.E. Biopolymer-Based Wound Dressings with Biochemical Cues for Cell-Instructive Wound Repair. Polymers 2022, 14, 5371. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Augustine, A.; Dalvi, Y.B.; Varghese, R.; Unni, R.N.; Kalarikkal, N.; Al Moustafa, A.E.; Thomas, S. Titanium Nanorods Loaded PCL Meshes with Enhanced Blood Vessel Formation and Cell Migration for Wound Dressing Applications. Macromol. Biosci. 2019, 19, e1900058. [Google Scholar] [CrossRef]
- Begum, S.; Hassan, Z.; Bräse, S.; Wöll, C.; Tsotsalas, M. Metal-Organic Framework-Templated Biomaterials: Recent Progress in Synthesis, Functionalization, and Applications. Acc. Chem. Res. 2019, 52, 1598–1610. [Google Scholar] [CrossRef]
- Nowak, M.; Barańska-Rybak, W. Nanomaterials as a Successor of Antibiotics in Antibiotic-Resistant, Biofilm Infected Wounds? Antibiotics 2021, 10, 941. [Google Scholar] [CrossRef]
- Zhao, F.; Su, Y.; Wang, J.; Romanova, S.; DiMaio, D.J.; Xie, J.; Zhao, S. A Highly Efficacious Electrical Biofilm Treatment System for Combating Chronic Wound Bacterial Infections. Adv. Mater. 2023, 35, e2208069. [Google Scholar] [CrossRef]
- Huanbutta, K.; Burapapadh, K.; Sriamornsak, P.; Sangnim, T. Practical Application of 3D Printing for Pharmaceuticals in Hospitals and Pharmacies. Pharmaceutics 2023, 15, 1877. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Álvarez-Echazú, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef]
- Hooshmand, S.; Mollazadeh, S.; Akrami, N.; Ghanad, M.; El-Fiqi, A.; Baino, F.; Nazarnezhad, S.; Kargozar, S. Mesoporous Silica Nanoparticles and Mesoporous Bioactive Glasses for Wound Management: From Skin Regeneration to Cancer Therapy. Materials 2021, 14, 3337. [Google Scholar] [CrossRef]
- Kim, N.; Lee, H.; Han, G.; Kang, M.; Park, S.; Kim, D.E.; Lee, M.; Kim, M.; Na, Y.; Oh, S.; et al. 3D-Printed Functional Hydrogel by DNA-Induced Biomineralization for Accelerated Diabetic Wound Healing. Adv. Sci. 2023, 10, e2300816. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tang, Y.; Pang, L.; Lin, C.; Huang, W.; Wang, D.; Jia, W. Angiogenesis and Full-Thickness Wound Healing Efficiency of a Copper-Doped Borate Bioactive Glass/Poly(Lactic-Co-Glycolic Acid) Dressing Loaded with Vitamin E in Vivo and in Vitro. ACS Appl. Mater. Interfaces 2018, 10, 22939–22950. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Pei, G.; Wu, Z.; Zhuang, H.; Zhang, Z.; Huan, Z.; Wu, C.; Chang, J. Bioactive Self-Pumping Composite Wound Dressings with Micropore Array Modified Janus Membrane for Enhanced Diabetic Wound Healing. Adv. Funct. Mater. 2020, 30, 2005422. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, e2106049. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Tomé, I.; Francisco, V.; Fernandes, H.; Ferreira, L. High-Throughput Screening of Nanoparticles in Drug Delivery. APL Bioeng. 2021, 5, 031511. [Google Scholar] [CrossRef]
- Suthar, J.K.; Vaidya, A.; Ravindran, S. Toxic Implications of Silver Nanoparticles on the Central Nervous System: A Systematic Literature Review. J. Appl. Toxicol. 2023, 43, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Lin, B.; Wu, L.; Li, K.; Liu, H.; Yan, J.; Liu, X.; Xi, Z. Neurotoxicity Induced by Zinc Oxide Nanoparticles: Age-Related Differences and Interaction. Sci. Rep. 2015, 5, 16117. [Google Scholar] [CrossRef]
- Boraschi, D.; Canesi, L.; Drobne, D.; Kemmerling, B.; Pinsino, A.; Prochazkova, P. Interaction between Nanomaterials and the Innate Immune System across Evolution. Biol. Rev. 2023, 98, 747–774. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sonu; Chowdhury, R.; Thukral, P.; Pathania, D.; Saklani, S.; Lucky; Rustagi, S.; Gautam, A.; Mishra, Y.K.; et al. Biogenic Green Metal Nano Systems as Efficient Anti-Cancer Agents. Environ. Res. 2023, 229, 115933. [Google Scholar] [CrossRef]
- Shukla, R.K.; Badiye, A.; Vajpayee, K.; Kapoor, N. Genotoxic Potential of Nanoparticles: Structural and Functional Modifications in DNA. Front. Genet. 2021, 12, 728250. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, A.M.; Tawfik, E.A.; Alomary, M.N.; Alsudir, S.A.; Alfahad, A.J.; Alshehri, A.A.; Almughem, F.A.; Mohammed, R.Y.; Alzaydi, M.M. Recent Advances in Mitochondrial Diseases: From Molecular Insights to Therapeutic Perspectives. Saudi Pharm. J. 2022, 30, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.; Al-Ayed, M.S.; Moussa, S.A. The effects of intraperitoneal administration of gold nanoparticles size and exposure duration on oxidative and antioxidants levels in various rat organs. Pak. J. Pharm. Sci. 2015, 28, 705–712. [Google Scholar] [PubMed]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; de Bem Silveira, G.; de Souza, D.L.; de Abreu Brandolfi, J.; de Souza, C.T.; Paula, M.M.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of Alzheimer’s type. Mater. Sci. Eng. C 2017, 77, 476–483. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangnim, T.; Puri, V.; Dheer, D.; Venkatesh, D.N.; Huanbutta, K.; Sharma, A. Nanomaterials in the Wound Healing Process: New Insights and Advancements. Pharmaceutics 2024, 16, 300. https://doi.org/10.3390/pharmaceutics16030300
Sangnim T, Puri V, Dheer D, Venkatesh DN, Huanbutta K, Sharma A. Nanomaterials in the Wound Healing Process: New Insights and Advancements. Pharmaceutics. 2024; 16(3):300. https://doi.org/10.3390/pharmaceutics16030300
Chicago/Turabian StyleSangnim, Tanikan, Vivek Puri, Divya Dheer, D. Nagasamy Venkatesh, Kampanart Huanbutta, and Ameya Sharma. 2024. "Nanomaterials in the Wound Healing Process: New Insights and Advancements" Pharmaceutics 16, no. 3: 300. https://doi.org/10.3390/pharmaceutics16030300
APA StyleSangnim, T., Puri, V., Dheer, D., Venkatesh, D. N., Huanbutta, K., & Sharma, A. (2024). Nanomaterials in the Wound Healing Process: New Insights and Advancements. Pharmaceutics, 16(3), 300. https://doi.org/10.3390/pharmaceutics16030300






