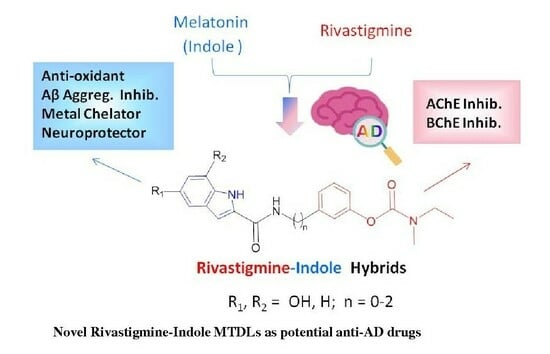
New Multitarget Rivastigmine–Indole Hybrids as Potential Drug Candidates for Alzheimer’s Disease
Abstract
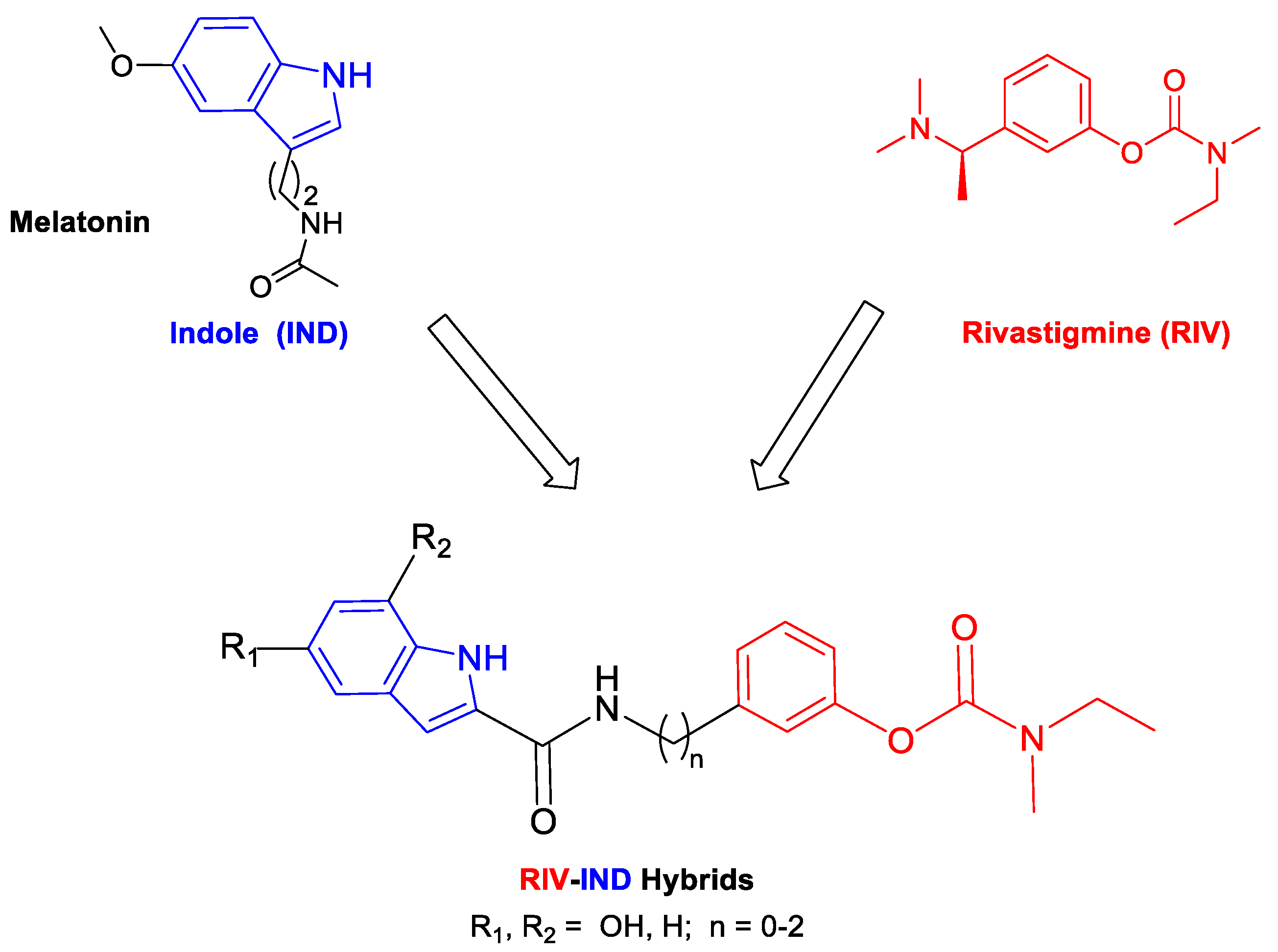
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
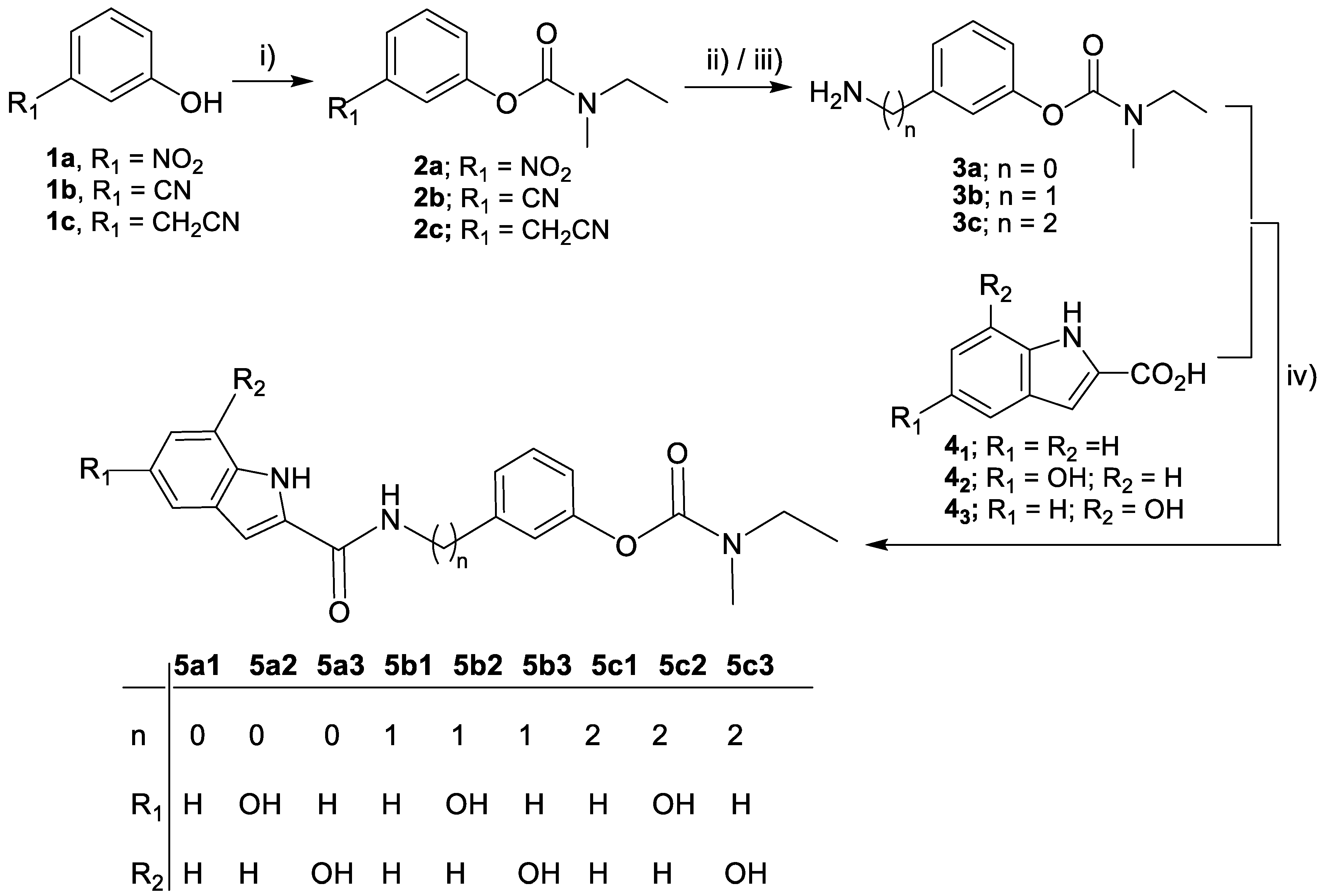
2.2. Synthesis of the RIV-IND Hybrids
2.2.1. General Procedure for the Synthesis of the Carbamates (2a, 2b, 2c)
3-Nitrophenyl ethylmethylcarbamate (2a)
3-Cyanophenyl ethylmethylcarbamate (2b)
3-(Cyanomethyl)phenyl ethylmethylcarbamate (2c)
2.2.2. General Procedure for the Synthesis of the Amino-phenylcarbamates (3a, 3b, 3c)
3-Aminophenyl ethylmethylcarbamate (3a)
3-(Aminomethyl)phenyl ethylmethylcarbamate (3b)
3-(2-Aminoethyl) phenyl ethyl methylcarbamate (3c)
2.2.3. General Procedure for the Synthesis of the Rivastigmine–Indole Hybrids (5a1-3; 5b1-3; 5c1-3)
3-[(1H-indole-2-carboxamido)phenyl ethyl(methyl)carbamate (5a1)
3-(5-Hydroxy-1H-indole-2-carboxamido)phenyl ethyl(methyl)carbamate (5a2)
3-(7-Hydroxy-1H-indole-2-carboxamido)phenyl ethyl(methyl)carbamate (5a3)
3-((1H-indole-2-carboxamido)methyl)phenyl ethylmethylcarbamate (5b1)
3-((5-Hydroxy-1H-indole-2-carboxamido)methyl)phenyl-ethyl(methyl)carbamate (5b2)
3-((7-Hydroxy-1H-indole-2-carboxamido)methyl)phenyl-ethyl(methyl)carbamate (5b3)
3-(2-(1H-indole-2-carboxamido)ethyl)phenyl ethylmethylcarbamate (5c1)
3-(2-(5-Hydroxy-1H-indole-2-carboxamido)ethyl)phenyl ethyl(methyl)carbamate (5c2)
3-(2-(7-Hydroxy-1H-indole-2-carboxamido)ethyl)phenyl ethyl(methyl)carbamate (5c3)
2.3. Molecular Modeling
2.4. Radical Scavenging Activity
2.5. Cholinesterase Inhibition
2.6. Inhibition of Self Aβ1-42 Aggregation
2.7. Cell Viability and In Vitro Neuroprotection
2.8. Prediction of Pharmacokinetic Properties
3. Results and Discussion
3.1. Synthesis of the RIV-IND Hybrids
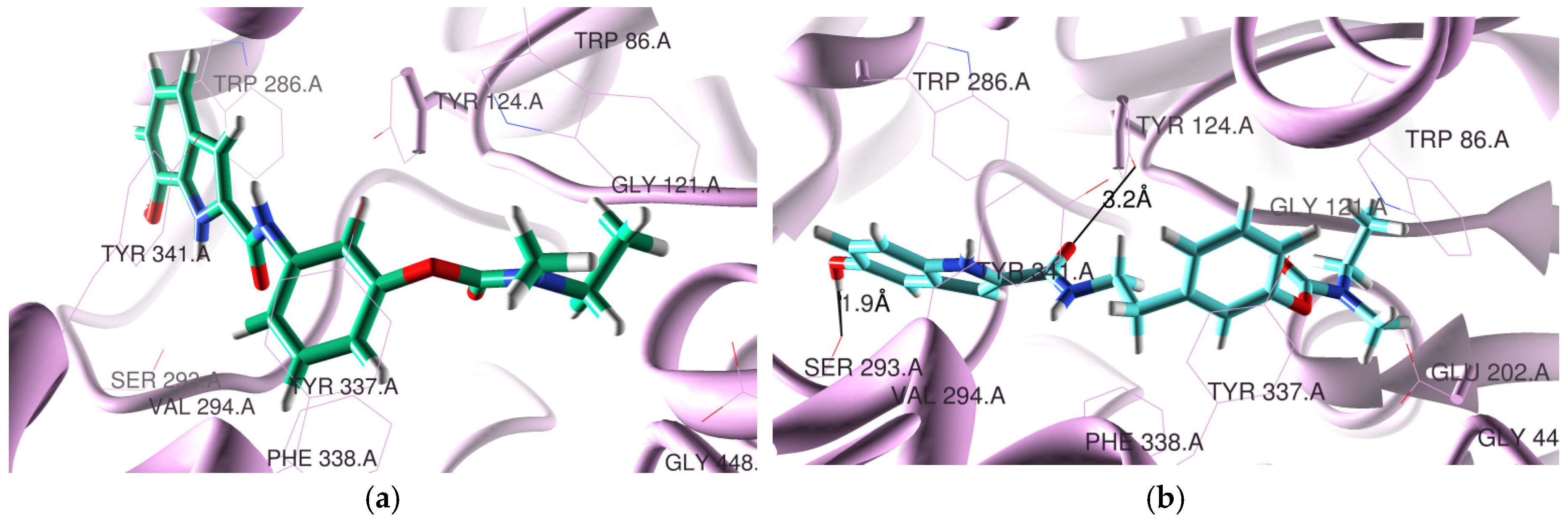
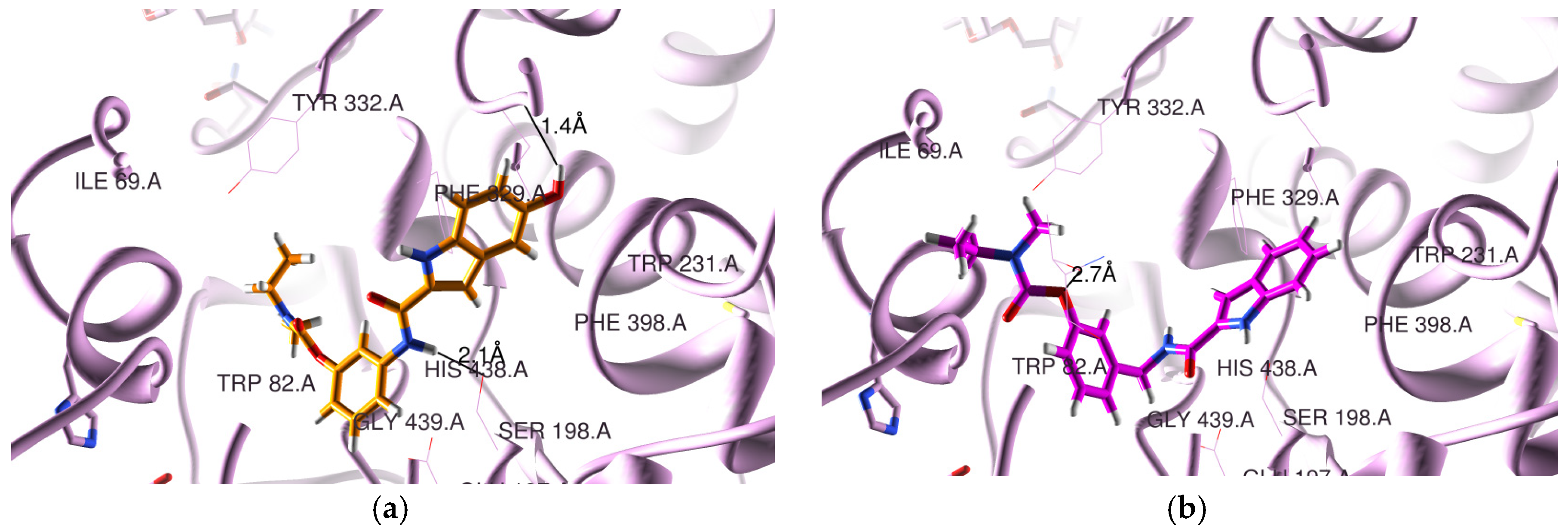
3.2. Molecular Modeling Studies
3.3. Free Radical Scavenging Activity
3.4. Inhibition of Cholinesterases
3.5. Inhibition of Aβ1-42 Self-Aggregation
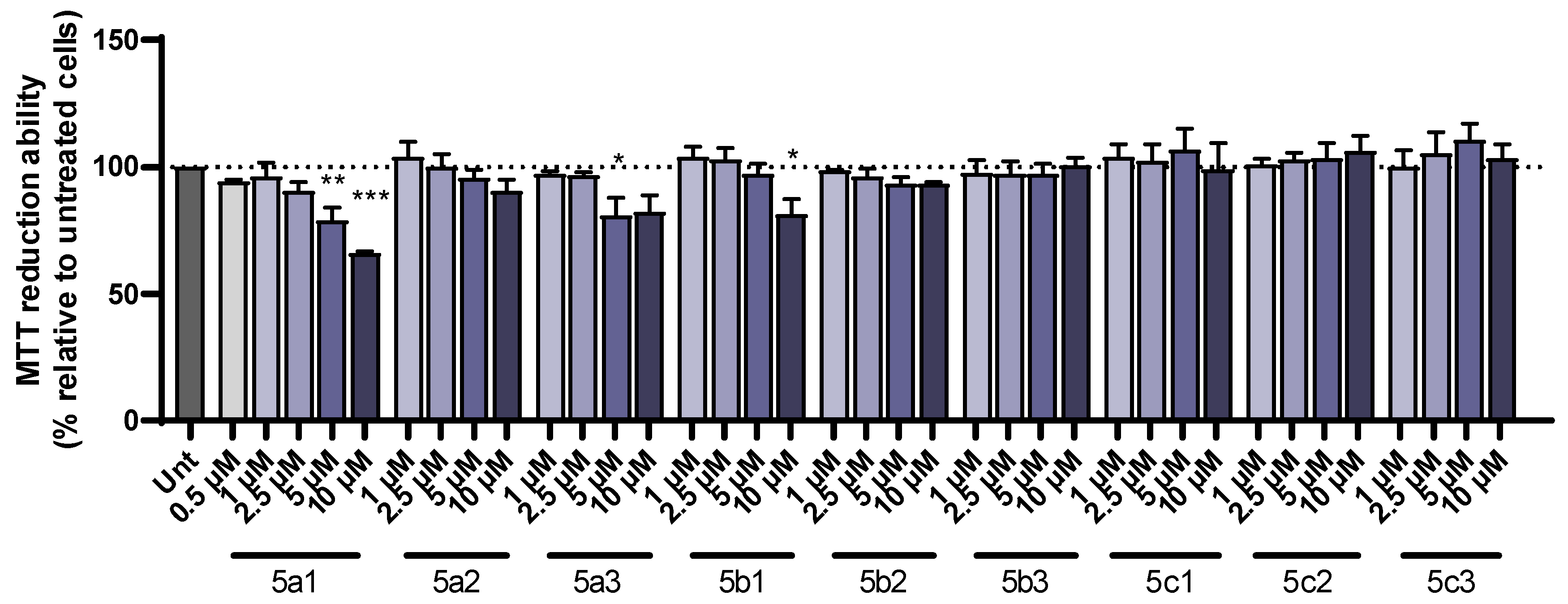
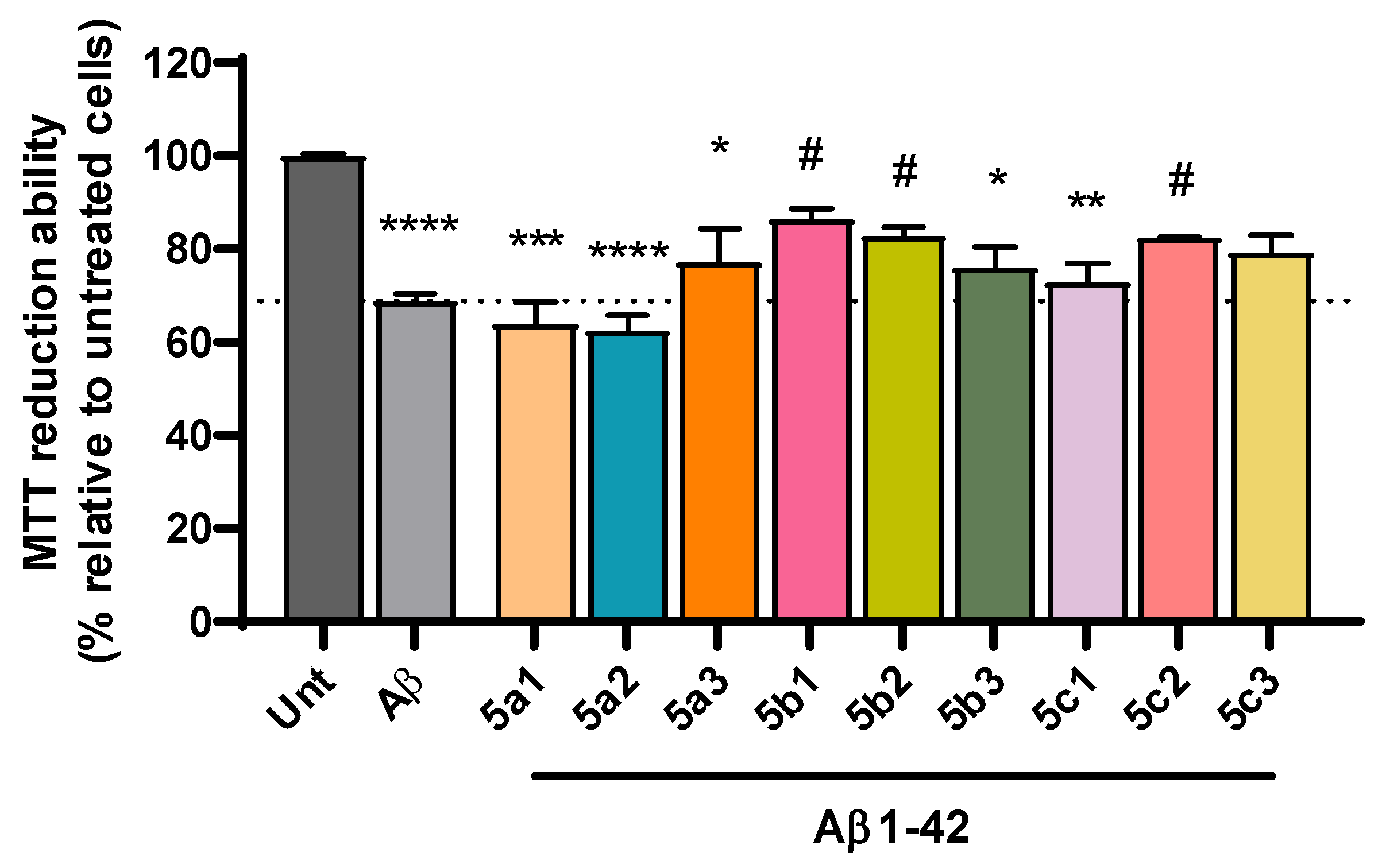
3.6. Cell Viability and Neuroprotection
3.7. Prediction of Pharmacokinetic Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hampel, H.; Au, R.; Mattke, S.; van der Flier, W.M.; Aisen, P.; Apostolova, L.; Chen, C.; Cho, M.; De Santi, S.; Gao, P.; et al. Designing the next-generation clinical care pathway for Alzheimer’s disease. Nat. Aging 2022, 2, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A. Treating Alzheimer´s before it takes hold. Nature 2022, 603, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal Dyshomeostasis and Oxidative Stress in Alzheimer’s Disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lim, M.H. Current understanding of metal-dependent amyloid-b aggregation and toxicity. RSC Chem. Biol. 2023, 4, 121–131. [Google Scholar] [CrossRef]
- Muñoz-Torrero, D. Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimer’s disease. Curr. Med. Chem. 2008, 15, 2433–2455. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Hey, J.A.; Porsteinsson, A.; Sabbagh, M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimer’s Res. Ther. 2021, 12, 95. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer´s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.D.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Nery, E.S.M.; et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLASER-ALZ 2 randomized clinical trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Young, A.B. Four decades of neurodegenerative disease research: How far we have come! J. Neurosci. 2009, 29, 12722–12728. [Google Scholar] [CrossRef]
- Hiremathad, A.; Chand, K.; Esteves, A.R.; Cardoso, S.M.; Ramsay, R.R.; Chaves, S.; Keri, R.S.; Santos, M.A. Tacrine-allyl/propargylcysteine-benzothiazole trihybrids as potential anti-Alzheimer´s drug candidates. RSC Adv. 2016, 6, 53519–53532. [Google Scholar] [CrossRef]
- Wu, M.-Y.; Esteban, G.; Brogi, S.; Shionoya, M.; Wang, L.; Campiani, G.; Unzeta, M.; Inokuchi, T.; Butini, S.; Marco-Contelles, J. Donepezil-like multifunctional agents: Design, synthesis, molecular modeling and biological evaluation. Eur. J. Med. Chem. 2016, 121, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From Combinations to Multitarget-Directed Ligands: A Continuum in Alzheimer’s Disease Polypharmacology. Med. Res. Revs. 2021, 41, 2606–2633. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef] [PubMed]
- Guiselin, T.; Lecoutey, C.; Rochais, C.; Dallemagne, P. Conceptual Framework of the Design of Pleiotropic Drugs against Alzheimer’s Disease. Pharmaceutics 2023, 15, 2382. [Google Scholar] [CrossRef]
- Chadha, N.; Silakari, O. Indoles as therapeutics of interest in medicinal chemistry: Bird´s eye view. Eur. J. Med. Chem. 2017, 134, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimers´s Disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef]
- Shukla, M.; Govitrapong, P.; Boontem, P.; Reiter, R.J.; Satayavivad, J. Mechanisms of Melatonin in Alleviating Alzheimer’s Disease. Curr. Neuropharmacol. 2017, 15, 1010–1031. [Google Scholar] [CrossRef]
- George, N.; Akhtar, M.J.; Al Balushi, K.A.; Khan, S.A. Rational drug design strategies for the development of promising multi-target directed indole hybrids as Anti-Alzheimer agents. Bioorg. Chem. 2022, 127, 105941. [Google Scholar] [CrossRef]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Brunetti, L.; Piemontese, L.; Pereira-Santos, A.R.; Cardoso, S.M.; Madrid, Y.; Chaves, S.; Santos, M.A. Novel Rivastigmine Derivatives As Promising Multi-Target Compounds For Potential Treatment of Alzheimer’s Disease. Biomedicines 2022, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Benchekroun, M.; Romero, A.; Egea, J.; Le´on, R.; Michalska, P.; Buendía, I.; Jimeno, M.L.; Jun, D.; Janockova, J.; Sepsova, V. The antioxidant additive approach for Alzheimer’s disease therapy: New ferulic (lipoic) acid plus melatonin modified tacrines as cholinesterases inhibitors, direct antioxidants, and nuclear factor (erythroid-derived 2)-like 2 activators. J. Med. Chem. 2016, 59, 9967–9973. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann; Oxford, Press: Oxford, UK, 1999. [Google Scholar]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.-P.; Gobec, S. Discovery, biological evaluation, and crystal structure of a novel nanomolar selective butyrylcholinesterase inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef] [PubMed]
- Maestro, Version 9.3; Schrödinger Inc.: Portland, OR, USA, 2012.
- Acton, A.; Banck, M.; Bréfort, J.; Cruz, M.; Curtis, D.; Hassinen, T.; Heikkilä, V.; Hutchison, G.; Huuskonen, J.; Jensen, J.; et al. Ghemical, version 3.0; GPL: Burbank, CA, USA, 2011.
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.; Goddard, T.; Huang, C.; Couch, G.; Greenblatt, D.; Meng, E.; Ferrin, T. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Quintanova, C.; Keri, R.S.; Marques, S.M.; Fernandes, M.G.; Cardoso, S.M.; Serralheiro, M.L.; Santos, M.A. Design, synthesis and bioevaluation of tacrine hybrids with cinnamate and cinnamylidene acetate derivatives as potential anti-Alzheimer drugs. Med. Chem. Comm. 2015, 6, 1969–1977. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Bolognesi, M.L.; Cavalli, A.; Melchiorre, C.; Andrisano, V. Insight into the kinetic of amyloid beta (1–42) peptide self-aggregation: Elucidation of inhibitors’ mechanism of action. ChemBioChem 2007, 8, 2152–2161. [Google Scholar] [CrossRef]
- Chao, X.; He, X.; Yang, Y.; Zhou, X.; Jin, M.; Liu, S.; Cheng, Z.; Liu, P.; Wang, Y.; Yu, J.; et al. Design, synthesis and pharmacological evaluation of novel tacrine-caffeic acid hybrids as multi-targeted compounds against Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2012, 22, 6498–6502. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- QikProp, Version 2.5; Schrödinger, LLC.: New York, NY, USA, 2005.
- Ballard, C.G. Advances in the treatment of Alzheimer´s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002, 47, 64–70. [Google Scholar] [CrossRef]
- Harel, M.; Sussman, J.L.; Krejci, E.; Bon, S.; Chanal, P.; Massoulie, J.; Silman, I. Conversion of acetylcholinesterase to butyrylcholinesterase: Modelling and mutagenesis. Proc. Natl. Acad. Sci. USA 1992, 89, 10827–10831. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, A.; Lockridge, O. Comparison of butyrylcholinesterase and acetylcholinesterase. Biochem. J. 1989, 260, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- Dighe, S.N.; Deora, G.S.; Mora, E.; Nachon, F.; Chan, S.; Parat, M.O.; Brazzolotto, X.; Ross, B.P. Discovery and structure-activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Zhao, Y.; Dou, J.; Wu, T.; Aisa, H.A. Investigating the Antioxidant and Acetylcholinesterase Inhibition Activities of Gossypium herbaceam. Molecules 2013, 18, 951–962. [Google Scholar] [CrossRef]
- Chaves, S.; Canário, S.; Carrasco, M.; Mira, L.; Santos, M.A. Hydroxy(thio)pyrone and hydroxy(thio)pyridinone iron chelators: Physico-chemical properties and antioxidant activity. J. Inorg. Biochem. 2012, 114, 38. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Selfridge, J.E.; Lu, J.; Lezi, E.; Cardoso, S.M.; Swerdlow, R.H. Mitochondrial abnormalities in Alzheimer’s disease: Possible targets for therapeutic intervention. Adv. Pharmacol. 2012, 64, 83–126. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, S.M.; Santos, S.; Swerdlow, R.H.; Oliveira, C.R. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 2001, 15, 1439–1441. [Google Scholar] [CrossRef] [PubMed]
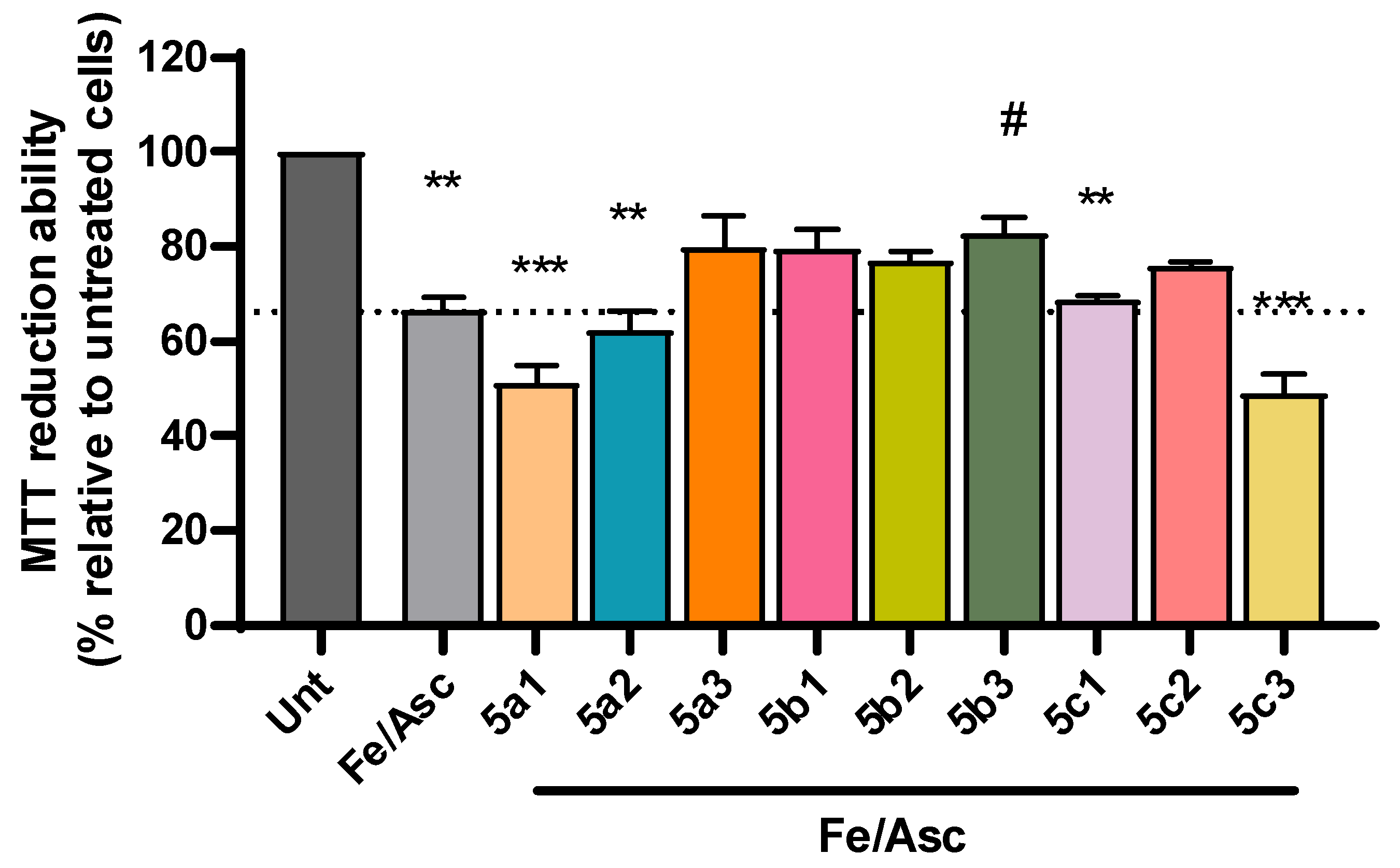
| Compound | n | R1 | R2 | Antioxidant Activity a EC50 (µM) | AChE Inhib b IC50 (µM) | BChE Inhib b IC50 (µM) | SI c | Aβ42 Self-Aggreg. Inhib d (%) |
|---|---|---|---|---|---|---|---|---|
| 5a1 | 0 | H | H | >2000 | (18 ± 2) × 10 | 29.9 ± 0.4 | 6.0 | 26.9 |
| 5a2 | 0 | OH | H | 8.4 ± 0.2 | 206 ± 4 | 3.22 ± 0.02 | 64.0 | 25.0 |
| 5a3 | 0 | H | OH | 14.5 ± 0.5 | 10.9 ± 0.1 | 10.4 ± 0.4 | 1.05 | 50.3 |
| 5b1 | 1 | H | H | >2000 | 78.4± 0.8 | 8.3 ± 0.1 | 9.4 | 19.9 |
| 5b2 | 1 | OH | H | 7.8 ± 0.5 | 80 ± 2 | 27.1 ± 0.1 | 3.0 | 29.4 |
| 5b3 | 1 | H | OH | 20.2 ± 0.4 | 80.7 ± 0.4 | 5.7 ± 0.3 | 14.2 | 47.8 |
| 5c1 | 2 | H | H | >2000 | 40 ± 2 | 14.5 ± 0.7 | 2.8 | 25.6 |
| 5c2 | 2 | OH | H | 8.5 ± 0.6 | 58.6 ± 0.3 | 15.2 ± 0.4 | 3.8 | 49.9 |
| 5c3 | 2 | H | OH | 20.7 ± 0.4 | 26.8 ± 0.3 | 14.9 ± 0.7 | 1.8 | 55.5 |
| Deferiprone | - | - | - | 148 ± 3 | - | - | ||
| Trolox | - | - | - | 13.8 ± 0.2 | - | - | ||
| Rivastigmine e | - | - | - | - | 32.1 | 0.39 | 82.3 | |
| Curcumin e | - | - | - | - | - | - | - | 65.7 |
| Comp. | Mol. Weight a | PSA b | Clog Po/w c | Log K (HSA) Serum Protein Binding d | log BB e | Caco-2 Permeab. (nm s−1) f | MDCK Permeab. (nm s−1) g | Oral Absorpt. h |
|---|---|---|---|---|---|---|---|---|
| 5a1 | 337.377 | 84.640 | 3.427 | 0.335 | −0.773 | 987 | 487 | 95 |
| 5a2 | 353.377 | 107.334 | 2.659 | 0.162 | −1.424 | 292 | 130 | 78 |
| 5a3 | 353.377 | 105.423 | 2.724 | 0.159 | −1.323 | 362 | 165 | 81 |
| 5b1 | 351.404 | 85.621 | 3.836 | 0.459 | −0.879 | 975 | 481 | 95 |
| 5b2 | 367.404 | 108.316 | 3.075 | 0.281 | −1.558 | 288 | 129 | 79 |
| 5b3 | 367.404 | 106.407 | 3.133 | 0.274 | −1.446 | 357 | 162 | 81 |
| 5c1 | 365.431 | 82.971 | 4.065 | 0.518 | −0.842 | 1104 | 550 | 94 |
| 5c2 | 381.430 | 104.664 | 3.302 | 0.339 | −1.501 | 326 | 147 | 78 |
| 5c3 | 381.430 | 103.752 | 3.360 | 0.332 | −1.392 | 405 | 186 | 81 |
| RIV | 250.340 | 38.483 | 2.488 | −0.133 | 0.475 | 1381 | 776 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bon, L.; Banaś, A.; Dias, I.; Melo-Marques, I.; Cardoso, S.M.; Chaves, S.; Santos, M.A. New Multitarget Rivastigmine–Indole Hybrids as Potential Drug Candidates for Alzheimer’s Disease. Pharmaceutics 2024, 16, 281. https://doi.org/10.3390/pharmaceutics16020281
Bon L, Banaś A, Dias I, Melo-Marques I, Cardoso SM, Chaves S, Santos MA. New Multitarget Rivastigmine–Indole Hybrids as Potential Drug Candidates for Alzheimer’s Disease. Pharmaceutics. 2024; 16(2):281. https://doi.org/10.3390/pharmaceutics16020281
Chicago/Turabian StyleBon, Leo, Angelika Banaś, Inês Dias, Inês Melo-Marques, Sandra M. Cardoso, Sílvia Chaves, and M. Amélia Santos. 2024. "New Multitarget Rivastigmine–Indole Hybrids as Potential Drug Candidates for Alzheimer’s Disease" Pharmaceutics 16, no. 2: 281. https://doi.org/10.3390/pharmaceutics16020281
APA StyleBon, L., Banaś, A., Dias, I., Melo-Marques, I., Cardoso, S. M., Chaves, S., & Santos, M. A. (2024). New Multitarget Rivastigmine–Indole Hybrids as Potential Drug Candidates for Alzheimer’s Disease. Pharmaceutics, 16(2), 281. https://doi.org/10.3390/pharmaceutics16020281