Exploring the Impact of Model-Informed Precision Dosing on Procalcitonin Concentrations in Critically Ill Patients: A Secondary Analysis of the DOLPHIN Trial
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Populations
2.3. Data Collection and Definitions
2.4. Laboratory Methods
2.5. Outcomes
2.6. Statistical Analyses
3. Results
3.1. Patient Characteristics
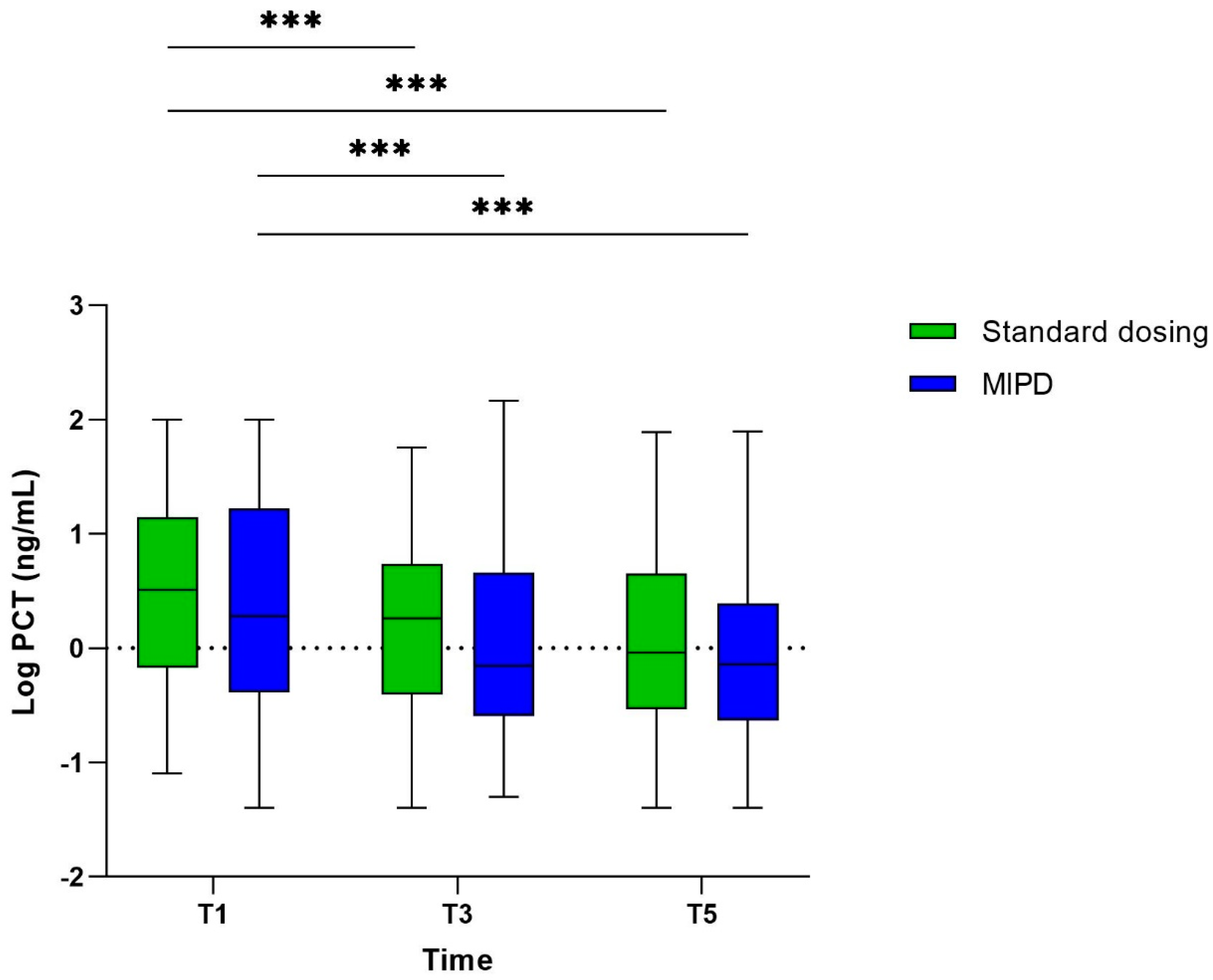
3.2. The Course of PCT
3.3. Course of PCT in 28-Day Survivors and Non-Survivors (Study Population I)
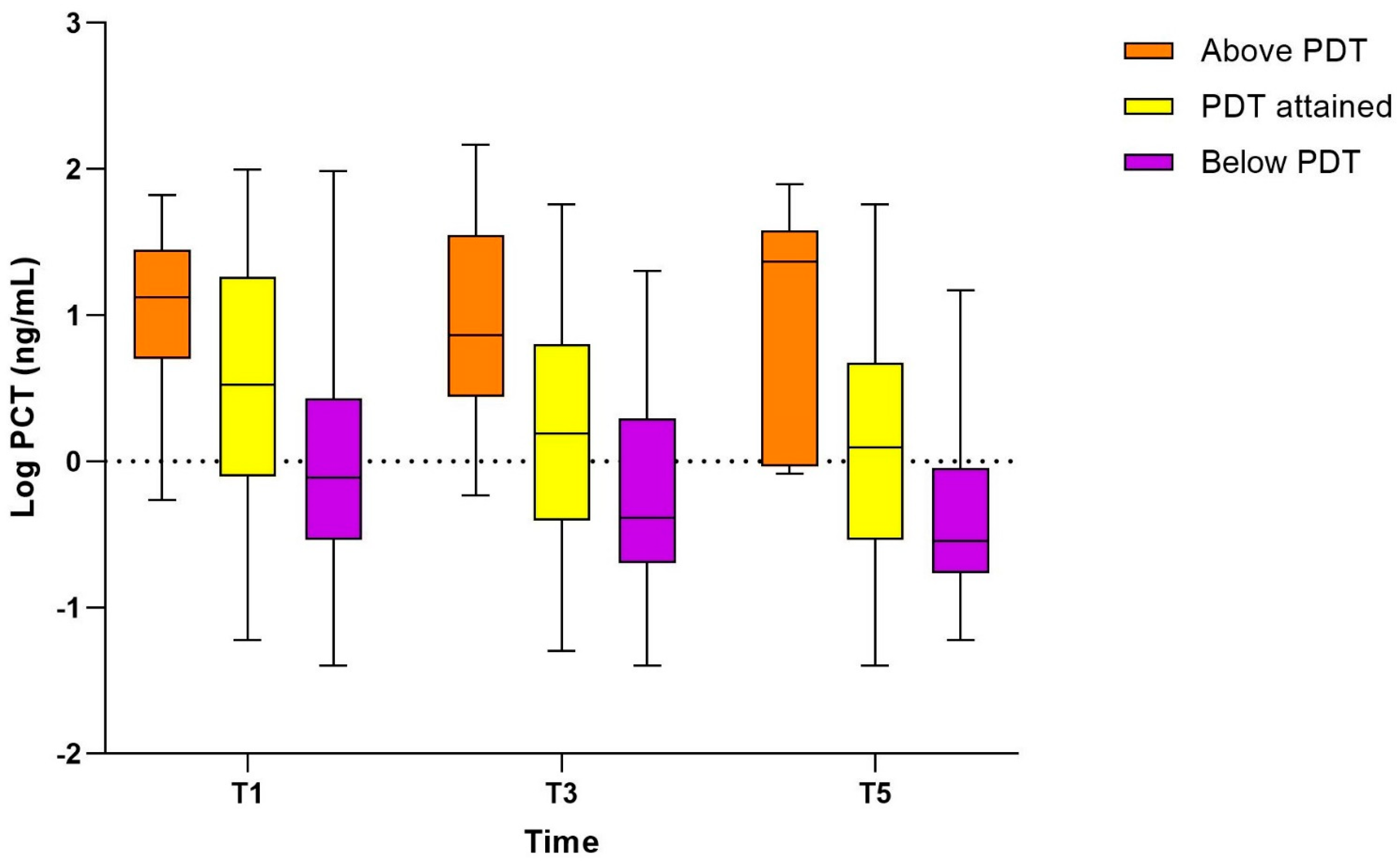
3.4. Association of PCT with Pharmacodynamic Target Attainment (Study Population II)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APACHE IV | Acute physiology and chronic health evaluation version 4 |
| BMI | Body mass index |
| CRP | C-reactive protein |
| GFR | Glomerular filtration rate |
| ICU | Intensive Care Unit |
| LOS | Length of stay |
| MIC | Minimal inhibitory concentration |
| MIPD | Model informed precision dosing |
| PCT | Procalcitonin |
| PK/PD | pharmacokinetic/pharmacodynamic |
| PDT | pharmacodynamic target |
| RCT | Randomised controlled trial |
| SOFA | Sequential organ failure assessment |
| TDM | Therapeutic drug monitoring |
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Smekal, A.K.; Furebring, M.; Eliasson, E.; Lipcsey, M. Low attainment to PK/PD-targets for beta-lactams in a multi-center study on the first 72 h of treatment in ICU patients. Sci. Rep. 2022, 12, 21891. [Google Scholar] [CrossRef] [PubMed]
- Haeseker, M.; Stolk, L.; Nieman, F.; Hoebe, C.; Neef, C.; Bruggeman, C.; Verbon, A. The ciprofloxacin target AUC: MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br. J. Clin. Pharmacol. 2013, 75, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Hope, W.W.; Roberts, J.A. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: Optimizing efficacy and reducing resistance development. Semin. Respir. Crit. Care Med. 2015, 36, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: An observational prospective cohort study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Tangden, T.; Ramos Martin, V.; Felton, T.W.; Nielsen, E.I.; Marchand, S.; Bruggemann, R.J.; Bulitta, J.B.; Bassetti, M.; Theuretzbacher, U.; Tsuji, B.T.; et al. The role of infection models and PK/PD modelling for optimising care of critically ill patients with severe infections. Intensive Care Med. 2017, 43, 1021–1032. [Google Scholar] [CrossRef]
- Wicha, S.G.; Martson, A.G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.W.; Minichmayr, I.K.; International Society of Anti-Infective Pharmacology (ISAP), the PK/PD Study Group of the European Society of Clinical Microbiology, Infectious Diseases (EPASG). From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef]
- Del Valle-Moreno, P.; Suarez-Casillas, P.; Mejias-Trueba, M.; Ciudad-Gutierrez, P.; Guisado-Gil, A.B.; Gil-Navarro, M.V.; Herrera-Hidalgo, L. Model-Informed Precision Dosing Software Tools for Dosage Regimen Individualization: A Scoping Review. Pharmaceutics 2023, 15, 1859. [Google Scholar] [CrossRef]
- Scott, J.; Deresinski, S. Use of biomarkers to individualize antimicrobial therapy duration: A narrative review. Clin. Microbiol. Infect. 2023, 29, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Azzini, A.M.; Dorizzi, R.M.; Sette, P.; Vecchi, M.; Coledan, I.; Righi, E.; Tacconelli, E. A 2020 review on the role of procalcitonin in different clinical settings: An update conducted with the tools of the Evidence Based Laboratory Medicine. Ann. Transl. Med. 2020, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Roberts, J.A. Individualising Therapy to Minimize Bacterial Multidrug Resistance. Drugs 2018, 78, 621–641. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwkoop, C.; Bonten, T.N.; van’t Wout, J.W.; Kuijper, E.J.; Groeneveld, G.H.; Becker, M.J.; Koster, T.; Wattel-Louis, G.H.; Delfos, N.M.; Ablij, H.C.; et al. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: A prospective observational study. Crit. Care 2010, 14, R206. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Gregoriano, C.; Heilmann, E.; Molitor, A.; Schuetz, P. Role of procalcitonin use in the management of sepsis. J. Thorac. Dis. 2020, 12, S5–S15. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C. Using Procalcitonin to Guide Antibiotic Therapy. Open Forum Infect. Dis. 2017, 4, ofw249. [Google Scholar] [CrossRef]
- Covington, E.W.; Roberts, M.Z.; Dong, J. Procalcitonin Monitoring as a Guide for Antimicrobial Therapy: A Review of Current Literature. Pharmacotherapy 2018, 38, 569–581. [Google Scholar] [CrossRef]
- Velicer, C.M.; Heckbert, S.R.; Lampe, J.W.; Potter, J.D.; Robertson, C.A.; Taplin, S.H. Antibiotic use in relation to the risk of breast cancer. JAMA 2004, 291, 827–835. [Google Scholar] [CrossRef]
- Garnacho-Montero, J.; Gutierrez-Pizarraya, A.; Escoresca-Ortega, A.; Corcia-Palomo, Y.; Fernandez-Delgado, E.; Herrera-Melero, I.; Ortiz-Leyba, C.; Marquez-Vacaro, J.A. De-escalation of empirical therapy is associated with lower mortality in patients with severe sepsis and septic shock. Intensive Care Med. 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Meier, M.A.; Branche, A.; Neeser, O.L.; Wirz, Y.; Haubitz, S.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; et al. Procalcitonin-guided Antibiotic Treatment in Patients With Positive Blood Cultures: A Patient-level Meta-analysis of Randomized Trials. Clin. Infect. Dis. 2019, 69, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Nobre, V.; Harbarth, S.; Graf, J.D.; Rohner, P.; Pugin, J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Thorsted, A.; Nielsen, E.I.; Friberg, L.E. Pharmacodynamics of immune response biomarkers of interest for evaluation of treatment effects in bacterial infections. Int. J. Antimicrob. Agents 2020, 56, 106059. [Google Scholar] [CrossRef] [PubMed]
- Aulin, L.B.S.; de Lange, D.W.; Saleh, M.A.A.; van der Graaf, P.H.; Voller, S.; van Hasselt, J.G.C. Biomarker-Guided Individualization of Antibiotic Therapy. Clin. Pharmacol. Ther. 2021, 110, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.J.; Haringman, J.; et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Ewoldt, T.M.J.; Hunfeld, N.G.M.; Muller, A.E.; Rietdijk, W.J.R.; Polinder, S.; van Gelder, T.; Endeman, H.; Koch, B.C.P. The effect of therapeutic drug monitoring of beta-lactam and fluoroquinolones on clinical outcome in critically ill patients: The DOLPHIN trial protocol of a multi-centre randomised controlled trial. BMC Infect. Dis. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Gonzalez Del Castillo, J.; Jensen, J.U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Rietdijk, W.J.R.; Drager, S.; Endeman, H.; Koch, B.C.P. Beta-lactam therapeutic drug monitoring in critically ill patients: Learnings for future research. Clin. Infect. Dis. 2023, 77, 663–664. [Google Scholar] [CrossRef]
- Liebchen, U.; Briegel, J.; Brinkmann, A.; Frey, O.; Wicha, S.G. Individualised dosing of antibiotics in ICU patients: Timing, target and model selection matter. Intensive Care Med. 2023, 49, 475–476. [Google Scholar] [CrossRef]
- Cotta, M.O.; Lipman, J.; De Waele, J. Advancing precision-based antimicrobial dosing in critically ill patients. Intensive Care Med. 2023, 49, 324–326. [Google Scholar] [CrossRef]
- Sanz-Codina, M.; Bozkir, H.O.; Jorda, A.; Zeitlinger, M. Individualized antimicrobial dose optimization: A systematic review and meta-analysis of randomized controlled trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef]
- Aldaz, A.; Idoate Grijalba, A.I.; Ortega, A.; Aquerreta, I.; Monedero, P. Effectiveness of Pharmacokinetic/Pharmacodynamic-Guided Meropenem Treatment in Critically Ill Patients: A Comparative Cohort Study. Ther. Drug Monit. 2021, 43, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, M.; Kohler, T.; Schweiger, A.M.; Keck, F.S.; Bein, B.; von Spiegel, T.; Schroeder, S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: A randomized prospective controlled trial. Crit. Care 2009, 13, R83. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Birkhahn, R.; Sherwin, R.; Jones, A.E.; Singer, A.; Kline, J.A.; Runyon, M.S.; Self, W.H.; Courtney, D.M.; Nowak, R.M.; et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit. Care Med. 2017, 45, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Wanner, G.A.; Keel, M.; Steckholzer, U.; Beier, W.; Stocker, R.; Ertel, W. Relationship between procalcitonin plasma levels and severity of injury, sepsis, organ failure, and mortality in injured patients. Crit. Care Med. 2000, 28, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Rehm, S.; Guertler, N.; Hinic, V.; Drager, S.; Bassetti, S.; Rentsch, K.M.; Sendi, P.; Osthoff, M. Probability of pharmacological target attainment with flucloxacillin in Staphylococcus aureus bloodstream infection: A prospective cohort study of unbound plasma and individual MICs. J. Antimicrob. Chemother. 2021, 76, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.C.; Frey, O.; Rohr, A.; Roberts, J.A.; Koberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of beta-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef]
- DeMerle, K.M.; Angus, D.C.; Baillie, J.K.; Brant, E.; Calfee, C.S.; Carcillo, J.; Chang, C.H.; Dickson, R.; Evans, I.; Gordon, A.C.; et al. Sepsis Subclasses: A Framework for Development and Interpretation. Crit. Care Med. 2021, 49, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Vutipongsatorn, K.; Jimenez, B.; Antcliffe, D.B. Patient Stratification in Sepsis: Using Metabolomics to Detect Clinical Phenotypes, Sub-Phenotypes and Therapeutic Response. Metabolites 2022, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Fornaro, G.; Pasquini, Z.; Zanoni, A.; Bartoletti, M.; Viale, P.; Pea, F. Impact of Inflammation on Voriconazole Exposure in Critically ill Patients Affected by Probable COVID-19-Associated Pulmonary Aspergillosis. Antibiotics 2023, 12, 764. [Google Scholar] [CrossRef]
- Rawson, T.M.; Charani, E.; Moore, L.S.P.; Gilchrist, M.; Georgiou, P.; Hope, W.; Holmes, A.H. Exploring the Use of C-Reactive Protein to Estimate the Pharmacodynamics of Vancomycin. Ther. Drug Monit. 2018, 40, 315–321. [Google Scholar] [CrossRef]
- Ramos-Martin, V.; Neely, M.N.; McGowan, P.; Siner, S.; Padmore, K.; Peak, M.; Beresford, M.W.; Turner, M.A.; Paulus, S.; Hope, W.W. Population pharmacokinetics and pharmacodynamics of teicoplanin in neonates: Making better use of C-reactive protein to deliver individualized therapy. J. Antimicrob. Chemother. 2016, 71, 3168–3178. [Google Scholar] [CrossRef]


| Standard Dosing (n = 177) | MIPD (n = 174) | Total (n = 351) | p-Value | |
|---|---|---|---|---|
| Age, median (IQR) | 64 (54–70) | 65 (56–72) | 64 (55–71) | 0.301 |
| Female sex, n (%) | 66 (37.3) | 66 (37.9) | 132 (37.6) | 0.913 |
| BMI, median (IQR), kg/m2 | 25.9 (23.0–29.4) | 26.3 (23.4–31.1) | 26.1 (23.1–30.6) | 0.292 |
| CCI, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | 0.222 |
| APACHE IV Score, median (IQR) | 70 (51–90) | 70 (51–89) | 70 (51–89) | 0.703 |
| SOFA Score T1, median (IQR) | 7 (4–9) | 7 (4–10) | 7 (4–10) | 0.363 |
| SOFA Score T3, median (IQR) | 4 (2–8) | 5 (2–8) | 5 (2–8) | 0.425 |
| SOFA Score T5, median (IQR) | 1.5 (0–6) | 3 (0–6) | 2 (0–6) | 0.057 |
| Sepsis, n (%) | 0.333 | |||
| No | 77 (44) | 84 (48) | 161 (46) | |
| Sepsis | 56 (32) | 58 (33) | 114 (33) | |
| Septic shock | 44 (25) | 32 (18) | 76 (22) | |
| Antibiotic class, n (%) | 0.901 | |||
| Beta-lactam | 135 (76) | 131 (75) | 266 (76) | |
| Fluoroquinolone | 42 (24) | 43 (25) | 85 (24) | |
| Main focus of infection, n (%) | 0.921 | |||
| Pulmonary | 117 (66) | 117 (67) | 234 (67) | |
| Intra-abdominal | 27 (15) | 29 (17) | 56 (16) | |
| Skin and soft tissue | 6 (3) | 3 (2) | 9 (3) | |
| Central nervous system | 5 (3) | 4 (2) | 9 (3) | |
| Urinary tract | 3 (2) | 6 (3) | 9 (3) | |
| Bacteraemia | 6 (3) | 2 (1) | 8 (2) | |
| Catheter-related infection | 2 (1) | 2 (1) | 4 (1) | |
| Ear, nose, throat | 1 (1) | 2 (1) | 3 (1) | |
| Endocarditis | 1 (1) | 1 (1) | 2 (1) | |
| Unknown focus | 6 (3) | 5 (3) | 11 (3) | |
| Other | 3 (2) | 3 (2) | 6 (2) | |
| Laboratory values, median (IQR) | ||||
| PCT T1, ng/mL | 3.22 (0.71–14.0) * | 1.92 (0.41–16.2) ** | 2.35 (0.54–14.25) | 0.153 |
| PCT T3, ng/mL | 1.83 (0.39–5.30) * | 0.7 (0.26–4.43) ** | 1.15 (0.34–4.96) | 0.057 |
| PCT T5, ng/mL | 0.91 (0.29–4.44) * | 0.72 (0.24–2.42) ** | 0.89 (0.25–3.35) | 0.333 |
| WBC T1, ×109/L | 13.0 (9.2–18.0) | 13.6 (8.8–17.4) | 13.2 (8.9–17.7) | 0.978 |
| WBC T3, ×109/L | 12.6 (8.7–16.0) | 11.7 (8.7–18.0) | 12.2 (8.7–17.1) | 0.918 |
| WBC T5, ×109/L | 12.8 (9.8–16.6) | 13.5 (9.8–18.7) | 13.1 (9.7–18.3) | 0.439 |
| CRP T1, mg/L | 216 (123–329) | 197 (104–304) | 213 (110–321) | 0.176 |
| CRP T3, mg/L | 128 (71–223) | 122.5 (67–191) | 123 (68–200) | 0.536 |
| CRP T5, mg/L | 84 (42–180) | 80 (43–160) | 82 (42–169) | 0.801 |
| Creatinine T1, µmol/L | 94 (63–146) | 89 (58–163) | 91 (60–153) | 0.602 |
| Creatinine T3, µmol/L | 85 (59–128) | 75 (54–140) | 80 (55–135) | 0.742 |
| Creatinine T5, µmol/L | 84 (55–119) | 70 (50–122) | 77 (53–120) | 0.424 |
| Outcome | ||||
| ICU LOS, median (IQR) | 8 (3–19) | 11 (5–20.75) | 10 (4–20) | 0.052 |
| Hospital LOS, median (IQR) | 21 (10–36.25) | 26 (14–43.75) | 23 (12.00, 40.75) | 0.035 |
| Mortality 28 days, n (%) | 44 (24.9) | 45 (25.9) | 89 (25.4) | 0.902 |
| Mortality 6 months, n (%) | 57 (32.2) | 62 (35.6) | 119 (33.9) | 0.501 |
| Below Target (n = 100) | Attained Target (n = 178) | Above Target (n = 28) | Total (n = 306) | p Value | |
|---|---|---|---|---|---|
| Age, median (IQR) | 61 (49–68) | 65 (57–70) | 68 (61–73) | 64 (55–71) | 0.003 |
| Female sex, n (%) | 33 (33.0) | 69 (38.8) | 15 (53.6) | 117 (38.2) | 0.141 |
| BMI, median (IQR), kg/m2 | 25.7 (23.0–29.5) | 26.5 (23.2–30.9) | 24.0 (20.2–26.2) | 26.2 (22.9–30.6) | 0.063 |
| CCI, median (IQR) | 2 (1–4) | 3 (2–4) | 4 (3–5) | 3 (2–4) | <0.001 |
| APACHE IV Score, median (IQR) | 64 (48–85) | 73 (56–89) | 78 (60–95) | 70 (51–89) | 0.046 |
| SOFA Score T0, median (IQR) | 6 (4–8) | 8 (5–11) | 9 (8–11) | 8 (5–10) | <0.001 |
| SOFA Score T1, median (IQR) | 5 (4–8) | 8 (4–11) | 7 (5–9) | 7 (4–10) | <0.001 |
| SOFA Score T3, median (IQR) | 4 (2–6) | 6 (3–11) | 6 (4–10) | 5 (3–9) | <0.001 |
| SOFA Score T5, median (IQR) | 3 (2–5) | 6 (3–10) | 6 (3–9) | 5 (3–8) | <0.001 |
| Sepsis, n (%) | <0.001 | ||||
| No | 64 (64) | 69 (39) | 12 (43) | 145 (47) | |
| Sepsis | 29 (29) | 63 (35) | 9 (32) | 101 (33) | |
| Septic shock | 7 (7) | 46 (26) | 7 (25) | 60 (20) | |
| Antibiotic class, n (%) | <0.001 | ||||
| Beta-lactam | 53 (53) | 147 (83) | 28 (100) | 228 (75) | |
| Fluoroquinolone | 47 (47) | 31 (17) | 0 (0) | 78 (26) | |
| Main focus of infection, n (%) | <0.001 | ||||
| Pulmonary | 82 (82) | 113 (64) | 11 (39) | 206 (67) | |
| Intra-abdominal | 4 (4) | 33 (19) | 10 (36) | 47 (15) | |
| Skin and soft tissue | 3 (3) | 5 (3) | 1 (4) | 9 (3) | |
| Central nervous system | 2 (2) | 6 (3) | 0 (0) | 8 (3) | |
| Urinary tract | 1 (1) | 6 (3) | 1 (4) | 8 (3) | |
| Bacteraemia | 0 (0) | 4 (2) | 2 (7) | 6 (2) | |
| Catheter-related infection | 0 (0) | 4 (2) | 0 (0) | 4 (1) | |
| Ear, nose, throat | 1 (1) | 1 (1) | 1 (4) | 3 (1) | |
| Endocarditis | 1 (1) | 1 (1) | 0 (0) | 2 (1) | |
| Other | 3 (3) | 1 (1) | 2 (7) | 6 (2) | |
| Unknown focus | 3 (3) | 4 (2) | 0 (0) | 7 (2) | |
| Laboratory values, median (IQR) | |||||
| PCT T1, ng/mL | 0.76 (0.29–2.41) * | 3.35 (0.80–17.52) ** | 13.15 (5.43–22.75) *** | 2.34 (0.53–14.28) | <0.001 |
| PCT T3, ng/mL | 0.42 (0.22–1.80) * | 1.46 (0.46–5.92) ** | 3.96 (2.82–10.82) *** | 1.07 (0.33–4.18) | <0.001 |
| PCT T5, ng/mL | 0.27 (0.17–0.84) * | 1.04 (0.28–3.61) ** | 1.07 (0.89–1.70) *** | 0.78 (0.23–2.36) | <0.001 |
| WBC T1, ×109/L | 13.6 (9.3–17.8) | 12.85 (8.8–17.3) | 16.1 (13.6–22.0) | 13.6 (9.2–17.9) | 0.02 |
| WBC T3, ×109/L | 12.0 (8.8–15.4) | 12.1 (8.5–17.6) | 16.6 (12.3–20.3) | 12.4 (8.7–17.2) | 0.039 |
| WBC T5, ×109/L | 13 (10.3–16.3) | 13.7 (9.9–18.9) | 16.7 (12.5–24.7) | 13.4 (10.0–18.7) | 0.169 |
| CRP T1, mg/L | 188 (94–288) | 214 (107–332) | 245 (195–307) | 213 (110–322) | 0.117 |
| CRP T3, mg/L | 112 (58–204) | 129 (72–198) | 114 (89–177) | 121 (67–193) | 0.658 |
| CRP T5, mg/L | 70 (39–162) | 82 (42–175) | 78 (68–119) | 76 (42–165) | 0.875 |
| Creatinine T1, µmol/L | 64 (52–91) | 105 (68–155) | 173 (97–233) | 90 (60–149) | <0.001 |
| Creatinine T3, µmol/L | 60 (48–82) | 89 (59–135) | 144 (87–236) | 78 (54–128) | <0.001 |
| Creatinine T5, µmol/L | 59 (44–81) | 82 (56–120) | 191 (142–253) | 76 (52–118) | <0.001 |
| Outcome | |||||
| ICU LOS, median (IQR) | 10 (4–19) | 10 (4–21) | 4 (2.75–9) | 9.5 (3.25–19) | 0.006 |
| ICU LOS, median (IQR) 28 d survivors | 11 (4–21.5) | 10 (3–21.5) | 4 (2–6.25) $ | 10 (3–20.5) | 0.028 |
| Hospital LOS, median (IQR) | 25 (12.5–41.0) | 24 (12.25–43.0) | 14.5 (8.5–22.25) $ | 23 (12.0–41.0) | 0.027 |
| Hospital LOS, median (IQR) 28 d survivors | 28 (14.25–44.5) | 29 (15–49) | 18 (11.5–38) | 28.5 (14.25–47.75) | 0.336 |
| Mortality 28 d, n (%) | 17 (17) | 50 (28.1) | 12 (42.9) | 79 (25.8) | 0.010 |
| Mortality 6 months, n (%) | 21 (21) | 71 (39.9) | 14 (50.0) | 106 (34.6) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dräger, S.; Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Verkaik, N.; Ramakers, C.; de Jong, E.; Osthoff, M.; Koch, B.C.P.; Endeman, H., on behalf of the DOLPHIN Investigators. Exploring the Impact of Model-Informed Precision Dosing on Procalcitonin Concentrations in Critically Ill Patients: A Secondary Analysis of the DOLPHIN Trial. Pharmaceutics 2024, 16, 270. https://doi.org/10.3390/pharmaceutics16020270
Dräger S, Ewoldt TMJ, Abdulla A, Rietdijk WJR, Verkaik N, Ramakers C, de Jong E, Osthoff M, Koch BCP, Endeman H on behalf of the DOLPHIN Investigators. Exploring the Impact of Model-Informed Precision Dosing on Procalcitonin Concentrations in Critically Ill Patients: A Secondary Analysis of the DOLPHIN Trial. Pharmaceutics. 2024; 16(2):270. https://doi.org/10.3390/pharmaceutics16020270
Chicago/Turabian StyleDräger, Sarah, Tim M. J. Ewoldt, Alan Abdulla, Wim J. R. Rietdijk, Nelianne Verkaik, Christian Ramakers, Evelien de Jong, Michael Osthoff, Birgit C. P. Koch, and Henrik Endeman on behalf of the DOLPHIN Investigators. 2024. "Exploring the Impact of Model-Informed Precision Dosing on Procalcitonin Concentrations in Critically Ill Patients: A Secondary Analysis of the DOLPHIN Trial" Pharmaceutics 16, no. 2: 270. https://doi.org/10.3390/pharmaceutics16020270
APA StyleDräger, S., Ewoldt, T. M. J., Abdulla, A., Rietdijk, W. J. R., Verkaik, N., Ramakers, C., de Jong, E., Osthoff, M., Koch, B. C. P., & Endeman, H., on behalf of the DOLPHIN Investigators. (2024). Exploring the Impact of Model-Informed Precision Dosing on Procalcitonin Concentrations in Critically Ill Patients: A Secondary Analysis of the DOLPHIN Trial. Pharmaceutics, 16(2), 270. https://doi.org/10.3390/pharmaceutics16020270






