Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer
Abstract
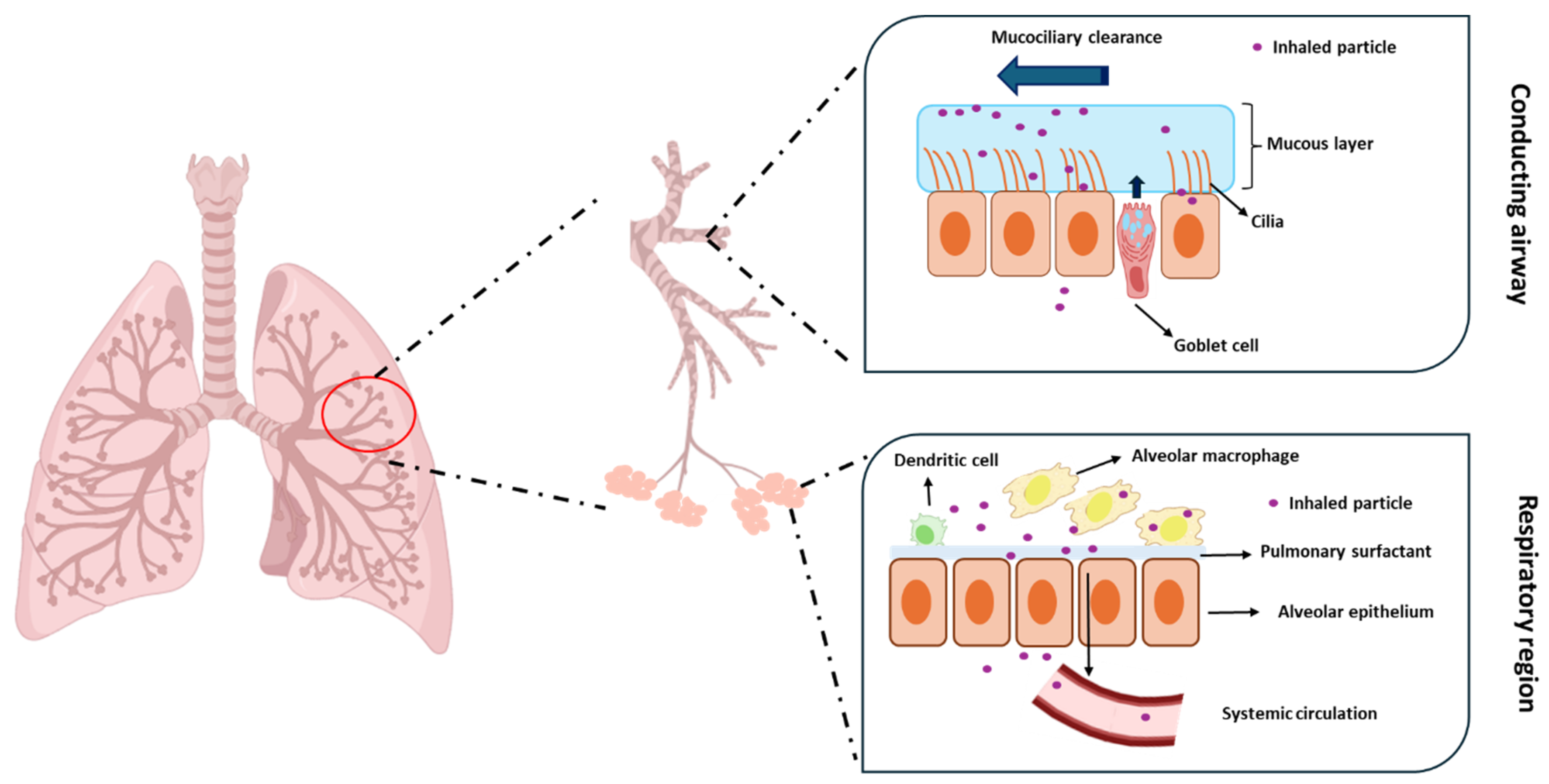
1. Introduction
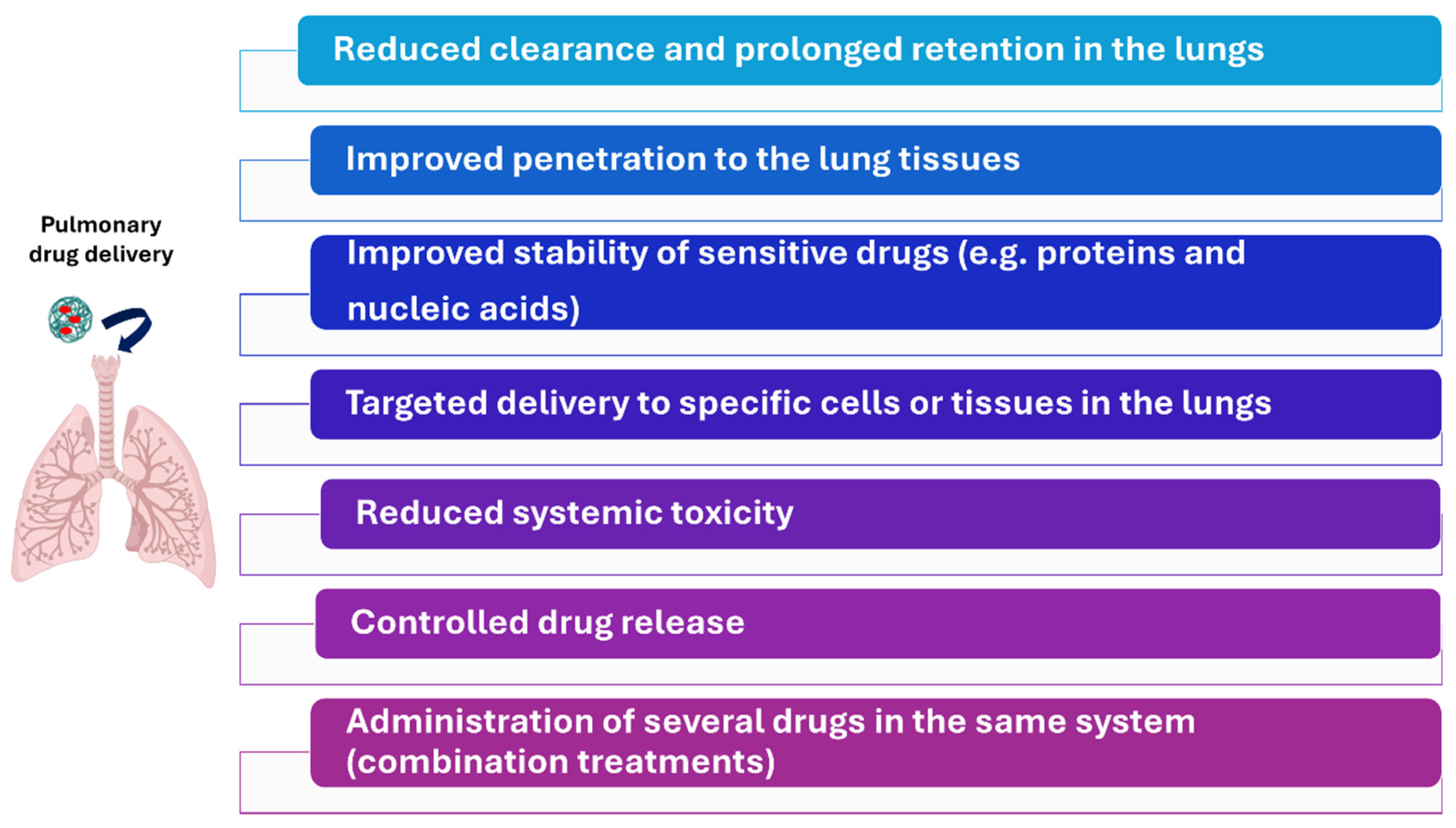
2. Nanomedicine in Pulmonary Drug Delivery
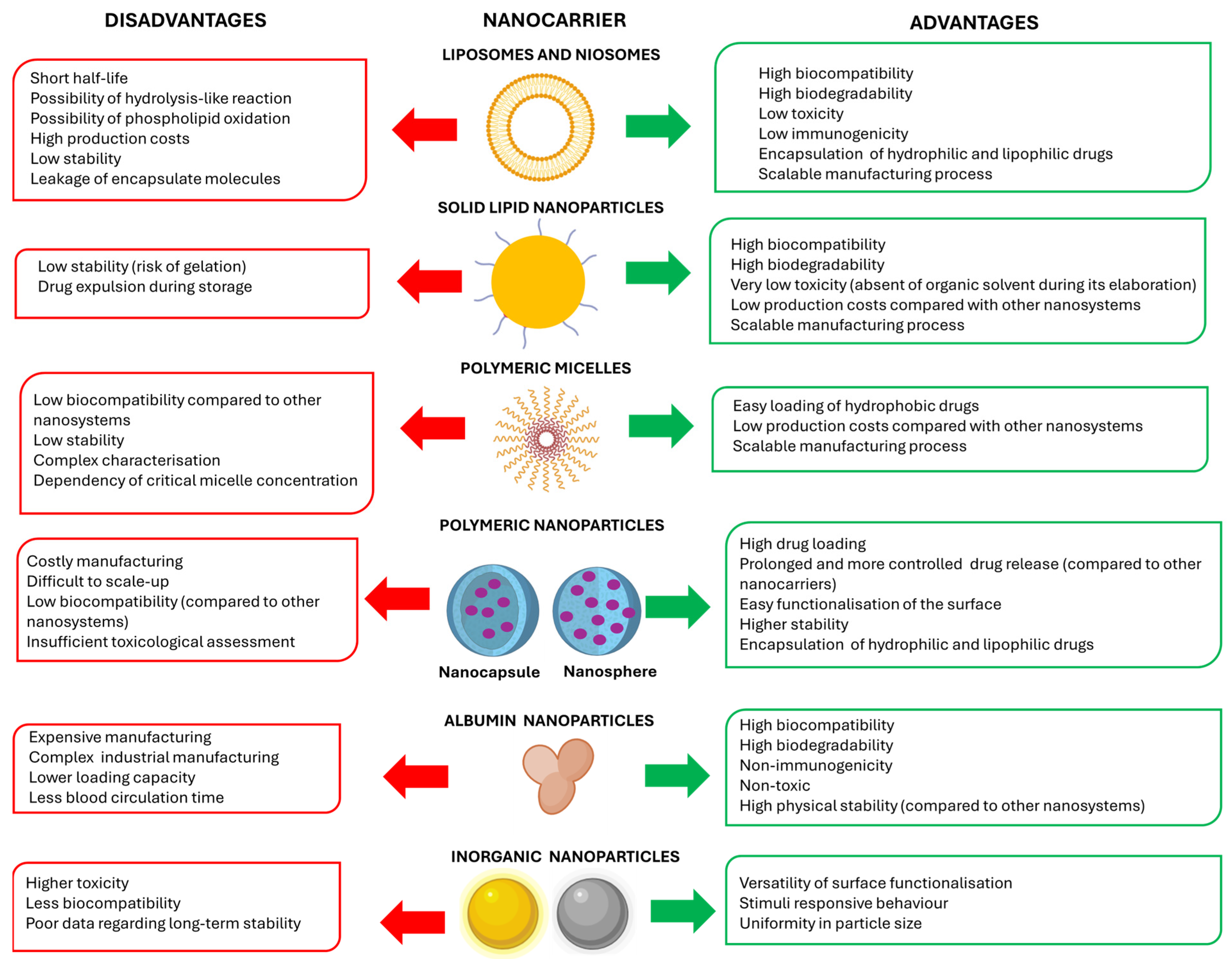
2.1. Types of Nanocarriers
2.2. Impact of Physicochemical Properties of Nanoparticles on Pulmonary Drug Delivery
2.2.1. Influence of Particle Size
2.2.2. Influence of Particle Morphology
2.2.3. Influence of the Surface Charge
2.2.4. Influence of Surface Hydrophobicity
2.3. Impact of Patient-Specific Factors
2.3.1. Smoking
2.3.2. Chronic Obstructive Pulmonary Disease
2.3.3. Cystic Fibrosis
2.3.4. Asthma
2.3.5. Lung Cancer
3. Pulmonary-Administered Nanomedicines to Combat Respiratory Tract Infections
3.1. Bacterial Infections
3.1.1. Tuberculosis
3.1.2. Bacterial Pneumonia
3.2. Fungal Infections
| Formulation | Drugs | Mechanism of Action | Disease | Excipients | Comments | Reference |
|---|---|---|---|---|---|---|
| Solid lipid nanoparticles | Isoniazid | Inhibition of the synthesis of mycolic acid | TB | PP SA Poloxamer 188 Mannose Leucine | pH-sensitive response achieved by adding isoniazid as a prodrug | [118] |
| Liposomes | Liquorice extract (glycyrrhizin) | Membrane disruption | TB | Lipoid® 100 Cholesterol | In total, 46% of the drug reached the lungs, with 16% remaining in the lungs 24 h post-administration | [119] |
| Nanogels | LLKKK18 | Membrane disruption | TB | HA | Infection levels significantly reduced after just five to ten doses | [120] |
| Nanogels | Isoniazid Rifampicin | Inhibition of the synthesis of mycolic acid (isoniazid) + inhibition of RNA synthesis (rifampicin) | TB | Carboxymethyl chitosan Genipin | Selective targeting of lungs, maintaining drug concentrations of 40–60% after 24 h | [121] |
| Polymeric nanoparticles | Rifampicin Ascorbic acid | Inhibition of RNA synthesis (rifampicin) + adjuvant (ascorbic acid) | TB | Sodium alginate Tween® 80 Sucrose Mannitol | Demonstrated activity against nine clinical strains of M. tuberculosis | [123] |
| Polymeric nanoparticles | Ciprofloxacin | Inhibition of type IV topoisomerase | Lower respiratory tract infections—unspecified | PEtOx Tannic acid | Sustained release over the course of 7 days | [127] |
| Polymeric nanoparticles | SET-M33 | Binding to LPS | Infection caused by P. aeruginosa | Dextran | Prolonged residence time (12-fold higher) compared to free peptide; demonstrated efficacy and safety in vivo | [128] |
| Anionic liposomes | Levofloxacin | Inhibition of type IV topoisomerase | Infection caused by P. aeruginosa in cystic fibrosis patients | DSPC Cholesterol DSPE-PEG 2000 | Sustained release over 72 h and demonstrated activity against five strains of P. aeruginosa | [129] |
| Liposomes | Levofloxacin Lysozyme | Inhibition of type IV topoisomerase (levofloxacin) + hydrolysis of peptidoglycan in bacterial cell wall (lysozyme) | Infection caused by S. aureus | Phospholipon® 90G Phospholipon® 90H Cholesterol Lactose | Demonstrated decrease in microbial burden in lungs, bronchoalveolar lavage fluid, and nasal fluid | [130] |
| Liposomes | Colistin | Displacement of magnesium and calcium in LPS | Infection caused by P. aeruginosa | Sodium cholesteryl sulphate Lipoid® S75 | Prolonged drug retention in the lung and enhanced in vivo efficacy | [131] |
| Formulation | Drugs | Mechanism of Action | Excipients | Comments | Reference |
|---|---|---|---|---|---|
| Polymeric nanoparticles | Voriconazole | Inhibition of ergosterol demethylation | Chitosan | Enhanced lung deposition and demonstrated in vitro efficacy against Aspergillus | [138] |
| Inorganic nanoparticles | Leaf extract of Artemisia sieberi | Not reported | Silver (AgNO3) | Demonstrated efficacy against Aspergillus, reducing lung tissue damage, and reduced oxidative stress | [139] |
| Liposomes | Dapsone | Inhibition of synthesis of dihydrofolic acid | DPPC Cholesterol | Prolonged in vitro release up to 16 h | [140] |
| Micelles | Amphotericin B | Membrane disruption | Chitosan Stearic acid | Aerosolisation of amphotericin B with improved activity compared to the free drug | [141] |
| Liposomes | Amphotericin B | Membrane disruption | Egg PC Cholesterol OPM OPP | Improved in vivo airway penetration in rats and accumulation in lung tissue for over 24 h | [143] |
| Nanostructured aggregates | Itraconazole | Inhibition of ergosterol demethylation | Mannitol Lecithin | Achieved lung deposition and systemic levels in mice | [144] |
3.3. Viral Infections
3.3.1. SARS-CoV-2
3.3.2. Other Viral Infections
| Formulation | Drugs | Mechanism of Action | Disease | Excipients | Comments | Reference |
|---|---|---|---|---|---|---|
| Polymeric nanoparticles | Remdesivir | Inhibition of RNA polymerase | COVID-19 | PLGA PCL | Selective targeting to ACE2 membrane receptor and enhanced antiviral effect compared to free drug | [156] |
| Nanovesicles | Dexamethasone | Agonist of the glucocorticoid receptor | COVID-19 | Nanovesicles were obtained from neutrophils from bone marrow of mice or rhesus macaques | Better outcome when the formulation was inhaled instead of injected. Improved targeting to macrophages. | [157] |
| Nanosuspension | Remdesivir | Inhibition of RNA polymerase | COVID-19 | PCL Pluronic® F127 HA Mannitol | Enhanced drug release compared to free drug (1.5-fold increase at 24 h and 1.9-fold increase at 48 h) and better safety index (1.3-fold higher). | [158] |
| Dendritic nanocarriers | Remdesivir | Inhibition of RNA polymerase | COVID-19 | Hyperbranched G4-PEG6k-OH | First-order release kinetics with total release of remdesivir after 24 h and similar toxicity profile than free drug. | [159] |
| Nanostructured lipid carriers | Hydroxychloroquine | Inhibition of TLR9 | COVID-19 | Sweet almond oil Glyceryl behenate PC Gelucire® | Improved lung tissue targeting of hydroxychloroquine, but further investigation needed to confirm its potential for COVID-19 treatment | [160] |
| Nanoparticles | Resveratrol | Activation of Sirt-1 | Infection caused by RSV | Unspecified | In vivo models demonstrated extended lung residence and reduced viral load compared to free drug | [161] |
| Liposomes | Oxymatrine | Antiviral activity induced by promoting difference cytokines | Infection caused by RSV | DPPC HSPC DPPG Cholesterol DSPE-PEG Chitosan | Selective distribution and improved retention in lung tissue compared to the free drug | [162] |
| Lipid nanoparticles | IFN-λ | Recruitment of neutrophils and NK cells | Influenza | Protamine Unspecified lipids Unspecified proteins | Improved delivery of IFN-λ to lungs and superior efficacy compared to recombinant IFN | [163] |
4. Nanovaccines
| Formulation | Infection | Excipients | Comments | Reference |
|---|---|---|---|---|
| Nanovesicles + liposomes | COVID-19 | DPPC, DPPE, DPPG, Cholesterol | Demonstrated neutralisation of multiple coronavirus variants and effective at generating mucosal immunity | [178] |
| Liposomes | COVID-19 | DPPC, DPPG, DPPE-PEG-COOH | Elicited stronger mucosal protective immunity compared to intramuscular or subcutaneous vaccination | [179] |
| Lipid nanoparticles | Influenza A | DMPE, DPSE, Cholesterol, DOPE, DSPC, DOTAP, DOTMA, DODA | All the mentioned lipids were tested, but the composition of the optimised composition is unspecified. Successfully delivered mRNA encoding antibodies against influenza A | [180] |
| Polymeric nanoparticles | Influenza A | CPTEG, CPH | Elicited robust systemic and mucosal humoral immune responses and enhanced systemic and lung-resident cellular immunity | [181] |
5. Pulmonary-Administered Nanomedicines to Combat Lung Cancer
| Nanocarrier | Drug | Observations | Reference |
|---|---|---|---|
| Liposomes | Curcumin | Better aerosolization properties. Selective cytotoxicity against lung cancer cells compared to healthy lung cells. Higher in vivo anticancer activity. | [196] |
| Pirfenidone | Good aerosolization performance More cytotoxic effect against A549 cells than non-encapsulated drug. | [208] | |
| Paclitaxel | Higher lung accumulation of paclitaxel compared to i.v. administration. Tumour reduction compared to non-treated animals. Higher survival rate compared to non-treated animals. | [197] | |
| Erlotinib | Good aerosolization performance using vibrating mesh nebulisers. | [201] | |
| Niosomes | Gemcitabine and paclitaxel | Aerosol output of 96.2%. Lower toxicity in healthy lung cells (MRC5) compared to free drugs (IC50 = 280 µg/mL vs IC50 < 1.6 µg/mL). Lower cytotoxic activity in lung cancer (A529 cells) compared to free drugs (IC50 = 46 µg/mL vs IC50 < 1.6 µg/mL). | [207] |
| Nanostructured lipid particles | Paclitaxel | Higher distribution in the lungs of the pulmonary route compared to intravenous injection. No signs of systemic toxicity after pulmonary administration. | [198] |
| Paclitaxel | Better lung accumulation compared to free paclitaxel. Higher anticancer activity than free paclitaxel. | [199] | |
| Paclitaxel and Doxorrubicin | Higher antiproliferative effect in A549 cells. Higher distribution in the lungs compared to non-encapsulated drugs. | [206] | |
| Solid lipid nanoparticles | Doxorubicin | Higher deposition of administered doses compared to inhaled free doxorubicin. Reach deeper regions in the lungs. Higher plasmatic level of doxorubicin compared to the administration of inhaled free doxorubicin. | [202] |
| Polymeric nanoparticles | Quinacrine (mepacrine) | Nanoparticles incorporating albumin on their surface. Good aerosolization properties. Improved in vitro anticancer activity in NSCLC compared to the free drug. Higher apoptosis induction. | [205] |
| Sorafenib | Appropriate aerosolization properties. Higher in vivo anticancer activity in NSCLC | [203] |
6. Challenges in Clinical Translation
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mansour, H.M.; Rhee, Y.S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Dames, P.; Gleich, B.; Flemmer, A.; Hajek, K.; Seidl, N.; Wiekhorst, F.; Eberbeck, D.; Bittmann, I.; Bergemann, C.; Weyh, T.; et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2007, 2, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Seresirikachorn, B.; Ghadiri, M. Chapter 7—Absorption enhancement of macromolecule-administered intrapulmonary. In Targeting Chronic Inflammatory Lung Diseases Using Advanced Drug Delivery Systems; Dua, K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 147–161. [Google Scholar]
- Lam, J.K.W.; Zhou, Q. Advances in Pulmonary Drug Delivery Systems and Inhalation Formulations. Pharm. Res. 2023, 40, 1013–1014. [Google Scholar] [CrossRef] [PubMed]
- Jain, H.; Bairagi, A.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Recent advances in the development of microparticles for pulmonary administration. Drug Discov. Today 2020, 25, 1865–1872. [Google Scholar] [CrossRef]
- Anaya, B.J.; D’Angelo, D.; Bettini, R.; Molina, G.; Sanz-Perez, A.; Dea-Ayuela, M.A.; Galiana, C.; Rodríguez, C.; Tirado, D.F.; Lalatsa, A.; et al. Heparin-azithromycin microparticles show anti-inflammatory effects and inhibit SARS-CoV-2 and bacterial pathogens associated to lung infections. Carbohydr. Polym. 2025, 348, 122930. [Google Scholar] [CrossRef]
- Torres-Suárez, A.I.; Martín-Sabroso, C.; Fraguas-Sánchez, A.I.; Rojo, M.Á.; Garrosa, M.; Fernández-Carballido, A. Chapter 7—Design of dosage forms: Influences of anatomy and administration routes. In Dosage Forms, Formulation Developments and Regulations; Nayak, A.K., Sen, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 183–221. [Google Scholar] [CrossRef]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J. Nanobiotechnology 2022, 20, 101. [Google Scholar] [CrossRef]
- Stannard, W.; O’Callaghan, C. Ciliary function and the role of cilia in clearance. J. Aerosol Med. 2006, 19, 110–115. [Google Scholar] [CrossRef]
- Araujo, F.; Martins, C.; Azevedo, C.; Sarmento, B. Chemical modification of drug molecules as strategy to reduce interactions with mucus. Adv. Drug Deliv. Rev. 2018, 124, 98–106. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov Today 2020, 25, 150–159. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. The biology of mucus: Composition, synthesis and organization. Adv. Drug Deliv. Rev. 2018, 124, 3–15. [Google Scholar] [CrossRef]
- Hill, D.B.; Button, B.; Rubinstein, M.; Boucher, R.C. Physiology and pathophysiology of human airway mucus. Physiol. Rev. 2022, 102, 1757–1836. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef] [PubMed]
- Taherali, F.; Varum, F.; Basit, A.W. A slippery slope: On the origin, role and physiology of mucus. Adv. Drug Deliv. Rev. 2018, 124, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.; Ackermann, M.; Lachmann, N. Beyond “Big Eaters”: The Versatile Role of Alveolar Macrophages in Health and Disease. Int. J. Mol. Sci. 2021, 22, 3308. [Google Scholar] [CrossRef]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef]
- Hidalgo, A.; Cruz, A.; Pérez-Gil, J. Barrier or carrier? Pulmonary surfactant and drug delivery. Eur. J. Pharm. Biopharm. 2015, 95, 117–127. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef]
- García-Díaz, M.; Birch, D.; Wan, F.; Nielsen, H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv. Drug Deliv. Rev. 2018, 124, 107–124. [Google Scholar] [CrossRef]
- Kole, E.; Jadhav, K.; Shirsath, N.; Dudhe, P.; Verma, R.K.; Chatterjee, A.; Naik, J. Nanotherapeutics for pulmonary drug delivery: An emerging approach to overcome respiratory diseases. J. Drug Deliv. Sci. Technol. 2023, 81, 104261. [Google Scholar] [CrossRef]
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef]
- Kumar, M.; Hilles, A.R.; Almurisi, S.H.; Bhatia, A.; Mahmood, S. Micro and nano-carriers-based pulmonary drug delivery system: Their current updates, challenges, and limitations—A review. JCIS Open 2023, 12, 100095. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Lozza, I.; Torres-Suárez, A.I. Nanomedicine Applications in Cancer Treatment. In Handbook of Cancer and Immunology; Rezaei, N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–37. [Google Scholar] [CrossRef]
- Rubey, K.M.; Brenner, J.S. Nanomedicine to fight infectious disease. Adv. Drug Deliv. Rev. 2021, 179, 113996. [Google Scholar] [CrossRef]
- Khan, A.; Alsahli, M.A.; Aljasir, M.A.; Maswadeh, H.; Mobark, M.A.; Azam, F.; Allemailem, K.S.; Alrumaihi, F.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; et al. Safety, Stability, and Therapeutic Efficacy of Long-Circulating TQ-Incorporated Liposomes: Implication in the Treatment of Lung Cancer. Pharmaceutics 2022, 14, 153. [Google Scholar] [CrossRef]
- Hu, T.; Gong, H.; Xu, J.; Huang, Y.; Wu, F.; He, Z. Nanomedicines for Overcoming Cancer Drug Resistance. Pharmaceutics 2022, 14, 1606. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, W.; Qi, Y.; Ying, Y.; Xia, Z. Overcoming Multidrug Resistance in Bacteria Through Antibiotics Delivery in Surface-Engineered Nano-Cargos: Recent Developments for Future Nano-Antibiotics. Front. Bioeng. Biotechnol. 2021, 9, 696514. [Google Scholar] [CrossRef] [PubMed]
- Kamat, S.; Kumari, M. Emergence of microbial resistance against nanoparticles: Mechanisms and strategies. Front Microbiol 2023, 14, 1102615. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D Printing Technologies in Personalized Medicine, Nanomedicines, and Biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Osouli-Bostanabad, K.; Puliga, S.; Serrano, D.R.; Bucchi, A.; Halbert, G.; Lalatsa, A. Microfluidic Manufacture of Lipid-Based Nanomedicines. Pharmaceutics 2022, 14, 1940. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- He, K.; Tang, M. Safety of novel liposomal drugs for cancer treatment: Advances and prospects. Chem.-Biol. Interact. 2018, 295, 13–19. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Lozza, I.; Torres-Suárez, A.I. Actively Targeted Nanomedicines in Breast Cancer: From Pre-Clinal Investigation to Clinic. Cancers 2022, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current status of nanomedicine in the chemotherapy of breast cancer. In Cancer Chemotherapy and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 84, pp. 689–706. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Izhar, M.P.; Hafeez, A.; Kushwaha, P.; Simrah. Drug Delivery Through Niosomes: A Comprehensive Review with Therapeutic Applications. J. Clust. Sci. 2023, 34, 2257–2273. [Google Scholar] [CrossRef]
- Moammeri, A.; Chegeni, M.M.; Sahrayi, H.; Ghafelehbashi, R.; Memarzadeh, F.; Mansouri, A.; Akbarzadeh, I.; Abtahi, M.S.; Hejabi, F.; Ren, Q. Current advances in niosomes applications for drug delivery and cancer treatment. Mater. Today Bio 2023, 23, 100837. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.W.; Guzman, E.B.; Menon, N.; Langer, R.S. Lipid nanoparticles for nucleic acid delivery to endothelial cells. Pharm. Res. 2023, 40, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Miguel, R.D.A.; Hirata, A.S.; Jimenez, P.C.; Lopes, L.B.; Costa-Lotufo, L.V. Beyond Formulation: Contributions of Nanotechnology for Translation of Anticancer Natural Products into New Drugs. Pharmaceutics 2022, 14, 1722. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Zwain, T.; Taneja, N.; Zwayen, S.; Shidhaye, A.; Palshetkar, A.; Singh, K.K. Chapter 9—Albumin nanoparticles—A versatile and a safe platform for drug delivery applications. In Nanoparticle Therapeutics; Kesharwani, P., Singh, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 327–358. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Ji, Q.; Zhu, H.; Qin, Y.; Zhang, R.; Wang, L.; Zhang, E.; Zhou, X.; Meng, R. GP60 and SPARC as albumin receptors: Key targeted sites for the delivery of antitumor drugs. Front. Pharmacol. 2024, 15, 1329636. [Google Scholar] [CrossRef]
- Hartley, C.; Rowan, D.; Chen, X.; Gomez-Arellano, L.; West, A.M.; Oshima, K.; Mackinnon, A.C. Increased SPARC expression is associated with neoadjuvant therapy in resectable pancreatic ductal adenocarcinoma. Pract. Lab. Med. 2020, 21, e00171. [Google Scholar] [CrossRef]
- Qu, N.; Song, K.; Ji, Y.; Liu, M.; Chen, L.; Lee, R.J.; Teng, L. Albumin Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2024, 19, 6945–6980. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of inorganic nanoparticles for revolutionary drug delivery applications: A critical review. Discov. Nano 2023, 18, 157. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Morillas-Becerill, L.; De Cola, L.; Zuidema, J.M. Inorganic nanoparticle empowered biomaterial hybrids: Engineered payload release. Front. Nanotechnol. 2022, 4, 999923. [Google Scholar] [CrossRef]
- de Pablo, E.; Fernández-García, R.; Ballesteros, M.P.; Torrado, J.J.; Serrano, D.R. Nebulised antibiotherapy: Conventional versus nanotechnology-based approaches, is targeting at a nano scale a difficult subject? Ann. Transl. Med. 2017, 5, 448. [Google Scholar] [CrossRef] [PubMed]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar] [PubMed]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.S.; Xu, Q.; Boylan, N.J.; Chisholm, J.; Tang, B.C.; Schuster, B.S.; Henning, A.; Ensign, L.M.; Lee, E.; Adstamongkonkul, P.; et al. Nanoparticles that do not adhere to mucus provide uniform and long-lasting drug delivery to airways following inhalation. Sci. Adv. 2017, 3, e1601556. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, A.; Kanno, S.; Kobayashi, T.; Hirano, S. Extrapulmonary translocation of intratracheally instilled fine and ultrafine particles via direct and alveolar macrophage-associated routes. Arch. Toxicol. 2009, 83, 429–437. [Google Scholar] [CrossRef]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Influence of particle size on drug delivery to rat alveolar macrophages following pulmonary administration of ciprofloxacin incorporated into liposomes. J. Drug Target. 2006, 14, 557–566. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.-F.; Quan, L.-H.; Liu, C.-Y.; Liu, X.-M.; Ehrhardt, C.; Liao, Y.-H. Pulmonary delivered polymeric micelles—Pharmacokinetic evaluation and biodistribution studies. Eur. J. Pharm. Biopharm. 2014, 88, 1064–1075. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler, M.; Erbe, F.; Mayer, P.; Takenaka, S.; Schulz, H.; Oberdörster, G.; Ziesenis, A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health—Part A 2002, 65, 1513–1530. [Google Scholar] [CrossRef]
- Choi, H.S.; Ashitate, Y.; Lee, J.H.; Kim, S.H.; Matsui, A.; Insin, N.; Bawendi, M.G.; Semmler-Behnke, M.; Frangioni, J.V.; Tsuda, A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 2010, 28, 1300–1303. [Google Scholar] [CrossRef]
- Mohammad, A.K.; Amayreh, L.K.; Mazzara, J.M.; Reineke, J.J. Rapid Lymph Accumulation of Polystyrene Nanoparticles Following Pulmonary Administration. Pharm. Res. 2013, 30, 424–434. [Google Scholar] [CrossRef]
- Garapaty, A.; Champion, J.A. Tunable particles alter macrophage uptake based on combinatorial effects of physical properties. Bioeng. Transl. Med. 2017, 2, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zellnitz, S.; Zellnitz, L.; Müller, M.T.; Meindl, C.; Schröttner, H.; Fröhlich, E. Impact of drug particle shape on permeability and cellular uptake in the lung. Eur. J. Pharm. Sci. 2019, 139, 105065. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Lau, R.W.M. Effect of Particle Shape on Dry Particle Inhalation: Study of Flowability, Aerosolization, and Deposition Properties. AAPS PharmSciTech 2009, 10, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Sarode, A.; Kanabar, D.D.; Muth, A.; Kunda, N.K.; Mitragotri, S.; Gupta, V. Bioinspired particle engineering for non-invasive inhaled drug delivery to the lungs. Mater. Sci. Eng. C 2021, 128, 112324. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kłodzińska, S.N.; Wan, F.; Nielsen, H.M. Nanoparticle-mediated pulmonary drug delivery: State of the art towards efficient treatment of recalcitrant respiratory tract bacterial infections. Drug Deliv. Transl. Res. 2021, 11, 1634–1654. [Google Scholar] [CrossRef]
- Murgia, X.; Loretz, B.; Hartwig, O.; Hittinger, M.; Lehr, C.-M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv. Drug Deliv. Rev. 2018, 124, 82–97. [Google Scholar] [CrossRef]
- Dawson, M.; Wirtz, D.; Hanes, J. Enhanced Viscoelasticity of Human Cystic Fibrotic Sputum Correlates with Increasing Microheterogeneity in Particle Transport. J. Biol. Chem. 2003, 278, 50393–50401. [Google Scholar] [CrossRef]
- Seydoux, E.; Rodriguez-Lorenzo, L.; Blom, R.A.M.; Stumbles, P.A.; Petri-Fink, A.; Rothen-Rutishauser, B.M.; Blank, F.; von Garnier, C. Pulmonary delivery of cationic gold nanoparticles boost antigen-specific CD4+ T Cell Proliferation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1815–1826. [Google Scholar] [CrossRef]
- Areny-Balagueró, A.; Mekseriwattana, W.; Camprubí-Rimblas, M.; Stephany, A.; Roldan, A.; Solé-Porta, A.; Artigas, A.; Closa, D.; Roig, A. Fluorescent PLGA Nanocarriers for Pulmonary Administration: Influence of the Surface Charge. Pharmaceutics 2022, 14, 1447. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Hirn, S.; Möller, W.; Schleh, C.; Wenk, A.; Celik, G.; Lipka, J.; Schäffler, M.; Haberl, N.; Johnston, B.D.; et al. Air–Blood Barrier Translocation of Tracheally Instilled Gold Nanoparticles Inversely Depends on Particle Size. ACS Nano 2014, 8, 222–233. [Google Scholar] [CrossRef]
- George, I.; Naudin, G.; Boland, S.; Mornet, S.; Contremoulins, V.; Beugnon, K.; Martinon, L.; Lambert, O.; Baeza-Squiban, A. Metallic oxide nanoparticle translocation across the human bronchial epithelial barrier. Nanoscale 2015, 7, 4529–4544. [Google Scholar] [CrossRef] [PubMed]
- Fromen, C.A.; Rahhal, T.B.; Robbins, G.R.; Kai, M.P.; Shen, T.W.; Luft, J.C.; DeSimone, J.M. Nanoparticle surface charge impacts distribution, uptake and lymph node trafficking by pulmonary antigen-presenting cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Witten, J.; Samad, T.; Ribbeck, K. Selective permeability of mucus barriers. Curr. Opin. Biotechnol. 2018, 52, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG Mucoadhesivity Paradox to Engineer Nanoparticles that “Slip” through the Human Mucus Barrier. Angew. Chem. Int. Ed. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Huang, X.; Chisholm, J.; Zhuang, J.; Xiao, Y.; Duncan, G.; Chen, X.; Suk, J.S.; Hanes, J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl. Acad. Sci. USA 2017, 114, E6595–E6602. [Google Scholar] [CrossRef]
- Murata, M.; Tahara, K.; Takeuchi, H. Real-time in vivo imaging of surface-modified liposomes to evaluate their behavior after pulmonary administration. Eur. J. Pharm. Biopharm. 2014, 86, 115–119. [Google Scholar] [CrossRef]
- Weers, J.; Clark, A. The Impact of Inspiratory Flow Rate on Drug Delivery to the Lungs with Dry Powder Inhalers. Pharm. Res. 2017, 34, 507–528. [Google Scholar] [CrossRef]
- Ahmadi, K.; Gharibi, Z.; Davoodian, P.; Gouklani, H.; Hassaniazad, M.; Ahmadi, N. The Effect of Smoking on the Increase of Infectious Diseases. Tob. Health 2022, 1, 100–106. [Google Scholar] [CrossRef]
- Jo, Y.S. Long-Term Outcome of Chronic Obstructive Pulmonary Disease: A Review. Tuberc. Respir. Dis. 2022, 85, 289–301. [Google Scholar] [CrossRef]
- Shah, B.K.; Singh, B.; Wang, Y.; Xie, S.; Wang, C. Mucus Hypersecretion in Chronic Obstructive Pulmonary Disease and Its Treatment. Mediat. Inflamm. 2023, 2023, 8840594. [Google Scholar] [CrossRef]
- Taccetti, G.; Francalanci, M.; Pizzamiglio, G.; Messore, B.; Carnovale, V.; Cimino, G.; Cipolli, M. Cystic Fibrosis: Recent Insights into Inhaled Antibiotic Treatment and Future Perspectives. Antibiotics 2021, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Blasi, F.; Canonica, G.W.; Morandi, L.; Richeldi, L.; Rossi, A. Treatment strategies for asthma: Reshaping the concept of asthma management. Allergy Asthma Clin. Immunol. 2020, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, P.; Manjappa, A.; Shah, R.; Jha, N.K.; Singh, S.K.; Dua, K.; Disouza, J.; Patravale, V. Inhalation delivery of repurposed drugs for lung cancer: Approaches, benefits and challenges. J. Control. Release 2022, 341, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elahe, D.-N. An Overview of Bacterial Respiratory Tract Infections and their Etiologies. J. Med. Bacteriol. 2023, 11, 36–46. [Google Scholar] [CrossRef]
- Thomas, M.; Bomar, P.A. Upper Respiratory Tract Infection. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mariyam, N.; Reji, S.; Saijan, S.; George, S.; Subscription, C. Assessment of Antibiotic Sensitivity Pattern in RTI Patients in a Secondary Care Hospital. J. Pharma Innov. 2020, 1, 41–47. [Google Scholar]
- Troeger, C.; Blacker, B.; Khalil, I.A.; Rao, P.C.; Cao, J.; Zimsen, S.R.; Albertson, S.B.; Deshpande, A.; Farag, T.; Abebe, Z.; et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef]
- Chee, E.; García, A.J. Biomaterial therapeutic strategies for treatment of bacterial lung infections. Biofilm 2023, 5, 100111. [Google Scholar] [CrossRef]
- Cantón, R.; Gottlieb, T.; Coombs, G.W.; Woo, P.C.Y.; Korman, T.M.; Garcia-Castillo, M.; Daley, D.; Bauer, K.A.; Wong, M.; Wolf, D.J.; et al. Antimicrobial surveillance: A 20-year history of the SMART approach to addressing global antimicrobial resistance into the future. Int. J. Antimicrob. Agents 2023, 62, 107014. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Leung, S.S.Y. Pulmonary Delivery of Emerging Antibacterials for Bacterial Lung Infections Treatment. Pharm. Res. 2023, 40, 1057–1072. [Google Scholar] [CrossRef]
- Muthu, V.; Sehgal, I.S.; Agarwal, R. Aerosolized Antifungals for the Treatment of Pulmonary Fungal Diseases. Curr. Fungal Infect. Rep. 2024, 18, 154–162. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Zhang, Y.; Lei, F.; Ren, R.; Tang, X. Opportunities and Challenges for Inhalable Nanomedicine Formulations in Respiratory Diseases: A Review. Int. J. Nanomed. 2024, 19, 1509–1538. [Google Scholar] [CrossRef] [PubMed]
- Khan, O.; Chaudary, N. The Use of Amikacin Liposome Inhalation Suspension (Arikayce) in the Treatment of Refractory Nontuberculous Mycobacterial Lung Disease in Adults. Drug Des. Dev. Ther. 2020, 14, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Amikacin Liposome Inhalation Suspension: A Review in Mycobacterium avium Complex Lung Disease. Drugs 2019, 79, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Pneumonia. 2022. Available online: https://medlineplus.gov/spanish/pneumonia.html (accessed on 25 April 2023).
- Lawn, S.D.; Zumla, A.I. Tuberculosis. Lancet 2011, 378, 57–72. [Google Scholar] [CrossRef]
- Villar-Hernández, R.; Ghodousi, A.; Konstantynovska, O.; Duarte, R.; Lange, C.; Raviglione, M. Tuberculosis: Current challenges and beyond. Breathe 2023, 19, 220166. [Google Scholar] [CrossRef]
- WHO. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Tuberculosis. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tuberculosis (accessed on 21 November 2022).
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020—Reflections on the Global TB burden, treatment and prevention efforts. Int. J. Infect. Dis. 2021, 113, S7–S12. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.; Amiano, N.; Cagel, M.; Bernabeu, E.; Chiappetta, D.A.; Moretton, M.A. Nanotechnology in Tuberculosis: State of the Art and the Challenges Ahead. Pharm. Res. 2018, 35, 213. [Google Scholar] [CrossRef]
- Eldholm, V.; Balloux, F. Antimicrobial Resistance in Mycobacterium tuberculosis: The Odd One Out. Trends Microbiol. 2016, 24, 637–648. [Google Scholar] [CrossRef]
- Grange, J.M.; Zumla, A. The global emergency of tuberculosis: What is the cause? J. R. Soc. Promot. Health 2002, 122, 78–81. [Google Scholar] [CrossRef]
- Garcia-Prats, A.J.; Willemse, M.; Seifart, H.I.; Jordaan, A.M.; Werely, C.J.; Donald, P.R.; Schaaf, H.S. Acquired drug resistance during inadequate therapy in a young child with tuberculosis. Pediatr. Infect. Dis. J. 2014, 33, 883–885. [Google Scholar] [CrossRef] [PubMed]
- Nasiruddin, M.; Neyaz, M.K.; Das, S. Nanotechnology-Based Approach in Tuberculosis Treatment. Tuberc. Res. Treat. 2017, 2017, 4920209. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Chau, E.; Mishra, A.; DeAnda, A.; Hegde, V.L.; Sastry, J.K.; Haviland, D.; Jagannath, C.; Godin, B.; Khan, A. CD44 receptor targeted nanoparticles augment immunity against tuberculosis in mice. J. Control. Release 2022, 349, 796–811. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Kong, Y. Tuberculosis Host-Pathogen Interactions; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Chai, Q.; Zhang, Y.; Liu, C.H. Mycobacterium tuberculosis: An adaptable pathogen associated with multiple human diseases. Front. Cell. Infect. Microbiol. 2018, 8, 158. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.L.; Salgueiro, J.; Bernabeu, E.; Gonzalez, L.; Manca, M.L.; Amiano, N.; Valenti, D.; Manconi, M.; García, V.; et al. Pulmonary delivery of rifampicin-loaded soluplus micelles against Mycobacterium tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 53, 101170. [Google Scholar] [CrossRef]
- Chae, J.; Choi, Y.; Tanaka, M.; Choi, J. Inhalable nanoparticles delivery targeting alveolar macrophages for the treatment of pulmonary tuberculosis. J. Biosci. Bioeng. 2021, 132, 543–551. [Google Scholar] [CrossRef]
- Baranyai, Z.; Soria-Carrera, H.; Alleva, M.; Millán-Placer, A.C.; Lucía, A.; Martín-Rapún, R.; Aínsa, J.A.; de la Fuente, J.M. Nanotechnology-Based Targeted Drug Delivery: An Emerging Tool to Overcome Tuberculosis. Adv. Ther. 2021, 4, 2000113. [Google Scholar] [CrossRef]
- Ma, C.; Wu, M.; Ye, W.; Huang, Z.; Ma, X.; Wang, W.; Wang, W.; Huang, Y.; Pan, X.; Wu, C. Inhalable solid lipid nanoparticles for intracellular tuberculosis infection therapy: Macrophage-targeting and pH-sensitive properties. Drug Deliv. Transl. Res. 2021, 11, 1218–1235. [Google Scholar] [CrossRef]
- Viswanathan, V.; Pharande, R.; Bannalikar, A.; Gupta, P.; Gupta, U.; Mukne, A. Inhalable liposomes of Glycyrrhiza glabra extract for use in tuberculosis: Formulation, in vitro characterization, in vivo lung deposition, and in vivo pharmacodynamic studies. Drug Dev. Ind. Pharm. 2019, 45, 11–20. [Google Scholar] [CrossRef]
- Silva, J.P.; Gonçalves, C.; Costa, C.; Sousa, J.; Silva-Gomes, R.; Castro, A.G.; Pedrosa, J.; Appelberg, R.; Gama, F.M. Delivery of LLKKK18 loaded into self-assembling hyaluronic acid nanogel for tuberculosis treatment. J. Control. Release 2016, 235, 112–124. [Google Scholar] [CrossRef]
- Wu, T.; Liao, W.; Wang, W.; Zhou, J.; Tan, W.; Xiang, W.; Zhang, J.; Guo, L.; Chen, T.; Ma, D.; et al. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydr. Polym. 2018, 197, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Vilchèze, C.; Kim, J.; Jacobs, W.R., Jr. Vitamin C Potentiates the Killing of Mycobacterium tuberculosis by the First-Line Tuberculosis Drugs Isoniazid and Rifampin in Mice. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Sánchez-Borzone, M.E.; Granero, G.E. Promising Chitosan-Coated Alginate-Tween 80 Nanoparticles as Rifampicin Coadministered Ascorbic Acid Delivery Carrier Against Mycobacterium tuberculosis. AAPS PharmSciTech 2019, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.B.A.; Nguyen, A.D.; Sharma, S. Bacterial Pneumonia. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Garg, M.; Prabhakar, N.; Gulati, A.; Agarwal, R.; Dhooria, S. Spectrum of imaging findings in pulmonary infections. Part 1: Bacterial and viral. Pol. J. Radiol. 2019, 84, e205. [Google Scholar] [CrossRef]
- Eshwara, V.K.; Mukhopadhyay, C.; Rello, J. Community-acquired bacterial pneumonia in adults: An update. Indian J. Med. Res. 2020, 151, 287–302. [Google Scholar] [CrossRef]
- Sabuj, M.Z.R.; Dargaville, T.R.; Nissen, L.; Islam, N. Inhaled ciprofloxacin-loaded poly(2-ethyl-2-oxazoline) nanoparticles from dry powder inhaler formulation for the potential treatment of lower respiratory tract infections. PLoS ONE 2021, 16, e0261720. [Google Scholar] [CrossRef]
- Falciani, C.; Zevolini, F.; Brunetti, J.; Riolo, G.; Gracia, R.; Marradi, M.; Loinaz, I.; Ziemann, C.; Cossío, U.; Llop, J.; et al. Antimicrobial Peptide-Loaded Nanoparticles as Inhalation Therapy for Pseudomonas aeruginosa Infections. Int. J. Nanomed. 2020, 15, 1117–1128. [Google Scholar] [CrossRef]
- Derbali, R.M.; Aoun, V.; Moussa, G.; Frei, G.; Tehrani, S.F.; Del’Orto, J.C.; Hildgen, P.; Roullin, V.G.; Chain, J.L. Tailored Nanocarriers for the Pulmonary Delivery of Levofloxacin against Pseudomonas aeruginosa: A Comparative Study. Mol. Pharm. 2019, 16, 1906–1916. [Google Scholar] [CrossRef]
- Gupta, P.V.; Nirwane, A.M.; Nagarsenker, M.S. Inhalable Levofloxacin Liposomes Complemented with Lysozyme for Treatment of Pulmonary Infection in Rats: Effective Antimicrobial and Antibiofilm Strategy. AAPS PharmSciTech 2018, 19, 1454–1467. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; Zhang, E.; Yang, L. Electrostatically entrapped colistin liposomes for the treatment of Pseudomonas aeruginosa infection. Pharm. Dev. Technol. 2017, 22, 436–444. [Google Scholar] [CrossRef]
- Palmieri, F.; Koutsokera, A.; Bernasconi, E.; Junier, P.; von Garnier, C.; Ubags, N. Recent Advances in Fungal Infections: From Lung Ecology to Therapeutic Strategies with a Focus on Aspergillus spp. Front. Med. 2022, 9, 832510. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ami, R. Systemic Antifungal Therapy for Invasive Pulmonary Infections. J. Fungi 2023, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- McKeny, P.T.; Nessel, T.A.; Zito, P.M. Antifungal Antibiotics. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jenks, J.D.; Hoenigl, M. Treatment of Aspergillosis. J. Fungi 2018, 4, 98. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, R.; de Pablo, E.; Ballesteros, M.P.; Serrano, D.R. Unmet clinical needs in the treatment of systemic fungal infections: The role of amphotericin B and drug targeting. Int. J. Pharm. 2017, 525, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Dennison, S.R.; Burrow, A.J.; Rudramurthy, S.M.; Swami, R.; Gorki, V.; Katare, O.P.; Kaushik, A.; Singh, B.; Singh, K.K. Nebulised surface-active hybrid nanoparticles of voriconazole for pulmonary Aspergillosis demonstrate clathrin-mediated cellular uptake, improved antifungal efficacy and lung retention. J. Nanobiotechnology 2021, 19, 19. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdallah, B.M. Effective Inhibition of Invasive Pulmonary Aspergillosis by Silver Nanoparticles Biosynthesized with Artemisia sieberi Leaf Extract. Nanomaterials 2021, 12, 51. [Google Scholar] [CrossRef]
- Chougule, M.; Padhi, B.; Misra, A. Development of Spray Dried Liposomal Dry Powder Inhaler of Dapsone. AAPS PharmSciTech 2008, 9, 47–53. [Google Scholar] [CrossRef]
- Gilani, K.; Moazeni, E.; Ramezanli, T.; Amini, M.; Fazeli, M.R.; Jamalifar, H. Development of Respirable Nanomicelle Carriers for Delivery of Amphotericin B by Jet Nebulization. J. Pharm. Sci. 2011, 100, 252–259. [Google Scholar] [CrossRef]
- Nasr, M.; Nawaz, S.; Elhissi, A. Amphotericin B lipid nanoemulsion aerosols for targeting peripheral respiratory airways via nebulization. Int. J. Pharm. 2012, 436, 611–616. [Google Scholar] [CrossRef]
- Vyas, S.P.; Quraishi, S.; Gupta, S.; Jaganathan, K.S. Aerosolized liposome-based delivery of amphotericin B to alveolar macrophages. Int. J. Pharm. 2005, 296, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tam, J.; Miller, D.A.; Zhou, J.; McConville, J.T.; Johnston, K.P.; Williams, R.O. High bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizers. Int. J. Pharm. 2008, 361, 177–188. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, H.R.; Yu, H. Viral Respiratory Infections. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elservier: Amsterdam, The Netherlands, 2020; pp. 284–288. [Google Scholar] [CrossRef]
- Zhou, J.; Krishnan, N.; Jiang, Y.; Fang, R.H.; Zhang, L. Nanotechnology for virus treatment. Nano Today 2021, 36, 101031. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Tian, R.; Chen, X. Cell-membrane-mimicking nanodecoys against infectious diseases. ACS Nano 2020, 14, 2569–2574. [Google Scholar] [CrossRef]
- Symptoms of COVID-19. 2022. Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/symptoms-causes/syc-20479963 (accessed on 24 April 2023).
- McKinnon, T.; Watson, A.; Richards, L.; Sears, J.; Brookes, M.J.; Green, C.A. The Volunteers in Research Programme: Supporting COVID-19 research and improving medical training in parallel. Clin. Med. 2021, 21, 182–188. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. Bmj 2020, 369, m1985. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Tawfeek, H.M.; Abdelfattah, A.; El-Saber Batiha, G.; Hetta, H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: Nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021, 63, 102435. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X.; et al. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Deb, S.; Reeves, A.A.; Hopefl, R.; Bejusca, R. ADME and Pharmacokinetic Properties of Remdesivir: Its Drug Interaction Potential. Pharmaceuticals 2021, 14, 655. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Sanna, V.; Satta, S.; Hsiai, T.; Sechi, M. Development of targeted nanoparticles loaded with antiviral drugs for SARS-CoV-2 inhibition. Eur. J. Med. Chem. 2022, 231, 114121. [Google Scholar] [CrossRef]
- Meng, Q.-F.; Tai, W.; Tian, M.; Zhuang, X.; Pan, Y.; Lai, J.; Xu, Y.; Xu, Z.; Li, M.; Zhao, G.; et al. Inhalation delivery of dexamethasone with iSEND nanoparticles attenuates the COVID-19 cytokine storm in mice and nonhuman primates. Sci. Adv. 2023, 9, eadg3277. [Google Scholar] [CrossRef]
- Fouad, S.A.; Malaak, F.A.; Teaima, M.H.; Omar, S.; Kutkat, O.; Elhabal, S.F.; El-Nabarawi, M. Novel inhalable nano-based/microparticles for enhanced sustained pulmonary delivery of remdesivir—A patient malleable treatment for coronaviruses infection: In vitro aerosolization, cytotoxicity assays and antiviral activity studies. J. Drug Deliv. Sci. Technol. 2024, 101, 106196. [Google Scholar] [CrossRef]
- Halevas, E.; Mavroidi, B.; Kokotidou, C.; Moschona, A.; Sagnou, M.; Mitraki, A.; Litsardakis, G.; Pelecanou, M. Remdesivir-loaded bis-MPA hyperbranched dendritic nanocarriers for pulmonary delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103625. [Google Scholar] [CrossRef]
- Ali, A.S.; Alrashedi, M.G.; Ahmed, O.A.; Ibrahim, I.M. Pulmonary Delivery of Hydroxychloroquine Nanostructured Lipid Carrier as a Potential Treatment of COVID-19. Polymers 2022, 14, 2616. [Google Scholar] [CrossRef]
- Chang, C.; Lu, C.; Zheng, Y.; Ji, J.; Lin, L.; Chen, L.; Chen, Z.; Chen, R. Sonication-Assisted Self-Assembled Resveratrol Nanoparticles with Enhanced Antiviral and Anti-inflammatory Activity against Respiratory Syncytial Virus-Induced Pneumonia. ACS Appl. Mater. Interfaces 2024, 16, 50442–50458. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Guo, M.; Liu, C.; Chen, X.; Tao, L.; Zhang, K.; Shen, X. Development of Inhalable Chitosan-Coated Oxymatrine Liposomes to Alleviate RSV-Infected Mice. Int. J. Mol. Sci. 2022, 23, 15909. [Google Scholar] [CrossRef]
- Gil, C.H.; Oh, C.; Lee, J.; Jang, M.; Han, J.; Cho, S.-D.; Park, S.-H.; Park, J.-H.; Kim, H.J. Inhalation Delivery of Interferon-λ-Loaded Pulmonary Surfactant Nanoparticles Induces Rapid Antiviral Immune Responses in the Lung. ACS Appl. Mater. Interfaces 2024, 16, 11147–11158. [Google Scholar] [CrossRef]
- Cojocaru, F.-D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials Designed for Antiviral Drug Delivery Transport across Biological Barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef]
- Chapter 14—Vaccination. In Primer to the Immune Response, 2nd ed.; Mak, T.W., Saunders, M.E., Jett, B.D., Eds.; Academic Cell: Boston, MA, USA, 2014; pp. 333–375. [Google Scholar] [CrossRef]
- Gholizadeh, O.; Akbarzadeh, S.; Ghazanfari Hashemi, M.; Gholami, M.; Amini, P.; Yekanipour, Z.; Tabatabaie, R.; Yasamineh, S.; Hosseini, P.; Poortahmasebi, V. Hepatitis A: Viral Structure, Classification, Life Cycle, Clinical Symptoms, Diagnosis Error, and Vaccination. Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 4263309. [Google Scholar] [CrossRef]
- Poon, C.; Patel, A.A. Organic and inorganic nanoparticle vaccines for prevention of infectious diseases. Nano Express 2020, 1, 012001. [Google Scholar] [CrossRef]
- Shichinohe, S.; Watanabe, T. Advances in Adjuvanted Influenza Vaccines. Vaccines 2023, 11, 1391. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.N.; Kelly, H.G.; Kent, S.J.; Wheatley, A.K. Current and future nanoparticle vaccines for COVID-19. eBioMedicine 2021, 74, 103699. [Google Scholar] [CrossRef] [PubMed]
- Spikevax, Data Sheet. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 24 April 2023).
- Comirnaty, Data Sheet. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 24 April 2023).
- Klimek, L.; Novak, N.; Hamelmann, E.; Werfel, T.; Wagenmann, M.; Taube, C.; Bauer, A.; Merk, H.; Rabe, U.; Jung, K.; et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: Position statement of the German Allergy Societies: Medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA). Allergo J. Int. 2021, 30, 51–55. [Google Scholar] [CrossRef]
- Hatziantoniou, S.; Maltezou, H.C.; Tsakris, A.; Poland, G.A.; Anastassopoulou, C. Anaphylactic reactions to mRNA COVID-19 vaccines: A call for further study. Vaccine 2021, 39, 2605–2607. [Google Scholar] [CrossRef]
- Lavelle, E.C.; Ward, R.W. Mucosal vaccines—Fortifying the frontiers. Nat. Rev. Immunol. 2022, 22, 236–250. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Zhu, G. Pulmonary delivery of mucosal nanovaccines. Nanoscale 2022, 14, 263–276. [Google Scholar] [CrossRef]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef]
- Wei, C.-J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Wang, S.; Ding, P.; Shen, L.; Fan, D.; Cheng, H.; Huo, J.; Wei, X.; He, H.; Zhang, G. Inhalable hybrid nanovaccines with virus-biomimetic structure boost protective immune responses against SARS-CoV-2 variants. J. Nanobiotechnology 2024, 22, 76. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, W.; Guo, M.; Huang, M.; Gu, Y.; Wang, T.; Ni, G.; Ming, D. Inhalable nanovaccine with biomimetic coronavirus structure to trigger mucosal immunity of respiratory tract against COVID-19. Chem. Eng. J. 2021, 418, 129392. [Google Scholar] [CrossRef] [PubMed]
- Lokugamage, M.P.; Vanover, D.; Beyersdorf, J.; Hatit, M.Z.C.; Rotolo, L.; Echeverri, E.S.; Peck, H.E.; Ni, H.; Yoon, J.-K.; Kim, Y.; et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 2021, 5, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.E.; Zacharias, Z.R.; Ross, K.A.; Narasimhan, B.; Waldschmidt, T.J.; Legge, K.L. Polyanhydride nanovaccine against H3N2 influenza A virus generates mucosal resident and systemic immunity promoting protection. Npj Vaccines 2024, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Seguin, L.; Durandy, M.; Feral, C.C. Lung Adenocarcinoma Tumor Origin: A Guide for Personalized Medicine. Cancers 2022, 14, 1759. [Google Scholar] [CrossRef]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Sardeli, C.; Zarogoulidis, P.; Kosmidis, C.; Amaniti, A.; Katsaounis, A.; Giannakidis, D.; Koulouris, C.; Hohenforst-Schmidt, W.; Huang, H.; Bai, C.; et al. Inhaled chemotherapy adverse effects: Mechanisms and protection methods. Lung Cancer Manag. 2020, 8, Lmt19. [Google Scholar] [CrossRef]
- Lemarie, E.; Vecellio, L.; Hureaux, J.; Prunier, C.; Valat, C.; Grimbert, D.; Boidron-Celle, M.; Giraudeau, B.; le Pape, A.; Pichon, E.; et al. Aerosolized Gemcitabine in Patients with Carcinoma of the Lung: Feasibility and Safety Study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 261–270. [Google Scholar] [CrossRef]
- Otterson, G.A.; Villalona-Calero, M.A.; Sharma, S.; Kris, M.G.; Imondi, A.; Gerber, M.; White, D.A.; Ratain, M.J.; Schiller, J.H.; Sandler, A.; et al. Phase I study of inhaled Doxorubicin for patients with metastatic tumors to the lungs. Clin Cancer Res 2007, 13, 1246–1252. [Google Scholar] [CrossRef]
- Tatsumura, T.; Koyama, S.; Tsujimoto, M.; Kitagawa, M.; Kagamimori, S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br. J. Cancer 1993, 68, 1146–1149. [Google Scholar] [CrossRef]
- Zarogoulidis, P.; Eleftheriadou, E.; Sapardanis, I.; Zarogoulidou, V.; Lithoxopoulou, H.; Kontakiotis, T.; Karamanos, N.; Zachariadis, G.; Mabroudi, M.; Zisimopoulos, A.; et al. Feasibility and effectiveness of inhaled carboplatin in NSCLC patients. Investig. New Drugs 2012, 30, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Wittgen, B.P.; Kunst, P.W.; van der Born, K.; van Wijk, A.W.; Perkins, W.; Pilkiewicz, F.G.; Perez-Soler, R.; Nicholson, S.; Peters, G.J.; Postmus, P.E. Phase I study of aerosolized SLIT cisplatin in the treatment of patients with carcinoma of the lung. Clin. Cancer Res. 2007, 13, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Verschraegen, C.F.; Gilbert, B.E.; Loyer, E.; Huaringa, A.; Walsh, G.; Newman, R.A.; Knight, V. Clinical Evaluation of the Delivery and Safety of Aerosolized Liposomal 9-Nitro-20(S)-Camptothecin in Patients with Advanced Pulmonary Malignancies. Clin. Cancer Res. 2004, 10, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, R.; Jiang, T.; Gao, Y.; Zhong, K.; Cheng, H.; Chen, X.; Li, S. Inhalable nanomedicine for lung cancer treatment. Smart Mater. Med. 2024, 5, 261–280. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Zheng, X.; Song, X.; Zhu, G.; Pan, D.; Li, H.; Hu, J.; Xiao, K.; Gong, Q.; Gu, Z.; Luo, K.; et al. Nanomedicine Combats Drug Resistance in Lung Cancer. Adv. Mater. 2024, 36, 2308977. [Google Scholar] [CrossRef]
- Kaur, G.; Bose, S.; Kataria, T.; Tyagi, A.; Singla, K.; Sharma, S.; Ghosh, S.; Jha, C.B. Curcumin as a Potential Phytoconstituent used for Cancer Treatment: An Overview. Nat. Prod. J. 2024, 14, 1–13. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Ge, Y.; Hu, Y.; Li, M.; Jin, Y. Inhalation treatment of primary lung cancer using liposomal curcumin dry powder inhalers. Acta Pharm. Sin. B 2018, 8, 440–448. [Google Scholar] [CrossRef]
- Koshkina, N.V.; Waldrep, J.C.; Roberts, L.E.; Golunski, E.; Melton, S.; Knight, V. Paclitaxel Liposome Aerosol Treatment Induces Inhibition of Pulmonary Metastases in Murine Renal Carcinoma Model1. Clin. Cancer Res. 2001, 7, 3258–3262. [Google Scholar]
- Garbuzenko, O.B.; Kuzmov, A.; Taratula, O.; Pine, S.R.; Minko, T. Strategy to enhance lung cancer treatment by five essential elements: Inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics 2019, 9, 8362–8376. [Google Scholar] [CrossRef]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.; Goyal, A.K. Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box-Behnken design. Drug Deliv. 2016, 23, 1912–1925. [Google Scholar] [CrossRef] [PubMed]
- Abdelgalil, A.A.; Al-Kahtani, H.M.; Al-Jenoobi, F.I. Chapter Four—Erlotinib. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 45, pp. 93–117. [Google Scholar]
- Szabová, J.; Mišík, O.; Fučík, J.; Mrázová, K.; Mravcová, L.; Elcner, J.; Lízal, F.; Krzyžánek, V.; Mravec, F. Liposomal form of erlotinib for local inhalation administration and efficiency of its transport to the lungs. Int. J. Pharm. 2023, 634, 122695. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jia, Y.; Wen, D. Preparation and characterization of solid lipid nanoparticles loaded with epirubicin for pulmonary delivery. Pharmazie 2010, 65, 585–587. [Google Scholar] [PubMed]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef]
- Oien, D.B.; Pathoulas, C.L.; Ray, U.; Thirusangu, P.; Kalogera, E.; Shridhar, V. Repurposing quinacrine for treatment-refractory cancer. Semin Cancer Biol. 2021, 68, 21–30. [Google Scholar] [CrossRef]
- Vaidya, B.; Kulkarni, N.S.; Shukla, S.K.; Parvathaneni, V.; Chauhan, G.; Damon, J.K.; Sarode, A.; Garcia, J.V.; Kunda, N.; Mitragotri, S.; et al. Development of inhalable quinacrine loaded bovine serum albumin modified cationic nanoparticles: Repurposing quinacrine for lung cancer therapeutics. Int. J. Pharm. 2020, 577, 118995. [Google Scholar] [CrossRef]
- Kaur, P.; Mishra, V.; Shunmugaperumal, T.; Goyal, A.K.; Ghosh, G.; Rath, G. Inhalable spray dried lipidnanoparticles for the co-delivery of paclitaxel and doxorubicin in lung cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101502. [Google Scholar] [CrossRef]
- Mohamad Saimi, N.I.; Salim, N.; Ahmad, N.; Abdulmalek, E.; Abdul Rahman, M.B. Aerosolized Niosome Formulation Containing Gemcitabine and Cisplatin for Lung Cancer Treatment: Optimization, Characterization and In Vitro Evaluation. Pharmaceutics 2021, 13, 59. [Google Scholar] [CrossRef]
- Parvathaneni, V.; Kulkarni, N.S.; Shukla, S.K.; Farrales, P.T.; Kunda, N.K.; Muth, A.; Gupta, V. Systematic development and optimization of inhalable pirfenidone liposomes for non-small cell lung cancer treatment. Pharmaceutics 2020, 12, 206. [Google Scholar] [CrossRef]
- Wang, W.; Zhong, Z.; Huang, Z.; Hiew, T.N.; Huang, Y.; Wu, C.; Pan, X. Nanomedicines for targeted pulmonary delivery: Receptor-mediated strategy and alternatives. Nanoscale 2024, 16, 2820–2833. [Google Scholar] [CrossRef]
- Bosetti, R.; Jones, S.L. Cost–Effectiveness of Nanomedicine: Estimating the Real Size of Nano-Costs. Nanomedicine 2019, 14, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. Nanomedicine for the poor: A lost cause or an idea whose time has yet to come? Nanomedicine 2021, 16, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Kendal, E. Ethical, Legal and Social Implications of Emerging Technology (ELSIET) Symposium. J. Bioethical Inq. 2022, 19, 363–370. [Google Scholar] [CrossRef] [PubMed]
- McClements, J.; McClements, D.J. Standardization of Nanoparticle Characterization: Methods for Testing Properties, Stability, and Functionality of Edible Nanoparticles. Crit. Rev. Food Sci. Nutr. 2016, 56, 1334–1362. [Google Scholar] [CrossRef]

| Formulation | Drug | Pharmaceutical Dosage Form | Indication |
|---|---|---|---|
| Tobi® * | Tobramycin | Solution for inhalation | Cystic fibrosis patients infected with Pseudomonas aeruginosa |
| Betkis® * | Tobramycin | Solution for inhalation | Cystic fibrosis patients infected with P. aeruginosa |
| Cayston® * | Aztreonam | Solution for inhalation | Cystic fibrosis patients infected with P. aeruginosa |
| Tobi® Podhaler * | Tobramycin | Dry powder inhaler | Cystic fibrosis patients infected with P. aeruginosa |
| Kitabis® Pak * | Tobramycin | Solution for inhalation | Cystic fibrosis patients infected with P. aeruginosa |
| Arikayce® * | Amikacin | Liposomal inhalation suspension | Nontuberculous mycobacterial lung disease |
| Opelconazole | Opelconazole | Dry powder inhaler | Aspergillosis |
| PUR1900 | Itraconazole | Dry powder inhaler | Fungal pulmonary infections |
| Voriconazole | Voriconazole | Dry powder inhaler | Fungal pulmonary infections |
| Apulmiq | Ciprofloxacin | Liposomal inhalation suspension | Chronic lung infections with P. aeruginosa |
| MRT5005 | Ciprofloxacin | Lipid nanoparticles | Cystic fibrosis |
| Drug | Dose | Cancer Type | Reference |
|---|---|---|---|
| Gemcitabine | 1–4 mg/kg | NSCLC | [186] |
| Doxorrubicin | 0.4–9.4 mg/m2 | Primary and metastases in the lungs | [187] |
| 5-Fluorouracil | 250 mg | NSCLC | [188] |
| Carboplatin (iv or inhaled) +Docetaxel (iv) | Docetaxel: 100 mg/m2 Carboplatin: AUC 5.5 | NSCLC | [189] |
| Liposomal cisplatin | 1.5–48 mg/m2 | NSCLC | [190] |
| Liposomal 9-Nitrocamptothecin | 6.7–26.6 µg/kg/day | Primary lung cancer Metastases in the lungs | [191] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, R.; Fraguas-Sánchez, A.I. Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics 2024, 16, 1584. https://doi.org/10.3390/pharmaceutics16121584
Fernández-García R, Fraguas-Sánchez AI. Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics. 2024; 16(12):1584. https://doi.org/10.3390/pharmaceutics16121584
Chicago/Turabian StyleFernández-García, Raquel, and Ana I. Fraguas-Sánchez. 2024. "Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer" Pharmaceutics 16, no. 12: 1584. https://doi.org/10.3390/pharmaceutics16121584
APA StyleFernández-García, R., & Fraguas-Sánchez, A. I. (2024). Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics, 16(12), 1584. https://doi.org/10.3390/pharmaceutics16121584







