5-Aminosalicylic Acid Distribution into the Intestinal Membrane Along the Gastrointestinal Tract After Oral Administration in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AC-5-ASA, N-Propionyl-5-ASA (Prop-5-ASA), and N-Propionyl-4-ASA (Prop-4-ASA)
2.3. 5-ASA Solubility
2.4. 5-ASA Membrane Distribution Study in Rats
2.5. Analysis of 5-ASA and AC-5-ASA Using HPLC
2.6. Statistical Analysis
3. Results
3.1. Synthesis of N-Acetyl-5-ASA (AC-5-ASA), N-Propionyl-5-ASA (Prop-5-ASA), and N-Propionyl-4-ASA (Prop-4-ASA)
3.2. 5-ASA Solubility
3.3. 5-ASA Membrane Distribution After Oral Administration in Rats
3.3.1. Comparison of 5-ASA Membrane Distribution Between 5-ASA Suspension in Water in Untreated Control Rats and 5-ASA Solution in 5% NaHCO3 in Lansoprazole-Pretreated Rats
3.3.2. Comparison of 5-ASA Regional Membrane Distribution Between 5-ASA Suspension in Water and 5-ASA Solution in 5% NaHCO3 3 h After Administration
3.3.3. Comparison of Regional Membrane Distribution of 5-ASA Between 5-ASA Suspension in Water, 0.1 M HCl, or 0.1 M NaOH in Untreated Control Rats and 5-ASA Solution in 5% NaHCO3 in Lansoprazole-Pretreated Rats 8 h After Administration
4. Discussion
4.1. Factors That Affected the 5-ASA Dissolution Amount In Vivo (Luminal pH, Water Volume)
4.2. 5-ASA Membrane Distribution Along the Digestive Tract
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.S.; Perisetti, A.; Kichloo, A.; Singh, A.; Goyal, H.; Rotundo, L.; Vennikandam, M.; Shaka, H.; Singh, G.; Singh, J.; et al. Increasing thirty-day readmissions of Crohn’s disease and ulcerative colitis in the United States: A national dilemma. World J. Gastrointest. Pathophysiol. 2022, 13, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.Y.; Peppercorn, M.A. MMX mesalamine: A novel high-dose, once-daily 5-aminosalicylate formulation for the induction of remission in mild to moderate UC and has a favorable safety profile. The treatment of ulcerative colitis. Expert Opin. Pharmacother. 2008, 9, 1049–1058. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Gomollón, F.; Hinojosa, J.; López San Román, A. Adherence of gastroenterologists to European Crohn’s and Colitis Organisation consensus on ulcerative colitis: A real-life survey in Spain. J. Crohn’s Colitis 2010, 4, 567–574. [Google Scholar] [CrossRef]
- Punchard, N.A.; Greenfield, S.M.; Thompson, R.P. Mechanism of action of 5-arninosalicylic acid. Mediat. Inflamm. 1992, 1, 151–165. [Google Scholar] [CrossRef]
- Greenfield, S.M.; Punchard, N.A.; Teare, J.P.; Thompson, R.P.H. Review article: The mode of action of the aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 1993, 7, 369–383. [Google Scholar] [CrossRef]
- Miyake, M.; Fujishima, M.; Nakai, D. Inhibitory Potency of Marketed Drugs for Ulcerative Colitis and Crohn’s Disease on PEPT1. Biol. Pharm. Bull. 2017, 40, 1572–1575. [Google Scholar] [CrossRef][Green Version]
- Shafii, A.; Chowdhury, J.R.; Das, K.M. Absorption, enterohepatic circulation, and excretion of 5-aminosalicylic acid in rats. Am. J. Gastroenterol. 1982, 77, 297–299. [Google Scholar]
- Nielsen, O.H.; Bondensen, S. Kinetics of 5-aminosalicylic acid after jejunal instillation in man. Br. J. Clin. Pharmacol. 1983, 16, 738–740. [Google Scholar] [CrossRef]
- Ireland, A.; Priddle, J.D.; Jewell, D.P. Acetylation of 5-aminosalicylic acid by isolated human colonic epithelial cells. Clin. Sci. 1990, 78, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Smetanová, L.; Stětinová, V.; Kholová, D.; Kuneš, M.; Nobilis, M.; Svoboda, Z.; Květina, J. Transintestinal transport mechanisms of 5-aminosalicylic acid (in situ rat intestine perfusion, Caco-2 cells) and Biopharmaceutics Classification System. Gen. Physiol. Biophys. 2013, 32, 361–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myers, B.; Evans, D.N.; Rhodes, J.; Evans, B.K.; Hughes, B.R.; Lee, M.G.; Richens, A.; Richards, D. Metabolism and urinary excretion of 5-amino salicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut 1987, 28, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef]
- Martir, J.; Flanagan, T.; Mann, J.; Fotaki, N. Impact of Food and Drink Administration Vehicles on Paediatric Formulation Performance: Part 1—Effects on Solubility of Poorly Soluble Drugs. AAPS Pharm. SciTech 2020, 21, 177. [Google Scholar] [CrossRef]
- Zhang, Y.; Wo, S.K.; Leng, W.; Gao, F.; Yan, X.; Zuo, Z. Population pharmacokinetics and IVIVC for mesalazine enteric-coated tablets. J. Control. Release 2022, 346, 275–288. [Google Scholar] [CrossRef]
- Kamm, M.A.; Sandborn, W.J.; Gassull, M.; Schreiber, S.; Jackowski, L.; Butler, T.; Lyne, A.; Stephenson, D.; Palmen, M.; Joseph, R.E. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology 2007, 132, 66–75. [Google Scholar] [CrossRef]
- Georgaka, D.; Butler, J.; Kesisoglou, F.; Reppas, C.; Vertzoni, M. Evaluation of dissolution in the lower intestine and its impact on the absorption process of high dose low solubility drugs. Mol. Pharm. 2017, 14, 4181–4191. [Google Scholar] [CrossRef]
- Le Berre, C.; Roda, G.; Nedeljkovic Protic, M.; Danese, S.; Peyrin-Biroulet, L. Modern use of 5-aminosalicylic acid compounds for ulcerative colitis. Expert Opin. Biol. Ther. 2020, 20, 363–378. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Piyapolrungroj, N.; Pao, L.; Li, C.; Liu, G.; Zimmermann, E.; Fleisher, D. Regulation of paracellular absorption of cimetidine and 5-aminosalicylate in rat intestine. Pharm. Res. 1999, 16, 1781–1785. [Google Scholar] [CrossRef]
- Li, P.; Luo, J.; Jiang, Y.; Pan, X.; Dong, M.; Chen, B.; Wang, J.; Zhou, H.; Jiang, H.; Duan, Y.; et al. Downregulation of OATP2B1 by proinflammatory cytokines leads to 5-ASA hyposensitivity in Ulcerative colitis. Chem. Biol. Interact. 2024, 398, 111074. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.W.; Schwab, M.; Klotz, U. Transport studies with 5-aminosalicylate. Eur. J. Clin. Pharmacol. 2006, 62, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Kawano, K.; Matsumura, R.; Sugihara, N.; Furuno, K. Inhibitory effect of flavonoids on the efflux of N-acetyl 5-aminosalicylic acid intracellularly formed in Caco-2 cells. J. Biomed. Biotechnol. 2009, 2009, 467489. [Google Scholar]
- Kamishikiryo, J.; Matsumura, R.; Takamori, T.; Sugihara, N. Effect of quercetin on the transport of N-acetyl 5-aminosalicylic acid. J. Pharm. Pharmacol. 2013, 65, 1037–1043. [Google Scholar] [CrossRef]
- Vos, M.D.; Verdievel, H.; Schoonjans, R.; Beke, R.; Weerdt, G.A.D.; Barbier, F. High-performance liquid chromatographic assay for the determination of 5-aminosalicylic acid and acetyl-5-aminosalicylic acidconcentrations in endoscopic intestinal biopsy in humans. J. Chromatogr. 1991, 564, 296–302. [Google Scholar] [CrossRef]
- Fukuda, T.; Naganuma, M.; Takabayashi, K.; Hagihara, Y.; Tanemoto, S.; Nomura, E.; Yoshimatsu, Y.; Sugimoto, S.; Nanki, K.; Mizuno, S.; et al. Mucosal concentrations of N-acetyl-5-aminosalicylic acid related to endoscopic activity in ulcerative colitis patients with mesalamine. J. Gastroenterol. Hepatol. 2020, 35, 1878–1885. [Google Scholar] [CrossRef]
- Allgayer, H.; Sonnenbichler, J.; Kruis, W.; Paumgartner, G. Determination of the pK values of 5-aminosalicylic acid and N-acetylaminosalicylic acid and comparison of the pH dependent lipid-water partition coefficients of sulphasalazine and its metabolites. Arzneimittelforschung 1985, 35, 1457–1459. [Google Scholar]
- French, D.L.; Mauger, J.W. Evaluation of the physicochemical properties and dissolution characteristics of mesalamine: Relevance to controlled intestinal drug delivery. Pharm. Res. 1993, 10, 1285–1290. [Google Scholar] [CrossRef]
- Goebell, H.; Klotz, U.; Nehlsen, B.; Layer, P. Oroileal transit of slow release 5-aminosalicylic acid. Gut 1993, 34, 669–675. [Google Scholar] [CrossRef]
- Japanese Standard Product Classification Number 872399; Interview Form of PENTASA Tablets 250 mg, 500 mg. PENTASA Granules 94%. 2024 (May) Revision. Kyorin Pharmaceutical Co., Ltd.: Tokyo, Japan, 2024. Available online: https://www.pmda.go.jp/PmdaSearch/iyakuSearch (accessed on 15 May 2024).
- Merchant, H.A.; Afonso-Pereira, F.; Rabbie, S.C.; Youssef, S.A.; Basit, A.W. Gastrointestinal characterization and drug solubility determination in animals. J. Pharm. Pharmacol. 2015, 67, 630–639. [Google Scholar] [CrossRef]
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Baker, J.R.; Fioritto, A.F.; Wang, Y.; Luo, R.; Li, S.; Wen, B.; Bly, M.; Tsume, Y.; Koenigsknecht, M.J.; et al. Measurement of in vivo gastrointestinal release and dissolution of three locally acting mesalamine formulations in regions of the human gastrointestinal tract. Mol. Pharm. 2017, 14, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Hens, B.; Tsume, Y.; Bermejo, M.; Paixao, P.; Koenigsknecht, M.J.; Baker, J.R.; Hasler, W.L.; Lionberger, R.; Fan, J.; Dickens, J.; et al. Low Buffer Capacity and Alternating Motility along the Human Gastrointestinal Tract: Implications for in Vivo Dissolution and Absorption of Ionizable Drugs. Mol. Pharm. 2017, 14, 4281–4294. [Google Scholar] [CrossRef] [PubMed]
- Mennah-Govela, Y.A.; Bornhorst, G.M. Food buffering capacity: Quantification methods and its importance in digestion and health. Food Funct. 2021, 12, 543–563. [Google Scholar] [CrossRef]
- Fadda, H.M.; Sousa, T.; Carlsson, A.S.; Abrahamsson, B.; Williams, J.G.; Kumar, D.; Basit, A.W. Drug solubility in luminal fluids from different regions of the small and large intestine of humans. Mol. Pharm. 2010, 7, 31527–31532. [Google Scholar] [CrossRef]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef]
- Grimm, M.; Koziolek, M.; Kühn, J.P.; Weitschies, W. Interindividual and intraindividual variability of fasted state gastric fluid volume and gastric emptying of water. Eur. J. Pharm. Biopharm. 2018, 127, 309–317. [Google Scholar] [CrossRef]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic resonance imaging quantification of fasted state colonic liquid pockets in healthy humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef]
- De Vos, M. Clinical pharmacokinetics of slow release mesalazine. Clin. Pharmacokinet. 2000, 39, 85–97. [Google Scholar] [CrossRef]
- Schreiber, S.; Kamm, M.A.; Lichtenstein, G.R. Mesalamine with MMX technology for the treatment of ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 299–314. [Google Scholar] [CrossRef]
- Ogata, H.; Aoyama, N.; Mizushima, S.; Hagino, A.; Hibi, T. Comparison of efficacy of ultimatrix mesalazine 4.8 g/day once-daily with other high-dose mesalazine in active ulcerative colitis: A randomized, double-blind study. Intest. Res. 2017, 15, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kijima, A.; Masuo, Y.; Ishimoto, T.; Sugiura, T.; Takahashi, S.; Nakamichi, N.; Kato, Y. Gene ablation of carnitine/organic cation transporter 1 reduces gastrointestinal absorption of 5-aminosalicylate in mice. Biol. Pharm. Bull. 2015, 38, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Alcántara, V.; Montrose, M.H. Acute murine colitis reduces colonic 5-aminosalicylic acid metabolism by regulation of N-acetyltransferase-2. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G1002-10. [Google Scholar] [CrossRef] [PubMed]
- Hickman, D.; Pope, J.; Patil, S.D.; Fakis, G.; Smelt, V.; Stanley, L.A.; Payton, M.; Unadkat, J.D.; Sim, E. Expression of arylamine N-acetyltransferase in human intestine. Gut 1998, 42, 402–409. [Google Scholar] [CrossRef]
 30 min incubation,
30 min incubation,  12 h incubation with shaking.
12 h incubation with shaking.
 30 min incubation,
30 min incubation,  12 h incubation with shaking.
12 h incubation with shaking.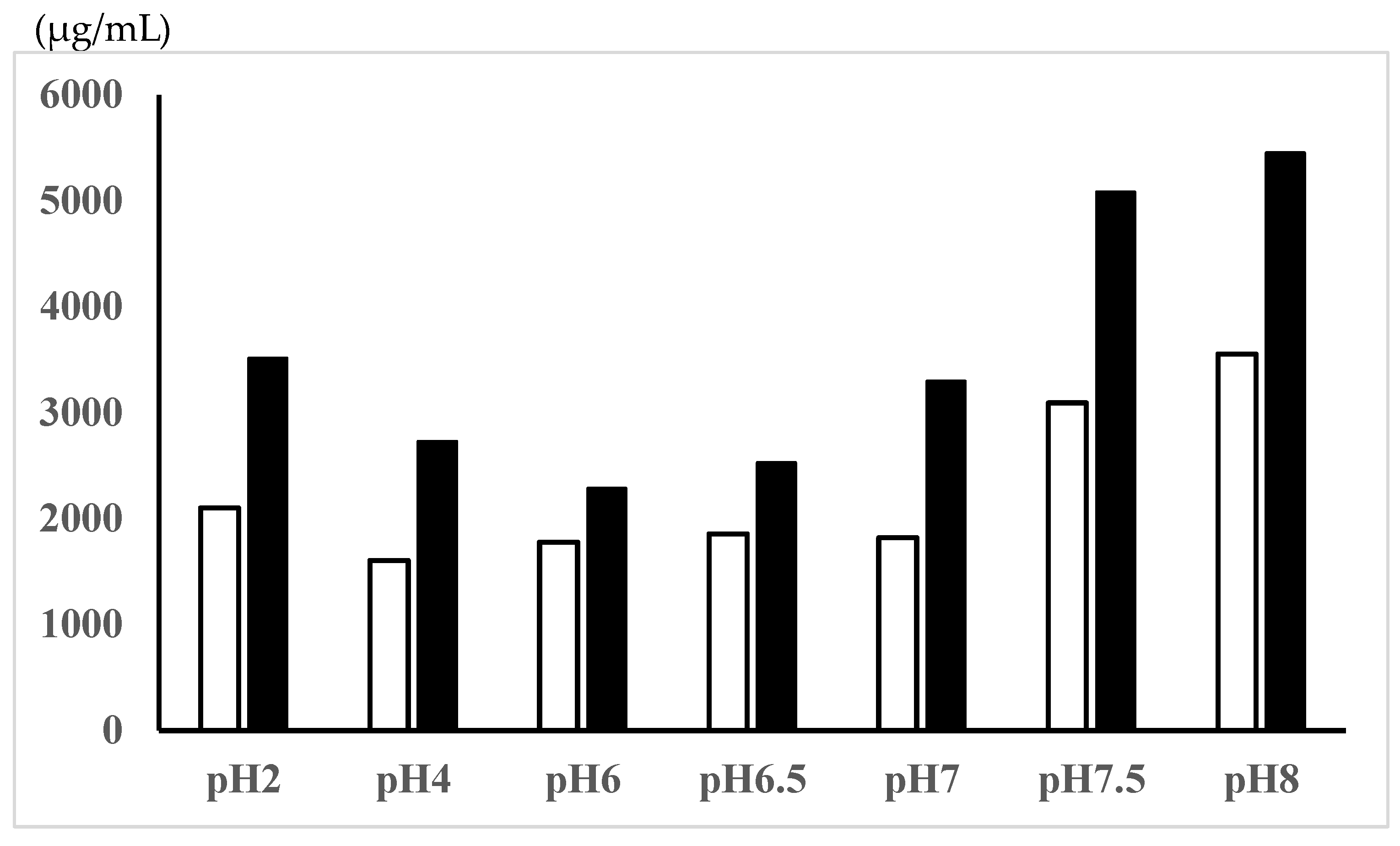
 ; concn. of AC-5-ASA,
; concn. of AC-5-ASA,  ; total (5-ASA + AC-5-ASA) concn.,
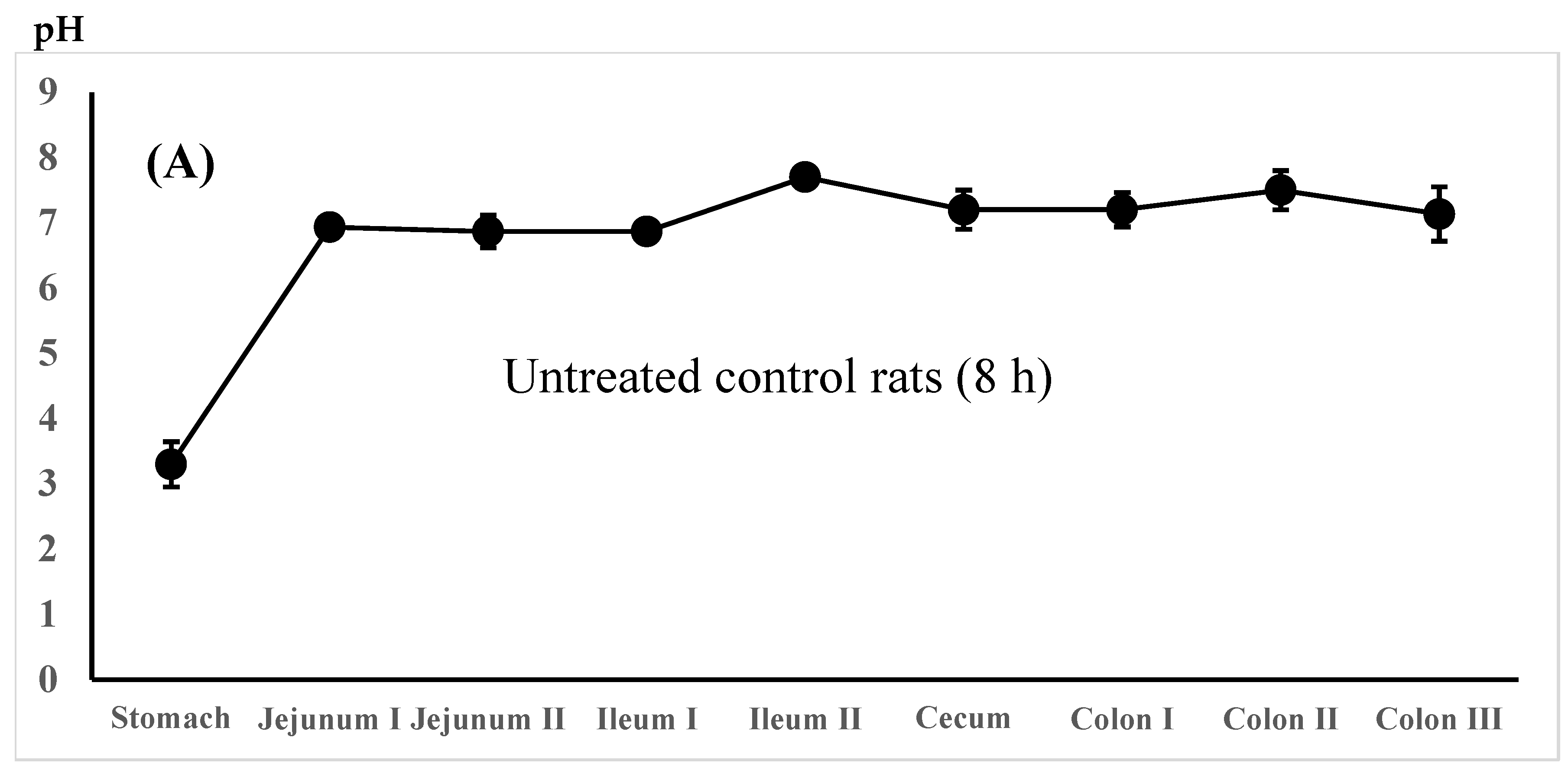
; total (5-ASA + AC-5-ASA) concn.,  . Regional luminal fluid pH along the GI tract 3 h after the administration of the 5-ASA suspension in water to the untreated control rats (C) and the 5-ASA solution in 5% NaHCO3 to the lansoprazole-pretreated rats (D) at a 30 mg/2.2 mL/kg dose. Each value represents the mean ± S.D. of 3 trials. There was no significant difference in each corresponding value between the untreated control and the lansoprazole-treated rats.
. Regional luminal fluid pH along the GI tract 3 h after the administration of the 5-ASA suspension in water to the untreated control rats (C) and the 5-ASA solution in 5% NaHCO3 to the lansoprazole-pretreated rats (D) at a 30 mg/2.2 mL/kg dose. Each value represents the mean ± S.D. of 3 trials. There was no significant difference in each corresponding value between the untreated control and the lansoprazole-treated rats.
 ; concn. of AC-5-ASA,
; concn. of AC-5-ASA,  ; total (5-ASA + AC-5-ASA) concn.,
; total (5-ASA + AC-5-ASA) concn.,  . Regional luminal fluid pH along the GI tract 3 h after the administration of the 5-ASA suspension in water to the untreated control rats (C) and the 5-ASA solution in 5% NaHCO3 to the lansoprazole-pretreated rats (D) at a 30 mg/2.2 mL/kg dose. Each value represents the mean ± S.D. of 3 trials. There was no significant difference in each corresponding value between the untreated control and the lansoprazole-treated rats.
. Regional luminal fluid pH along the GI tract 3 h after the administration of the 5-ASA suspension in water to the untreated control rats (C) and the 5-ASA solution in 5% NaHCO3 to the lansoprazole-pretreated rats (D) at a 30 mg/2.2 mL/kg dose. Each value represents the mean ± S.D. of 3 trials. There was no significant difference in each corresponding value between the untreated control and the lansoprazole-treated rats.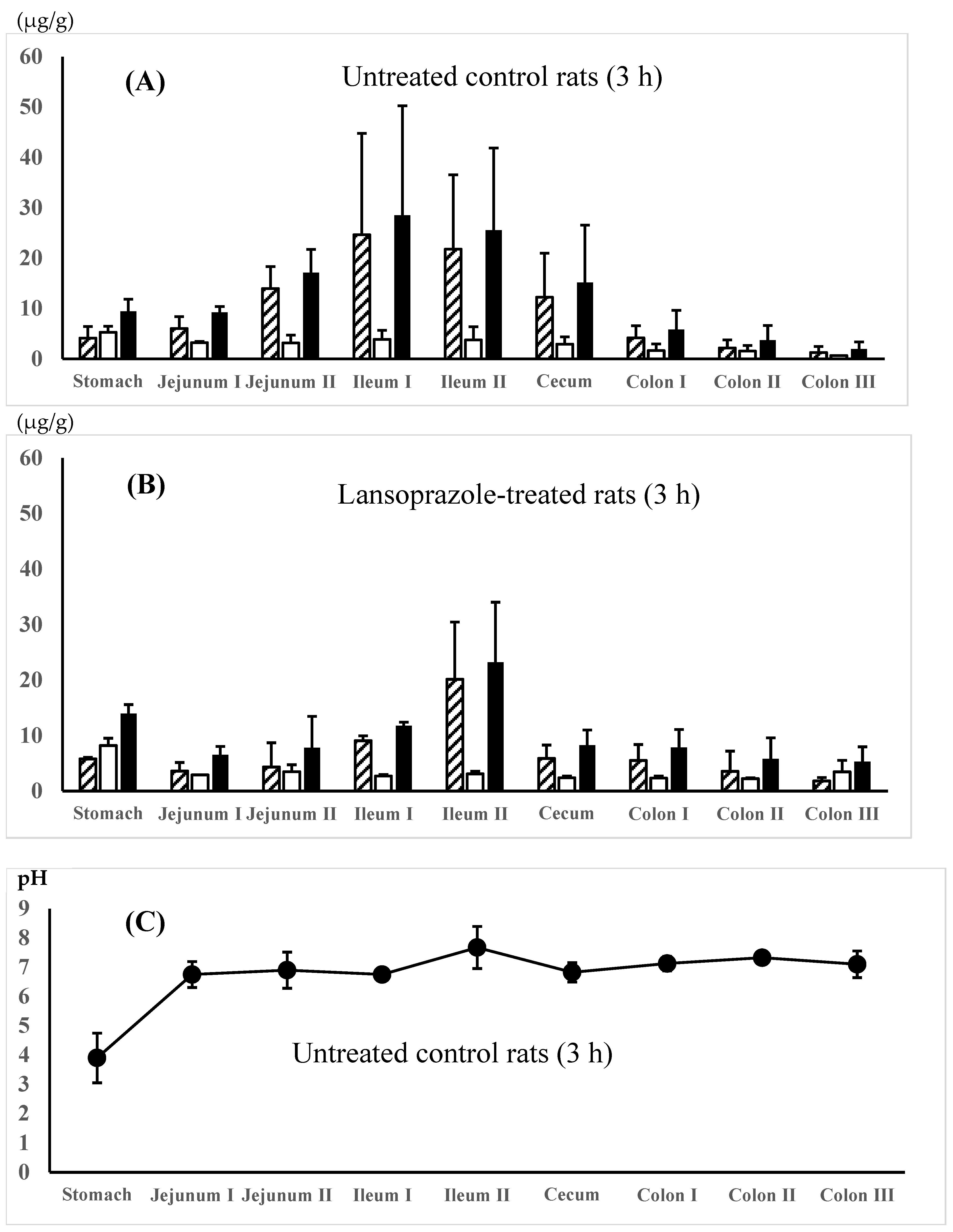

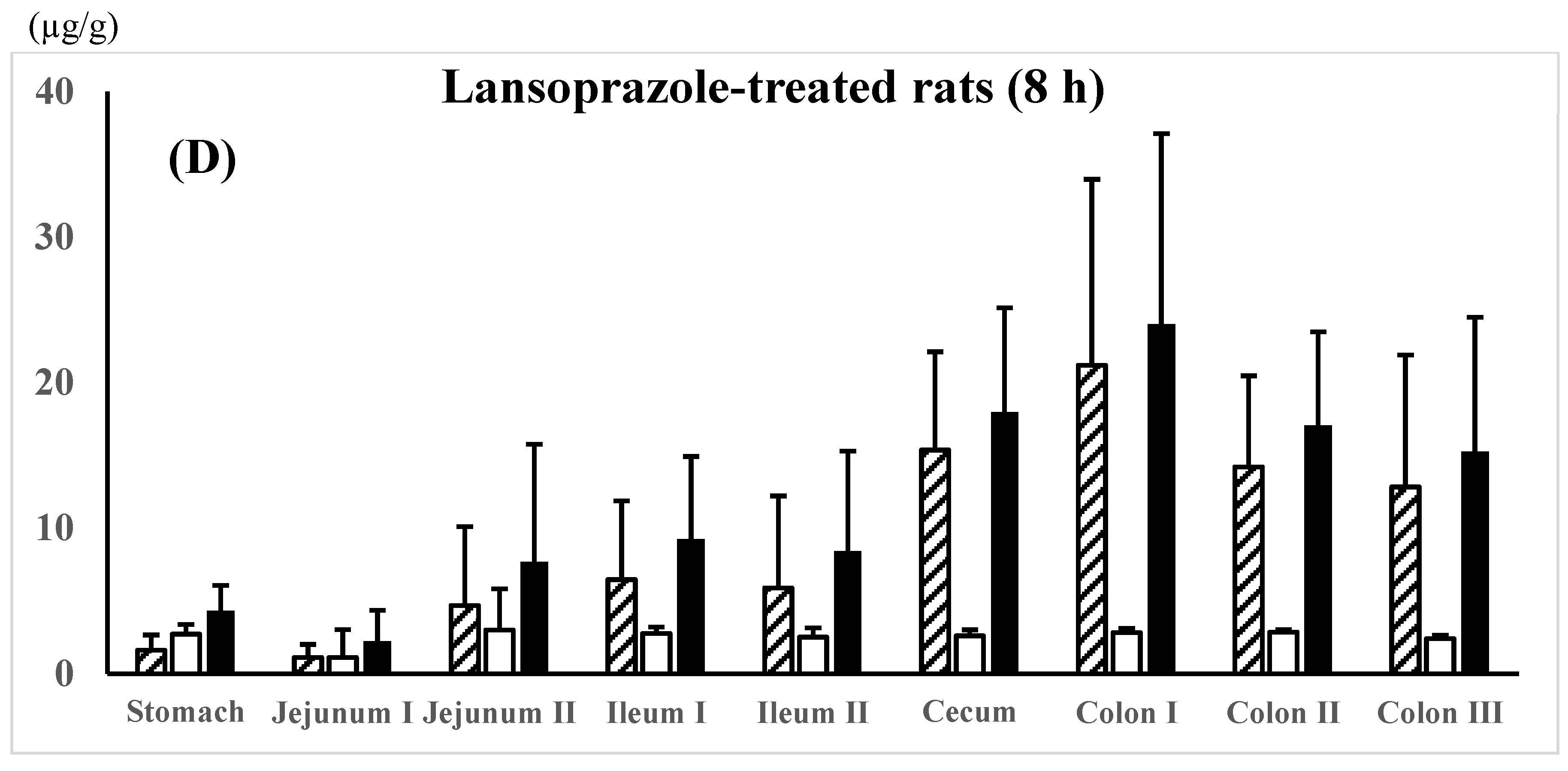
 ; AC-5-ASA concn.,
; AC-5-ASA concn.,  ; total (5-ASA + AC-5-ASA) concn.,
; total (5-ASA + AC-5-ASA) concn.,  . Each value represents the mean ± S.D. of three trials. There was no significant difference in each corresponding value between the different dosing preparations.
. Each value represents the mean ± S.D. of three trials. There was no significant difference in each corresponding value between the different dosing preparations.
 ; AC-5-ASA concn.,
; AC-5-ASA concn.,  ; total (5-ASA + AC-5-ASA) concn.,
; total (5-ASA + AC-5-ASA) concn.,  . Each value represents the mean ± S.D. of three trials. There was no significant difference in each corresponding value between the different dosing preparations.
. Each value represents the mean ± S.D. of three trials. There was no significant difference in each corresponding value between the different dosing preparations.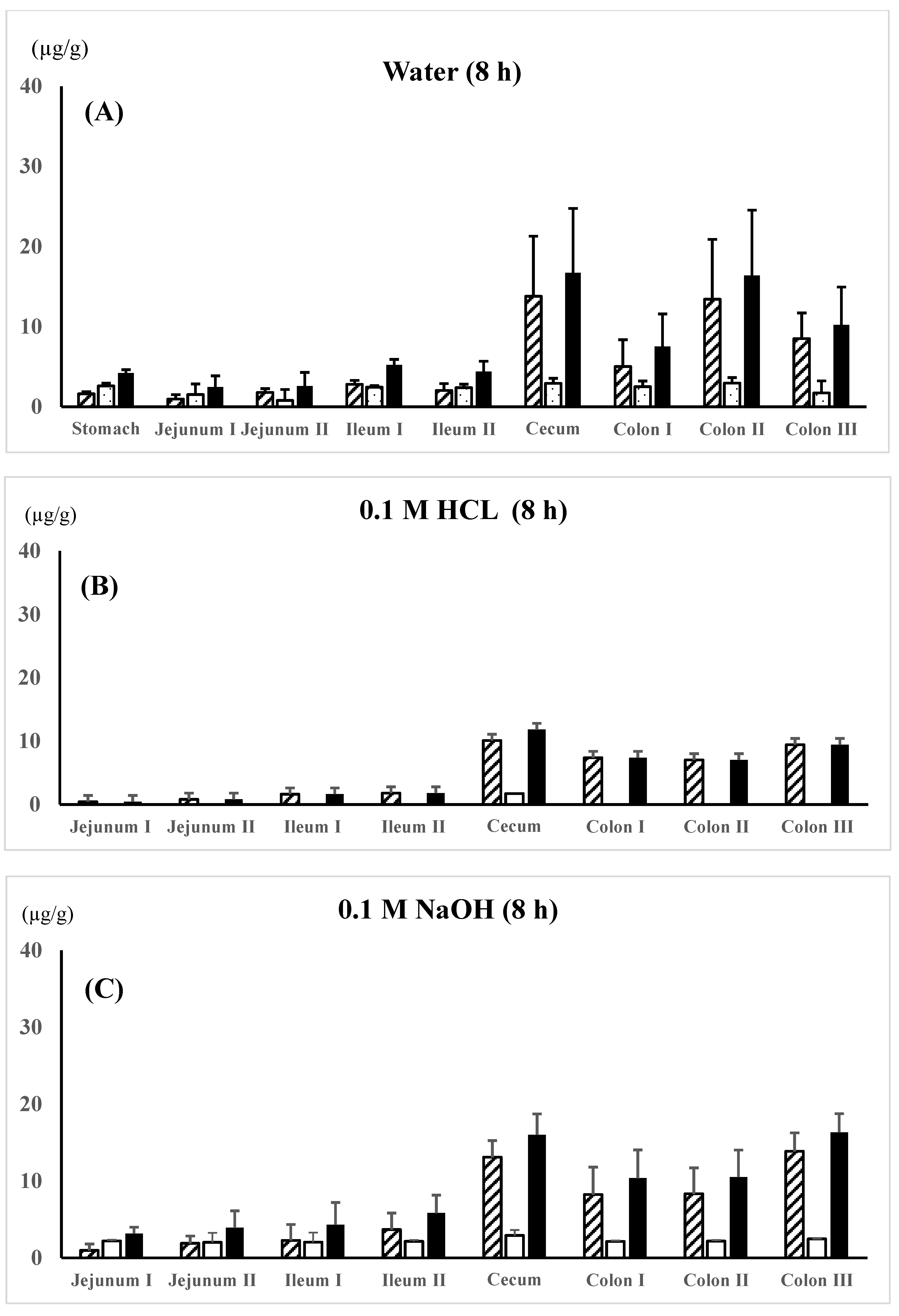

| Vehicle | Solubility (mg/mL) | Dissolved Fraction (%) | Solid Fraction (%) |
|---|---|---|---|
| Water | 1.6 | 11.7 | 88.3 |
| 0.1 M HCl | 3.9 | 28.6 | 71.4 |
| 0.1 M NaOH | 13.1 | 96.1 | 3.9 |
| 5% NaHCO3 | 58.3 | 100 | 0 |
| 5-ASA Suspension in Water | 5-ASA Solution in 5%NaHCO3 | |||||
|---|---|---|---|---|---|---|
| (Untreated Control Rats) | (Lansoprazole-Treated Rats) | |||||
| 5-ASA | AC-5-ASA | Total | 5-ASA | AC-5-ASA | Total | |
| Concentration in plasma (µg/mL) | ||||||
| At 0.5 h | 5.3 ± 4.3 | 12.0 ± 3.1 | 17.2 ± 7.2 | 6.2 ± 2.9 | 9.4 ± 1.9 | 15.6 ± 4.8 |
| At 3 h | 0.3 ± 0.0 | 1.9 ± 0.7 | 2.1 ± 0.8 | 0.4 ± 0.1 | 2.0 ± 0.4 | 2.4 ± 0.4 |
| At 8 h | 0.3 ± 0.3 | 0.7 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.0 | 0.8 ± 0.4 | 1.3 ± 0.4 |
| Concentration in urine (µg/mL) | ||||||
| At 0.5 h | 40.6 ± 28.6 | 1028.5 ± 642.7 | 1069.1 ± 669.9 | 21.3 ± 6.2 | 824.9 ± 196.5 | 846.2 ± 195.7 |
| At 3 h | 4.8 ± 2.8 | 443.6 ± 0.7 | 448.3 ± 169.5 | 8.2 ± 7.5 | 522.8 ± 86.9 | 531.0 ± 86.0 |
| At 8 h | 6.6 ± 6.7 | 320.4 ± 174.3 | 327.0 ± 180.8 | 3.2 ± 0.7 | 320.1 ± 66.9 | 323.4 ± 67.5 |
| Concentration in GI membrane at 3 h (µg/g) | ||||||
| Small intestine | 17.5 ± 3.6 | 69.1 ± 32.1 | 86.6 ± 31.5 | 19.4 ± 2.5 | 38.7 ± 9.2 | 58.1 ± 9.9 |
| Large intestine | 6.8 ± 6.1 | 19.8 ± 13.3 | 26.6 ± 19.3 | 7.8 ± 5.0 | 16.7 ± 6.8 | 24.5 ± 10.0 |
| Whole GI tract | 24.3 ± 9.7 | 89.0 ± 26.9 | 113.2 ± 29.5 | 27.2 ± 6.4 | 55.4 ± 14.5 | 82.1 ± 18.8 |
| Concentration in GI membrane at 8 h (µg/g) | ||||||
| Small intestine | 9.7 ± 2.2 | 9.2 ± 1.6 | 18.9 ± 2.3 | 12.1 ± 4.8 | 19.8 ± 14.9 | 31.9 ± 19.4 |
| Large intestine | 10.1 ± 3.1 | 40.7 ± 18.8 | 50.9 ± 21.9 | 10.7 ± 0.9 | 63.6 ± 30.4 | 74.3 ± 31.3 |
| Whole GI tract | 19.9 ± 3.9 | 49.9 ± 17.5 | 64.1 ± 20.3 | 22.9 ± 5.6 | 83.4 ± 41.1 | 106.3 ± 46.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, Y.; Goto, Y.; Ohnishi, F.; Koga, S.; Kawano, S.; Hieda, Y.; Goromaru, T.; Murakami, T. 5-Aminosalicylic Acid Distribution into the Intestinal Membrane Along the Gastrointestinal Tract After Oral Administration in Rats. Pharmaceutics 2024, 16, 1567. https://doi.org/10.3390/pharmaceutics16121567
Maeda Y, Goto Y, Ohnishi F, Koga S, Kawano S, Hieda Y, Goromaru T, Murakami T. 5-Aminosalicylic Acid Distribution into the Intestinal Membrane Along the Gastrointestinal Tract After Oral Administration in Rats. Pharmaceutics. 2024; 16(12):1567. https://doi.org/10.3390/pharmaceutics16121567
Chicago/Turabian StyleMaeda, Yorinobu, Yuta Goto, Fumiya Ohnishi, Syoutarou Koga, Satoshi Kawano, Yuhzo Hieda, Takeshi Goromaru, and Teruo Murakami. 2024. "5-Aminosalicylic Acid Distribution into the Intestinal Membrane Along the Gastrointestinal Tract After Oral Administration in Rats" Pharmaceutics 16, no. 12: 1567. https://doi.org/10.3390/pharmaceutics16121567
APA StyleMaeda, Y., Goto, Y., Ohnishi, F., Koga, S., Kawano, S., Hieda, Y., Goromaru, T., & Murakami, T. (2024). 5-Aminosalicylic Acid Distribution into the Intestinal Membrane Along the Gastrointestinal Tract After Oral Administration in Rats. Pharmaceutics, 16(12), 1567. https://doi.org/10.3390/pharmaceutics16121567






