Model-Based Dose Selection of a Sphingosine-1-Phosphate Modulator, Etrasimod, in Patients with Various Degrees of Hepatic Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Software and Information Resources
2.2. Model Development
| Parameter | Unit | Input Value | Reported Value | Reference |
|---|---|---|---|---|
| Physicochemical properties | ||||
| Molecular weight | g/mol | 457.493 | 457.493 | [24] |
| Effective molecular weight | g/mol | 406.5 | PK-Sim | |
| Lipophilicity | Log | 5.73 | 5.73, 6.45 | [24] |
| pKa | 4.26 | 4.26 | [24] | |
| Solubility | mg/mL | 0.000477 | 0.000477 | [25] |
| Plasma protein binding | % | 97.9 | 97.9 | [25] |
| Absorption | ||||
| Intestinal permeability | cm/min | 0.02 | Calculated | |
| Dissolution model | Weibull | PK-Sim | ||
| Dissolution time (50% dissolved) | min | 120 | Optimized | |
| Distribution | ||||
| Partition coefficient model | Rodger and Roland | PK-Sim | ||
| Cellular permeability model | PK-Sim | PK-Sim | ||
| Fraction unbound | % | 2.10 | [25] | |
| Blood to plasma ratio | 0.66 | 0.66 | [16] | |
| Enzymatic biotransformation Process type: In vitro metabolic rate in presence of recombinant enzymes | ||||
| CYP2C8 | µL/min/pmol | 0.01157 | [16] | |
| CYP2C9 | µL/min/pmol | 0.08525 | [16] | |
| CYP3A4 | µL/min/pmol | 0.01640 | [16] | |
| CYP2J2 | µL/min/pmol | 0.01872 | [16] | |
| CYP2C19 | µL/min/pmol | 0.01331 | [16] | |
| Parameterization of enzyme inhibition process | ||||
| Ki for fluconazole-CYP3A4 complex | µmol/L | 10.7 | [32] | |
| Ki for fluconazole-CYP2C9 complex | µmol/L | 19.60 | [33] | |
| Ki for fluconazole-CYP2C19 complex | µmol/L | 1.74 | [33] | |
| Parameterization of enzyme induction process | ||||
| EC50 for rifampicin-CYP3A4 complex | µmol/L | 0.34 | 0.34 | [34] |
| Emax for rifampicin-CYP3A4 complex | 9 | 9 | [34] | |
| EC50 for rifampicin-CYP2C8 complex | µmol/L | 0.34 | 0.34 | [35] |
| Emax for rifampicin-CYP2C8 complex | 3.2 | 3.2 | [35] | |
2.3. Etrasimod PBPK Model Development and Verification in Adult Healthy Population
2.4. Modeling the Effect of Drug Interactions on the Etrasimod Pharmacokinetic
2.5. Modeling the Effect of Hepatic Impairment on the Etrasimod Pharmacokinetic
| Model Parameter | Severity of Liver Disease | ||
|---|---|---|---|
| Child–Pugh A | Child–Pugh B | Child–Pugh C | |
| Portal vein blood flow a | 0.40 | 0.36 | 0.04 |
| Hepatic arterial blood flow a | 1.3 | 2.3 | 3.4 |
| Renal blood flow a | 0.88 | 0.65 | 0.48 |
| Cardiac index a | 1.11 | 1.27 | 1.36 |
| Blood flow in other organs a | 1.75 | 2.25 | 2.75 |
| Albumin a | 0.81 | 0.68 | 0.50 |
| Alpha-1-acid glycoprotein a | 0.60 | 0.56 | 0.30 |
| Hematocrit a | 0.39 | 0.37 | 0.35 |
| Functional liver mass a | 0.69 | 0.55 | 0.28 |
| GFR a | 1 | 0.70 | 0.36 |
| CYP3A4 activity b | 1 | 0.40 | 0.40 |
| CYP2J2 activity b | 1 | 1 | 1 |
| CYP2C8 activity c | 0.69 | 0.52 | 0.33 |
| CYP2C9 activity c | 0.69 | 0.52 | 0.33 |
| CYP2C19 activity c | 0.32 | 0.26 | 0.12 |
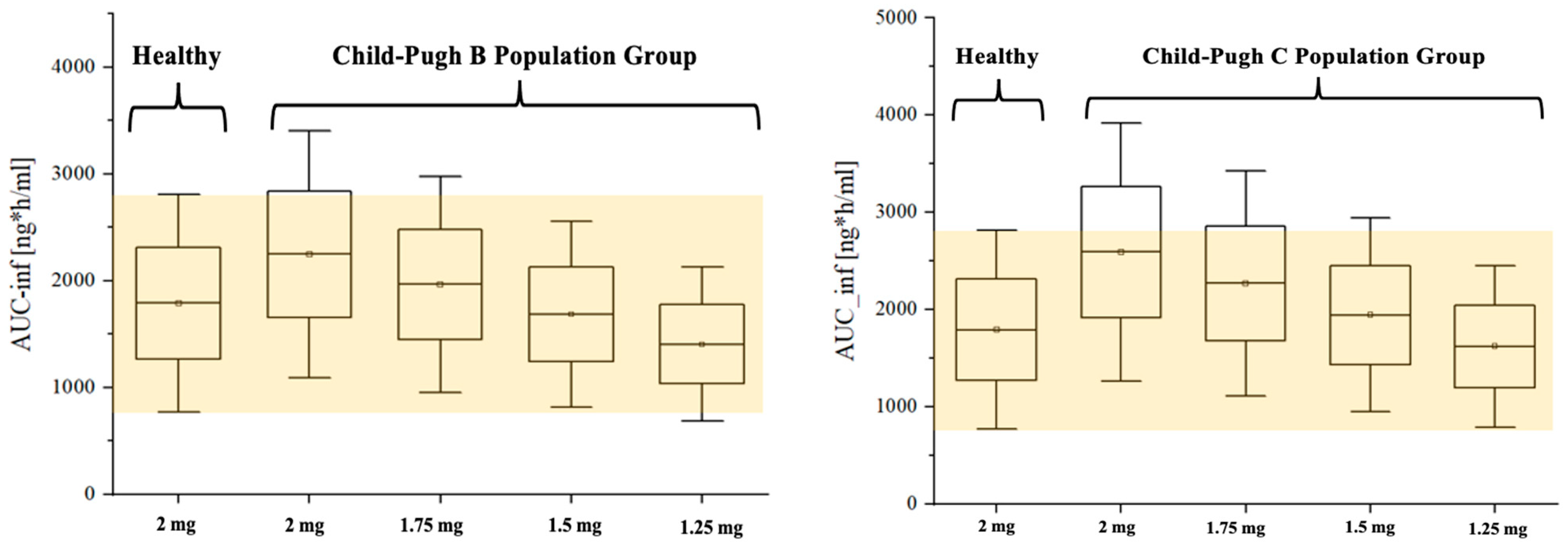
2.6. Dosing Adjustment of Etrasimod Based on the Severity of the Liver Impairment
3. Results
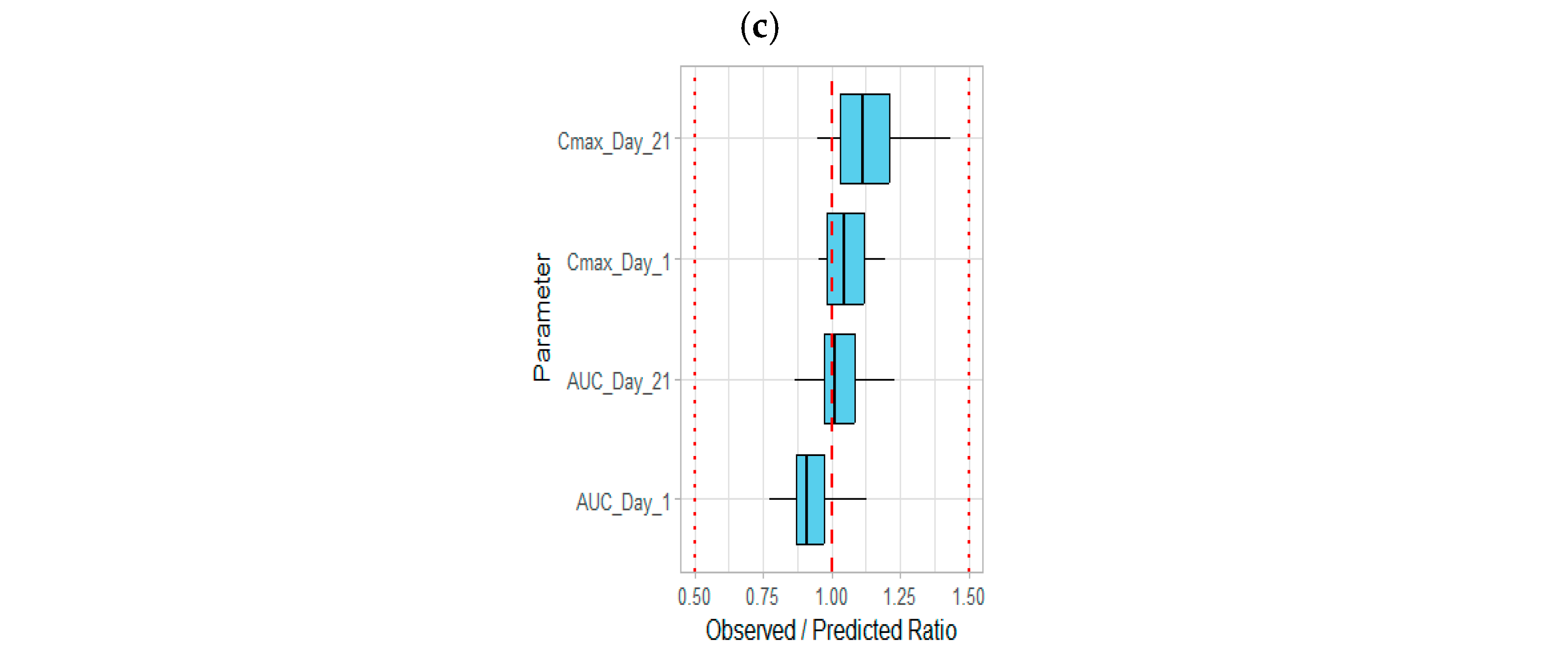
3.1. Development of the Etrasimod PBPK Model in an Adult Healthy Population
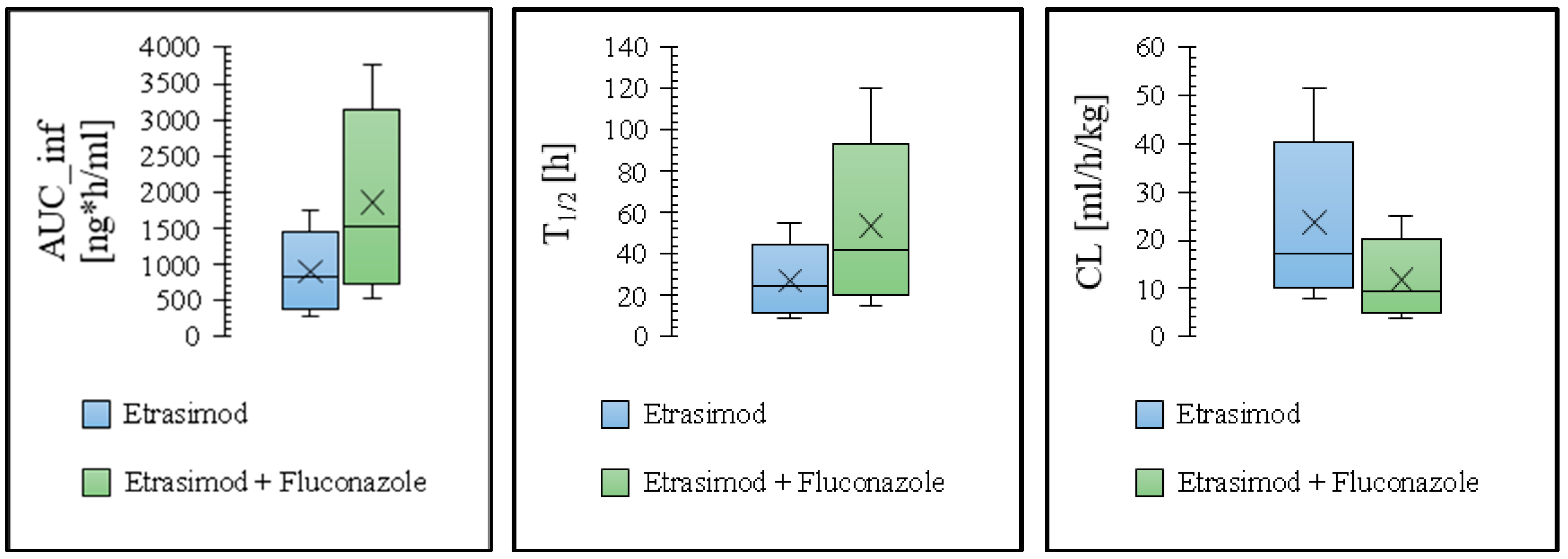
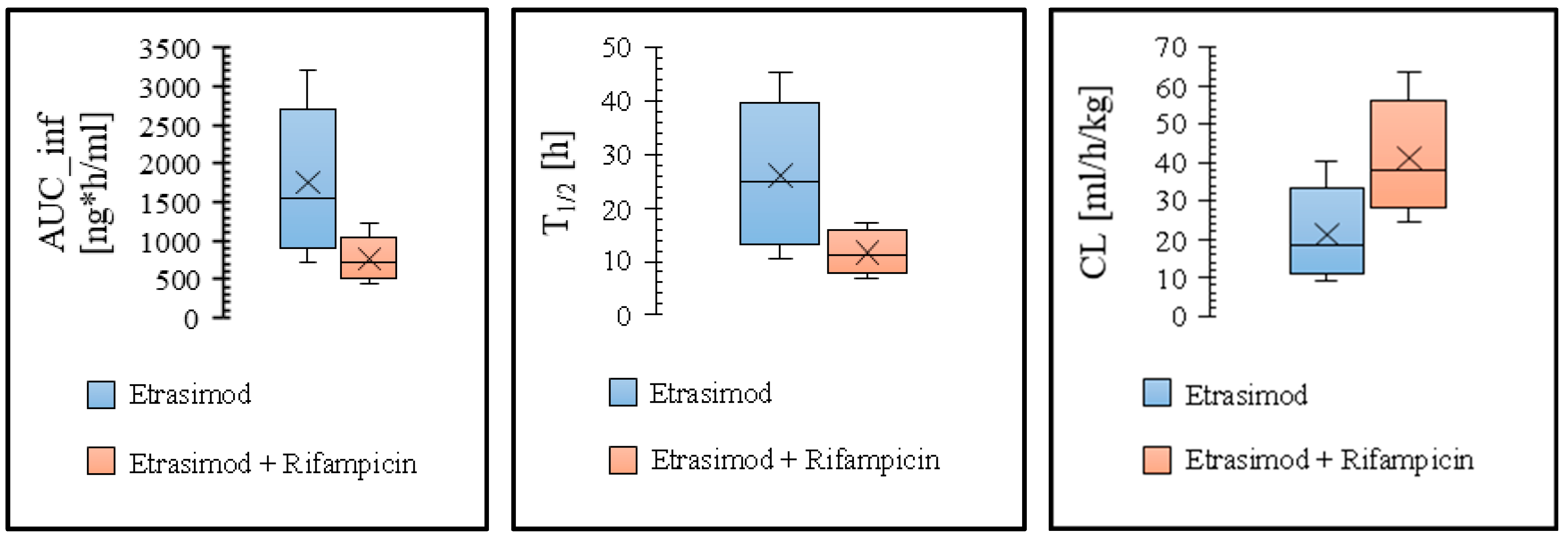
3.2. Modeling the Effect of Drug Interaction on the Etrasimod Exposure
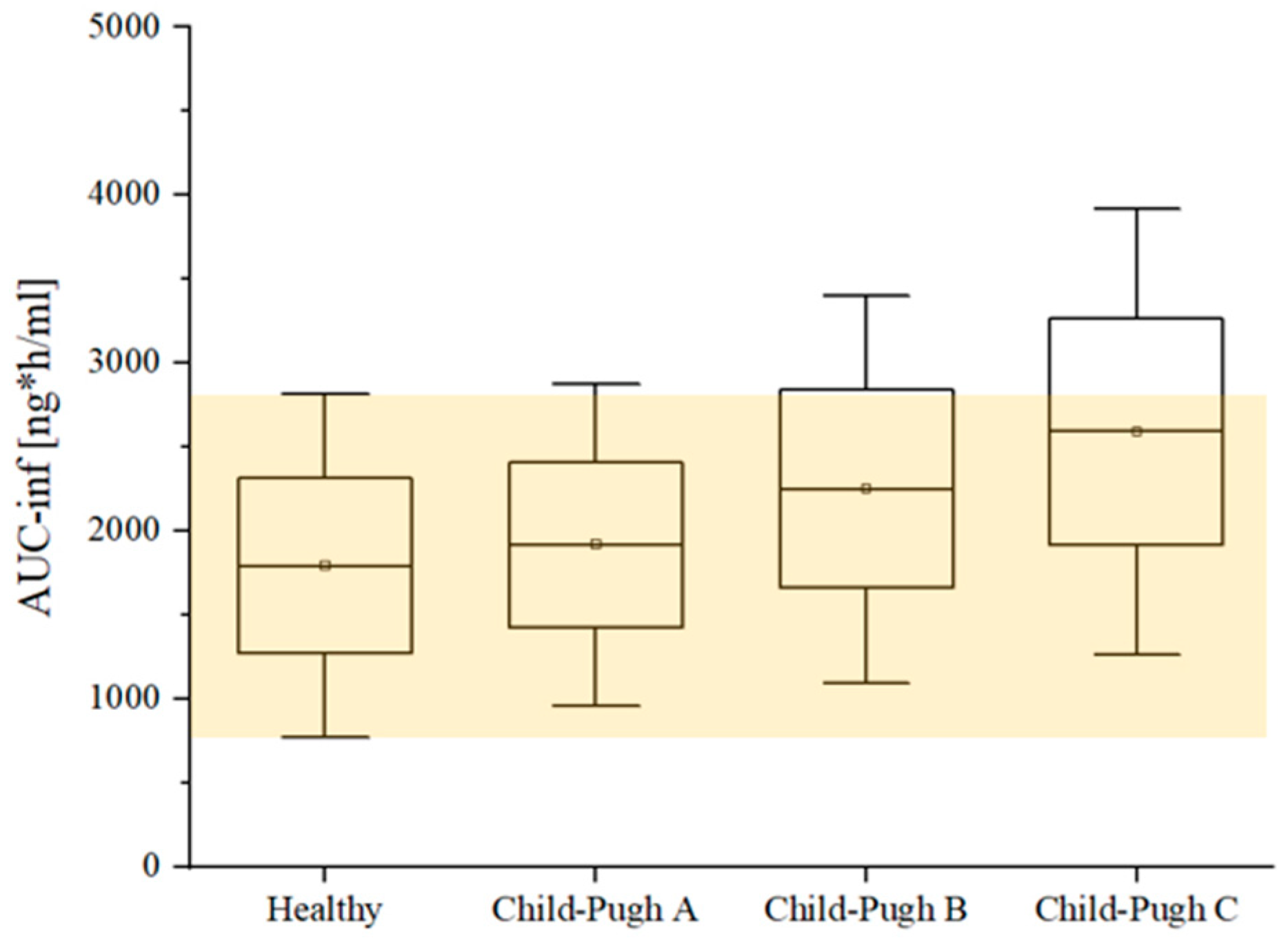
3.3. Modeling the Effect of Hepatic Impairment on the Etrasimod Pharmacokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gowerrousseau, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794.e4. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Loftus, E.V.; Isaacs, K.L.; Regueiro, M.D.; Gerson, L.B.; Sands, B.E. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am. J. Gastroenterol. 2018, 113, 481–517. [Google Scholar] [CrossRef]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Choy, M.C.; Visvanathan, K.; De Cruz, P. An Overview of the Innate and Adaptive Immune System in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Gersemann, M.; Wehkamp, J.; Stange, E.F. Innate Immune Dysfunction in Inflammatory Bowel Disease. J. Intern. Med. 2012, 271, 421–428. [Google Scholar] [CrossRef]
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Singh, S.; Allegretti, J.R.; Siddique, S.M.; Terdiman, J.P. AGA Technical Review on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1465–1496.e17. [Google Scholar] [CrossRef]
- Bryan, A.M.; Del Poeta, M. Sphingosine-1-Phosphate Receptors and Innate Immunity. Cell. Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA) VELSIPITYTM (Etrasimod) Tablets. 2023. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216956s000lbl.pdf (accessed on 22 November 2024).
- Vermeire, S.; Chiorean, M.; Panés, J.; Peyrin-Biroulet, L.; Zhang, J.; Sands, B.E.; Lazin, K.; Klassen, P.; Naik, S.U.; Cabell, C.H.; et al. Long-Term Safety and Efficacy of Etrasimod for Ulcerative Colitis: Results from the Open-Label Extension of the OASIS Study. J. Crohns Colitis 2021, 15, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as Induction and Maintenance Therapy for Ulcerative Colitis (ELEVATE): Two Randomised, Double-Blind, Placebo-Controlled, Phase 3 Studies. Lancet 2023, 401, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef]
- Gilardi, D.; Gabbiadini, R.; Allocca, M.; Correale, C.; Fiorino, G.; Furfaro, F.; Zilli, A.; Peyrin-Biroulet, L.; Danese, S. PK, PD, and Interactions: The New Scenario with JAK Inhibitors and S1P Receptor Modulators, Two Classes of Small Molecule Drugs, in IBD. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 797–806. [Google Scholar] [CrossRef]
- Lee, C.A.; Oh, D.A.; Tang, Y.; Yi, P.; Bashir, M.; English, S.; Croft, M.; Blackburn, A.; Bloom, S.; Gilder, K.; et al. Disposition and Mass Balance of Etrasimod in Healthy Subjects and In Vitro Determination of the Enzymes Responsible for Its Oxidative Metabolism. Clin. Pharmacol. Drug Dev. 2023, 12, 553–571. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Draft Guidance for Industry. In Vitro Metabolism and Transporter Mediated Drug-Drug Interactions. 2017. Available online: https://www.fda.gov/files/drugs/published/In-Vitro-Metabolism--and-Transporter--Mediated-Drug-Drug-Interaction-Studies-Guidance-for-Industry.pdf (accessed on 22 November 2024).
- Verbeeck, R.K. Pharmacokinetics and Dosage Adjustment in Patients with Hepatic Dysfunction. Eur. J. Clin. Pharmacol. 2008, 64, 1147–1161. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.; Unadkat, J.D.; Zhang, X.; Wu, D.; Heimbach, T. Applications, Challenges, and Outlook for PBPK Modeling and Simulation: A Regulatory, Industrial and Academic Perspective. Pharm. Res. 2022, 39, 1701–1731. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Grimstein, M.; Fan, J.; Grillo, J.A.; Huang, S.M.; Zhu, H.; Wang, Y. Application of PBPK Modeling and Simulation for Regulatory Decision Making and Its Impact on US Prescribing Information: An Update on the 2018–2019 Submissions to the US FDA’s Office of Clinical Pharmacology. J. Clin. Pharmacol. 2020, 60, S160–S178. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, T.; Chen, Y.; Chen, J.; Dixit, V.; Parrott, N.; Peters, S.A.; Poggesi, I.; Sharma, P.; Snoeys, J.; Shebley, M.; et al. Physiologically-Based Pharmacokinetic Modeling in Renal and Hepatic Impairment Populations: A Pharmaceutical Industry Perspective. Clin. Pharmacol. Ther. 2021, 110, 297–310. [Google Scholar] [CrossRef]
- Kuepfer, L.; Niederalt, C.; Wendl, T.; Schlender, J.F.; Willmann, S.; Lippert, J.; Block, M.; Eissing, T.; Teutonico, D. Applied Concepts in PBPK Modeling: How to Build a PBPK/PD Model. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 516–531. [Google Scholar] [CrossRef]
- Wojtyniak, J.G.; Britz, H.; Selzer, D.; Schwab, M.; Lehr, T. Data Digitizing: Accurate and Precise Data Extraction for Quantitative Systems Pharmacology and Physiologically-Based Pharmacokinetic Modeling. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.A.; Ramaiya, A.; Tang, Y.; Sapone, A.; Gilder, K.; Randle, A.; Grundy, J.S. S959 A Phase 1 Drug Interaction Study Evaluating the Effects of Itraconazole on the Pharmacokinetics, Safety, and Tolerability of Etrasimod in Healthy Volunteers. Am. J. Gastroenterol. 2022, 117, e694–e695. [Google Scholar] [CrossRef]
- Lee, C.A.; Tang, Y.; Villa-Caballero, L.; Shan, K.; Randle, A.; Grundy, J.S. S774 Pharmacokinetics, Safety, and Tolerability of Etrasimod: Results From a Phase 1 Drug-Drug Interaction Study in Healthy Volunteers. Am. J. Gastroenterol. 2022, 117, e550. [Google Scholar] [CrossRef]
- Lee, C.A.; Schreiber, S.; Bressler, B.; Adams, J.W.; Oh, D.A.; Tang, Y.Q.; Zhang, J.; Komori, H.K.; Grundy, J.S. Safety, Pharmacokinetics, and Pharmacodynamics of Etrasimod: Single and Multiple Ascending Dose Studies in Healthy Adults. Clin. Pharmacol. Drug Dev. 2024, 13, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Darpo, B.; Connor, K.; Cabell, C.H.; Grundy, J.S. Cardiovascular Evaluation of Etrasimod, a Selective Sphingosine 1-Phosphate Receptor Modulator, in Healthy Adults: Results of a Randomized, Thorough QT/QTc Study. Clin. Pharmacol. Drug Dev. 2024, 13, 326–340. [Google Scholar] [CrossRef]
- Thelen, K.; Coboeken, K.; Willmann, S.; Dressman, J.B.; Lippert, J. Evolution of a Detailed Physiological Model to Simulate the Gastrointestinal Transit and Absorption Process in Humans, Part II: Extension to Describe Performance of Solid Dosage Forms. J. Pharm. Sci. 2012, 101, 1267–1280. [Google Scholar] [CrossRef]
- Thelen, K.; Coboeken, K.; Willmann, S.; Burghaus, R.; Dressman, J.B.; Lippert, J. Evolution of a Detailed Physiological Model to Simulate the Gastrointestinal Transit and Absorption Process in Humans, Part 1: Oral Solutions. J. Pharm. Sci. 2011, 100, 5324–5345. [Google Scholar] [CrossRef]
- Niwa, T.; Shiraga, T.; Takagia, A. Effect of Antifungal Drugs on Cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 Activities in Human Liver Microsomes. Biol. Pharm. Bull. 2005, 28, 1805–1808. [Google Scholar] [CrossRef]
- Kunze, K.L.; Wienkers, L.C.; Thummel, K.E.; Trager, W.F. Warfarin-Fluconazole I-Inhibition of the Human Cytochrome P450-Dependent Metabolism of Warfarin by Fluconazole: In Vitro Studies. Drug Metab. Dispos. 1996, 24, 414–421. [Google Scholar]
- Templeton, I.E.; Houston, J.B.; Galetin, A. Predictive Utility of in Vitro Rifampin Induction Data Generated in Fresh and Cryopreserved Human Hepatocytes, Fa2N-4,and HepaRG Cells. Drug Metab. Dispos. 2011, 39, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.B.; Wiegand, C.; Prentiss, P.L.; Fahmi, O.A. Time-Course of Cytochrome P450 (CYP450) Induction in Cultured Human Hepatocytes: Evaluation of Activity and MRNA Expression Profiles for Six Inducible CYP450 Enzymes [poster no. P186]. In Proceedings of the 10th International ISSX Meeting, Toronto, ON, Canada, 29 September–3 October 2013; p. P186. [Google Scholar]
- Jones, H.M.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.A.; Snoeys, J.; Upreti, V.V.; Zheng, M.; Hall, S.D. Physiologically Based Pharmacokinetic Modeling in Drug Discovery and Development: A Pharmaceutical Industry Perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Sager, J.E.; Yu, J.; Ragueneau-Majlessi, I.; Isoherranen, N. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification. Drug Metab. Dispos. 2015, 43, 1823–1837. [Google Scholar] [CrossRef]
- Lippert, J.; Burghaus, R.; Edginton, A.; Frechen, S.; Karlsson, M.; Kovar, A.; Lehr, T.; Milligan, P.; Nock, V.; Ramusovic, S.; et al. Open Systems Pharmacology Community—An Open Access, Open Source, Open Science Approach to Modeling and Simulation in Pharmaceutical Sciences. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 878–882. [Google Scholar] [CrossRef]
- Frechen, S.; Solodenko, J.; Wendl, T.; Dallmann, A.; Ince, I.; Lehr, T.; Lippert, J.; Burghaus, R. A Generic Framework for the Physiologically-based Pharmacokinetic Platform Qualification of PK-Sim and Its Application to Predicting Cytochrome P450 3A4–Mediated Drug–Drug Interactions. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 633–644. [Google Scholar] [CrossRef]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A Semi-Mechanistic Model to Predict the Effects of Liver Cirrhosis on Drug Clearance. Clin. Pharmacokinet. 2010, 49, 189–206. [Google Scholar] [CrossRef]
- Edginton, A.N.; Willmann, S. Physiology-Based Simulations of a Pathological Condition: Prediction of Pharmacokinetics in Patients with Liver Cirrhosis. Clin. Pharmacokinet. 2008, 47, 743–752. [Google Scholar] [CrossRef]
- Lee, C.; Tang, Y.; Schroeder, C.; Zhang, J.; Nguyen-Cleary, T.; Ma, M.; Lyon, N.; Grundy, J.S. S720 Pharmacokinetics (PK), Safety, and Tolerability of Etrasimod (APD334) in Participants With Mild, Moderate, or Severe Hepatic Impairment: A Single-Dose, Open-Label, Parallel-Group Study. Am. J. Gastroenterol. 2021, 116, S327–S328. [Google Scholar] [CrossRef]
- Willmann, S.; Coboeken, K.; Kapsa, S.; Thelen, K.; Mundhenke, M.; Fischer, K.; Hügl, B.; Mück, W. Applications of Physiologically Based Pharmacokinetic Modeling of Rivaroxaban—Renal and Hepatic Impairment and Drug-Drug Interaction Potential. J. Clin. Pharmacol. 2021, 61, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Scheff, J.D.; Almon, R.R.; Dubois, D.C.; Jusko, W.J.; Androulakis, I.P. Assessment of Pharmacologic Area under the Curve When Baselines Are Variable. Pharm. Res. 2011, 28, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Hanke, N.; Frechen, S.; Moj, D.; Britz, H.; Eissing, T.; Wendl, T.; Lehr, T. PBPK Models for CYP3A4 and P-gp DDI Prediction: A Modeling Network of Rifampicin, Itraconazole, Clarithromycin, Midazolam, Alfentanil, and Digoxin. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 647–659. [Google Scholar] [CrossRef]
- Strougo, A.; Yassen, A.; Krauwinkel, W.; Danhof, M.; Freijer, J. A Semiphysiological Population Model for Prediction of the Pharmacokinetics of Drugs under Liver and Renal Disease Conditions. Drug Metab. Dispos. 2011, 39, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Han, A.N.; Han, B.R.; Zhang, T.; Heimbach, T. Hepatic Impairment Physiologically Based Pharmacokinetic Model Development: Current Challenges. Curr. Pharmacol. Rep. 2021, 7, 213–226. [Google Scholar] [CrossRef]
- Jones, H.M.; Mayawala, K.; Poulin, P. Dose Selection Based on Physiologically Based Pharmacokinetic (PBPK) Approaches. AAPS J. 2013, 15, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.C.; Hildbrand, C.; Born, C.; Egger, S.; Rätz Bravo, A.E.; Krähenbühl, S. Dose Adjustment in Patients with Liver Cirrhosis: Impact on Adverse Drug Reactions and Hospitalizations. Eur. J. Clin. Pharmacol. 2013, 69, 1565–1573. [Google Scholar] [CrossRef][Green Version]
- Delcò, F.; Tchambaz, L.; Schlienger, R.; Drewe, J.; Krähenbühl, S. Dose Adjustment in Patients with Liver Disease. Drug Saf. 2005, 28, 529–545. [Google Scholar] [CrossRef]

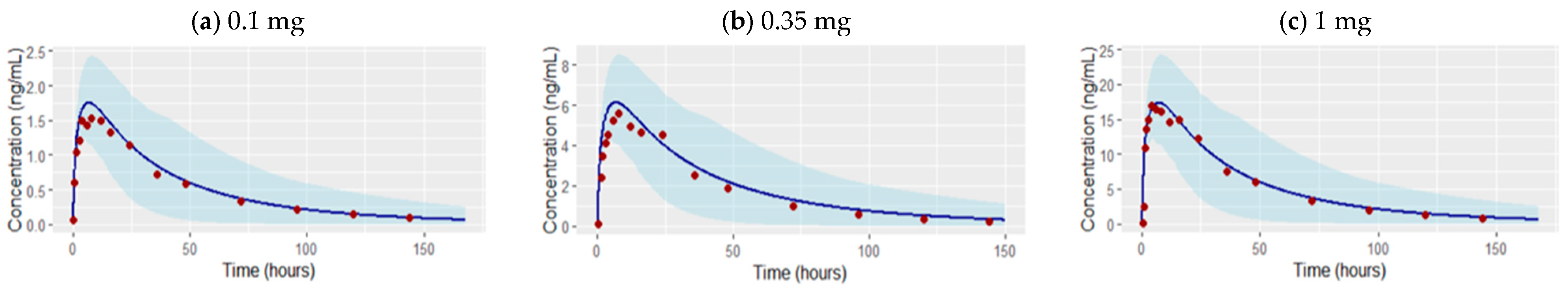
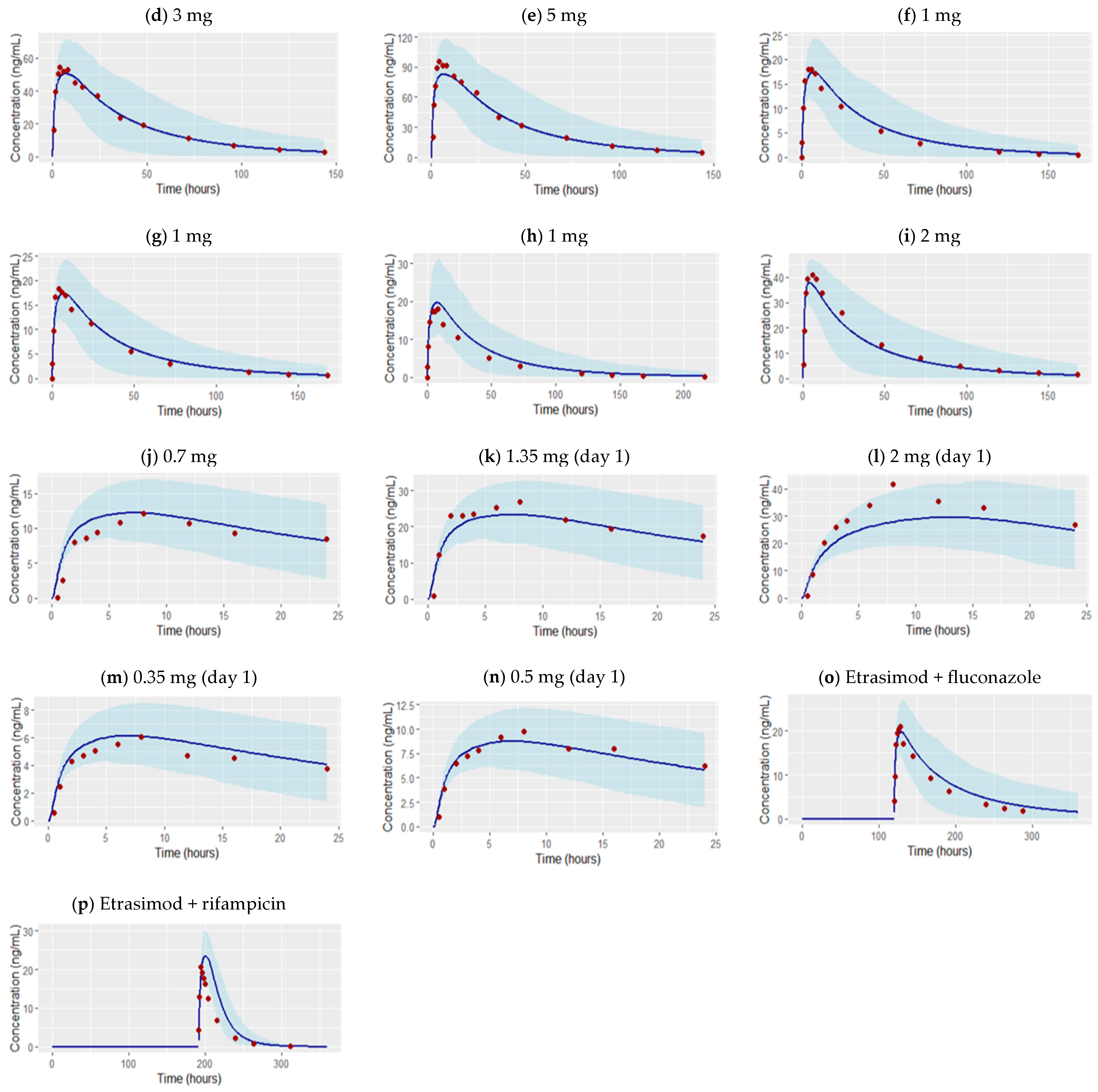

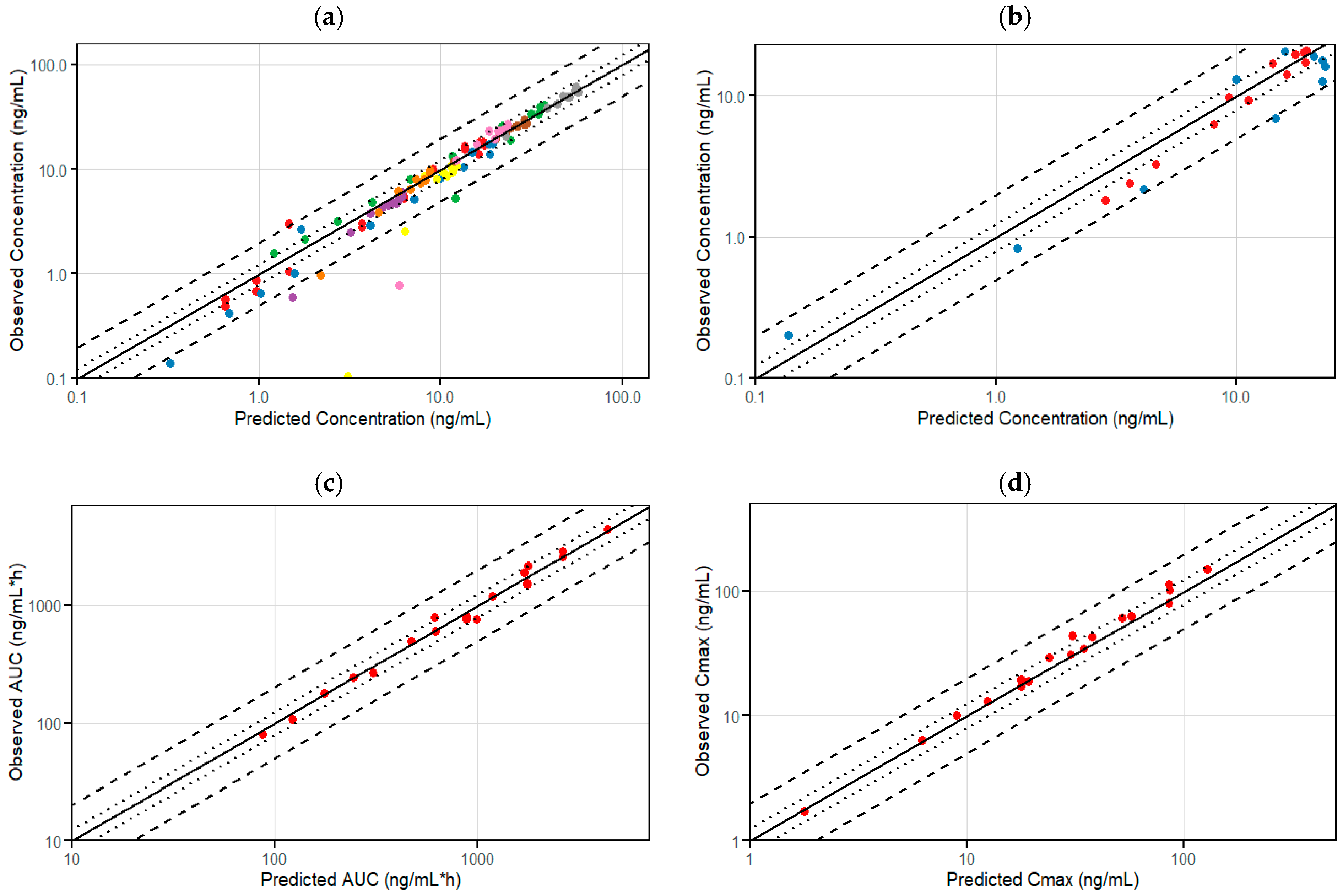
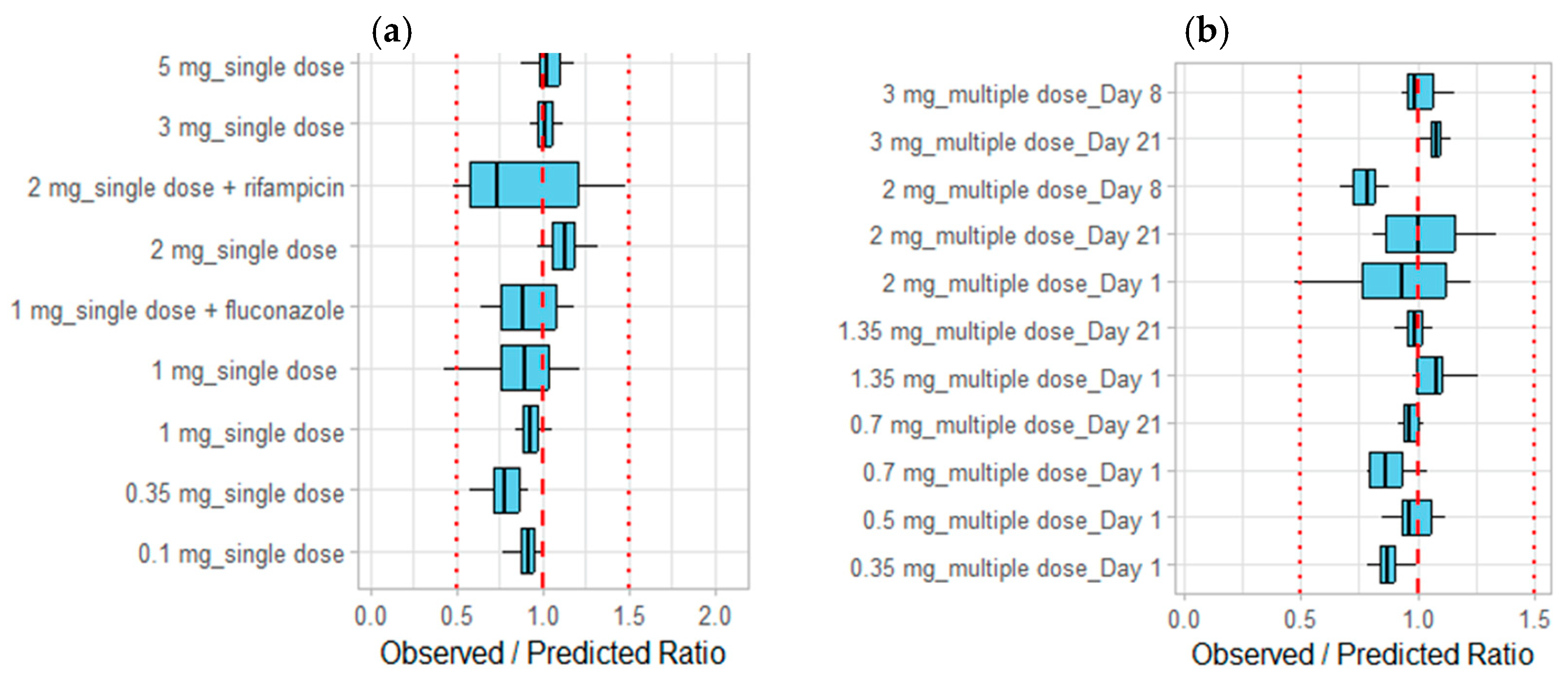
| Dose | N (male %) | Age (year) | Weight (kg) | AUC_inf [ng·h/mL] | Cmax [ng/mL] | Ref. |
|---|---|---|---|---|---|---|
| Phase 1, open label, single dose study to build and refine the model | ||||||
| 1 mg | 18 (68.4) | 32 (8.1) | 75.7 (11.4) | 763 (207) | 18.8 (3.3) | [26] |
| Phase 1, open label, single dose study to build and refine the model a | ||||||
| 1 mg | 19 (73.7) | 35.3 (10.4) | 73.5 (14.2) | 759 (196) | 19 (4.4) | [27] |
| 1 mg | 19 (36.8) | 36.4 (10.6) | 77.1 (16.5) | 802 (232) | 19.5 (5.0) | |
| 2 mg | 18 (83.3) | 39.4 (9.1) | 80.6 (9.5) | 1510 (488) | 34.2 (11.1) | |
| Phase 1, open label, single dose study to build and refine the model | ||||||
| 2 mg | 8 (100) | 31 (6.6) | 77.3 (11.48) | 1900 (596) | 42.5 (10.2) | [16] |
| Phase 1, randomized, double-blind, SAD study to verify the model | ||||||
| 0.1 mg | 6 (66.7) | 27.3 (5.8) | 27.8 ± 4.6 (BMI) | 79.8 (21.3) | 1.7 (0.6) | [28] |
| 0.35 mg | 6 (33.3) | 25.8 (4.4) | 30.0 ± 4.9 (BMI) | 268 (31.0) | 6.3 (0.4) | |
| 1 mg | 6 (50.0) | 31.5 (6.4) | 29.3 ± 3.8 (BMI) | 793 (168) | 17.2 (5.5) | |
| 3 mg | 6 (66.7) | 31.0 (7.1) | 27.2 ± 4.7 (BMI) | 2600 (840) | 60.5 (11.7) | |
| 5 mg | 6 (33.3) | 33.0 (9.4) | 27.2 ± 6.4 (BMI) | 4390 (610) | 102 (19.1) | |
| Phase 1, randomized, double-blind, MAD study to verify the model. PK parameters after the last dose | ||||||
| 0.7 mg | 10 (50.0) | 34.2 (8.8) | 28.9 ± 4.5 (BMI) | 596 (121) | 30.8 (6.6) | [28] |
| 1.35 mg | 10 (30.0) | 31.4 (9.0) | 26.9 ± 3.1 (BMI) | 1197 (226) | 63.5 (11.8) | |
| 2 mg | 10 (40.0) | 30.1 (7.0) | 27.0 ± 5.8 (BMI) | 2163 (489) | 113 (27.5) | |
| 0.35 mg/2 mg | 10 (50.0) | 32.8 (6.0) | 27.2 ± 2.1 (BMI) | 1513 (359) | 80.5 (17.4) | |
| 0.5 mg/3 mg | 10 (40.0) | 29.0 (7.2) | 27.7 ± 2.2 (BMI) | 2867 (376) | 151 (19.0) | |
| Phase 1, randomized, double-blind, MAD study to verify the model. PK parameters after the last dose | ||||||
| 2 mg/3 mg/4 mg | 30 | 18–55 | 50–100 | 2885 (793) | 163 (46.7) | [29] |
| Group | Data | AUC_inf [ng·h/mL] | Cmax [ng/mL] | T½ [h] |
|---|---|---|---|---|
| Effect of enzymes inhibition | ||||
| Control † | Predicted | 875 | 17.7 | 44.0 |
| Observed | 759 | 19.0 | 42.5 | |
| Fold error | 1.15 | 0.93 | 1.04 | |
| Etrasimod + Fluconazole | Predicted | 1772 | 20.0 | 82.1 |
| Observed | 1440 | 21.4 | 88.2 | |
| Fold error | 1.23 | 0.93 | 0.70 | |
| Pred. AUCR | 2.03 | |||
| Obs. AUCR | 1.90 | |||
| Pred/Obs AUCR | 1.07 | |||
| Pred. CmaxR | 1.13 | |||
| Obs. CmaxR | 1.13 | |||
| Pred/Obs CmaxR | 1.0 | |||
| Pred. T½R | 1.87 | |||
| Obs. T½R | 2.08 | |||
| Pred/Obs T½R | 0.90 | |||
| Effect of enzymes induction | ||||
| Control ‡ | Predicted | 1750 | 34.3 | 44.0 |
| Observed | 1510 | 34.2 | 41.1 | |
| Fold error | 1.16 | 1.0 | 1.10 | |
| Etrasimod + Rifampicin | Predicted | 754 | 24.0 | 17.0 |
| Observed | 770 | 36.0 | 21.0 | |
| Fold error | 0.98 | 0.67 | 0.81 | |
| Pred. AUCR | 0.43 | |||
| Obs. AUCR | 0.51 | |||
| Pred/Obs AUCR | 0.84 | |||
| Pred. CmaxR | 0.70 | |||
| Obs. CmaxR | 1.05 | |||
| Pred/Obs CmaxR | 0.67 | |||
| Pred. T½R | 0.40 | |||
| Obs. T½R | 0.51 | |||
| Pred/Obs T½R | 0.78 | |||
| Parameter | Population | Observed | Predicted | Fold Error |
|---|---|---|---|---|
| AUC_inf (ng·h/mL) | Healthy | 1510 a | 1750 | 1.16 |
| CP-A | 1706 (13% ↑) b | 1894 (12.50% ↑) | 1.11 | |
| CP-B | 1948 (29% ↑) b | 2208 (28.40% ↑) | 1.13 | |
| CP-C | 2371 (57%↑) b | 2526 (52.4% ↑) | 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alasmari, M.S.; Alqahtani, F.; Alasmari, F.; Alsultan, A. Model-Based Dose Selection of a Sphingosine-1-Phosphate Modulator, Etrasimod, in Patients with Various Degrees of Hepatic Impairment. Pharmaceutics 2024, 16, 1540. https://doi.org/10.3390/pharmaceutics16121540
Alasmari MS, Alqahtani F, Alasmari F, Alsultan A. Model-Based Dose Selection of a Sphingosine-1-Phosphate Modulator, Etrasimod, in Patients with Various Degrees of Hepatic Impairment. Pharmaceutics. 2024; 16(12):1540. https://doi.org/10.3390/pharmaceutics16121540
Chicago/Turabian StyleAlasmari, Mohammed S., Faleh Alqahtani, Fawaz Alasmari, and Abdullah Alsultan. 2024. "Model-Based Dose Selection of a Sphingosine-1-Phosphate Modulator, Etrasimod, in Patients with Various Degrees of Hepatic Impairment" Pharmaceutics 16, no. 12: 1540. https://doi.org/10.3390/pharmaceutics16121540
APA StyleAlasmari, M. S., Alqahtani, F., Alasmari, F., & Alsultan, A. (2024). Model-Based Dose Selection of a Sphingosine-1-Phosphate Modulator, Etrasimod, in Patients with Various Degrees of Hepatic Impairment. Pharmaceutics, 16(12), 1540. https://doi.org/10.3390/pharmaceutics16121540







