Two in One: Size Characterization and Accelerated Short-Term Physical Stability of Dual-Drug Suspensions with Two Acidic Compounds (Indomethacin and Naproxen)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Suspension Preparation by Dual Centrifugation
2.3. Particle Size Measurements
Analysis of Particle Size Profiles of Dual-Drug Suspensions
2.4. Thermodynamic Solubility in Dispersion Media
3. Results and Discussion
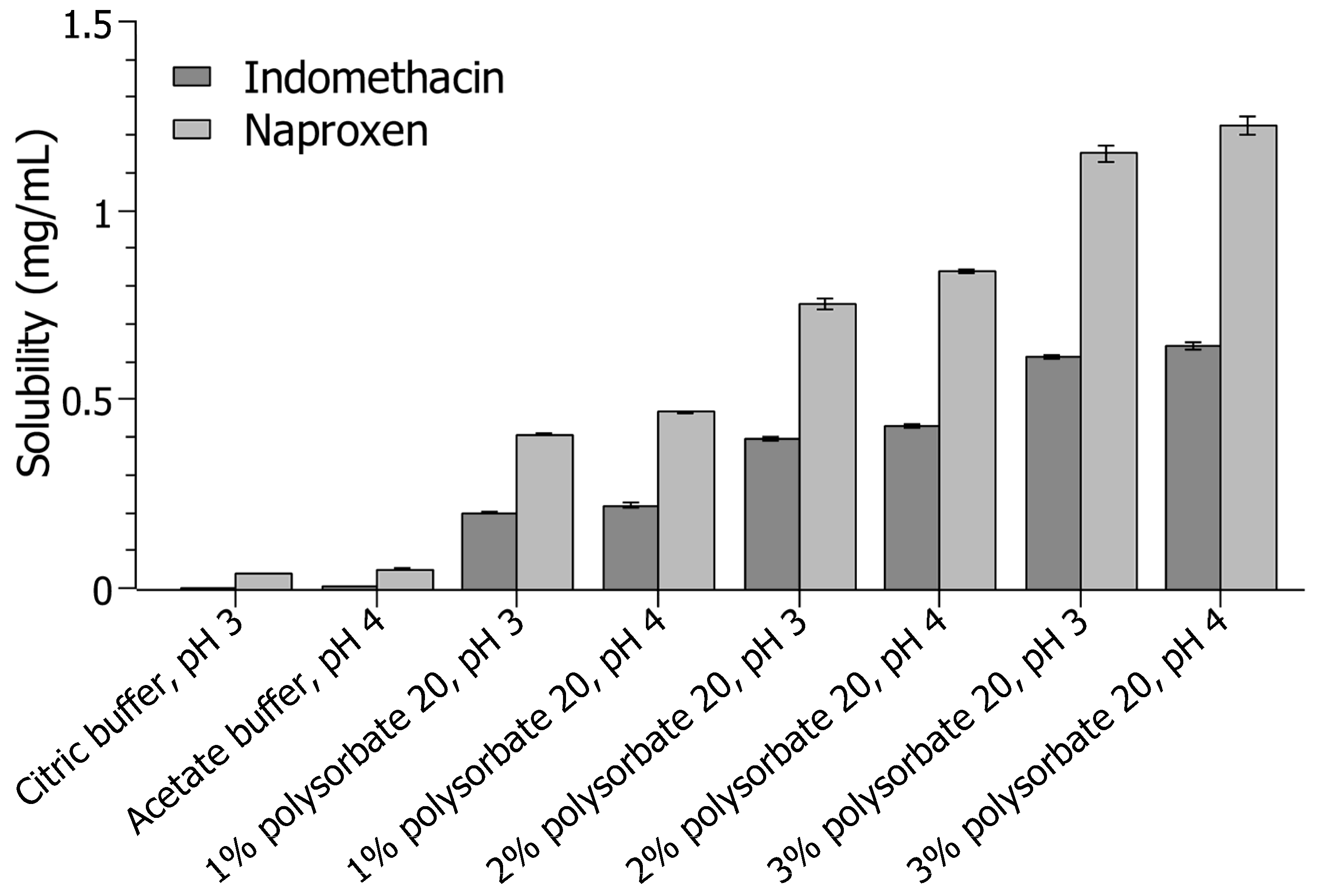
3.1. Preliminary Solubility Investigation
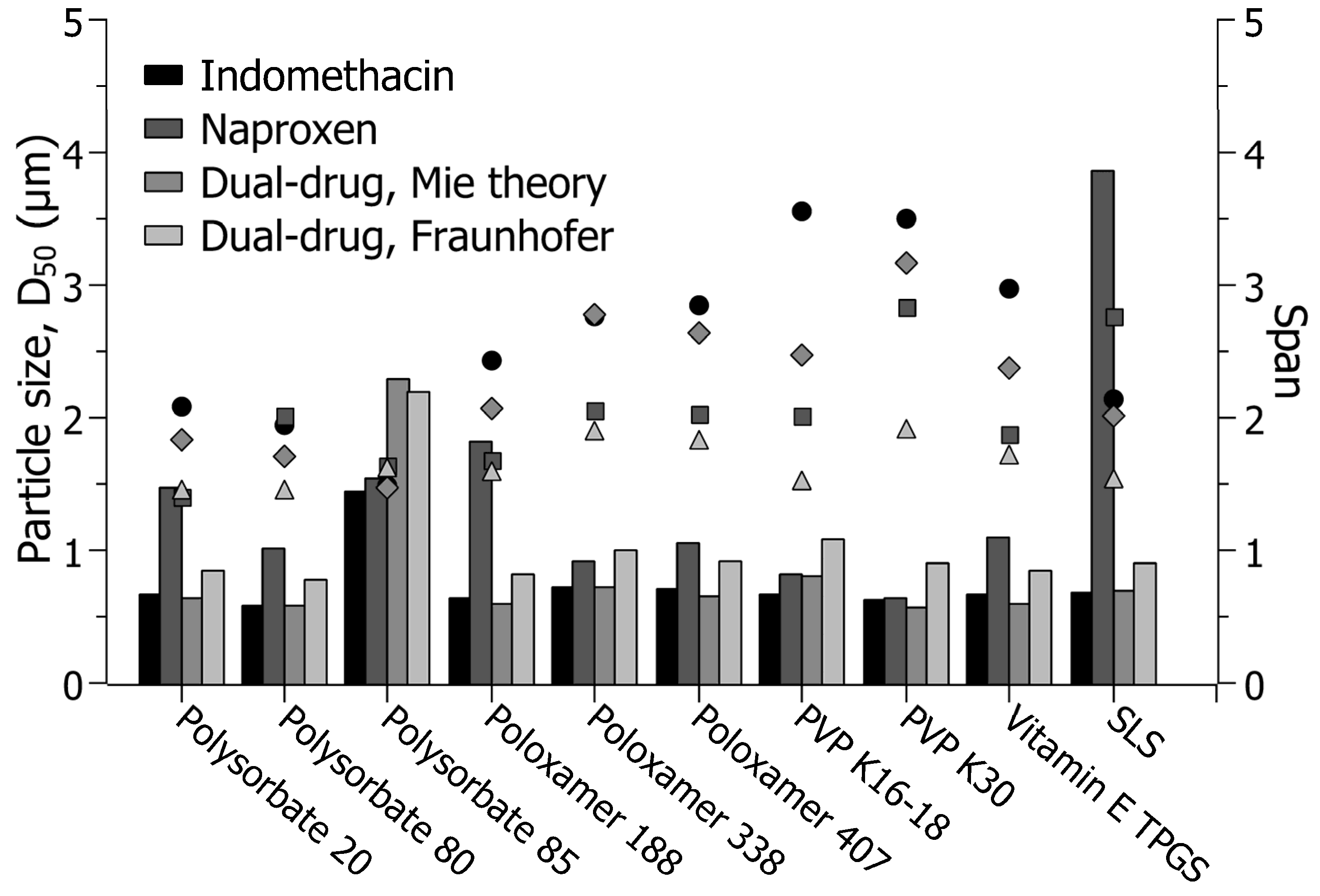
3.2. Screening of Stabilizers
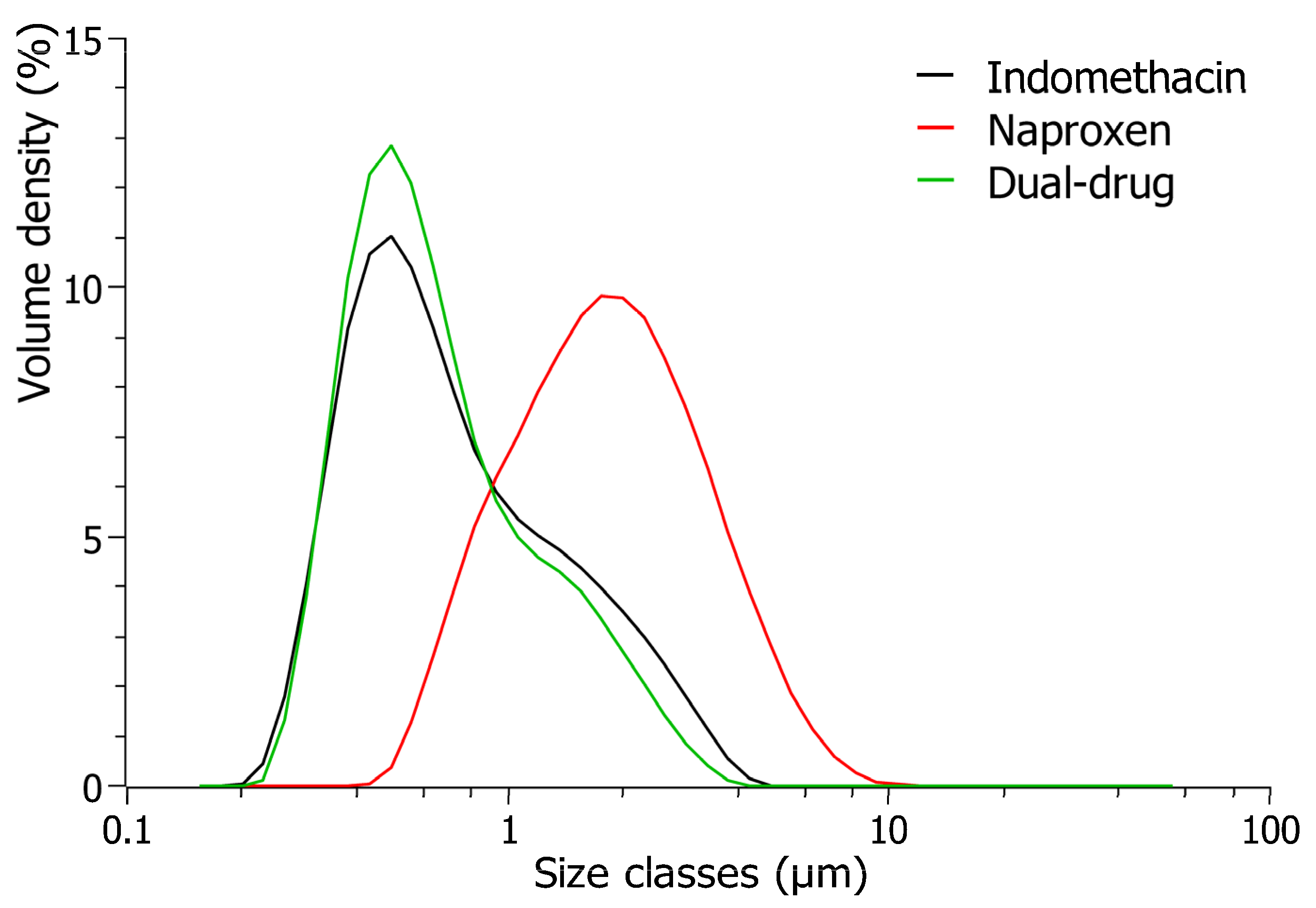
3.3. Interpretation of Dual-Drug Suspensions
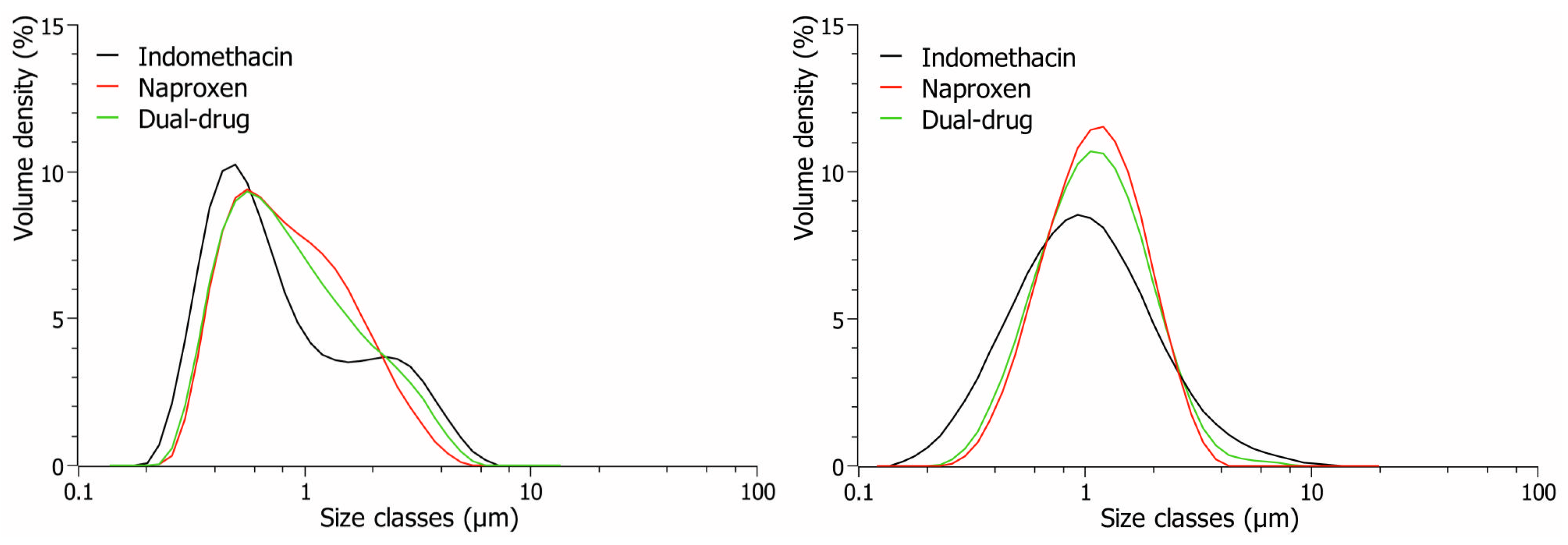
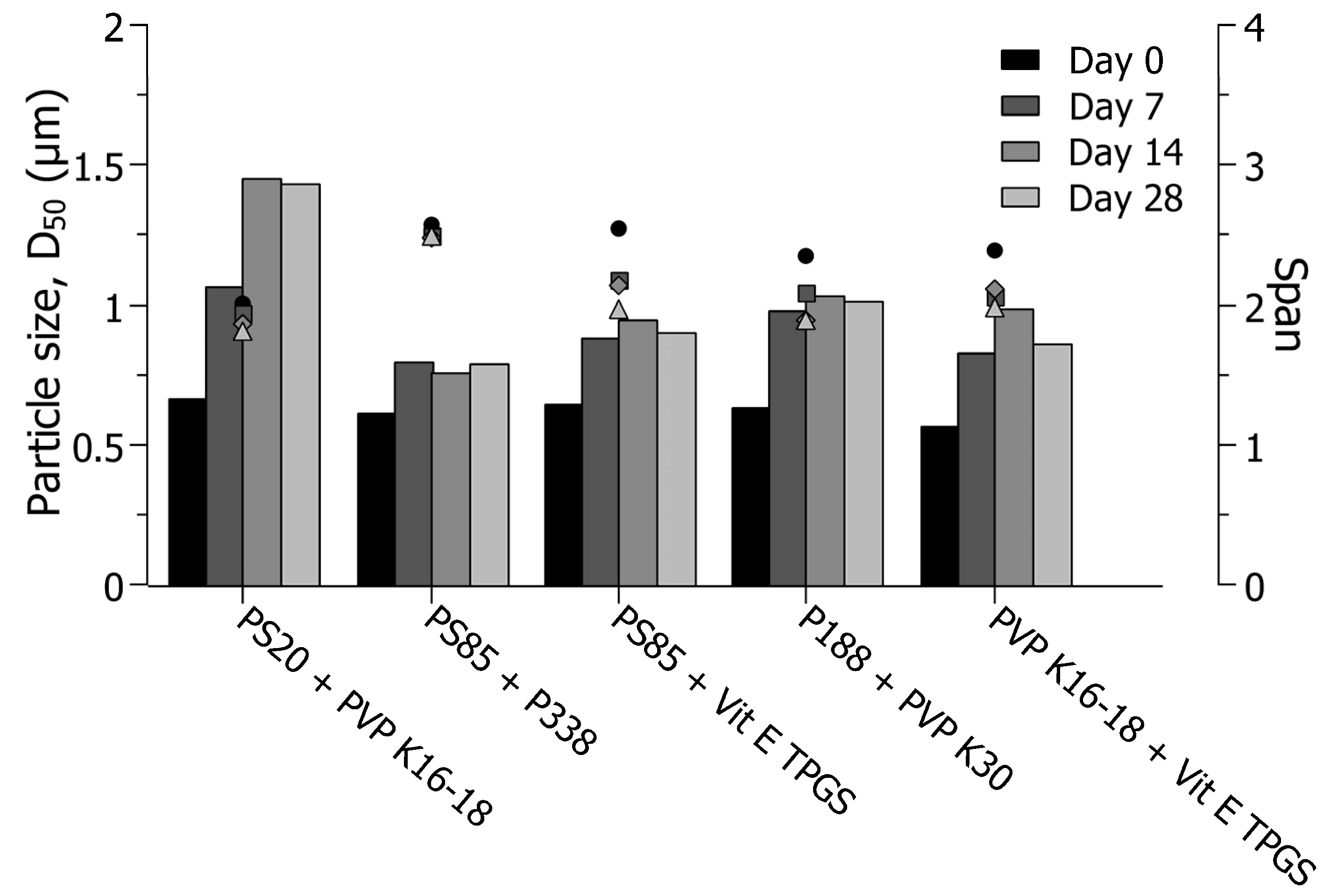
3.4. Short-Term Physical Stability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, E.J.; Amatya, S.; Kim, M.S.; Park, J.H.; Seol, E.; Lee, H.; Shin, Y.H.; Na, D.H. Long-acting injectable formulations of antipsychotic drugs for the treatment of schizophrenia. Arch. Pharmacal Res. 2013, 36, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.; Rannard, S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv. Drug Deliv. Rev. 2016, 103, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, I.; Tiihonen, J.; Kotzalidis, G.D.; Verdolini, N.; Murru, A.; Goikolea, J.M.; Valentí, M.; Aedo, A.; Vieta, E. Long-acting injectable antipsychotics (LAIs) for maintenance treatment of bipolar and schizoaffective disorders: A systematic review. Eur. Neuropsychopharmacol. 2019, 29, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Fisch, A.; Rad-Malekshahi, M.; Romic, M.D.; Kittel, B.; Ullrich, T.; Wang, J.; Krause, R.W.M.; Adler, S.; Lammers, T.; et al. Clinically established biodegradable long acting injectables: An industry perspective. Adv. Drug Deliv. Rev. 2020, 167, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.T.C.; Kappi, A.; Wang, T.; Makowski, A.; Cooley, A.T. The effect of long-acting injectable antipsychotic medications compared with oral antipsychotic medications among people with schizophrenia: A systematic review and meta-analysis. Int. J. Ment. Health Nurs. 2022, 31, 469–535. [Google Scholar] [CrossRef]
- O’Brien Brien, M.N.; Jiang, W.; Wang, Y.; Loffredo, D.M. Challenges and opportunities in the development of complex generic long-acting injectable drug products. J. Control. Release 2021, 336, 144–158. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. Eur. J. Pharm. Biopharm. 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Bao, Q.; Zou, Y.; Wang, Y.; Choi, S.; Burgess, D.J. Impact of Formulation Parameters on In Vitro Release from Long-Acting Injectable Suspensions. AAPS J. 2021, 23, 42. [Google Scholar] [CrossRef]
- Wilkinson, J.; Ajulo, D.; Tamburrini, V.; Gall, G.L.; Kimpe, K.; Holm, R.; Belton, P.; Qi, S. Lipid based intramuscular long-acting injectables: Current state of the art. Eur. J. Pharm. Sci. 2022, 178, 106253. [Google Scholar] [CrossRef]
- Bauer, A.; Berben, P.; Chakravarthi, S.S.; Chattorraj, S.; Garg, A.; Gourdon, B.; Heimbach, T.; Huang, Y.; Morrison, C.; Mundhra, D.; et al. Current State and Opportunities with Long-acting Injectables: Industry Perspectives from the Innovation and Quality Consortium “Long-Acting Injectables” Working Group. Pharm. Res. 2023, 40, 1601–1631. [Google Scholar] [CrossRef]
- Holm, R.; Lee, R.W.; Glassco, J.; DiFranco, N.; Bao, Q.; Burgess, D.J.; Lukacova, V.; Alidori, S. Long-Acting Injectable Aqueous Suspensions-Summary From an AAPS Workshop. AAPS J. 2023, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Alidori, S.; Subramanian, R.; Holm, R. Patient-Centric Long-Acting Injectable and Implantable Platforms—An Industrial Perspective. Mol. Pharm. 2024, 21, 4238–4258. [Google Scholar] [CrossRef] [PubMed]
- Remenar, J.F. Making the leap from daily oral dosing to long-acting injectables: Lessons from the antipsychotics. Mol. Pharm. 2014, 11, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing–Oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G. A screening study of surface stabilization during the production of drug nanocrystals. J. Pharm. Sci. 2009, 98, 2091–2103. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Combinative Particle Size Reduction Technologies for the Production of Drug Nanocrystals. J. Pharm. 2014, 2014, 265754. [Google Scholar] [CrossRef]
- Brunaugh, A.; Smyth, H.D.C. Process optimization and particle engineering of micronized drug powders via milling. Drug Deliv. Transl. Res. 2018, 8, 1740–1750. [Google Scholar] [CrossRef]
- Afolabi, A.; Akinlabi, O.; Bilgili, E. Impact of process parameters on the breakage kinetics of poorly water-soluble drugs during wet stirred media milling: A microhydrodynamic view. Eur. J. Pharm. Sci. 2014, 51, 75–86. [Google Scholar] [CrossRef]
- Nakach, M.; Authelin, J.R.; Tadros, T.; Galet, L.; Chamayou, A. Engineering of nano-crystalline drug suspensions: Employing a physico-chemistry based stabilizer selection methodology or approach. Int. J. Pharm. 2014, 476, 277–288. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Krautstrunk, I.; Dengler, M.; Juhnke, M.; Grandeury, A.; Bunjes, H.; Kwade, A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur. J. Pharm. Biopharm. 2015, 92, 171–179. [Google Scholar] [CrossRef]
- Hagedorn, M.; Bögershausen, A.; Rischer, M.; Schubert, R.; Massing, U. Dual centrifugation—A new technique for nanomilling of poorly soluble drugs and formulation screening by an DoE-approach. Int. J. Pharm. 2017, 530, 79–88. [Google Scholar] [CrossRef]
- Hagedorn, M.; Liebich, L.; Bögershausen, A.; Massing, U.; Hoffmann, S.; Mende, S.; Rischer, M. Rapid development of API nano-formulations from screening to production combining dual centrifugation and wet agitator bead milling. Int. J. Pharm. 2019, 565, 187–198. [Google Scholar] [CrossRef]
- Bahadur, K.C.R.; Xu, P. Multicompartment intracellular self-expanding nanogel for targeted delivery of drug cocktail. Adv. Mater. 2012, 24, 6479–6483. [Google Scholar] [CrossRef]
- Zachariah, R.; Harries, A.D.; Luo, C.; Bachman, G.; Graham, S.M. Scaling-up co-trimoxazole prophylaxis in HIV-exposed and HIV-infected children in high HIV-prevalence countries. Lancet Infect. Dis. 2007, 7, 686–693. [Google Scholar] [CrossRef]
- Lee, J.H.; Nan, A. Combination drug delivery approaches in metastatic breast cancer. J. Drug Deliv. 2012, 2012, 915375. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.M.; Khan, M.W.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Dudhipala, N.; Majumdar, S. Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications. Int. J. Nanomed. 2022, 17, 2283–2299. [Google Scholar] [CrossRef]
- Haloi, P.; Lokesh, B.S.; Chawla, S.; Konkimalla, V.B. Formulation of a dual drug-loaded nanoparticulate co-delivery hydrogel system and its validation in rheumatoid arthritis animal model. Drug Deliv. 2023, 30, 2184307. [Google Scholar] [CrossRef]
- Jin, S.; Lan, Z.; Yang, G.; Li, X.; Shi, J.Q.; Liu, Y.; Zhao, C. Computationally guided design and synthesis of dual-drug loaded polymeric nanoparticles for combination therapy. Aggregate 2024, 5, e606. [Google Scholar] [CrossRef]
- Shelke, R.; Velagacherla, V.; Nayak, U.Y. Recent advances in dual-drug co-amorphous systems. Drug Discov. Today 2024, 29, 103863. [Google Scholar] [CrossRef]
- De Cleyn, E.; Holm, R.; Van den Mooter, G. Size Analysis of Small Particles in Wet Dispersions by Laser Diffractometry: A Guidance to Quality Data. J. Pharm. Sci. 2019, 108, 1905–1914. [Google Scholar] [CrossRef]
- Kerr, H.E.; Softley, L.K.; Suresh, K.; Hodgkinson, P.; Evans, I.R. Structure and physicochemical characterization of a naproxen-picolinamide cocrystal. Acta Crystallogr. Sect. C Struct. Chem. 2017, 73 Pt 3, 168–175. [Google Scholar] [CrossRef]
- O’Brien, M.; McCauley, J.; Cohen, E. Indomethacin. Anal. Profiles Drug Subst. 1984, 13, 211–238. [Google Scholar] [CrossRef]
- Zulbeari, N.; Hansen, M.; Morgen, P.; Holm, R. Impact of Drug Compounds Mechanical/Deformation Properties on the Preparation of Nano- and Microsuspensions. J. Drug Deliv. Sci. Technol. 2024, 95, 105605. [Google Scholar] [CrossRef]
- Jones, A.R. Error contour charts relevant to particle sizing by forward-scattered lobe methods. J. Phys. D Appl. Phys. 1977, 10, L163. [Google Scholar] [CrossRef]
- Xie, H.; Xu, L.; Niu, H.; Cao, Z. Particle sizing from Fraunhofer diffraction pattern using a digital micro-mirror device and a single photodiode. Powder Technol. 2018, 332, 351–358. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef]
- Zulbeari, N.; Mustafova, S.S.; Simonsen, A.C.; Lund, F.W.; Holm, R. The Langmuir-Blodgett trough (Langmuir film balance) can be used to understand the stabilizer concentrations in aqueous nano- and microsuspensions. Int. J. Pharm. 2024, 665, 124726. [Google Scholar] [CrossRef] [PubMed]
- Sastry, N.V.; Hoffmann, H. Interaction of amphiphilic block copolymer micelles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 247–261. [Google Scholar] [CrossRef]
- Zheng, Y.; Davis, H.T. Mixed Micelles of Nonionic Surfactants and Uncharged Block Copolymers in Aqueous Solutions: Microstructure Seen by Cryo-TEM. Langmuir 2000, 16, 6453–6459. [Google Scholar] [CrossRef]
| Indomethacin | Naproxen | Dual-Drug | ||||
|---|---|---|---|---|---|---|
| Stabilizer | Monomodal | Bimodal | Monomodal | Bimodal | Monomodal | Bimodal |
| Polysorbate 20 | ||||||
| Polysorbate 80 | ||||||
| Polysorbate 85 | ||||||
| Poloxamer 188 | ||||||
| Poloxamer 338 | ||||||
| Poloxamer 407 | ||||||
| PVP K16-18 | ||||||
| PVP K30 | ||||||
| Vitamin E TPGS | ||||||
| SLS | ||||||
| Indomethacin | Naproxen | Dual-Drug | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | |
| Polysorbate 20 | 0.662 µm | 1.230 µm | 1.470 µm | 3.950 µm | 0.632 µm | 1.720 µm |
| Polysorbate 80 | 0.590 µm | 0.912 µm | 1.020 µm | 2.400 µm | 0.578 µm | 0.910 µm |
| Polysorbate 85 | 1.440 µm | 2.620 µm | 1.540 µm | 4.880 µm | 2.290 µm | 2.950 µm |
| Poloxamer 188 | 0.642 µm | 0.739 µm | 1.820 µm | 3.230 µm | 0.596 µm | 0.836 µm |
| Poloxamer 338 | 0.722 µm | 0.857 µm | 0.919 µm | 2.460 µm | 0.722 µm | 0.927 µm |
| Poloxamer 407 | 0.705 µm | 0.778 µm | 1.060 µm | 3.100 µm | 0.650 µm | 0.843 µm |
| PVP K16-18 | 0.665 µm | 1.220 µm | 0.816 µm | 2.020 µm | 0.803 µm | 1.170 µm |
| PVP K30 | 0.619 µm | 0.692 µm | 0.637 µm | 1.880 µm | 0.565 µm | 0.784 µm |
| Vitamin E TPGS | 0.672 µm | 0.757 µm | 1.100 µm | 1.790 µm | 0.592 µm | 0.878 µm |
| SLS | 0.674 µm | 0.858 µm | 3.860 µm | 4.120 µm | 0.695 µm | 1.870 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulbeari, N.; Hansen, S.M.; Holm, R. Two in One: Size Characterization and Accelerated Short-Term Physical Stability of Dual-Drug Suspensions with Two Acidic Compounds (Indomethacin and Naproxen). Pharmaceutics 2024, 16, 1495. https://doi.org/10.3390/pharmaceutics16121495
Zulbeari N, Hansen SM, Holm R. Two in One: Size Characterization and Accelerated Short-Term Physical Stability of Dual-Drug Suspensions with Two Acidic Compounds (Indomethacin and Naproxen). Pharmaceutics. 2024; 16(12):1495. https://doi.org/10.3390/pharmaceutics16121495
Chicago/Turabian StyleZulbeari, Nadina, Signe Malig Hansen, and René Holm. 2024. "Two in One: Size Characterization and Accelerated Short-Term Physical Stability of Dual-Drug Suspensions with Two Acidic Compounds (Indomethacin and Naproxen)" Pharmaceutics 16, no. 12: 1495. https://doi.org/10.3390/pharmaceutics16121495
APA StyleZulbeari, N., Hansen, S. M., & Holm, R. (2024). Two in One: Size Characterization and Accelerated Short-Term Physical Stability of Dual-Drug Suspensions with Two Acidic Compounds (Indomethacin and Naproxen). Pharmaceutics, 16(12), 1495. https://doi.org/10.3390/pharmaceutics16121495







