Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Predictive In Silico Modeling of Physicochemical Properties
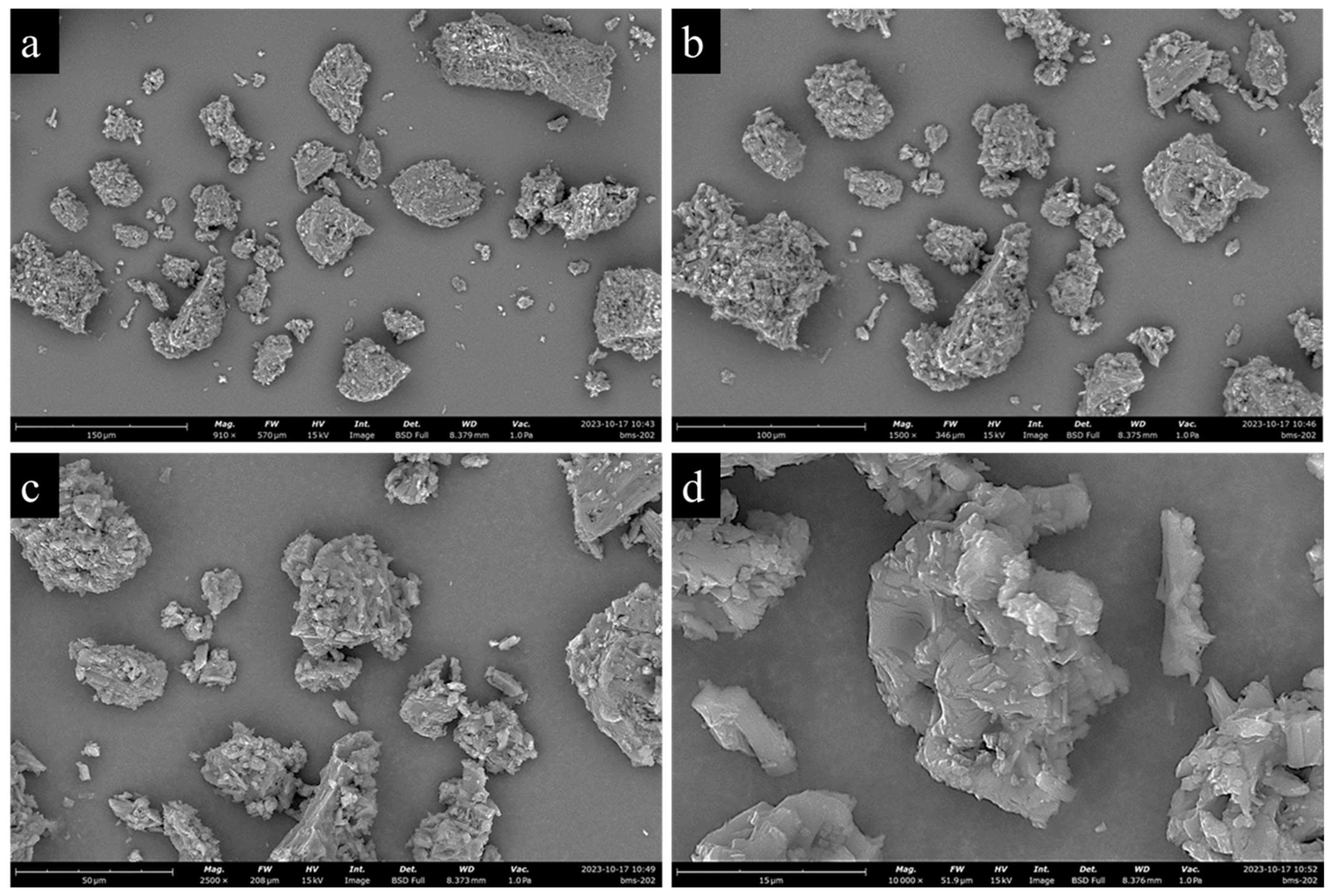
2.2.2. Scanning Electron Microscopy (SEM)
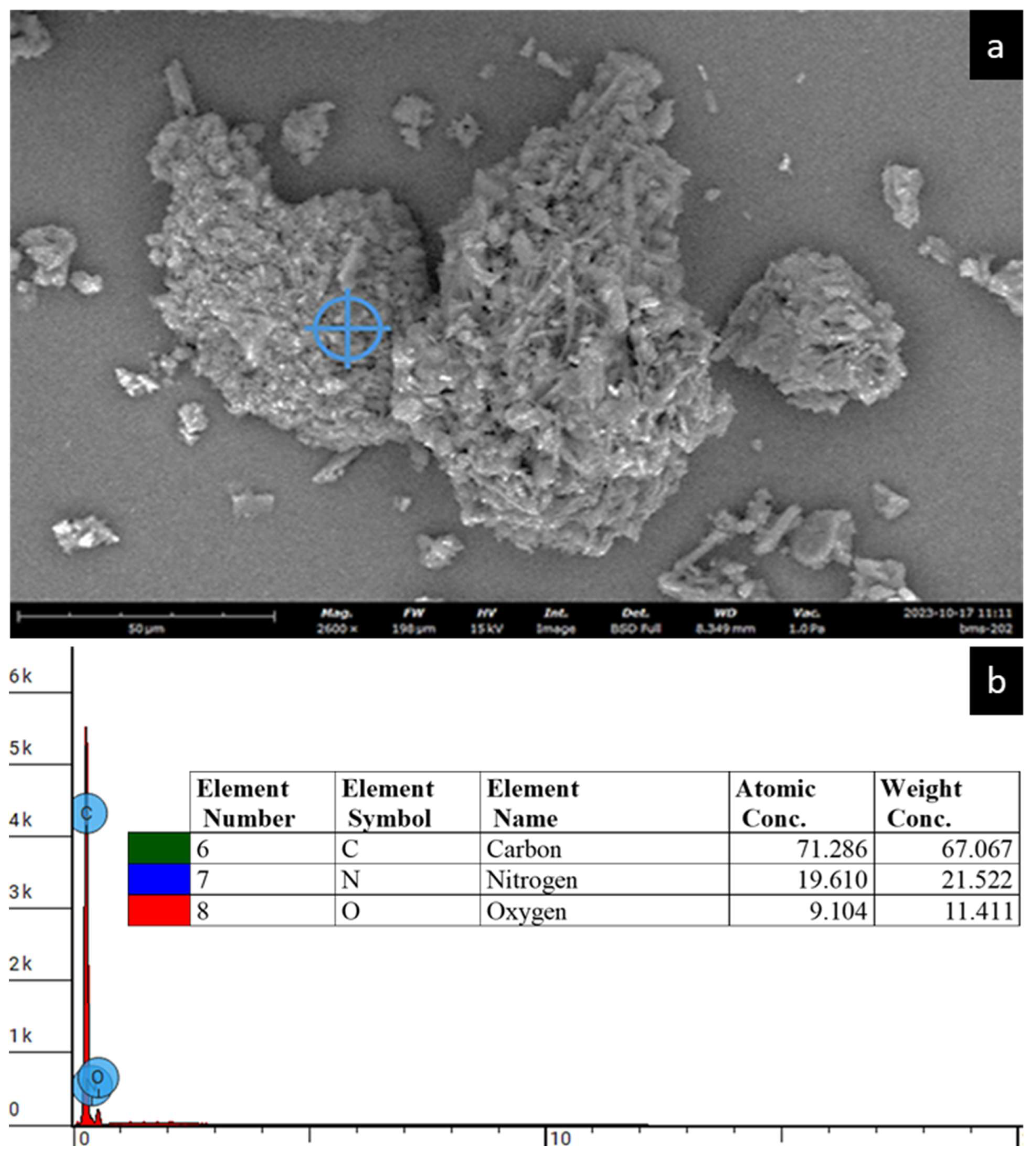
2.2.3. Energy-Dispersive X-Ray (EDX) Spectroscopy
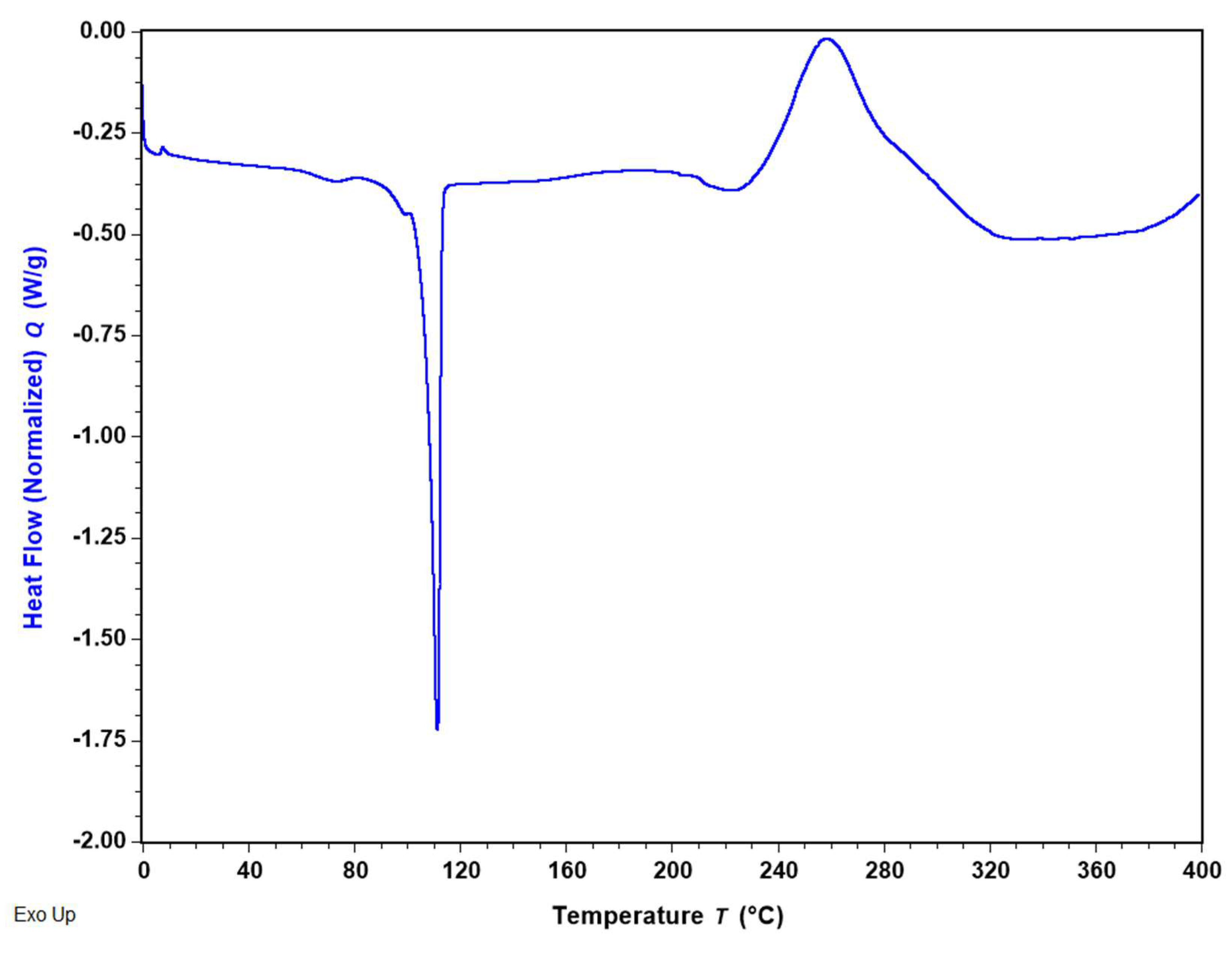
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Hot-Stage Microscopy (HSM) Under Cross-Polarizers
2.2.6. Raman Spectroscopy
2.2.7. Confocal Raman Microscopy (CRM)
2.2.8. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.2.9. Fourier Transform Infrared (FTIR) Microscopy
2.2.10. Karl Fisher (KF) Coulometric Titration
2.2.11. Gravimetric Vapor Sorption (GVS)
2.2.12. Dose-Dependent In Vitro Cell Viability on Skin Epithelial Cells
2.2.13. In Vitro Transepithelial Electrical Resistance (TEER) with Skin Epithelial Cells at the Air–Liquid Interface (ALI)
3. Results
3.1. Predictive In Silico Modeling of Physicochemical Properties
3.2. Scanning Electron Microscopy (SEM)
3.3. Energy-Dispersive X-Ray (EDX) Spectroscopy
3.4. Differential Scanning Calorimetry (DSC)
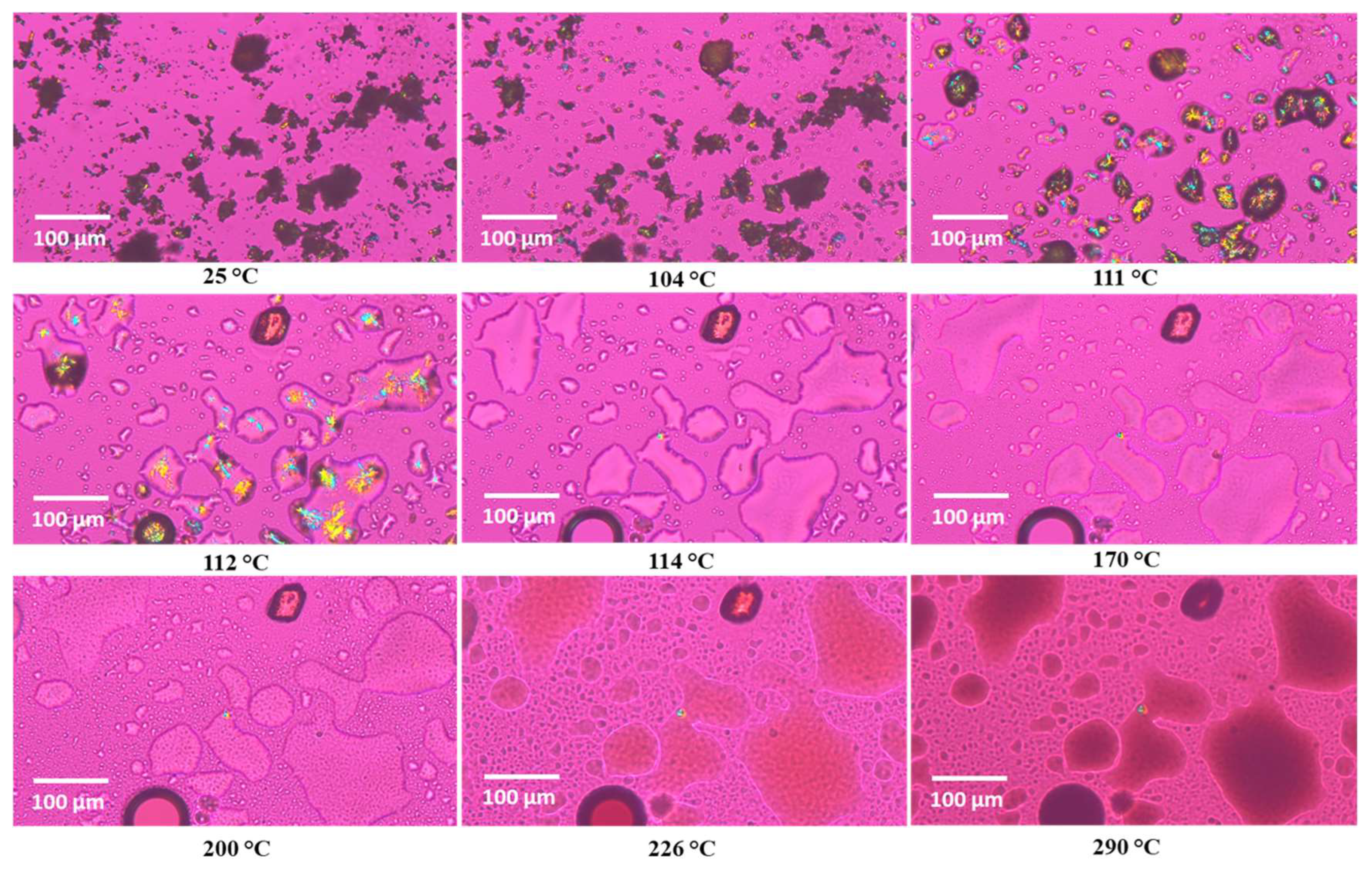
3.5. Hot-Stage Microscopy (HSM) Under Cross-Polarizers
3.6. Raman Spectroscopy
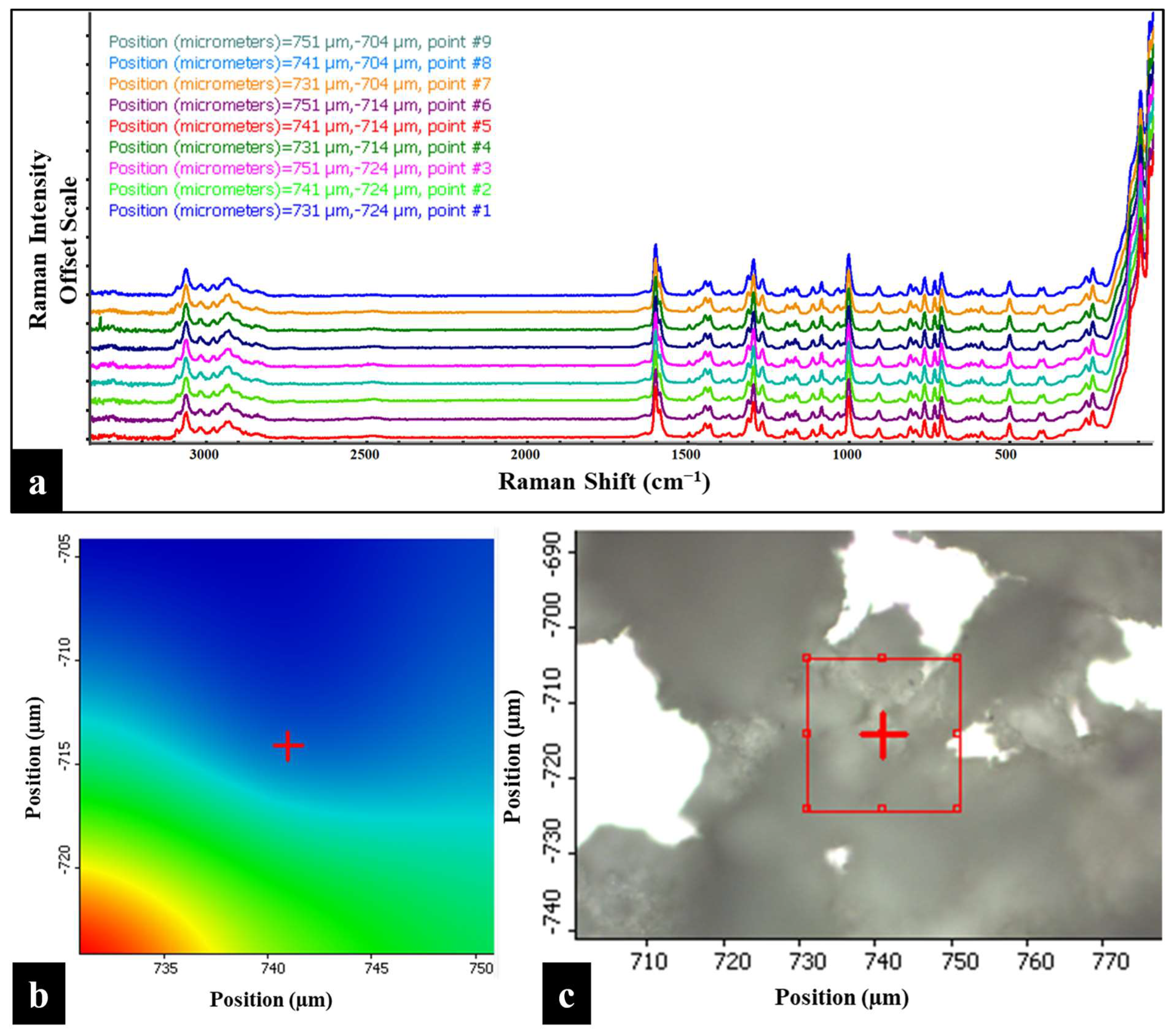
3.7. Raman Mapping
3.8. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.9. FTIR Microscopy
3.10. Karl Fisher (KF) Coulometric Titration
3.11. Gravimetric Vapor Sorption (GVS)
3.12. Dose-Dependent In Vitro Cell Viability on Skin Epithelial Cells
3.13. In Vitro Transepithelial Electrical Resistance (TEER) with Skin Epithelial Cells at the Air–Liquid Interface (ALI)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasikumar, P.G.; Ramachandra, M. Small molecule agents targeting PD-1 checkpoint pathway for cancer immunotherapy: Mechanisms of action and other considerations for their advanced development. Front. Immunol. 2022, 13, 752065. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, K.; Anagnostou, T.; Boussiotis, V.A. The PD1: PD-L1/2 pathway from discovery to clinical implementation. Front. Immunol. 2016, 7, 550. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Lee, S.H.; Heo, Y.-S. Molecular interactions of antibody drugs targeting PD-1, PD-L1, and CTLA-4 in immuno-oncology. Molecules 2019, 24, 1190. [Google Scholar] [CrossRef]
- Guzik, K.; Zak, K.M.; Grudnik, P.; Magiera, K.; Musielak, B.; Torner, R.; Skalniak, L.; Domling, A.; Dubin, G.; Holak, T.A. Small-molecule inhibitors of the programmed cell death-1/programmed death-ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J. Med. Chem. 2017, 60, 5857–5867. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Schadendorf, D. Update in the treatment of non-melanoma skin cancers: The use of PD-1 inhibitors in basal cell carcinoma and cutaneous squamous-cell carcinoma. J. Immunother. Cancer 2022, 10, e005082. [Google Scholar] [CrossRef]
- Vaishampayan, P.; Curiel-Lewandrowski, C.; Dickinson, S.E. PD-L1 as an emerging target in the treatment and prevention of keratinocytic skin cancer. Mol. Carcinog. 2023, 62, 52–61. [Google Scholar] [CrossRef]
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin cancer screening: Updated evidence report and systematic review for the US preventive services task force. JAMA 2023, 329, 1296–1307. [Google Scholar] [CrossRef]
- Dickinson, S.E.; Vaishampayan, P.; Jandova, J.; Ai, Y.E.; Kirschnerova, V.; Zhang, T.; Calvert, V.; Petricoin, E., III; Chow, H.S.; Hu, C. Inhibition of UV-Induced Stress Signaling and Inflammatory Responses in SKH-1 Mouse Skin by Topical Small-Molecule PD-L1 Blockade. JID Innov. 2024, 4, 100255. [Google Scholar] [CrossRef]
- Dickinson, S.E.; Khawam, M.; Kirschnerova, V.; Vaishampayan, P.; Centuori, S.M.; Saboda, K.; Calvert, V.S.; Petricoin, E.F., III; Curiel-Lewandrowski, C. Increased PD-L1 expression in human skin acutely and chronically exposed to UV irradiation. Photochem. Photobiol. 2021, 97, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Kaufman, H.L.; Emerick, K.S.; Miller, D.M. Immunotherapy for nonmelanoma skin cancer: Facts and hopes. Clin. Cancer Res. 2022, 28, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Blum, V.; Müller, B.; Hofer, S.; Pardo, E.; Zeidler, K.; Diebold, J.; Strobel, K.; Brand, C.; Aebi, S.; Gautschi, O. Nivolumab for recurrent cutaneous squamous cell carcinoma: Three cases. Eur. J. Dermatol. 2018, 28, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Constantinidou, A.; Alifieris, C.; Trafalis, D.T. Targeting programmed cell death-1 (PD-1) and ligand (PD-L1): A new era in cancer active immunotherapy. Pharmacol. Ther. 2019, 194, 84–106. [Google Scholar] [CrossRef]
- Imai, K.; Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Michielin, O.; Cervera, C.; Ribi, C.; Aguado, J.M.; Fernández-Ruiz, M.; Manuel, O. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: An infectious diseases perspective (Immune checkpoint inhibitors, cell adhesion inhibitors, sphingosine-1-phosphate receptor modulators and proteasome inhibitors). Clin. Microbiol. Infect. 2018, 24, S95–S107. [Google Scholar]
- Schneider, C.K. Monoclonal antibodies-regulatory challenges. Curr. Pharm. Biotechnol. 2008, 9, 431–438. [Google Scholar] [CrossRef]
- Vandivort, T.C.; Horton, D.B.; Johnson, S.B. Regulatory and strategic considerations for addressing immunogenicity and related responses in biopharmaceutical development programs. J. Clin. Transl. Sci. 2020, 4, 547–555. [Google Scholar] [CrossRef]
- OuYang, Y.; Gao, J.; Zhao, L.; Lu, J.; Zhong, H.; Tang, H.; Jin, S.; Yue, L.; Li, Y.; Guo, W. Design, synthesis, and evaluation of o-(biphenyl-3-ylmethoxy) nitrophenyl derivatives as PD-1/PD-L1 inhibitors with potent anticancer efficacy in vivo. J. Med. Chem. 2021, 64, 7646–7666. [Google Scholar] [CrossRef]
- Hu, Z.; Yu, P.; Du, G.; Wang, W.; Zhu, H.; Li, N.; Zhao, H.; Dong, Z.; Ye, L.; Tian, J. PCC0208025 (BMS202), a small molecule inhibitor of PD-L1, produces an antitumor effect in B16-F10 melanoma-bearing mice. PLoS ONE 2020, 15, e0228339. [Google Scholar] [CrossRef]
- Yang, X.; Wang, W.; Ji, T. Metabolic remodeling by the PD-L1 inhibitor BMS-202 significantly inhibits cell malignancy in human glioblastoma. Cell Death Dis. 2024, 15, 186. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Vogt, F.G.; Li, X.; Hayes, D., Jr.; Mansour, H.M. Design, characterization, and aerosolization of organic solution advanced spray-dried moxifloxacin and ofloxacin dipalmitoylphosphatidylcholine (DPPC) microparticulate/nanoparticulate powders for pulmonary inhalation aerosol delivery. Int. J. Nanomed. 2013, 8, 3489–3505. [Google Scholar]
- Li, X.; Vogt, F.G.; Hayes, D., Jr.; Mansour, H.M. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Vogt, F.G.; Hayes, D., Jr.; Mansour, H.M. Design, characterization, and aerosol dispersion performance modeling of advanced spray-dried microparticulate/nanoparticulate mannitol powders for targeted pulmonary delivery as dry powder inhalers. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 81–93. [Google Scholar] [CrossRef]
- Meenach, S.A.; Vogt, F.G.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly (ethylene glycol)(DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols. Int. J. Nanomed. 2013, 8, 275–293. [Google Scholar]
- Muralidharan, P.; Hayes, D., Jr.; Fineman, J.R.; Black, S.M.; Mansour, H.M. Advanced Microparticulate/Nanoparticulate Respirable Dry Powders of a Selective RhoA/Rho Kinase (Rock) Inhibitor for Targeted Pulmonary Inhalation Aerosol Delivery. Pharmaceutics 2021, 13, 2188. [Google Scholar] [CrossRef]
- Wu, X.; Hayes, D., Jr.; Zwischenberger, J.B.; Kuhn, R.J.; Mansour, H.M. Design and physicochemical characterization of advanced spray-dried tacrolimus multifunctional particles for inhalation. Drug Des. Dev. Ther. 2013, 7, 59–72. [Google Scholar]
- Li, X.; Vogt, F.G.; Hayes, D., Jr.; Mansour, H.M. Design, characterization, and aerosol dispersion performance modeling of advanced co-spray dried antibiotics with mannitol as respirable microparticles/nanoparticles for targeted pulmonary delivery as dry powder inhalers. J. Pharm. Sci. 2014, 103, 2937–2949. [Google Scholar] [CrossRef]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. High-performing dry powder inhalers of paclitaxel DPPC/DPPG lung surfactant-mimic multifunctional particles in lung cancer: Physicochemical characterization, in vitro aerosol dispersion, and cellular studies. AAPS PharmSciTech 2014, 15, 1574–1587. [Google Scholar] [CrossRef]
- Acosta, M.F.; Abrahamson, M.D.; Encinas-Basurto, D.; Fineman, J.R.; Black, S.M.; Mansour, H.M. Inhalable nanoparticles/microparticles of an AMPK and Nrf2 activator for targeted pulmonary drug delivery as dry powder inhalers. AAPS J. 2021, 23, 2. [Google Scholar] [CrossRef]
- Acosta, M.F.; Muralidharan, P.; Grijalva, C.L.; Abrahamson, M.D.; Hayes, D., Jr.; Fineman, J.R.; Black, S.M.; Mansour, H.M. Advanced therapeutic inhalation aerosols of a Nrf2 activator and RhoA/Rho kinase (ROCK) inhibitor for targeted pulmonary drug delivery in pulmonary hypertension: Design, characterization, aerosolization, in vitro 2D/3D human lung cell cultures, and in vivo efficacy. Ther. Adv. Respir. Dis. 2021, 15, 1753466621998245. [Google Scholar] [PubMed]
- Alabsi, W.; Acosta, M.F.; Al-Obeidi, F.A.; Hay, M.; Polt, R.; Mansour, H.M. Synthesis, Physicochemical Characterization, In Vitro 2D/3D Human Cell Culture, and In Vitro Aerosol Dispersion Performance of Advanced Spray Dried and Co-Spray Dried Angiotensin (1—7) Peptide and PNA5 with Trehalose as Microparticles/Nanoparticles for Targeted Respiratory Delivery as Dry Powder Inhalers. Pharmaceutics 2021, 13, 1278. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.I.; Acosta, M.F.; Muralidharan, P.; Yuan, J.X.-J.; Black, S.M.; Hayes, D., Jr.; Mansour, H.M. Advanced spray dried proliposomes of amphotericin B lung surfactant-mimic phospholipid microparticles/nanoparticles as dry powder inhalers for targeted pulmonary drug delivery. Pulm. Pharmacol. Ther. 2020, 64, 101975. [Google Scholar] [CrossRef]
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.A.; Kumar, D.; Bolás, F.; Ballesteros, M. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.H.; Encinas-Basurto, D.; Sun, B.; Eedara, B.B.; Dickinson, S.E.; Wondrak, G.T.; Chow, H.-H.S.; Curiel-Lewandrowski, C.; Mansour, H.M. Design, physicochemical characterization, and in vitro permeation of innovative resatorvid topical formulations for targeted skin drug delivery. Pharmaceutics 2022, 14, 700. [Google Scholar] [CrossRef]
- Ruiz, V.H.; Encinas-Basurto, D.; Sun, B.; Eedara, B.B.; Roh, E.; Alarcon, N.O.; Curiel-Lewandrowski, C.; Bode, A.M.; Mansour, H.M. Innovative Rocuronium Bromide Topical Formulation for Targeted Skin Drug Delivery: Design, Comprehensive Characterization, In Vitro 2D/3D Human Cell Culture and Permeation. Int. J. Mol. Sci. 2023, 24, 8776. [Google Scholar] [CrossRef]
- Shafi, H.; Lora, A.J.; Donow, H.M.; Aggarwal, S.; Fu, P.; Wang, T.; Mansour, H.M. Advanced Spray-Dried Inhalable Microparticles/Nanoparticles of an Innovative Mitophagy Activator for Targeted Lung Delivery: Design, Comprehensive Characterization, Human Lung Cell Culture, and In Vitro Aerosol Dispersion Performance. ACS Pharmacol. Transl. Sci. 2024. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Kula, P.; Herman, J. High birefringence liquid crystals. Crystals 2013, 3, 443–482. [Google Scholar] [CrossRef]
- Colthup, N. Introduction to Infrared and Raman Spectroscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Colaianni, S.M.; Nielsen, O.F. Low-frequency Raman spectroscopy. J. Mol. Struct. 1995, 347, 267–283. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zografi, G. The quantitative analysis of crystallinity using FT-Raman spectroscopy. Pharm. Res. 1998, 15, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Rana, B.; Tomar, D.; Kaur, S.; Jena, K.C. Fundamentals of ATR-FTIR spectroscopy and its role for probing in-situ molecular-level interactions. In Modern Techniques of Spectroscopy: Basics, Instrumentation, and Applications; Springer: Singapore, 2021; pp. 3–37. [Google Scholar]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Shafi, H.; Rashid, R.; Rather, S.-U.; Reddy, D.S.; Azmi, L.; Abdal-hay, A.; Alrokayan, S.H.; Khan, H.A.; Khan, N.A.; Sheikh, F.A. Super disintegrating oromucosal nanofiber patch of zolmitriptan for rapid delivery and efficient brain targeting. Chem. Eng. J. 2023, 463, 142481. [Google Scholar] [CrossRef]
- Khan, T.; Ranjan, R.; Dogra, Y.; Pandya, S.M.; Shafi, H.; Singh, S.; Yadav, P.N.; Misra, A. Intranasal eutectic powder of zolmitriptan with enhanced bioavailability in the rat brain. Mol. Pharm. 2016, 13, 3234–3240. [Google Scholar] [CrossRef]
- Shafi, H.; Reddy, D.S.; Rashid, R.; Roy, T.; Kawoosa, S.; Bader, G.; Chakradhar, J.; Abdal-Hay, A.; Beigh, M.A.; Majeed, S. Optimizing the fabrication of electrospun nanofibers of prochlorperazine for enhanced dissolution and permeation properties. Biomater. Adv. 2024, 158, 213773. [Google Scholar] [CrossRef]
- Kim, S.; Day, C.M.; Song, Y.; Holmes, A.; Garg, S. Innovative topical patches for non-melanoma skin cancer: Current challenges and key formulation considerations. Pharmaceutics 2023, 15, 2577. [Google Scholar] [CrossRef]
- Brown, M.B.; Turner, R.; Lim, S.T. Topical product formulation development. In Transdermal and Topical Drug Delivery: Principles and Practice; Wiley: Hoboken, NJ, USA, 2012; pp. 255–284. [Google Scholar]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef]
- Bongioanni, A.; Bueno, M.S.; Mezzano, B.A.; Longhi, M.R.; Garnero, C. Pharmaceutical crystals: Development, optimization, characterization and biopharmaceutical aspects. In Crystal Growth and Chirality-Technologies and Applications; IntechOpen: London, UK, 2022. [Google Scholar]
- Chupak, L.S.; Zheng, X. Compounds Useful as Immunomodulators. WO2015034820A1, 12 March 2015. [Google Scholar]
- Zhang, H.; Xia, Y.; Yu, C.; Du, H.; Liu, J.; Li, H.; Huang, S.; Zhu, Q.; Xu, Y.; Zou, Y. Discovery of novel small-molecule inhibitors of PD-1/PD-L1 interaction via structural simplification strategy. Molecules 2021, 26, 3347. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Guzik, K.; Zieba, B.J.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1). Oncotarget 2016, 7, 30323. [Google Scholar] [CrossRef]
- Surmiak, E.; Magiera-Mularz, K.; Musielak, B.; Muszak, D.; Kocik-Krol, J.; Kitel, R.; Plewka, J.; Holak, T.A.; Skalniak, L. PD-L1 inhibitors: Different classes, activities, and mechanisms of action. Int. J. Mol. Sci. 2021, 22, 11797. [Google Scholar] [CrossRef]
- Basu, S.; Yang, J.; Xu, B.; Magiera-Mularz, K.; Skalniak, L.; Musielak, B.; Kholodovych, V.; Holak, T.A.; Hu, L. Design, synthesis, evaluation, and structural studies of C2-symmetric small molecule inhibitors of programmed cell death-1/programmed death-ligand 1 protein–protein interaction. J. Med. Chem. 2019, 62, 7250–7263. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, H.; Guo, W.; Liu, B.; Yan, W.; Zhang, C.; Qin, L.; Huang, J.; Wang, H.; Wu, S. Discovery of Highly Potent Small-Molecule PD-1/PD-L1 Inhibitors with a Novel Scaffold for Cancer Immunotherapy. J. Med. Chem. 2024, 67, 4083–4099. [Google Scholar] [CrossRef]
- Groenland, S.L.; Mathijssen, R.H.; Beijnen, J.H.; Huitema, A.D.; Steeghs, N. Individualized dosing of oral targeted therapies in oncology is crucial in the era of precision medicine. Eur. J. Clin. Pharmacol. 2019, 75, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.R.; Mayawala, K.; Gadamsetty, S.; Kang, S.P.; de Alwis, D.P. Optimal dosing for targeted therapies in oncology: Drug development cases leading by example. Clin. Cancer Res. 2016, 22, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef]
- Arumugasaamy, N.; Navarro, J.; Kent Leach, J.; Kim, P.C.; Fisher, J.P. In vitro models for studying transport across epithelial tissue barriers. Ann. Biomed. Eng. 2019, 47, 1–21. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
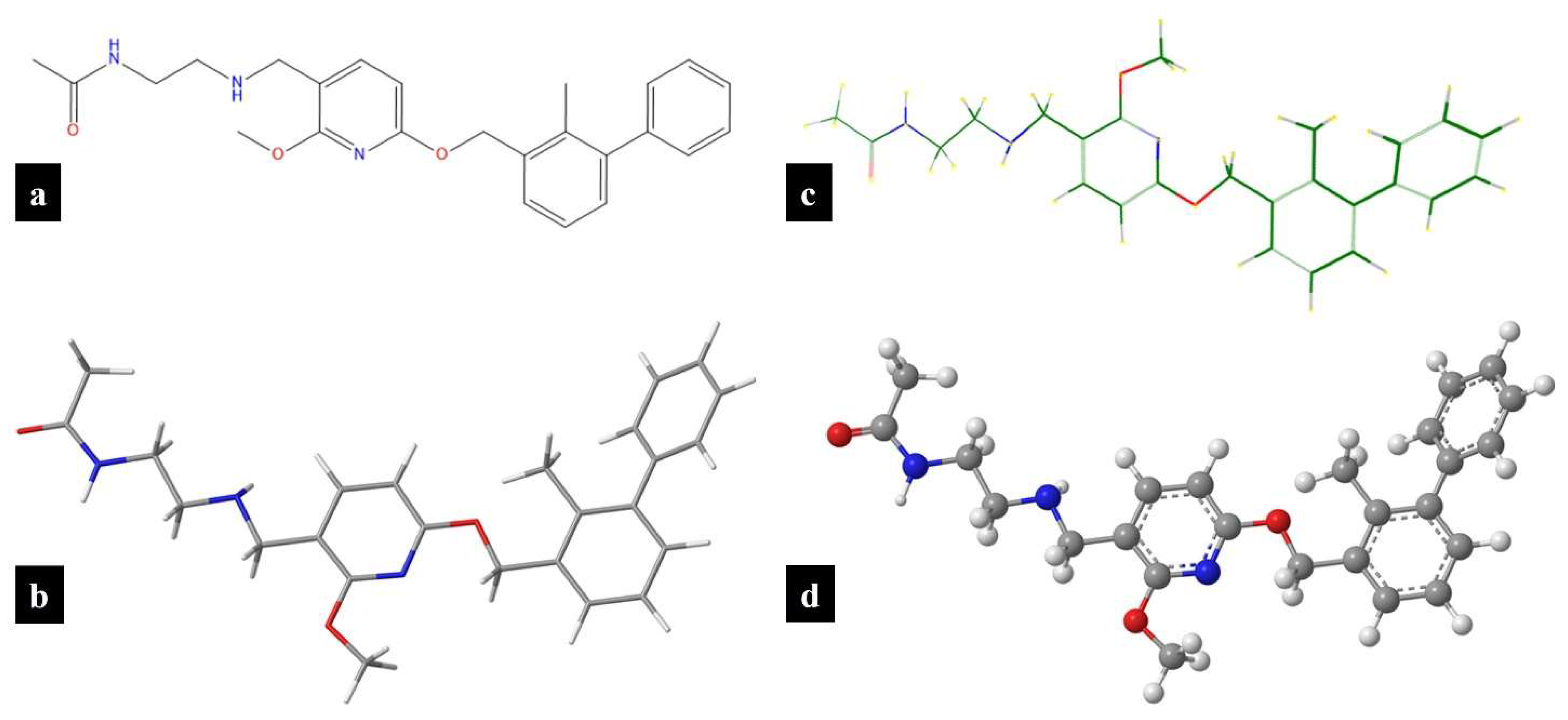






| Property | ChemDraw® | Chem3D® | Molecular Modeling Pro® |
|---|---|---|---|
| Exact Mass | 419.22 g/mol | 419.220891806 g/mol | N/A |
| Mol Weight | 419.53 g/mol | 419.525 g/mol | 419.4833 g/mol |
| Mol Formula | C25H29N3O3 | C25H29N3O | C25H29N3O |
| LogP | 4.37 Log Units | 5.10126 Log Units | 4.3251 Log Units |
| CLogP | 4.40815 Log Units | 4.40815 Log Units | 4.41308 Log Units |
| LogS | –6.084 Log Units | –6.08447 Log Units | –5.72508 Log Units |
| pKa | 8.080 Log Units | 8.080 Log Units | N/A |
| tPSA | 71.95 Å2 | 71.95 Å2 | 75.64 Å2 |
| Melting Point | 562.07 °C | N/A | N/A |
| Transition Type | Endothermic Peak | Exothermic Peak |
|---|---|---|
| Enthalpy (J/g) | 84.41 ± 0.38 | 198.92 ± 9.85 |
| Peak Temperature (°C) | 110.90 ± 0.54 | 260.87 ± 2.02 |
| Samples | Water Content (% w/w) |
|---|---|
| BMS-202 n = 1 | 1.909 |
| BMS-202 n = 2 | 2.028 |
| BMS-202 n = 3 | 4.338 |
| Average | 2.758 |
| Standard Deviation | ±1.369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafi, H.; Lora, A.J.; Donow, H.M.; Dickinson, S.E.; Wondrak, G.T.; Chow, H.-H.S.; Curiel-Lewandrowski, C.; Mansour, H.M. Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand. Pharmaceutics 2024, 16, 1409. https://doi.org/10.3390/pharmaceutics16111409
Shafi H, Lora AJ, Donow HM, Dickinson SE, Wondrak GT, Chow H-HS, Curiel-Lewandrowski C, Mansour HM. Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand. Pharmaceutics. 2024; 16(11):1409. https://doi.org/10.3390/pharmaceutics16111409
Chicago/Turabian StyleShafi, Hasham, Andrea J. Lora, Haley M. Donow, Sally E. Dickinson, Georg T. Wondrak, H.-H. Sherry Chow, Clara Curiel-Lewandrowski, and Heidi M. Mansour. 2024. "Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand" Pharmaceutics 16, no. 11: 1409. https://doi.org/10.3390/pharmaceutics16111409
APA StyleShafi, H., Lora, A. J., Donow, H. M., Dickinson, S. E., Wondrak, G. T., Chow, H.-H. S., Curiel-Lewandrowski, C., & Mansour, H. M. (2024). Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand. Pharmaceutics, 16(11), 1409. https://doi.org/10.3390/pharmaceutics16111409









