Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains
Abstract
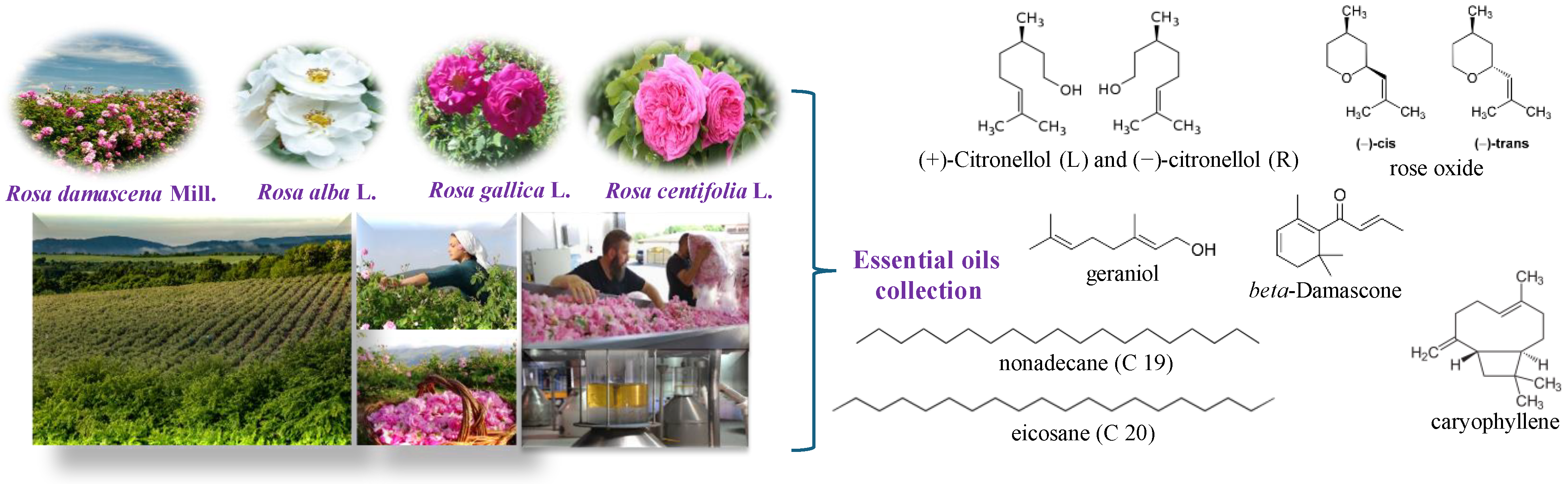
1. Introduction
2. Materials and Methods
2.1. Rose Oil Distillation
2.2. Chemical Analysis: Gas Chromatography (GC-FID/MS)
2.3. M. tuberculosis Strains
2.4. Resazurin Microtitre Plate Assay
2.5. Molecular Analysis of Bacterial Strains and Bioinformatics
3. Results and Discussion
3.1. Chemical Composition and Antimycobacterial Activity of the Essential Oils from Rosa spp.
3.2. Antibacterial Action and Potentially Underlying Genomic Variation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrigoni, R.; Ballini, A.; Topi, S.; Bottalico, L.; Jirillo, E.; Santacroce, L. Antibiotic Resistance to Mycobacterium tuberculosis and Potential Use of Natural and Biological Products as Alternative Anti-Mycobacterial Agents. Antibiotics 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, G.; Wen, S.; Huo, F.; Wang, F.; Huang, H.; Pang, Y. Para-aminosalicylic acid increases the susceptibility to isoniazid in clinical isolates of Mycobacterium tuberculosis. Infect. Drug. Resist. 2019, 12, 825–829. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2020: Executive Summary; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Low, W.L.; Kenward, K.; Britland, S.T.; Amin, M.C.; Martin, C. Essential oils and metal ions as alternative antimicrobial agents: A focus on tea tree oil and silver. Int. Wound J. 2017, 14, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Ginova, A.; Tsvetkov, I.; Kondakova, V. Rosa damascena Mill.—An overview for evaluation of propagation methods. Bulg. J. Agric. Sci. 2012, 18, 545–556. [Google Scholar]
- Kovatcheva, N.; Zheljazkov, V.D.; Astatkie, T. Productivity, oil content, composition, and bioactivity of oil-bearing rose accessions. HortScience 2011, 46, 710–714. [Google Scholar] [CrossRef]
- Younis, A.; Khan, M.; Ali, A.; Pervez, M. Performance of four Rosa species under Faisalabad agro-climatic conditions. Cad. Pesqui. J. 2006, 18, 8–15. [Google Scholar]
- Kirov, M.; Vankov, S. Rose, Rose oil and Jirozital; Publishing House “Medicine and Physical Culture”: Sofia, Bulgaria, 1986; p. 121. (In Bulgarian) [Google Scholar]
- Mileva, A.; Alexandrova, A.; Petrov, L. Rose Oil–Biological Effects and Application in Alternative and Conventional Medicine. Prime Arch. Biosci. 2022, 2, 1–22. [Google Scholar]
- Maia, W.M.N.; de Andrade, F.D.C.P.; Filgueiras, L.A.; Mendes, A.N.; Assunção, A.F.C.; Rodrigues, N.D.S.; Marques, R.B.; Maia Filho, A.L.M.; de Sousa, D.P.; Lopes, L.D.S. Antidepressant activity of rose oxide essential oil: Possible involvement of serotonergic transmission. Heliyon 2021, 7, e06620. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of Rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar]
- Yasa, N.; Masoumi, F.; Rouhani, R.S.; Haji, A.A. Chemical composition and antioxidant activity of the extract and essential oil of Rosa damascena from Iran, population of Guilan. DARU J. Pharm. Sci. 2009, 17, 175–180. [Google Scholar]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Önder, D. Variation in antioxidant capacity, antioxidant activity and mineral composition during flower development of oil-bearing rose (Rosa damascena Mill). Sci. Rep. 2023, 13, 17255. [Google Scholar] [CrossRef] [PubMed]
- Gochev, V.; Wlcek, K.; Buchbauer, G.; Stoyanova, A.; Dobreva, A.; Schmidt, E.; Jirovetz, L. Comparative Evaluation of Antimicrobial Activity and Composition of Rose Oils from Various Geographic Origins, in Particular Bulgarian Rose Oil. Nat. Prod. Comm. 2008, 3, 1063–1068. [Google Scholar] [CrossRef]
- Dinçoğlu, A.H.; Rugji, J. Use of rose oil in probiotic fermented whey as a functional food. J. Food Sci. Technol. 2021, 58, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils-Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Knobloch, K.; Pauli, A.; Iberl, B.; Weigand, H.; Weis, N. Antibacterial and antifungal properties of essential oil components. J. Essent. Oil Res. 1989, 1, 118–119. [Google Scholar] [CrossRef]
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Antimicrobial properties of basil and its possible application in food packaging. J. Agric. Food Chem. 2003, 51, 3197–3207. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Mileva, M.; Ilieva, Y.; Jovtchev, G.; Gateva, S.; Zaharieva, M.M.; Georgieva, A.; Dimitrova, L.; Dobreva, A.; Angelova, T.; Vilhelmova-Ilieva, N.; et al. Rose Flowers-A Delicate Perfume or a Natural Healer? Biomolecules 2021, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, A.; Nedeva, D.; Mileva, M. Comparative Study of the Yield and Chemical Profile of Rose Oils and Hydrosols Obtained by Industrial Plantations of Oil-Bearing Roses in Bulgaria. Resources 2023, 12, 83. [Google Scholar] [CrossRef]
- Huang, D.F.; Xu, J.G.; Liu, J.X.; Zhang, H.; Hu, Q.P. Chemical constituents, antibacterial activity and mechanism of action of the essential oil from Cinnamomum cassia bark against four food-related bacteria. Microbiology 2014, 83, 357–365. [Google Scholar] [CrossRef]
- Azhdarzadeh, F.; Hojjati, M. Chemical composition and antimicrobial activity of leaf, ripe and unripe peel of bitter orange (Citrus aurantium) essential oils. Nutr. Food Sci. Res. 2016, 3, 43–50. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.J.; Nikaido, H. The Envelope of Mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef]
- ISO 9842:2024; Essential oil of rose (Rosa x damascena Miller). International Organization for Standardization: Geneva, Switzerland, 2024. Available online: https://www.iso.org/standard/86897.html (accessed on 24 October 2024).
- NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 10 June 2024).
- Zalutskaya, A.; Wijkander, M.; Jureen, P.; Skrahina, A.; Hoffner, S. Multidrug-resistant Myobacterium tuberculosis caused by the Beijing genotype and a specific T1 genotype clone (SIT No. 266) is widely transmitted in Minsk. Int. J. Mycobacteriol. 2013, 2, 194–198. [Google Scholar] [CrossRef]
- Mokrousov, I.; Chernyaeva, E.; Vyazovaya, A.; Sinkov, V.; Zhuravlev, V.; Narvskaya, O. Next-Generation Sequencing of Mycobacterium tuberculosis. Emerg. Infect. Dis. 2016, 22, 1127–1129. [Google Scholar] [CrossRef]
- Mokrousov, I.; Vinogradova, T.; Dogonadze, M.; Zabolotnykh, N.; Vyazovaya, A.; Vitovskaya, M.; Solovieva, N.; Ariel, B. A multifaceted interplay between virulence, drug resistance, and the phylogeographic landscape of Mycobacterium tuberculosis. Microbiol. Spectr. 2023, 11, e0139223. [Google Scholar] [CrossRef]
- Coll, F.; McNerney, R.; Guerra-Assunção, J.A.; Glynn, J.R.; Perdigão, J.; Viveiros, M.; Portugal, I.; Pain, A.; Martin, N.; Clark, T.G. A robust SNP barcode for typing Mycobacterium tuberculosis complex strains. Nat. Commun. 2014, 5, 4812. [Google Scholar] [CrossRef]
- Vinogradova, T.; Dogonadze, M.; Zabolotnykh, N.; Badleeva, M.; Yarusova, I.; Vyazovaya, A.; Gerasimova, A.; Zhdanova, S.; Vitovskaya, M.; Solovieva, N.; et al. Extremely lethal and hypervirulent Mycobacterium tuberculosis strain cluster emerging in Far East, Russia. Emerg. Microbes Infect. 2021, 10, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Mokrousov, I.; Vyazovaya, A.; Shitikov, E.; Badleeva, M.; Belopolskaya, O.; Bespiatykh, D.; Gerasimova, A.; Ioannidis, P.; Jiao, W.; Khromova, P.; et al. Insight into pathogenomics and phylogeography of hypervirulent and highly-lethal Mycobacterium tuberculosis strain cluster. BMC Infect. Dis. 2023, 23, 426. [Google Scholar] [CrossRef] [PubMed]
- Shitikov, E.; Kolchenko, S.; Mokrousov, I.; Bespyatykh, J.; Ischenko, D.; Ilina, E.; Govorun, V. Evolutionary pathway analysis and unified classification of East Asian lineage of Mycobacterium tuberculosis. Sci. Rep. 2017, 7, 9227. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.S.; Kennedy, S.L.; Müller, R.; Stephens, R.H.; Holst, M.; Caffell, A.C.; Roberts, C.A.; Brown, T.A. Genotype of a historic strain of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2012, 109, 18511–18516. [Google Scholar] [CrossRef] [PubMed]
- Krasavin, M.; Lukin, A.; Vedekhina, T.; Manicheva, O.; Dogonadze, M.; Vinogradova, T.; Zabolotnykh, N.; Rogacheva, E.; Kraeva, L.; Sharoyko, V.; et al. Attachment of a 5-nitrofuroyl moiety to spirocyclic piperidines produces non-toxic nitrofurans that are efficacious in vitro against multidrug-resistant Mycobacterium tuberculosis. Eur. J. Med. Chem. 2019, 166, 125–135. [Google Scholar] [CrossRef]
- Ilieva, Y.; Dimitrova, L.; Georgieva, A.; Vilhelmova-Ilieva, N.; Zaharieva, M.M.; Kokanova-Nedialkova, Z.; Dobreva, A.; Nedialkov, P.; Kussovski, V.; Kroumov, A.D.; et al. In Vitro Study of the Biological Potential of Wastewater Obtained after the Distillation of Four Bulgarian Oil-Bearing Roses. Plants 2022, 11, 1073. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Franzblau, S.G.; Fischer, N.H. Antimycobacterial plant terpenoids. Planta Medica 2001, 67, 685–694. [Google Scholar] [CrossRef]
- Schön, T.; Werngren, J.; Machado, D.; Borroni, E.; Wijkander, M.; Lina, G.; Mouton, J.; Matuschek, E.; Kahlmeter, G.; Giske, C.; et al. Multicentre testing of the EUCAST broth microdilution reference method for MIC determination on Mycobacterium tuberculosis. Clin. Microbiol. Infect. 2021, 27, 288.e1–288.e4. [Google Scholar] [CrossRef]
- Mokrousov, I.; Slavchev, I.; Solovieva, N.; Dogonadze, M.; Vyazovaya, A.; Valcheva, V.; Masharsky, A.; Belopolskaya, O.; Dimitrov, S.; Zhuravlev, V.; et al. Molecular Insight into Mycobacterium tuberculosis Resistance to Nitrofuranyl Amides Gained through Metagenomics-like Analysis of Spontaneous Mutants. Pharmaceuticals 2022, 15, 1136. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, L.; Guo, L.; Wang, L.; Liu, Y. Antibacterial mechanism of rose essential oil against Pseudomonas putida isolated from white Hypsizygus marmoreus at cellular and metabolic levels. Ind. Crops Prod. 2023, 196, 116523. [Google Scholar] [CrossRef]
- Cao, D.; Jiang, X.; Wu, T.; Xiang, Y.; Liu, J.; Li, Z.; Yuan, X.; Bi, K.; Dong, X.; Tønjum, T.; et al. Identification of essential oils with strong activity against stationary phase Mycobacterium abscessus. Heliyon 2024, 10, e27073. [Google Scholar] [CrossRef] [PubMed]
- Baldin, V.P.; Bertin de Lima Scodro, R.; Mariano Fernandez, C.M.; Ieque, A.L.; Caleffi-Ferracioli, K.R.; Dias Siqueira, V.L.; de Almeida, A.L.; Gonçalves, J.E.; Garcia Cortez, D.A.; Cardoso, R.F. Ginger essential oil and fractions against Mycobacterium spp. J. Ethnopharmacol. 2019, 244, 112095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, C.; Zhang, N.; Peng, Y.; Ma, Y.; Gu, K.; Liu, X.; Liu, X.; Liu, X.; Liu, Y.; et al. Menthone Exerts its Antimicrobial Activity against Methicillin Resistant Staphylococcus aureus by Affecting Cell Membrane Properties and Lipid Profile. Drug Des. Dev. Ther. 2023, 17, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Camus, J.C.; Pryor, M.J.; Médigue, C.; Cole, S.T. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 2002, 148, 2967–2973. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, H.; Shao, Z.; Sun, L.; Li, C.; Lu, Y.; You, X.; Yang, X. Deletion of the Mycobacterium tuberculosis cyp138 gene leads to changes in membrane-related lipid composition and antibiotic susceptibility. Front. Microbiol. 2024, 15, 1301204. [Google Scholar] [CrossRef]
- Safi, H.; Gopal, P.; Lingaraju, S.; Ma, S.; Levine, C.; Dartois, V.; Yee, M.; Li, L.; Blanc, L.; Ho Liang, H.P.; et al. Phase variation in M. tuberculosis glpK produces transiently heritable drug tolerance. Proc. Natl. Acad. Sci. USA 2019, 116, 19665–19674. [Google Scholar] [CrossRef]
- Schempp, F.M.; Hofmann, K.E.; Mi, J.; Kirchner, F.; Meffert, A.; Schewe, H.; Schrader, J.; Buchhaupt, M. Investigation of monoterpenoid resistance mechanisms in Pseudomonas putida and their consequences for biotransformations. Appl. Microbiol. Biotechnol. 2020, 104, 5519–5533. [Google Scholar] [CrossRef]
- Laws, M.; Jin, P.; Rahman, K.M. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol. 2022, 30, 57–68. [Google Scholar] [CrossRef]
- Cassio Barreto de Oliveira, M.; Balan, A. The ATP-Binding Cassette (ABC) Transport Systems in Mycobacterium tuberculosis: Structure, Function, and Possible Targets for Therapeutics. Biology 2020, 9, 443. [Google Scholar] [CrossRef]
- Horváth, G.; Ács, K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. Flavour Fragr. J. 2015, 30, 331–341. [Google Scholar] [CrossRef]
- da Costa, R.H.S.; Rocha, J.E.; de Freitas, T.S.; Pereira, R.L.S.; Junior, F.N.P.; de Oliveira, M.R.C.; Batista, F.L.A.; Coutinho, H.D.M.; de Menezes, I.R.A. Evaluation of antibacterial activity and reversal of the NorA and MepA efflux pump of estragole against Staphylococcus aureus bacteria. Arch. Microbiol. 2021, 203, 3551–3555. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.-M.; Amaral, L.; Bolla, J.-M. Geraniol restores antibiotic activities against multidrug resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Duko, B.; Gebeyehu, A.; Ayano, G. Prevalence and correlates of depression and anxiety among patients with tuberculosis at Wolaita Sodo University Hospital and Sodo Health Center, Wolaita Sodo, South Ethiopia, Cross-sectional study. BMC Psychiatry 2015, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.O.; Dearman, S.P.; Chaudhry, I.B.; Rizvi, N.; Waheed, W. The relationship between anxiety, depression, and illness perception in tuberculosis patients in Pakistan. Clin. Pract. Epidemiol. Ment. Health 2008, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Ige, O.M.; Lasebikan, V.O. Prevalence of depression in tuberculosis patients in comparison with non-tuberculosis family contacts visiting the DOTS clinic in a Nigerian tertiary care hospital and its correlation with disease pattern. Ment. Health Fam. Med. 2011, 8, 235–241. [Google Scholar]
- Peltzer, K.; Naidoo, P.; Matseke, G.; Louw, J.; McHunu, G.; Tutshana, B. Prevalence of psychological distress and associated factors in tuberculosis patients in public primary care clinics in South Africa. BMC Psychiatry 2012, 12, 89. [Google Scholar] [CrossRef]

| Compound | RI Calc. | RI Lit. | R. damascena | R. alba | R. gallica | R. centifolia |
|---|---|---|---|---|---|---|
| Ethanol | 489 | 489 | 0.09 ± 0.02 | 0.10± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Phenylethanol | 1110 | 1110 | 0.48 ± 0.02 | 0.22 ± 0.02 | 0.29 ± 0.03 | 0.10 ± 0.01 |
| Citronellol | 1229 | 1228 | 29.77 ± 0.42 | 12.64 ± 0.92 | 8.38 ± 0.20 | 7.60 ± 0.22 |
| Nerol | 1751 * | 1751 * | 11.04 ± 0.12 | 10.92 ± 0.70 | 4.72 ± 0.52 | 4.36 ± 0.42 |
| Geraniol | 1248 | 1246 | 21.05 ± 0.90 | 30.98 ± 0.10 | 21.93 ± 0.04 | 15.35 ± 0.12 |
| Methyl eugenol | 1405 | 1405 | 1.30 ± 0.10 | 0.56 ± 0.04 | 0.94 ± 0.33 | 0.62 ± 0.10 |
| Heptadecane (C17) | 1700 | 1700 | 1.64 ± 0.00 | 1.98 ± 0.07 | 2.78 ± 0.10 | 1.38 ± 0.09 |
| Nonadecene(C19:1) | 1880 | 1880 | 2.30 ± 0.14 | 4.41 ± 0.24 | 1.71 ± 0.07 | 1.75 ± 0.02 |
| Nonadecane (C19) | 1901 | 1901 | 11.12 ± 0.06 | 11.70 ± 0.07 | 21.18 ± 0.10 | 18.62 ± 0.10 |
| Eicosane (C20) | 2000 | 2000 | 1.08 ± 0.05 | 1.21 ± 0.00 | 1.60 ± 0.12 | 0.96 ± 0.02 |
| Heneicosane (C21) | 2100 | 2100 | 7.02 ± 0.72 | 9.86 ± 0.09 | 9.07 ± 0.12 | 6.78 ± 0.04 |
| Essential Oils | H37Rv (Susceptible, L4.9) | 4542 (MDR, LAM Genotype) | 396 (MDR, Beijing Genotype) |
|---|---|---|---|
| R. alba L. | 0.16 | 0.31 | 0.31 |
| R. centifolia L. | 0.16 | 1.25 | 0.62 |
| R. damascena Mill. | 0.31 | 1.25 | 0.62 |
| R. gallica L. | 1.25 | 1.25 | 0.62 |
| Genome Pos. | Label | Gene | Codon | aa | PAM1 ** | SIFT P ** | Protein Function (Based on Information from Micobrowser and STRING Tool) |
|---|---|---|---|---|---|---|---|
| 222925 | Rv0191 | Rv0191 | 213 | A-T | 22 | 0.11 | MFS-type transporter. Probable conserved integral membrane protein; Active efflux pump that plays an important role in chloramphenicol resistance. Overexpression causes pyrazinamide resistance |
| 227098 * | Rv0194 | Rv0194 | 74 | M-T | 6 | 1.00 | Multidrug ABC transporter ATPase/permease Probable transmembrane multidrug efflux pump; Overexpression in M. smegmatis increases resistance to erythromycin, ampicillin, novobiocin, and vancomycin. |
| 230170 | Rv0194 | Rv0194 | 1098 | P-L | 3 | 0.00 | |
| 775639 * | Rv0676c | mmpL5 | 948 | I-V | 57 | 1.00 | Probable conserved transmembrane transport protein mmpl5; Part of an export system, which is required for biosynthesis and secretion of siderophores |
| 776100 | Rv0676c | mmpL5 | 794 | T-I | 7 | 1.00 | |
| 778743 | Rv0677c | mmpS5 | 55 | V-M | 4 | 0.08 | Possible conserved membrane protein mmps5; Part of an export system, which is required for biosynthesis and secretion of siderophores. Essential for virulence |
| 1361285 | Rv1217c | Rv1217c | 173 | A-T | 22 | 0.18 | Probable tetronasin-transport integral membrane protein ABC transporter; Probably part of the ABC transporter complex Rv1217c-Rv1218c involved in the resistance to a wide range of structurally unrelated drugs. Probably responsible for the translocation of the substrate across the membrane |
| 1362006 | Rv1218c | Rv1218c | 243 | Q-R | 10 | 0.60 | Probable tetronasin-transport atp-binding protein ABC transporter; Probably part of the ABC transporter complex Rv1217c-Rv1218c involved in the resistance to a wide range of structurally unrelated drugs. Could be involved in the efflux of substrates belonging to the diverse chemical classes of novobiocins, biarylpiperazines, pyridines, bisanilinopyrimidines, pyrroles and, to a smaller extent, pyrazolones. Probably responsible for energy coupling to the transport system |
| 1839759 | Rv1634 | Rv1634 | 198 | G-R | 0 | 0.52 | Possible drug efflux membrane protein; Could be involved in fluoroquinolones efflux |
| 3005014 | Rv2688c | Rv2688c | 213 | C-R | 1 | 0.00 | Antibiotic-transport ATP-binding protein ABC transporter; Part of the ABC transporter complex Rv2686c/Rv2687c/Rv2688c involved in fluoroquinolones export. Probably responsible for energy coupling to the transport system |
| 3005185 | Rv2688c | Rv2688c | 156 | P-T | 5 | 0.83 | |
| 2608117 | Rv2333c/Stp | Rv2333c/Stp | 69 | D-Y | 3 | 1.00 | Integral membrane drug efflux protein stp; Contributes to spectinomycin and tetracycline resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valcheva, V.; Mileva, M.; Dogonadze, M.; Dobreva, A.; Mokrousov, I. Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains. Pharmaceutics 2024, 16, 1393. https://doi.org/10.3390/pharmaceutics16111393
Valcheva V, Mileva M, Dogonadze M, Dobreva A, Mokrousov I. Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains. Pharmaceutics. 2024; 16(11):1393. https://doi.org/10.3390/pharmaceutics16111393
Chicago/Turabian StyleValcheva, Violeta, Milka Mileva, Marine Dogonadze, Ana Dobreva, and Igor Mokrousov. 2024. "Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains" Pharmaceutics 16, no. 11: 1393. https://doi.org/10.3390/pharmaceutics16111393
APA StyleValcheva, V., Mileva, M., Dogonadze, M., Dobreva, A., & Mokrousov, I. (2024). Antimycobacterial Activity of Essential Oils from Bulgarian Rosa Species Against Phylogenomically Different Mycobacterium tuberculosis Strains. Pharmaceutics, 16(11), 1393. https://doi.org/10.3390/pharmaceutics16111393






