Enhancing Gentamicin Antibacterial Activity by Co-Encapsulation with Thymoquinone in Liposomal Formulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Liposomal Formulation
Liposomal Gentamicin–Thymoquinone (Lipo-GEN-THQ) Preparation
2.3. Liposomal Gentamicin–Thymoquinone (Lipo-GEN-THQ) Characterization
2.3.1. Gentamicin Concentration, Encapsulation Efficacy (%EE), and Drug-Loading Capacity (%LC)
2.3.2. Dynamic Size, Polydispersity Index, and Z-Potential
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3.4. Biological Stability
2.4. Transmission Electron Microscopy
2.4.1. Lipo-GEN-THQ Liposome Morphology
2.4.2. Empty Liposome–Bacterial Membrane Fusion
2.4.3. Loaded Liposome–Bacterial Interaction
2.5. Biological Activity
2.5.1. Tested Bacteria and Growth Conditions
2.5.2. Free Drugs Checkerboard Assay
2.5.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
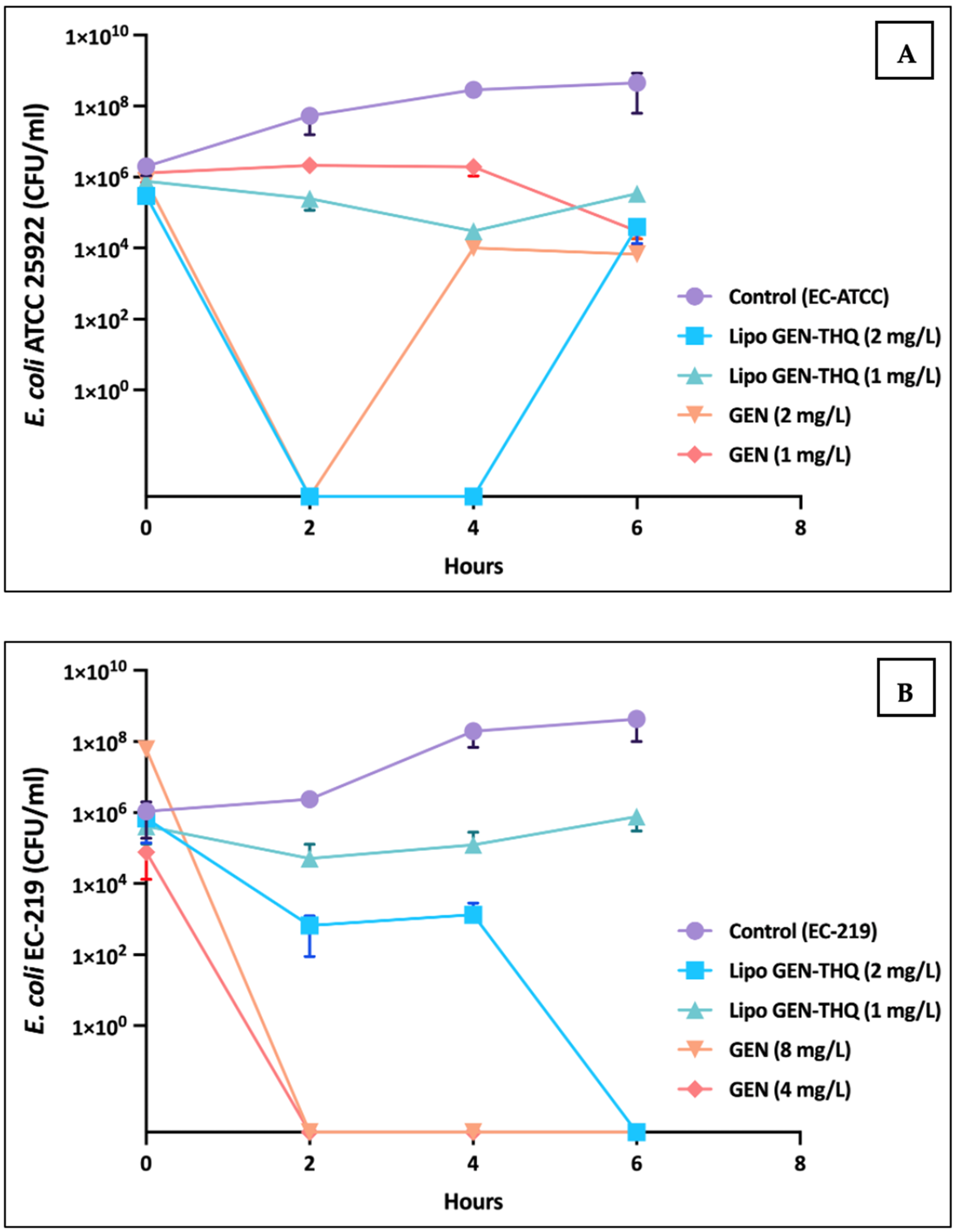
2.5.4. Time–Kill Curve Assay
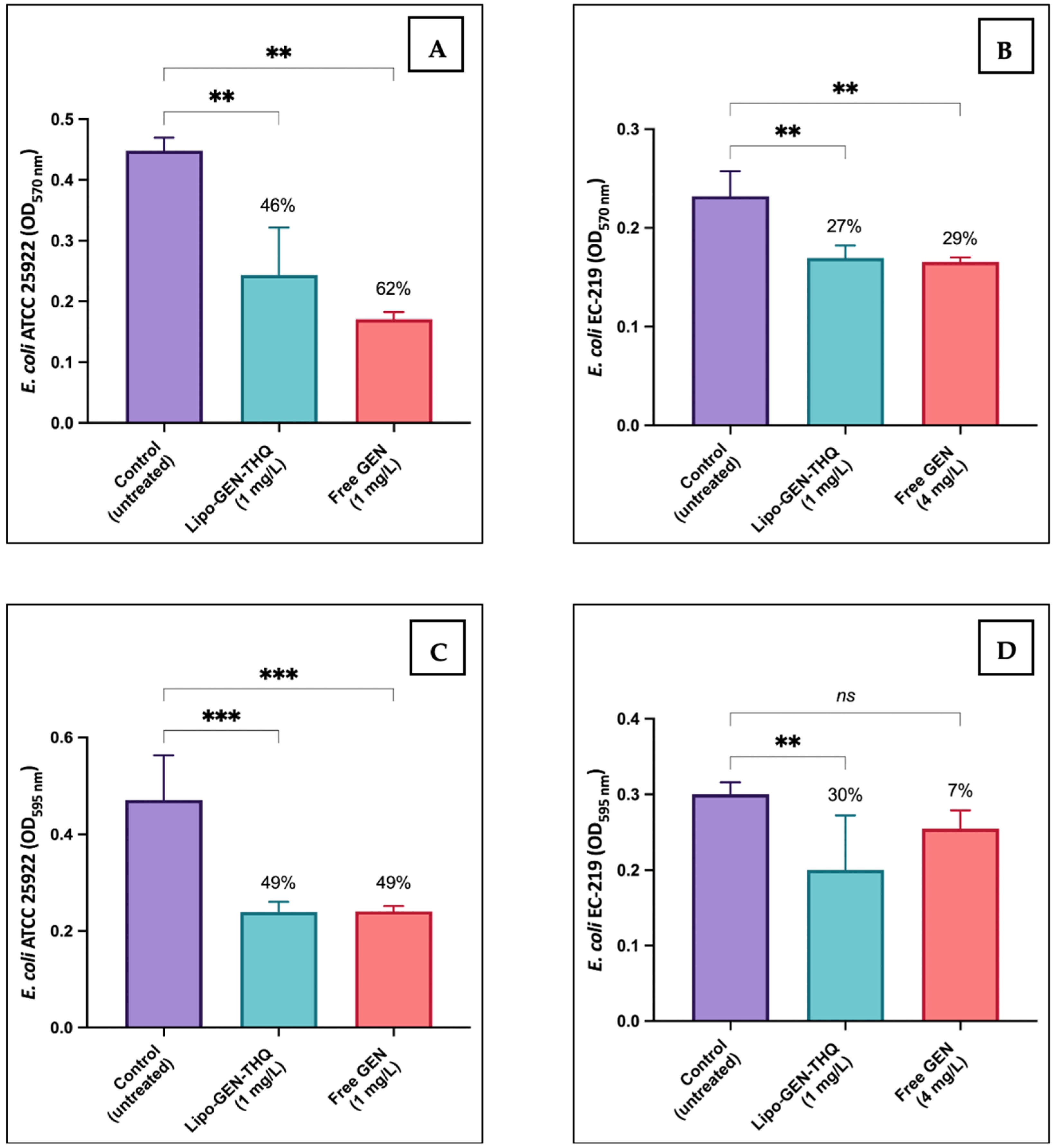
2.5.5. Biofilm Inhibition and Eradication
2.6. Lipo-GEN-THQ Cell Adhesion Prevention
2.6.1. Cell Line and Tested Bacteria
2.6.2. Antiadhesion Assay
2.7. Statistical Analysis
3. Results
3.1. Physical Characterization of Lipo-GEN-THQ Formulation: Size, Polydispersity Index, and Z-Potential
3.2. FTIR Analysis
3.3. GEN Retention and Lipo-GEN-THQ Formulation Stability
3.4. TEM Imaging of Empty Liposome–Bacterial Membrane Fusion
3.5. TEM Imaging of Loaded Liposomes (Lipo-GEN-THQ) Effect on Bacterial Ultrastructure
3.6. GEN Concentration, Checkerboard Assay, Minimum Inhibitory Concentrations (MICs), and Minimum Bactericidal Concentrations (MBCs)
3.7. Time–Dose Response of GEN vs Lipo-GEN-THQ Formulation at MIC and Sub-MIC Concentrations
3.8. Biofilm Inhibition and Eradication Assays
3.9. Antiadhesion Activity of Sub-Inhibitory Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. IScience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Childs-Kean, L.M.; Shaeer, K.M.; Varghese Gupta, S.; Cho, J.C. Aminoglycoside allergic reactions. Pharmacy 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Barker, C.I.; Sharland, M. What do I need to know about aminoglycoside antibiotics? Arch. Dis. Child. Educ. Pract. 2017, 102, 89–93. [Google Scholar] [CrossRef]
- Riedel, S.; Morse, S.A.; Mietzner, T.A.; Miller, S. Jawetz Melnick & Adelbergs Medical Microbiology 28 E; McGraw Hill Professional: New York, NY, USA, 2019. [Google Scholar]
- Rosenberg, C.R.; Fang, X.; Allison, K.R. Potentiating aminoglycoside antibiotics to reduce their toxic side effects. PLoS ONE 2020, 15, e0237948. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Mauricio, M.D.; Guerra-Ojeda, S.; Marchio, P.; Valles, S.L.; Aldasoro, M.; Escribano-Lopez, I.; Herance, J.R.; Rocha, M.; Vila, J.M.; Victor, V.M. Nanoparticles in medicine: A focus on vascular oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 6231482. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A review of the pharmacokinetics, pharmacodynamics, clinical experience and future directions. Drugs 2016, 76, 485. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef]
- Su, H.; Jia, J.; Mao, Y.; Zhu, R.; Li, Z. A real-world analysis of FDA Adverse Event Reporting System (FAERS) events for liposomal and conventional doxorubicins. Sci. Rep. 2024, 14, 5095. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.M.S.; Bento, E.B.; da Cunha Almeida, L.; de Sá, L.Z.C.M.; Lima, E.M. Preparation, characterization and in vitro antimicrobial activity of liposomal ceftazidime and cefepime against Pseudomonas aeruginosa strains. Braz. J. Microbiol. 2012, 43, 984. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Allemailem, K.S.; Alnuqaydan, A.M.; Almatroudi, A.; Alrumaihi, F.; Aljaghwani, A.; Khalilullah, H.; Younus, H.; Khan, A.; Khan, M.A. Safety and Therapeutic Efficacy of Thymoquinone-Loaded Liposomes against Drug-Sensitive and Drug-Resistant Acinetobacter baumannii. Pharmaceutics 2021, 13, 677. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950. [Google Scholar]
- Shaterzadeh-Yazdi, H.; Noorbakhsh, M.-F.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Immunomodulatory and Anti-inflammatory Effects of Thymoquinone. Cardiovasc. Hematol. Disord. Drug Targets 2018, 18, 52–60. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Alhariri, M.; Majrashi, M.A.; Bahkali, A.H.; Almajed, F.S.; Azghani, A.O.; Khiyami, M.A.; Alyamani, E.J.; Aljohani, S.M.; Halwani, M. Efficacy of neutral and negatively charged liposome-loaded gentamicin on planktonic bacteria and biofilm communities. Int. J. Nanomed. 2017, 12, 6949. [Google Scholar] [CrossRef]
- Rukholm, G.; Mugabe, C.; Azghani, A.O.; Omri, A. Antibacterial activity of liposomal gentamicin against Pseudomonas aeruginosa: A time–kill study. Int. J. Antimicrob. Agents 2006, 27, 247–252. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.M.; Khan, M.W.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. [Google Scholar] [CrossRef]
- Alarfaj, R.E.; Alkhulaifi, M.M.; Al-Fahad, A.J.; Aljihani, S.; Yassin, A.E.B.; Alghoribi, M.F.; Halwani, M.A. Antibacterial Efficacy of Liposomal Formulations Containing Tobramycin and N-Acetylcysteine against Tobramycin-Resistant Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii. Pharmaceutics 2022, 14, 130. [Google Scholar] [CrossRef]
- Mugabe, C.; Halwani, M.; Azghani, A.O.; Lafrenie, R.M.; Omri, A. Mechanism of Enhanced Activity of Liposome-Entrapped Aminoglycosides against Resistant Strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 50, 2016–2022. [Google Scholar] [CrossRef]
- Nguyen, H.T.; O’donovan, L.A.; Venter, H.; Russell, C.C.; McCluskey, A.; Page, S.W.; Trott, D.J.; Ogunniyi, A.D. Comparison of Two Transmission Electron Microscopy Methods to Visualize Drug-Induced Alterations of Gram-Negative Bacterial Morphology. Antibiotics 2021, 10, 307. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Marked synergistic bactericidal effects and mode of action of medium-chain fatty acids in combination with organic acids against Escherichia coli O157: H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Disease (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 1–7. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Kirby, J.E. Antimicrobial Synergy Testing by the Inkjet Printer-assisted Automated Checkerboard Array and the Manual Time-kill Method. J. Vis. Exp. 2019, 146, e58636. [Google Scholar] [CrossRef]
- NCCLS. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Haney, E.F.; Trimble, M.J.; Hancock, R.E.W. Microtiter plate assays to assess antibiofilm activity against bacteria. Nat. Protoc. 2021, 16, 2615–2632. [Google Scholar] [CrossRef]
- Molina Bertrán, S.d.C.; Monzote, L.; Cappoen, D.; Escalona Arranz, J.C.; Gordillo Pérez, M.J.; Rodríguez-Ferreiro, A.O.; Chill Nuñez, I.; Novo, C.P.; Méndez, D.; Cos, P.; et al. Inhibition of Bacterial Adhesion and Biofilm Formation by Seed-Derived Ethanol Extracts from Persea americana Mill. Molecules 2022, 27, 5009. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0. 2023. Sweden. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 20 November 2023).
- Flamm, R.K.; Rhomberg, P.R.; Lindley, J.M.; Sweeney, K.; Ellis-Grosse, E.J.; Shortridge, D. Evaluation of the bactericidal activity of fosfomycin in combination with selected antimicrobial comparison agents tested against Gram-negative bacterial strains by using time-kill curves. Antimicrob. Agents Chemother. 2019, 63, e02549-18. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Langa, E.; Valenzuela, A.; Ballestero, D.; Pino-Otín, M.R. Synergistic Activity of Thymol with Commercial Antibiotics against Critical and High WHO Priority Pathogenic Bacteria. Plants 2023, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Reeder, R. Antibiotic use on crops in low and middle-income countries based on recommendations made by agricultural advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- LiverTox. Aminoglycosides; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Rybak, L.P.; Ramkumar, V. Ototoxicity. Kidney Int. 2007, 72, 931–935. [Google Scholar] [CrossRef]
- Kushner, B.; Allen, P.D.; Crane, B.T. Frequency and Demographics of Gentamicin Use. Otol. Neurotol. 2016, 37, 190. [Google Scholar] [CrossRef]
- FDA. Liposome Drug Chemistry, Manufacturing, and Controls, Human Products Pharmacokinetics and Bioavailability, and Labeling Documentation; Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Imran, M.; Revol-Junelles, A.M.; Paris, C.; Guedon, E.; Linder, M.; Desobry, S. Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci. Technol. 2015, 62, 341–349. [Google Scholar] [CrossRef]
- Li, Z.; Peng, S.; Chen, X.; Zhu, Y.; Zou, L.; Zhou, W.; Liu, W.; Liu, C. Effect of dynamic high pressure microfluidization on structure and stability of pluronic F127 modified liposomes. J. Dispers. Sci. Technol. 2019, 40, 982–989. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, D.; Sui, G.; Wang, D.; Wu, M.; Han, L.; Mu, H.; Duan, J. Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria. Int. J. Biol. Macromol. 2020, 156, 640–647. [Google Scholar] [CrossRef]
- Mu, H.; Tang, J.; Liu, Q.; Sun, C.; Wang, T.; Duan, J. Potent Antibacterial Nanoparticles against Biofilm and Intracellular Bacteria. Sci. Rep. 2016, 6, 18877. [Google Scholar] [CrossRef]
- Sainaga Jyothi, V.G.S.; Bulusu, R.; Venkata Krishna Rao, B.; Pranothi, M.; Banda, S.; Kumar Bolla, P.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef]
- Roberts, S.A.; Lee, C.; Singh, S.; Agrawal, N. Versatile Encapsulation and Synthesis of Potent Liposomes by Thermal Equilibration. Membranes 2022, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Batul, R.; Bhave, M.; Mahon, P.J.; Yu, A. Polydopamine Nanosphere with In-Situ Loaded Gentamicin and Its Antimicrobial Activity. Molecules 2020, 25, 2090. [Google Scholar] [CrossRef] [PubMed]
- Pagola, S.; Benavente, A.; Raschi, A.; Romano, E.; Molina, M.A.A.; Stephens, P.W. Crystal structure determination of thymoquinone by high-resolution x-ray powder diffraction. AAPS PharmSciTech 2004, 5, e28. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018, 295, 119–132. [Google Scholar] [CrossRef]
- Mondal, R.; Bobde, Y.; Ghosh, B.; Giri, T.K. Development and Characterization of a Phospholipid Complex for Effective Delivery of Capsaicin. Indian J. Pharm. Sci. 2019, 81, 1011–1019. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Qatifi SAl Alessa, M.; Hajji, H.A.; Sarafroz, M. A bioanalytical UHPLC based method used for the quantification of thymoquinone-loaded-PLGA-nanoparticles in the treatment of epilepsy. BMC Chem. 2020, 14, 10. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Pande, S. Liposomes for drug delivery: Review of vesicular composition, factors affecting drug release and drug loading in liposomes. Artif. Cells Nanomed. Biotechnol. 2023, 51, 428–440. [Google Scholar] [CrossRef]
- Ramli, N.A.; Ali, N.; Hamzah, S.; Yatim, N.I. Physicochemical characteristics of liposome encapsulation of stingless bees’ propolis. Heliyon 2021, 7, e06649. [Google Scholar] [CrossRef]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef]
- Martin, N.L.; Beveridge, T.J. Gentamicin interaction with Pseudomonas aeruginosa cell envelope. Antimicrob. Agents Chemother. 1986, 29, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.W.; Keepers, T.; Andrews, L.; Krause, K.M. Aminoglycoside Revival: Review of a Historically Important Class of Antimicrobials Undergoing Rejuvenation. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Hilpert, K.; Gani, J.; Rumancev, C.; Simpson, N.; Lopez-Perez, P.M.; Garamus, V.M.; von Gundlach, A.R.; Markov, P.; Scocchi, M.; Mikut, R.; et al. Rational Designed Hybrid Peptides Show up to a 6-Fold Increase in Antimicrobial Activity and Demonstrate Different Ultrastructural Changes as the Parental Peptides Measured by BioSAXS. Front. Pharmacol. 2021, 12, 769739. [Google Scholar] [CrossRef] [PubMed]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Al Shahrani, M.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196. [Google Scholar] [CrossRef] [PubMed]
- Halawani, E. Antibacterial Activity of Thymoquinone and Thymohydroquinone of Nigella sativa L. and Their Interaction with Some Antibiotics. Adv. Biol. Res. 2009, 3, 148–152. [Google Scholar]
- Jankowski, G.; Sawicki, R.; Truszkiewicz, W.; Wolan, N.; Ziomek, M.; Hryć, B.; Sieniawska, E. Molecular insight into thymoquinone mechanism of action against Mycobacterium tuberculosis. Front. Microbiol. 2024, 15, 1353875. [Google Scholar] [CrossRef]
- Wang, S.; Deng, H.; Wang, Y.; Rui, W.; Zhao, P.; Yong, Q.; Guo, D.; Liu, J.; Guo, X.; Wang, Y.; et al. Antimicrobial Activity and Action Mechanism of Thymoquinone against Bacillus cereus and Its Spores. Foods 2021, 10, 3048. [Google Scholar] [CrossRef]
- Scriboni, A.B.; Couto, V.M.; De Morais Ribeiro, L.N.; Freires, I.A.; Groppo, F.C.; De Paula, E.; Franz-Montan, M.; Cogo-Müller, K. Fusogenic Liposomes Increase the Antimicrobial Activity of Vancomycin Against Staphylococcus aureus Biofilm. Front. Pharmacol. 2019, 10, 1401. [Google Scholar] [CrossRef]
- Bera, S.; Mondal, D. Antibacterial Efficacies of Nanostructured Aminoglycosides. ACS Omega 2022, 7, 4724–4734. [Google Scholar] [CrossRef]
- Hill, M.; Cunningham, R.N.; Hathout, R.M.; Johnston, C.; Hardy, J.G.; Migaud, M.E. Formulation of antimicrobial tobramycin loaded PLGA nanoparticles via complexation with AOT. J. Funct. Biomater. 2019, 10, 26. [Google Scholar] [CrossRef]
- Lu, E.; Franzblau, S.; Onyuksel, H.; Popescu, C. Preparation of aminoglycoside-loaded chitosan nanoparticles using dextran sulphate as a counterion. J. Microencapsul. 2009, 26, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Mohammadi, S.; Vaezi, Z.; Naderi-Manesh, H. Improvement of anti-biofilm activities via co-delivery of curcumin and gentamicin in lipid-polymer hybrid nanoparticle. J. Biomater. Sci. Polym. Ed. 2022, 33, 174–196. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.M.; Quinn, D.J.; Ingram, R.J.; Gilmore, B.F.; Donnelly, R.F.; Taggart, C.C.; Scott, J.J. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar] [CrossRef]
- Mostafa, M.; Alaaeldin, E.; Aly, U.F.; Sarhan, H.A. Optimization and Characterization of Thymoquinone-Loaded Liposomes with Enhanced Topical Anti-inflammatory Activity. AAPS PharmSciTech 2018, 19, 3490–3500. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2018, 47, 13–23. [Google Scholar] [CrossRef]
- Di Martino, P. Effects of Antibiotics on Adherence of Pseudomonas aeruginosa and Pseudomonas fluorescens to Human Fibronectin. Chemotherapy 2001, 47, 344–349. [Google Scholar] [CrossRef]
- Berim, I.; Sethi, S. Community-Acquired Pneumonia. In Clinical Respiratory Medicine, 4th ed.; Saunders: Philadelphia, PA, USA, 2012; pp. 296–308. [Google Scholar] [CrossRef]







| Characteristic | Lipo-GEN-THQ Formula | Empty Liposomes |
|---|---|---|
| Hydrodynamic size (nm) | 107.9 (2.45) | 104.5 (1.66) |
| Polydispersity index (PDI) | 0.15 (0.04) | 0.07 (0.01) |
| Geometric size (nm) | 109.2 (40.88) | 97.0 (35.48) |
| Zeta potential (Mv) | −16.89 (4.10) | −44.13 (9.30) |
| Bacteria | AST ** | FICI | Free GEN (mg/L) | Lipo-GEN-THQ (mg/L) | ||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |||
| Staphylococcus aureus ATCC 29213 | Susceptible | Indifferent | 2 | 4 | 2 | 4 |
| Escherichia coli ATCC 25922 | Susceptible | Indifferent | 2 | 4 | 2 | 1 |
| E. coli EC-83 * | Susceptible | Indifferent | 2 | 4 | 1 | 2 |
| E. coli EC-157 * | Susceptible | Indifferent | 2 | 4 | 1 | 2 |
| E. coli EC-219 * | Resistant | Synergy | 8 | 16 | 2 | 4 |
| E. coli EC-542 * | Susceptible | Indifferent | 2 | 4 | 1 | 2 |
| E. coli EC-543 * | Susceptible | Indifferent | 2 | 4 | 2 | 4 |
| Klebsiella pneumonia KP-19 * | Susceptible | Indifferent | 1 | 2 | 1 | 2 |
| K. pneumonia KP-47 * | Susceptible | Indifferent | 1 | 2 | 1 | 2 |
| K. pneumonia KP-86 * | Susceptible | Indifferent | 1 | 2 | 0.5 | 1 |
| K. pneumonia KP-160 * | Susceptible | Synergy | 2 | 4 | 2 | 4 |
| K. pneumonia KP-206 * | Susceptible | Indifferent | 0.5 | 1 | 0.5 | 1 |
| K. pneumonia KP-208 * | Susceptible | Indifferent | 1 | 2 | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, R.R.; Alkhulaifi, M.M.; Al Jeraisy, M.; Albekairy, A.M.; Ali, R.; Alrfaei, B.M.; Ehaideb, S.N.; Al-Asmari, A.I.; Qahtani, S.A.; Halwani, A.; et al. Enhancing Gentamicin Antibacterial Activity by Co-Encapsulation with Thymoquinone in Liposomal Formulation. Pharmaceutics 2024, 16, 1330. https://doi.org/10.3390/pharmaceutics16101330
Alzahrani RR, Alkhulaifi MM, Al Jeraisy M, Albekairy AM, Ali R, Alrfaei BM, Ehaideb SN, Al-Asmari AI, Qahtani SA, Halwani A, et al. Enhancing Gentamicin Antibacterial Activity by Co-Encapsulation with Thymoquinone in Liposomal Formulation. Pharmaceutics. 2024; 16(10):1330. https://doi.org/10.3390/pharmaceutics16101330
Chicago/Turabian StyleAlzahrani, Raghad R., Manal M. Alkhulaifi, Majed Al Jeraisy, Abdulkareem M. Albekairy, Rizwan Ali, Bahauddeen M. Alrfaei, Salleh N. Ehaideb, Ahmed I. Al-Asmari, Sultan Al Qahtani, Abdulaziz Halwani, and et al. 2024. "Enhancing Gentamicin Antibacterial Activity by Co-Encapsulation with Thymoquinone in Liposomal Formulation" Pharmaceutics 16, no. 10: 1330. https://doi.org/10.3390/pharmaceutics16101330
APA StyleAlzahrani, R. R., Alkhulaifi, M. M., Al Jeraisy, M., Albekairy, A. M., Ali, R., Alrfaei, B. M., Ehaideb, S. N., Al-Asmari, A. I., Qahtani, S. A., Halwani, A., Yassin, A. E. B., & Halwani, M. A. (2024). Enhancing Gentamicin Antibacterial Activity by Co-Encapsulation with Thymoquinone in Liposomal Formulation. Pharmaceutics, 16(10), 1330. https://doi.org/10.3390/pharmaceutics16101330








