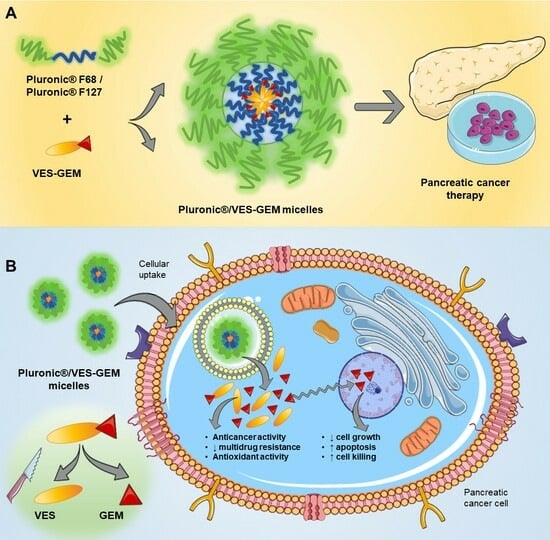
Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the VES-GEM Conjugate
2.3. Characterization of the VES-GEM Conjugate
2.4. HPLC Quantification Methods
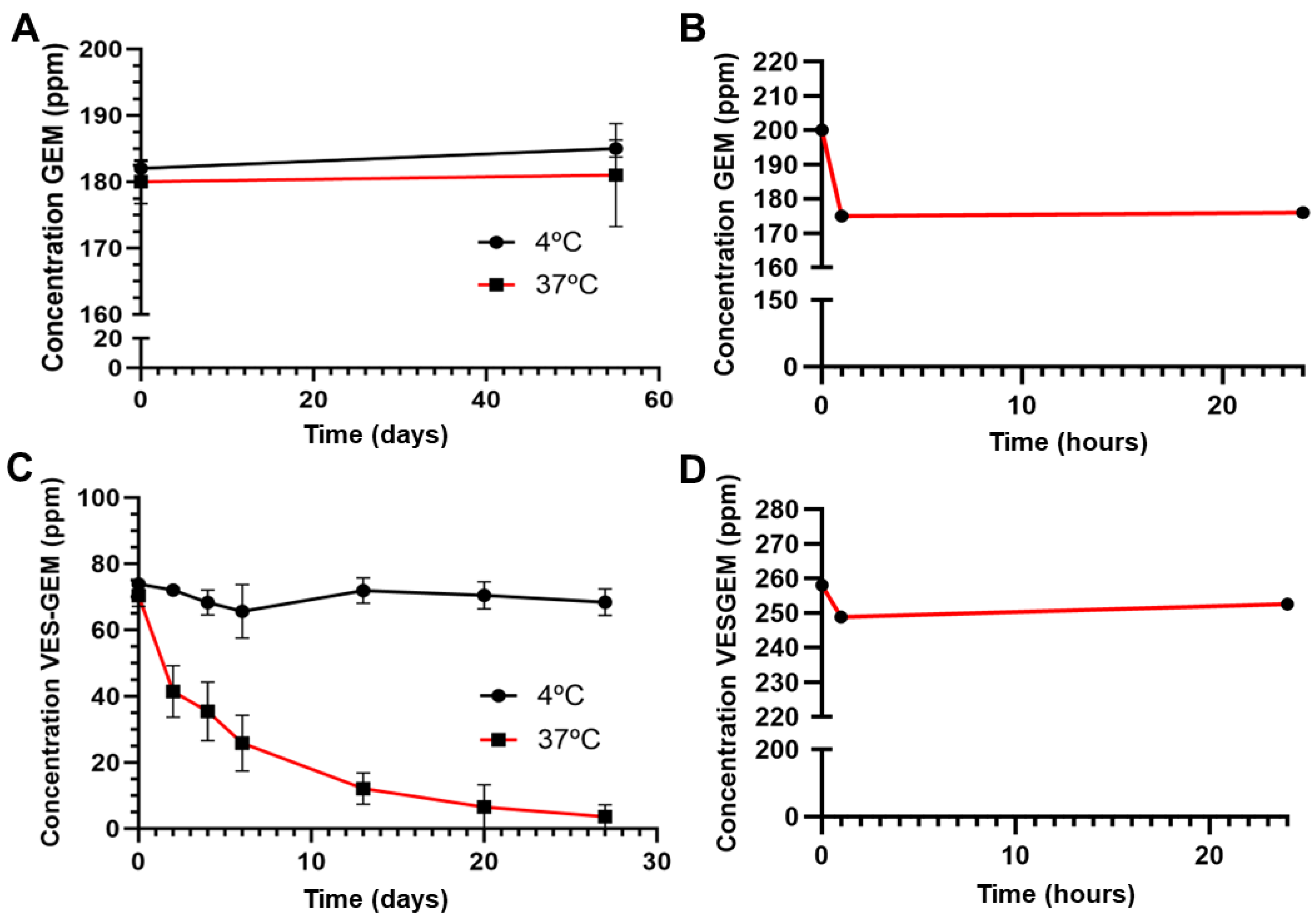
2.5. Stability of GEM and the VES-GEM Conjugate
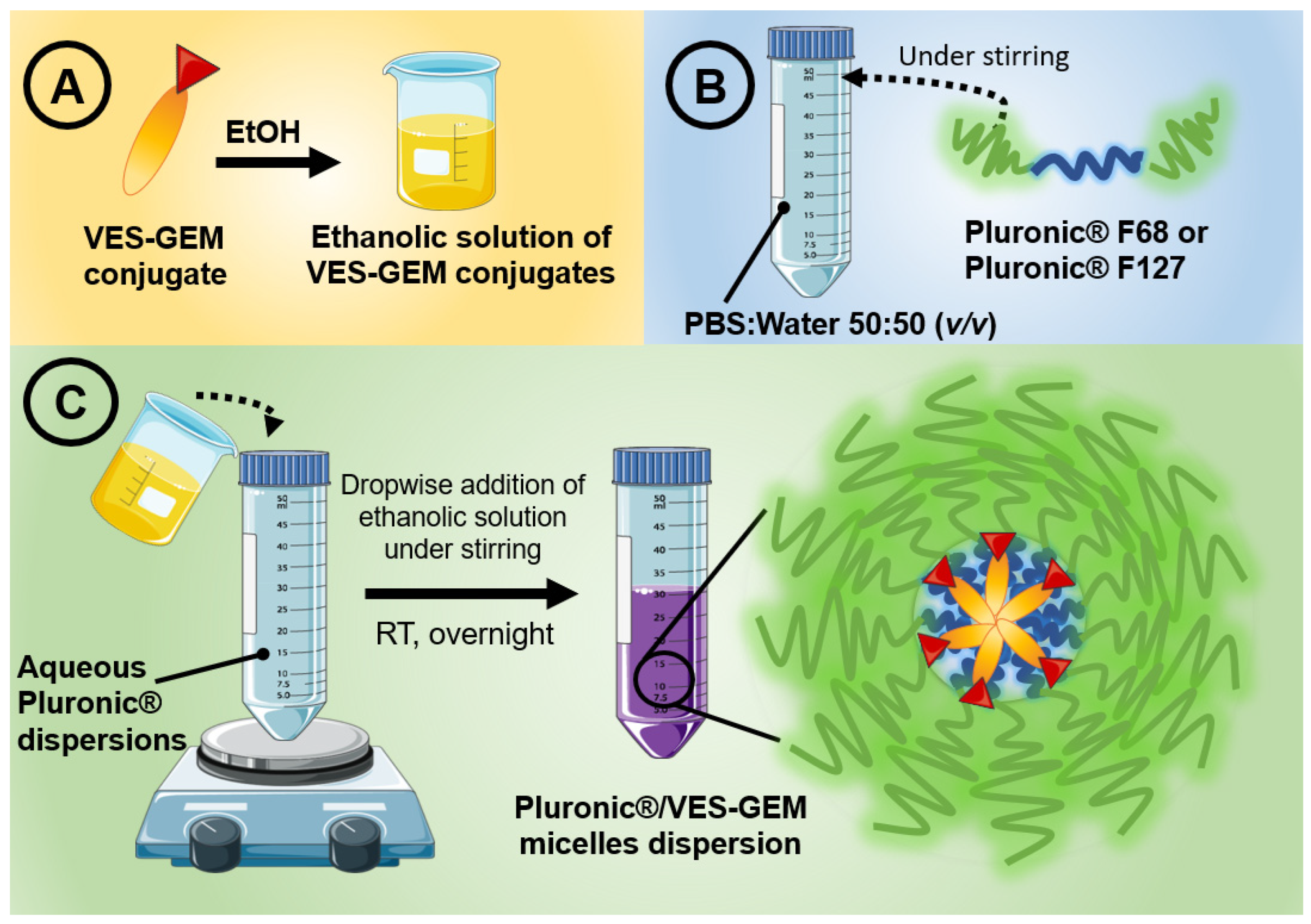
2.6. Pluronic®/VES-GEM Micelle Preparation
2.7. Influence of the VES-GEM Conjugate on the Micellization Process
2.8. Pluronic®/Soluplus®@VES-GEM Mixed Micelle Preparation
2.9. Micelle Characterization
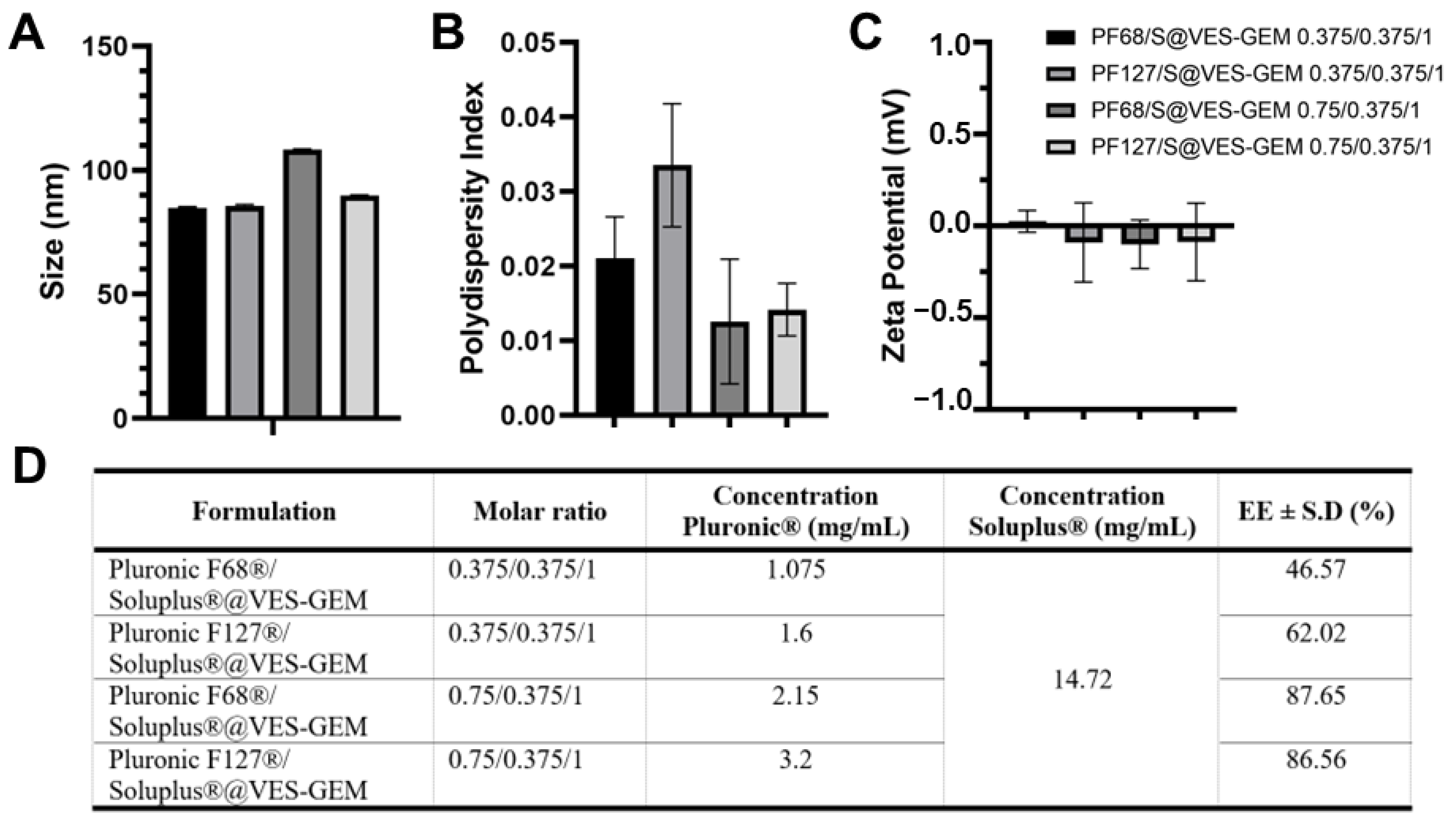
2.9.1. Micelle Size, ZP and PDI
2.9.2. Encapsulation Efficiency and Drug Loading
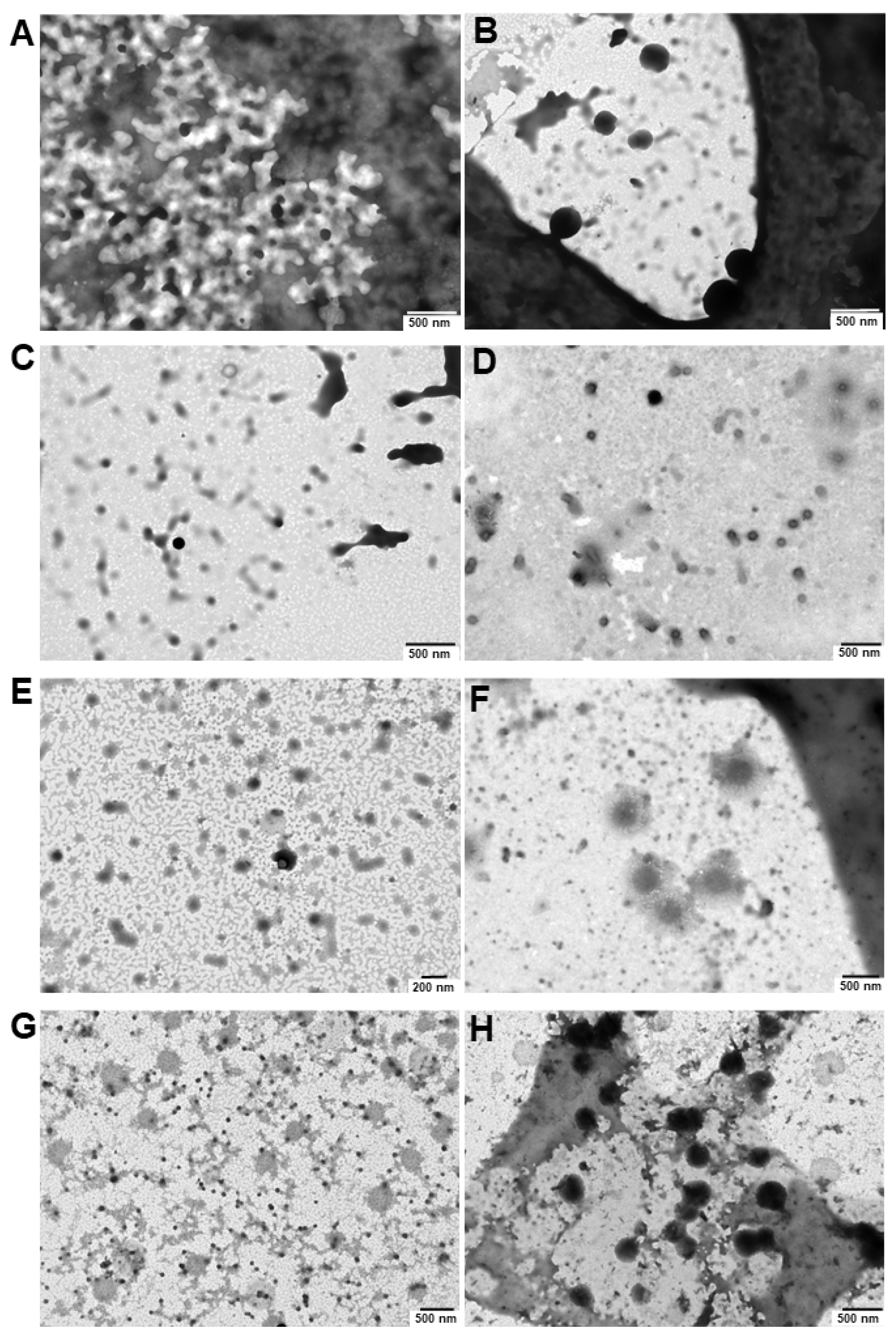
2.9.3. Transmission Electron Microscopy (TEM)
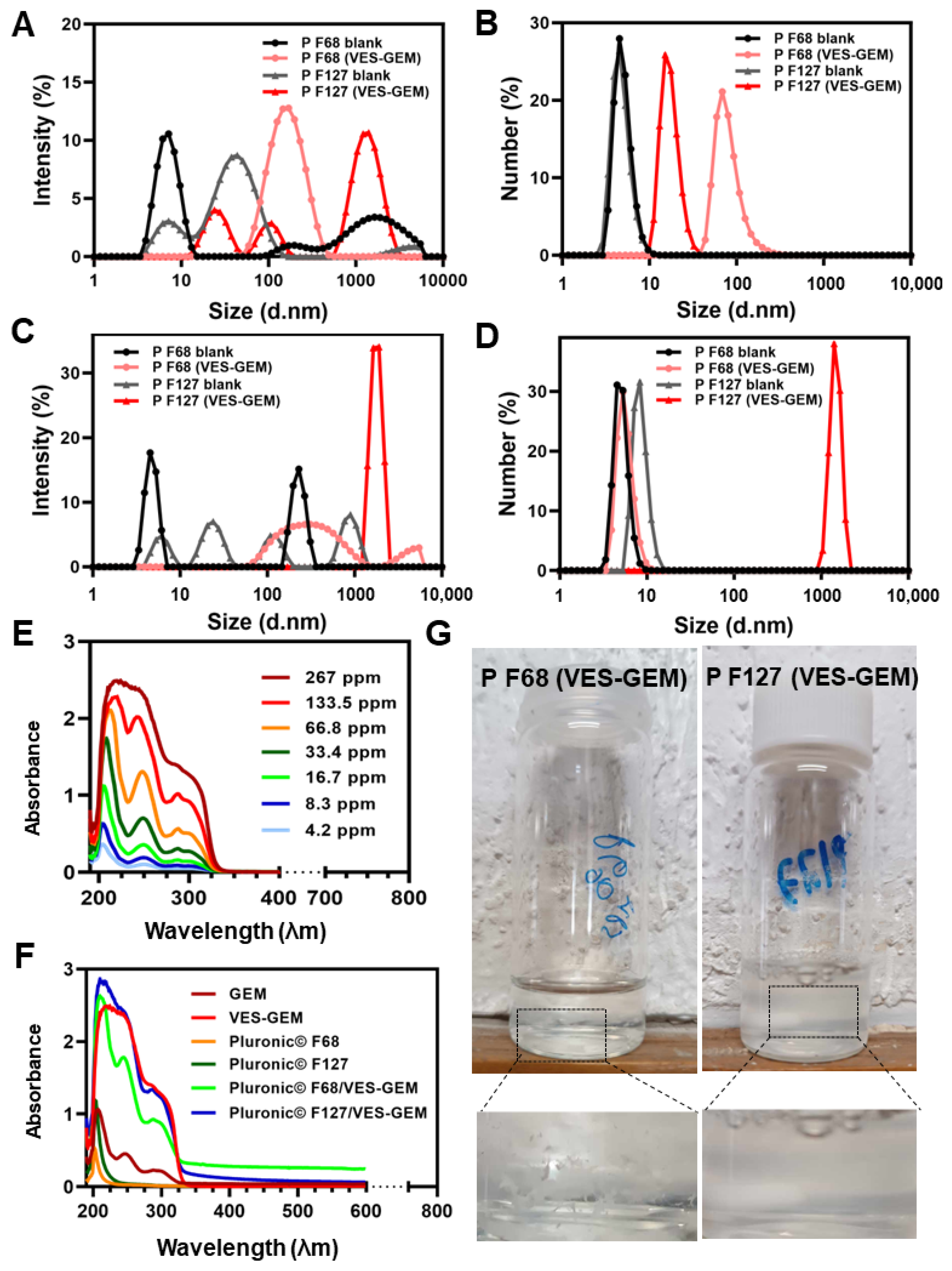
2.9.4. UV-VIS Spectra
2.10. VES-GEM Conjugate Release Profile
2.11. Solubilization Capacity of Tween 80
2.12. Stability of the Micelles
2.13. Blood Compatibility
2.14. Cell Viability
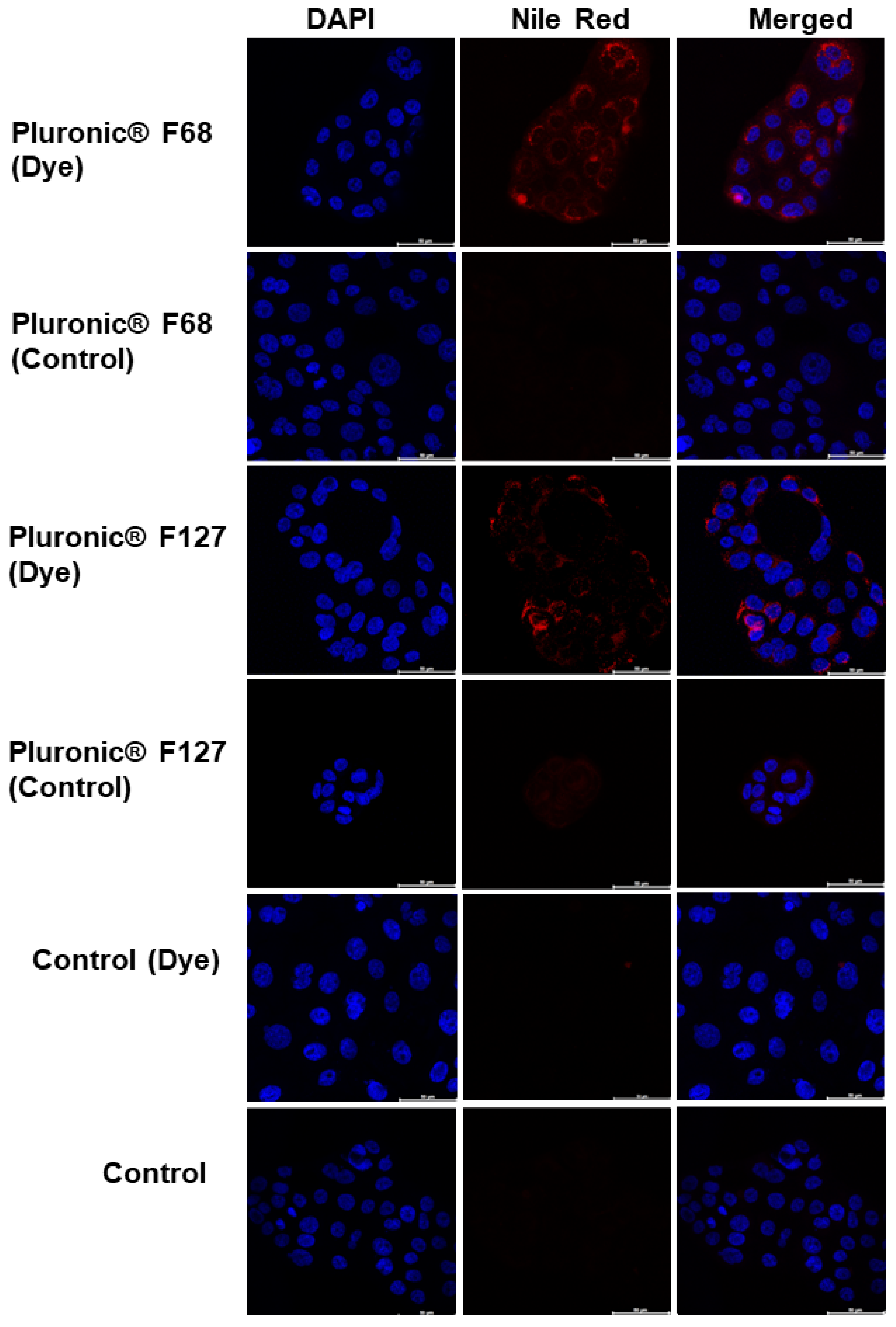
2.15. In Vitro Cell Uptake
2.16. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of VES-GEM
3.2. Stability of the Free Drug and Conjugate
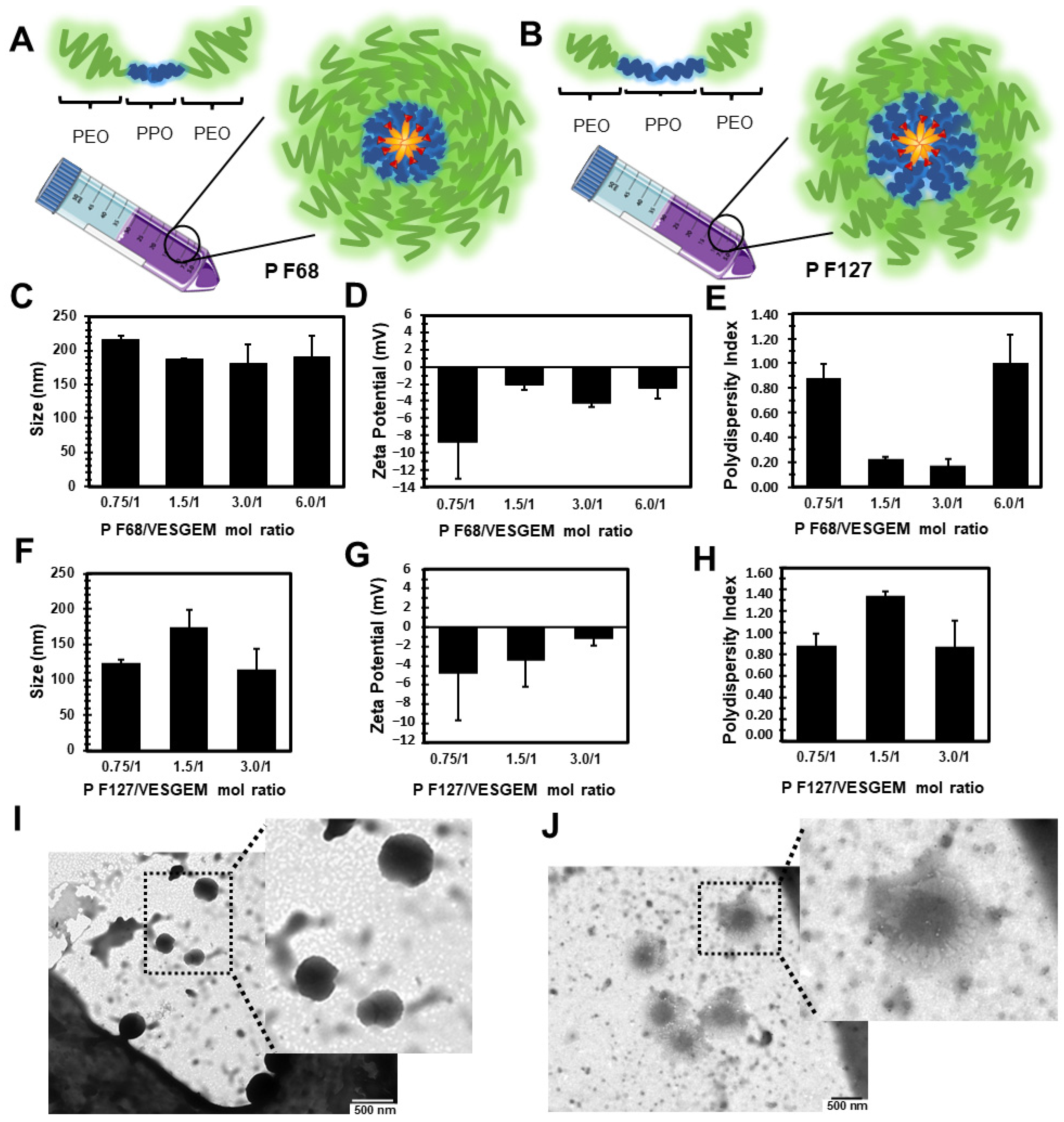
3.3. Pluronic®/VES-GEM Conjugate Micelle Preparation and Characterization
3.4. Critical Micelle Concentration of the Pluronic®/VES-GEM Micelles
3.5. Addition of Co-Surfactant
3.6. Drug Release from Pluronic®/VES-GEM Micelles
3.7. Stability of Pluronic®/VES-GEM Micelles
3.8. Blood Compatibility
3.9. Cell Viability Assay
3.10. Cell Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nevala-Plagemann, C.; Hidalgo, M.; Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat. Rev. Clin. Oncol. 2020, 17, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef]
- Xiao, Z.; Todd, L.; Huang, L.; Noguera-Ortega, E.; Lu, Z.; Huang, L.; Kopp, M.; Li, Y.; Pattada, N.; Zhong, W.; et al. Desmoplastic stroma restricts T cell extravasation and mediates immune exclusion and immunosuppression in solid tumors. Nat. Commun. 2023, 14, 5110. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, Y.; Na’ara, S.; Gil, Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2015, 23, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Hawrylkiewicz, A.; Ptaszynska, N. Gemcitabine Peptide-Based Conjugates and Their Application in Targeted Tumor Therapy. Molecules 2021, 26, 364. [Google Scholar] [CrossRef] [PubMed]
- Adiseshaiah, P.P.; Crist, R.M.; Hook, S.S.; McNeil, S.E. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 750–765. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Elnaggar, Y.S.R.; Abdallah, O.Y. Reviewing two decades of nanomedicine implementations in targeted treatment and diagnosis of pancreatic cancer: An emphasis on state of art. J. Control. Release Off. J. Control. Release Soc. 2019, 293, 21–35. [Google Scholar] [CrossRef]
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Cen, Y.; Lin, X.; Wu, Q. Antitumor gemcitabine conjugated micelles from amphiphilic comb-like random copolymers. Colloids Surf. B Biointerfaces 2016, 146, 707–715. [Google Scholar] [CrossRef]
- Coppens, E.; Desmaele, D.; Mougin, J.; Tusseau-Nenez, S.; Couvreur, P.; Mura, S. Gemcitabine Lipid Prodrugs: The Key Role of the Lipid Moiety on the Self-Assembly into Nanoparticles. Bioconjugate Chem. 2021, 32, 782–793. [Google Scholar] [CrossRef]
- Emamzadeh, M.; Desmaele, D.; Couvreur, P.; Pasparakis, G. Dual controlled delivery of squalenoyl-gemcitabine and paclitaxel using thermo-responsive polymeric micelles for pancreatic cancer. J. Mater. Chem. B 2018, 6, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lian, Y.; Du, L.; Wang, S.; Su, C.; Gao, C. Self-assembled drug delivery systems. Part 6: In vitro/in vivo studies of anticancer N-octadecanoyl gemcitabine nanoassemblies. Int. J. Pharm. 2012, 430, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lian, Y.; Du, L. Self-assembly of N-acyl derivatives of gemcitabine at the air/water interface and the formation of nanoscale structures in water. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 60–65. [Google Scholar] [CrossRef]
- Inkoom, A.; Ndemazie, N.; Affram, K.; Smith, T.; Zhu, X.; Underwood, P.; Krishnan, S.; Ofori, E.; Han, B.; Trevino, J.; et al. Enhancing efficacy of gemcitabine in pancreatic patient-derived xenograft mouse models. Int. J. Pharm. X 2020, 2, 100056. [Google Scholar] [CrossRef]
- Norouzi, P.; Amini, M.; Dinarvand, R.; Arefian, E.; Seyedjafari, E.; Atyabi, F. Co-delivery of gemcitabine prodrug along with anti NF-kappaB siRNA by tri-layer micelles can increase cytotoxicity, uptake and accumulation of the system in the cancers. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111161. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, W.; Dai, X.; Katragadda, U.; McKinley, D.; Teng, Q.; Tan, C. Enhanced tumor delivery of gemcitabine via PEG-DSPE/TPGS mixed micelles. Mol. Pharm. 2014, 11, 1140–1150. [Google Scholar] [CrossRef]
- Lu, Z.; Su, J.; Li, Z.; Zhan, Y.; Ye, D. Hyaluronic acid-coated, prodrug-based nanostructured lipid carriers for enhanced pancreatic cancer therapy. Drug Dev. Ind. Pharm. 2017, 43, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lansakara, P.D.; Rodriguez, B.L.; Cui, Z. Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. Int. J. Pharm. 2012, 429, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Daman, Z.; Ostad, S.; Amini, M.; Gilani, K. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: A comparison with its self-assembled nanoparticles. Int. J. Pharm. 2014, 468, 142–151. [Google Scholar] [CrossRef]
- Forciniti, S.; Dalla Pozza, E.; Greco, M.R.; Amaral Carvalho, T.M.; Rolando, B.; Ambrosini, G.; Carmona-Carmona, C.A.; Pacchiana, R.; Di Molfetta, D.; Donadelli, M.; et al. Extracellular Matrix Composition Modulates the Responsiveness of Differentiated and Stem Pancreatic Cancer Cells to Lipophilic Derivate of Gemcitabine. Int. J. Mol. Sci. 2020, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, F.; Chen, X.; Wan, J.; Wang, Y.; Li, T.; Wang, H. Self-Assembled Gemcitabine Prodrug Nanoparticles Show Enhanced Efficacy against Patient-Derived Pancreatic Ductal Adenocarcinoma. ACS Appl. Mater. Interfaces 2020, 12, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Geng, J.; An, P.; Xu, Y.; Huang, J.; Lu, W.; Liu, S.; Yu, J. Cathepsin B-sensitive cholesteryl hemisuccinate–gemcitabine prodrug nanoparticles: Enhanced cellular uptake and intracellular drug controlled release. RSC Adv. 2015, 5, 6985–6992. [Google Scholar] [CrossRef]
- Coppens, E.; Desmaele, D.; Naret, T.; Garcia-Argote, S.; Feuillastre, S.; Pieters, G.; Cailleau, C.; Paul, J.L.; Prost, B.; Solgadi, A.; et al. Gemcitabine lipid prodrug nanoparticles: Switching the lipid moiety and changing the fate in the bloodstream. Int. J. Pharm. 2021, 609, 121076. [Google Scholar] [CrossRef]
- Rejiba, S.; Reddy, L.H.; Bigand, C.; Parmentier, C.; Couvreur, P.; Hajri, A. Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 841–849. [Google Scholar] [CrossRef]
- Maksimenko, A.; Caron, J.; Mougin, J.; Desmaele, D.; Couvreur, P. Gemcitabine-based therapy for pancreatic cancer using the squalenoyl nucleoside monophosphate nanoassemblies. Int. J. Pharm. 2015, 482, 38–46. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, C.; Sebastian, V.; Irusta, S.; Desmaele, D.; Couvreur, P.; Blanco-Prieto, M.J. A unique multidrug nanomedicine made of squalenoyl-gemcitabine and alkyl-lysophospholipid edelfosine. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2019, 144, 165–173. [Google Scholar] [CrossRef]
- Lirussi, F.; Pyrshev, K.; Yesylevskyy, S.; Rivel, T.; Lopez, T.; Coppens, E.; Mura, S.; Couvreur, P.; Ramseyer, C. Plasma membrane lipid bilayer is druggable: Selective delivery of gemcitabine-squalene nano-medicine to cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166614. [Google Scholar] [CrossRef] [PubMed]
- Sobot, D.; Mura, S.; Yesylevskyy, S.O.; Dalbin, L.; Cayre, F.; Bort, G.; Mougin, J.; Desmaele, D.; Lepetre-Mouelhi, S.; Pieters, G.; et al. Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nat. Commun. 2017, 8, 15678. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nogales, C.; Moreno, H.; Zandueta, C.; Desmaele, D.; Lecanda, F.; Couvreur, P.; Blanco-Prieto, M.J. Combinatorial Nanomedicine Made of Squalenoyl-Gemcitabine and Edelfosine for the Treatment of Osteosarcoma. Cancers 2020, 12, 1895. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, C.; Mura, S.; Couvreur, P.; Blanco-Prieto, M.J. Squalenoyl-gemcitabine/edelfosine nanoassemblies: Anticancer activity in pediatric cancer cells and pharmacokinetic profile in mice. Int. J. Pharm. 2020, 582, 119345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Chen, Y.; Wang, K.; Luan, Y. Design, synthesis and antitumor activity study of a gemcitabine prodrug conjugated with a HDAC6 inhibitor. Bioorganic Med. Chem. Lett. 2022, 72, 128881. [Google Scholar] [CrossRef]
- Di, Y.; Gao, Y.; Gai, X.; Wang, D.; Wang, Y.; Yang, X.; Zhang, D.; Pan, W.; Yang, X. Co-delivery of hydrophilic gemcitabine and hydrophobic paclitaxel into novel polymeric micelles for cancer treatment. RSC Adv. 2017, 7, 24030–24039. [Google Scholar] [CrossRef]
- Rouco, H.; Diaz-Rodriguez, P.; Gaspar, D.P.; Goncalves, L.M.D.; Cuerva, M.; Remunan-Lopez, C.; Almeida, A.J.; Landin, M. Rifabutin-Loaded Nanostructured Lipid Carriers as a Tool in Oral Anti-Mycobacterial Treatment of Crohn’s Disease. Nanomaterials 2020, 10, 2138. [Google Scholar] [CrossRef]
- Xu, Y.; Meng, H.; Du, F.; Lu, W.; Liu, S.; Huang, J.; Yu, J. Preparation of intravenous injection nanoformulation of VESylated gemcitabine by co-assembly with TPGS and its anti-tumor activity in pancreatic tumor-bearing mice. Int. J. Pharm. 2015, 495, 792–797. [Google Scholar] [CrossRef]
- Fang, Y.; Du, F.; Xu, Y.; Meng, H.; Huang, J.; Zhang, X.; Lu, W.; Liu, S.; Yu, J. Enhanced cellular uptake and intracellular drug controlled release of VESylated gemcitabine prodrug nanocapsules. Colloids Surf. B Biointerfaces 2015, 128, 357–362. [Google Scholar] [CrossRef]
- Guo, R.; Long, Y.; Lu, Z.; Deng, M.; He, P.; Li, M.; He, Q. Enhanced stability and efficacy of GEM-TOS prodrug by co-assembly with antimetastatic shell LMWH-TOS. Acta Pharm. Sin. B 2020, 10, 1977–1988. [Google Scholar] [CrossRef]
- Abu-Fayyad, A.; Nazzal, S. Gemcitabine-vitamin E conjugates: Synthesis, characterization, entrapment into nanoemulsions, and in-vitro deamination and antitumor activity. Int. J. Pharm. 2017, 528, 463–470. [Google Scholar] [CrossRef]
- Gaudin, A.; Song, E.; King, A.R.; Saucier-Sawyer, J.K.; Bindra, R.; Desmaele, D.; Couvreur, P.; Saltzman, W.M. PEGylated squalenoyl-gemcitabine nanoparticles for the treatment of glioblastoma. Biomaterials 2016, 105, 136–144. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.; Wang, J.; Xu, X.; Zhang, A.; Li, Y.; Zhang, Z. Macrophage Membrane-Coated Nano-Gemcitabine Promotes Lymphocyte Infiltration and Synergizes AntiPD-L1 to Restore the Tumoricidal Function. ACS Nano 2023, 17, 322–336. [Google Scholar] [CrossRef]
- Logan, K.A.; Nesbitt, H.; Callan, B.; Gao, J.; McKaig, T.; Taylor, M.; Love, M.; McHale, A.P.; Callan, J.F. Synthesis of a gemcitabine-modified phospholipid and its subsequent incorporation into a single microbubble formulation loaded with paclitaxel for the treatment of pancreatic cancer using ultrasound-targeted microbubble destruction. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik 2021, 165, 374–382. [Google Scholar] [CrossRef]
- Bulanadi, J.C.; Xue, A.; Gong, X.; Bean, P.A.; Julovi, S.M.; de Campo, L.; Smith, R.C.; Moghaddam, M.J. Biomimetic Gemcitabine–Lipid Prodrug Nanoparticles for Pancreatic Cancer. ChemPlusChem 2020, 85, 1283–1291. [Google Scholar] [CrossRef]
- Rathod, S.; Bahadur, P.; Tiwari, S. Nanocarriers based on vitamin E-TPGS: Design principle and molecular insights into improving the efficacy of anticancer drugs. Int. J. Pharm. 2021, 592, 120045. [Google Scholar] [CrossRef]
- de Castro, K.C.; Coco, J.C.; Dos Santos, E.M.; Ataide, J.A.; Martinez, R.M.; do Nascimento, M.H.M.; Prata, J.; da Fonte, P.; Severino, P.; Mazzola, P.G.; et al. Pluronic(R) triblock copolymer-based nanoformulations for cancer therapy: A 10-year overview. J. Control. Release Off. J. Control. Release Soc. 2023, 353, 802–822. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric Drug Delivery System Based on Pluronics for Cancer Treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for Drug Delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Khaliq, N.U.; Lee, J.; Kim, S.; Sung, D.; Kim, H. Pluronic F-68 and F-127 Based Nanomedicines for Advancing Combination Cancer Therapy. Pharmaceutics 2023, 15, 2102. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Jia, X.; Zhang, Z.; Sun, D.; Xie, H.; Zang, D.; Liu, T. Effect of pharmaceutical excipients on micellization of Pluronic and the application as drug carrier to reverse MDR. J. Mol. Liq. 2023, 383, 122182. [Google Scholar] [CrossRef]
- Rarokar, N.; Agrawal, R.; Yadav, S.; Khedekar, P.; Ravikumar, C.; Telange, D.; Gurav, S. Pteroyl-γ-l-glutamate/Pluronic® F68 modified polymeric micelles loaded with docetaxel for targeted delivery and reduced toxicity. J. Mol. Liq. 2023, 369, 120842. [Google Scholar] [CrossRef]
- Ingrungruengluet, P.; Wang, D.; Li, X.; Yang, C.; Waiprib, Y.; Li, C. Preparation and Primary Bioactivity Evaluation of Novel Water-Soluble Curcumin-Loaded Polymeric Micelles Fabricated with Chitooligosaccharides and Pluronic F-68. Pharmaceutics 2023, 15, 2497. [Google Scholar] [CrossRef]
- Dantas Lopes Dos Santos, D.; Besegato, J.F.; de Melo, P.B.G.; Oshiro Junior, J.A.; Chorilli, M.; Deng, D.; Bagnato, V.S.; Rastelli, A.N.S. Curcumin-loaded Pluronic((R)) F-127 Micelles as a Drug Delivery System for Curcumin-mediated Photodynamic Therapy for Oral Application. Photochem. Photobiol. 2021, 97, 1072–1088. [Google Scholar] [CrossRef]
- Jung, S.Y.; Yoo, J.; Yang, K.J.; Jang, S.Y.; Yi, G.; Kim, D.K.; Koo, H. Intratympanic administration of alpha-lipoic acid-loaded pluronic F-127 nanoparticles ameliorates acute hearing loss. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102329. [Google Scholar] [CrossRef]
- Martins, J.N.L.; Lucredi, N.C.; Oliveira, M.C.; Oliveira, A.C.V.; Godoy, M.A.F.; Sá-Nakanishi, A.B.; Bracht, L.; Cesar, G.B.; Gonçalves, R.S.; Vicentini, V.E.P.; et al. Poloxamers-based nanomicelles as delivery vehicles of hypericin for hepatic photodynamic therapy. J. Drug Deliv. Sci. Technol. 2023, 79, 104043. [Google Scholar] [CrossRef]
- Uner, B.; Ergin, A.D. Enhanced mitochondrial co-localization of β-escin micelle and pancreatic tumor accumulation relation. J. Drug Deliv. Sci. Technol. 2023, 89, 104994. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, J.; Wu, Q.; Shen, W.; Yang, Y.; Yin, D. Targeting Lysosome for Enhanced Cancer Photodynamic/Photothermal Therapy in a “One Stone Two Birds” Pattern. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef]
- Zong, J.; Peng, H.; Qing, X.; Fan, Z.; Xu, W.; Du, X.; Shi, R.; Zhang, Y. pH-Responsive Pluronic F127–Lenvatinib-Encapsulated Halogenated Boron-Dipyrromethene Nanoparticles for Combined Photodynamic Therapy and Chemotherapy of Liver Cancer. ACS Omega 2021, 6, 12331–12342. [Google Scholar] [CrossRef]
- Kamya, E.; Yi, S.; Hussain, Z.; Lu, Z.; Li, W.; Yan, J.; Ma, F.; Ullah, I.; Cao, Y.; Pei, R. Donor-Acceptor Engineering for Tailoring Highly Efficient Photosensitizers for Image-Guided Antitumor Photodynamic Therapy. ACS Macro Lett. 2023, 12, 1549–1557. [Google Scholar] [CrossRef]
- Bonde, G.V.; Ajmal, G.; Yadav, S.K.; Mittal, P.; Singh, J.; Bakde, B.V.; Mishra, B. Assessing the viability of Soluplus(R) self-assembled nanocolloids for sustained delivery of highly hydrophobic lapatinib (anticancer agent): Optimisation and in-vitro characterisation. Colloids Surf. B Biointerfaces 2020, 185, 110611. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, C.; Wei, F.; Lv, H. Improved solubility and oral bioavailability of apigenin via Soluplus/Pluronic F127 binary mixed micelles system. Drug Dev. Ind. Pharm. 2017, 43, 1276–1282. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm. 2018, 15, 571–584. [Google Scholar] [CrossRef]
- Ch, S.; Padaga, S.G.; Ghosh, B.; Roy, S.; Biswas, S. Chitosan-poly(lactide-co-glycolide)/poloxamer mixed micelles as a mucoadhesive thermo-responsive moxifloxacin eye drop to improve treatment efficacy in bacterial keratitis. Carbohydr. Polym. 2023, 312, 120822. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Y.; Liu, L.; Pan, H.; Liu, H. Effect of 2-ethylbutyric acid on thermodynamics stability of various nonionic surfactants tanshione-loaded micelles. J. Mol. Liq. 2022, 362, 119775. [Google Scholar] [CrossRef]
- Bernabeu, E.; Gonzalez, L.; Cagel, M.; Gergic, E.P.; Moretton, M.A.; Chiappetta, D.A. Novel Soluplus((R))-TPGS mixed micelles for encapsulation of paclitaxel with enhanced in vitro cytotoxicity on breast and ovarian cancer cell lines. Colloids Surf. B Biointerfaces 2016, 140, 403–411. [Google Scholar] [CrossRef]
- Shi, Y.; Zhu, H.; Ren, Y.; Li, K.; Tian, B.; Han, J.; Feng, D. Preparation of protein-loaded PEG-PLA micelles and the effects of ultrasonication on particle size. Colloid Polym. Sci. 2017, 295, 259–266. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Zhang, C.; Yan, J.; Hou, X.; Du, S.; Zeng, C.; Zhao, W.; Deng, B.; McComb, D.W.; et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 2021, 12, 7264. [Google Scholar] [CrossRef]
- Estado, J.d. Ley 14/2007, de 3 de Julio, de Investigación Biomédica. 2007, BOE-A-2007-12945. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2007-12945 (accessed on 5 April 2023).
- Farto-Vaamonde, X.; Diaz-Gomez, L.; Parga, A.; Otero, A.; Concheiro, A.; Alvarez-Lorenzo, C. Perimeter and carvacrol-loading regulate angiogenesis and biofilm growth in 3D printed PLA scaffolds. J. Control. Release Off. J. Control. Release Soc. 2022, 352, 776–792. [Google Scholar] [CrossRef]
- Diaz-Rodriguez, P.; Gonzalez, P.; Serra, J.; Landin, M. Key parameters in blood-surface interactions of 3D bioinspired ceramic materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 232–239. [Google Scholar] [CrossRef]
- Zuccari, G.; Alfei, S.; Zorzoli, A.; Marimpietri, D.; Turrini, F.; Baldassari, S.; Marchitto, L.; Caviglioli, G. Increased Water-Solubility and Maintained Antioxidant Power of Resveratrol by Its Encapsulation in Vitamin E TPGS Micelles: A Potential Nutritional Supplement for Chronic Liver Disease. Pharmaceutics 2021, 13, 1128. [Google Scholar] [CrossRef]
- Thapa, R.K.; Cazzador, F.; Gronlien, K.G.; Tonnesen, H.H. Effect of curcumin and cosolvents on the micellization of Pluronic F127 in aqueous solution. Colloids Surf. B Biointerfaces 2020, 195, 111250. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef]
- Tehrani, S.F.; Bharadwaj, P.; Leblond Chain, J.; Roullin, V.G. Purification processes of polymeric nanoparticles: How to improve their clinical translation? J. Control. Release Off. J. Control. Release Soc. 2023, 360, 591–612. [Google Scholar] [CrossRef]
- Prasanthan, P.; Kishore, N. Self-assemblies of pluronic micelles in partitioning of anticancer drugs and effectiveness of this system towards target protein. RSC Adv. 2021, 11, 22057–22069. [Google Scholar] [CrossRef]
- Tripathi, N.; Singhvi, G.; Roy, A.; Kuperkar, K.; Bahadur, P. Nanoscale Pluronic® micellar templates with varying %EO content for controlled drug release and cytotoxicity. J. Mol. Liq. 2023, 384, 122215. [Google Scholar] [CrossRef]
- Ko, N.R.; Lee, S.J.; Chandrasekaran, A.P.; Tyagi, A.; Ramakrishna, S.; Kim, S.Y.; Kim, D.W.; Pack, C.G.; Oh, S.J. Smart Vitamin Micelles as Cancer Nanomedicines for Enhanced Intracellular Delivery of Doxorubicin. Int. J. Mol. Sci. 2021, 22, 1298. [Google Scholar] [CrossRef]
- Raval, A.; Pillai, S.A.; Bahadur, A.; Bahadur, P. Systematic characterization of Pluronic® micelles and their application for solubilization and in vitro release of some hydrophobic anticancer drugs. J. Mol. Liq. 2017, 230, 473–481. [Google Scholar] [CrossRef]
- Vivero-Lopez, M.; Sparacino, C.; Quelle-Regaldie, A.; Sanchez, L.; Candal, E.; Barreiro-Iglesias, A.; Huete-Toral, F.; Carracedo, G.; Otero, A.; Concheiro, A.; et al. Pluronic(R)/casein micelles for ophthalmic delivery of resveratrol: In vitro, ex vivo, and in vivo tests. Int. J. Pharm. 2022, 628, 122281. [Google Scholar] [CrossRef]
- Dang, L.H.; Vu, M.T.; Chen, J.; Nguyen, C.K.; Bach, L.G.; Tran, N.Q.; Le, V.T. Effect of Ultrasonication on Self-Assembled Nanostructures Formed by Amphiphilic Positive-Charged Copolymers and Negative-Charged Drug. ACS Omega 2019, 4, 4540–4552. [Google Scholar] [CrossRef]
- Song, Y.; Tian, Q.; Huang, Z.; Fan, D.; She, Z.; Liu, X.; Cheng, X.; Yu, B.; Deng, Y. Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int. J. Nanomed. 2014, 9, 2307–2317. [Google Scholar] [CrossRef][Green Version]
- Alvarez-Lorenzo, C.; Sosnik, A.; Concheiro, A. PEO-PPO block copolymers for passive micellar targeting and overcoming multidrug resistance in cancer therapy. Curr. Drug Targets 2011, 12, 1112–1130. [Google Scholar] [CrossRef]
- Dong, K.; Zhang, M.; Liu, Y.; Gao, X.; Wu, X.; Shi, D.; Guo, C.; Wang, J. Pterostilbene-Loaded Soluplus/Poloxamer 188 Mixed Micelles for Protection against Acetaminophen-Induced Acute Liver Injury. Mol. Pharm. 2023, 20, 1189–1201. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus((R)) polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef]
- Sanhaji, M.; Goring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Prina-Mello, A.; Stapf, M.; Ludwig, R.; Volkov, Y.; et al. The phenotype of target pancreatic cancer cells influences cell death by magnetic hyperthermia with nanoparticles carrying gemicitabine and the pseudo-peptide NucAnt. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 101983. [Google Scholar] [CrossRef]
- Fryer, R.A.; Barlett, B.; Galustian, C.; Dalgleish, A.G. Mechanisms underlying gemcitabine resistance in pancreatic cancer and sensitisation by the iMiD lenalidomide. Anticancer Res. 2011, 31, 3747–3756. [Google Scholar]
- Arranja, A.; Schroder, A.P.; Schmutz, M.; Waton, G.; Schosseler, F.; Mendes, E. Cytotoxicity and internalization of Pluronic micelles stabilized by core cross-linking. J. Control. Release Off. J. Control. Release Soc. 2014, 196, 87–95. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, F.; Liu, K.; Liu, S.; Xiao, T.; Zhu, Y.; Xu, L. Mitochondria-Targeting Polymer Micelles in Stepwise Response Releasing Gemcitabine and Destroying the Mitochondria and Nucleus for Combined Antitumor Chemotherapy. Int. J. Mol. Sci. 2022, 23, 12624. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Un, K.; Nanjo, K.; Nishiyama, N.; Kusuhara, H.; Kataoka, K.; Kawanishi, T.; Goda, Y.; Okuda, H. Elucidating the molecular mechanism for the intracellular trafficking and fate of block copolymer micelles and their components. Biomaterials 2014, 35, 1347–1358. [Google Scholar] [CrossRef]
- Loureiro, A.; Noro, J.; Abreu, A.S.; Nogueira, E.; Soares da Costa, D.; Silva, C.; Cavaco-Paulo, A. Absence of Albumin Improves in Vitro Cellular Uptake and Disruption of Poloxamer 407-Based Nanoparticles inside Cancer Cells. Mol. Pharm. 2018, 15, 527–535. [Google Scholar] [CrossRef]







| Formulations | Polymer-to-Conjugate Molar Ratio | % w/v of Pluronic® | % w/v of VES-GEM | % w/w VES-GEM in Pluronic® | Concentration of Pluronic® (mg/mL) | Concentration of Pluronic® (mM) | Concentration of VES-GEM (mg/mL) |
|---|---|---|---|---|---|---|---|
| Pluronic® F68/VES-GEM | 0.75/1 | 0.22 | 0.027 | 12.40 | 2.15 | 0.256 | 0.267 |
| 1.5/1 | 0.43 | 6.20 | 4.30 | 0.512 | |||
| 3/1 | 0.86 | 3.10 | 8.60 | 1.024 | |||
| 6/1 | 1.72 | 1.55 | 17.21 | 2.048 | |||
| Pluronic® F127/VES-GEM | 0.75/1 | 0.32 | 0.027 | 8.18 | 3.23 | 0.258 | |
| 1.5/1 | 0.65 | 4.13 | 6.45 | 0.517 | |||
| 3/1 | 1.30 | 2.07 | 12.90 | 1.034 |
| Parameter | Result |
|---|---|
| Molecular weight | 775.976 |
| Isotope formula | C42H63F2N3O8 |
| Isoelectric point | 6.26 |
| LogP | 8.93 |
| LogD | 8.93 |
| HLB (Chemaxon) | 9.53 |
| HLB (Davies) | 10.13 |
| HLB | 8.64 |
| Intrinsic solubility | −10.76 (logS) |
| Solubility at pH 7.4 | −10.76 (logS) |
| Solubility category | Low (lower than 0.01 mg/mL) |
| Predominant species (pH 4–9) | Non-ionized |
| Charge (pH 4–9) | ~0 |
| H bond donor/acceptor sites | 3/15 |
| Formulations | Molar Ratio | Filtered | Size (nm) ± S.D. | ZP (mV) ± S.D. | PDI ± S.D. |
|---|---|---|---|---|---|
| Pluronic® F68 | - | Yes | 34.24 ± 13.11 | −3.51 ± 0.88 | 0.486 ± 0.039 |
| Pluronic® F68 | - | No | 185.73 ± 43.16 | −4.33 ± 1.11 | 0.350 ± 0.024 |
| Pluronic® F68/VES-GEM | 3/1 | Yes | 140.01 ± 0.28 | −5.38 ± 1.60 | 0.213 ± 0.011 |
| Pluronic® F68/VES-GEM | 3/1 | No | 284.27 ± 1.04 | 0.04 ± 0.83 | 0.497 ± 0.074 |
| Pluronic® F127 | - | Yes | 25.57 ± 0.47 | −2.14 ± 0.14 | 0.493 ± 0.086 |
| Pluronic® F127 | - | No | 307.57 ± 49.54 | −9.11 ± 0.62 | 0.456 ± 0.025 |
| Pluronic® F127/VES-GEM | 1.5/1 | Yes | 136.67 ± 25.70 | −1.54 ± 1.08 | 1.234 ± 0.021 |
| Pluronic® F127/VES-GEM | 1.5/1 | No | 3282.33 ± 641.42 | −0.69 ± 0.28 | 0.477 ± 0.340 |
| Formulations | Molar Ratio | Sonication Time | Filtered before Measurement | Size (nm) ± S.D. | PDI ± S.D. |
|---|---|---|---|---|---|
| Pluronic® F127 | - | 0 min | Yes | 35.82 ± 0.50 | 0.536 ± 0.030 |
| No | 100.47 ± 24.63 | 0.359 ± 0.107 | |||
| 5 min | Yes | 23.30 ± 0.54 | 0.435 ± 0.011 | ||
| No | 270.03 ± 69.35 | 0.379 ± 0.031 | |||
| 10 min | Yes | 31.65 ± 2.98 | 0.421 ± 0.052 | ||
| No | 353.4 ± 45.8 | 0.380 ± 0.035 | |||
| 30 min | Yes | 33.16 ± 5.26 | 0.489 ± 0.060 | ||
| No | 379.33 ± 40.86 | 0.400 ± 0.025 | |||
| 90 min | Yes | 28.62 ± 0.21 | 0.419 ± 0.005 | ||
| No | 94.07 ± 13.84 | 0.378 ± 0.038 | |||
| Pluronic® F127/VES-GEM | 1.5/1 | 0 min | Yes | 131.33 ± 23.06 | 0.973 ± 0.083 |
| No | 2146.33 ± 276.06 | 0.945 ± 0.184 | |||
| 5 min | Yes | 111.27 ± 6.77 | 0.928 ± 0.011 | ||
| No | 2501.3 ± 117.3 | 0.827 ± 0.161 | |||
| 10 min | Yes | 103.36 ± 17.04 | 0.814 ± 0.039 | ||
| No | 2265.33 ± 147.82 | 0.589 ± 0.247 | |||
| 30 min | Yes | 227.80 ± 76.42 | 0.568 ± 0.194 | ||
| No | 2472.67 ± 267.82 | 0.640 ± 0.107 | |||
| 90 min | Yes | 66.45 ± 3.66 | 0.717 ± 0.012 | ||
| No | 2519.67 ± 298.92 | 0.617 ± 0.100 |
| Formulations | Molar Ratio | Ultrasonication Time | Filtered before Measurement | Size (nm) ± S.D. | PDI ± S.D. |
|---|---|---|---|---|---|
| Pluronic® F127 | - | 0 s | Yes | 35.82 ± 0.50 | 0.536 ± 0.030 |
| No | 100.47 ± 24.63 | 0.359 ± 0.107 | |||
| 3 s | Yes | 36.03 ± 0.93 | 0.435 ± 0.011 | ||
| No | 37.47 ± 6.27 | 0.402 ± 0.112 | |||
| 6 s | Yes | 31.89 ± 0.44 | 0.576 ± 0.047 | ||
| No | 282.27 ± 176.53 | 0.683 ± 0.055 | |||
| 12 s | Yes | 39.93 ± 0.10 | 0.782 ± 0.006 | ||
| No | 408.57 ± 84.89 | 0.556 ± 0.088 | |||
| 24 s (6 s on, 3 s off) | Yes | 46.09 ± 1.45 | 0.763 ± 0.027 | ||
| No | 91.21 ± 21.43 | 0.559 ± 0.127 | |||
| 36 s (6 s on, 3 s off) | Yes | 41.09 ± 1.03 | 0.883 ± 0.029 | ||
| No | 265.13 ± 109.23 | 0.623 ± 0.188 | |||
| Pluronic® F127/VES-GEM | 1.5/1 | 0 s | Yes | 131.33 ± 23.06 | 0.973 ± 0.083 |
| No | 2146.33 ± 276.06 | 0.945 ± 0.184 | |||
| 3 s | Yes | 146.9 ± 1.45 | 0.446 ± 0.033 | ||
| No | 863.97 ± 54.17 | 0.582 ± 0.026 | |||
| 6 s | Yes | 158.67 ± 6.84 | 0.400 ± 0.059 | ||
| No | 589.07 ± 10.44 | 0.541 ± 0.037 | |||
| 12 s | Yes | 169.00 ± 10.77 | 0.402 ± 0.015 | ||
| No | 506.23 ± 33.59 | 0.551 ± 0.045 | |||
| 24 s (6 s on, 3 s off) | Yes | 407.80 ± 7.80 | 0.310 ± 0.022 | ||
| No | 307.57 ± 5.46 | 0.461 ± 0.033 | |||
| 36 s (6 s on, 3 s off) | Yes | 186.50 ± 4.78 | 0.294 ± 0.013 | ||
| No | 395.83 ± 17.97 | 0.443 ± 0.053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira-Silva, M.; Miranda-Pastoriza, D.; Diaz-Gomez, L.; Sotelo, E.; Paiva-Santos, A.C.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy. Pharmaceutics 2024, 16, 95. https://doi.org/10.3390/pharmaceutics16010095
Pereira-Silva M, Miranda-Pastoriza D, Diaz-Gomez L, Sotelo E, Paiva-Santos AC, Veiga F, Concheiro A, Alvarez-Lorenzo C. Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy. Pharmaceutics. 2024; 16(1):95. https://doi.org/10.3390/pharmaceutics16010095
Chicago/Turabian StylePereira-Silva, Miguel, Darío Miranda-Pastoriza, Luis Diaz-Gomez, Eddy Sotelo, Ana Cláudia Paiva-Santos, Francisco Veiga, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2024. "Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy" Pharmaceutics 16, no. 1: 95. https://doi.org/10.3390/pharmaceutics16010095
APA StylePereira-Silva, M., Miranda-Pastoriza, D., Diaz-Gomez, L., Sotelo, E., Paiva-Santos, A. C., Veiga, F., Concheiro, A., & Alvarez-Lorenzo, C. (2024). Gemcitabine-Vitamin E Prodrug-Loaded Micelles for Pancreatic Cancer Therapy. Pharmaceutics, 16(1), 95. https://doi.org/10.3390/pharmaceutics16010095