Preparation of Dihydromyricetin-Loaded Self-Emulsifying Drug Delivery System and Its Anti-Alcoholism Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Analysis of DMY by HPLC-MS/MS
2.4. Analysis of DMY by HPLC
2.5. Analysis of Alcohol by GC
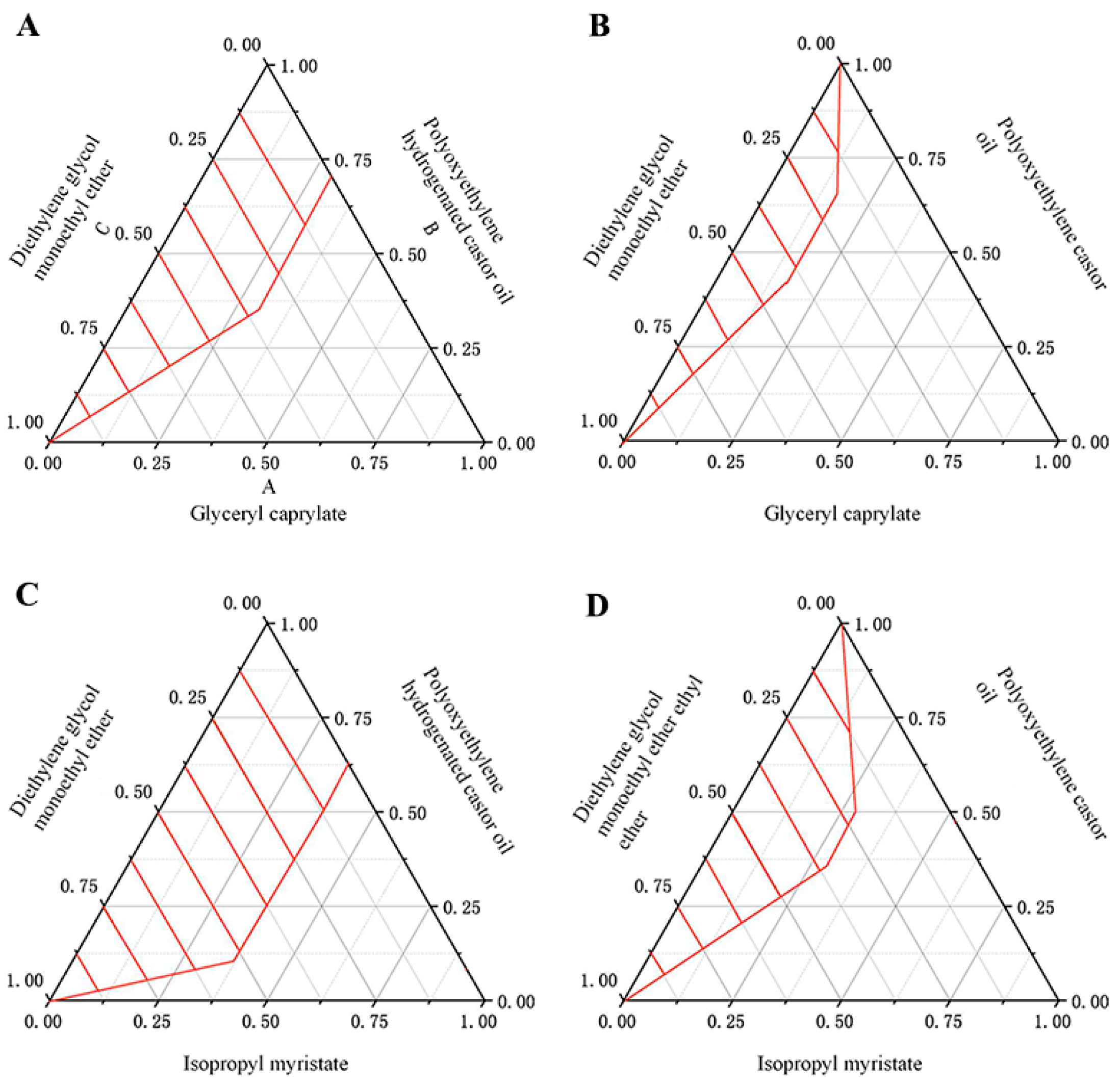
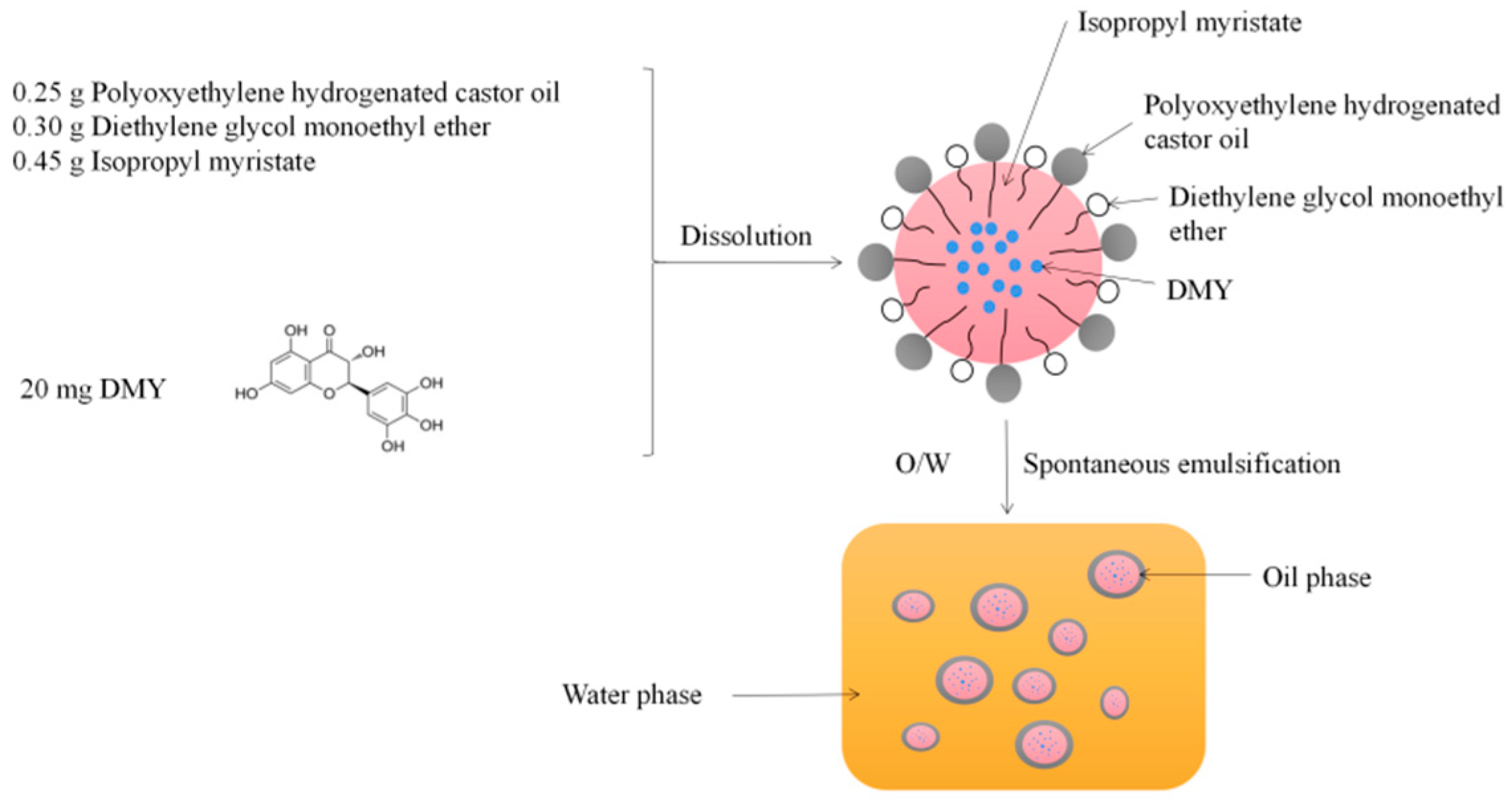
2.6. Preparation of DMY-SEDDS
2.7. Physicochemical Characterization of DMY-SEDDS
2.8. Determination of EE% and DL%
2.9. Effect of Medium pH on the Emulsification of DMY-SEDDS
2.10. Stability Analysis in Simulative Gastrointestinal Fluid
2.11. In-Situ Single-Pass Intestinal Perfusion
2.12. Pharmacokinetic Study
2.13. Study on Anti-Alcoholism of DMY-SEDDS
2.13.1. Loss of Righting Reflex Assay
2.13.2. Determination of Alcohol Concentration in Blood
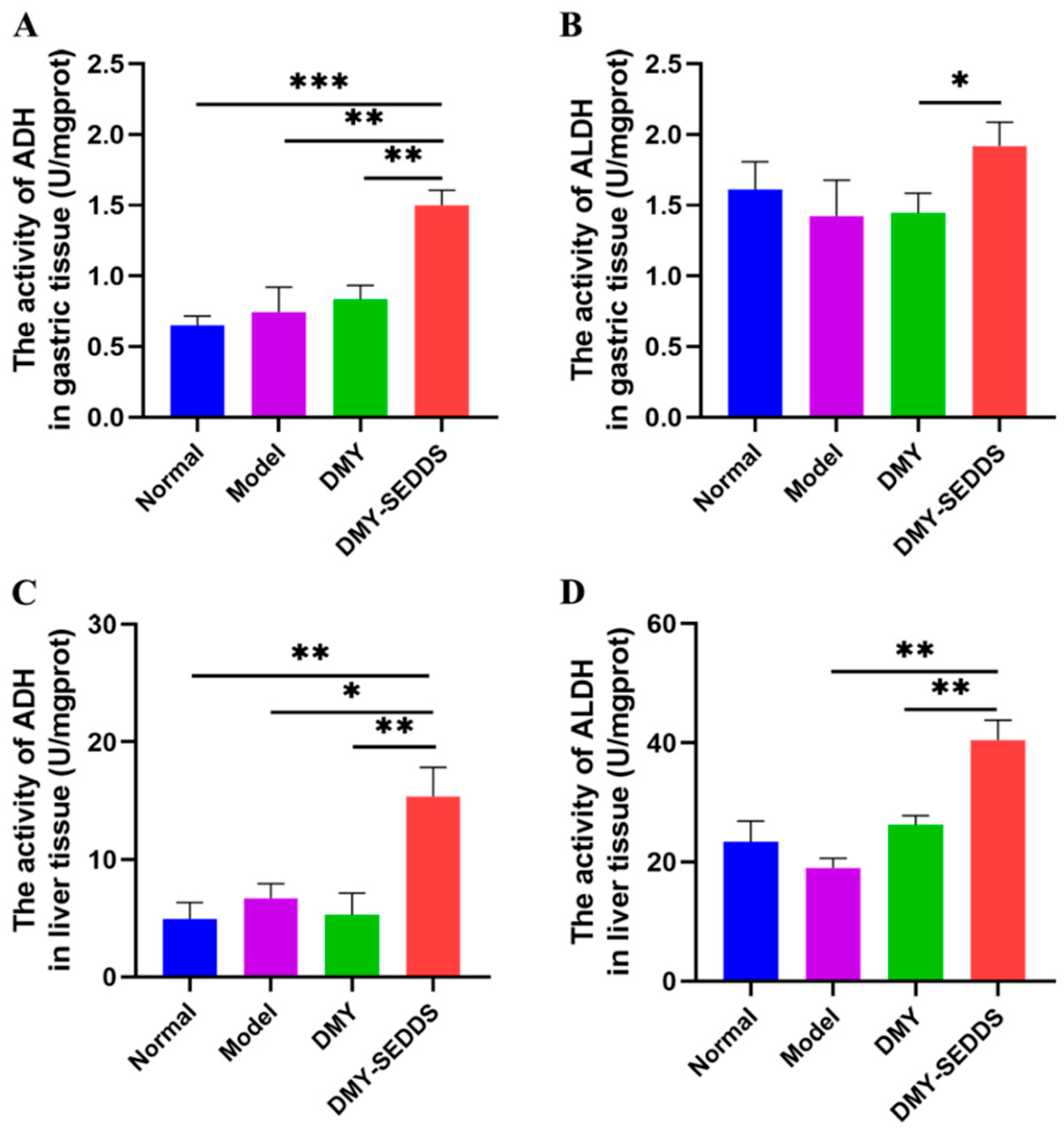
2.13.3. Effects of DMY-SEDDS on ADH and ALDH Activities in Gastric and Liver
Tissues in Mice
2.13.4. Protective Effect of DMY-SEDDS on Gastric Mucosal Injury Induced by Ethanol in Mice
2.14. Statistic Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of DMY-SEDDS
3.2. Effect of Medium pH on the Emulsification of DMY-SEDDS
3.3. Stability Analysis in Simulative Gastrointestinal Fluid
3.4. In-Situ Single-Pass Intestinal Perfusion
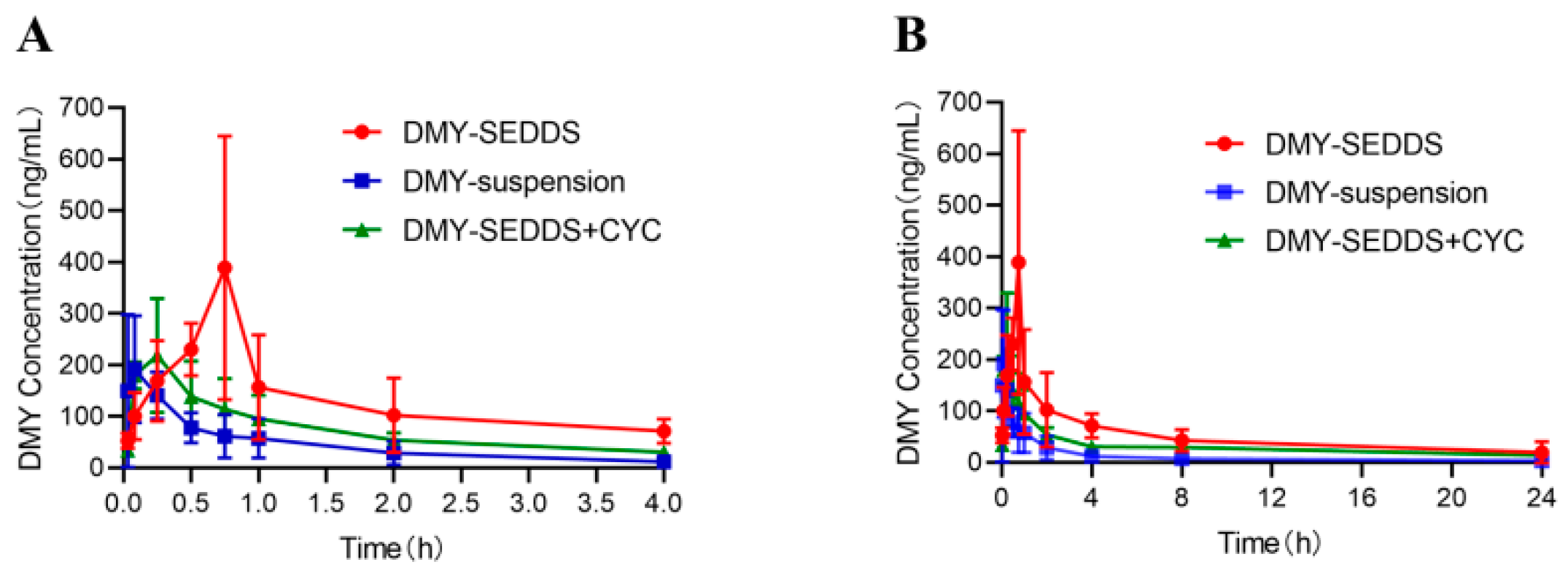
3.5. Pharmacokinetic Study
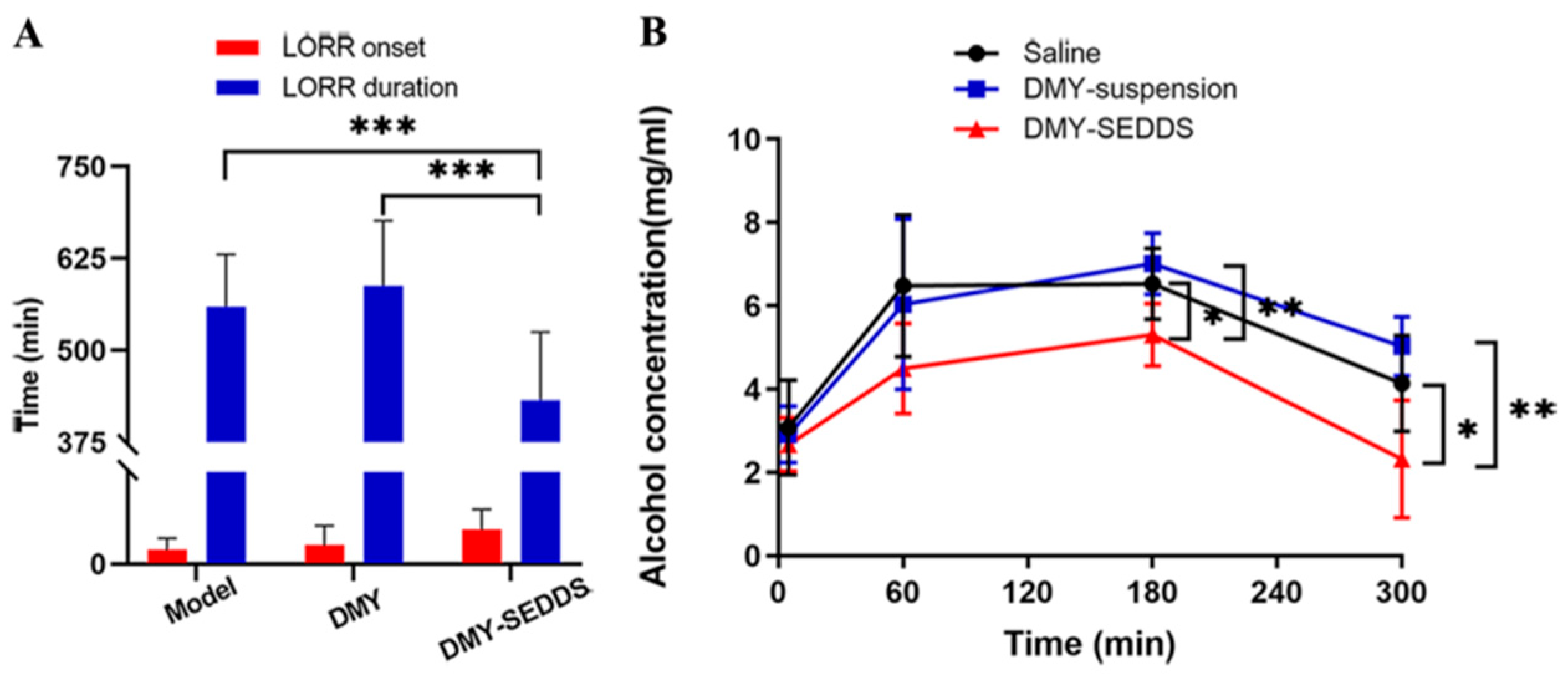
3.6. Study on Anti-Alcoholism of DMY-SEDDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- World Health Organization. Global Status Report On Alcohol and Health 2018; World Health Organization: Geneve, Switzerland, 2019. [Google Scholar]
- Julkunen, R.J.; Tannenbaum, L.; Baraona, E.; Lieber, C.S. First Pass Metabolism of Ethanol: An Important Determinant of Blood Levels After Alcohol Consumption. Alcohol 1985, 2, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.T.; Gentry, R.T.; Ito, D.; Yokoyama, H.; Baraona, E.; Lieber, C.S. First-Pass Metabolism of Ethanol is Predominantly Gastric. Alcohol. Clin. Exp. Res. 1993, 17, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Crabb, D.W.; Matsumoto, M.; Chang, D.; You, M. Overview of the Role of Alcohol Dehydrogenase and Aldehyde Dehydrogenase and their Variants in the Genesis of Alcohol-Related Pathology. Proc. Nutr. Soc. 2004, 63, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J. Alcohol Dehydrogenase: Enzymology and Metabolism. Alcohol Alcohol. Suppl. 1994, 2, 113–119. [Google Scholar] [PubMed]
- Vonghia, L.; Leggio, L.; Ferrulli, A.; Bertini, M.; Gasbarrini, G.; Addolorato, G. Acute Alcohol Intoxication. Eur. J. Intern. Med. 2008, 19, 561–567. [Google Scholar] [CrossRef]
- Burbige, E.J.; Lewis, D.J.; Halsted, C.H. Alcohol and the Gastrointestinal Tract. Med. Clin. N. Am. 1984, 68, 77–89. [Google Scholar] [CrossRef]
- Bao, S.; Zhang, Y.; Ye, J.; Zhu, Y.; Li, R.; Xu, X.; Zhang, Q. Self-Assembled Micelles Enhance the Oral Delivery of Curcumin for the Management of Alcohol-Induced Tissue Injury. Pharm. Dev. Technol. 2021, 26, 880–889. [Google Scholar] [CrossRef]
- Pihan, G.; Regillo, C.; Szabo, S. Free Radicals and Lipid Peroxidation in Ethanol- or Aspirin-Induced Gastric Mucosal Injury. Dig. Dis. Sci. 1987, 32, 1395–1401. [Google Scholar] [CrossRef]
- Silen, W.; Ito, S. Mechanisms for Rapid Re-Epithelialization of the Gastric Mucosal Surface. Annu. Rev. Physiol. 1985, 47, 217–229. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Alcohol, Oxidative Stress, and Free Radical Damage. Alcohol Res. -Curr. Rev. 2003, 27, 277–284. [Google Scholar]
- Nagy, L.E. Molecular Aspects of Alcohol Metabolism: Transcription Factors Involved in Early Ethanol-Induced Liver Injury. Annu. Rev. Nutr. 2004, 24, 55–78. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhao, S.; Zhu, Y.; Mao, J.; Liu, X.; Ye, J.; Zhang, Q.; Xu, X. Supramolecular Aggregates of Myricetin Improve its Bioavailability and its Role in Counteracting Alcoholism. J. Drug Deliv. Sci. Technol. 2022, 74, 103515. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; Lopez-Saez, J.A.; Cobianchi, R.C. Antibacterial Activity of Pure Flavonoids Isolated From Mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zhang, H.; Li, X.; Zhan, H.; Chen, F.; Han, L.; Xu, Y.; Cao, X. Ampelopsin Attenuates Lipopolysaccharide-Induced Inflammatory Response through the Inhibition of the Nf-Kappab and Jak2/Stat3 Signaling Pathways in Microglia. Int. Immunopharmacol. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, L.; Yu, J.; Zhang, J.; Xu, M.; Sun, L.; Luo, H.; Feng, Z.; Meng, G. Dihydromyricetin Attenuated Ang Ii Induced Cardiac Fibroblasts Proliferation Related to Inhibitory of Oxidative Stress. Eur. J. Pharmacol. 2017, 807, 159–167. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin Improves Glucose and Lipid Metabolism and Exerts Anti-Inflammatory Effects in Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L.; Zeng, X.A. Dihydromyricetin: A Review On Identification and Quantification Methods, Biological Activities, Chemical Stability, Metabolism and Approaches to Enhance its Bioavailability. Trends Food Sci. Technol. 2019, 91, 586–597. [Google Scholar] [CrossRef]
- Ruan, L.P.; Chen, S.; Yu, B.Y.; Zhu, D.N.; Cordell, G.A.; Qiu, S.X. Prediction of Human Absorption of Natural Compounds by the Non-Everted Rat Intestinal Sac Model. Eur. J. Med. Chem. 2006, 41, 605–610. [Google Scholar] [CrossRef]
- Kicinska, A.; Jarmuszkiewicz, W.; Liang, J. Flavonoids and Mitochondria: Activation of Cytoprotective Pathways. AlcoholMolecules 2020, 25, 1420–3049. [Google Scholar] [CrossRef]
- Shen, Y.; Lindemeyer, A.K.; Gonzalez, C.; Shao, X.M.; Spigelman, I.; Olsen, R.W.; Liang, J. Dihydromyricetin as a Novel Anti-Alcohol Intoxication Medication. J. Neurosci. 2012, 32, 390–401. [Google Scholar] [CrossRef]
- Ye, J.; Bao, S.; Zhao, S.; Zhu, Y.; Ren, Q.; Li, R.; Xu, X.; Zhang, Q. Self-Assembled Micelles Improve the Oral Bioavailability of Dihydromyricetin and Anti-Acute Alcoholism Activity. AAPS PharmSciTech 2021, 22, 111. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xin, Z.; Zhang, F.; Zhai, Z.; Ni, X.; Chen, J.; Yang, K.; Liao, P.; Zhang, L.; Xiao, Z.; et al. The Cytoprotective Effects of Dihydromyricetin and Associated Metabolic Pathway Changes On Deoxynivalenol Treated Ipec-J2 Cells. Food Chem. 2021, 338, 128116. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Hamed Almurisi, S.; Ahmed Mahdi Dukhan, A.; Mandal, U.K.; Sengupta, P. Controversies with Self-Emulsifying Drug Delivery System From Pharmacokinetic Point of View. Drug Deliv. 2016, 23, 3639–3652. [Google Scholar] [CrossRef] [PubMed]
- Dokania, S.; Joshi, A.K. Self-Microemulsifying Drug Delivery System (Smedds)--Challenges and Road Ahead. Drug Deliv. 2015, 22, 675–690. [Google Scholar] [CrossRef]
- Aungst, B.J. Intestinal Permeation Enhancers. J. Pharm. Sci. 2000, 89, 429–442. [Google Scholar] [CrossRef]
- Zafar, A.; Yasir, M.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Alshehri, S.; Ghoneim, M.M.; Alquraini, A.; Rawaf, A.; Ansari, M.J.; et al. Formulation of Self-Nanoemulsifying Drug Delivery System of Cephalexin: Physiochemical Characterization and Antibacterial Evaluation. Polymers 2022, 14, 1055. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Y.; Luo, H.; Sun, L.; Xu, M.; Yu, J.; Zhou, Q.; Meng, G.; Yang, S. Recent Update On the Pharmacological Effects and Mechanisms of Dihydromyricetin. Front. Pharmacol. 2018, 9, 1204. [Google Scholar] [CrossRef]
- Yan, B.; Ma, Y.; Guo, J.; Wang, Y. Self-Microemulsifying Delivery System for Improving Bioavailability of Water Insoluble Drugs. J. Nanopart. Res. 2020, 22, 18. [Google Scholar] [CrossRef]
- Avasarala, H.; Dinakaran, S.K.; Kakaraparthy, R.; Jayanti, V.R. Self-Emulsifying Drug Delivery System for Enhanced Solubility of Asenapine Maleate: Design, Characterization, in Vitro, Ex Vivo Andin Vivo Appraisal. Drug Dev. Ind. Pharm. 2019, 45, 548–559. [Google Scholar] [CrossRef]
- Li, F.; Hu, R.; Wang, B.; Gui, Y.; Cheng, G.; Gao, S.; Ye, L.; Tang, J. Self-Microemulsifying Drug Delivery System for Improving the Bioavailability of Huperzine a by Lymphatic Uptake. Acta Pharm. Sin. B 2017, 7, 353–360. [Google Scholar] [CrossRef]
- Hammond, G.L.; Nisker, J.A.; Jones, L.A.; Siiteri, P.K. Estimation of the Percentage of Free Steroid in Undiluted Serum by Centrifugal Ultrafiltration-Dialysis. J. Biol. Chem. 1980, 255, 5023–5026. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.A.; Woolhiser, M.M.; Ladics, G.S.; Korjagin, V.A.; Schafer, B.W.; Storer, N.P.; Green, S.B.; Kan, L. Stability of a Set of Allergens and Non-Allergens in Simulated Gastric Fluid. Int. J. Food Sci. Nutr. 2007, 58, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Rabel, M.; Warncke, P.; Grüttner, C.; Bergemann, C.; Kurland, H.; Müller, R.; Dugandžić, V.; Thamm, J.; Müller, F.A.; Popp, J.; et al. Simulation of the Long-Term Fate of Superparamagnetic Iron Oxide-Based Nanoparticles Using Simulated Biological Fluids. Nanomedicine 2019, 14, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.T.; Hiserodt, R.D.; Fukuda, E.K.; Ruiz, R.J.; Zhou, Z.; Lech, J.; Rosen, S.L.; Hartman, T.G. Determination of Allicin, S-Allylcysteine and Volatile Metabolites of Garlic in Breath, Plasma Or Simulated Gastric Fluids. J. Nutr. 2001, 131, 968S–971S. [Google Scholar] [CrossRef] [PubMed]
- Zakeri-Milani, P.; Valizadeh, H.; Tajerzadeh, H.; Azarmi, Y.; Islambolchilar, Z.; Barzegar, S.; Barzegar-Jalali, M. Predicting Human Intestinal Permeability Using Single-Pass Intestinal Perfusion in Rat. J. Pharm. Pharm. Sci. 2007, 10, 368–379. [Google Scholar]
- Satoh, H.; Guth, P.H.; Grossman, M.I. Role of Bacteria in Gastric Ulceration Produced by Indomethacin in the Rat: Cytoprotective Action of Antibiotics. Gastroenterology 1983, 84, 483–489. [Google Scholar] [CrossRef]
- Yonei, Y.; Guth, P.H. Ethanol-Induced Gastric Injury. Role of Submucosal Venoconstriction and Leukotrienes. Dig. Dis. Sci. 1991, 36, 601–608. [Google Scholar] [CrossRef]
- Guth, P.H. Current Concepts in Gastric Microcirculatory Pathophysiology. Yale J. Biol. Med. 1992, 65, 677–688. [Google Scholar]
- Kono, Y. Generation of Superoxide Radical During Autoxidation of Hydroxylamine and an Assay for Superoxide Dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Mathews, P.D.; Mertins, O.; Angelov, B.; Angelova, A. Cubosomal Lipid Nanoassemblies with Ph-Sensitive Shells Created by Biopolymer Complexes: A Synchrotron Saxs Study. J. Colloid Interface Sci. 2022, 607 Pt 1, 440–450. [Google Scholar] [CrossRef]
- Xiang, D.; Wang, C.; Wang, W.; Shi, C.; Xiong, W.; Wang, M.; Fang, J. Gastrointestinal Stability of Dihydromyricetin, Myricetin, and Myricitrin: An in Vitro Investigation. Int. J. Food Sci. Nutr. 2017, 68, 704–711. [Google Scholar] [CrossRef]
- Dahan, A.; Hoffman, A. Evaluation of a Chylomicron Flow Blocking Approach to Investigate the Intestinal Lymphatic Transport of Lipophilic Drugs. Eur. J. Pharm. Sci. 2005, 24, 381–388. [Google Scholar] [CrossRef]
- Sun, M.; Zhai, X.; Xue, K.; Hu, L.; Yang, X.; Li, G.; Si, L. Intestinal Absorption and Intestinal Lymphatic Transport of Sirolimus From Self-Microemulsifying Drug Delivery Systems Assessed Using the Single-Pass Intestinal Perfusion (Spip) Technique and a Chylomicron Flow Blocking Approach: Linear Correlation with Oral Bioavailabilities in Rats. Eur. J. Pharm. Sci. 2011, 43, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ryšánek, P.; Grus, T.; Lukáč, P.; Kozlík, P.; Křížek, T.; Pozniak, J.; Roušarová, J.; Královičová, J.; Kutinová Canová, N.; Boleslavská, T.; et al. Validity of Cycloheximide Chylomicron Flow Blocking Method for the Evaluation of Lymphatic Transport of Drugs. Br. J. Pharmacol. 2021, 178, 4663–4674. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Duvdevani, R.; Shapiro, I.; Elmann, A.; Finkelstein, E.; Hoffman, A. The Oral Absorption of Phospholipid Prodrugs: In Vivo and in Vitro Mechanistic Investigation of Trafficking of a Lecithin-Valproic Acid Conjugate Following Oral Administration. J. Control. Release 2008, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.H.; Eisenhofer, G.; Lambie, D.G. The Effects of Acute and Chronic Ingestion of Ethanol On the Autonomic Nervous System. Drug Alcohol Depend. 1986, 18, 319–328. [Google Scholar] [CrossRef]
- Estonius, M.; Svensson, S.; Hoog, J.O. Alcohol Dehydrogenase in Human Tissues: Localisation of Transcripts Coding for Five Classes of the Enzyme. Febs Lett. 1996, 397, 338–342. [Google Scholar] [CrossRef]
- Lieber, C.S.; Gentry, R.T.; Baraona, E. First Pass Metabolism of Ethanol. Alcohol Alcohol. Suppl. 1994, 2, 163–169. [Google Scholar] [PubMed]
- Teschke, R. Microsomal Ethanol-Oxidizing System: Success Over 50 Years and an Encouraging Future. Alcohol. Clin. Exp. Res. 2019, 43, 386–400. [Google Scholar] [CrossRef]
- Silva, J.; Yu, X.; Moradian, R.; Folk, C.; Spatz, M.H.; Kim, P.; Bhatti, A.A.; Davies, D.L.; Liang, J. Dihydromyricetin Protects the Liver Via Changes in Lipid Metabolism and Enhanced Ethanol Metabolism. Alcoholism 2020, 44, 1046–1060. [Google Scholar] [CrossRef]
- Pan, J.; He, S.; Xu, H.; Zhan, X.; Yang, X.; Xiao, H.; Shi, H.; Ren, J. Oxidative Stress Disturbs Energy Metabolism of Mitochondria in Ethanol-Induced Gastric Mucosa Injury. World J. Gastroenterol. 2008, 14, 5857. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, R.; Ribiere, C.; Rouach, H. Ethanol-Induced Lipid Peroxidation and Oxidative Stress in Extrahepatic Tissues. Alcohol Alcohol. 1990, 25, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhai, Q.; Shi, X. Alcohol-Induced Oxidative Stress and Cell Responses. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. S3), S26–S29. [Google Scholar] [CrossRef] [PubMed]



| Medium | Peak 1 | Percent (%) |
|---|---|---|
| pH 1.3 | 37.16 | 100 |
| pH 6.8 | 38.98 | 100 |
| pH 7.0 | 36.29 | 100 |
| Parameter | DMY Suspension | DMY-SEDDS | DMY-SEDDS + CYC |
|---|---|---|---|
| AUC0-t (μg∙h/L) | 304.06 ± 146.07 | 1256.59 ± 549.71 ** | 769.07 ± 73.19 |
| MRT0-t (h) | 3.94 ± 2.95 | 6.31 ± 1.67 * | 7.37 ± 1.67 |
| Tmax (h) | 0.13 ± 0.09 | 0.62 ± 0.21 * | 0.19 ± 0.09 |
| Cmax (μg/L) | 211.01 ± 114.71 | 438.76 ± 192.46 * | 236.29 ± 91.15 |
| Group | Number of Mice | Drunkenness Rate | Death Rate | LORR Onset (min) | LORR Duration (min) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Model | 12 | 100% | 33% | 10.50 ± 7.79 | 559.63 ± 71.03 |
| DMY | 12 | 100% | 25% | 13.58 ± 13.68 | 587.89 ± 88.69 |
| DMY-SEDDS | 12 | 75% | 0% | 24.75 ± 14.14 *,# | 432.38 ± 92.92 **,## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Wang, S.; Mao, J.; Wang, Z.; Zhao, S.; Ren, Q.; Kang, J.; Ye, J.; Xu, X.; Zhu, Y.; et al. Preparation of Dihydromyricetin-Loaded Self-Emulsifying Drug Delivery System and Its Anti-Alcoholism Effect. Pharmaceutics 2023, 15, 2296. https://doi.org/10.3390/pharmaceutics15092296
Dong J, Wang S, Mao J, Wang Z, Zhao S, Ren Q, Kang J, Ye J, Xu X, Zhu Y, et al. Preparation of Dihydromyricetin-Loaded Self-Emulsifying Drug Delivery System and Its Anti-Alcoholism Effect. Pharmaceutics. 2023; 15(9):2296. https://doi.org/10.3390/pharmaceutics15092296
Chicago/Turabian StyleDong, Jianxia, Shu Wang, Jiamin Mao, Zhidan Wang, Shiying Zhao, Qiao Ren, Jialing Kang, Jing Ye, Xiaohong Xu, Yujin Zhu, and et al. 2023. "Preparation of Dihydromyricetin-Loaded Self-Emulsifying Drug Delivery System and Its Anti-Alcoholism Effect" Pharmaceutics 15, no. 9: 2296. https://doi.org/10.3390/pharmaceutics15092296
APA StyleDong, J., Wang, S., Mao, J., Wang, Z., Zhao, S., Ren, Q., Kang, J., Ye, J., Xu, X., Zhu, Y., & Zhang, Q. (2023). Preparation of Dihydromyricetin-Loaded Self-Emulsifying Drug Delivery System and Its Anti-Alcoholism Effect. Pharmaceutics, 15(9), 2296. https://doi.org/10.3390/pharmaceutics15092296






