Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures
2.2. Eukaryotic Cell Culture
2.3. Bacterial Growth Curves
2.4. Crystal Violet Biofilm Staining
2.5. Cell-Free Supernatant (CFS) Production
2.6. Viability Assay
2.7. Wound Healing Assay
2.8. IL-6 ELISA Assay
2.9. Statistical Analysis
3. Results and Discussion
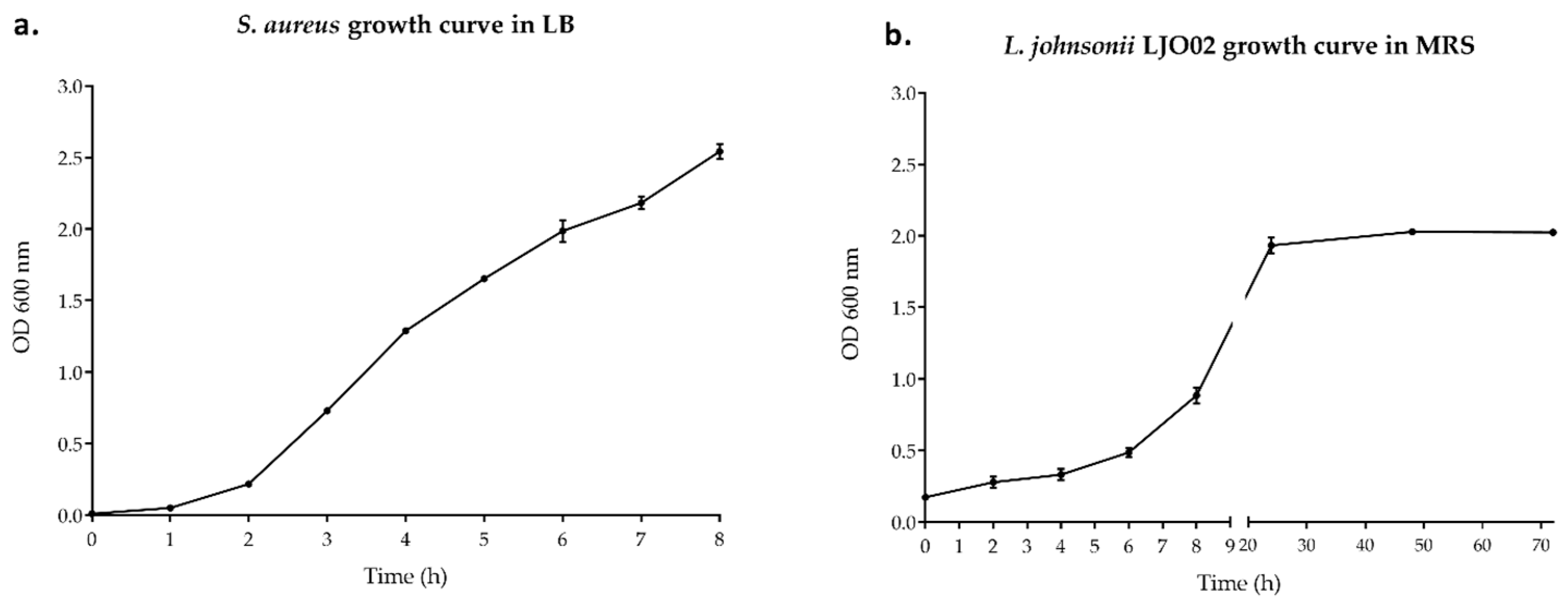
3.1. Bacterial Growth Curves
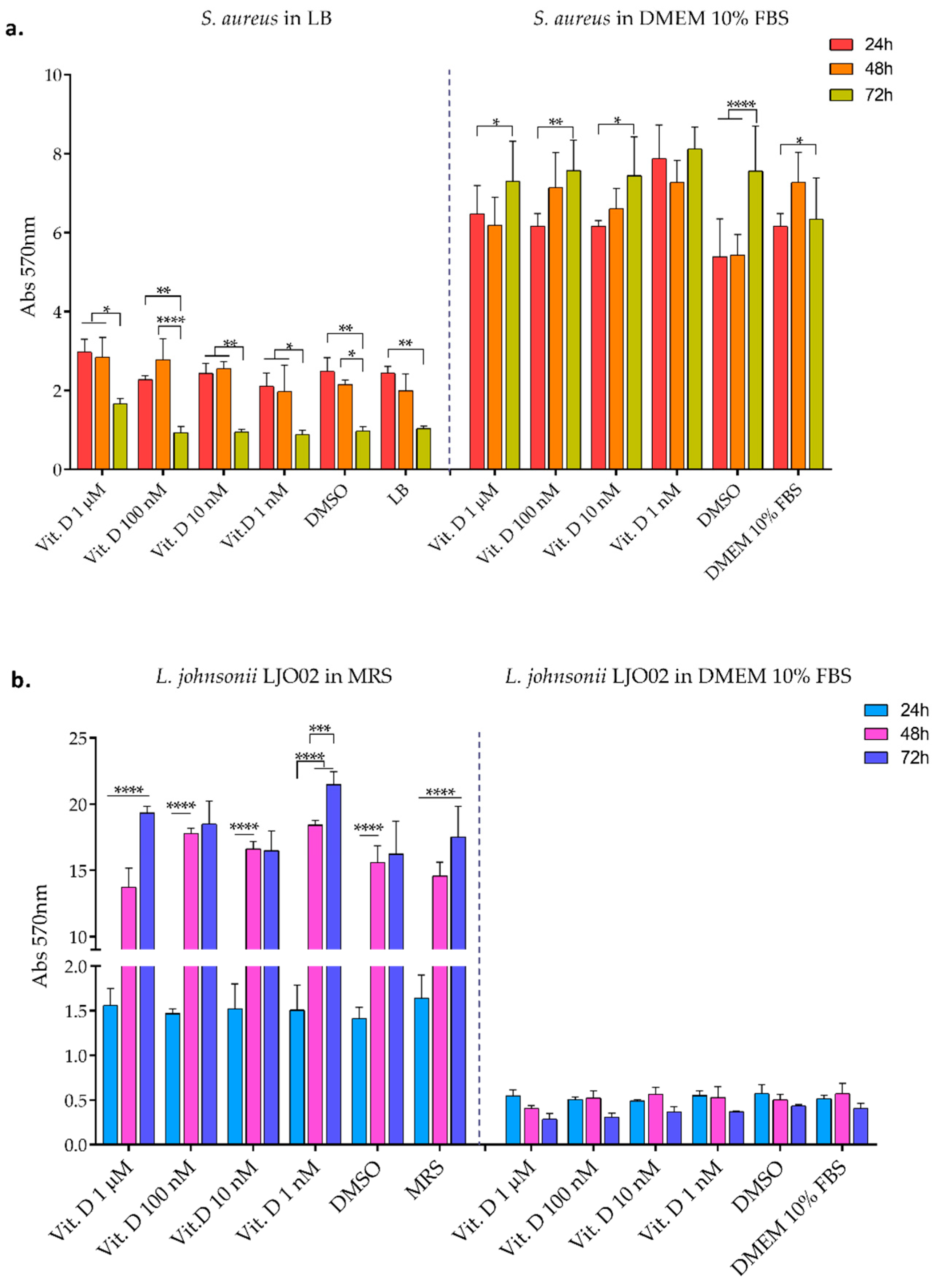
3.2. Bacterial Biofilm Formation
3.3. L. johnsonii LJO02 CFS Analysis
3.4. LJO02 CFS and Vitamin D Effects on HaCaT Cells Viability
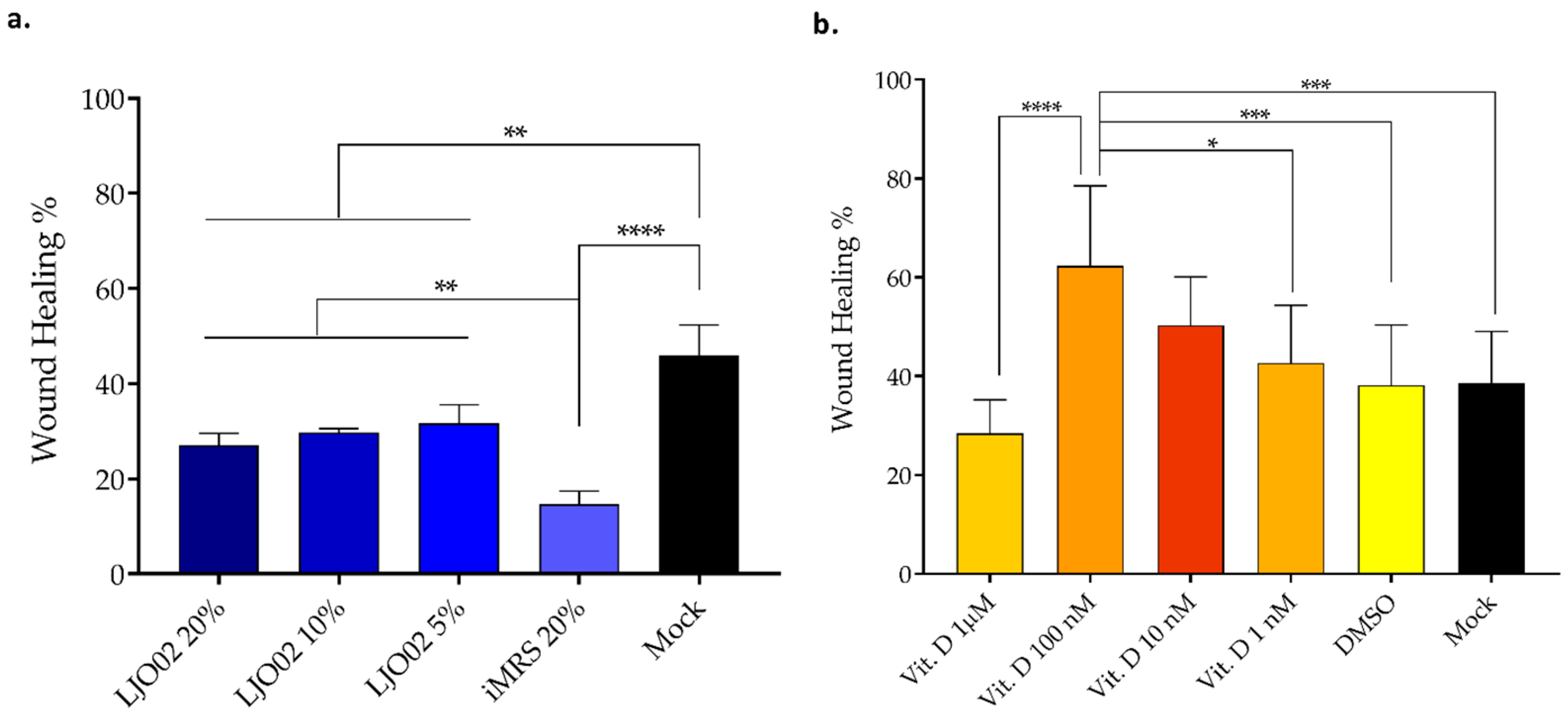
3.5. LJO02 CFS and Vitamin D Effects on HaCaT Cells Wound Healing
3.6. LJO02 CFS and Vitamin D Effects on S. aureus-Infected HaCaT Cell Viability
3.7. LJO02 CFS and Vitamin D Effects on S. aureus-Infected HaCaT Cell Wound Healing
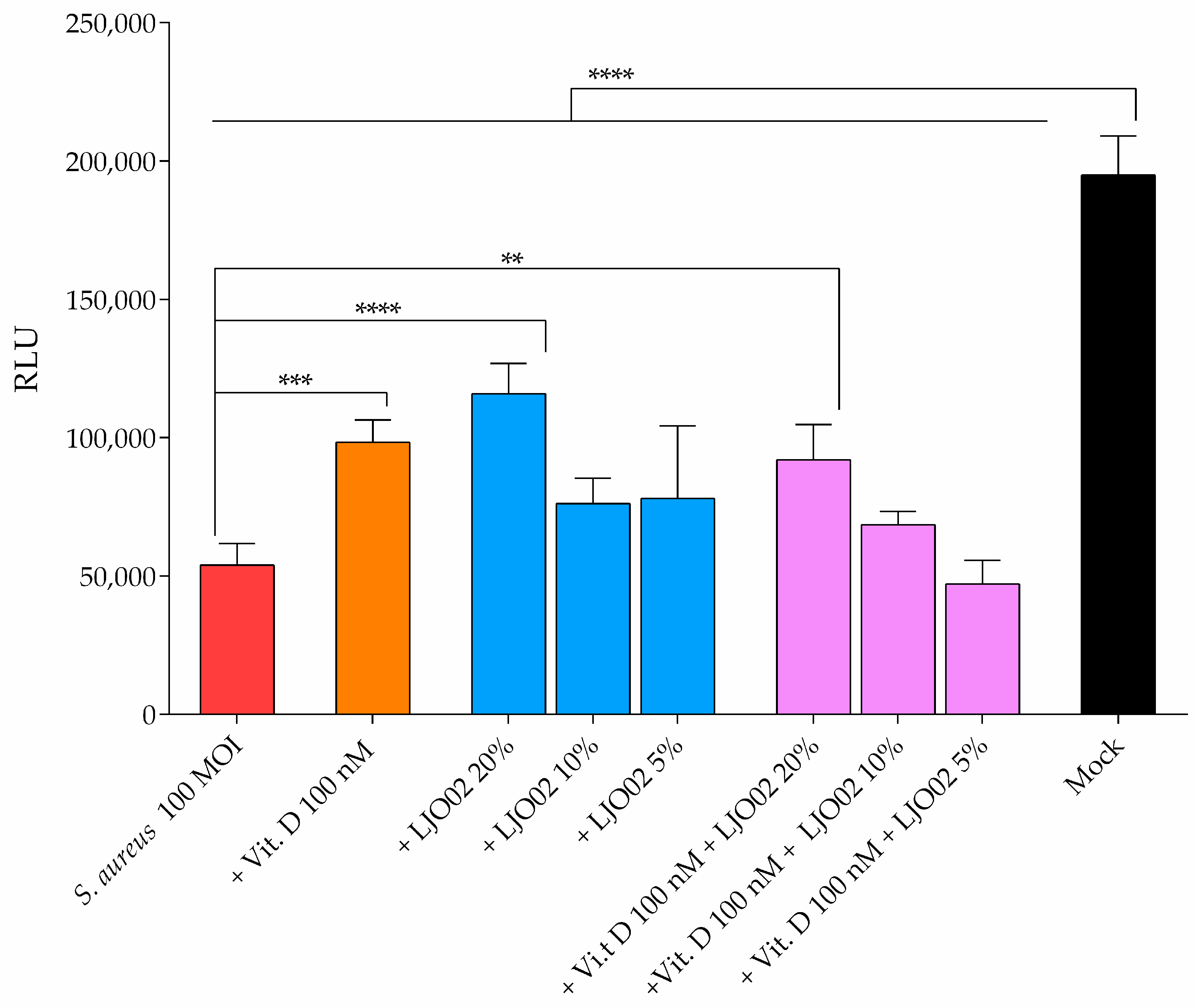
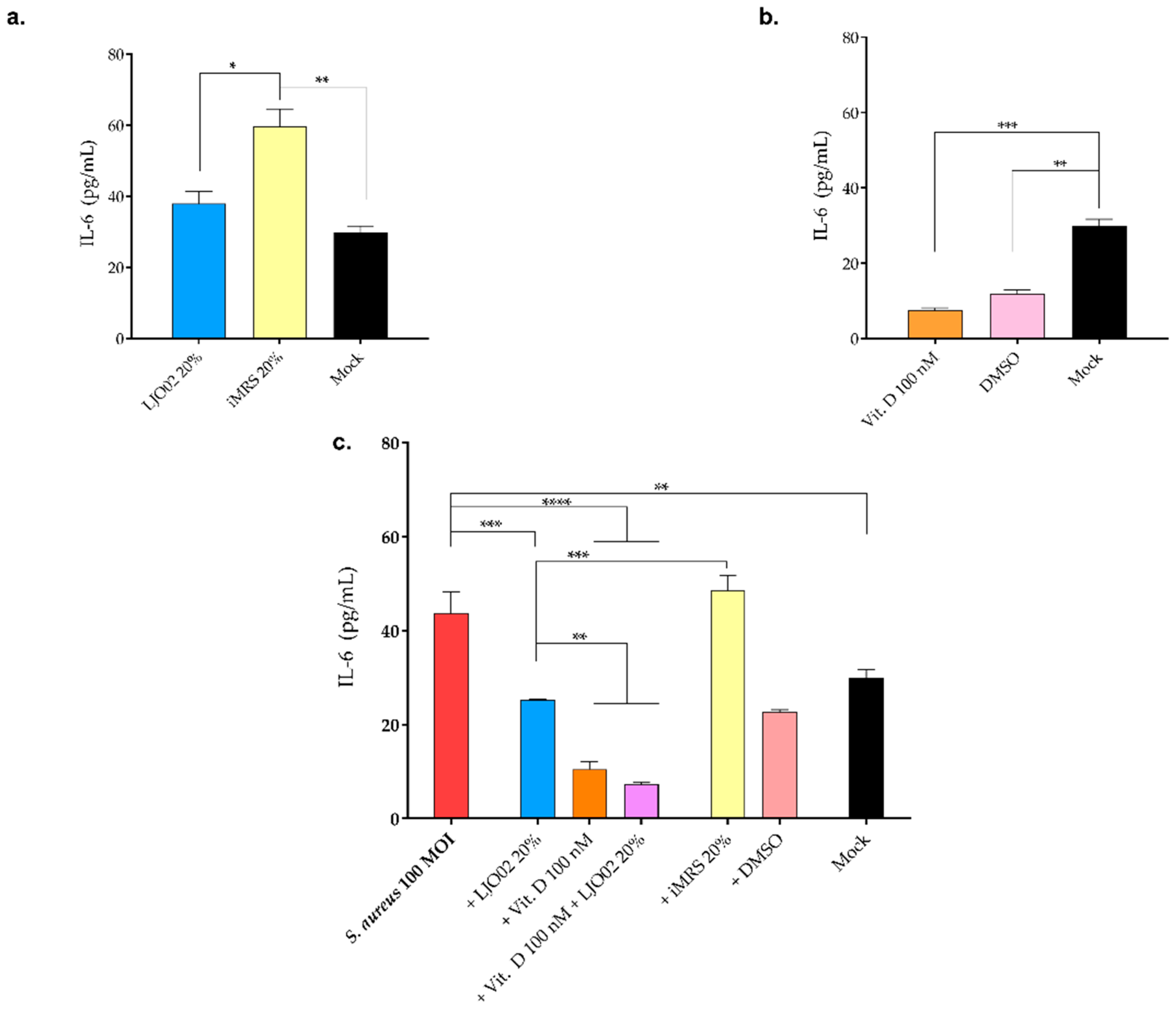
3.8. LJO02 CFS and Vitamin D Effect on IL-6 Production by S. aureus-Infected HaCaT Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut–Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Squarzanti, D.F.; Zanetta, P.; Ormelli, M.; Manfredi, M.; Barberis, E.; Vanella, V.V.; Amoruso, A.; Pane, M.; Azzimonti, B. An animal derivative-free medium enhances Lactobacillus johnsonii LJO02 supernatant selective efficacy against the methicillin (oxacillin)-resistant Staphylococcus aureus virulence through key-metabolites. Sci. Rep. 2022, 12, 8666. [Google Scholar] [CrossRef] [PubMed]
- Kullander, J.; Forslund, O.; Dillner, J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 2009, 18, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Squarzanti, D.F.; Zavattaro, E.; Pizzimenti, S.; Amoruso, A.; Savoia, P.; Azzimonti, B. Non-Melanoma Skin Cancer: News from microbiota research. Crit. Rev. Microbiol. 2020, 46, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Borges, A.; Simões, M. Staphylococcus aureus Toxins and Their Molecular Activity in Infectious Diseases. Toxins 2018, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Ciardiello, T.; Franzoni, M.; Pasini, F.; Giuliani, G.; Rinaldi, F. Effect of commonly used cosmetic preservatives on skin resident microflora dynamics. Sci. Rep. 2021, 11, 8695. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.-C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Ngo, Q.V.; Faass, L.; Sähr, A.; Hildebrand, D.; Eigenbrod, T.; Heeg, K.; Nurjadi, D. Inflammatory Response Against Staphylococcus aureus via Intracellular Sensing of Nucleic Acids in Keratinocytes. Front. Immunol. 2022, 13, 828626. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Santra, S.; Das, A.; Dixith, S.; Sinha, M.; Ghatak, S.; Ghosh, N.; Banerjee, P.; Khanna, S.; Mathew-Steiner, S.; et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann. Surg. 2020, 271, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Podia, M.; Priyanka; Raut, S.; Singh, S.; Pinnaka, A.K.; Khatri, N. Insight Into the Beneficial Role of Lactiplantibacillus plantarum Supernatant Against Bacterial Infections, Oxidative Stress, and Wound Healing in A549 Cells and BALB/c Mice. Front. Pharmacol. 2021, 12, 728614. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jiang, Z.; Li, D.; Jiang, D.; Wu, Y.; Ren, H.; Peng, H.; Lai, Y. Oral Antibiotic Treatment Induces Skin Microbiota Dysbiosis and Influences Wound Healing. Microb. Ecol. 2015, 69, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Kesavelu, D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 204993612311544. [Google Scholar] [CrossRef]
- Ng, W.Z.J.; van Hasselt, J.; Aggarwal, B.; Manoharan, A. Association Between Adult Antibiotic Use, Microbial Dysbiosis and Atopic Conditions—A Systematic Review. J. Asthma Allergy 2023, 16, 1115–1132. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Fumincelli, L.; Mazzo, A.; Caldeira, S.; Martins, J.C. Comfort, well-being and quality of life: Discussion of the differences and similarities among the concepts. Porto Biomed. J. 2017, 2, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Brandi, J.; Cheri, S.; Manfredi, M.; Di Carlo, C.; Vita Vanella, V.; Federici, F.; Bombiero, E.; Bazaj, A.; Rizzi, E.; Manna, L.; et al. Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 2020, 10, 11572. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Arrioja-Bretón, D.; López-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef] [PubMed]
- Aiba, Y.; Umeda, K.; Rahman, S.; Nguyen, S.V.; Komatsu, Y. Synergistic effect of anti-Helicobacter pylori urease immunoglobulin Y from egg yolk of immunized hens and Lactobacillus johnsonii No.1088 to inhibit the growth of Helicobacter pylori in vitro and in vivo. Vaccine 2019, 37, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Drumond, M.M.; Tapia-Costa, A.P.; Neumann, E.; Nunes, Á.C.; Barbosa, J.W.; Kassuha, D.E.; Mancha-Agresti, P. Cell-free supernatant of probiotic bacteria exerted antibiofilm and antibacterial activities against Pseudomonas aeruginosa: A novel biotic therapy. Front. Pharmacol. 2023, 14, 1152588. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; Miller, C.W.; El-Abbassi, A.M.; Cutchins, D.C.; Cutchins, C.; Grant, W.B.; Peiris, A.N. Antimicrobial implications of vitamin D. Dermato-Endocrinology 2011, 3, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Xu, H.-J.; Li, Y.; Hu, C.-M.; Yang, J.-Y.; Sun, M.-Y. The Anti-Inflammatory Effects of Vitamin D in Tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2736. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Thomason, J.; Rentsch, C.; Stenehjem, E.A.; Hidron, A.I.; Rimland, D. Association between vitamin D deficiency and methicillin-resistant Staphylococcus aureus infection. Infection 2015, 43, 715–722. [Google Scholar] [CrossRef]
- Yokosawa, E.B.; Arthur, A.E.; Rentschler, K.M.; Wolf, G.T.; Rozek, L.S.; Mondul, A.M. Vitamin D intake and survival and recurrence in head and neck cancer patients. Laryngoscope 2018, 128, E371–E376. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, L.; Lei, K.; Zeng, J.; Luo, J.; Yin, Y.; Li, Y.; Zhang, L.; Nie, X.; Zuo, D.; et al. Vitamin D3 analogue facilitates epithelial wound healing through promoting epithelial-mesenchymal transition via the Hippo pathway. J. Dermatol. Sci. 2020, 100, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Patria, F.F.; Ceccarini, M.R.; Codini, M.; Conte, C.; Perioli, L.; Beccari, T.; Albi, E. A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment. Int. J. Mol. Sci. 2019, 20, 3634. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Dias, K.; Barbugli, P.A.; de Patto, F.; Lordello, V.B.; de Aquino Penteado, L.; Medeiros, A.I.; Vergani, C.E. Soluble factors from biofilm of Candida albicans and Staphylococcus aureus promote cell death and inflammatory response. BMC Microbiol. 2017, 17, 146. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Hogan, P.G.; Hunstad, D.A.; Fritz, S.A. Vitamin D sufficiency and Staphylococcus aureus infection in children. Pediatr. Infect. Dis. J. 2015, 34, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21, 36–43. [Google Scholar] [CrossRef]
- Saravanan, P.; Pooja, R.; Balachander, N.; K., K.R.S.; S., S.; S., R. Anti-inflammatory and wound healing properties of lactic acid bacteria and its peptides. Folia Microbiol. 2023, 68, 337–353. [Google Scholar] [CrossRef]
- Zanetta, P.; Squarzanti, D.F.; Sorrentino, R.; Rolla, R.; Aluffi Valletti, P.; Garzaro, M.; Dell’Era, V.; Amoruso, A.; Azzimonti, B. Oral microbiota and vitamin D impact on oropharyngeal squamous cell carcinogenesis: A narrative literature review. Crit. Rev. Microbiol. 2021, 47, 224–239. [Google Scholar] [CrossRef]
- Mousa, A.; Misso, M.; Teede, H.; Scragg, R.; de Courten, B. Effect of vitamin D supplementation on inflammation: Protocol for a systematic review. BMJ Open 2016, 6, e010804. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Kang, C.-H. Immunostimulatory Activity of Lactic Acid Bacteria Cell-Free Supernatants through the Activation of NF-κB and MAPK Signaling Pathways in RAW 264.7 Cells. Microorganisms 2022, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Kasti, A.N.; Synodinou, K.D.; Pyrousis, I.A.; Nikolaki, M.D.; Triantafyllou, K.D. Probiotics Regulating Inflammation via NLRP3 Inflammasome Modulation: A Potential Therapeutic Approach for COVID-19. Microorganisms 2021, 9, 2376. [Google Scholar] [CrossRef] [PubMed]
- Alva-Murillo, N.; Téllez-Pérez, A.D.; Medina-Estrada, I.; Álvarez-Aguilar, C.; Ochoa-Zarzosa, A.; López-Meza, J.E. Modulation of the inflammatory response of bovine mammary epithelial cells by cholecalciferol (vitamin D) during Staphylococcus aureus internalization. Microb. Pathog. 2014, 77, 24–30. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid.-Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]






| Protein Concentration (mg/mL) | pH | Lactic Acid Concentration (g/L) |
|---|---|---|
| 8.52 | 4 | 7.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanetta, P.; Ballacchino, C.; Squarzanti, D.F.; Amoruso, A.; Pane, M.; Azzimonti, B. Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes. Pharmaceutics 2024, 16, 18. https://doi.org/10.3390/pharmaceutics16010018
Zanetta P, Ballacchino C, Squarzanti DF, Amoruso A, Pane M, Azzimonti B. Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes. Pharmaceutics. 2024; 16(1):18. https://doi.org/10.3390/pharmaceutics16010018
Chicago/Turabian StyleZanetta, Paola, Chiara Ballacchino, Diletta Francesca Squarzanti, Angela Amoruso, Marco Pane, and Barbara Azzimonti. 2024. "Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes" Pharmaceutics 16, no. 1: 18. https://doi.org/10.3390/pharmaceutics16010018
APA StyleZanetta, P., Ballacchino, C., Squarzanti, D. F., Amoruso, A., Pane, M., & Azzimonti, B. (2024). Lactobacillus johnsonii LJO02 (DSM 33828) Cell-Free Supernatant and Vitamin D Improve Wound Healing and Reduce Interleukin-6 Production in Staphylococcus aureus-Infected Human Keratinocytes. Pharmaceutics, 16(1), 18. https://doi.org/10.3390/pharmaceutics16010018









