Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile
Abstract
1. Introduction
2. Methods
2.1. In Vitro Studies
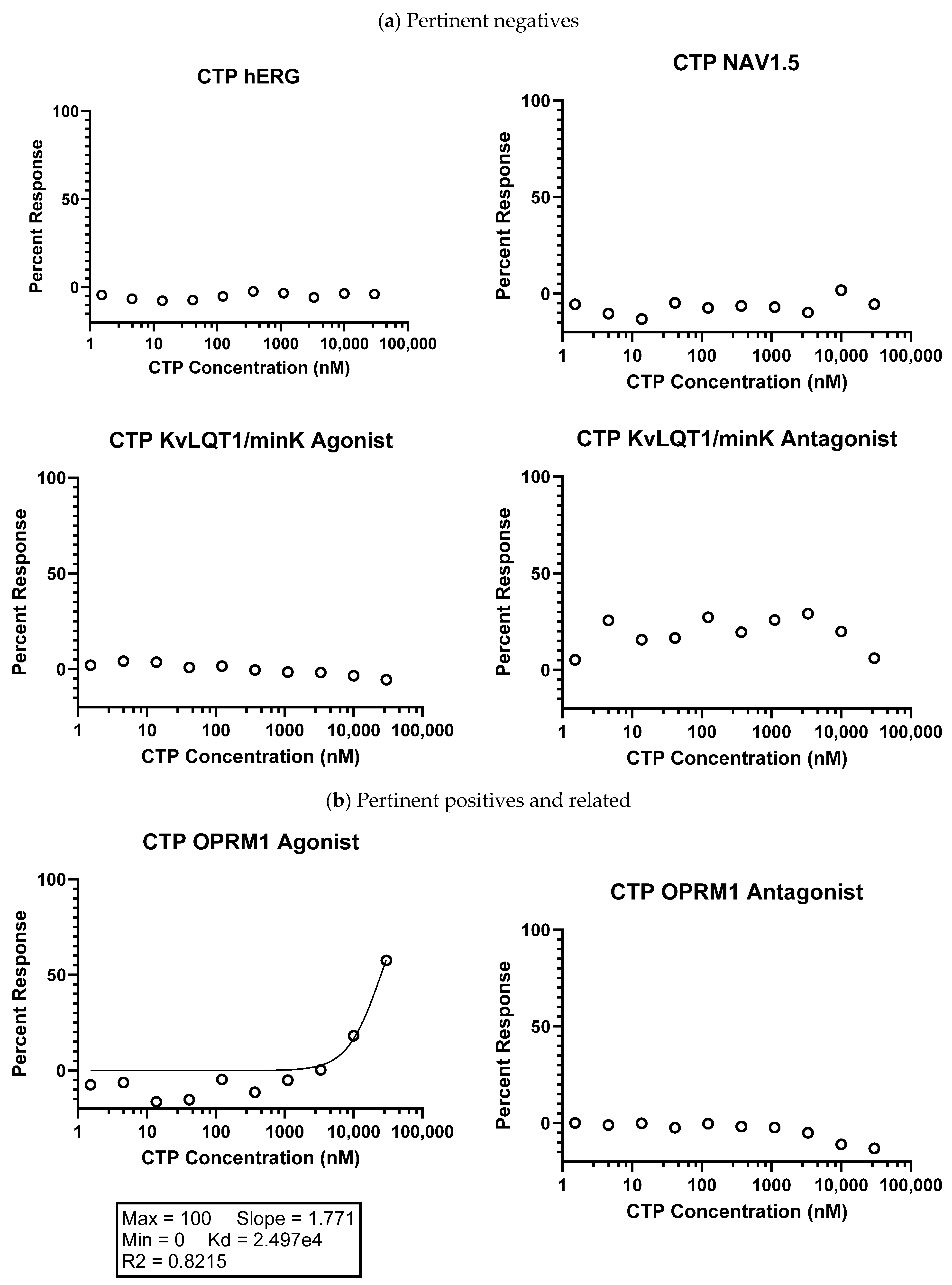
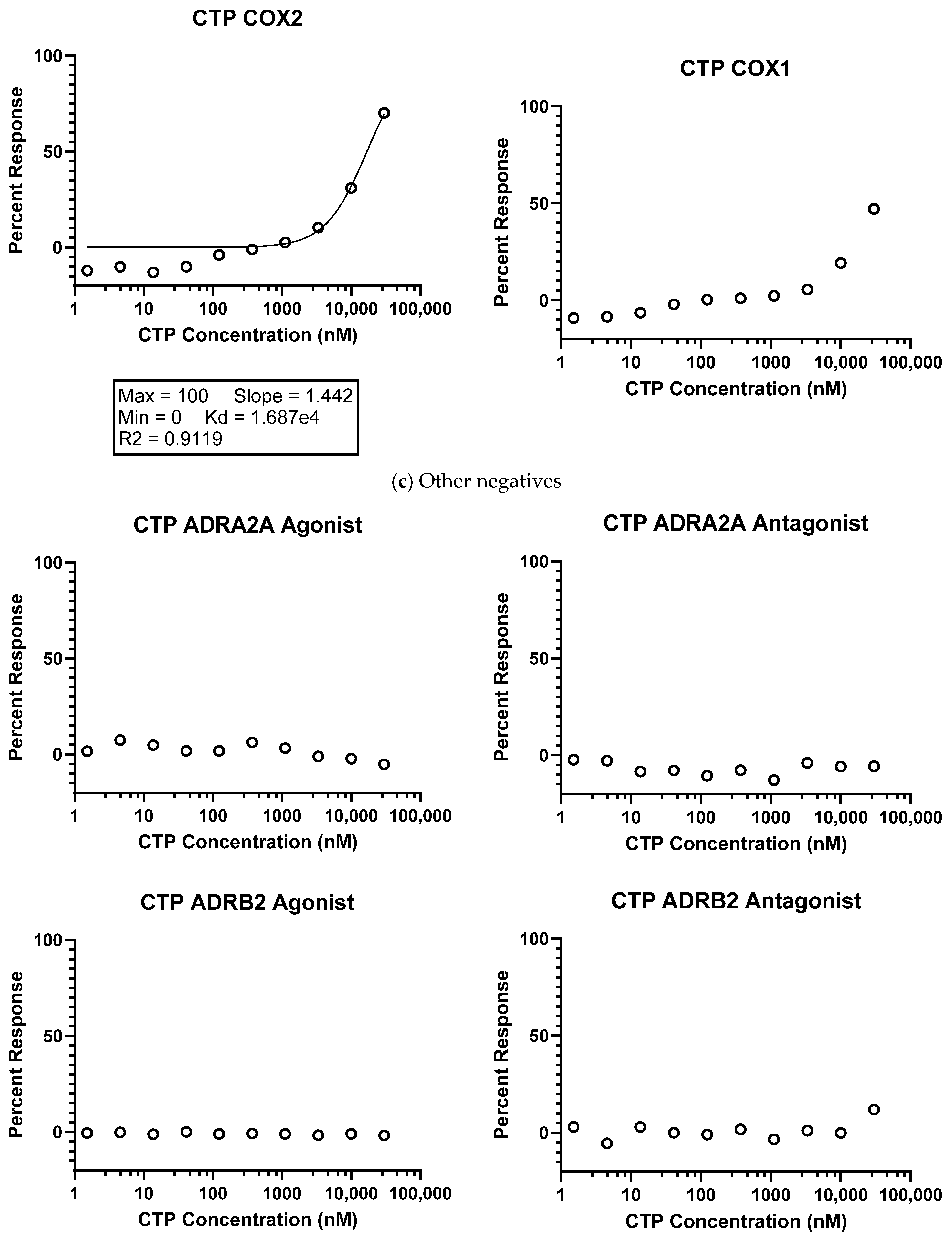
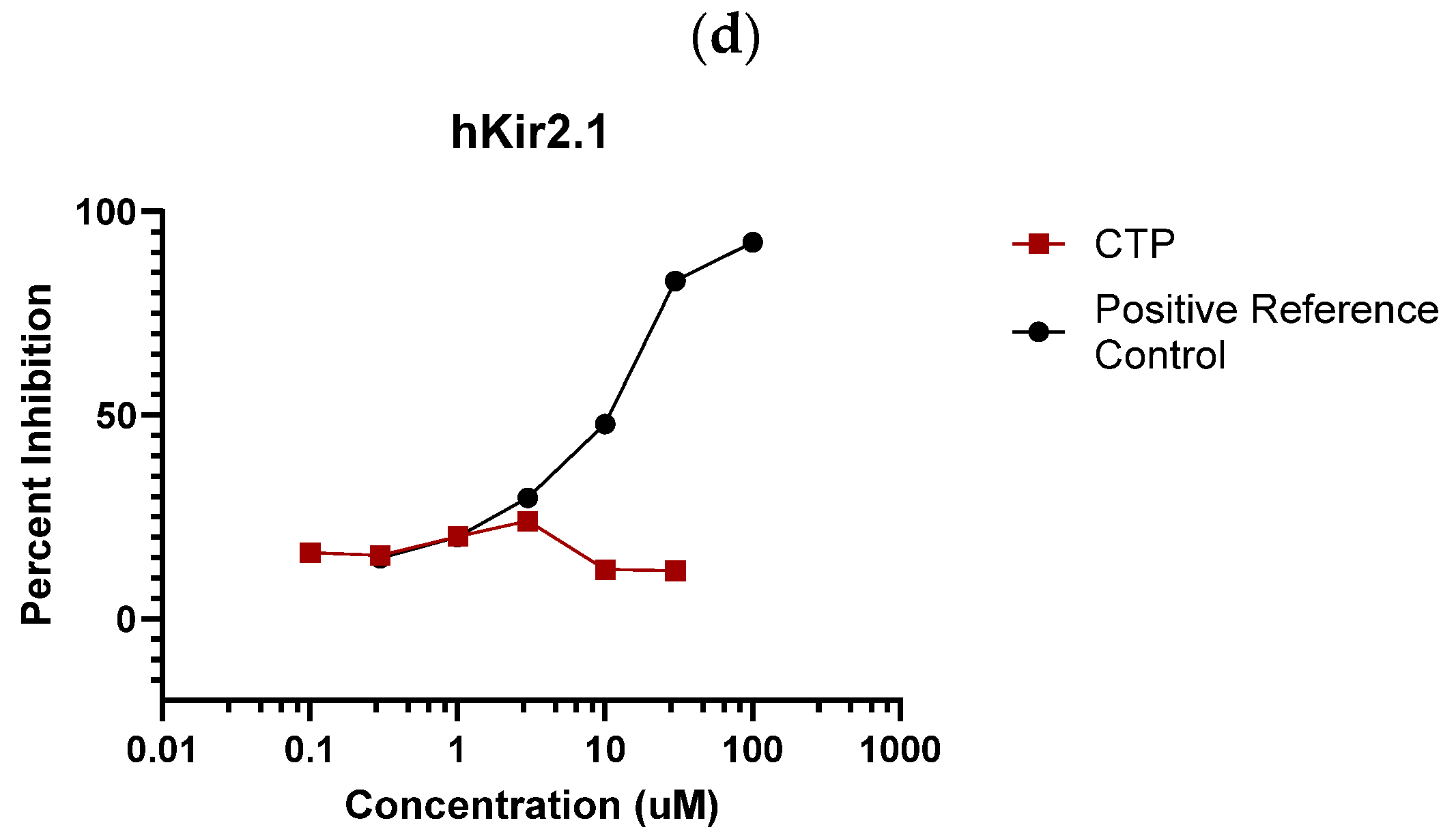
2.1.1. Eurofins Ion Channel Cardiac Profiler Panel
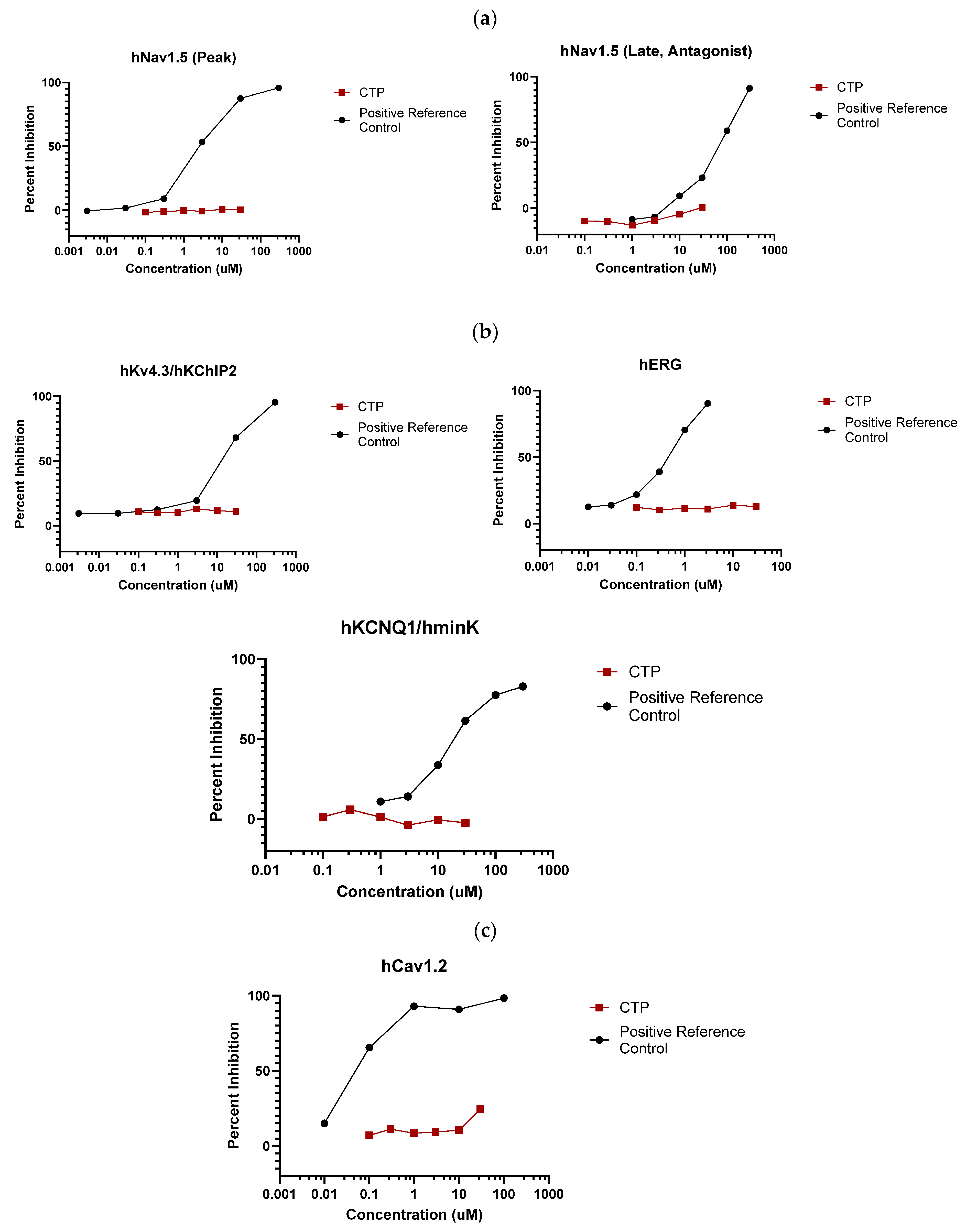
2.1.2. Eurofins SAFETYscan E/IC50 ELECT Service
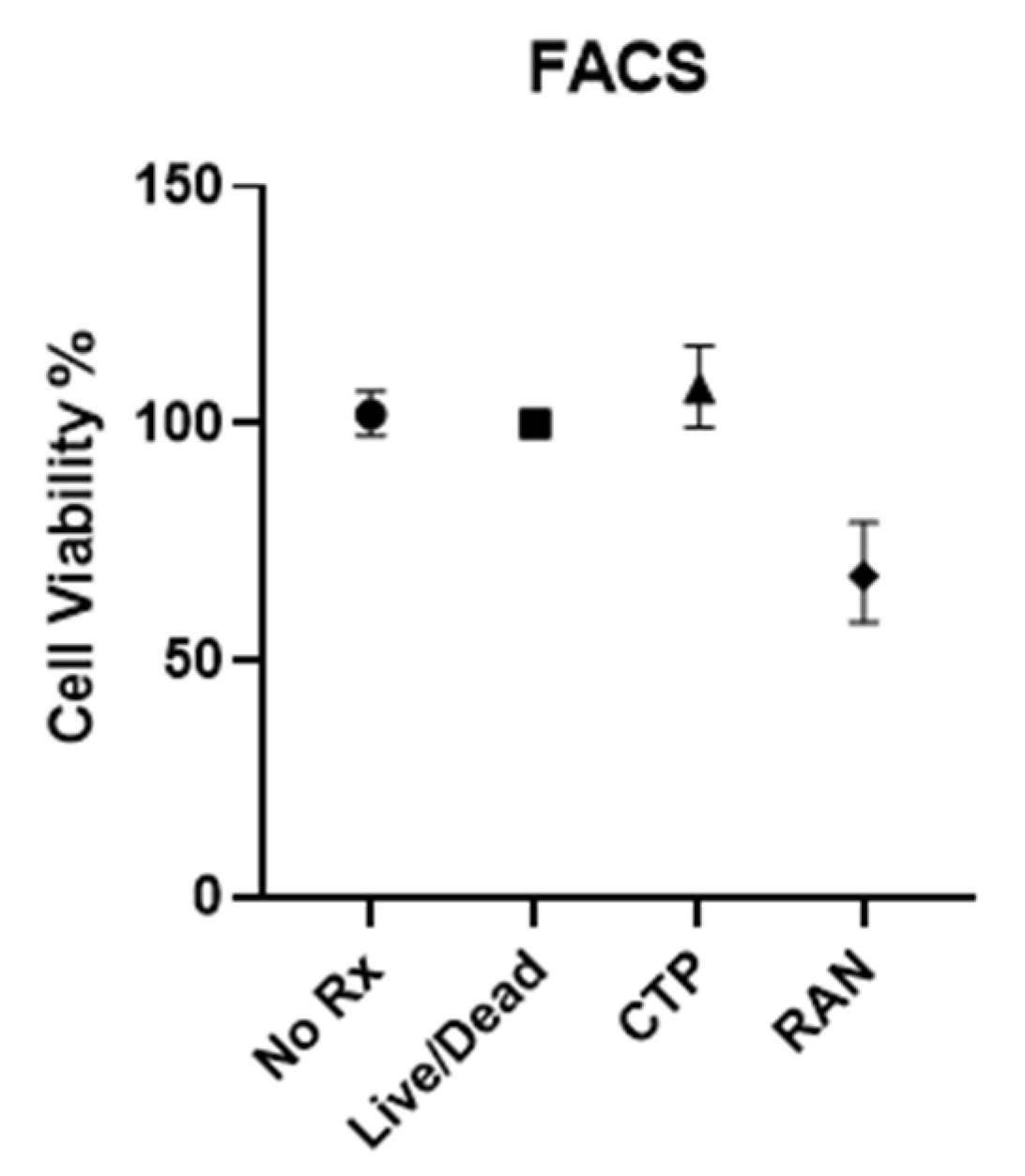
2.2. In Vivo Studies
3. Discussion
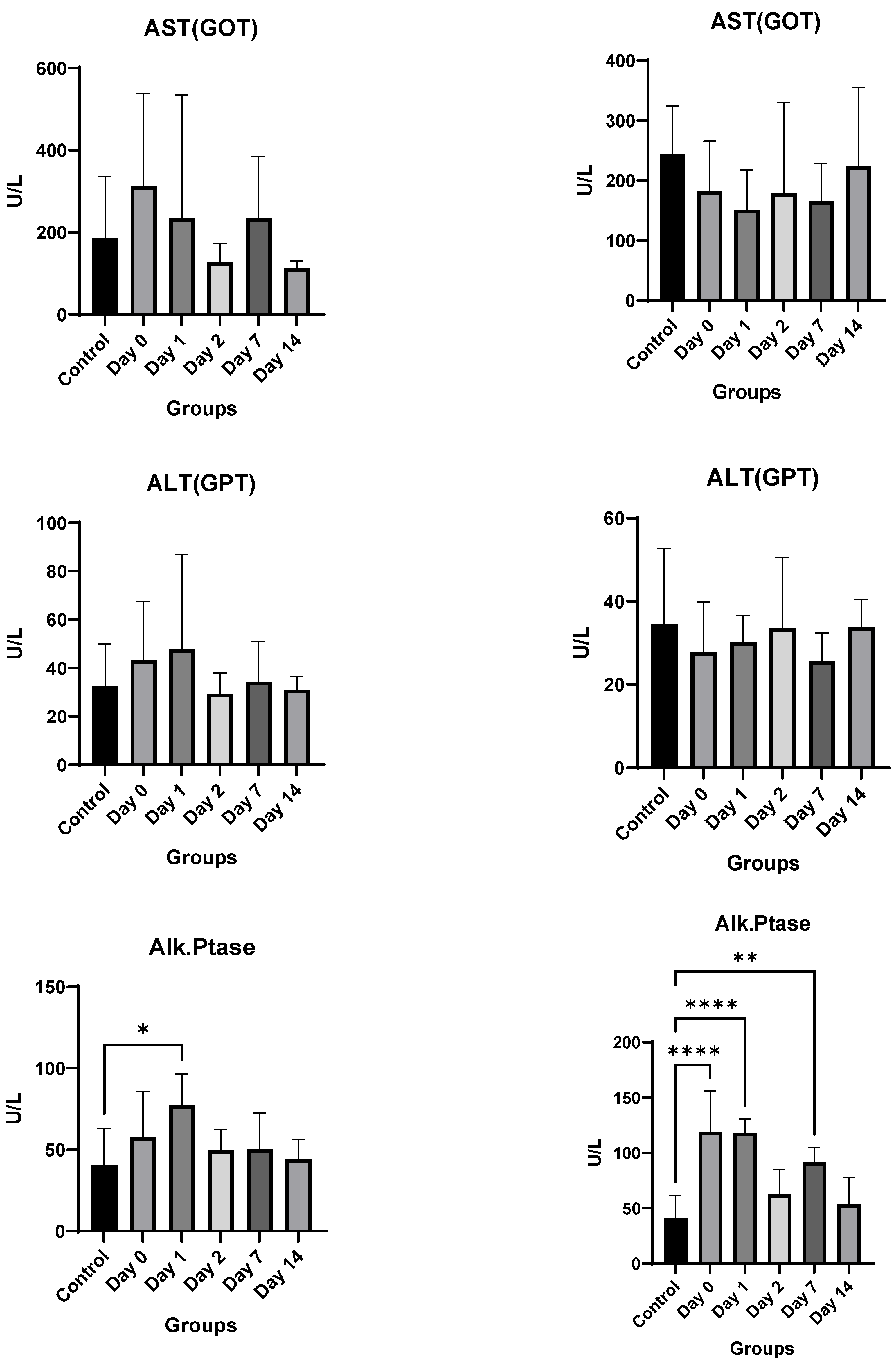
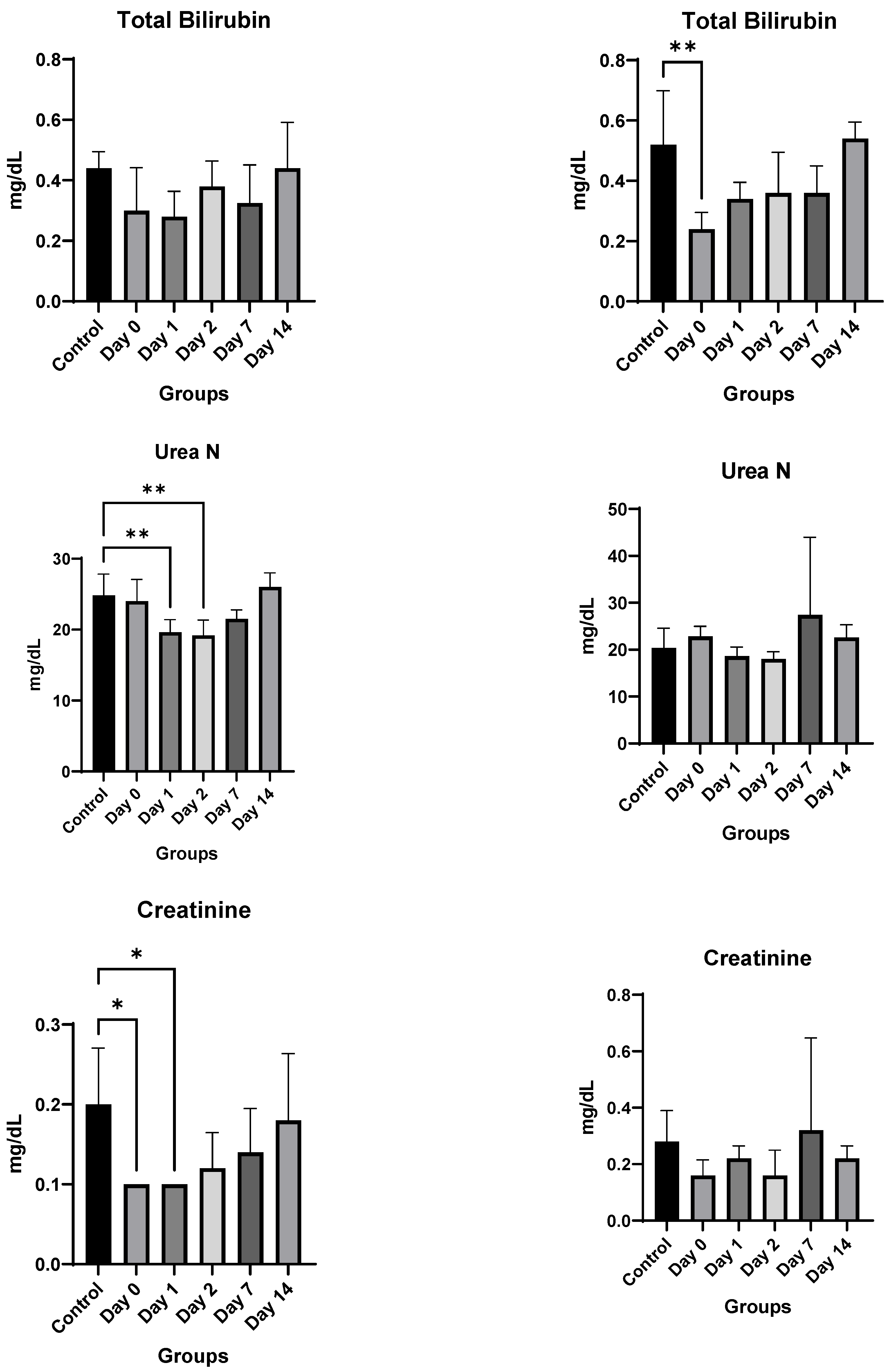
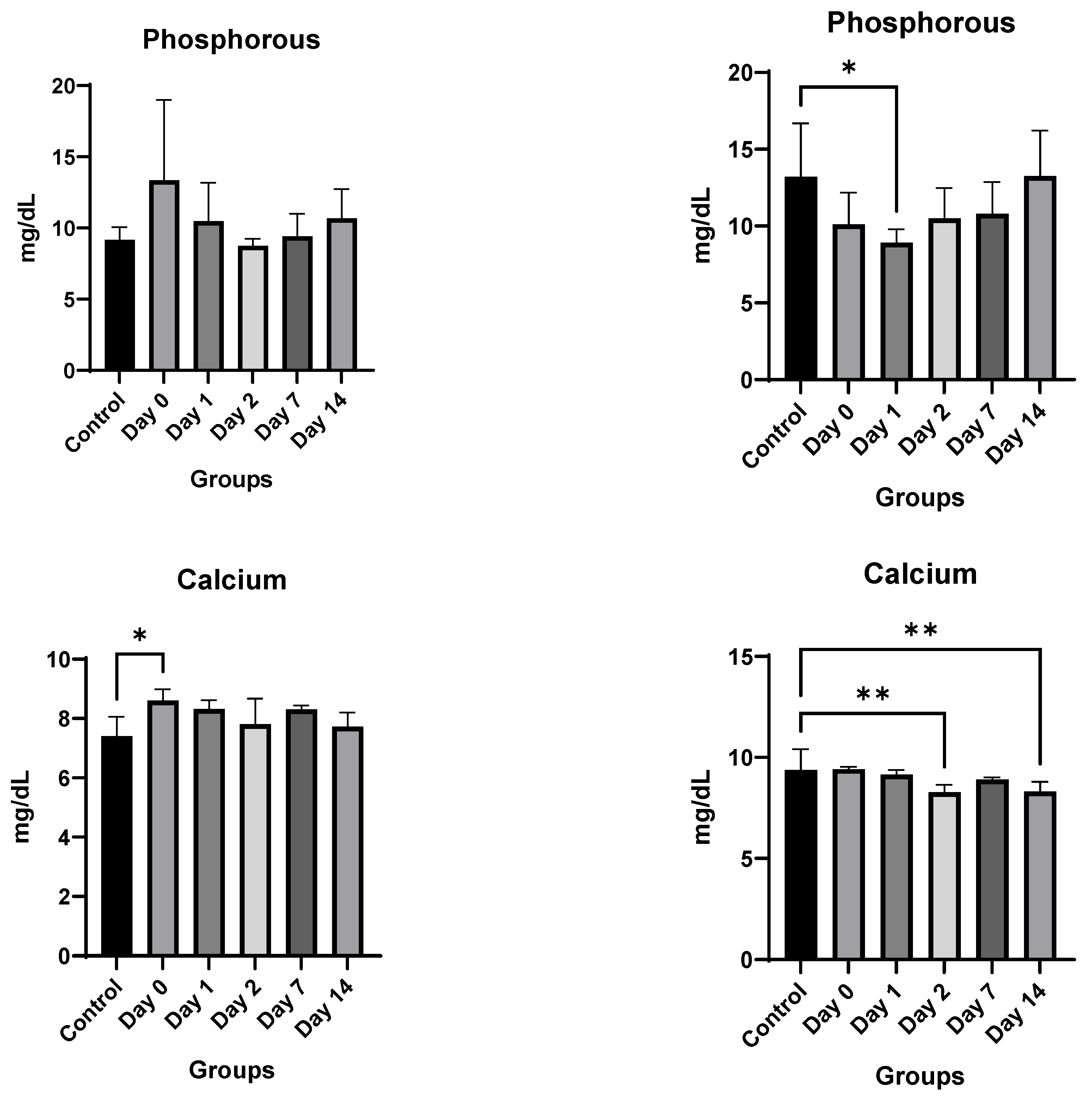
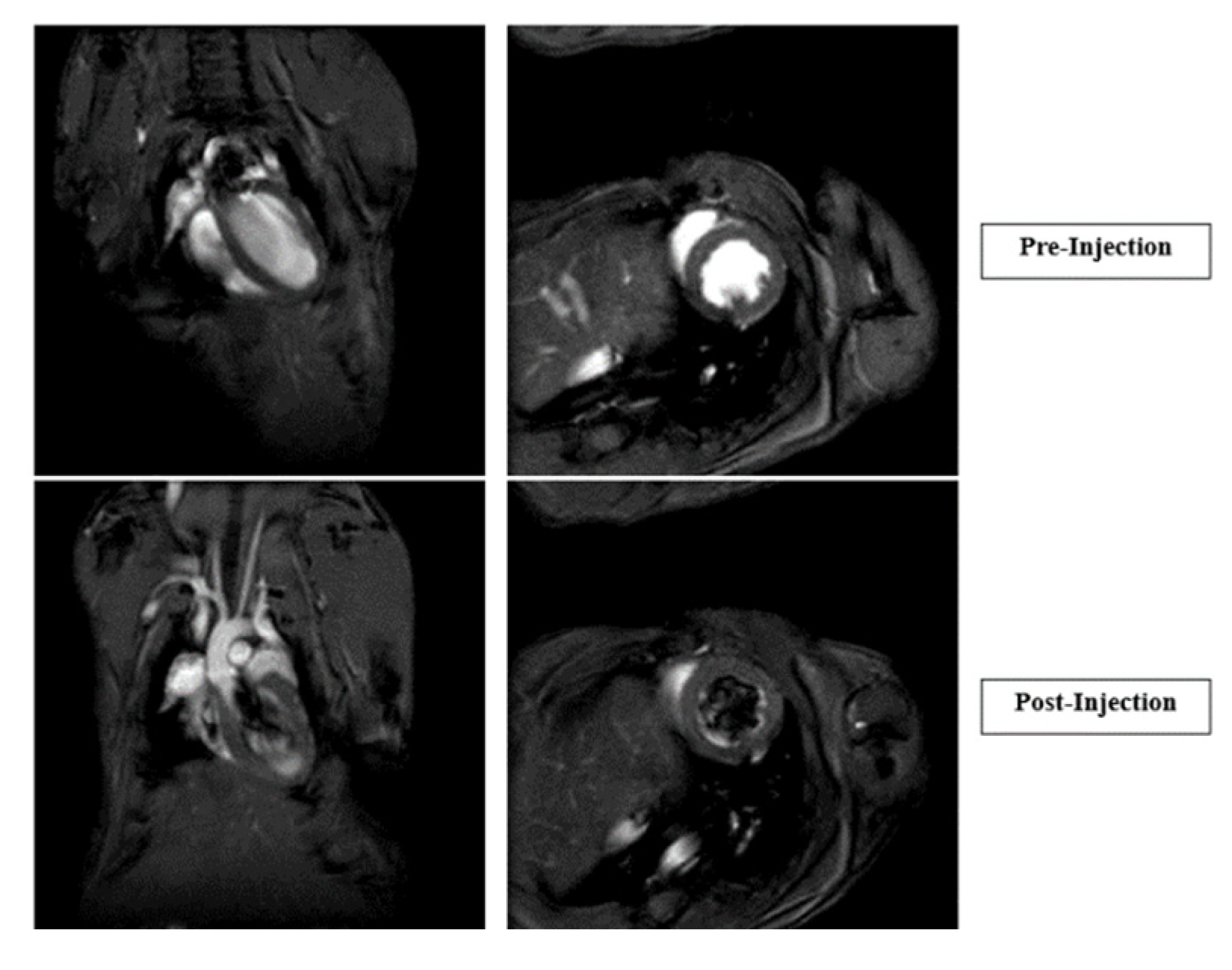
4. Results
4.1. In Vitro Studies
4.2. Eurofins Toxicology Study
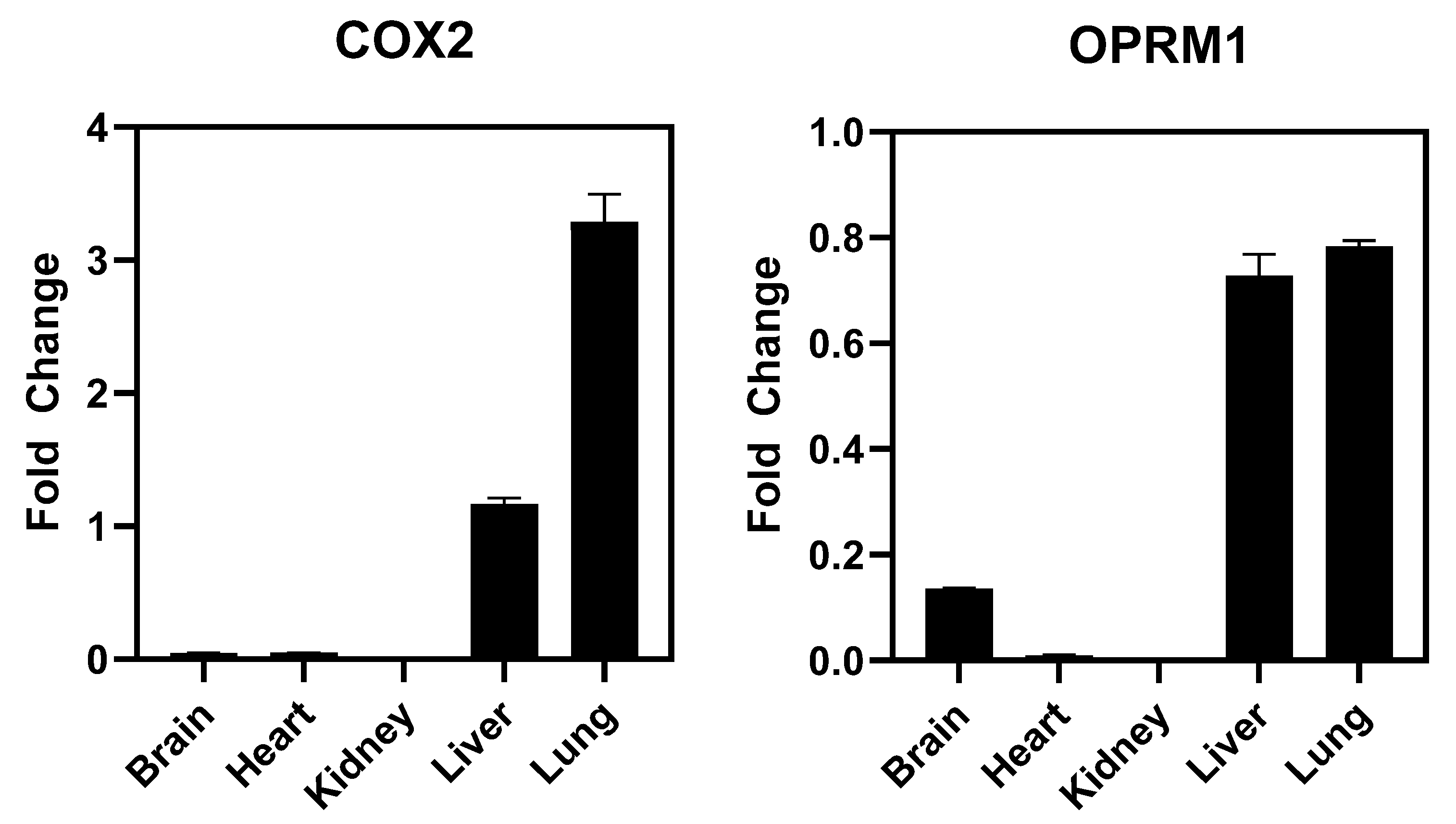
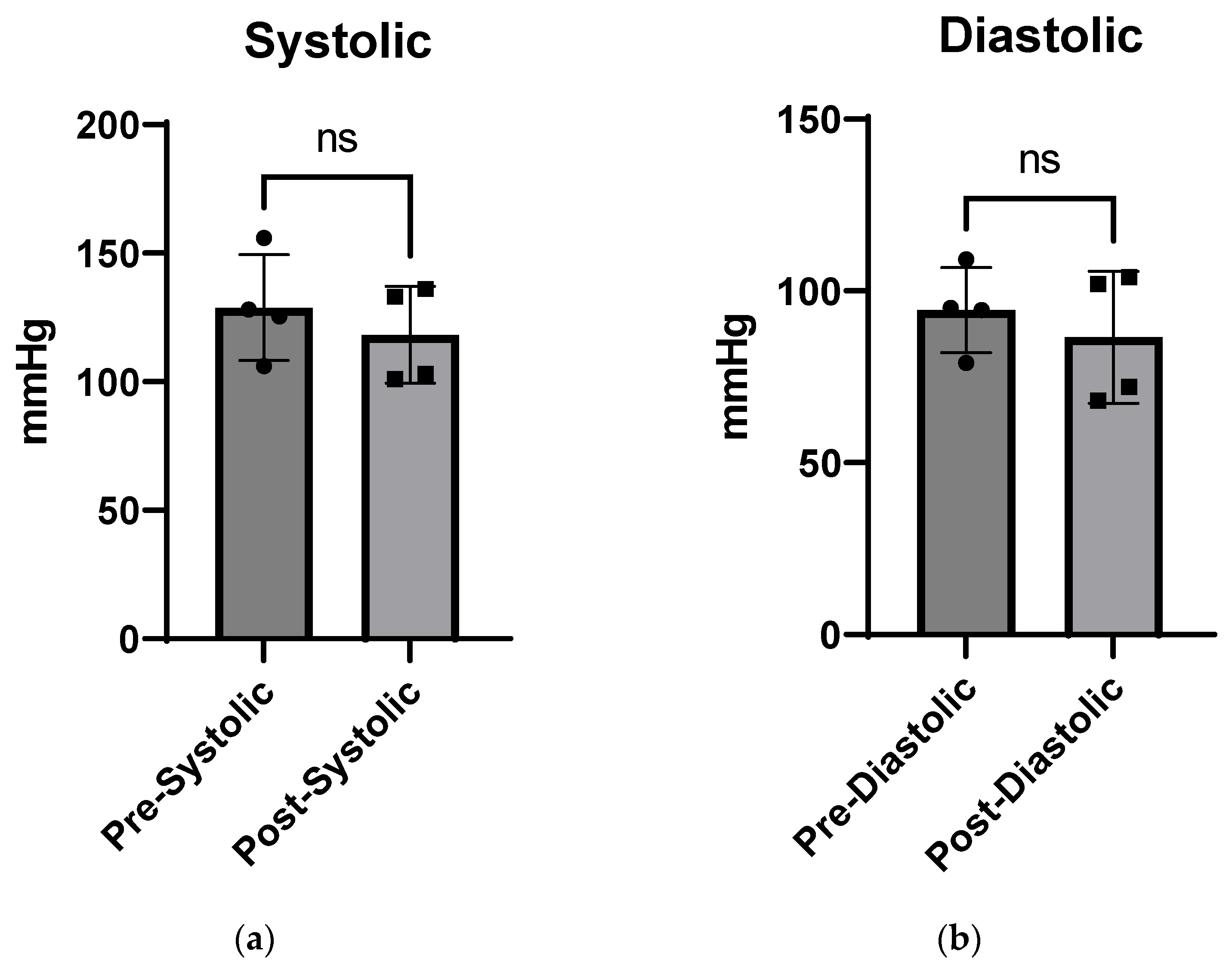
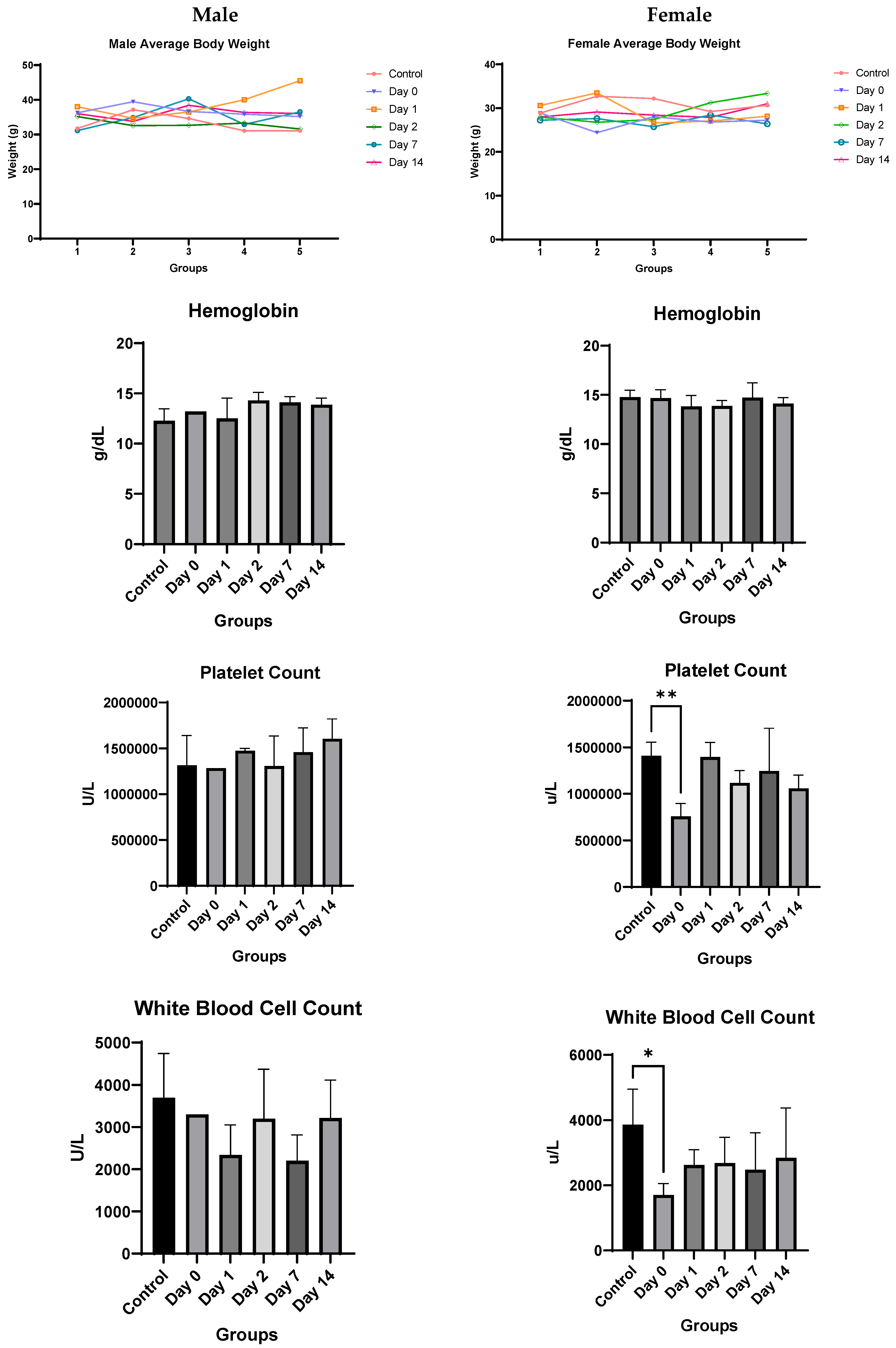
4.3. In Vivo Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| Alk. Ptase | Alkaline Phosphatase |
| BP | Blood Pressure |
| CPP | Cell-Penetrating Peptide |
| CTP | Cardiac-Targeting Peptide |
| FACS | Fluorescence-Activated Cell Sorting |
| GLP | Good Laboratory Practices |
| GPCR | G-Protein Coupled Receptor |
| hCMC | Human Cardiomyocyte Cells |
| HR | Heart Rate |
| PBS | Phosphate Buffered Saline |
| MRI | Magnetic Resonance Imaging |
| RT-qPCR | Real-Time Quantitative Polymerase Chain Reaction |
| TAT | Trans-Activator of Transcription |
| RAN | Random Peptide |
References
- Derakhshankhah, H.; Jafari, S. Cell penetrating peptides: A concise review with emphasis on biomedical applications. Biomed. Pharmacother. 2018, 108, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Zahid, M.; Phillips, B.E.; Albers, S.M.; Giannoukakis, N.; Watkins, S.C.; Robbins, P.D. Identification of a cardiac specific protein transduction domain by in vivo biopanning using a M13 phage peptide display library in mice. PLoS ONE 2010, 5, e12252. [Google Scholar] [CrossRef]
- Pasqualini, R.; Koivunen, E.; Ruoslahti, E. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J. Cell Biol. 1995, 130, 1189–1196. [Google Scholar] [CrossRef]
- Rajotte, D.; Arap, W.; Hagedorn, M.; Koivunen, E.; Pasqualini, R.; Ruoslahti, E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Investig. 1998, 102, 430–437. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Mi, Z.; Lu, X.; Mai, J.C.; Ng, B.G.; Wang, G.; Lechman, E.R.; Watkins, S.C.; Rabinowich, H.; Robbins, P.D. Identification of a synovial fibroblast-specific protein transduction domain for delivery of apoptotic agents to hyperplastic synovium. Mol. Ther. 2003, 8, 295–305. [Google Scholar] [CrossRef]
- Mai, J.C.; Mi, Z.; Kim, S.H.; Ng, B.; Robbins, P.D. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar] [PubMed]
- Zahid, M.; Robbins, P.D. Protein transduction domains: Applications for molecular medicine. Curr. Gene Ther. 2012, 12, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Sahagun, D.; Zahid, M. Cardiac-Targeting Peptide: From Discovery to Applications. Biomolecules 2023, 13, 1690. [Google Scholar] [CrossRef] [PubMed]
- Parn, K.; Eriste, E.; Langel, U. The Antimicrobial and Antiviral Applications of Cell-Penetrating Peptides. Methods Mol. Biol. 2015, 1324, 223–245. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Feldman, K.S.; Garcia-Borrero, G.; Feinstein, T.N.; Pogodzinski, N.; Xu, X.; Yurko, R.; Czachowski, M.; Wu, Y.L.; Mason, N.S.; et al. Cardiac Targeting Peptide, a Novel Cardiac Vector: Studies in Bio-Distribution, Imaging Application, and Mechanism of Transduction. Biomolecules 2018, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Anderson, R.N. The Leading Causes of Death in the US for 2020. J. Am. Med. Assoc. 2021, 325, 1829–1830. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Gundam, S.R.; Bansal, A.; Kethamreddy, M.; Lowe, V.; Zahid, M. Synthesis and evaluation of novel [68Ga]Ga-NOTA-CTP in normal CD-1 mice as a myocardial perfusion imaging PET probe. J. Nucl. Med. 2023, 64, P1066. [Google Scholar]
- Kim, H.; Mun, D.; Kang, J.Y.; Lee, S.H.; Yun, N.; Joung, B. Improved cardiac-specific delivery of RAGE siRNA within small extracellular vesicles engineered to express intense cardiac targeting peptide attenuates myocarditis. Mol. Ther. Nucleic Acids 2021, 24, 1024–1032. [Google Scholar] [CrossRef]
- Avula, U.M.; Yoon, H.K.; Lee, C.H.; Kaur, K.; Ramirez, R.J.; Takemoto, Y.; Ennis, S.R.; Morady, F.; Herron, T.; Berenfeld, O.; et al. Cell-selective arrhythmia ablation for photomodulation of heart rhythm. Sci. Transl. Med. 2015, 7, 311ra172. [Google Scholar] [CrossRef]
- Gallicano, G.I.; Fu, J.; Mahapatra, S.; Sharma, M.V.R.; Dillon, C.; Deng, C.; Zahid, M. Reversing Cardiac Hypertrophy at the Source Using a Cardiac Targeting Peptide Linked to miRNA106a: Targeting Genes That Cause Cardiac Hypertrophy. Pharmaceuticals 2022, 15, 871. [Google Scholar] [CrossRef]
- Yurko, R.; Islam, K.; Weber, B.; Salama, G.; Zahid, M. Conjugation of amiodarone to a novel cardiomyocyte cell penetrating peptide for potential targeted delivery to the heart. Front. Chem. 2023, 11, 1220573. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Weber, B.; Yurko, R.; Islam, K.; Agrawal, V.; Lopuszynski, J.; Yagi, H.; Salama, G. Cardiomyocyte-Targeting Peptide to Deliver Amiodarone. Pharmaceutics 2023, 15, 2107. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, S.R.; Ho, A.; Vocero-Akbani, A.; Dowdy, S.F. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science 1999, 285, 1569–1572. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Lohcharoenkal, W.; Gotz, F.; Werner, R.G.; Manosroi, W.; Manosroi, A. Transdermal absorption and stability enhancement of salmon calcitonin by Tat peptide. Drug Dev. Ind. Pharm. 2013, 39, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V.; Lee, K.H.; Huang, Y.; Shin, M.C.; Park, Y.S.; Kim, H.; Moon, C. Topical Delivery of Cell-Penetrating Peptide-Modified Human Growth Hormone for Enhanced Wound Healing. Pharmaceutics 2023, 16, 394. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Rao, P.; Bradshaw, J.; Weller, R. Topical application of superoxide dismutase mediated by HIV-TAT peptide attenuates UVB-induced damages in human skin. Eur. J. Pharm. Biopharm. 2016, 107, 286–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mailhiot, S.E.; Thompson, M.A.; Eguchi, A.E.; Dinkel, S.E.; Lotz, M.K.; Dowdy, S.F.; June, R.K. The TAT Protein Transduction Domain as an Intra-Articular Drug Delivery Technology. Cartilage 2021, 13, 1637S–1645S. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Chen, X.; Zheng, X.; Qu, N.; Zhang, B.; Xia, C. IL-1beta receptor antagonist (IL-1Ra) combined with autophagy inducer (TAT-Beclin1) is an effective alternative for attenuating extracellular matrix degradation in rat and human osteoarthritis chondrocytes. Arthritis Res. Ther. 2019, 21, 171. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Lin, T.; Yin, H.; Pan, Y.; Zhu, M.; Zhang, M. A cell-permeable peptide inhibitor of p55PIK signaling alleviates suture-induced corneal neovascularization and inflammation. Heliyon 2023, 9, e14869. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakazawa, M.; Yamashita, T.; Sorimachi, H.; Hata, S.; Tomita, H.; Isago, H.; Baba, A.; Ishiguro, S. Intravitreal injection or topical eye-drop application of a mu-calpain C2L domain peptide protects against photoreceptor cell death in Royal College of Surgeons’ rats, a model of retinitis pigmentosa. Biochim. Biophys. Acta 2012, 1822, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Gomara, M.J.; Haro, I. Atorvastatin-loaded peptide amphiphiles against corneal neovascularization. Nanomedicine 2023, 18, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.S.; Aguilera, T.A.; Jiang, T.; Ellies, L.G.; Nguyen, Q.T.; Wong, E.H.; Gross, L.A.; Tsien, R.Y. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol. 2009, 1, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lin, X.; Feng, Q.; Pan, X.; Song, S.; Yang, J. Inhibition of human lung cancer cells by anti-p21Ras scFv mediated by the activatable cell-penetrating peptide. Anticancer Drugs 2022, 33, e562–e572. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.H.; Jang, J.; Cheon, D.H.; Chong, S.E.; Ahn, J.H.; Hyun, S.; Yu, J.; Lee, Y. pH-Activatable cell penetrating peptide dimers for potent delivery of anticancer drug to triple-negative breast cancer. J. Control Release 2021, 330, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, D.V.; Camargo, M.F.; Quraishi, M.A.; Adams, S.R.; Advani, S.J. Tumor Activated Cell Penetrating Peptides to Selectively Deliver Immune Modulatory Drugs. Pharmaceutics 2021, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zu, C.; He, D.; Li, Y.; Chen, Q.; Chen, Q.; Wang, H.; Wang, R.; Chaurasiya, B.; Zaro, J.L.; et al. pH-dependent reversibly activatable cell-penetrating peptides improve the antitumor effect of artemisinin-loaded liposomes. J. Colloid Interface Sci. 2021, 586, 391–403. [Google Scholar] [CrossRef]
- Lee, J.; Oh, E.T.; Lee, H.J.; Lee, E.; Kim, H.G.; Park, H.J.; Kim, C. Tuning of Peptide Cytotoxicity with Cell Penetrating Motif Activatable by Matrix Metalloproteinase-2. ACS Omega 2022, 7, 29684–29691. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Pei, X.; Cao, W.; Ye, J.; Wang, J.; Sun, L.; Yu, F.; Wang, J.; Li, N.; et al. Antibody-siRNA conjugates (ARCs) using multifunctional peptide as a tumor enzyme cleavable linker mediated effective intracellular delivery of siRNA. Int. J. Pharm. 2021, 606, 120940. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Q.; Chen, X.; Wang, Q.; Yan, C.; Zhao, X.; Zhao, W.; Zhu, W.H. An Enzyme-Activatable Aggregation-Induced-Emission Probe: Intraoperative Pathological Fluorescent Diagnosis of Pancreatic Cancer via Specific Cathepsin E. Adv. Mater. 2022, 34, e2107444. [Google Scholar] [CrossRef]
- Whitney, M.; Crisp, J.L.; Olson, E.S.; Aguilera, T.A.; Gross, L.A.; Ellies, L.G.; Tsien, R.Y. Parallel in vivo and in vitro selection using phage display identifies protease-dependent tumor-targeting peptides. J. Biol. Chem. 2010, 285, 22532–22541. [Google Scholar] [CrossRef]
- Koivunen, E.; Arap, W.; Valtanen, H.; Rainisalo, A.; Medina, O.P.; Heikkila, P.; Kantor, C.; Gahmberg, C.G.; Salo, T.; Konttinen, Y.T.; et al. Tumor targeting with a selective gelatinase inhibitor. Nat. Biotechnol. 1999, 17, 768–774. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef]
- Sabouri-Rad, S.; Oskuee, R.K.; Mahmoodi, A.; Gholami, L.; Malaekeh-Nikouei, B. The effect of cell penetrating peptides on transfection activity and cytotoxicity of polyallylamine. Bioimpacts 2017, 7, 139–145. [Google Scholar] [CrossRef]
- Zakeri-Milani, P.; Najafi-Hajivar, S.; Sarfraz, M.; Nokhodchi, A.; Mohammadi, H.; Montazersaheb, S.; Niazi, M.; Hemmatzadeh, M.; Soleymani-Goloujeh, M.; Baradaran, B.; et al. Cytotoxicity and Immunogenicity Evaluation of Synthetic Cell-penetrating Peptides for Methotrexate Delivery. Iran J. Pharm. Res. 2021, 20, 506–515. [Google Scholar] [CrossRef]
- Reveret, L.; Leclerc, M.; Morin, F.; Emond, V.; Calon, F. Pharmacokinetics, biodistribution and toxicology of novel cell-penetrating peptides. Sci. Rep. 2023, 13, 11081. [Google Scholar] [CrossRef]
- Jones, S.W.; Christison, R.; Bundell, K.; Voyce, C.J.; Brockbank, S.M.; Newham, P.; Lindsay, M.A. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 2005, 145, 1093–1102. [Google Scholar] [CrossRef]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019, 9, 6298. [Google Scholar] [CrossRef]
- Hoffmann, K.; Milech, N.; Juraja, S.M.; Cunningham, P.T.; Stone, S.R.; Francis, R.W.; Anastasas, M.; Hall, C.M.; Heinrich, T.; Bogdawa, H.M.; et al. A platform for discovery of functional cell-penetrating peptides for efficient multi-cargo intracellular delivery. Sci. Rep. 2018, 8, 12538. [Google Scholar] [CrossRef]
- Saar, K.; Lindgren, M.; Hansen, M.; Eiriksdottir, E.; Jiang, Y.; Rosenthal-Aizman, K.; Sassian, M.; Langel, U. Cell-penetrating peptides: A comparative membrane toxicity study. Anal. Biochem. 2005, 345, 55–65. [Google Scholar] [CrossRef]
- Suhorutsenko, J.; Oskolkov, N.; Arukuusk, P.; Kurrikoff, K.; Eriste, E.; Copolovici, D.M.; Langel, U. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug. Chem. 2011, 22, 2255–2262. [Google Scholar] [CrossRef]
- Kilk, K.; Mahlapuu, R.; Soomets, U.; Langel, U. Analysis of in vitro toxicity of five cell-penetrating peptides by metabolic profiling. Toxicology 2009, 265, 87–95. [Google Scholar] [CrossRef]
- Janssen, B.J.; Smits, J.F. Autonomic control of blood pressure in mice: Basic physiology and effects of genetic modification. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1545–R1564. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahagun, D.A.; Lopuszynski, J.B.; Feldman, K.S.; Pogodzinski, N.; Zahid, M. Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile. Pharmaceutics 2024, 16, 73. https://doi.org/10.3390/pharmaceutics16010073
Sahagun DA, Lopuszynski JB, Feldman KS, Pogodzinski N, Zahid M. Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile. Pharmaceutics. 2024; 16(1):73. https://doi.org/10.3390/pharmaceutics16010073
Chicago/Turabian StyleSahagun, Daniella A., Jack B. Lopuszynski, Kyle S. Feldman, Nicholas Pogodzinski, and Maliha Zahid. 2024. "Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile" Pharmaceutics 16, no. 1: 73. https://doi.org/10.3390/pharmaceutics16010073
APA StyleSahagun, D. A., Lopuszynski, J. B., Feldman, K. S., Pogodzinski, N., & Zahid, M. (2024). Toxicity Studies of Cardiac-Targeting Peptide Reveal a Robust Safety Profile. Pharmaceutics, 16(1), 73. https://doi.org/10.3390/pharmaceutics16010073






