1. Introduction
Onychomycosis (ONC), the most common worldwide nail infection, is a fungal infection caused by both dermatophytes and non-dermatophytes [
1,
2]. Its incidence is rising due to the ageing population (ONC prevalence increases with age, affecting 20% to 50% of individuals over 60 years of age) [
1,
2,
3,
4,
5] and increasing numbers of diabetic patients, who are 2.5 times more likely to develop ONC [
2,
5,
6,
7]. The challenges found in the development of efficacious topical treatments for onychomycosis have stimulated research on new formulations and enhancement approaches that may lead to more efficient topical medicines [
8,
9,
10,
11]. ONC topical treatments are time-consuming, requiring daily or weekly applications for up to one year [
12], which leads to low compliance and treatment failure [
13,
14]. Ideally, new approaches should be developed that enable longer intervals between doses as to facilitate compliance.
Medicated lacquers including off-patent and more recent actives (ciclopirox, terbinafine, amorolfine, tavaborole, and efinaconazole) represent the topical medicines most sold globally for the treatment of ONC [
15]. Most of these products are prepared with a blend of organic solvents that evaporate or quickly permeate the nail plate, so an impermeable hydrophobic polymeric film is formed on the nail surface [
16]. Because this process is fast, whether drug delivery can take place from this film becomes crucial for a formulation to be efficient. Yet, it has been suggested that, as solvents disappear, they leave behind a residue of crystallized drug that is unable to partition into, or diffuse across, the nail plate [
17,
18]. In addition, because an antifungal must attain effective concentrations across the thickness (from 0.38 ± 0.05 mm to 0.63 ± 0.10 mm) of fingernails [
19] and toenails (0.72 ± 0.20 mm) [
20], nail poration [
21,
22] has been suggested as a tool with which to facilitate the penetration of drugs into the nail plate. However, the efficiency of this approach to deliver antifungals has been rarely explored [
21]. In addition, for poration drug delivery to be effective, the approach requires formulations that flow through the pores without drying quickly and that avoid drug crystallization. Because of their quick metamorphosis, it is anticipated that solvent-based lacquers will not be a suitable choice for this application.
Nail lacquers containing ciclopirox are widely manufactured and prescribed globally, yet, as with most topical products for ONC, their clinical efficacy based on the cure rate is far from optimal [
23,
24,
25]. The aim of this work was to develop aqueous-based, simple, and cost-effective vehicles, including permeation enhancers, that provide high solubility for CPO and are capable of delivering high fluxes of the drug, whether or not they are associated with microporation devices.
2. Materials and Methods
2.1. Chemicals
Ciclopirox olamine (CPO) batch 19D11-B023-0486 was purchased from Fagron (Rotterdam, The Netherlands). Micolamina® lacquer batch T266 (CPO 8%, isopropyl alcohol, ethyl acetate, polymeth-acryliccopolyethylacrylate and dimethylsulfoxide) was purchased from a local distributor in Recife, Brazil. Isopropyl myristate, polyethylene glycol 400, Tween® 20 (Polyoxy-ethylene sorbitan monolaurate), Span® 80 (Sorbitan monooleate), Tween® 80 (Polyox-yethylene sorbitan monooleate), ethyl alcohol, Kolliphor® EL (Macrogolglycerol ricinoleate), Poloxamer 407, propylene glycol, isopropyl alcohol, potassium hydroxide, urea, hydroxypropylmethylcellulose (HPMC), sodium chloride, potassium chloride, sodium phosphate, potassium phosphate, sodium azide, oleic acid, BRIJ®20 (Polyoxy-ethylene 20 oleyl ether), sodium lauryl sulfate (SLS), and HPLC solvents methanol and acetonitrile from JT Baker, Phillipsburg, NJ, USA, were purchased from Casa do laboratório, Pernambuco, Brazil. Sabouraud dextrose agar and mycosel agar (PL1340) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Plastlabor (Rio de Janeiro, Brazil), respectively.
2.2. Nail Clippings Collection and Preparation for Tests
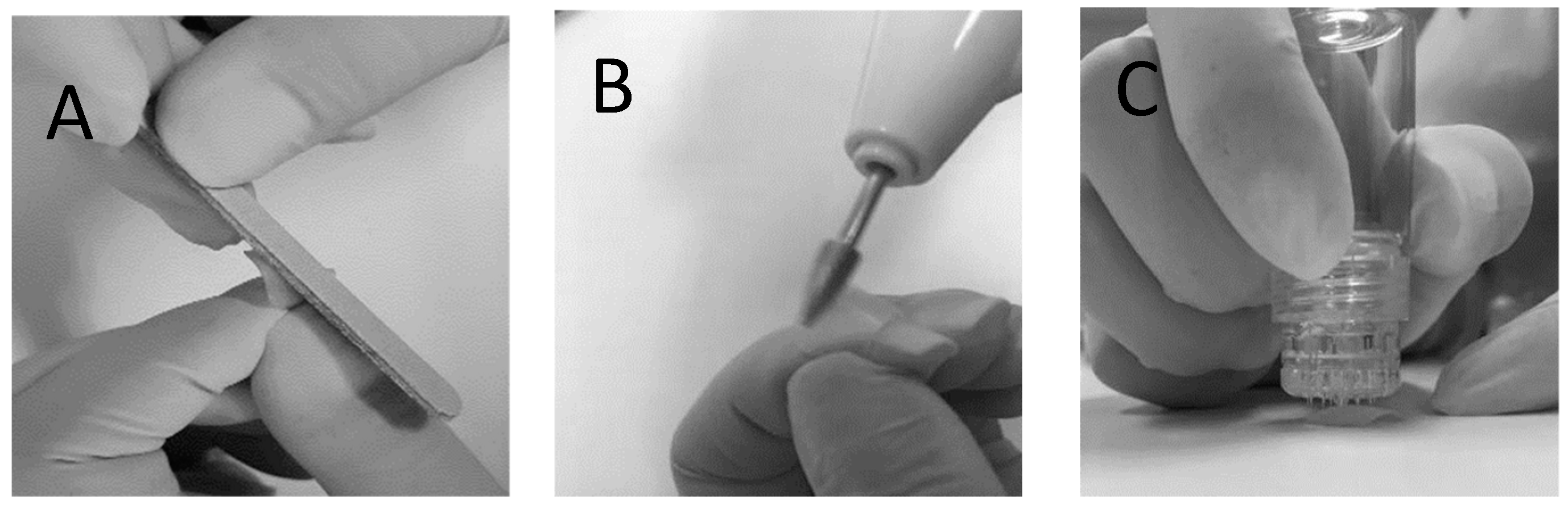
Human fingernail clippings at least 8 mm in length were donated by healthy volunteers after informed consent. Ethical approval was granted by the Human Ethics Committee of the Universidade Federal de Pernambuco (CAAE: 27554719.1.0005208). Following donation, the nail clippings were kept frozen at −20 °C until use. Before being used for in vitro permeation tests (IVPTs) and microbiological tests, the nail clippings were thawed and submitted to one of two physical treatments: The first nail treatment involved 2 h (pure water) hydration followed by sanding of the dorsal surface of the nails backwards and forwards 5 times using a nail file (
Figure 1A); these nails are referred to as the non-porated (NP) nails henceforth. The second nail treatment involved 2 h of hydration followed by sanding of the dorsal surface of the nails using an electric nail file (
Figure 1B), followed by microporating the nails 5 times using a commercially available device (Hydra.needle™, Guangzhou Ekai Electronic Technology Co., Ltd., Guangzhou, China) with titanium needles 0.60 mm in length (
Figure 1C). The process resulted in approximately 20 pores being present in the diffusion area used for IVPT and microbiological tests. These nails are referred to as the microporated (MP) nails henceforth.
To assess the depth of the pores formed using the Hydra.needle™, three porated nails were cut in half and placed on a cryostat holder with the cut surface placed upwards. Blocking was performed in gel (Tissue-Tek
® O.C.T.™ Compound by Sakura) after which the samples were frozen and cut in a cryostat (Leica CM1860UV, Leica Mycrosystems, Wetzlar, Germany) at −27 °C. Five nail serial cross sections were obtained from each nail with a thickness of 7 µm and mounted on microscopy slides. During these sequential nail sections, one nail section was collected and placed on microscopy slides, whereas the next two were discarded. This cycle was repeated 5 times until 5 samples from each nail were collected for imaging. On the same day, the samples were observed under a fluorescence microscope (Leica DMI4000 B, Germany) using a 20x objective. The nails were photomicrographed and the thickness of the nails and the depth of the pores, when found, were measured. Data were evaluated using ImageJ software (National Institutes of Health, version 1.41 NIH).
Figure 2 shows representative transversal cuts of a non-porated nail, following hydration and sanding only, and of a microporated nail following hydration, filing, and microporation. When pores were found, their depth was always less than the total thickness of the plate. For example, in the specimens shown in
Figure 2, the total nail thickness was 259.7 µm, and the pore depth was 143.1 µm, suggesting that the microporation procedure did not create channels throughout the whole plate.
2.3. High Performance Liquid Chromatography (HPLC)
Samples were analyzed for CPO using an HPLC Nexera X2, Shimadzu
® (Kyoto, Japan), equipped with a diode array detector (DAD). The mobile phase combined acetonitrile (Pump A) and 1 mM EDTA aqueous solution containing 20 mM of phosphoric acid (Pump B) used in gradient mode. The cycle started with 10% of the organic phase (Pump A) and 90% of the aqueous phase (Pump B) as the initial condition, which was kept for 2.5 min, followed by 6.5 min comprising 60% Pump B and 40% Pump A, and by 1 min during which the column was rebalanced to the initial condition. A Gemini-NX C18 chromatographic column (150 × 4.6 mm 5 µm) was used, the flow rate was 1 mL/min, and the injection volume was 40 µL. CPO was detected at 303 nm. The total analysis time was 10 min, and the retention time for CPO was 6.2 min. The data were obtained using the LC Solution
® software version 1.25. The CLAE-DAD assay for CPO was partially validated following the guidelines of RDC No. 166/17 [
26] and RDC No. 27/2012 [
27] of the Brazilian National Health Surveillance Agency (ANVISA) for analytical and bioanalytical methods, respectively, with respect to the selectivity, linearity, accuracy, and precision of the method. The selectivity of the assay was verified using mobile phase, phosphate buffered saline (PBS) (pH = 7.4), PBS with sodium azide (30 mg/L), and human nail blank and nail extraction solution (70:30 ethanol/water), which was compared to a standard CPO solution (5 μg/mL) in the same solvent. Three samples of blank (untreated) nails from three different donors were cut into small pieces with scissors and placed in 1.5 mL microtubes containing 1 mL of ethanol/water (70:30), which was agitated using a shaking table (FinePCR
®, Gunpo-si, South Korea) for 7 days after which the extracting solutions were filtered (0.45 μm) and quantified by HPLC-DAD. The linearity of the method was verified at concentrations of 5, 10, 20, 30, 50, and 100 μg/mL of CPO. Accuracy was determined by the repeatability test, in the same run (intraday) and in different runs on two different days (interdays) run by different analysts. In both cases, triplicate samples of three different concentrations (low, medium, and high) were used.
2.4. Solubility Study
The solubility of CPO in different vehicles was determined by adding excess drug (600 mg) to 1 mL to pure solvents, as well as to binary and ternary mixtures (
Table 1) in a centrifuge microtube (Eppendorf
®, Hamburg, Germany). The dispersions were homogenized by stirring at a controlled temperature (32 ± 2 °C), in a water bath (Quimis
®, Diadema, Brazil) for a period of 72 h and then centrifuged (Eppendorf
®, Germany) at 20,000×
g rpm for 10 min. An aliquot was removed from the supernatant, filtered through a 0.45 µm PVDF membrane filter (Millex
®, Darmstadt, Germany), and diluted with mobile phase (if necessary), after which the concentration of CPO was determined using the HPLC-DAD method described above.
2.5. Formulations
Based on the results from the solubility test, a series of gel (GH) and thermogel (GP) formulations were prepared according to the compositions described in
Table 2. Based on the IVRT results obtained with these vehicles, some additional modifications on gel GH6 were tested (
Table 3). To prepare gel formulations with HPMC, the polymer was added to ultrapure water, and the mixture was vortexed (Phoenix
®, Garbsen, Germany) until the polymer was completely solubilized and a clear solution was formed. Subsequently, urea, potassium hydroxide, or SLS was added when relevant (
Table 2 and
Table 3). Isopropyl alcohol (IPA), propylene glycol (PG), or transcutol was added to the final total volume, and the drug was finally incorporated into the formulation using a magnetic stir bar. In the case of gels with Poloxamer
® (Darmstadt, Germany), the gelling agent was initially added to the water/isopropyl alcohol mixture. Subsequently, either PG or transcutol was added, and the preparation was vortexed and kept at a temperature of 22 °C for 24 h for complete solubilization of the polymer. Finally, the rest of the constituents and the drug were incorporated into the formulation using a magnetic stir bar. To assess the presence of crystals in the formulations chosen for the microbiological assay, 50 µL of the gels GH6 and GH6-GK were placed on glass slides at a temperature of 32 ± 1 °C and imaged in an optical microscope (Bioval, Curitiba, Brazil) after 10, 20, 30, 60, 90, 120, and 240 min (see representative images in the
Supplementary Material, Section S2).
2.6. In Vitro Release Tests (IVRTs)
CPO release from the gel and thermogel formulations in
Table 2 and
Table 3 and from the marketed Micolamina
® was studied using vertical diffusion cells (PermeGear Inc. Bethlehem, PA, USA, diffusion area = 0.65 cm
2). Donor formulations were 10 µL of 8% and 16% gel and thermogel formulations (corresponding to 1.23 mg/cm
2 and 2.46 mg/cm
2 of CPO respectively) and Micolamina
® (TheraSkin
® Farmacêutica, São Bernardo do Campo, Brazil). The receptor compartment was filled with 5 mL of phosphate buffered saline (PBS) (pH = 7.4). These series of experiments used three different membranes: a mixed cellulose esters membrane GSWP (0.22 µm pore size) from Merck Millipore Ltd. Millipore
® (Merck, Darmstadt, Germany), batch R5PA92282 (hydrophilic); a synthetic silicone elastomer membrane (Dow Corning
® 7-4107 (Corning, Corning, NY, USA)) (0.75 μm), and a hydrophobic PTFE membrane (0.45 µm pore size) from Filtrilo Ltd. (Colombo, Paraná, Brazil). The hydrophilic cellulose esters membranes were conditioned in phosphate buffered saline (PBS) for 12 h before use. The assembled system was maintained under magnetic stirring (300 rpm) (Fisatom 713d, Fisatom, São Paulo, Brazil) and kept at 32 ± 1 °C throughout the experiment. At 5, 10, and 20 min and at 0.5, 0.75, 1, 2, and 4 h, 0.5 mL were sampled from the receptor solution and replaced with fresh receptor medium. The samples were filtered (Millipore
® 0.45 µm) and analyzed by HPLC/DAD.
Comparisons among formulations were done by one-way ANOVA followed by Bonferroni’s multiple comparison tests. The level of statistical significance was established at p < 0.05.
2.7. In Vitro Permeation Tests (IVPTs)
IVPTs were performed using vertical diffusion cells with a nail adaptor (PermeGear Inc. Bethlehem, PA, USA, diffusion area = 0.196 cm2). The dorsal nail surface was placed facing the donor compartment, and the ventral surface was in contact with the receptor compartment, which was filled with 5 mL of PBS (pH = 7.4), ensuring sink conditions throughout the experiment.
The first type of IVPT experiment (MD-NP) involved multiple doses and filed/non-porated nails. In this case, 10 µL (equivalent to approximately 4.1 mg/cm2 and 8.2 mg/cm2 of CPO for formulations containing 8% and 16% of the active, respectively, were applied every 24 h. The surface of the nail was cleansed using two Johnson’s® dry cotton swabs (Nova Brunswick, NJ, USA) between applying each new dose.
The second type of IVPT experiment (SD-MP) involved a single dose and filed/micro-porated nails. In this case, 50 µL (equivalent to approximately 20.41 mg/cm2 and 40.82 mg/cm2 of CPO) of formulations containing 8% and 16% CPO, respectively, were applied once at the beginning of the experiment.
In both experiments, the donor was occluded with Parafilm® (Chicago, IL, USA); the cells were incubated at 32 ± 1 °C for 14 days, and the receptor was magnetically stirred (IKA®, Staufen, Germany). Subsequently, 0.5 mL of the receptor solution were sampled after 7 days (and replaced with fresh buffer) and at the end of the experiments (14 days). The samples were filtered (Millipore® 0.45 µm) and analyzed using the previously described (HPLC-DAD) method.
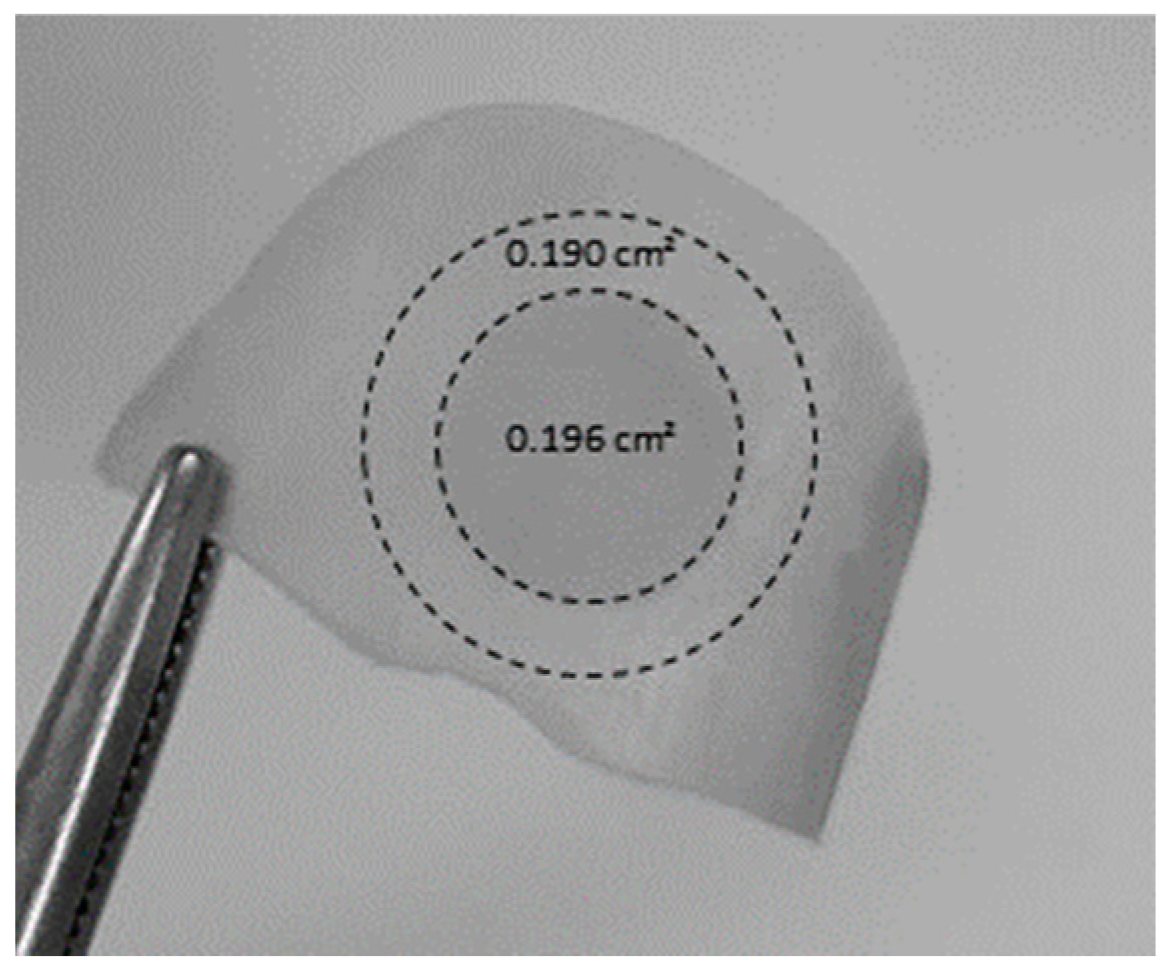
To evaluate the amount of drug present in the nail plate at the end of the experiments, the nail samples were removed from the nail adaptor and cleaned twice with isopropyl alcohol prep swabs (bio-Soma
®, São Paulo, Brazil). The central circular region corresponding to the diffusional area (
Figure 3) was cut out from the peripheral area and weighed, cut into small pieces, and placed in a centrifuge microtube (Eppendorf
®, Germany) with extraction solution (1 mL of 70% ethanol). The centrifuge microtube was then shaken for 7 days, after which the solutions were filtered and analyzed using HPLC-UV as described above. An attempt was made to evaluate lateral diffusion, i.e., outside the diffusional area. For this, a ring (outer radius–inner radius ~2 mm) of nail specimen immediately outside the diffusion area was cut (
Figure 3). The stability of CPO during the extraction procedure was previously established.
Comparisons among formulations were done by one-way and two-way ANOVA followed by Bonferroni’s multiple comparison tests and an unpaired Student t-test. The level of statistical significance was established at p < 0.05.
2.8. Microbiological Assay
The method described by Sleven et al. (2015) [
28] with minor modifications as shown in
Figure 4 was used. The bottom end of a 2 mL glass vial (Sigma-Aldrich
®, EUA) was cut so that the vial could be used as a “donor chamber” for the formulations tested (see below). All material used was autoclaved at 121 °C for 15 min before mounting the system. Porated and non-porated nails were cleaned with isopropyl alcohol prep swabs (bio-Soma
®, São Paulo, Brazil) and then sterilized for 30 min using an ultraviolet lamp (Veco, Campinas, Brazil). The following assembly procedure was carried out in a laminar flow hood: A nail clipping was mounted on the opening (5 mm diameter, 0.196 cm
2) of the polypropylene cap of the vial with the nail dorsal side upwards; a rubber ring was placed on the ventral surface of the nail to prevent leakage, and the screw-on cap of the glass vial was then attached (
Figure 4A). The ensemble was placed direct in contact with Sabouraud dextrose (SDA) agar, which simulated the nail bed (
Figure 4B) located at the bottom of a 50 mL glass beaker and the set covered with Parafilm
®.
For the preload step, a 50 μL single dose of the formulations was applied to the dorsal surface of the nails. After incubation for 14 days in an oven at 36.5 ± 1 °C, the vial–nail ensemble was transferred to a medium Sabouraud dextrose (SDA) agar contaminated with freshly inoculated
T. rubrum (clinical isolate from internal collection provided by Sylvio Campos Medical Mycology Laboratory of the Biosciences Center—CB/UFPE, Recife, Brazil) as shown in
Figure 4C and kept incubated in an oven at 36.5 ± 1 °C for another 7 days. Afterwards, whether an inhibition halo had been formed in the contaminated medium was verified (
Figure 4D). The percentage inhibition was calculated as the area of inhibition halo relative to the total area of potential growth (11.94 cm
2 in all cases). The area of the inhibition zone was estimated as that of a circle with the diameter measured with a digital caliper (0–150 mm). When an inhibition halo was formed, an additional step was carried to verify the watertightness of the setup. For this, the vial–nail ensemble was transferred at the end of the experiment to a new non-inoculated agar, and 400 μL of a 1% methylene blue aqueous solution were added to the donor chamber and observed for another 7 days [
28]. If no inhibition halo formed, a direct mycological examination was performed on the nails [
29,
30]. If main fungal structures were not found in the microscopic evaluation, the fragments samples of this nail were cultivated on mycosel agar (selective medium for dermatophyte fungi) to check for potential
T. rubrum growth.
Comparisons among formulations were done by two-way ANOVA followed by Bonferroni’s multiple comparison tests. The level of statistical significance was established at p < 0.05.
4. Discussion
This work aimed to provide further insight on the potential of a microporation–formulation combination approach as a tool to improve the efficiency of topical therapies for onychomycosis. A proof-of-concept for this approach was provided in 2015 by Chiu et al. [
22], who demonstrated the lateral diffusion of a marker across the nail plate from nanoparticle reservoirs immobilized in created pores; the nanoparticles were observed 70 µm deep into the nail. Further work combining microporation with tioconazole nanocapsules found that a single poration step enhanced delivery of the antifungal when combined with a single dose but not when followed by multiple doses of the nanocapsules [
21]. Lastly, some work [
37] testing dissolvable microneedle (circa 1000 µm depth × 500 µm middle dm × 700 µm external dm) array patches containing terbinafine and methylhydroxy-4-benzoate reported an extremely fast permeation across bovine hooves, comparable to that observed across a cellulosic filter membrane. However, the hooves had been soaked in 70% ethanol for 24 h prior to the studies, and the impact of this step on the poration effectiveness and permeation results is unclear. Prior work performed with human nail clippings [
21,
22] used a dermaroller (Infinite Beauty
®) with 250 µm titanium needles, and given this “roller” poration action, trench-like structures or elongated cracks were created on the nail structure in addition to pores. Thus, a device (
Figure 1) enabling a microporation action perpendicular with respect to the nail surface was used for this study, with the hope of creating channels that enter deeper into the nail structure. The Hydra.needle
TM device with 0.6 mm long titanium needles was operated manually, and an approximate number of pores (~20) was created in the IVPT diffusion area (0.196 cm
2), which corresponds to ~102 pores/cm
2. The surface area of fingernail and toenail plates ranges between ~1.5 and 5.3 cm
2 [
38,
39], so to recreate an equivalent pore density in practical applications, a nail poration device would need to create 153–540 pores across the whole nail plate, respectively. The microscopic images of porated nails (
Figure 2) suggest that the pores created were less than 150 µm deep, suggesting that less than half of the length of the titanium needles penetrated the nail plate. While poration was effective in this study, there is no information with which the results reported here can be compared. Overall, there is very little information regarding the optimum depth and density of nail poration required for the treatment of onychomycosis, so this is an area requiring further research. Importantly, an antifungal must reach effective concentrations across the whole nail plate as well as at the nail bed. This is in contrast with skin poration, which aims to bypass the stratum corneum barrier to deliver drugs to the live skin layers. A successful approach will need to consider pore density as well as how much a specific drug can diffuse laterally from the pores into the surrounding plate structure.
An important objective was to develop simple formulations with a high capacity to solubilize CPO, so the first stage of this project measured the solubility of CPO in a series of solvents and solvent mixtures. Based on this data (
Table 1), a series of mixtures based on water/IPA, transcutol, and PG were selected as bases for the gel and thermogel formulations (
Table 2 and
Table 3). Because of the high solubility of CPO in the solvent mixtures selected, the new formulations could incorporate 16% CPO, doubling the drug content in the marketed product. Other additional components were based on prior reports [
40] regarding their potential use in transungual delivery. The application sought in this work enabled flexibility in their formulation compared to traditional lacquers, which facilitated the attainment of this high solubility capacity. Organic solvents that evaporate fast have been preferred for the preparation of traditional medicated lacquers, so, once applied, they form rapidly dried films with a prolonged residence on the nail plate. However, as solvents evaporate, the drug may come out of the solution and crystallize, which stops further drug delivery. Indeed, when aqueous-based CPO lacquers have been prepared, their ability to maintain the drug in solution and to delivery it is highly increased compared to traditional organic lacquers, as recently demonstrated [
41,
42]. In the formulation–microporation approach, the new pores provide internal localization sites for the formulation; thus, adhesiveness properties of the formulation become secondary to their capacity to flow in and remain in the channels created. While the results attained in this work suggest that the formulations developed were able to enter the pores and to keep CPO solubilized for efficient delivery, further work is required to establish the formulation properties for optimum performance, among which viscosity, surface tension, and microfluidics seem the obvious starting point.
The next stage in this work explored the in vitro release of CPO across artificial membranes. IVRTs are an integral part of the quality testing of topical products, although there were no expectations that IVRT and IVPT performance will be correlated. However, IVPTs on nails are usually long (7–14 days), which significantly slows the screening of vehicles for further development. Thus, the availability of faster tests that identify best formulations for further characterization, even in the absence of quantitative correlations, would be advantageous. Previous work [
21] performed with silicone membranes suggested that formulations that can extend the in vitro release of the drug provide better nail delivery compared to those showing a burst release profile. For this reason, short 4 h IVRTs were performed with PTFE and silicone membranes (
Figure 5 and
Figure 6), which demonstrated the important role of membrane choice. With regard to the commercial Micolamina
®, very similar release profiles were obtained with both membranes, suggesting possibly that CPO release was primarily rate-limited by the lacquer in both experiments. In case of the gel formulations, CPO release seemed to be rate-controlled by the drug diffusion across the silicone membrane, as very little CPO reached the receptor in all cases. Similar behavior was observed for thermogel vehicles and silicone membranes. PTFE membranes enabled the differentiation of formulations, regarding both the total percentage released (10–91%) and the shape of the profile, with clear burst effects being observed for the GH2 and GH4 vehicles. Thus, it could be argued that CPO release across the PTFE membranes were primarily rate-limited by the drug release from the vehicles tested. Thermogel vehicles released little (1.6–5% in 4 h) CPO across the PTFE membranes, which, given the above discussion, could reflect an actual slower CPO release from the thermogel vehicles. These results underline how choosing an artificial membrane, the diffusion across which is not the rate-limiting process in the IVRT, is crucial for these tests to be meaningful.
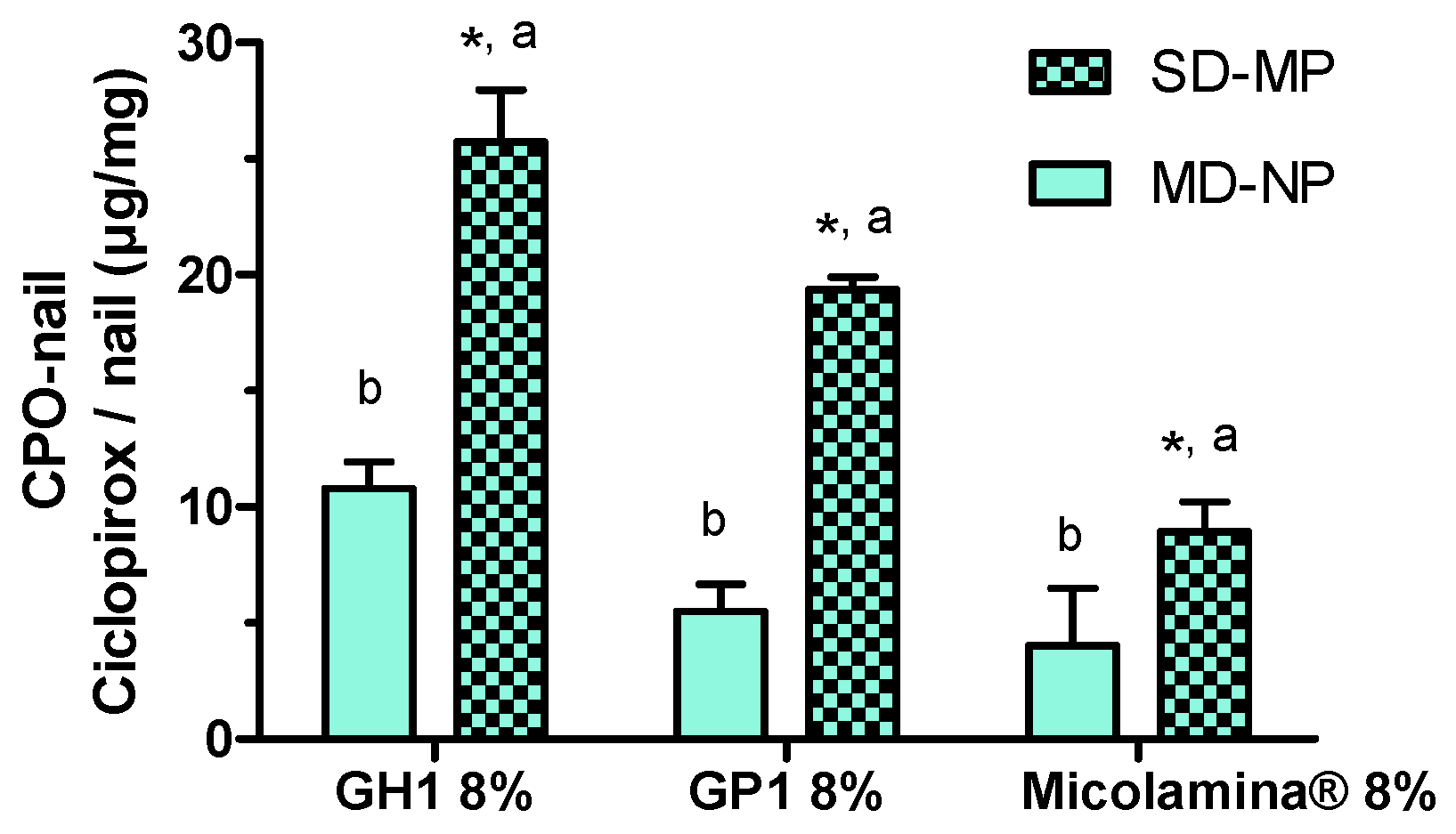
Next, a first series of IVPTs aimed to assess the effect of poration on the amount of CPO present in the nail after 14-day IVPTs. Importantly, only one dose of the formulations was applied to the porated nails (SD-MP), whereas a daily dose was applied to the non-porated nails. (MD-NP). Despite the smaller (~a third) dose applied, CPO-Nail was significantly enhanced by poration in all cases. This is a significant finding, suggesting that formulation–poration combination approaches could simultaneously reduce the dosing frequency, facilitating adherence, reduce drug wastage, and improve antifungal delivery. Finally, the new formulations GH1 and GP1 provided a higher CPO-Nail than Micolamina® in both the MD-NP and SD-MP conditions.
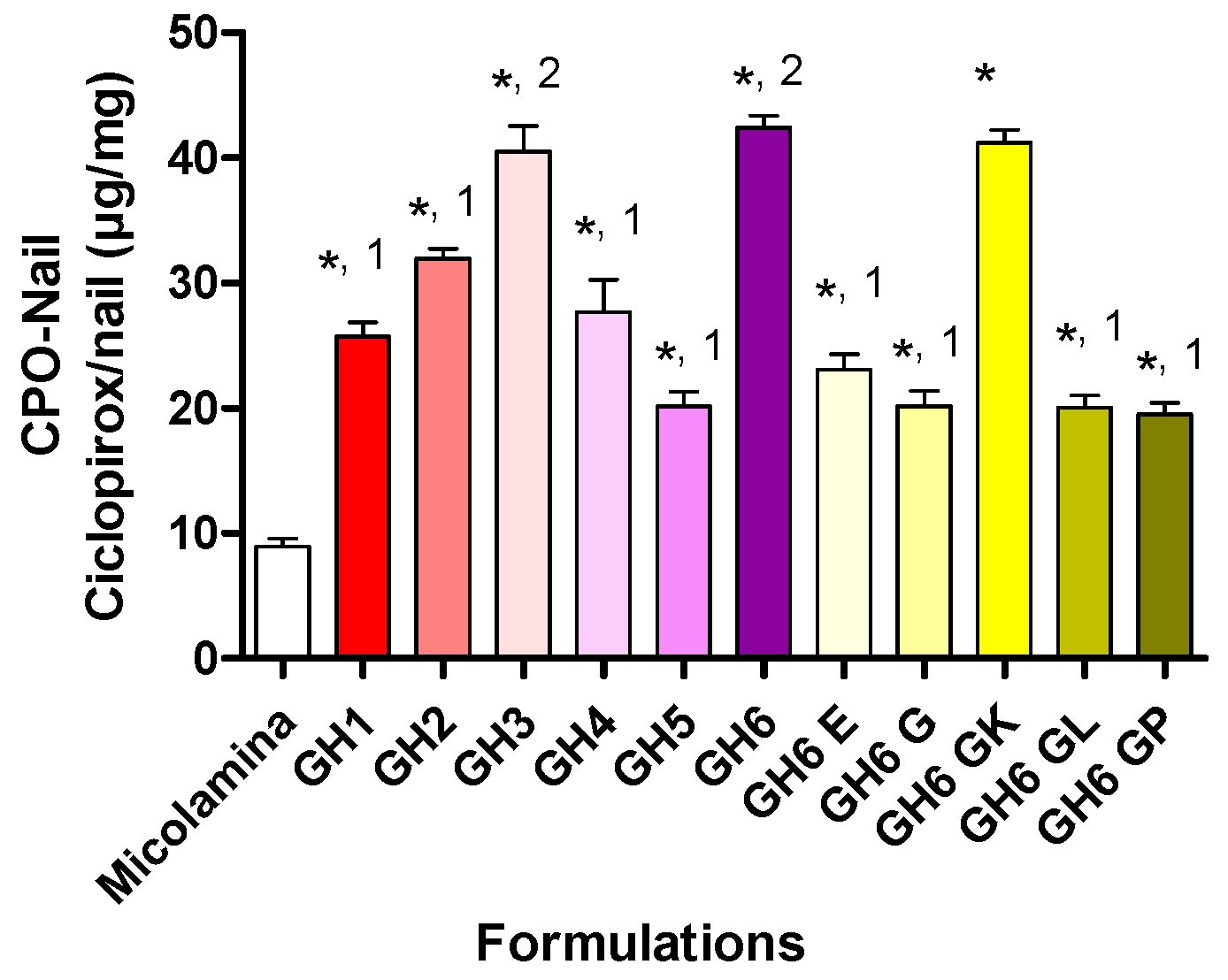
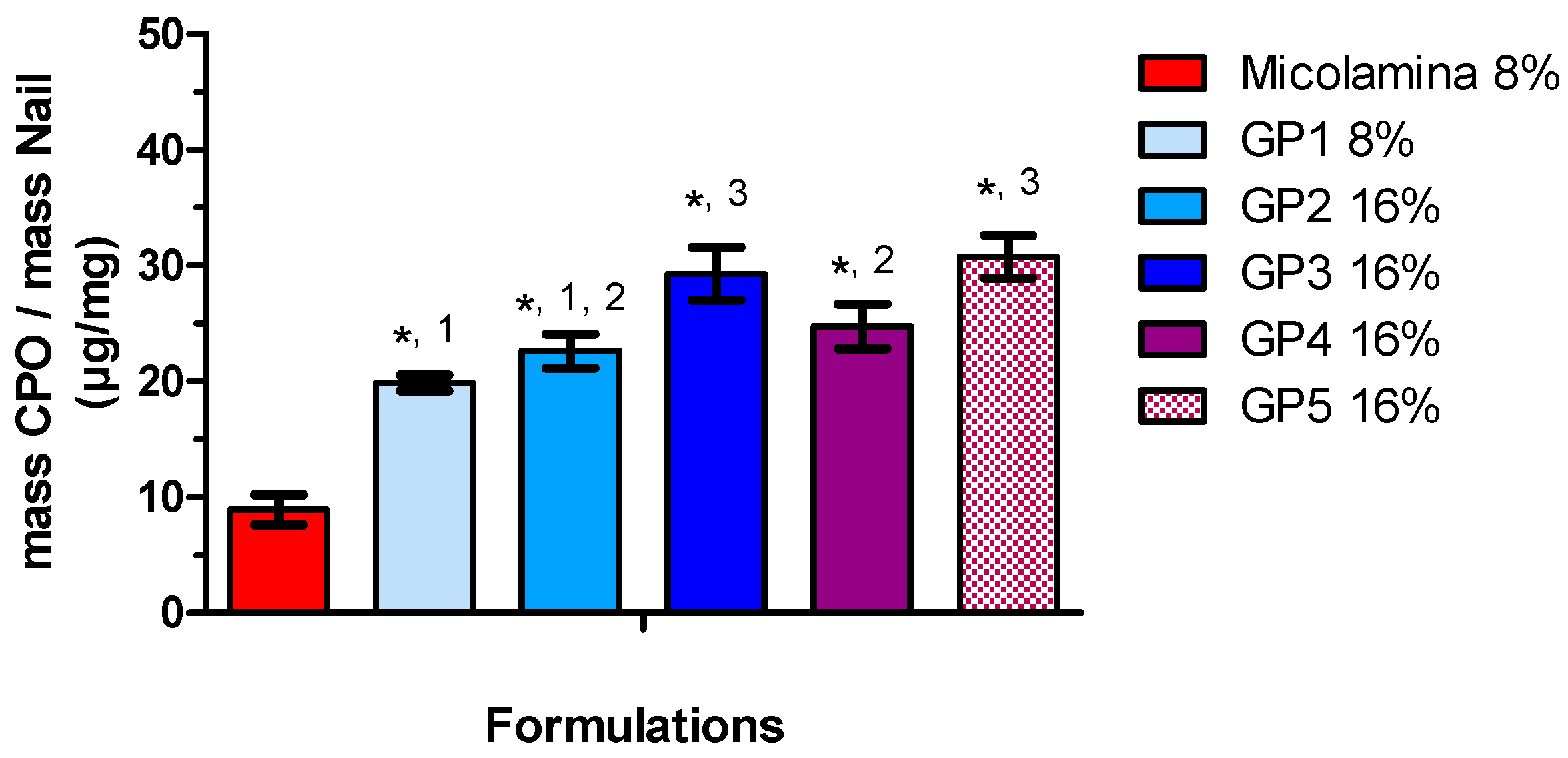
Following these positive results, all new vehicles were screened in SD-MP conditions using CPO-Nail as the metric for comparisons (
Figure 8 and
Figure 9). Overall, incorporating either transcutol or propylene glycol (GH3, GH6) to the water/IPA solvent system (GH2 resulted in better nail recovery, although this enhancing effect was not observed for the 8% CPO gels (GH1 and GH5). Doubling CPO content increased CPO-Nail proportionally for GH5 (20.1 ± 2.4 µg/mg) and GH6 (42.4 ± 2.0 µg/mg) but not for GH1 (25.7 ± 22.2 µg/mg) and GH2 (32.0 ± 1.5 µg/mg). All gel formulations, including those with 8% CPO, delivered more CPO to the nail than Micolamina
®, which is not surprising, as the latter is formulated as a lacquer rather than a vehicle with the capacity to flow into the created channels. Unfortunately, no clear correlations were found between the IVRT results and CPO-Nail. For example, among the 16% CPO vehicles, the gels providing a burst release, GH2 and GH4, provided a smaller CPO-Nail than GH3, GH6, and GH6-GK but a similar or higher one compared to GH6-E, GH6-G, GH6-GL, and GH6-GP. Thermogels also provided higher CPO-Nail than Micolamina
® (
Figure 9), and the highest nail recovery was observed also for vehicles (GP3 and GP5) containing transcutol and propylene glycol. Despite significant work on transungual drug delivery [
36], predicting the enhancement that will be observed for a specific formulation and active pharmaceutical ingredient is still challenging. In addition, it is unclear how drug delivery from a film placed on top of a non-porated nail can be extrapolated to delivery from the formulation localized in the pores created in this work.
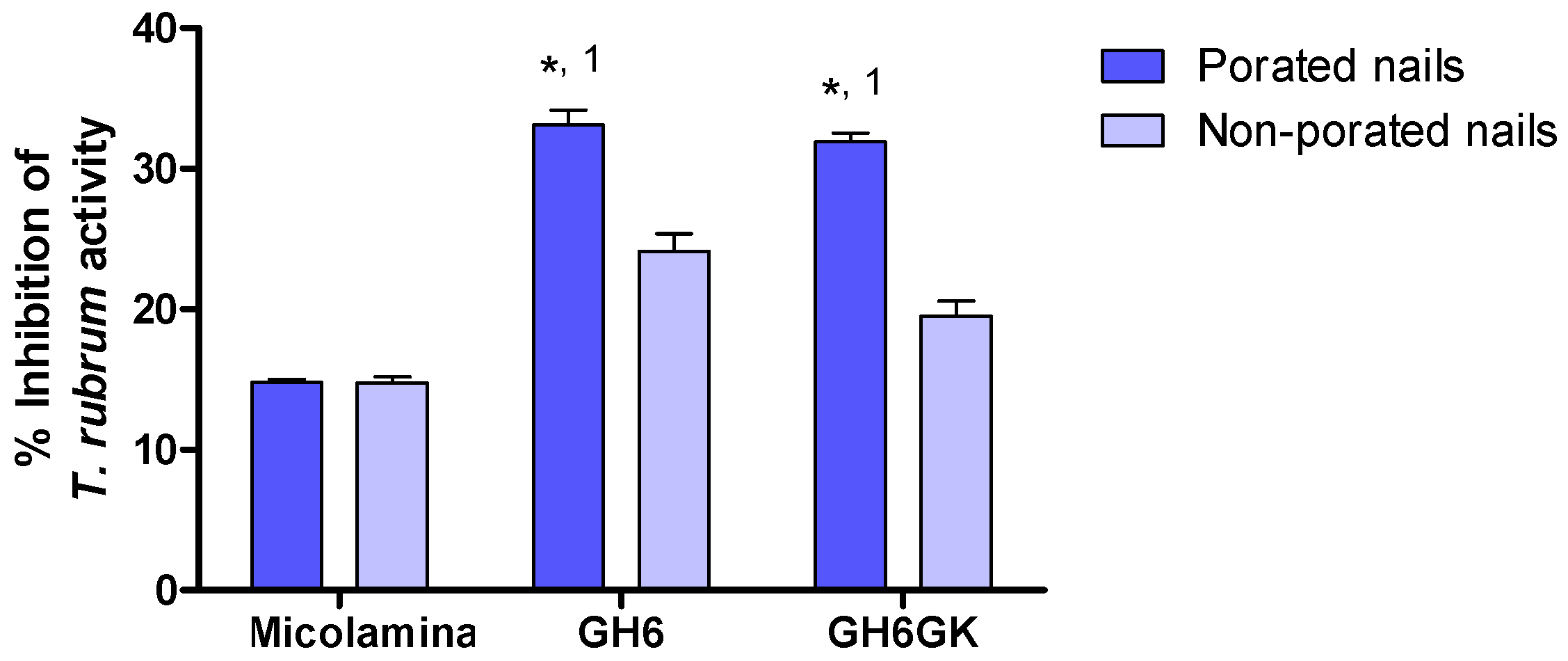
Because gel vehicles provided a larger CPO-Nail than the thermogel vehicles, they were selected for subsequent IVPTs and microbiological tests. CPO-Nail and CPO-Rec were significantly higher for GH6 and GH6-GK than for Micolamina
® (
Figure 10), with no differences being observed between the gels. Thus, in this work, the higher nail recovery observed for the gels was linked to a higher delivery to the receptor. This is important because, to treat onychomycosis effectively, a sufficient amount of the drug must be delivered across the nail plate (CPO-Nail) and must reach the nail bed (CPO-Rec). A limitation of the CPO-Nail metric is that it provides an average amount of drug across the nail, whereas information on the drug distribution across different layers of the plate would be more informative regarding therapy outcomes. Thus, to complete assessment of the new approach, a microbiological test based on a prior method [
28] was performed using porated and non-porated nails (
Table 4;
Figure 11). In this test, the 14-day pre-load stage provided time for the drug to be released and diffused across the nail, so inhibitory activity could be seen when the nail was placed on the
T. rubrum-contaminated medium, provided that sufficient delivery had taken place. These results further substantiate that the proposed formulation–microporation approach has the potential to improve the treatment of onychomycosis. The smaller but significant superiority observed for the gels with non-porated nails could be related to their capacity to keep the drug solubilized for a longer period of time or to their higher drug load. The larger superiority observed in the case of porated nails suggest that, in addition to these advantages, the gels were able to penetrate the channels and keep the drug solubilized to facilitate delivery from these sites. Poration did not improve the inhibitory effect of Micolamina
®, probably because the organic-base lacquer dries before significant penetration into the pores can occur. Generally speaking, there was a very clear correlation between the results of the IVPTs (
Figure 10) and the microbiological (
Figure 11) tests, whether CPO-Nail or CPO-Rec were considered.
The results here indicate that formulation–poration combination approaches have a clear potential to improve the treatment of nail fungal infections. However, significant questions remain to be solved regarding the design of a nail poration device that adapts to the nail plate geometry and variability in its thickness and surface, the length and number of microneedles required, the best properties for a formulation to enter the pores and provide extended release, and finally the impact of poration on diseased nails.