Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Collection of Saliva
2.3. Western Blot Analysis
2.4. Apoptosis Analysis by TUNEL Staining
2.5. Histopathological Examination
2.6. Immunohistochemistry
2.7. Measurement of DPP-4 and GLP-1
2.8. Measurement of Amylase Alpha 1
2.9. Measurement of AGEs
2.10. Statistical Analysis
3. Results
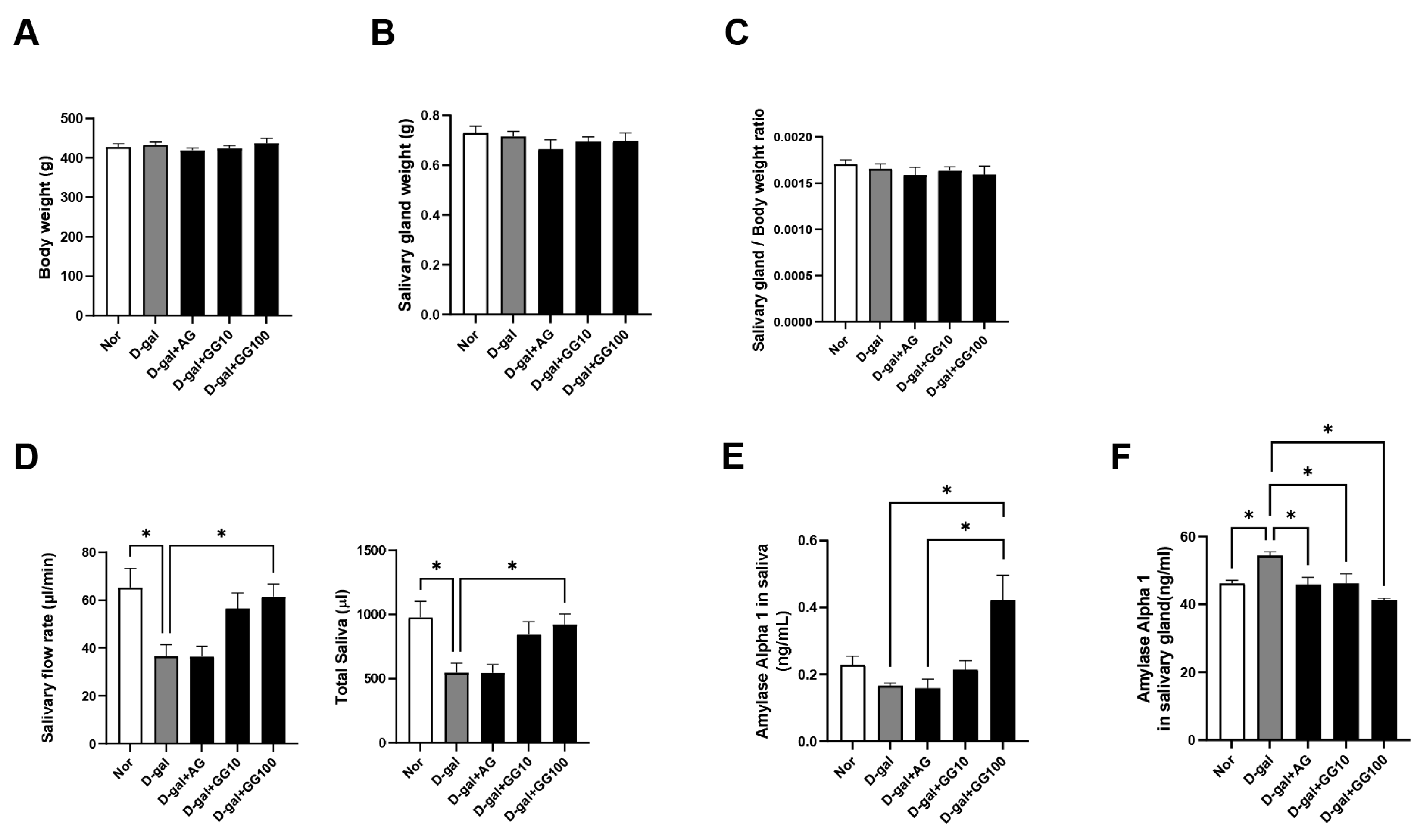
3.1. Gemigliptin Prevented D-Gal-Induced Salivary Gland Dysfunction and Pathological Changes
3.2. Effect of Gemigliptin on DPP-4 Activity and GLP-1 Levels in the Salivary Gland and Serum
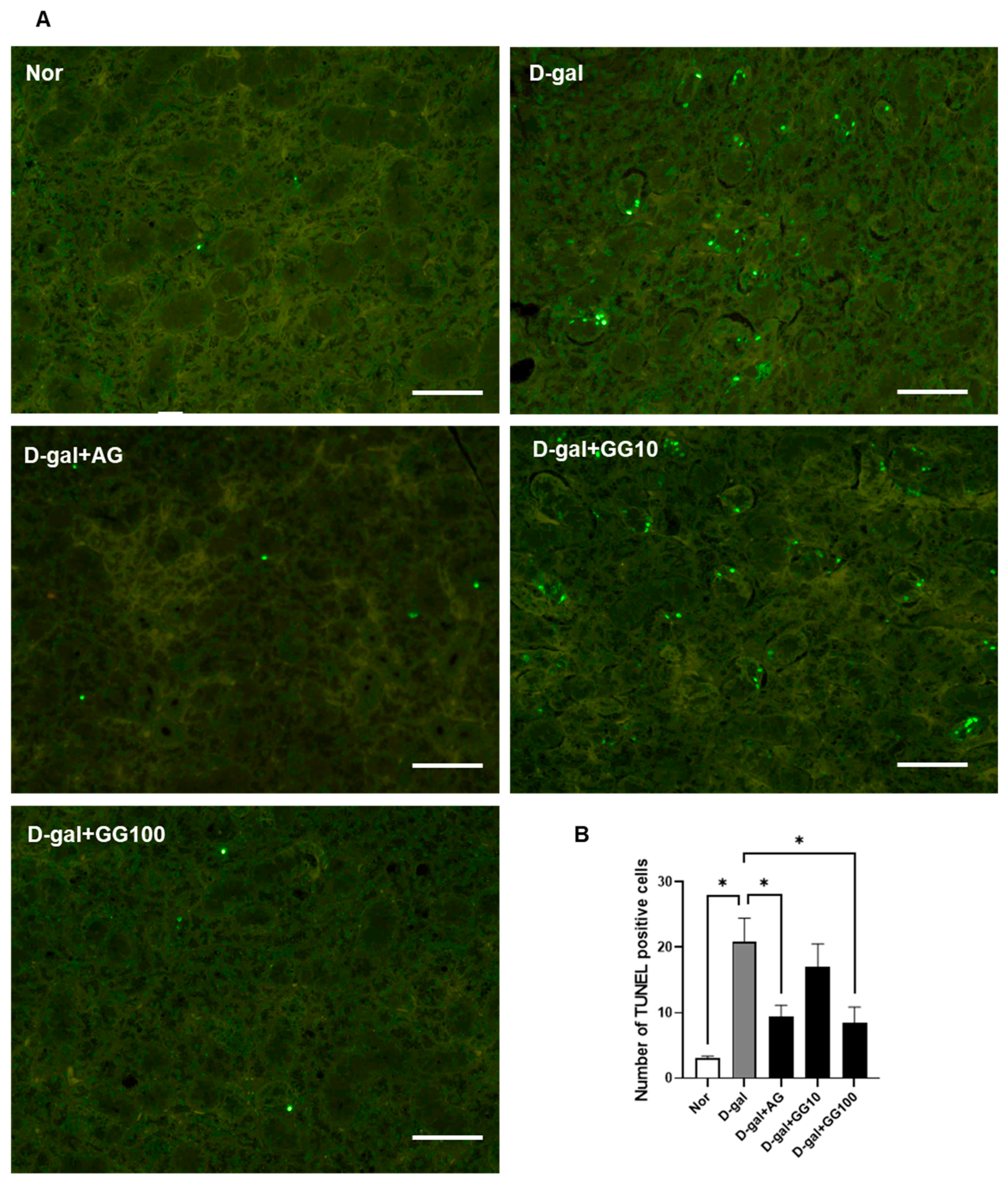
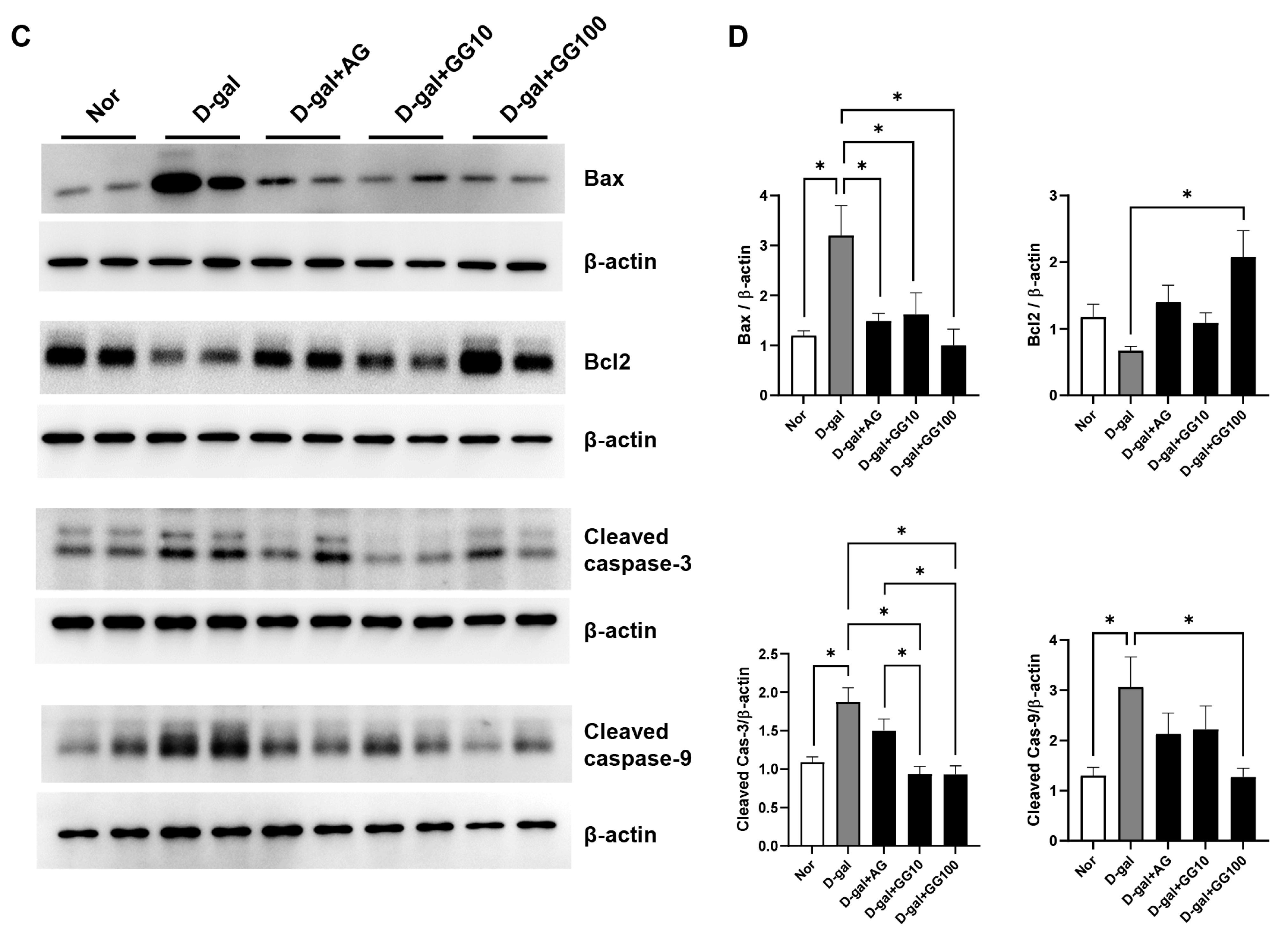
3.3. Gemigliptin Inhibits Apoptosis in the Salivary Glands of D-Gal-Injected Rats
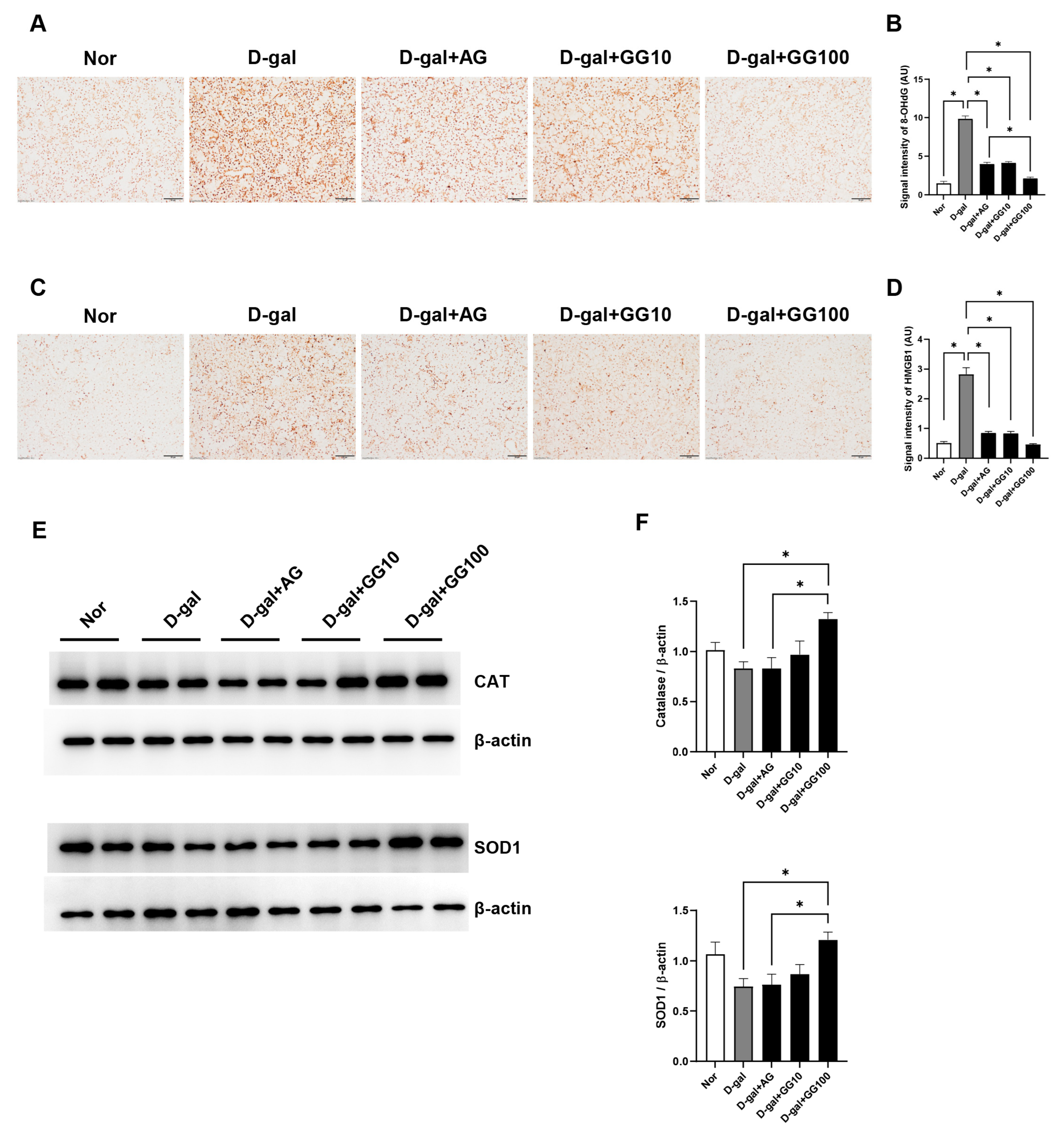
3.4. Gemigliptin Inhibits the Expression of Oxidative Stress Markers in the Salivary Glands of D-Gal-Injected Rats
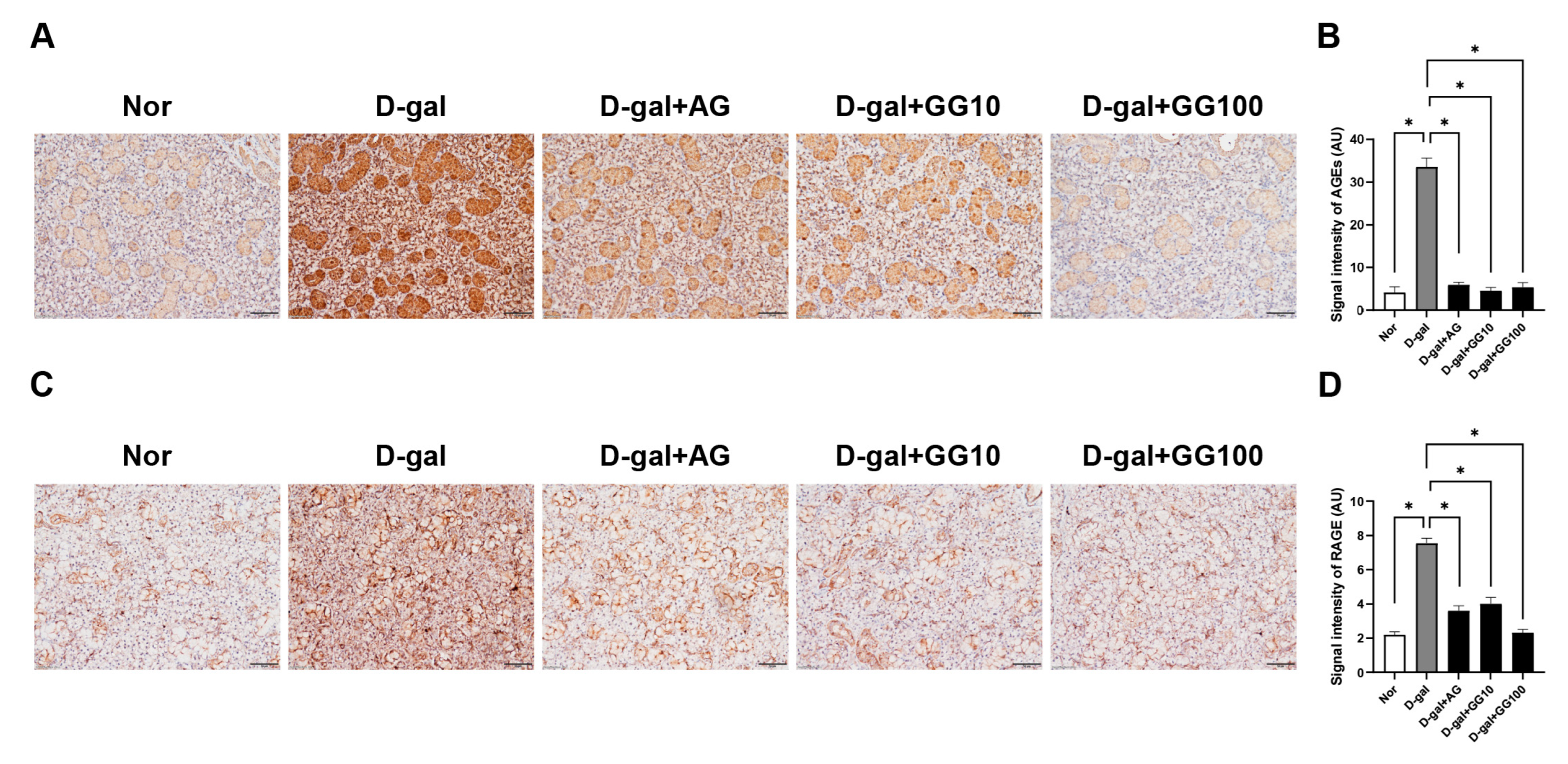
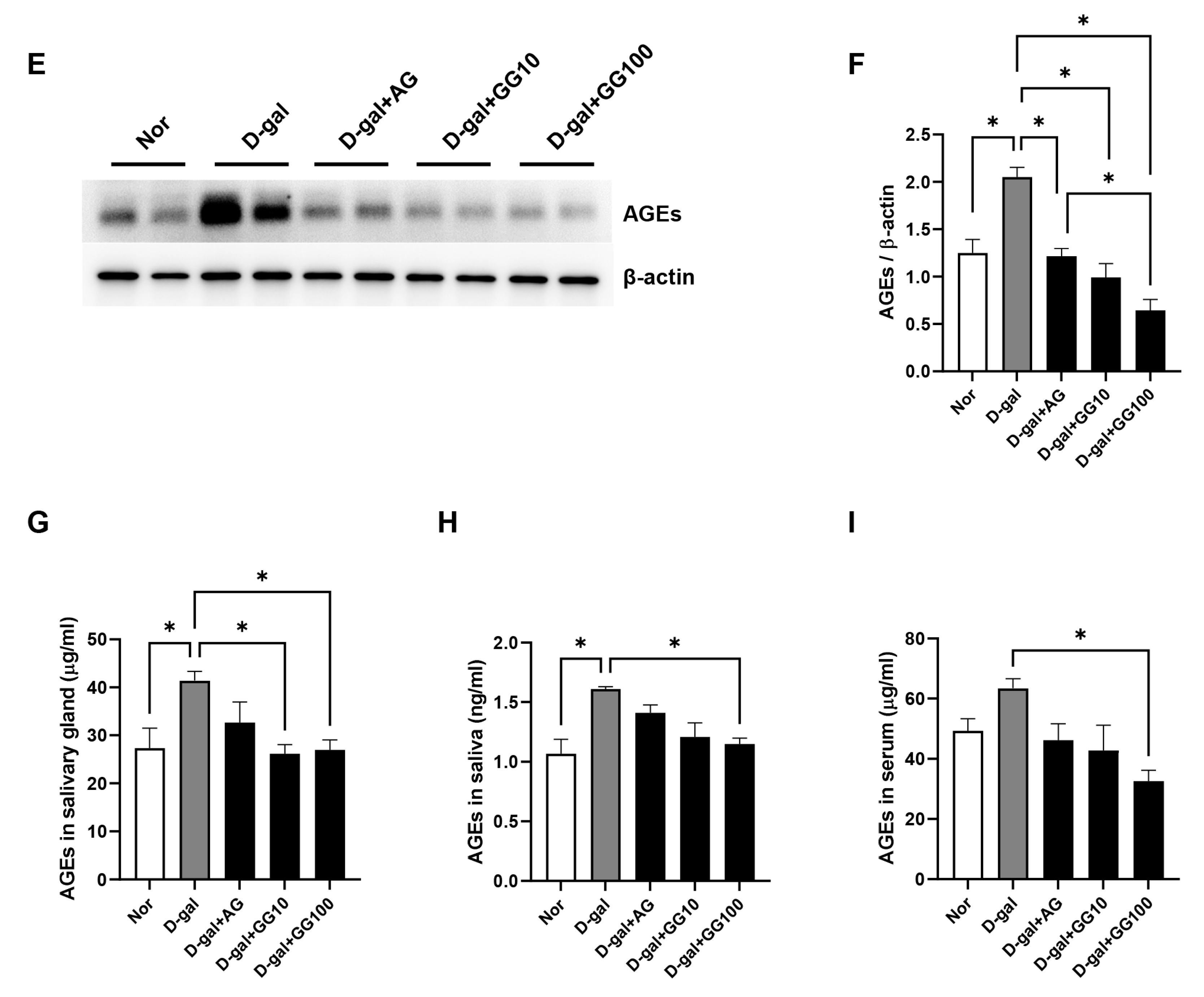
3.5. Gemigliptin Reduces AGE Accumulation and RAGE Expression in the Salivary Glands of D-Gal-Injected Rats
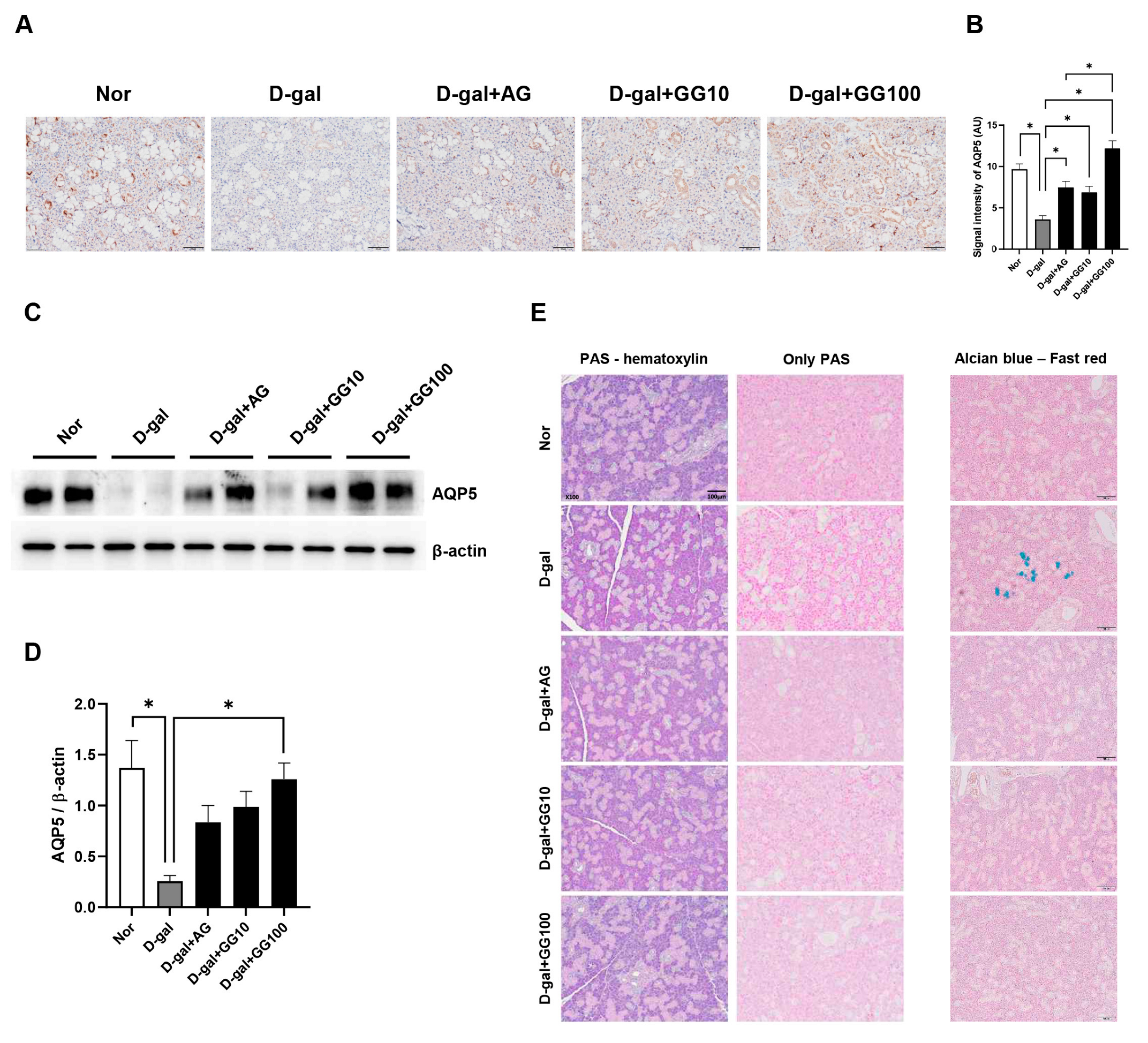
3.6. Gemigliptin Increases AQP5 Expression and Mucin Secretion in the Salivary Glands of Rats Injected with D-Galactose
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desa, U. United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2015 Revision, Key Findings and Advance Tables, online ed.; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2015; Available online: https://scholar.google.co.kr/scholar?hl=ko&as_sdt=0%2C5&q=World+Population+Prospects+The+2015+Revision+2015&btnG= (accessed on 13 October 2023).
- Osterberg, T.; Landahl, S.; Hedegård, B. Salivary flow, saliva, pH and buffering capacity in 70-year-old men and women. Correlation to dental health, dryness in the mouth, disease and drug treatment. J. Oral Rehabil. 1984, 11, 157–170. [Google Scholar] [CrossRef]
- Mese, H.; Matsuo, R. Salivary secretion, taste and hyposalivation. J. Oral Rehabil. 2007, 34, 711–723. [Google Scholar] [CrossRef]
- Vissink, A.; Spijkervet, F.K.L.; Amerongen, A.V.N. Aging and saliva: A review of the literature. Spec. Care Dent. 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Vandenberghe-Descamps, M.; Labouré, H.; Prot, A.; Septier, C.; Tournier, C.; Feron, G.; Sulmont-Rossé, C. Salivary flow decreases in healthy elderly people independently of dental status and drug intake. J. Texture Stud. 2016, 47, 353–360. [Google Scholar] [CrossRef]
- Ahmed, N. Advanced glycation endproducts—Role in pathology of diabetic complications. Diabetes Res. Clin. Pract. 2005, 67, 3–21. [Google Scholar] [CrossRef]
- Bengmark, S. Impact of nutrition on ageing and disease. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 2–7. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- Jung, W.K.; Park, S.-B.; Kim, H.R.; Ryu, H.Y.; Kim, Y.H.; Kim, J. Advanced glycation end products increase salivary gland hypofunction in d-galactose-induced aging rats and its prevention by physical exercise. Curr. Issues Mol. Biol. 2021, 43, 2059–2067. [Google Scholar] [CrossRef]
- Wu, M.; Huang, B.; Hu, L.; Zhang, T.; Zhang, B.; Zhao, X.; Lu, R.; Xiong, W.; Zhang, S.; Li, J. Ganoderma lucidum polysaccharides ameliorates D-galactose-induced aging salivary secretion disorders by upregulating the rhythm and aquaporins. Exp. Gerontol. 2023, 175, 112147. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, W.K.; Park, S.-B.; Ryu, H.Y.; Kim, Y.H.; Kim, J. Polydatin alleviates diabetes-induced hyposalivation through anti-glycation activity in db/db mouse. Pharmaceutics 2021, 14, 51. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J. Preventive effect of polydatin on diabetes-related hypofunction of salivary gland in streptozotocin-induced diabetic rats. J. Biomed. Transl. Res. 2021, 22, 159–167. [Google Scholar] [CrossRef]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, S.-H.; Yim, H.-J. Gemigliptin, a novel dipeptidyl peptidase 4 inhibitor: First new anti-diabetic drug in the history of Korean pharmaceutical industry. Arch. Pharmacal Res. 2013, 36, 1185–1188. [Google Scholar] [CrossRef]
- Bae, E.J. DPP-4 inhibitors in diabetic complications: Role of DPP-4 beyond glucose control. Arch. Pharmacal Res. 2016, 39, 1114–1128. [Google Scholar] [CrossRef]
- Kenawy, S.; Hegazy, R.; Hassan, A.; El-Shenawy, S.; Gomaa, N.; Zaki, H.; Attia, A. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: Protective role of metformin and saxagliptin. PLoS ONE 2017, 12, e0183565. [Google Scholar] [CrossRef]
- Dietrich, N.; Kolibabka, M.; Busch, S.; Bugert, P.; Kaiser, U.; Lin, J.; Fleming, T.; Morcos, M.; Klein, T.; Schlotterer, A. The DPP4 inhibitor linagliptin protects from experimental diabetic retinopathy. PLoS ONE 2016, 11, e0167853. [Google Scholar] [CrossRef]
- Nakashima, S.; Matsui, T.; Takeuchi, M.; Yamagishi, S.-I. Linagliptin blocks renal damage in type 1 diabetic rats by suppressing advanced glycation end products-receptor axis. Horm. Metab. Res. 2014, 46, 717–721. [Google Scholar] [CrossRef]
- Matsui, T.; Nishino, Y.; Takeuchi, M.; Yamagishi, S.-I. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product–receptor axis. Pharmacol. Res. 2011, 63, 383–388. [Google Scholar] [CrossRef]
- Sakata, K.; Hayakawa, M.; Yano, Y.; Tamaki, N.; Yokota, N.; Eto, T.; Watanabe, R.; Hirayama, N.; Matsuo, T.; Kuroki, K. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE)–receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes/Metab. Res. Rev. 2013, 29, 624–630. [Google Scholar] [CrossRef]
- Jung, E.; Kim, J.; Kim, S.H.; Kim, S.; Cho, M.-H. Gemigliptin, a novel dipeptidyl peptidase-4 inhibitor, exhibits potent anti-glycation properties in vitro and in vivo. Eur. J. Pharmacol. 2014, 744, 98–102. [Google Scholar] [CrossRef]
- Oh, H.; Nguyen, H.D.; Yoon, I.M.; Ahn, B.R.; Kim, M.S. Antidiabetic effect of gemigliptin: A systematic review and meta-analysis of randomized controlled trials with Bayesian inference through a quality management system. Sci. Rep. 2021, 11, 20938. [Google Scholar] [CrossRef]
- Kang, W.S.; Jung, W.K.; Park, S.-B.; Kim, H.R.; Kim, J. Gemigliptin suppresses salivary dysfunction in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2021, 137, 111297. [Google Scholar] [CrossRef]
- D’Aiuto, F.; Gable, D.; Syed, Z.; Allen, Y.; Wanyonyi, K.; White, S.; Gallagher, J. Evidence summary: The relationship between oral diseases and diabetes. Br. Dent. J. 2017, 222, 944–948. [Google Scholar] [CrossRef]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Yu, Y.; Bai, F.; Wang, W.; Liu, Y.; Yuan, Q.; Qu, S.; Zhang, T.; Tian, G.; Li, S.; Li, D. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol. Biochem. Behav. 2015, 133, 122–131. [Google Scholar] [CrossRef]
- Zhou, Y.-Y.; Ji, X.-F.; Fu, J.-P.; Zhu, X.-J.; Li, R.-H.; Mu, C.-K.; Wang, C.-L.; Song, W.-W. Gene transcriptional and metabolic profile changes in mimetic aging mice induced by D-galactose. PLoS ONE 2015, 10, e0132088. [Google Scholar] [CrossRef]
- Palma-Duran, S.A.; Kontogianni, M.D.; Vlassopoulos, A.; Zhao, S.; Margariti, A.; Georgoulis, M.; Papatheodoridis, G.; Combet, E. Serum levels of advanced glycation end-products (AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify non-alcoholic fatty liver disease in age-, sex-and BMI-matched normo-glycemic adults. Metabolism 2018, 83, 120–127. [Google Scholar] [CrossRef]
- Saleh, D.O.; Mansour, D.F.; Hashad, I.M.; Bakeer, R.M. Effects of sulforaphane on D-galactose-induced liver aging in rats: Role of keap-1/nrf-2 pathway. Eur. J. Pharmacol. 2019, 855, 40–49. [Google Scholar] [CrossRef]
- Aydin, F.; Kalaz, E.B.; Kucukgergin, C.; Coban, J.; Dogru-Abbasoglu, S.; Uysal, M. Carnosine treatment diminished oxidative stress and glycation products in serum and tissues of D-galactose-treated rats. Curr. Aging Sci. 2018, 11, 10–15. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, L.; Peng, Y.; Yuan, D.; Zhang, S.; Gong, Z.; Xiao, W. l-Theanine attenuates liver aging by inhibiting advanced glycation end products in d-galactose-induced rats and reversing an imbalance of oxidative stress and inflammation. Exp. Gerontol. 2020, 131, 110823. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Silljé, H.H. Glucagon-like peptide 1 prevents reactive oxygen species–induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef]
- Cappetta, D.; Ciuffreda, L.P.; Cozzolino, A.; Esposito, G.; Scavone, C.; Sapio, L.; Naviglio, S.; D’Amario, D.; Crea, F.; Rossi, F. Dipeptidyl peptidase 4 inhibition ameliorates chronic kidney disease in a model of salt-dependent hypertension. Oxidative Med. Cell. Longev. 2019, 2019, 8912768. [Google Scholar] [CrossRef]
- Matsui, T.; Nakashima, S.; Nishino, Y.; Ojima, A.; Nakamura, N.; Arima, K.; Fukami, K.; Okuda, S.; Yamagishi, S.-i. Dipeptidyl peptidase-4 deficiency protects against experimental diabetic nephropathy partly by blocking the advanced glycation end products-receptor axis. Lab. Investig. 2015, 95, 525–533. [Google Scholar] [CrossRef][Green Version]
- Sarker, M.K.; Lee, J.H.; Lee, D.H.; Chun, K.-H.; Jun, H.-S. Attenuation of diabetic kidney injury in DPP4-deficient rats; role of GLP-1 on the suppression of AGE formation by inducing glyoxalase 1. Aging 2020, 12, 593. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Geng, X. Protective role of three differently processed corn bran on glucose and lipid concentrations in d-galactose-induced mice model. J. Food Biochem. 2020, 44, e13281. [Google Scholar] [CrossRef]
- Walvekar, M.; Sarvalkar, P.; Pol, S. Effect of curcumin supplementation on the submandibular salivary glands of D-galactose induced male mice. Int. J. Pharm. Sci. Res. 2012, 3, 4796. [Google Scholar] [CrossRef]
- Enoki, N.; Kiyoshima, T.; Sakai, T.; Kobayashi, I.; Takahashi, K.; Terada, Y.; Sakai, H. Age-dependent changes in cell proliferation and cell death in the periodontal tissue and the submandibular gland in mice: A comparison with other tissues and organs. J. Mol. Histol. 2007, 38, 321–332. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.M.; Choi, M.E.; Jeon, E.J.; Park, J.-M.; Kim, Y.-M.; Choi, J.-S. Adiponectin is associated with inflammaging and age-related salivary gland lipid accumulation. Aging 2023, 15, 1840. [Google Scholar] [CrossRef]
- Miao, N.; Zhan, Y.; Xu, Y.; Yuan, H.; Qin, C.; Lin, F.; Xie, X.; Mu, S.; Yuan, M.; Mu, H. Loss of Fam20c causes defects in the acinar and duct structure of salivary glands in mice. Int. J. Mol. Med. 2019, 43, 2103–2117. [Google Scholar] [CrossRef]
- Martínez Allo, V.C.; Hauk, V.; Sarbia, N.; Pinto, N.A.; Croci, D.O.; Dalotto-Moreno, T.; Morales, R.M.; Gatto, S.G.; Manselle Cocco, M.N.; Stupirski, J.C. Suppression of age-related salivary gland autoimmunity by glycosylation-dependent galectin-1-driven immune inhibitory circuits. Proc. Natl. Acad. Sci. USA 2020, 117, 6630–6639. [Google Scholar] [CrossRef]
- Watanabe, M.; Yamagishi-Wang, H.; Kawaguchi, M. Lowered susceptibility of muscarinic receptor involved in salivary secretion of streptozotocin-induced diabetic rats. Jpn. J. Pharmacol. 2001, 87, 117–124. [Google Scholar] [CrossRef]
- Santos, J.; Saus, E.; Smalley, S.; Cataldo, L.; Alberti, G.; Parada, J.; Gratacòs, M.; Estivill, X. Copy number polymorphism of the salivary amylase gene: Implications in human nutrition research. J. Nutr. Nutr. 2012, 5, 117–131. [Google Scholar] [CrossRef]
- Walvekar, M.; Pillai, M.; Methe, K.; Bhopale, L. Modulatory effects of centrophenoxine on salivary glands in D-galactose induced aged female mice. J. Endocrinol. Reprod. 2008, 12, 90–95. Available online: https://scholar.google.co.kr/scholar?hl=ko&as_sdt=0%2C5&q=Modulatory+effects+of+centrophenoxine+on+salivary+glands+in+D-galactose+induced+aged+female+mice&btnG= (accessed on 15 October 2023).
- Ungureanu, L.B.; Grădinaru, I.; Ghiciuc, C.M.; Amălinei, C.; Gelețu, G.L.; Petrovici, C.G.; Stănescu, R.Ș. Atrophy and inflammatory changes in salivary glands induced by oxidative stress after exposure to drugs and other chemical substances: A systematic review. Medicina 2023, 59, 1692. [Google Scholar] [CrossRef]
- Pedersen, A.; Bardow, A.; Jensen, S.B.; Nauntofte, B. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002, 8, 117–129. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Q.Y.; Shi, X.J.; Cong, X.; Zhang, Y.; Wu, L.L.; Yu, G.Y.; Xiang, R.L. Disruption of tight junctions contributes to hyposalivation of salivary glands in a mouse model of type 2 diabetes. J. Anat. 2020, 237, 556–567. [Google Scholar] [CrossRef]
- Delporte, C.; Bryla, A.; Perret, J. Aquaporins in salivary glands: From basic research to clinical applications. Int. J. Mol. Sci. 2016, 17, 166. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Nieman, M.L.; Boivin, G.P.; Melvin, J.E.; Kikuchi, K.-I.; Hand, A.R.; Lorenz, J.N.; Menon, A.G. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc. Natl. Acad. Sci. USA 2007, 104, 3621–3626. [Google Scholar] [CrossRef]
- Krane, C.M.; Melvin, J.E.; Nguyen, H.-V.; Richardson, L.; Towne, J.E.; Doetschman, T.; Menon, A.G. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J. Biol. Chem. 2001, 276, 23413–23420. [Google Scholar] [CrossRef]
- Kuraji, M.; Matsuno, T.; Satoh, T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J. Clin. Biochem. Nutr. 2016, 59, 79–85. [Google Scholar] [CrossRef]



| Gemigliptin | Saxagliptin | Linagliptin | Vildagliptin | Alogliptin | |
|---|---|---|---|---|---|
| Formula | C18H19F8N5O2 | C18H25N3O2 | C25H27N8O2 | C17H25N3O2 | C18H21N5O2 |
| Chemical structure | 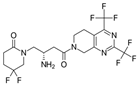 | 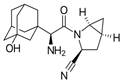 | 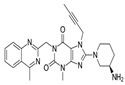 |  | 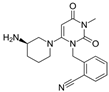 |
| Therapeutic dose | 50 mg/day | 5 mg/day | 5 mg/day | 100 mg/day | 25 mg/kg |
| DPP-4 inhibition IC50 | 6.3 nmol/L | 50 nmol/L | 1 nmol/L | 62 nmol/L | 24 nmol/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.K.; Park, S.-B.; Yu, H.Y.; Kim, J. Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats. Pharmaceutics 2024, 16, 35. https://doi.org/10.3390/pharmaceutics16010035
Jung WK, Park S-B, Yu HY, Kim J. Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats. Pharmaceutics. 2024; 16(1):35. https://doi.org/10.3390/pharmaceutics16010035
Chicago/Turabian StyleJung, Woo Kwon, Su-Bin Park, Hwa Young Yu, and Junghyun Kim. 2024. "Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats" Pharmaceutics 16, no. 1: 35. https://doi.org/10.3390/pharmaceutics16010035
APA StyleJung, W. K., Park, S.-B., Yu, H. Y., & Kim, J. (2024). Gemigliptin Improves Salivary Gland Dysfunction in D-Galactose-Injected Aging Rats. Pharmaceutics, 16(1), 35. https://doi.org/10.3390/pharmaceutics16010035







