Lipid-Based Nanotechnology: Liposome
Abstract
:1. Introduction
2. Characterization and Major Components of Liposomes
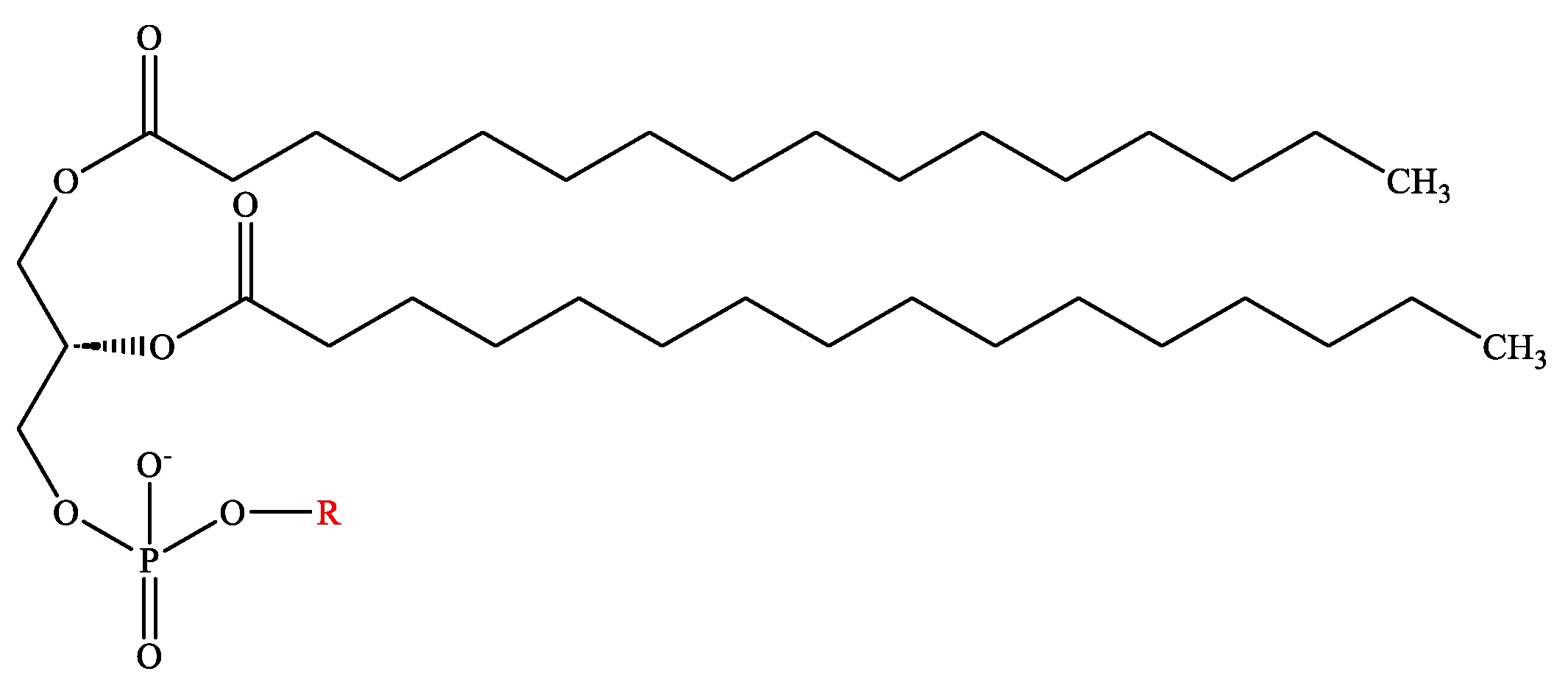
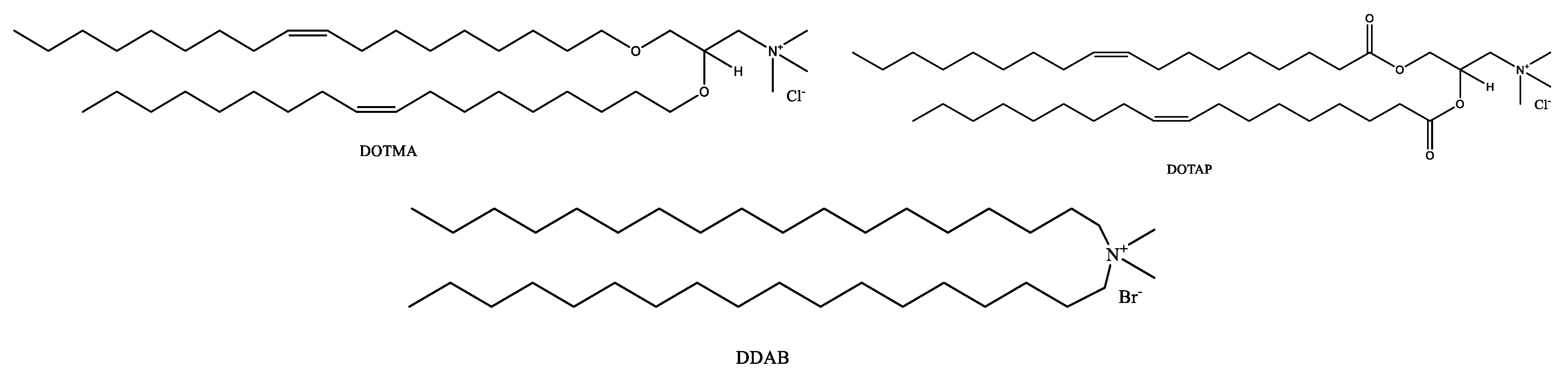
2.1. Phospholipids
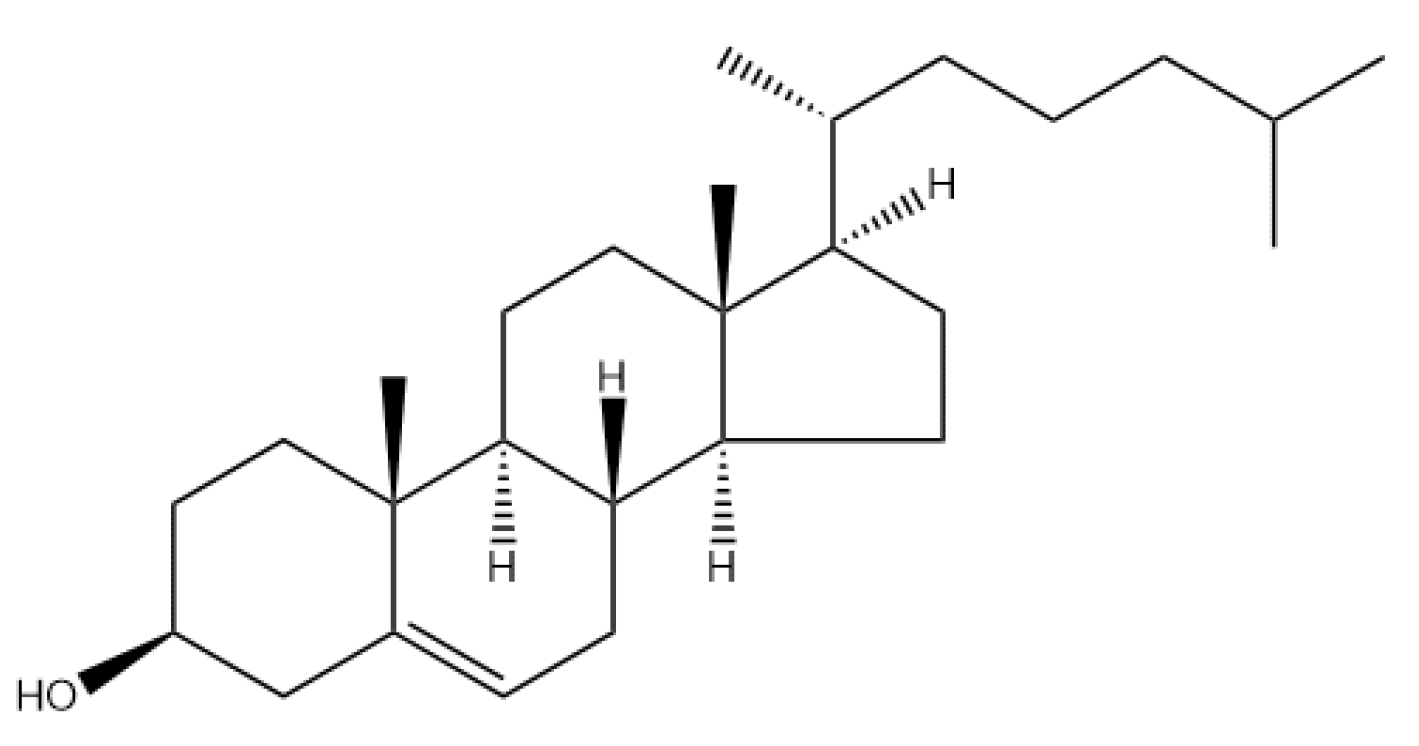
2.2. Cholesterol
2.3. Polyethylene Glyco
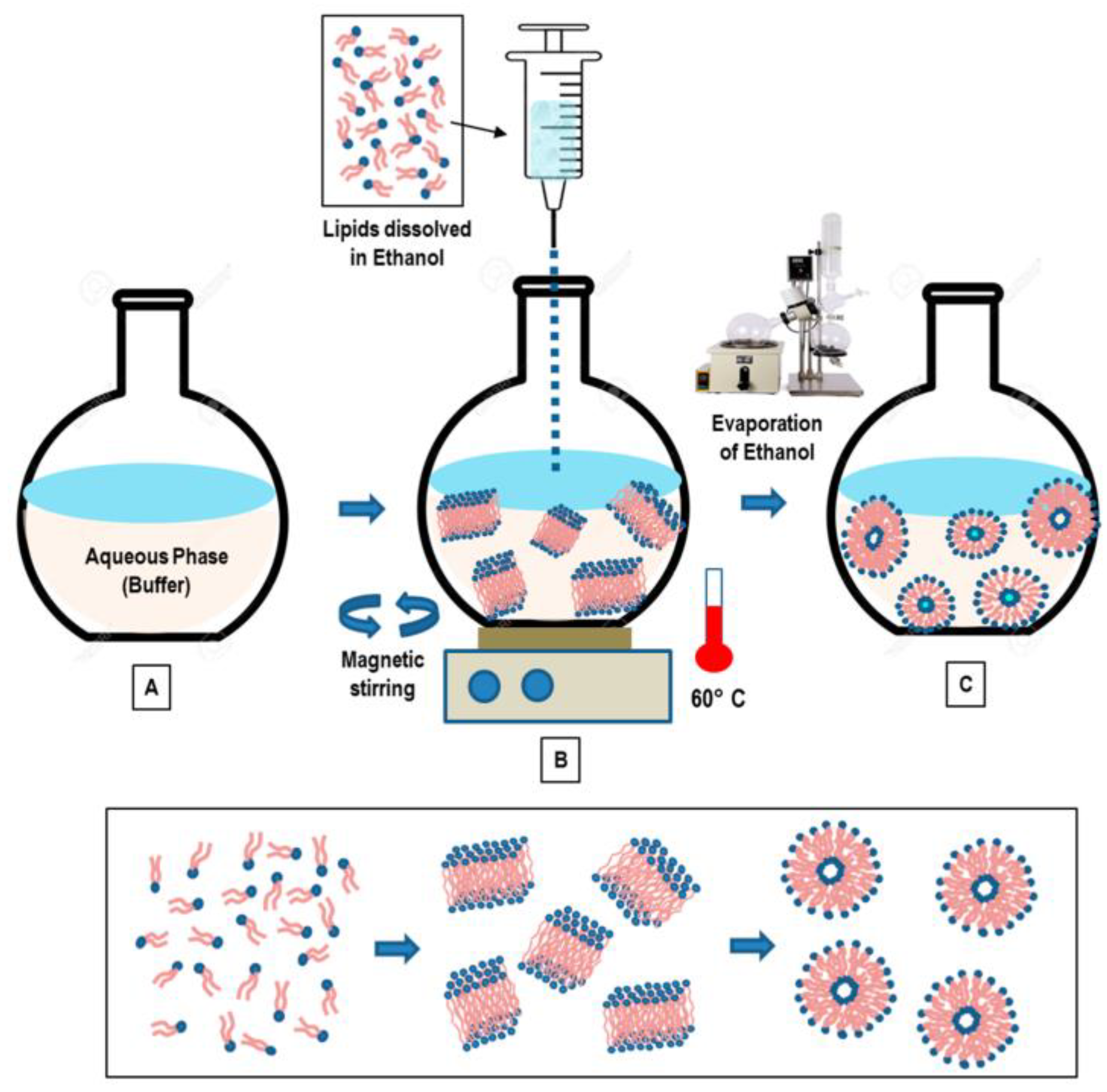
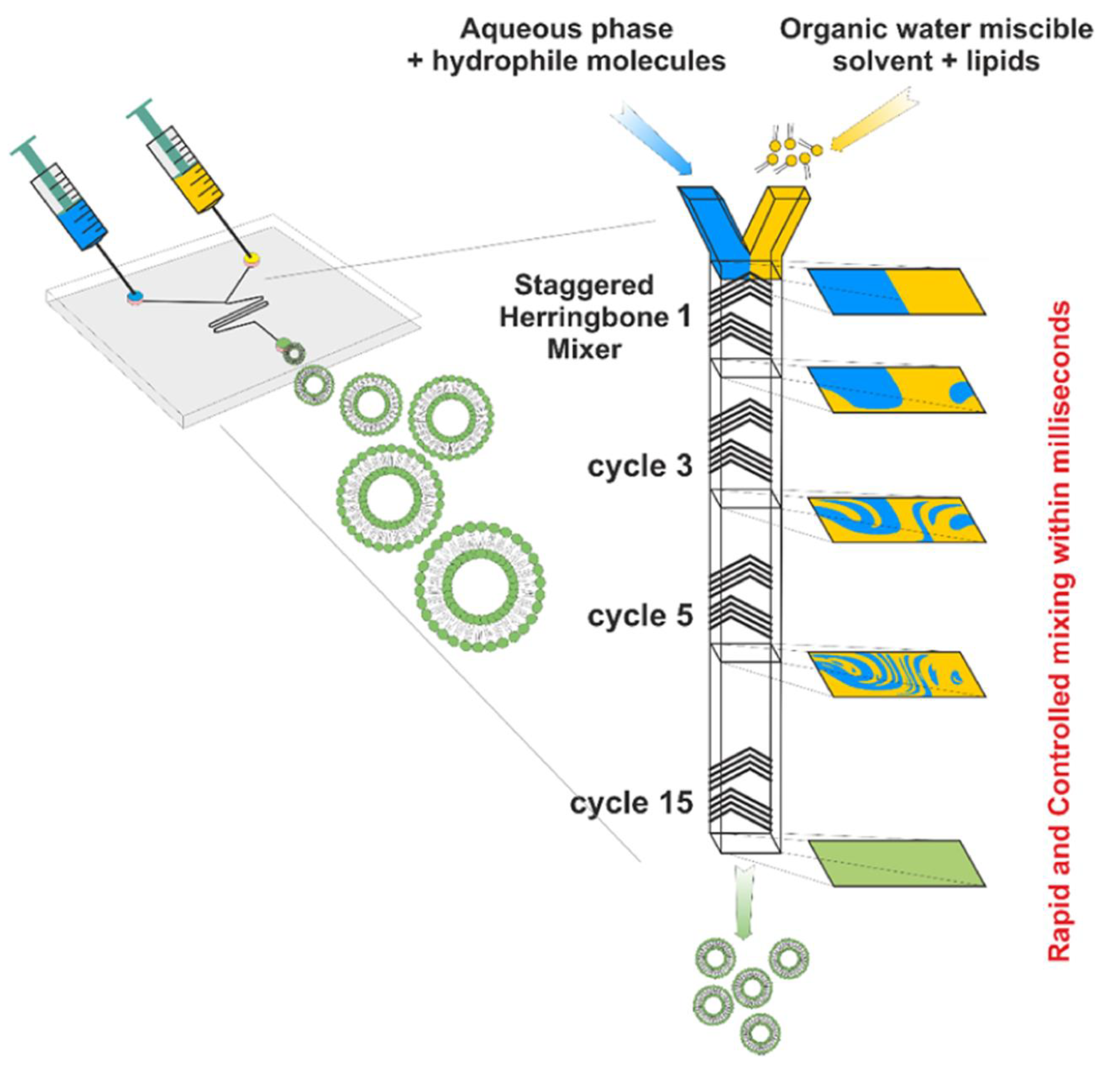
2.4. Major Methods of Liposome Preparation
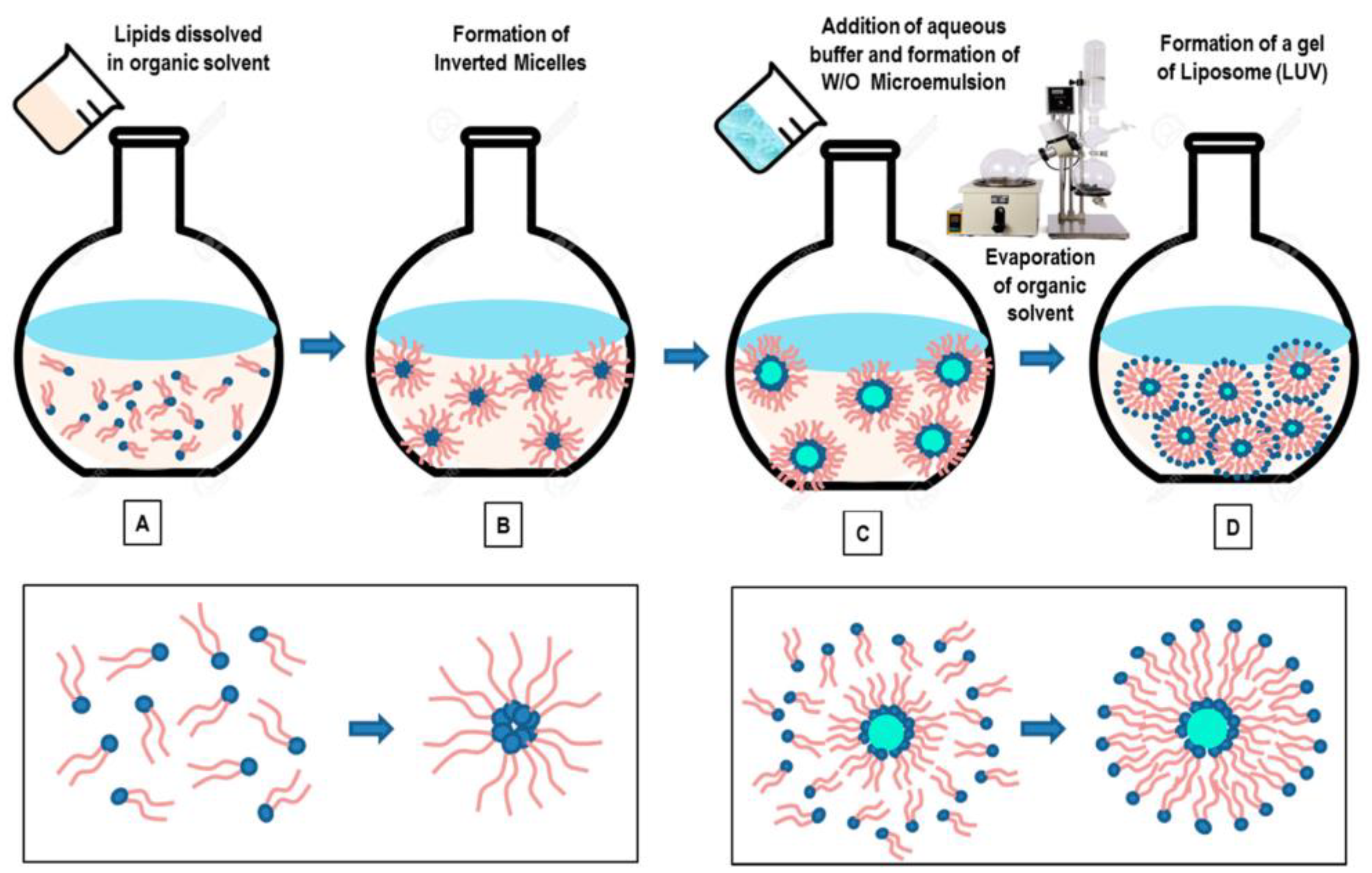
2.4.1. Thin Film Hydration
2.4.2. Reverse Phase Evaporation
2.4.3. Solvent Injection
2.4.4. Detergent Removal
2.4.5. Micro Hydrodynamic Focusing
2.5. Major Methods of Liposome Characterization
2.5.1. Size Distribution and Zeta-Potential
2.5.2. Stability and Drug Leakage
2.5.3. Phase Transition
2.5.4. Fluorescence Microscopy
2.5.5. Fourier Transform Infrared Spectroscopy
2.6. Stimuli-Responsive Liposomes
2.6.1. pH-Responsive Liposomes
2.6.2. Temperature-Responsive Liposomes
2.6.3. Enzyme-Responsive Liposomes
3. Pharmaceutical Applications of Liposomes
3.1. Anti-Cancer
3.1.1. Doxil
3.1.2. Onivyde
3.1.3. Liposome-Peptide Conjugated Drugs
3.2. Anti-Fungal
3.2.1. Amphotericin B and Ambisome
3.2.2. Nystatin and Nyotran
3.2.3. Inhaled Liposomal Antimicrobial Medications
3.3. Pain Management
3.3.1. Exparel
3.3.2. Liposomal Cannabidiol
3.4. Vaccination
3.4.1. Liposomal Vaccines
3.4.2. Lipid-Based mRNA Nanovaccines
4. Discussion and Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.M.; Van Zanten, S.E.M.V.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): A collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963. [Google Scholar] [CrossRef] [PubMed]
- Bureš, J.; Kohoutová, D.; Zavoral, M. Gastrointestinal toxicity of systemic oncology immunotherapy. Klin. Onkol. 2022, 35, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Shastry, M.; Jacob, S.; Rugo, H.S.; Hamilton, E. Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer. Breast 2022, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W.M. General perception of liposomes: Formation, manufacturing and applications. In Liposomes-Advances and Perspectives; IntechOpen: London, UK, 2019. [Google Scholar]
- Kiaie, S.H.; Mojarad-Jabali, S.; Khaleseh, F.; Allahyari, S.; Taheri, E.; Zakeri-Milani, P.; Valizadeh, H. Axial pharmaceutical properties of liposome in cancer therapy: Recent advances and perspectives. Int. J. Pharm. 2020, 581, 119269. [Google Scholar] [CrossRef]
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Touti, R.; Noun, M.; Guimberteau, F.; Lecomte, S.; Faure, C. What is the fate of multi-lamellar liposomes of controlled size, charge and elasticity in artificial and animal skin? Eur. J. Pharm. Biopharm. 2020, 151, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zou, Y.; Bhattacharya, A.; Zhang, D.; Lang, S.Q.; Houk, K.; Devaraj, N.K. Enzyme-free synthesis of natural phospholipids in water. Nat. Chem. 2020, 12, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Cífková, E.; Hájek, R.; Lísa, M.; HolĿapek, M. Hydrophilic interaction liquid chromatography mass spectrometry of (lyso) phosphatidic acids, (lyso) phosphatidylserines and other lipid classes. J. Chromatogr. A 2016, 1439, 65–73. [Google Scholar] [CrossRef]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Sousa-Pinto, I.; Malcata, F.X. Chapter 16—Algal spent biomass—A pool of applications. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.-J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–433. [Google Scholar]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Saha, R.N.; Alexander, A.; Singhvi, G. Tailoring the multi-functional properties of phospholipids for simple to complex self-assemblies. J. Control. Release 2022, 349, 460–474. [Google Scholar] [CrossRef]
- Jin, Y.; Tan, Y.; Wu, J.; Ren, Z. Lipid droplets: A cellular organelle vital in cancer cells. Cell Death Discov. 2023, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Surdu, A.M.; Pînzariu, O.; Ciobanu, D.-M.; Negru, A.-G.; Căinap, S.-S.; Lazea, C.; Iacob, D.; Săraci, G.; Tirinescu, D.; Borda, I.M. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol effects on cholesterol metabolism via bile acid biosynthesis, CYP7A1: A review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinué, Á.; Martínez-Hervás, S.; González-Navarro, H. Impact of cholesterol metabolism in immune cell function and atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef]
- Albuquerque, H.M.T.; Santos, C.M.M.; Silva, A.M.S. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2018, 24, 116. [Google Scholar] [CrossRef]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.X. Polymer properties and characterization. In Developing Solid Oral Dosage Forms; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–223. [Google Scholar]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Woodle, M.C.; Newman, M.S.; Martin, F.J. Liposome leakage and blood circulation: Comparison of adsorbed block copolymers with covalent attachment of PEG. Int. J. Pharm. 1992, 88, 327–334. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Sun, M.; Zhang, J.; Bi, Y. Coating liposomes with ring-like PEG: The synthesis and stealth effect of cholesterol–PEG–cholesterol. Mater. Adv. 2022, 3, 2417–2424. [Google Scholar] [CrossRef]
- Fischer, C.; Lamer, T.; Wang, W.; McKinnie, S.M.; Iturrioz, X.; Llorens-Cortes, C.; Oudit, G.Y.; Vederas, J.C. Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl-and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur. J. Med. Chem. 2019, 166, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Cui, H.; Sun, P.; Yang, X.; Chen, Q. Circumvent PEGylation dilemma by implementing matrix metalloproteinase-responsive chemistry for promoted tumor gene therapy. Chin. Chem. Lett. 2020, 31, 3143–3148. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.; De Gier, J.; Greville, G. Osmotic properties and water permeability of phospholipid liquid crystals. Chem. Phys. Lipids 1967, 1, 225–246. [Google Scholar] [CrossRef]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef]
- Gregoriadis, G.; McCormack, B.; Obrenovich, M.; Perrie, Y. Entrapment of plasmid DNA vaccines into liposomes by dehydration/rehydration. DNA Vaccines Methods Protoc. 2000, 29, 305–311. [Google Scholar]
- Andra, V.V.S.N.L.; Pammi, S.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Doskocz, J.; Dałek, P.; Foryś, A.; Trzebicka, B.; Przybyło, M.; Mesarec, L.; Iglič, A.; Langner, M. The effect of lipid phase on liposome stability upon exposure to the mechanical stress. Biochim. Et Biophys. Acta-Biomembr. 2020, 1862, 183361. [Google Scholar] [CrossRef]
- Xiang, B.; Cao, D.-Y. Preparation of drug liposomes by thin-film hydration and homogenization. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 25–35. [Google Scholar]
- Shi, N.-Q.; Qi, X.-R. Preparation of drug liposomes by reverse-phase evaporation. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 37–46. [Google Scholar]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Gouda, A.; Sakr, O.S.; Nasr, M.; Sammour, O. Ethanol injection technique for liposomes formulation: An insight into development, influencing factors, challenges and applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102174. [Google Scholar] [CrossRef]
- Lin, M.; Qi, X.-R. Purification method of drug-loaded liposome. In Liposome-Based Drug Delivery Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 111–121. [Google Scholar]
- Kanda, H.; Katsube, T.; Wahyudiono; Goto, M. Preparation of liposomes from soy lecithin using liquefied dimethyl ether. Foods 2021, 10, 1789. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. Removal of detergent and solvent traces from liposomes. In Liposome Technology; CRC Press: Boca Raton, FL, USA, 2019; Volume I, p. 1109. [Google Scholar]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sun, J. Lipid in chips: A brief review of liposomes formation by microfluidics. Int. J. Nanomed. 2021, 16, 7391–7416. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, P.; Li, J.; Tornillo, G.; McCloy, T.; Barrow, D. Droplet Microfluidics for Tumor Drug-Related Studies and Programmable Artificial Cells. Glob. Chall. 2021, 5, 2000123. [Google Scholar] [CrossRef] [PubMed]
- Kotouček, J.; Hubatka, F.; Mašek, J.; Kulich, P.; Velínská, K.; Bezděková, J.; Fojtíková, M.; Bartheldyová, E.; Tomečková, A.; Stráská, J.; et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci. Rep. 2020, 10, 5595. [Google Scholar] [CrossRef]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Yang, K.; Mesquita, B.; Horvatovich, P.; Salvati, A. Tuning liposome composition to modulate corona formation in human serum and cellular uptake. Acta Biomater. 2020, 106, 314–327. [Google Scholar] [CrossRef]
- Cauzzo, J.; Jayakumar, N.; Ahluwalia, B.S.; Ahmad, A.; Škalko-Basnet, N. Characterization of liposomes using quantitative phase microscopy (QPM). Pharmaceutics 2021, 13, 590. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Salt, L.J.; Ridout, M.J.; Han, J.; Wilde, P.J. Structural stability of liposome-stabilized oil-in-water pickering emulsions and their fate during in vitro digestion. Food Funct. 2019, 10, 7262–7274. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Yang, G.; van Der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Liposomes with Water as a pH-Responsive Functionality for Targeting of Acidic Tumor and Infection Sites. Angew. Chem. 2021, 133, 17855–17860. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent advances in tumor targeting via EPR effect for cancer treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of liposome size, surface charge, and PEGylation on rheumatoid arthritis targeting therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022. [Google Scholar] [CrossRef]
- Eskandari, V.; Sadeghi, M.; Hadi, A. Physical and chemical properties of nano-liposome, application in nano medicine. J. Comput. Appl. Mech. 2021, 52, 751–767. [Google Scholar]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, biomedical applications, and stability parameters with emphasis on cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Demetzos, C. Differential scanning calorimetry (DSC): A tool to study the thermal behavior of lipid bilayers and liposomal stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Patel, N.; Panda, S. Liposome drug delivery system: A critic review. JPSBR 2012, 2, 169–175. [Google Scholar]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.R.; Rezler, E.M.; Lauer-Fields, J.; Fields, G.B. Effects of drug hydrophobicity on liposomal stability. Chem. Biol. Drug Des. 2008, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Haneef, J.; Amir, M.; Sheikh, N.A.; Chadha, R. Mitigating Drug Stability Challenges Through Cocrystallization. AAPS PharmSciTech 2023, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Liposomes for the treatment of brain cancer—A review. Pharmaceuticals 2023, 16, 1056. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D.; Ploch-Jankowska, A.; Zięba, A.; Kozik, V. The advances and challenges of liposome-assisted drug release in the presence of serum albumin molecules: The influence of surrounding pH. Materials 2022, 15, 1586. [Google Scholar] [CrossRef] [PubMed]
- Faroux, J.M.; Ureta, M.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A. An overview of peroxidation reactions using liposomes as model systems and analytical methods as monitoring tools. Colloids Surf. B Biointerfaces 2020, 195, 111254. [Google Scholar] [CrossRef] [PubMed]
- Smaisim, G.F.; Mohammed, K.J.; Hadrawi, S.K.; Koten, H.; Kianfar, E. Properties and application of nanostructure in liquid crystals. BioNanoScience 2023, 13, 819–839. [Google Scholar] [CrossRef]
- Kianfar, E.; Salimi, M.; Koohestani, B. Methanol to gasoline conversion over CuO/ZSM-5 catalyst synthesized and influence of water on conversion. Fine Chem. Eng. 2020, 1, 75–82. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Mohammed, D.B.; Abdulhadi, A.M.; Uktamov, K.F.; Alsultany, F.H.; Izzat, S.E.; Ansari, M.J.; Kzar, H.H.; Al-Gazally, M.E.; Kianfar, E. Nanofluids: Properties and applications. J. Sol-Gel Sci. Technol. 2022, 104, 1–35. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Li, Y.; Gu, R.; Yan, X. Characterization of lipid-based nanomedicines at the single-particle level. Fundam. Res. 2023, 3, 488–504. [Google Scholar] [CrossRef]
- Dey, A.; Gare, S.; Swain, S.; Bhattacharya, P.; Dhyani, V.; Giri, L.; Neogi, S. 3D imaging and quantification of PLL coated fluorescent ZnO NP distribution and ROS accumulation using laser scanning confocal microscopy. AIChE J. 2022, 68, e17801. [Google Scholar] [CrossRef]
- Oleksiievets, N.; Mathew, C.; Thiele, J.C.; Gallea, J.I.; Nevskyi, O.; Gregor, I.; Weber, A.; Tsukanov, R.; Enderlein, J.R. Single-molecule fluorescence lifetime imaging using wide-field and confocal-laser scanning microscopy: A comparative analysis. Nano Lett. 2022, 22, 6454–6461. [Google Scholar] [CrossRef] [PubMed]
- Karakas, C.Y.; Ustundag, C.B.; Sahin, A.; Karadag, A. Co-axial electrospinning of liposomal propolis loaded gelatin-zein fibers as a potential wound healing material. J. Appl. Polym. Sci. 2023, 140, e54683. [Google Scholar] [CrossRef]
- Ciobanasu, C. Confocal Laser Scanning Microscopy and Model Membranes to Study Translocation Mechanisms of Membrane Active Peptides. Pharmaceutics 2022, 14, 1699. [Google Scholar] [CrossRef] [PubMed]
- Colson, L.; Kwon, Y.; Nam, S.; Bhandari, A.; Maya, N.M.; Lu, Y.; Cho, Y. Trends in Single-Molecule Total Internal Reflection Fluorescence Imaging and Their Biological Applications with Lab-on-a-Chip Technology. Sensors 2023, 23, 7691. [Google Scholar] [CrossRef]
- Scheeder, A.; Brockhoff, M.; Ward, E.; Kaminski Schierle, G.S.; Mela, I.; Kaminski, C.F. Molecular mechanisms of liposome interactions with bacterial envelopes. bioRxiv 2023. [Google Scholar] [CrossRef]
- Ashby, G.; Keng, K.E.; Hayden, C.C.; Gollapudi, S.; Houser, J.R.; Jamal, S.; Stachowiak, J.C. Selective Endocytic Uptake of Targeted Liposomes Occurs within a Narrow Range of Liposome Diameters. ACS Appl. Mater. Interfaces 2023, 15, 49988–50001. [Google Scholar] [CrossRef]
- Naeeni, N.B.; Tabrizi, M.H.; Karimi, E.; Ghafaripour, H. Synthesis and characterization of liposomal nanoparticles coated with chitosan–folate for efficient delivery of lawsone to pancreatic cancer cells. Polym. Bull. 2023. [Google Scholar] [CrossRef]
- Goh, C.F.; Lane, M.E. Advanced structural characterisation of pharmaceuticals using nano-thermal analysis (nano-TA). Adv. Drug Deliv. Rev. 2022, 180, 114077. [Google Scholar] [CrossRef]
- Meng, J.; Wang, Y.-Y.; Hao, Y.-P. Application of two glycosylated Lactobacillus surface layer proteins in coating cationic liposomes. World J. Microbiol. Biotechnol. 2023, 39, 108. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-responsive nanocarriers in cancer therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liang, C.; Zhao, Y.; Xue, X.; Ma, Z.; Qi, J.; Shen, H.; Yang, S.; Zhang, J.; Jia, Q. Multistage pH-responsive codelivery liposomal platform for synergistic cancer therapy. J. Nanobiotechnol. 2022, 20, 177. [Google Scholar] [CrossRef] [PubMed]
- Shinn, J.; Kwon, N.; Lee, S.A.; Lee, Y. Smart pH-responsive nanomedicines for disease therapy. J. Pharm. Investig. 2022, 52, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Bami, M.S.; Estabragh, M.A.R.; Khazaeli, P.; Ohadi, M.; Dehghannoudeh, G. pH-responsive drug delivery systems as intelligent carriers for targeted drug therapy: Brief history, properties, synthesis, mechanism and application. J. Drug Deliv. Sci. Technol. 2022, 70, 102987. [Google Scholar] [CrossRef]
- Zhao, F.; Sharma, G.; Kim, J.-C. Temperature and oxidation-sensitive dioleoylphosphatidylethanolamine liposome stabilized with poly (ethyleneimine)/(phenylthio) acetic acid ion pair. J. Biomater. Sci. Polym. Ed. 2023, 34, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Alle, M.; Park, S.C.; Zhao, F.; Long, W.; Samala, S.; Kim, J.-C. Self-assembly prepared using an ion pair of poly (ethylene imine) and (phenylthio) acetic acid as a drug carrier for oxidation, temperature, and NIR-responsive release. Chem. Eng. J. 2021, 415, 128954. [Google Scholar] [CrossRef]
- Zhao, F.; Kim, J.-C. Thermo-sensitive self-assembly of poly (ethylene imine)/(phenylthio) acetic acid ion pair in surfactant solutions. Drug Deliv. 2022, 29, 2245–2257. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, S.; Tong, S.; Zhao, H.; Chen, R.; Song, B.; Wu, J. Advances in the Study of Liposomes Gel with Stimulus Responsiveness in Disease Treatment. J. Clust. Sci. 2023, 1–14. [Google Scholar] [CrossRef]
- Razmimanesh, F.; Sodeifian, G. Evaluation of a temperature-responsive magnetotocosome as a magnetic targeting drug delivery system for sorafenib tosylate anticancer drug. Heliyon 2023, 9, e21794. [Google Scholar] [CrossRef]
- Chasteen, J.L.; Padilla-Coley, S.; Li, D.-H.; Smith, B.D. Palladium responsive liposomes for triggered release of aqueous contents. Bioorganic Med. Chem. Lett. 2023, 84, 129215. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, Q.; Wang, X.; Wu, J.; Hu, X.; Liu, M.; Zhang, X.; Wei, Y.; Liu, Z.; Liu, H. Bioorthogonal catalytic nanozyme-mediated lysosomal membrane leakage for targeted drug delivery. Theranostics 2022, 12, 1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for tumor targeted therapy: A review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Fassas, A.; Anagnostopoulos, A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 795–802. [Google Scholar] [CrossRef]
- Mischler, R.; Metcalfe, I.C. Inflexal®V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20, B17–B23. [Google Scholar] [CrossRef]
- Gardikis, K.; Tsimplouli, C.; Dimas, K.; Micha-Screttas, M.; Demetzos, C. New chimeric advanced Drug Delivery nano Systems (chi-aDDnSs) as doxorubicin carriers. Int. J. Pharm. 2010, 402, 231–237. [Google Scholar] [CrossRef]
- Carvalho, B.; Roland, L.M.; Chu, L.F.; Campitelli, V.A., III; Riley, E.T. Single-dose, extended-release epidural morphine (DepoDur™) compared to conventional epidural morphine for post-cesarean pain. Anesth. Analg. 2007, 105, 176–183. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D. FDA Approval Summary:(Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid LeukemiaFDA Approval:(Daunorubicin and Cytarabine). Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Faro-Viana, J.; Bergman, M.-L.; Gonçalves, L.A.; Duarte, N.; Coutinho, T.P.; Borges, P.C.; Diwo, C.; Castro, R.; Matoso, P.; Malheiro, V.; et al. Population homogeneity for the antibody response to COVID-19 BNT162b2/Comirnaty vaccine is only reached after the second dose across all adult age ranges. Nat. Commun. 2022, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Kirste, I.; Hortsch, S.; Grunert, V.P.; Legault, H.; Maglinao, M.; Eichenlaub, U.; Kashlan, B.; Pajon, R.; Jochum, S. Quantifying the Vaccine-Induced Humoral Immune Response to Spike-Receptor Binding Domain as a Surrogate for Neutralization Testing Following mRNA-1273 (Spikevax) Vaccination Against COVID-19. Infect. Dis. Ther. 2023, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Qian, F.; Wang, X.; Li, H.; Wang, L. Targeted delivery of 20 (S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer. Biomed. Microdevices 2019, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23. [Google Scholar] [CrossRef]
- Yang, B.; Song, B.-P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243. [Google Scholar] [CrossRef]
- Bompaire, F.; Durand, T.; Léger-Hardy, I.; Psimaras, D.; Ricard, D. Chemotherapy-related cognitive impairment or «chemobrain»: Concept and state of art. Geriatr. Psychol. Neuropsychiatr. Vieil. 2017, 15, 89–98. [Google Scholar] [CrossRef]
- Lim, Z.F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR effect: Combined strategies to improve the EPR effect in the tumor microenvironment. Theranostics 2019, 9, 8073. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Zhao, G.; Yin, J.; Ouyang, X.; Wang, Y.; Yang, C.; Wang, B.; Dong, P.; Wang, Z.; Watari, H.; et al. Lentiviral CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the epithelial to mesenchymal transition in ovarian cancer cells. J. Cancer 2017, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Maggio, I.; Zittersteijn, H.A.; Wang, Q.; Liu, J.; Janssen, J.M.; Ojeda, I.T.; van der Maarel, S.M.; Lankester, A.C.; Hoeben, R.C.; Gonçalves, M. Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components. Gene Ther. 2020, 27, 209–225. [Google Scholar] [CrossRef]
- Zununi Vahed, S.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1327–1341. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Jin, Z.; Chenghao, Y.; Cheng, P. Anticancer effect of tanshinones on female breast cancer and gynecological cancer. Front. Pharmacol. 2022, 12, 4144. [Google Scholar] [CrossRef]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022, 113, 2224–2231. [Google Scholar] [CrossRef]
- Hong, K.; Drummond, D.C.; Kirpotin, D. Liposomes Useful for Drug Delivery. U.S. Patent US20160030341A1, 4 February 2016. [Google Scholar]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Sis, M.J.; Webber, M.J. Drug delivery with designed peptide assemblies. Trends Pharmacol. Sci. 2019, 40, 747–762. [Google Scholar] [CrossRef]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mai, R.; Zhang, C.; Yu, D.; Ren, Y.; Li, G.; Cheng, B.; Li, L.; Yu, Z.; Chen, J. Discovery of novel cell-penetrating and tumor-targeting peptide-drug conjugate (PDC) for programmable delivery of paclitaxel and cancer treatment. Eur. J. Med. Chem. 2021, 213, 113050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Hu, Y.; Jiang, K.; Li, Z.; Lin, Y.-Z.; Wei, G.; Lu, W. Cell-permeable NF-κB inhibitor-conjugated liposomes for treatment of glioma. J. Control. Release 2018, 289, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, K.; Sánchez-Navarro, M.; Tapia-Arellano, A.; Giralt, E.; Kogan, M.J.; Araya, E. Oligoarginine peptide conjugated to bsa improves cell penetration of gold nanorods and nanoprisms for biomedical applications. Pharmaceutics 2021, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, İ.; İbrahim, M. Liposome–ligand conjugates: A review on the current state of art. J. Drug Target. 2020, 28, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Cole, A.; Huang, D.; Wang, Q.; Guo, Z.; Yang, W.; Lu, J. Clinical significance of hepsin and underlying signaling pathways in prostate cancer. Biomolecules 2022, 12, 203. [Google Scholar] [CrossRef]
- Kang, M.H.; Park, M.J.; Yoo, H.J.; Lee, S.G.; Kim, S.R.; Yeom, D.W.; Kang, M.J.; Choi, Y.W. RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes for enhanced intracellular drug delivery to hepsin-expressing cancer cells. Eur. J. Pharm. Biopharm. 2014, 87, 489–499. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, L.; Zhang, L.; Shi, K.; Cun, X.; Yang, Y.; Liu, Y.; Gao, H.; He, Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. 2016, 6, 19800. [Google Scholar] [CrossRef]
- Dellière, S.; Rivero-Menendez, O.; Gautier, C.; Garcia-Hermoso, D.; Alastruey-Izquierdo, A.; Alanio, A. Emerging mould infections: Get prepared to meet unexpected fungi in your patient. Med. Mycol. 2020, 58, 156–162. [Google Scholar]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar] [CrossRef]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Breitkopf, R.; Treml, B.; Rajsic, S. Invasive Fungal Infections after Liver Transplantation. J. Clin. Med. 2023, 12, 3238. [Google Scholar] [CrossRef] [PubMed]
- Scolarici, M.; Jorgenson, M.; Saddler, C.; Smith, J. Fungal infections in liver transplant recipients. J. Fungi 2021, 7, 524. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Vahedi-Shahandashti, R.; Dietl, A.M.; Binder, U.; Nagl, M.; Würzner, R.; Lass-Flörl, C. Aspergillus terreus and the Interplay with Amphotericin B: From Resistance to Tolerance? Antimicrob. Agents Chemother. 2022, 66, e0227421. [Google Scholar] [CrossRef] [PubMed]
- Paajanen, J.; Halme, M.; Palomäki, M.; Anttila, V.-J. Disseminated Scedosporium apiospermum central nervous system infection after lung transplantation: A case report with successful recovery. Med. Mycol. Case Rep. 2019, 24, 37–40. [Google Scholar] [CrossRef]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and other polyenes—Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Readio, J.D.; Bittman, R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim. Et Biophys. Acta-Biomembr. 1982, 685, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.J. Amphotericin B formulations: A comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics 2023, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mei, Z.; Mei, L.; Tang, J.; Yuan, W.; Srinivasan, S.; Ackermann, R.; Schwendeman, A.S. Analytical method development and comparability study for AmBisome® and generic Amphotericin B liposomal products. Eur. J. Pharm. Biopharm. 2020, 157, 241–249. [Google Scholar] [CrossRef]
- Adler-Moore, J.P.; Proffitt, R.T. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J. Liposome Res. 1993, 3, 429–450. [Google Scholar] [CrossRef]
- Walsh, T.J.; Yeldandi, V.; McEvoy, M.; Gonzalez, C.; Chanock, S.; Freifeld, A.; Seibel, N.I.; Whitcomb, P.O.; Jarosinski, P.; Boswell, G. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 1998, 42, 2391–2398. [Google Scholar] [CrossRef]
- Sousa, F.; Nascimento, C.; Ferreira, D.; Reis, S.; Costa, P. Reviving the interest in the versatile drug nystatin: A multitude of strategies to increase its potential as an effective and safe antifungal agent. Adv. Drug Deliv. Rev. 2023, 199, 114969. [Google Scholar] [CrossRef]
- Anim, V.C.E. Antifungal drug therapy in avian species. Vet. Clin. Exot. Anim. Pract. 2003, 6, 337–350. [Google Scholar]
- Belakhov, V.; Garabadzhiu, A.; Chistyakova, T. Polyene Macrolide Antibotic Derivatives: Preparation, Overcoming Drug Resistance, and Prospects for Use in Medical Practice. Pharm. Chem. J. 2019, 52, 890–901. [Google Scholar] [CrossRef]
- Johnson, E.M.; Ojwang, J.O.; Szekely, A.; Wallace, T.L.; Warnock, D.W. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob. Agents Chemother. 1998, 42, 1412–1416. [Google Scholar] [CrossRef]
- Krishna, S.S.; Sudheesh, M.; Viswanad, V. Liposomal drug delivery to the lungs: A post COVID-19 scenario. J. Liposome Res. 2023, 33, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Nano-delivery to the lung-by inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Sarvaiya, J.; Mohindroo, P. A drift on liposomes to proliposomes: Recent advances and promising approaches. J. Liposome Res. 2022, 32, 317–331. [Google Scholar] [CrossRef]
- Vuong, N.N.; Hammond, D.; Kontoyiannis, D.P. Clinical Uses of Inhaled Antifungals for Invasive Pulmonary Fungal Disease: Promises and Challenges. J. Fungi 2023, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Alabdullah, M.N.; Yousfan, A. Is low dose of liposomal amphotericin B effective in management of acute invasive fungal rhinosinusitis? Our conclusions from Al-Mowassat University Hospital, Syria: A prospective observational study. BMC Infect. Dis. 2023, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Muthu, V.; Gogineni, R.R.; Agarwal, R.; Prasad, K.T.; Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Rudramurthy, S.M.; Singh, H.; Garg, M. Treatment of pulmonary mucormycosis with adjunctive nebulized amphotericin B (MUCONAB trial): Results of an open-label randomized controlled trial. Mycoses 2023, 66, 688–696. [Google Scholar] [CrossRef]
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.; Kumar, D.; Bolás, F.; Ballesteros, M. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788. [Google Scholar] [CrossRef]
- Celi, S.S.; Fernández-García, R.; Afonso-Urich, A.I.; Ballesteros, M.P.; Healy, A.M.; Serrano, D.R. Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics 2023, 15, 2601. [Google Scholar] [CrossRef]
- Saalbach, K.P. Nasal and pulmonary routes of drug delivery. In Novel Platforms for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 569–606. [Google Scholar]
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52. [Google Scholar] [CrossRef]
- El-Tallawy, S.N.; Nalamasu, R.; Pergolizzi, J.V.; Gharibo, C. Pain management during the COVID-19 pandemic. Pain Ther. 2020, 9, 453–466. [Google Scholar] [CrossRef]
- Hollmann, M.W.; Rathmell, J.P.; Lirk, P. Optimal postoperative pain management: Redefining the role for opioids. Lancet 2019, 393, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Imani, F.; Zaman, B.; De Negri, P. Postoperative pain management: Role of dexmedetomidine as an adjuvant. Anesthesiol. Pain Med. 2020, 10, e112176. [Google Scholar] [CrossRef]
- Ghai, B.; Jafra, A.; Bhatia, N.; Chanana, N.; Bansal, D.; Mehta, V. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: A narrative review. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 3. [Google Scholar] [PubMed]
- Gu, J.-H.; Liu, C.-C.; Xie, J.-L.; Ma, B.; Cui, S.-M.; Yang, G.-Z.; He, S.-C. The Local Anesthetic Bupivacaine Inhibits the Progression of Non-Small Cell Lung Cancer by Inducing Autophagy Through Akt/mTOR Signaling. Front. Oncol. 2021, 11, 616445. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Xu, X.; Wang, A. An injectable mesoporous silica-based analgesic delivery system prolongs the duration of sciatic nerve block in mice with minimal toxicity. Acta Biomater. 2021, 135, 638–649. [Google Scholar] [CrossRef]
- Podgorski, E.; Driver, L.; Gulati, A.; Abdi, S. Catheter-based techniques for terminal cancer pain: A review of nonneuraxial interventions with clinical implications for end-of-life pain management. Pain Physician 2021, 24, E1137. [Google Scholar]
- Patel, S.; Habib, A. Anaesthesia for the parturient with obesity. BJA Educ. 2021, 21, 180. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Label Approved on 10/28/2011 (PDF) for EXPAREL; US Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Davidson, E.M.; Barenholz, Y.; Cohen, R.; Haroutiunian, S.; Kagan, L.; Ginosar, Y. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth. Analg. 2010, 110, 1018–1023. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163. [Google Scholar] [CrossRef]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a therapeutic target: Evidence of its neuroprotective and neuromodulatory function in Parkinson’s disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef]
- Boyaji, S.; Merkow, J.; Elman, R.N.M.; Kaye, A.D.; Yong, R.J.; Urman, R.D. The role of cannabidiol (CBD) in chronic pain management: An assessment of current evidence. Curr. Pain Headache Rep. 2020, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E.; Bialer, M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs 2020, 34, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation-mediated cancer signaling. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 1086–1104. [Google Scholar]
- Shilo-Benjamini, Y.; Cern, A.; Zilbersheid, D.; Hod, A.; Lavy, E.; Barasch, D.; Barenholz, Y. A case report of subcutaneously injected liposomal cannabidiol formulation used as a compassion therapy for pain management in a dog. Front. Vet. Sci. 2022, 9, 892306. [Google Scholar] [CrossRef] [PubMed]
- Blair, E. Next generation of liposomal delivery for cannabidiol from a hemp extract: A safety study. Am. J. Endocan. Med. 2019, 1, 20–22. [Google Scholar]
- Canouï, E.; Launay, O. History and principles of vaccination. Rev. Des Mal. Respir. 2019, 36, 74–81. [Google Scholar] [CrossRef]
- Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Narasimhan, B.; Mallapragada, S.K. Respiratory nanoparticle-based vaccines and challenges associated with animal models and translation. J. Control. Release 2015, 219, 622–631. [Google Scholar] [CrossRef]
- Song, C.; Li, F.; Wang, S.; Wang, J.; Wei, W.; Ma, G. Recent advances in particulate adjuvants for cancer vaccination. Adv. Ther. 2020, 3, 1900115. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Q.; Zou, Y.; Miao, C.; Ma, G.; Wu, J. Chitosan particle-emulsion complex adjuvants: The effect of particle distribution on the immune intensity and response type. Carbohydr. Polym. 2023, 309, 120673. [Google Scholar] [CrossRef]
- Andersson, L.; Häyry, P.; Bach, M.; Bach, J. Differences in the effects of adult thymectomy on T-cell mediated responses in vitro. Nature 1974, 252, 252–254. [Google Scholar] [CrossRef]
- Tretiakova, D.; Vodovozova, E. Liposomes as adjuvants and vaccine delivery systems. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef]
- Di, J.; Xie, F.; Xu, Y. When liposomes met antibodies: Drug delivery and beyond. Adv. Drug Deliv. Rev. 2020, 154, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Fahrmann, J.F.; Hanash, S.M.; Vykoukal, J. Extracellular vesicles mediate B cell immune response and are a potential target for cancer therapy. Cells 2020, 9, 1518. [Google Scholar] [CrossRef] [PubMed]
- van Dissel, J.T.; Joosten, S.A.; Hoff, S.T.; Soonawala, D.; Prins, C.; Hokey, D.A.; O’Dee, D.M.; Graves, A.; Thierry-Carstensen, B.; Andreasen, L.V. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 2014, 32, 7098–7107. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.J.; Wilkinson, A.; Bramwell, V.W.; Christensen, D.; Perrie, Y. Th1 immune responses can be modulated by varying dimethyldioctadecylammonium and distearoyl-sn-glycero-3-phosphocholine content in liposomal adjuvants. J. Pharm. Pharmacol. 2014, 66, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Chand, M.; Brown, K.; Ladhani, S.N. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv 2021. [Google Scholar] [CrossRef]
- van den Berg, A.I.; Yun, C.-O.; Schiffelers, R.M.; Hennink, W.E. Polymeric delivery systems for nucleic acid therapeutics: Approaching the clinic. J. Control. Release 2021, 331, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.A.; Essa, E.A.; Elebyary, T.T.; Faheem, A.M.; Elkordy, A.A. Brief on recent application of liposomal vaccines for lower respiratory tract viral infections: From influenza to COVID-19 vaccines. Pharmaceuticals 2021, 14, 1173. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y. Multi-functional liposome: A powerful theranostic nano-platform enhancing photodynamic therapy. Adv. Sci. 2021, 8, 2100876. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Y.; Van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Anju, V.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef]
- Sforzi, J.; Palagi, L.; Aime, S. Liposome-based bioassays. Biology 2020, 9, 202. [Google Scholar] [CrossRef]
- Hernandez, C.; Shukla, S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1190. [Google Scholar]
- Che, J.; Najer, A.; Blakney, A.K.; McKay, P.F.; Bellahcene, M.; Winter, C.W.; Sintou, A.; Tang, J.; Keane, T.J.; Schneider, M.D. Neutrophils enable local and non-invasive liposome delivery to inflamed skeletal muscle and ischemic heart. Adv. Mater. 2020, 32, 2003598. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lin, Y.-C.; Huang, C.-Y.; Wu, S.-R.; Chen, C.-M.; Liu, H.-L. Ultrasound-responsive neurotrophic factor-loaded microbubble-liposome complex: Preclinical investigation for Parkinson’s disease treatment. J. Control. Release 2020, 321, 519–528. [Google Scholar] [CrossRef]
- Kahana, M.; Weizman, A.; Gabay, M.; Loboda, Y.; Segal-Gavish, H.; Gavish, A.; Barhum, Y.; Offen, D.; Finberg, J.; Allon, N. Liposome-based targeting of dopamine to the brain: A novel approach for the treatment of Parkinson’s disease. Mol. Psychiatry 2021, 26, 2626–2632. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, S.; Singh, M.R.; Singh, D.; Tekwani, B.L. Chapter 11—Novel perspectives for delivery of bioactives through blood–brain barrier and treatment of brain diseases. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 317–341. [Google Scholar]
- Chen, Z.-J.; Yang, S.-C.; Liu, X.-L.; Gao, Y.; Dong, X.; Lai, X.; Zhu, M.-H.; Feng, H.-Y.; Zhu, X.-D.; Lu, Q. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020, 20, 4177–4187. [Google Scholar] [CrossRef]
- Rommasi, F.; Esfandiari, N. Liposomal nanomedicine: Applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P. Liposomal nanostructures: Properties and applications. In Nanoscale Processing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 163–179. [Google Scholar]
- Magar, K.T.; Boafo, G.F.; Li, X.; Chen, Z.; He, W. Liposome-based delivery of biological drugs. Chin. Chem. Lett. 2022, 33, 587–596. [Google Scholar] [CrossRef]
- Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon 2022, 8, e08934. [Google Scholar]
- Ferrer, J.R.; Sinegra, A.J.; Ivancic, D.; Yeap, X.Y.; Qiu, L.; Wang, J.-J.; Zhang, Z.J.; Wertheim, J.A.; Mirkin, C.A. Structure-dependent biodistribution of liposomal spherical nucleic acids. ACS Nano 2020, 14, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.S.; Fernandes, R.S.; Cavalcante, C.H.; da Costa César, I.; Leite, E.A.; Lopes, S.C.A.; Ferretti, A.; Rubello, D.; Townsend, D.M.; de Oliveira, M.C. Influence of PEG coating on the biodistribution and tumor accumulation of pH-sensitive liposomes. Drug Deliv. Transl. Res. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Dong, T.; Phung, A.T.; Shah, J.R.; Larson, C.; Sanchez, A.B.; Blair, S.L.; Oronsky, B.; Trogler, W.C.; Reid, T. Full Remission of CAR-Deficient Tumors by DOTAP-Folate Liposome Encapsulation of Adenovirus. ACS Biomater. Sci. Eng. 2022, 8, 5199–5209. [Google Scholar] [CrossRef]
- Guan, J.; Guo, H.; Tang, T.; Wang, Y.; Wei, Y.; Seth, P.; Li, Y.; Dehm, S.M.; Ruoslahti, E.; Pang, H.B. iRGD-liposomes enhance tumor delivery and therapeutic efficacy of antisense oligonucleotide drugs against primary prostate cancer and bone metastasis. Adv. Funct. Mater. 2021, 31, 2100478. [Google Scholar] [CrossRef]
- Khadke, S.; Roces, C.B.; Cameron, A.; Devitt, A.; Perrie, Y. Formulation and manufacturing of lymphatic targeting liposomes using microfluidics. J. Control. Release 2019, 307, 211–220. [Google Scholar] [CrossRef]
- Moon, D.; Park, H.; Hwang, I.; Cha, A.; Yun, H.; Lee, J.; Park, S.H.; Lee, E.J.; Kim, H.S. Smart gene therapeutics for selective targeting of myofibroblasts derived from hepatic stellate cells and limited expression under inflamed conditions. Clin. Transl. Med. 2022, 12, e991. [Google Scholar] [CrossRef]
- Patel, U.; Rathnayake, K.; Jani, H.; Jayawardana, K.W.; Dhakal, R.; Duan, L.; Jayawardena, S.N. Near-infrared responsive targeted drug delivery system that offer chemo-photothermal therapy against bacterial infection. Nano Sel. 2021, 2, 1750–1769. [Google Scholar] [CrossRef]
- Almeida, B.; Nag, O.K.; Rogers, K.E.; Delehanty, J.B. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules 2020, 25, 5672. [Google Scholar] [CrossRef] [PubMed]
- Moosavian, S.A.; Kesharwani, P.; Singh, V.; Sahebkar, A. Aptamer-functionalized liposomes for targeted cancer therapy. Aptamers Eng. Nanocarriers Cancer Ther. 2023, 448, 141–172. [Google Scholar]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893. [Google Scholar] [CrossRef]
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557. [Google Scholar] [CrossRef]
- de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; Cassali, G.D.; de Paula Sabino, A.; Townsend, D.M.; Oliveira, M.C.; de Barros, A.L.B. Evaluation of acute toxicity and in vitro antitumor activity of a novel doxorubicin-loaded folate-coated pH-sensitive liposome. Biomed. Pharmacother. 2023, 165, 115280. [Google Scholar] [CrossRef]
- Oros-Pantoja, R.; Córdoba-Adaya, J.C.; Torres-García, E.; Morales-Avila, E.; Aranda-Lara, L.; Santillán-Benítez, J.G.; Sánchez-Holguín, M.; Hernández-Herrera, N.O.; Otero, G.; Isaac-Olivé, K. Preclinical evaluation of early multi-organ toxicity induced by liposomal doxorubicin using 67Ga-citrate. Nanotoxicology 2022, 16, 247–264. [Google Scholar] [CrossRef]
- Ranjbar, S.; Zhong, X.-B.; Manautou, J.; Lu, X. A holistic analysis of the intrinsic and delivery-mediated toxicity of siRNA therapeutics. Adv. Drug Deliv. Rev. 2023, 201, 115052. [Google Scholar] [CrossRef]
- Batist, G.; Ramakrishnan, G.; Rao, C.S.; Chandrasekharan, A.; Gutheil, J.; Guthrie, T.; Shah, P.; Khojasteh, A.; Nair, M.K.; Hoelzer, K. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001, 19, 1444–1454. [Google Scholar] [CrossRef]


| Advantages of Liposome: |
|---|
|
|
|
|
|
| Name of the Phospholipids | R-Group |
|---|---|
| Phosphatidic acid | -H |
| Phosphatidylethanolamine | -CH2-CH2-NH3+ |
| Phosphatidylglycerol | -CH2-CHOH-CH2-OH |
| Phosphatidylcholine | -CH2-CH2-N(CH3)3+ |
| Phosphatidylserine | -CH2-CH-NH2COOH |
| Phosphatidylinositol |  |
| Name | Clinical Approval Year | Liposomal Composition | Drug Encapsulated | Drug Type | Route of Administration | Company | References |
|---|---|---|---|---|---|---|---|
| Doxil | 1995 | HSPC:Cholesterol:DSPE-PEG2000 | Doxorubicin | Chemotherapeutic | I.V. | Johnson & Johnson, Milpitas, CA, USA | [101,102] |
| Abelcet | 1995 | DMPC:DMPG | Amphotericin B | Antifungal | I.V. | Leadiant Biosciences. Inc., Rockville, MD, USA | [103,104] |
| DaunoXome | 1996 | DSPC:Cholesterol | Daunorubicin | Chemotherapeutic | I.V. | Galen US, Inc., Souderton, PA, USA | [101,105] |
| Amphotec | 1996 | Cholesteryl sulphate:Amphotericin B | Amphotericin B | Antifungal | I.V. | Sequus Pharmaceuticals Inc., Menlo Park, CA, USA | [101] |
| Inflexal V | 1997 | 70% Lecithin, 20% Cephalin and 10% Phospholipids | Influenza virus antigen, strain A and B | Vaccine | I.M. | Sun Pharmaceutical Industries Ltd., Princeton, NJ, USA | [101,106] |
| Ambisome | 1997 | HSPC:DSPG:Cholesterol:Amphotericin B | Amphotericin B | Antifungal | I.V. | Fujisawa Healthcare, Inc. and Gilead Sciences, Inc., Foster City, CA, USA | [101] |
| Myocet | 2000 | EPG:Cholesterol | Doxorubicin | Chemotherapeutic | I.V. | Zeneus Pharma Ltd., Oxford, UK | [101,107] |
| Visudyne | 2000 | Verteporfin:DMPC and EPG | Verteporfin | Photosensitizer | I.V. | Novartis International AG, Basel, Switzerland | [101] |
| DepoDur | 2004 | DOPC:DPPG:Cholesterol:Tricaprylin and Triolein | Morphine sulfate | Narcotic Analgesic | Epidural | Pacira Pharmaceuticals, Inc., Watford, UK | [101,108] |
| Mepact | 2004 | DOPS:POPC | Mifamurtide | Immunomodulator/Antitumor | I.V. | Takeda Pharmaceutical Limited, Tokyo, Japan | [101] |
| Exparel | 2011 | DEPC:DPPG:Cholesterol:Tricaprylin | Bupivacaine | Anesthetic | I.V. | Pacira Pharmaceuticals, Inc., Parsippany-Troy Hills, NJ, USA | [101] |
| Onivyde | 2015 | DSPC:MPEG-2000:DSPE | Irinotecan | Chemotherapeutic | I.V. | Merrimack Pharmaceuticals, Inc., Cambridge, MA, USA | [101,109] |
| Vyxeos | 2017 | DSPC:DSPG:Cholesterol | Daunorubicin + Cytarabine | Antineoplastic | I.V. | Jazz Pharmaceuticals, Inc., Dublin, Ireland | [110] |
| Onpattro | 2018 | Cholesterol, DLin-MC3-DMA:DSPC:PEG2000-C-DMG | Patisiran | RNAi agent | I.V. | Alnylam Pharmaceuticals, Cambridge, MA, USA | [111] |
| Comirnaty | 2021 | ALC-0315:ALC-0159:cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Pfizer-BioNTech, Mainz, Germany | [112] |
| Spikevax | 2022 | SM-102:mPEG2000-DMG:Cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Moderna, Cambridge, MA, USA | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics 2024, 16, 34. https://doi.org/10.3390/pharmaceutics16010034
Jiang Y, Li W, Wang Z, Lu J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics. 2024; 16(1):34. https://doi.org/10.3390/pharmaceutics16010034
Chicago/Turabian StyleJiang, Yanhao, Wenpan Li, Zhiren Wang, and Jianqin Lu. 2024. "Lipid-Based Nanotechnology: Liposome" Pharmaceutics 16, no. 1: 34. https://doi.org/10.3390/pharmaceutics16010034
APA StyleJiang, Y., Li, W., Wang, Z., & Lu, J. (2024). Lipid-Based Nanotechnology: Liposome. Pharmaceutics, 16(1), 34. https://doi.org/10.3390/pharmaceutics16010034






