Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
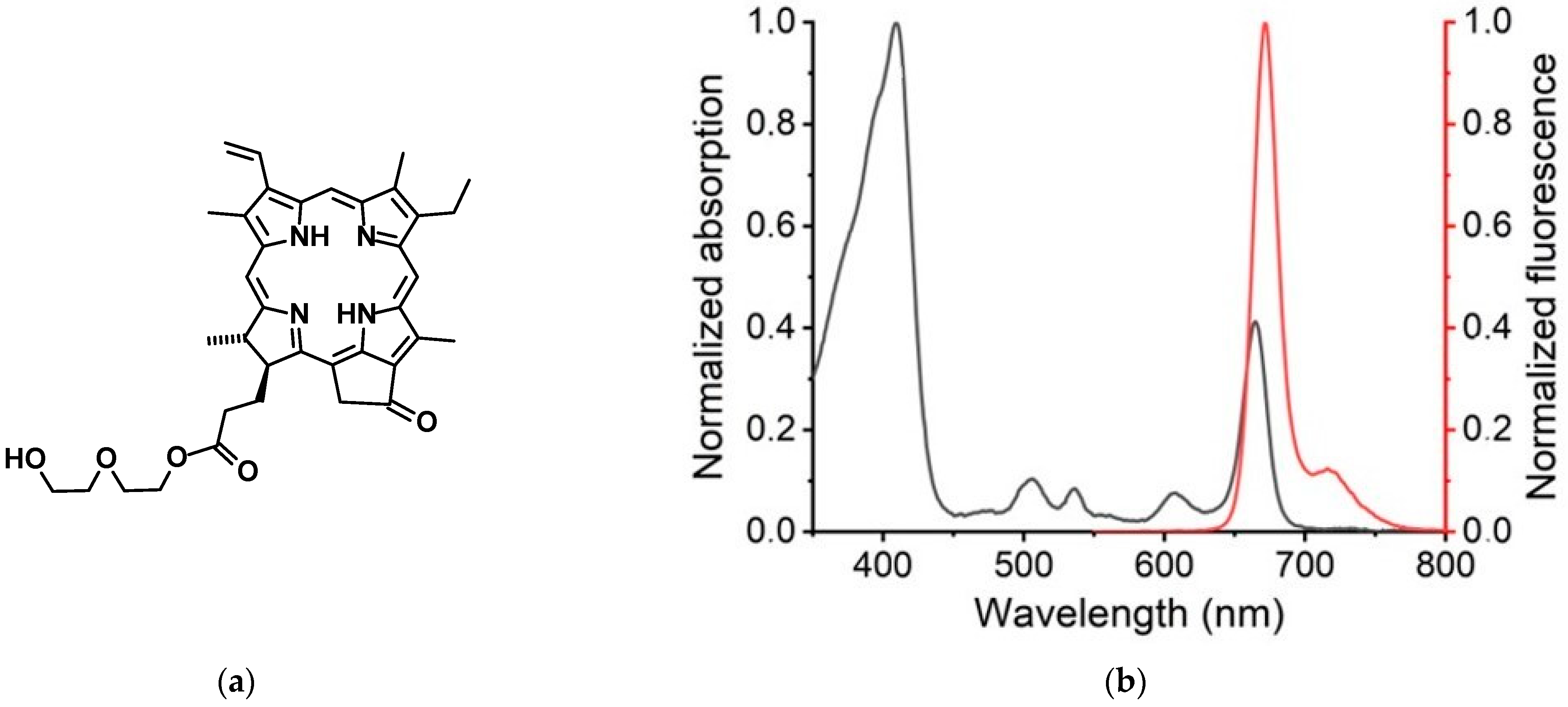
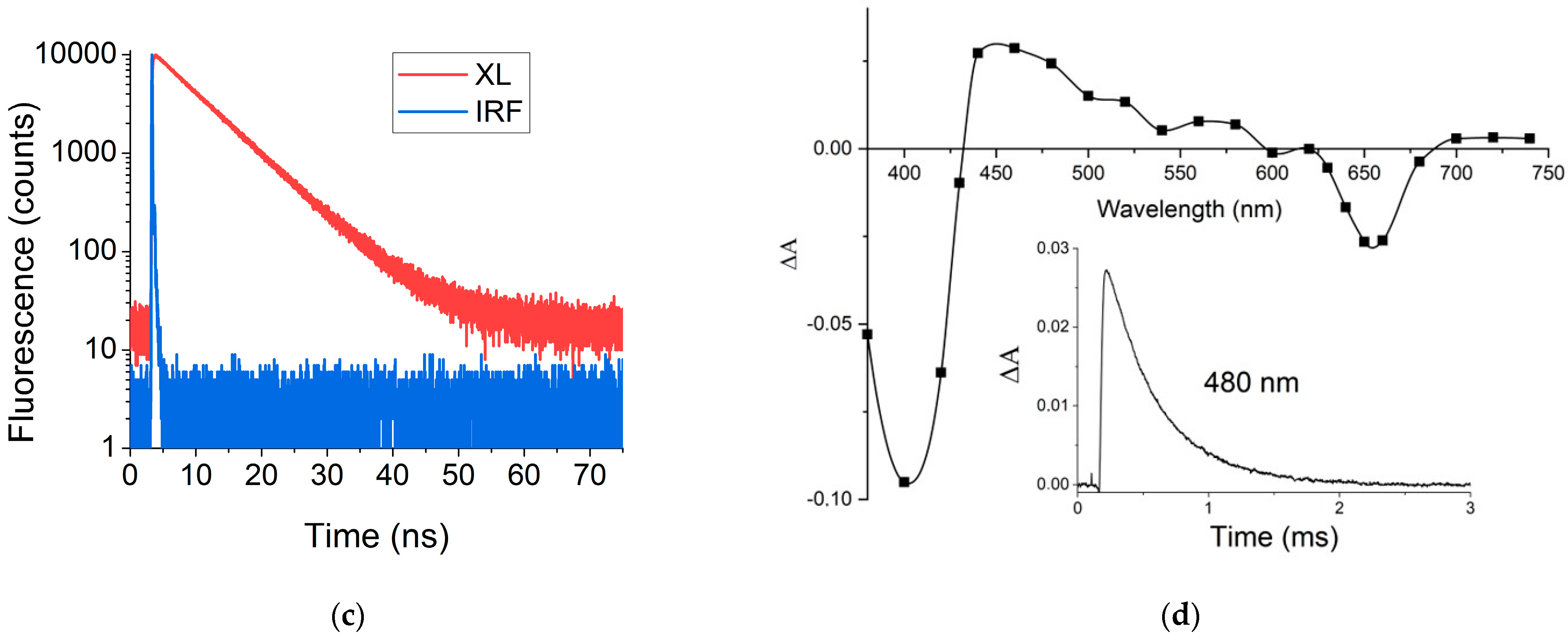
2.2.1. Photophysical Properties of XL
Fluorescence and Singlet Oxygen Measurements
Flash Photolysis
Laser Flash Photolysis
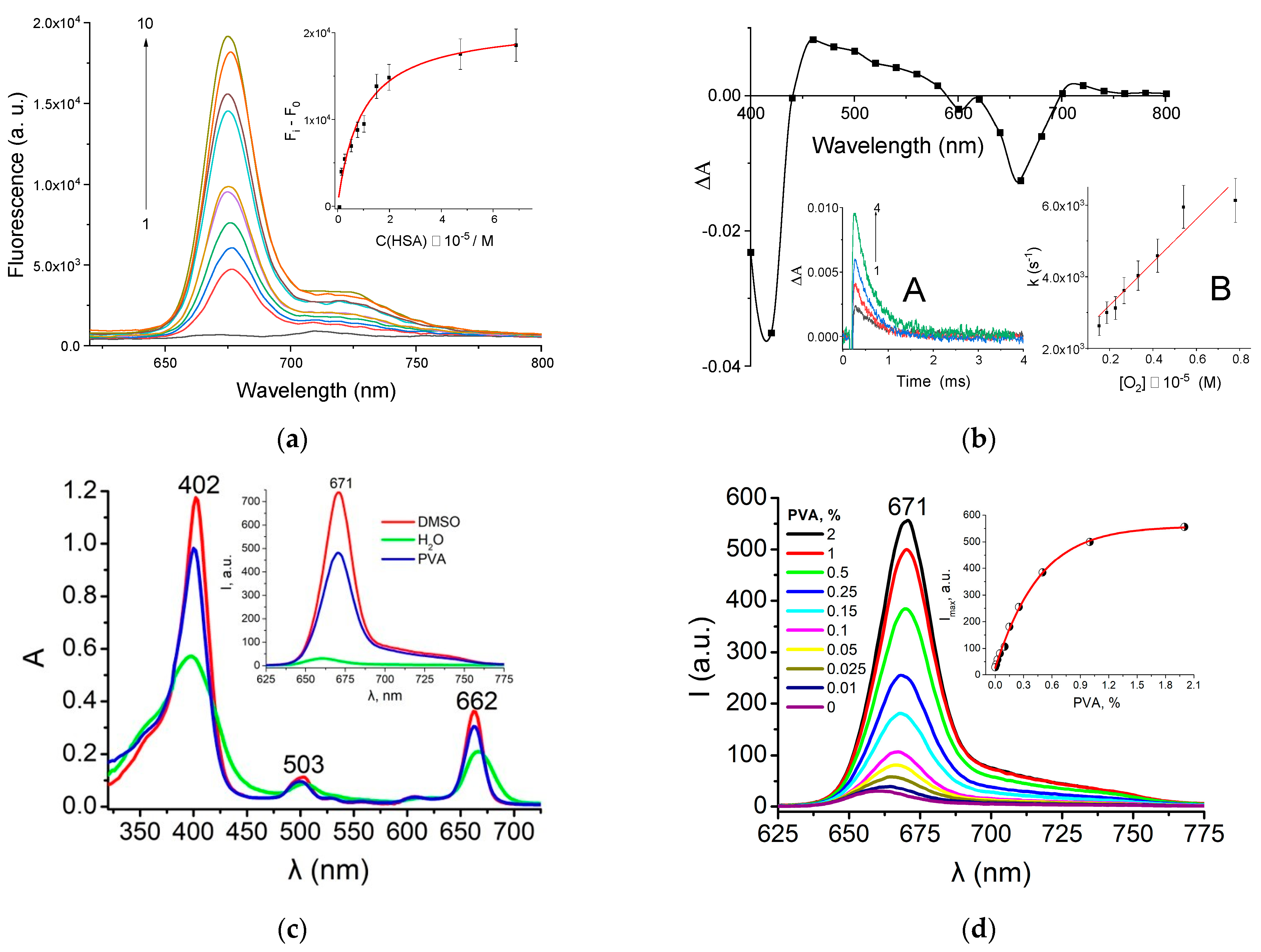
XL–HSA Complex Formation
2.2.2. XL Binding to PVA in Solution
2.2.3. Synthesis and Scale-Up of NP–XL
2.2.4. Characterization of NP–XL
Size, ζ-Potential-Potential, and Polydispersity Index Measurement
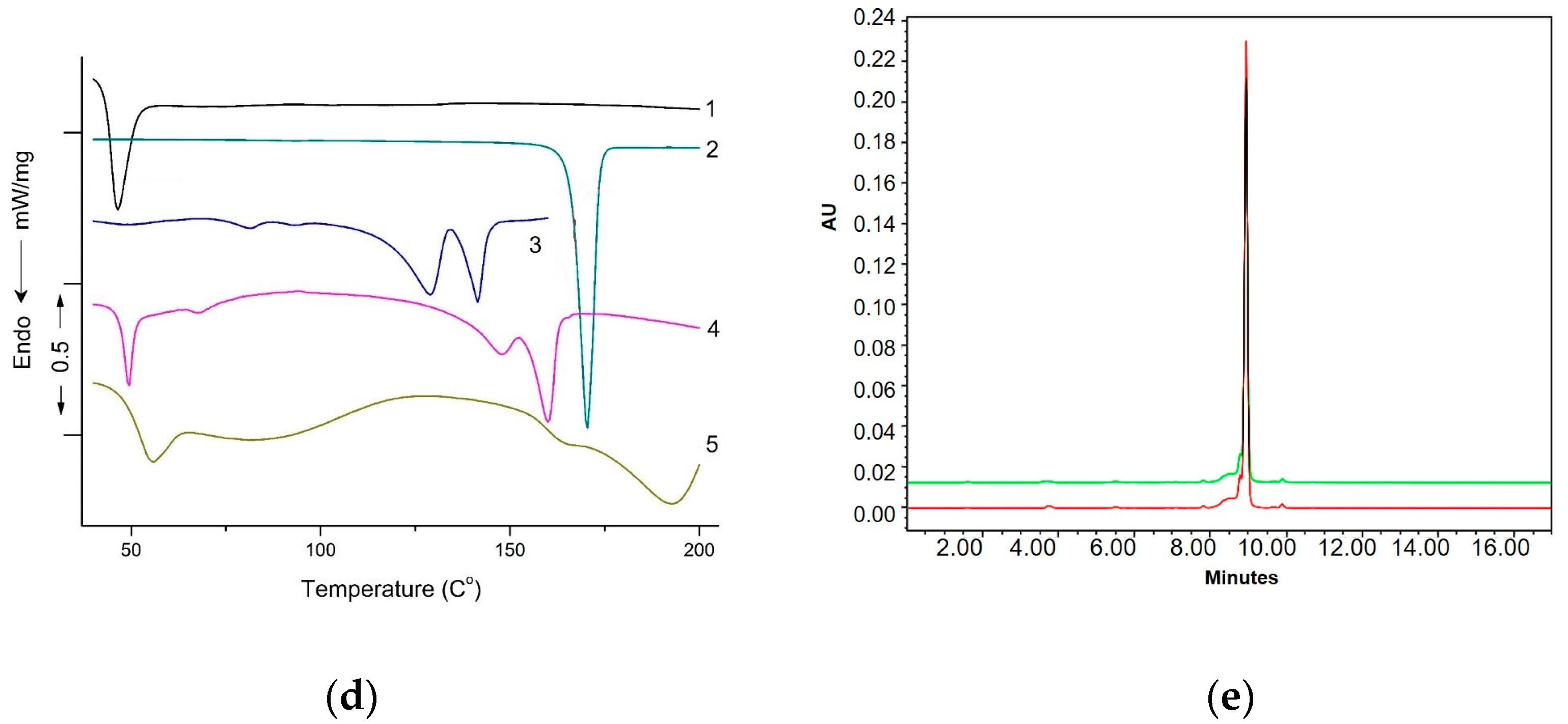
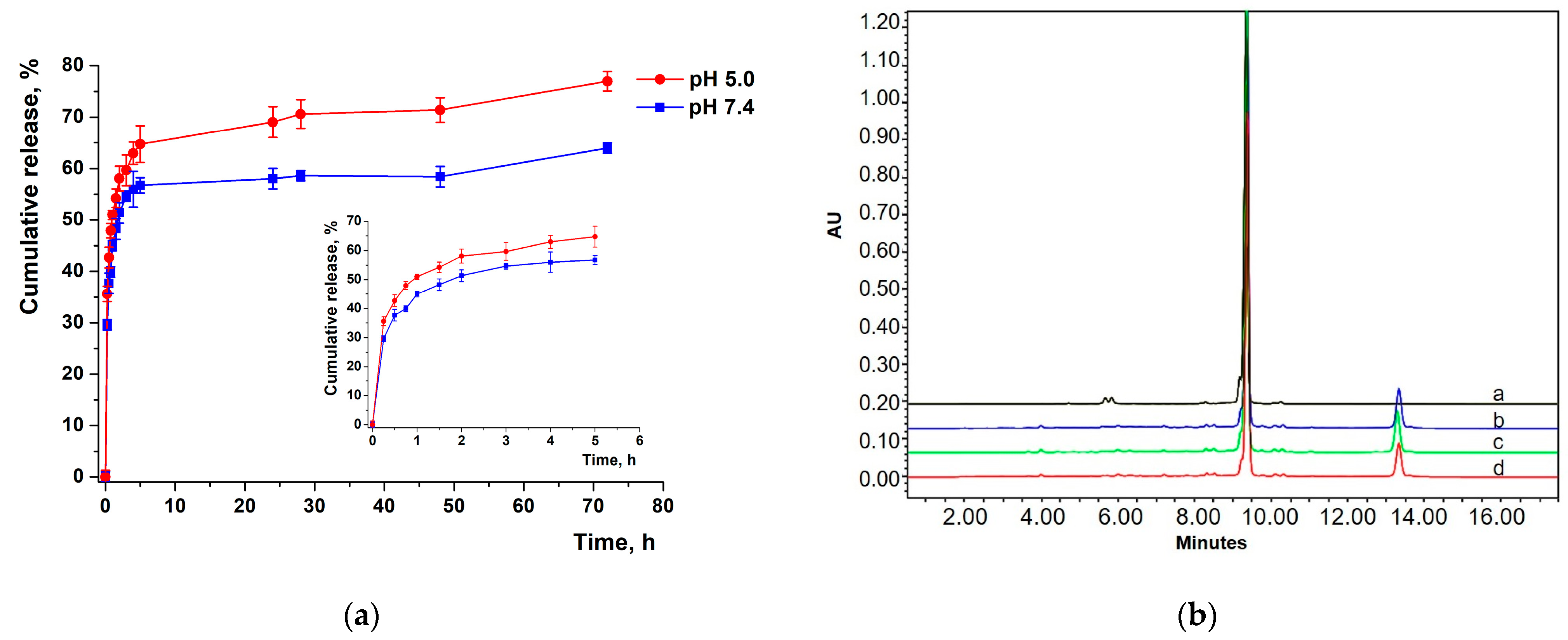
HPLC Analysis of XL
DL and EE Measurement
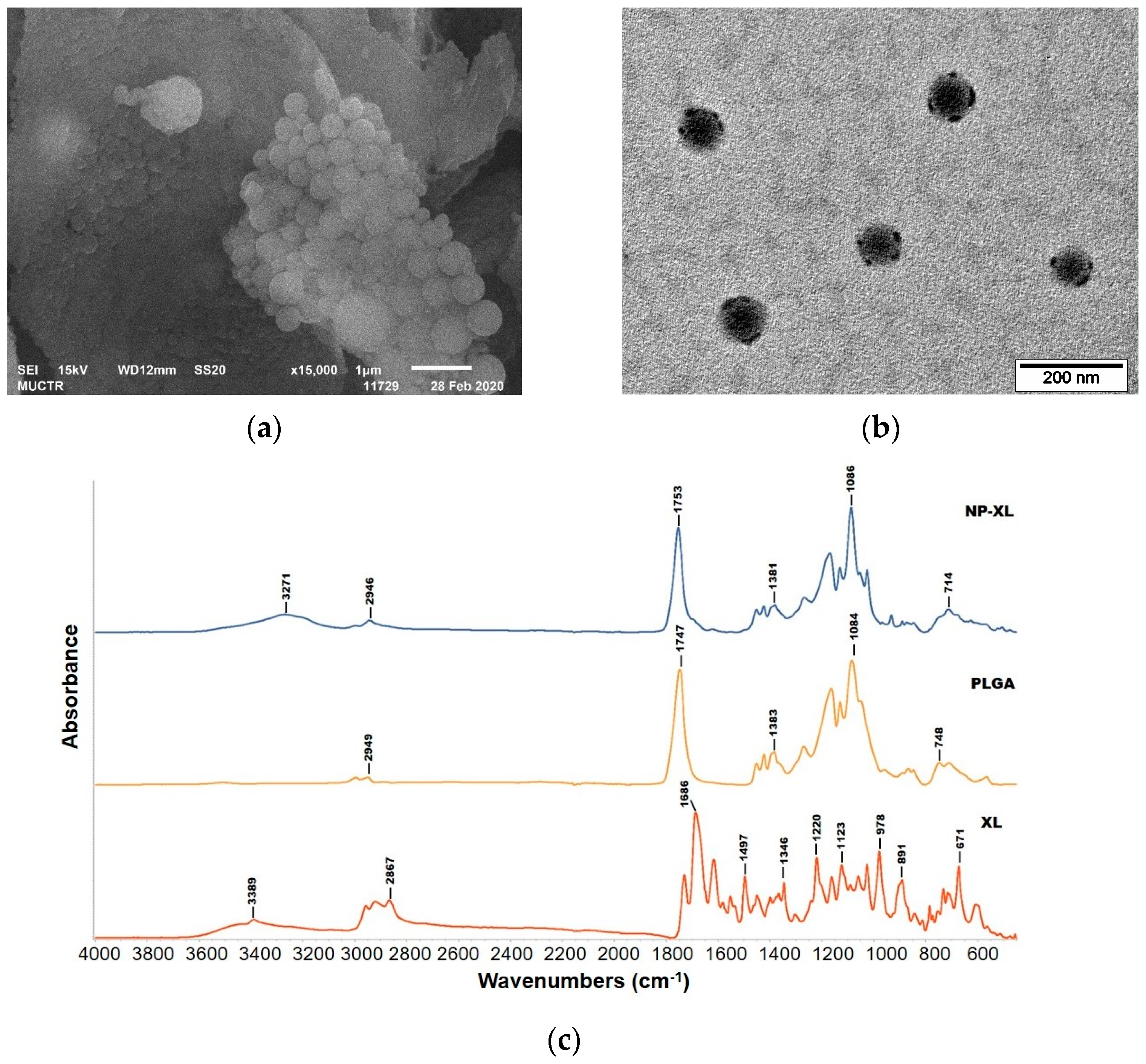
Morphological Study
Determination of PVA Residual Content
Fourier Transform Infrared Spectroscopy (FTIR)
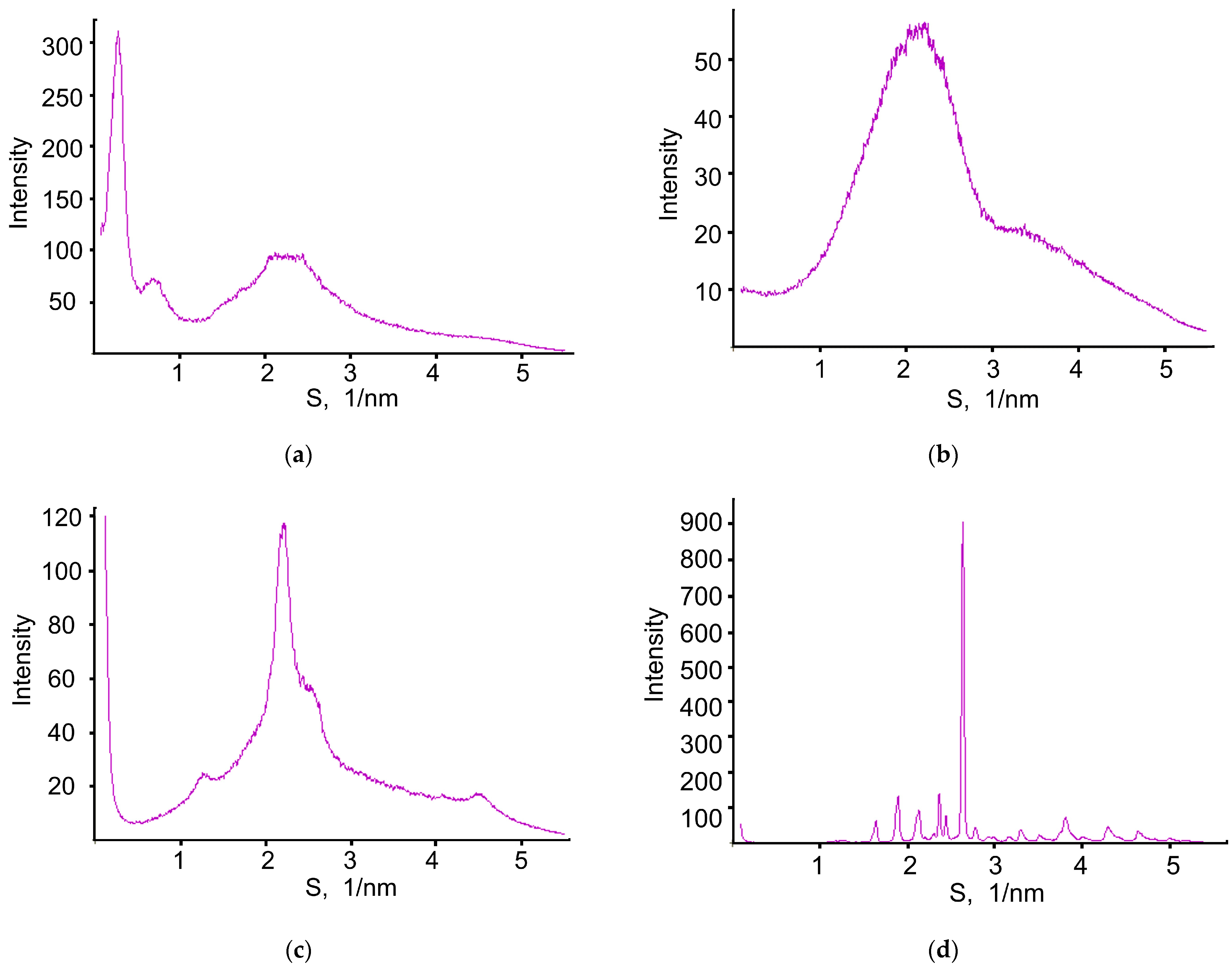
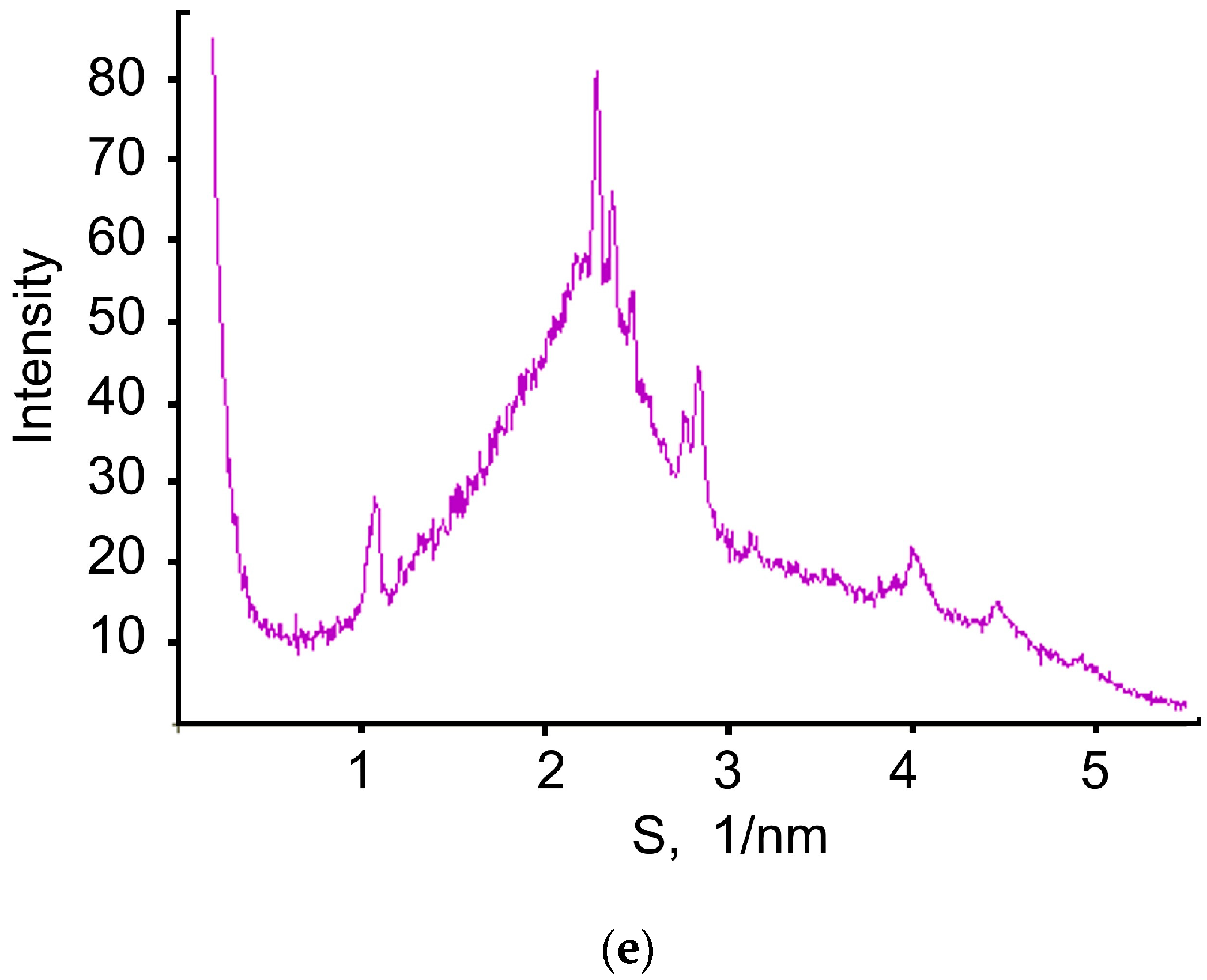
X-ray Diffraction (XRD)
Differential Scanning Calorimetry
2.2.5. Modeling of XL Release Kinetics
2.2.6. Sterilization
Gamma Irradiation
Membrane Filtration and Sterility Testing
2.2.7. Cell Culture
2.2.8. HeLa TI Cell-Based Assay
2.2.9. Cytotoxic Activity (2D)
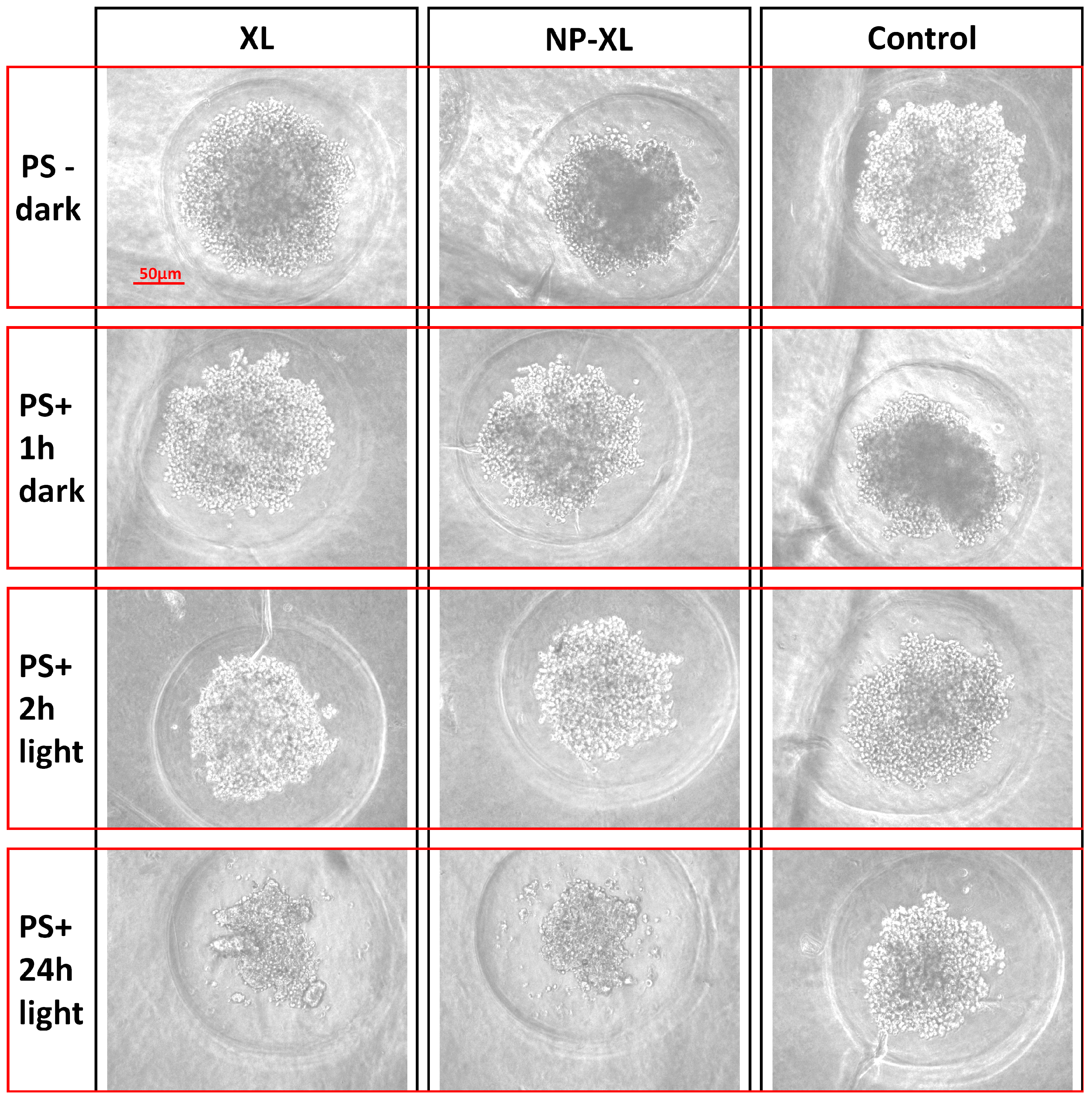
2.2.10. Growth of MDA-MB-231 Spheroids (3D) and Cytotoxic Activity
2.2.11. ROS and XL and NP–XL Subcellular Localization
2.2.12. Western Blotting
2.2.13. Data Analysis and Statistical Evaluation
3. Results and Discussion
3.1. Photophysical Properties of XL
3.2. XL–HSA Complex Formation
3.3. XL Binding with PVA
3.4. Synthesis and Scale-Up of NP–XL
3.5. Morphological Analysis
3.6. Determination of PVA Residual Content
3.7. Fourier Transform Infrared Spectroscopy
3.8. DSC Studies
- interactions between polymer and drugs,
- crystalline morphology of the polymer and the state of encapsulated drug (solid or molecular dispersion),
- intermolecular interactions between drugs and pharmaceutical adjuvants.
3.9. X-ray Diffraction Study
3.10. Modeling of XL Release Kinetics
3.11. Sterilization
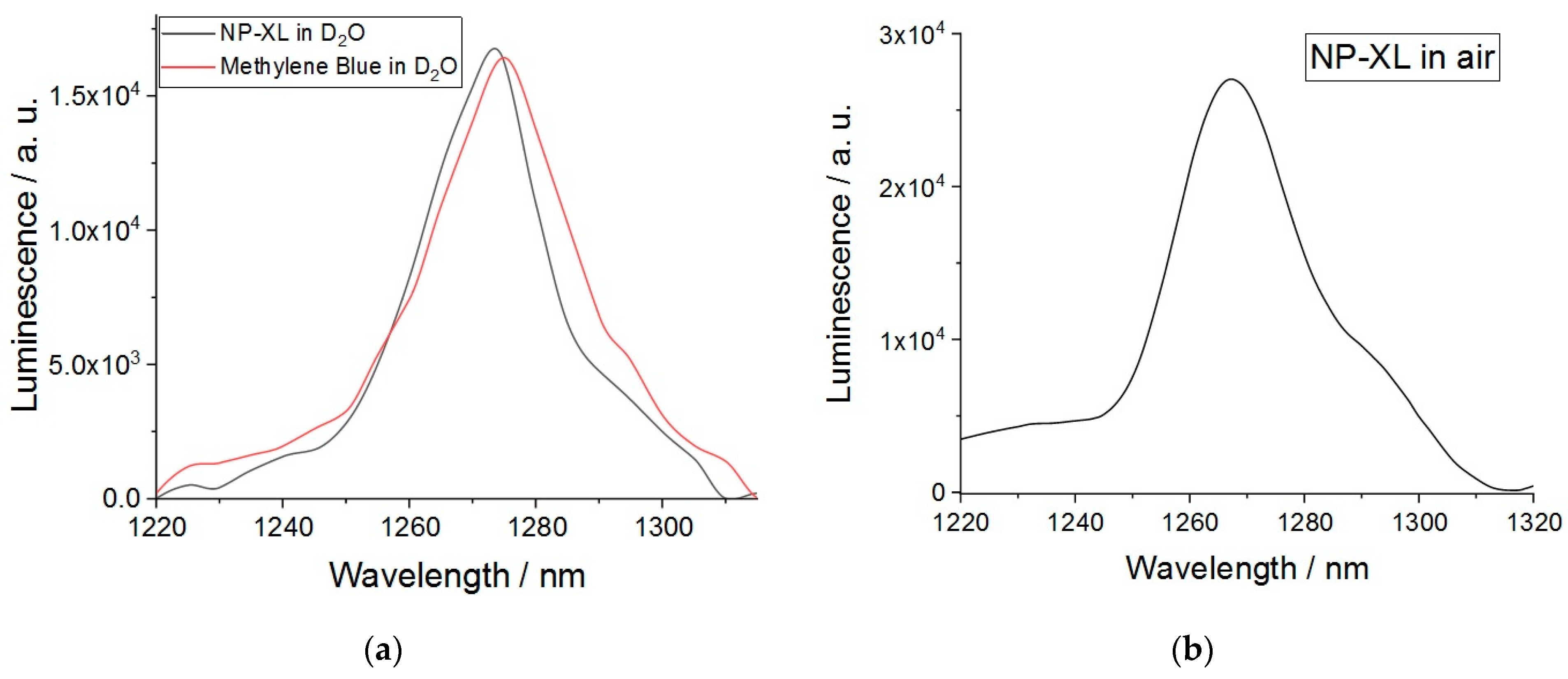
3.12. Singlet Oxygen Generation
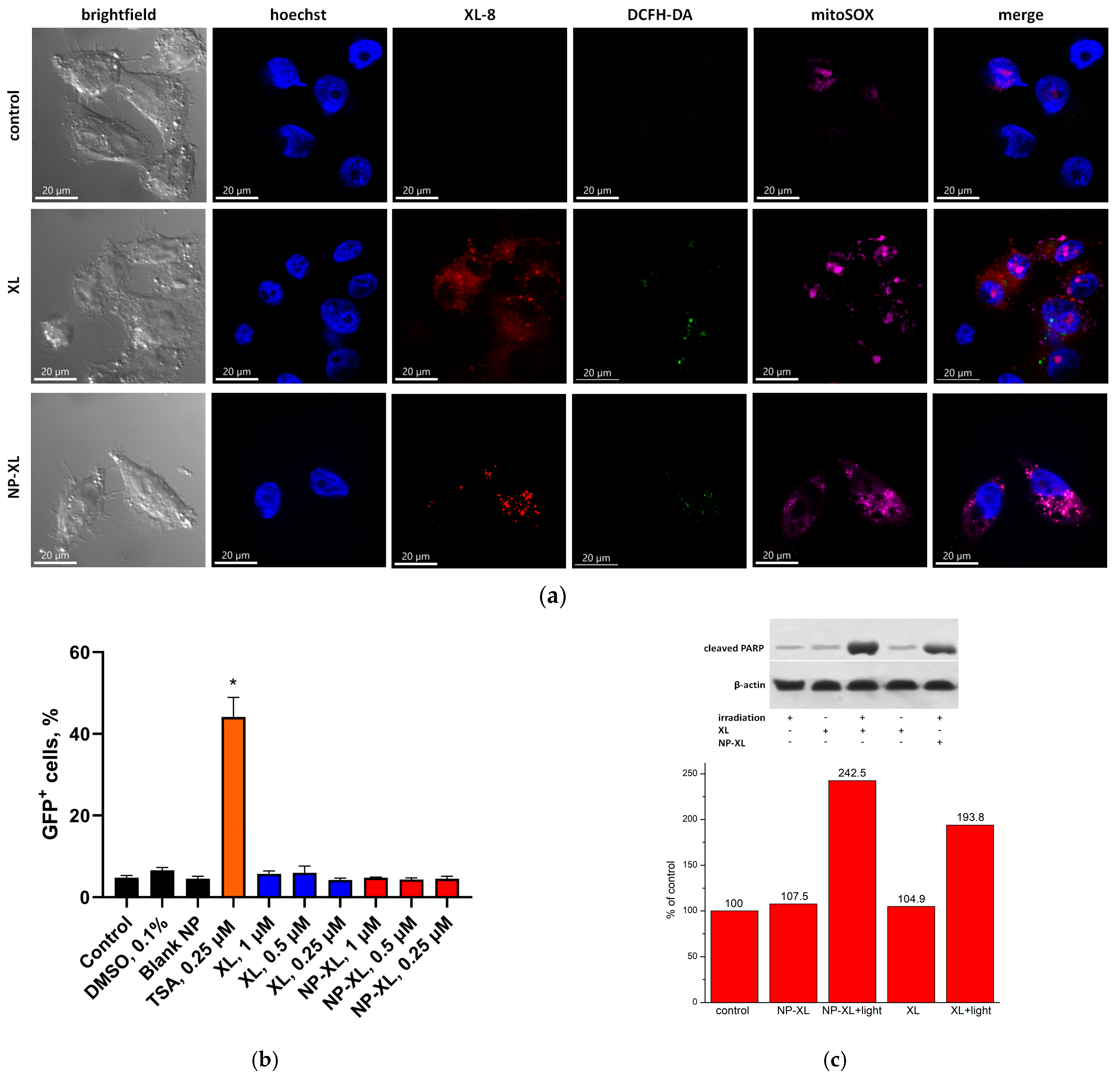
3.13. NP–XL Epigenetic Activity
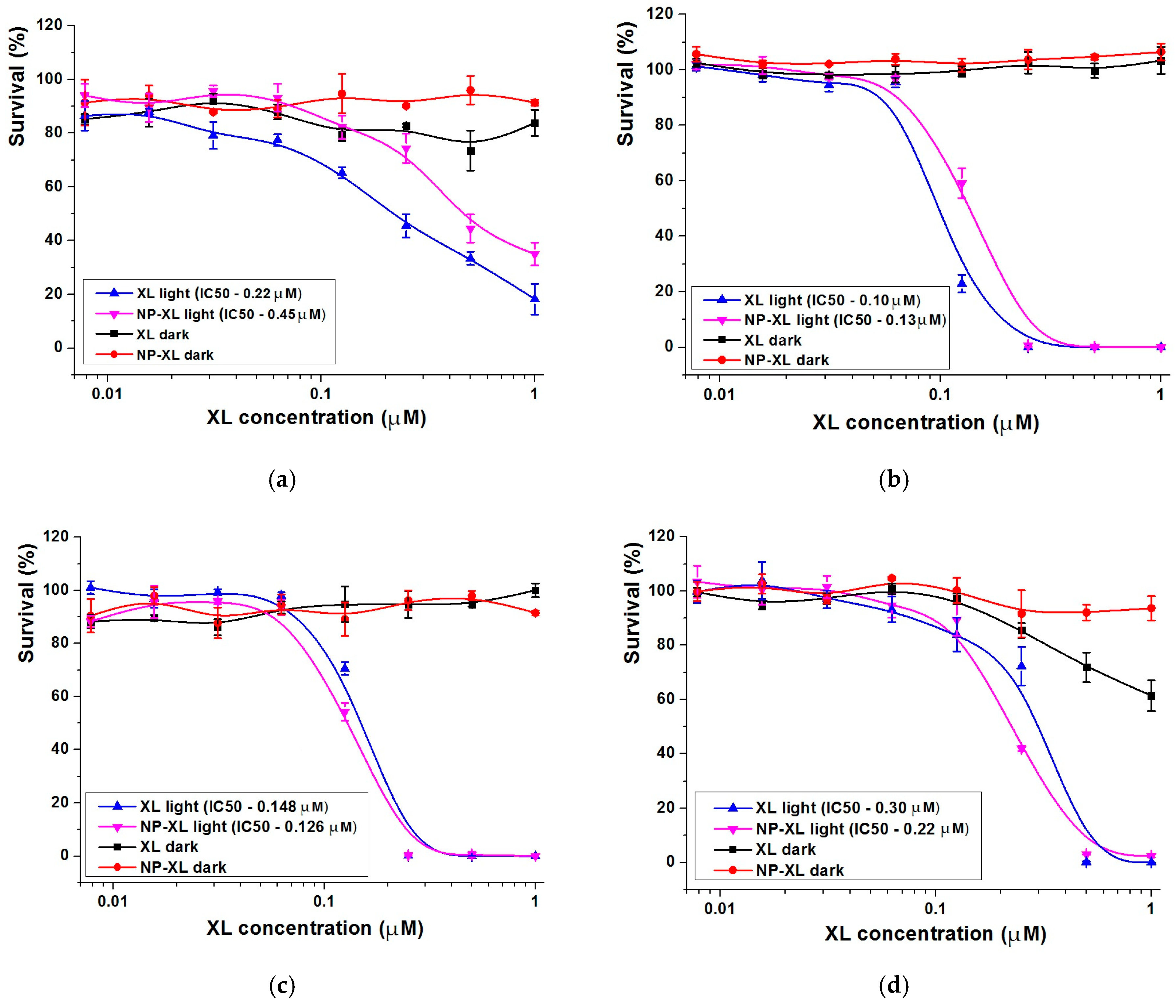
3.14. In Vitro Cytotoxicity of XL and NP–XL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front. Chem. 2021, 9, 691697. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Aihara, Y.; Oda, Y.; Fukui, A.; Tsuzuki, S.; Saito, T.; Nitta, M.; Muragaki, Y.; Kawamata, T. Photodynamic Therapy for Malignant Brain Tumors in Children and Young Adolescents. Front. Oncol. 2022, 12, 957267. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic Therapy for Solid Tumors: A Review of the Literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Potential Application of Photosensitizers with High-Z Elements for Synergic Cancer Therapy. Front. Pharmacol. 2022, 13, 921729. [Google Scholar] [CrossRef]
- Belykh, D.V.; Startseva, O.M.; Patov, S.A. Novel p H-Independent Amphiphilic Chlorophyll a Derivatives with Oligoethylene Glycol Substituents as a Hydrophilic Part: Synthesis and Hydrophilicity Estimation. Macroheterocycles 2014, 7, 401–413. [Google Scholar]
- Mollaeva, M.R.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.D.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef]
- Naskar, S.; Das, S.K.; Sharma, S.; Kuotsu, K. A Review on Designing Poly (Lactic-Co-Glycolic Acid) Nanoparticles as Drug Delivery Systems. Pharm. Nanotechnol. 2021, 9, 36–50. [Google Scholar] [CrossRef]
- Alvi, M.; Yaqoob, A.; Rehman, K.; Shoaib, S.M.; Akash, M.S.H. PLGA-Based Nanoparticles for the Treatment of Cancer: Current Strategies and Perspectives. AAPS Open 2022, 8, 12. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Zabolotsky, A.; Mollaev, M.; Zhunina, O.; Fomicheva, M.; Lobanov, A.; Severin, E.; Yabbarov, N. High-Effective Reactive Oxygen Species Inducer Based on Mn-Tetraphenylporphyrin Loaded PLGA Nanoparticles in Binary Catalyst Therapy. Free Radic. Biol. Med. 2019, 143, 522–533. [Google Scholar] [CrossRef]
- Faustova, M.R.; Nikolskaya, E.D.; Mollaev, M.D.; Sokol, M.B.; Zabolotsky, A.I.; Zhunina, O.A.; Fomicheva, M.V.; Schvets, V.I.; Lobanov, A.V.; Yabbarov, N.G. Polymer Particles Containing Fe-Based Metalloporphyrin as a Highly Efficient Stimulator of Reactive Oxygen Species Formation in Vitro and in Vivo. Russ. Chem. Bull. 2019, 68, 2216–2224. [Google Scholar] [CrossRef]
- Faustova, M.; Nikolskaya, E.; Sokol, M.; Fomicheva, M.; Petrov, R.; Yabbarov, N. Metalloporphyrins in Medicine: From History to Recent Trends. ACS Appl. Bio Mater. 2020, 3, 8146–8171. [Google Scholar] [CrossRef]
- Faustova, M.R.; Nikolskaya, E.D.; Zhunina, O.A.; Mollaev, M.D.; Yabbarov, N.G.; Lobanov, A.V.; Melnikov, M.Y.; Severin, E.S. Polymer Nanoparticles Loaded with FeCl-Tetraphenylporphyrin for Binary Catalytic Therapy of Neoplasms. Russ. Chem. Bull. 2018, 67, 359–365. [Google Scholar] [CrossRef]
- Vallecorsa, P.; Di Venosa, G.; Ballatore, M.B.; Ferreyra, D.; Mamone, L.; Sáenz, D.; Calvo, G.; Durantini, E.; Casas, A. Novel meso-substituted porphyrin derivatives and its potential use in photodynamic therapy of cancer. BMC Cancer 2021, 21, 547. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.N.; Alam, M.S.; Waziri, A.; Pottoo, F.H.; Yadav, A.K.; Hasnain, M.S.; Almalki, F.A. Chapter 12—QbD Applications for the Development of Nanopharmaceutical Products. In Pharmaceutical Quality by Design; Beg, S., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 229–253. ISBN 978-0-12-815799-2. [Google Scholar]
- Giribabu, L.; Kandhadi, J.; Kanaparthi, R.K. Phosphorus(V)Corrole-Porphyrin Based Hetero Trimers: Synthesis, Spectroscopy and Photochemistry. J. Fluoresc. 2014, 24, 569–577. [Google Scholar] [CrossRef]
- Chin, K.K.; Trevithick-Sutton, C.C.; McCallum, J.; Jockusch, S.; Turro, N.J.; Scaiano, J.C.; Foote, C.S.; Garcia-Garibay, M.A. Quantitative Determination of Singlet Oxygen Generated by Excited State Aromatic Amino Acids, Proteins, and Immunoglobulins. J. Am. Chem. Soc. 2008, 130, 6912–6913. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Sokol, M.B.; Yabbarov, N.G.; Mollaeva, M.R.; Chirkina, M.V.; Mollaev, M.D.; Zabolotsky, A.I.; Kuznetsov, S.L.; Nikolskaya, E.D. Alpha-Fetoprotein Mediated Targeting of Polymeric Nanoparticles to Treat Solid Tumors. Nanomedicine 2022, 17, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.P.; Lan-Chun-Fung, Y.L.; Pritchard, J.G. Determination of Poly(Vinyl Alcohol) via Its Complex with Boric Acid and Iodine. Anal. Chim. Acta 1979, 104, 153–160. [Google Scholar] [CrossRef]
- Krivandin, A.; Solovieva, A.; Glagolev, N.; Shatalova, O.V.; Kotova, S. Structure Alteraitions of Perfluorinated Sulfocationic Membrans under the Action of Ethylene Glycol (SAXS and WAXS Studies). Polymer 2003, 44, 5789–5796. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Naumenko, A.; Kutsevol, N. Spectroscopic Investigation of Chlorin-Based Photosensitizers in Polymer Matrix. Int. J. Polym. Sci. 2021, 2021, e8842052. [Google Scholar] [CrossRef]
- Reynolds, E.W.; Demas, J.N.; DeGraff, B.A. Viscosity and Temperature Effects on the Rate of Oxygen Quenching of Tris-(2,2′-Bipyridine)Ruthenium(II). J. Fluoresc. 2013, 23, 237–241. [Google Scholar] [CrossRef]
- Militello, M.P.; Hernández-Ramírez, R.E.; Lijanova, I.V.; Previtali, C.M.; Bertolotti, S.G.; Arbeloa, E.M. Novel PAMAM Dendrimers with Porphyrin Core as Potential Photosensitizers for PDT Applications. J. Photochem. Photobiol. Chem. 2018, 353, 71–76. [Google Scholar] [CrossRef]
- Borissevitch, I.E.; Tominaga, T.T.; Schmitt, C.C. Photophysical Studies on the Interaction of Two Water-Soluble Porphyrins with Bovine Serum Albumin. Effects upon the Porphyrin Triplet State Characteristics. J. Photochem. Photobiol. Chem. 1998, 114, 201–207. [Google Scholar] [CrossRef]
- Gradova, M.; Gradov, O.; Lobanov, A.; Movchan, T.; Plotnikova, E.; Chernyadyev, A.; Startseva, O.; Belykh, D. Photophysical Properties and Aggregation Behavior of Diethylene Glycol Substituted Pyropheophorbide-a in Cationic Surfactant Solutions. J. Porphyr. Phthalocyanines 2022, 26, 708–718. [Google Scholar] [CrossRef]
- Gavrina, A.I.; Shirmanova, M.V.; Aksenova, N.A.; Yuzhakova, D.V.; Snopova, L.B.; Solovieva, A.B.; Тimashev, P.S.; Dudenkova, V.V.; Zagaynova, E.V. Photodynamic Therapy of Mouse Tumor Model Using Chlorin E6- Polyvinyl Alcohol Complex. J. Photochem. Photobiol. B 2018, 178, 614–622. [Google Scholar] [CrossRef]
- Umemoto, Y.; Uchida, S.; Yoshida, T.; Shimada, K.; Kojima, H.; Takagi, A.; Tanaka, S.; Kashiwagura, Y.; Namiki, N. An Effective Polyvinyl Alcohol for the Solubilization of Poorly Water-Soluble Drugs in Solid Dispersion Formulations. J. Drug Deliv. Sci. Technol. 2020, 55, 101401. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic Emulsification: An Overview on the Preparation of Different Emulsifiers-Stabilized Emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Maeda, H.; Sawa, T.; Konno, T. Mechanism of Tumor-Targeted Delivery of Macromolecular Drugs, Including the EPR Effect in Solid Tumor and Clinical Overview of the Prototype Polymeric Drug SMANCS. J. Control. Release 2001, 74, 47–61. [Google Scholar] [CrossRef]
- Roces, C.B.; Christensen, D.; Perrie, Y. Translating the Fabrication of Protein-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles from Bench to Scale-Independent Production Using Microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. [Google Scholar] [CrossRef]
- Feng, S.; Huang, G. Effects of Emulsifiers on the Controlled Release of Paclitaxel (Taxol) from Nanospheres of Biodegradable Polymers. J. Control. Release Off. J. Control. Release Soc. 2001, 71, 53–69. [Google Scholar] [CrossRef]
- Mares, A.G.; Pacassoni, G.; Marti, J.S.; Pujals, S.; Albertazzi, L. Formulation of Tunable Size PLGA-PEG Nanoparticles for Drug Delivery Using Microfluidic Technology. PLoS ONE 2021, 16, e0251821. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual Polyvinyl Alcohol Associated with Poly (D,L-Lactide-Co-Glycolide) Nanoparticles Affects Their Physical Properties and Cellular Uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Singh, G.; Tanurajvir, K.; Ravinder, K.; Kaur, A. Recent Biomedical Applications and Patents on Biodegradable Polymer-PLGA. Int. J. Pharmacol. Pharm. Sci. 2014, 1, 30–42. [Google Scholar]
- Berezin, K.; Nechaev, V. Calculation of Chlorin IR Spectrum by Density Functional Theory. Chem. Nat. Compd. 2003, 39, 540–548. [Google Scholar] [CrossRef]
- Gladkova, O.L.; Parkhats, M.V.; Gorbachova, A.N.; Terekhov, S.N. FTIR Spectra and Normal-Mode Analysis of Chlorin E6 and Its Degradation-Induced Impurities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 76, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Passerini, N.; Craig, D.Q.M. An Investigation into the Effects of Residual Water on the Glass Transition Temperature of Polylactide Microspheres Using Modulated Temperature DSC. J. Control. Release 2001, 73, 111–115. [Google Scholar] [CrossRef]
- D’Souza, S.; Dorati, R.; DeLuca, P.P. Effect of Hydration on Physicochemical Properties of End-Capped PLGA. Adv. Biomater. 2014, 2014, e834942. [Google Scholar] [CrossRef]
- Mainardes, R.; Gremiao, M.; Evangelista, R. Thermoanalytical Study of Praziquantel-Loaded PLGA Nanoparticles. Rev. Bras. Cienc. Farm.—RBCF 2006, 42, 523–530. [Google Scholar] [CrossRef]
- Jamil, N.; Husin, H.; ALFIDA, A.; Aman, Z.; Hassan, Z. Characterization and Preparation of Polyvinyl Alcohol (PVA) as Inhibitor in Formation of Hydrates. Int. J. Curr. Res. Sci. Eng. Technol. 2018, 1, 578. [Google Scholar] [CrossRef]
- Bassett, D.C. Principles of Polymer Morphology, 1st ed.; Cambridge University Press: New York, NY, USA, 1981; ISBN 978-0-521-23270-8. [Google Scholar]
- Ye, P.; Byron, T. Characterization of D-Mannitol by Thermal Analysis, FTIR, and Raman Spectroscopy. Am. Lab. 2008, 40, 24–27. [Google Scholar]
- Hodge, I.M.; Berens, A.R. Effects of Annealing and Prior History on Enthalpy Relaxation in Glassy Polymers. 2. Mathematical Modeling. Macromolecules 1982, 15, 762–770. [Google Scholar] [CrossRef]
- Park, K.; Otte, A.; Sharifi, F.; Garner, J.; Skidmore, S.; Park, H.; Jhon, Y.K.; Qin, B.; Wang, Y. Potential Roles of the Glass Transition Temperature of PLGA Microparticles in Drug Release Kinetics. Mol. Pharm. 2021, 18, 18–32. [Google Scholar] [CrossRef]
- Ige, P.P.; Dipsingh, S.N. Preparation and in Vitro–in Vivo Evaluation of Surface-Modified Poly(Lactide-Co-Glycolide) Nanoparticles as Controlled Release Carriers for Flutamide Delivery. J. Microencapsul. 2015, 32, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Pool, H.; Quintanar, D.; Figueroa, J.d.D.; Marinho Mano, C.; Bechara, J.E.H.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, e145380. [Google Scholar] [CrossRef]
- Kavanagh, O.; Hogan, F.; Murphy, C.; Croker, D.; Walker, G. Formulating a Stable Mannitol Infusion While Maintaining Hyperosmolarity. Pharmaceutics 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.J.; Pygall, S.R.; Cooper, V.B.; Mann, J.C. Overcoming Sink Limitations in Dissolution Testing: A Review of Traditional Methods and the Potential Utility of Biphasic Systems. J. Pharm. Pharmacol. 2012, 64, 1549–1559. [Google Scholar] [CrossRef]
- Kim, H.-G.; Gater, D.L.; Kim, Y.-C. Development of Transdermal Vitamin D3 (VD3) Delivery System Using Combinations of PLGA Nanoparticles and Microneedles. Drug Deliv. Transl. Res. 2018, 8, 281–290. [Google Scholar] [CrossRef]
- Poltavets, Y.I.; Zhirnik, A.S.; Zavarzina, V.V.; Semochkina, Y.P.; Shuvatova, V.G.; Krasheninnikova, A.A.; Aleshin, S.V.; Dronov, D.O.; Vorontsov, E.A.; Balabanyan, V.Y.; et al. In Vitro Anticancer Activity of Folate-Modified Docetaxel-Loaded PLGA Nanoparticles against Drug-Sensitive and Multidrug-Resistant Cancer Cells. Cancer Nanotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched With Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch—Stärke 2017, 70, 1700238. [Google Scholar] [CrossRef]
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood–Brain Barrier for Drug Delivery: From R&D to cGMP. Pharmaceutics 2021, 13, 500. [Google Scholar] [CrossRef]
- Hasanain, F.; Guenther, K.; Mullett, W.M.; Craven, E. Gamma Sterilization of Pharmaceuticals—A Review of the Irradiation of Excipients, Active Pharmaceutical Ingredients, and Final Drug Product Formulations. PDA J. Pharm. Sci. Technol. 2014, 68, 113–137. [Google Scholar] [CrossRef]
- Yaman, A. Alternative Methods of Terminal Sterilization for Biologically Active Macromolecules. Curr. Opin. Drug Discov. Devel. 2001, 4, 760–763. [Google Scholar] [PubMed]
- EMA Guide on Sterilisation for Medicinal Products, API Excipients and Primary Packaging Published—ECA Academy. Available online: https://www.gmp-compliance.org/gmp-news/ema-guide-on-sterilisation-for-medicinal-products-api-excipients-and-primary-packaging-published (accessed on 25 August 2023).
- Williams, H.; Huxley, J.; Claybourn, M.; Booth, J.; Hobbs, M.; Meehan, L.; Clark, B. The Effect of γ-Irradiation and Polymer Composition on the Stability of PLG Polymer and Microspheres. Polym. Degrad. Stab. 2006, 91, 2171–2181. [Google Scholar] [CrossRef]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-Loaded PLGA Nanoparticles for the Chemotherapy of Glioblastoma: Towards the Pharmaceutical Development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef]
- Wachowska, M.; Muchowicz, A.; Golab, J. Targeting Epigenetic Processes in Photodynamic Therapy-Induced Anticancer Immunity. Front. Oncol. 2015, 5, 176. [Google Scholar]
- Wachowska, M.; Gabrysiak, M.; Golab, J. Epigenetic Remodeling Combined with Photodynamic Therapy Elicits Anticancer Immune Responses. OncoImmunology 2014, 3, e28837. [Google Scholar] [CrossRef][Green Version]
- Halaburková, A.; Jendželovský, R.; Kovaľ, J.; Herceg, Z.; Fedoročko, P.; Ghantous, A. Histone Deacetylase Inhibitors Potentiate Photodynamic Therapy in Colon Cancer Cells Marked by Chromatin-Mediated Epigenetic Regulation of CDKN1A. Clin. Epigenetics 2017, 9, 62. [Google Scholar] [CrossRef]
- Salva, K.A.; Wood, G.S. Epigenetically Enhanced Photodynamic Therapy (ePDT) Is Superior to Conventional Photodynamic Therapy for Inducing Apoptosis in Cutaneous T-Cell Lymphoma. Photochem. Photobiol. 2015, 91, 1444–1451. [Google Scholar] [CrossRef]
- Maksimova, V.; Shalginskikh, N.; Vlasova, O.; Usalka, O.; Beizer, A.; Bugaeva, P.; Fedorov, D.; Lizogub, O.; Lesovaya, E.; Katz, R.; et al. HeLa TI Cell-Based Assay as a New Approach to Screen for Chemicals Able to Reactivate the Expression of Epigenetically Silenced Genes. PLoS ONE 2021, 16, e0252504. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Shou, D.; Huang, R.; Khuc, T.; Dai, S.; Zheng, W.; Klumpp-Thomas, C.; Xia, M. Identification of HDAC Inhibitors Using a Cell-Based HDAC I/II Assay. J. Biomol. Screen. 2016, 21, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Poleshko, A.; Einarson, M.B.; Shalginskikh, N.; Zhang, R.; Adams, P.D.; Skalka, A.M.; Katz, R.A. Identification of a Functional Network of Human Epigenetic Silencing Factors. J. Biol. Chem. 2010, 285, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, V.; Makus, J.; Popova, V.; Prus, A.; Usalka, O.; Trapeznikova, E.; Zhidkova, E.; Belitsky, G.; Yakubovskaya, M.; Kirsanov, K. Histone Methyltransferases as a New Target for Epigenetic Action of Vorinostat. Biochem. Mosc. 2023, 88, 968–978. [Google Scholar] [CrossRef]
- Pola, M.; Kolarova, H.; Ruzicka, J.; Zholobenko, A.; Modriansky, M.; Mosinger, J.; Bajgar, R. Effects of zinc porphyrin and zinc phthalocyanine derivatives in photodynamic anticancer therapy under different partial pressures of oxygen in vitro. Investig. New Drugs 2021, 39, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pucelik, B.; Sułek, A.; Dąbrowski, J.M. Bacteriochlorins and their metal complexes as NIR-absorbing photosensitizers: Properties, mechanisms, and applications. Coord. Chem. Rev. 2020, 416, 213340. [Google Scholar]
- Voloboueva, L.A.; Emery, J.F.; Sun, X.; Giffard, R.G. Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett. 2013, 587, 756–762. [Google Scholar]
- Roelofs, B.A.; Shealinna, X.G.; Studlack, P.E.; Polster, B.M. Low micromolar concentrations of the superoxide probe MitoSOX uncouple neural mitochondria and inhibit complex IV. Free Radic. Biol. Med. 2015, 86, 250–258. [Google Scholar] [CrossRef]
- da Silva, C.L.; Del Ciampo, J.O.; Rossetti, F.C.; Bentley, M.V.; Pierre, M.B. PLGA nanoparticles as delivery systems for protoporphyrin IX in topical PDT: Cutaneous penetration of photosensitizer observed by fluorescence microscopy. J. Nanosci. Nanotechnol. 2013, 13, 6533–6540. [Google Scholar] [CrossRef]
- Yu, Y.; Dai, M.; Xu, W.; Chen, S.; Lu, Q. Close to Real: Large-Volume 3D Cell Spheroids on a Superamphiphobic Surface. Adv. Mater. Interfaces 2021, 8, 2100039. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Shipunova, V.O.; Kovalenko, V.L.; Kotelnikova, P.A.; Sogomonyan, A.S.; Shilova, O.N.; Komedchikova, E.N.; Zvyagin, A.V.; Nikitin, M.P.; Deyev, S.M. Targeting Cancer Cell Tight Junctions Enhances PLGA-Based Photothermal Sensitizers’ Performance In Vitro and In Vivo. Pharmaceutics 2022, 14, 43. [Google Scholar] [CrossRef] [PubMed]

| Lab Scale | Size, nm | PDI | ζ-Potential, mV | DL, % | EE, % |
|---|---|---|---|---|---|
| 1-folds | 182 ± 19 | 0.129 ± 0.010 | −22.2 ± 3.8 | 4.6 ± 0.2 | 88 ± 3 |
| 3-folds | 222 ± 10 | 0.243 ± 0.012 | −12.8 ± 1.4 | 6.6 ± 0.3 | 77 ± 2 |
| 6-folds | 295 ± 7 | 0.310 ± 0.010 | −12.8 ± 2.6 | 5.3 ± 0.3 | 63 ± 4 |
| Initial PVA Concentration, % w/w | Blank NPs without Centrifugation, % w/w | Blank NPs after Centrifugation, % w/w | NP–XL after Centrifugation, % w/w |
|---|---|---|---|
| 88.8 ± 0.3 | 63.9 ± 0.5 | 9.4 ± 0.4 * | 10.9 ± 0.5 * |
| Mathematical Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | Weibull | ||||||
| R2 | R2 | R2 | R2 | R2 | n | K | R2 | α | β | Ti | |
| NP–XL | −9.4276 | −3.9656 | −4.9590 | −7.3557 | 0.9905 | 0.089 | 44.222 | 0.6754 | 1.594 | 0.115 | 0.244 |
| γ-Irradiation Dose, kGy | Size, nm | PDI | ζ-Potential, mV | DL, % | EE, % |
|---|---|---|---|---|---|
| Before irradiation | 222 ± 10 | 0.243 ± 0.012 | −12.8 ± 4 | 6.6 ± 0.3 | 77 ± 2 |
| 10 | 259 ± 92; 2836 ± 1182 * | 0.345 ± 0.024 * | −12.0 ± 3 | 5.4 ± 0.3 * | 66 ± 2 |
| 15 | 255 ± 114; 1567 ± 662; 4470 ± 880 * | 0.484 ± 0.020 * | −12.4 ± 3 | 5.1 ± 0.4 * | 65 ± 3 |
| 25 | 334 ± 207; 2642 ± 1315 * | 0.490 ± 0.031 * | −11.5 ± 3 | 4.8 ± 0.4 * | 65 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokol, M.B.; Beganovskaya, V.A.; Mollaeva, M.R.; Yabbarov, N.G.; Chirkina, M.V.; Belykh, D.V.; Startseva, O.M.; Egorov, A.E.; Kostyukov, A.A.; Kuzmin, V.A.; et al. Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative. Pharmaceutics 2024, 16, 126. https://doi.org/10.3390/pharmaceutics16010126
Sokol MB, Beganovskaya VA, Mollaeva MR, Yabbarov NG, Chirkina MV, Belykh DV, Startseva OM, Egorov AE, Kostyukov AA, Kuzmin VA, et al. Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative. Pharmaceutics. 2024; 16(1):126. https://doi.org/10.3390/pharmaceutics16010126
Chicago/Turabian StyleSokol, Maria B., Veronika A. Beganovskaya, Mariia R. Mollaeva, Nikita G. Yabbarov, Margarita V. Chirkina, Dmitry V. Belykh, Olga M. Startseva, Anton E. Egorov, Alexey A. Kostyukov, Vladimir A. Kuzmin, and et al. 2024. "Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative" Pharmaceutics 16, no. 1: 126. https://doi.org/10.3390/pharmaceutics16010126
APA StyleSokol, M. B., Beganovskaya, V. A., Mollaeva, M. R., Yabbarov, N. G., Chirkina, M. V., Belykh, D. V., Startseva, O. M., Egorov, A. E., Kostyukov, A. A., Kuzmin, V. A., Lomakin, S. M., Shilkina, N. G., Krivandin, A. V., Shatalova, O. V., Gradova, M. A., Abakumov, M. A., Nikitin, A. A., Maksimova, V. P., Kirsanov, K. I., & Nikolskaya, E. D. (2024). Pharmaceutical Approach to Develop Novel Photosensitizer Nanoformulation: An Example of Design and Characterization Rationale of Chlorophyll α Derivative. Pharmaceutics, 16(1), 126. https://doi.org/10.3390/pharmaceutics16010126









