Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
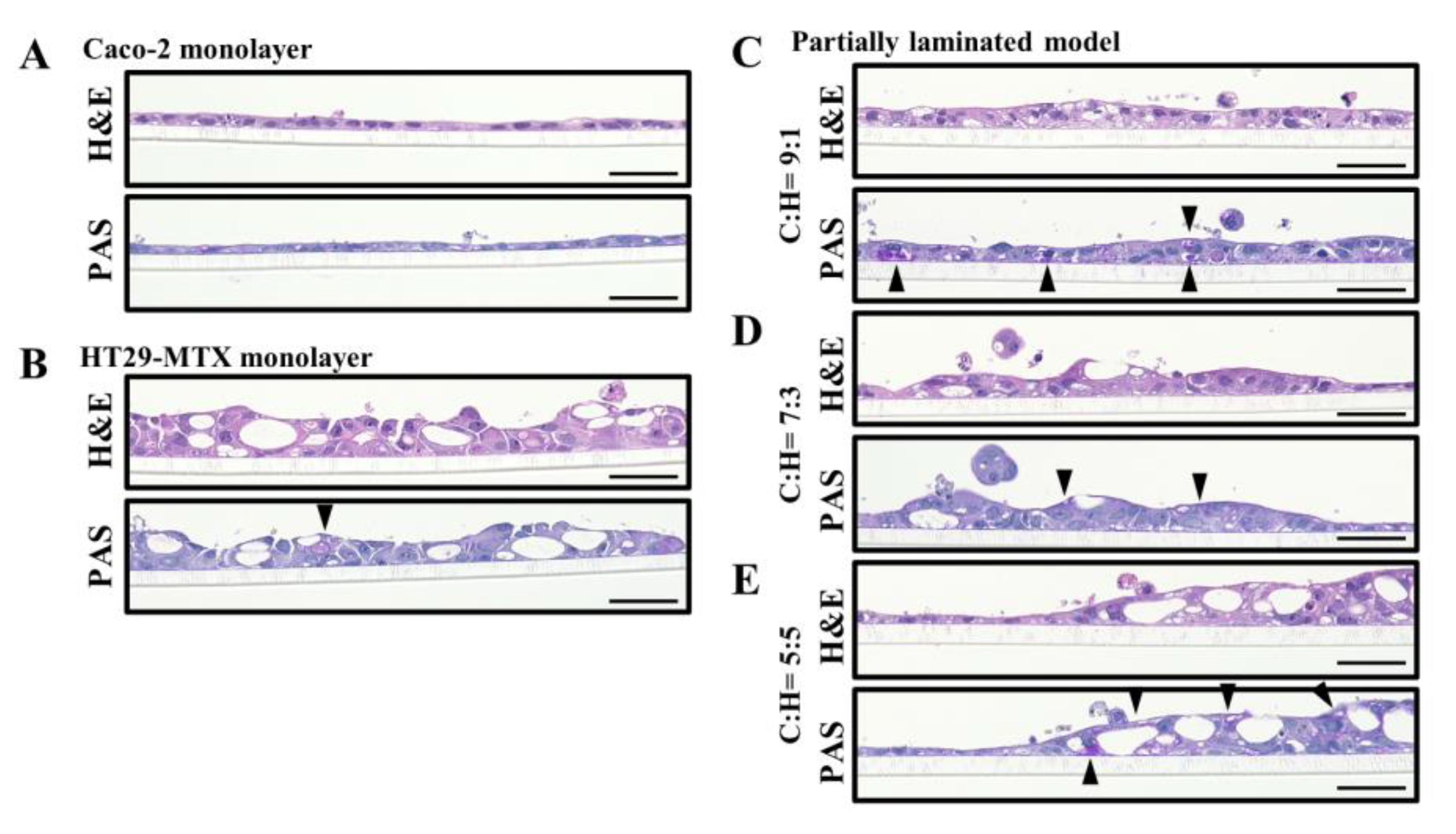
2.3. Histological Assessment
2.4. Mucin Production
2.5. TEER Measurements
2.6. Permeability Study
2.7. Analytical Methods
2.8. Statistics
3. Results
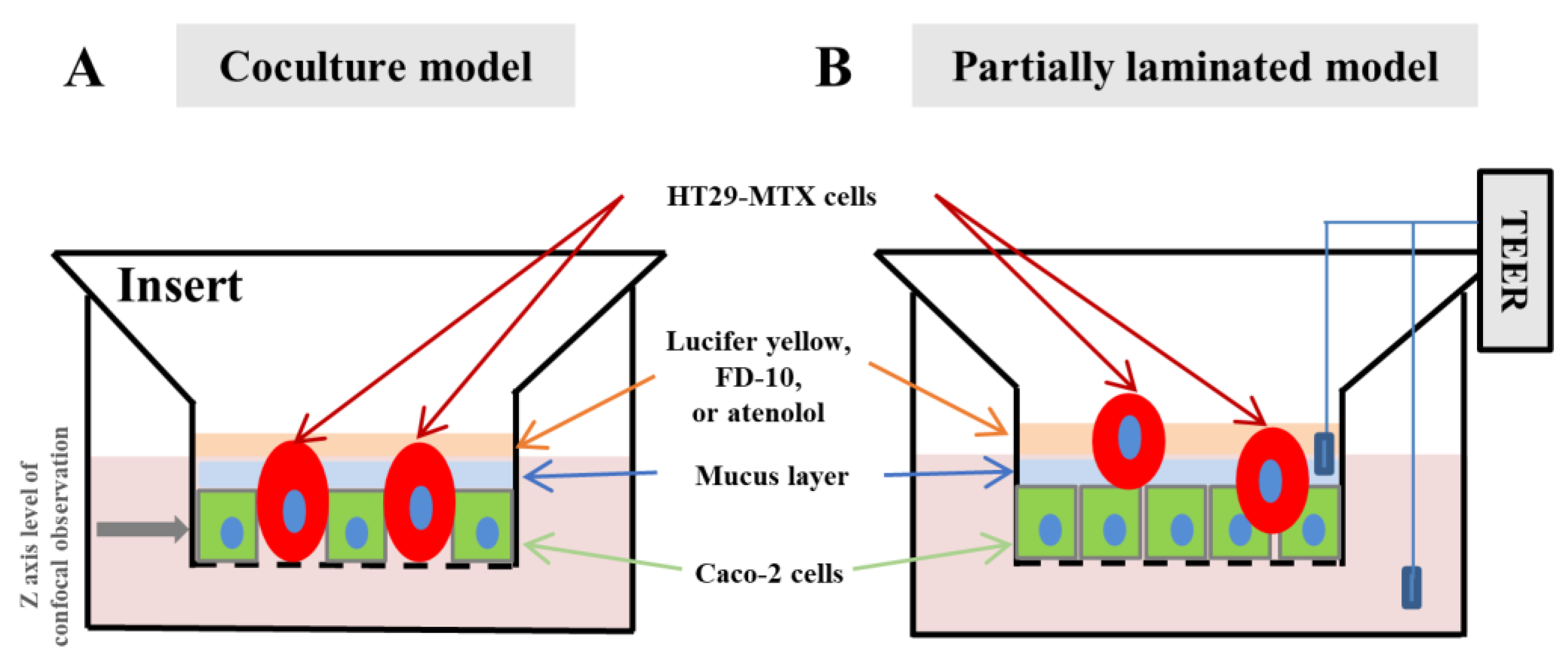
3.1. Effect of Caco-2/HT29-MTX Ratio on the Morphology of the Partially Laminated and Coculture Models
3.2. Mucus Layer in the Partially Laminated Model
3.3. TEER Evaluation of Partially Laminated and Coculture Model Integrities
3.4. Paracellular Markers Showing Permeability in the Partially Laminated and Coculture Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilgers, A.R.; Conradi, R.A.; Burton, P.S. Caco-2 cell monolayers as a model for drug transport across the intestinal mucosa. Pharm. Res. 1990, 7, 902–910. [Google Scholar] [CrossRef]
- Rubas, W.; Jezyk, N.; Grass, G.M. Comparison of the permeability characteristics of a human colonic epithelial (Caco-2) cell line to colon of rabbit, monkey, and dog intestine and human drug absorption. Pharm. Res. 1993, 10, 113–118. [Google Scholar] [CrossRef]
- Stewart, B.H.; Chan, O.H.; Lu, R.H.; Reyner, E.L.; Schmid, H.L.; Hamilton, H.W.; Steinbaugh, B.A.; Taylor, M.D. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: Relationship to absorption in humans. Pharm. Res. 1995, 12, 693–699. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Goll, R.; van Beelen Granlund, A. Intestinal barrier homeostasis in inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 3–12. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Chen, X.M.; Elisia, I.; Kitts, D.D. Defining conditions for the C/H-C of Caco-2 and HT29-MTX cells using Taguchi design. J. Pharmacol. Toxicol. Methods 2010, 61, 334–342. [Google Scholar] [CrossRef]
- Sun, H.; Chow, E.C.Y.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef]
- Macedo, H.M.; Baião, A.; Pinto, S.; Barros, A.S.; Almeida, H.; Almeida, A.; Neves, J.D.; Sarmento, B. Mucus-producing 3D cell culture models. Adv. Drug Deliv. Rev. 2021, 178, 113993. [Google Scholar] [CrossRef]
- Béduneau, A.; Tempesta, C.; Fimbel, S.; Pellequer, Y.; Jannin, V.; Demarne, F.; Lamprecht, A. A tunable Caco-2/HT29-MTX C/H-C model mimicking variable permeabilities of the human intestine obtained by an original seeding procedure. Eur. J. Pharm. Biopharm. 2014, 87, 290–298. [Google Scholar] [CrossRef]
- Reale, O.; Huguet, A.; Fessard, V. Co-culture model of Caco-2/HT29-MTX cells: A promising tool for investigation of phycotoxins toxicity on the intestinal barrier. Chemosphere 2020, 273, 128497. [Google Scholar] [CrossRef] [PubMed]
- Lesuffleur, T.; Barbat, A.; Luccioni, C.; Beaumatin, J.; Clair, M.; Kornowski, A.; Dussaulx, E.; Dutrillaux, B.; Zweibaum, A. Dihydrofolate reductase gene amplification-associated shift of differentiation in methotrexate-adapted HT29 cells. J. Cell Biol. 1991, 115, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, C.; Spahn-Langguth, H.; Regårdh, C.G.; Lipka, E.; Amidon, G.L.; Langguth, P. Caco-2 versus Caco-2/HT29-MTX co-cultured cell lines: Permeabilities via diffusion, inside- and outside-directed carrier-mediated transport. J. Pharm. Sci. 2000, 89, 63–75. [Google Scholar] [CrossRef]
- Behrens, I.; Stenberg, P.; Artursson, P.; Kissel, T. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm. Res. 2001, 18, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Dando, S.A.; Morrison, R.A. Evaluation of Biocoat intestinal epithelium differentiation environment (3-day cultured Caco-2 cells) as an absorption screening model with improved productivity. Pharm. Res. 1997, 14, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Lakeram, M.; Lockley, D.J.; Sanders, D.J.; Pendlington, R.; Forbes, B. Paraben transport and metabolism in the biomimetic artificial membrane permeability assay (BAMPA) and 3-day and 21-day Caco-2 cell systems. J. Biomol. Screen. 2007, 12, 84–91. [Google Scholar] [CrossRef]
- Gupta, V.; Doshi, N.; Mitragotri, S. Permeation of insulin, calcitonin and exenatide across Caco-2 monolayers: Measurement using a rapid, 3-day system. PLoS ONE 2013, 8, e57136. [Google Scholar] [CrossRef]
- Yamashita, S.; Konishi, K.; Yamazaki, Y.; Taki, Y.; Sakane, T.; Sezaki, H.; Furuyama, Y. New and Better protocols for a Short-Term Caco-2 Cell Culture System. J. Pharm. Sci. 2002, 91, 669–679. [Google Scholar] [CrossRef]
- Yang, T.; Arnold, J.J.; Ahsan, F. Tetradecylmaltoside (TDM) enhances in vitro and in vivo intestinal absorption of enoxaparin, a low molecular weight heparin. J. Drug Target. 2005, 13, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, O.; Iriarte, G.; Rico, E.; Nerea Ferreirós, N.; Maguregui, M.I.; Alonso, R.M.; Jiménez, R.M. LC-MS/MS method for the determination of several drugs used in combined cardiovascular therapy in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 2685–2692. [Google Scholar] [CrossRef]
- Larsson, J.M.H.; Thomsson, K.A.; Rodríguez-Piñeiro, A.M.; Karlsson, H.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. III. Gastrointestinal Muc5ac and Muc2 mucin O-glycan patterns reveal a regiospecific distribution. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G357–G363. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Macedo, M.H.; Barros, A.S.; Martínez, E.; Barrias, C.C.; Sarmento, B. All layers matter: Innovative three-dimensional epithelium-stroma-endothelium intestinal model for reliable permeability outcomes. J. Control. Release 2022, 341, 414–430. [Google Scholar] [CrossRef]
- Van Dongen, J.M.; Kooyman, J.; Visser, W.J. The influence of 400 r x-irradiation on the number and the localization of mature and immature goblet cells and Paneth cells in intestinal crypt and villus. Cell Tissue Kinet. 1976, 9, 65–75. [Google Scholar] [CrossRef]
- Gordon, J.I. Intestinal epithelial differentiation: New insights from chimeric and transgenic mice. J. Cell Biol. 1989, 108, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Gordon, J.I. The intestinal stem cell niche: There grows the neighborhood. Proc. Natl. Acad. Sci. USA 2001, 98, 12334–12336. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Al-Jawadi, A.; Kishida, K.; Yu, S.; Hu, M.; Fritzky, L.F.; Edelblum, K.L.; Gao, N.; Ferraris, R.P. Marked differences in tight junction composition and macromolecular permeability among different intestinal cell types. BMC Biol. 2018, 16, 19. [Google Scholar] [CrossRef]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef]
- Dossou-Yovo, F.; Mamadou, G.; Soudy, I.D.; Limas-Nzouzi, N.; Miantezila, J.; Desjeux, J.F.; Eto, B. Metronidazole or cotrimoxazole therapy is associated with a decrease in intestinal bioavailability of common antiretroviral drugs. PLoS ONE 2014, 9, e89943. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Griesser, J.; Hetényi, G.; Kadas, H.; Demarne, F.; Jannin, V.; Bernkop-Schnürch, A. Self-emulsifying peptide drug delivery systems: How to make them highly mucus permeating. Int. J. Pharm. 2018, 538, 159–166. [Google Scholar] [CrossRef]
- Boegh, M.; García-Díaz, M.; Müllertz, A.; Nielsen, H.M. Steric and interactive barrier properties of intestinal mucus elucidated by particle diffusion and peptide permeation. Eur. J. Pharm. Biopharm. 2015, 95, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lee, B.C.; Pou, S.; Ciccolella, D. Effects of activation of polymorphonuclear leukocytes on airway goblet cell mucin release in a coculture system. Inflamm. Res. 2003, 52, 258–262. [Google Scholar] [CrossRef]
- Smirnova, M.G.; Guo, L.; Birchall, J.P.; Pearson, J.P. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell. Immunol. 2003, 221, 42–49. [Google Scholar] [CrossRef]
- Navabi, N.; McGuckin, M.A.; Lindén, S.K. Gastrointestinal cell lines form polarized epithelia with an adherent mucus layer when cultured in semi-wet interfaces with mechanical stimulation. PLoS ONE 2013, 8, e68761. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Martinez, M.N.; Dao, K.; Gabriel, V.; Zdyrski, C.; Jergens, A.E.; Atherly, T.; Iennarella-Servantez, C.A.; Burns, L.E.; Schrunk, D.; et al. Canine intestinal organoids as a novel in vitro model of intestinal drug permeability: A proof-of-concept study. Cells 2023, 12, 1269–1299. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Watanabe, C.; Ando, Y.; Kitaoka, S.; Egawa, Y.; Takashima, T.; Matsumoto, A.; Murakami, M. Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability. Pharmaceutics 2023, 15, 2338. https://doi.org/10.3390/pharmaceutics15092338
Cheng Y, Watanabe C, Ando Y, Kitaoka S, Egawa Y, Takashima T, Matsumoto A, Murakami M. Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability. Pharmaceutics. 2023; 15(9):2338. https://doi.org/10.3390/pharmaceutics15092338
Chicago/Turabian StyleCheng, Yi, Chie Watanabe, Yusuke Ando, Satoshi Kitaoka, Yuya Egawa, Tomoya Takashima, Akihiro Matsumoto, and Masahiro Murakami. 2023. "Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability" Pharmaceutics 15, no. 9: 2338. https://doi.org/10.3390/pharmaceutics15092338
APA StyleCheng, Y., Watanabe, C., Ando, Y., Kitaoka, S., Egawa, Y., Takashima, T., Matsumoto, A., & Murakami, M. (2023). Caco-2 Cell Sheet Partially Laminated with HT29-MTX Cells as a Novel In Vitro Model of Gut Epithelium Drug Permeability. Pharmaceutics, 15(9), 2338. https://doi.org/10.3390/pharmaceutics15092338






