Cutaneous Cell Therapy Manufacturing Timeframe Rationalization: Allogeneic Off-the-Freezer Fibroblasts for Dermo-Epidermal Combined Preparations (DE-FE002-SK2) in Burn Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Compliance of the Study
2.2. Materials and Consumables Used for the Study
2.3. Equipment Used for the Study
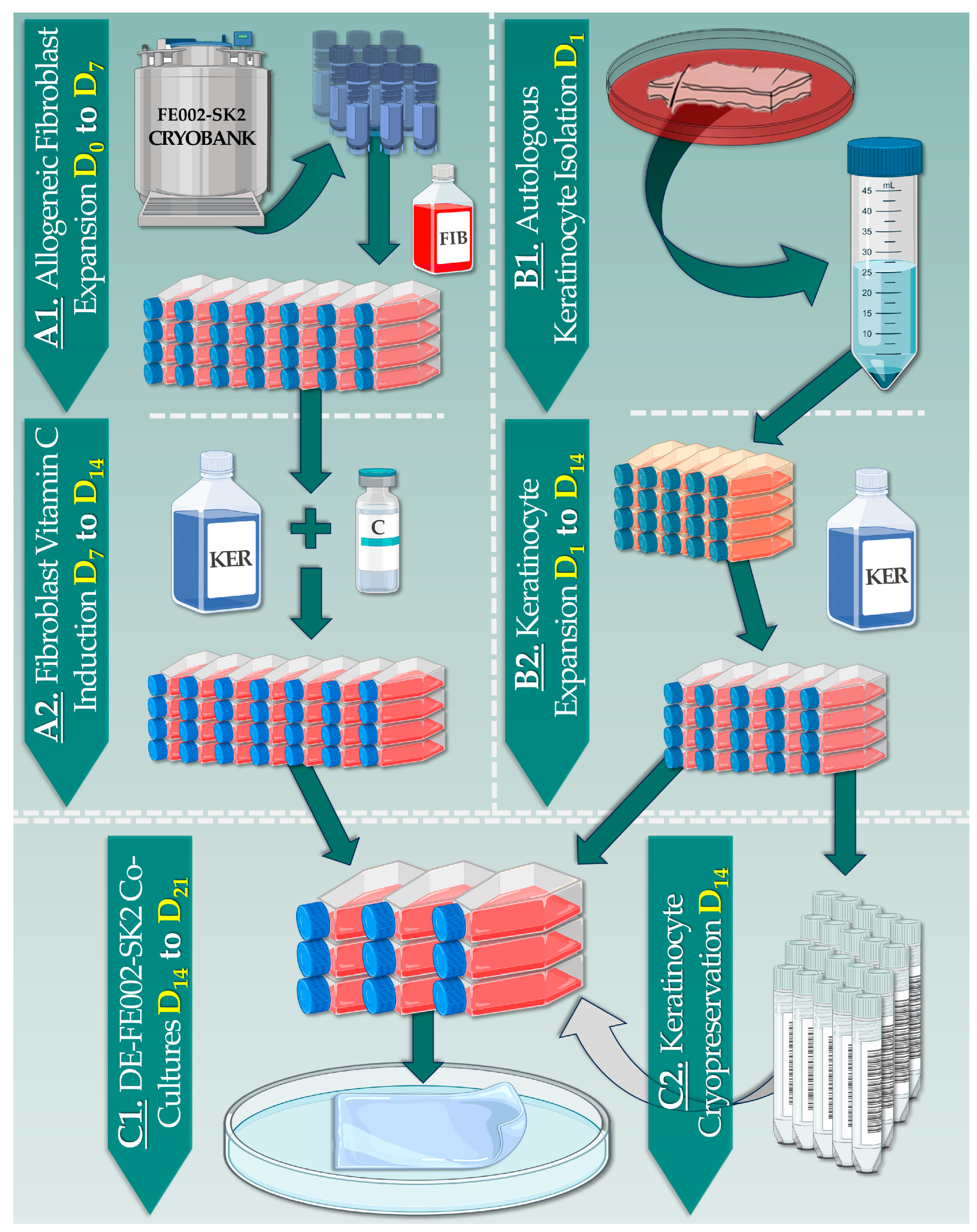
2.4. Primary Cell Sourcing and Cellular Raw Material Manufacture
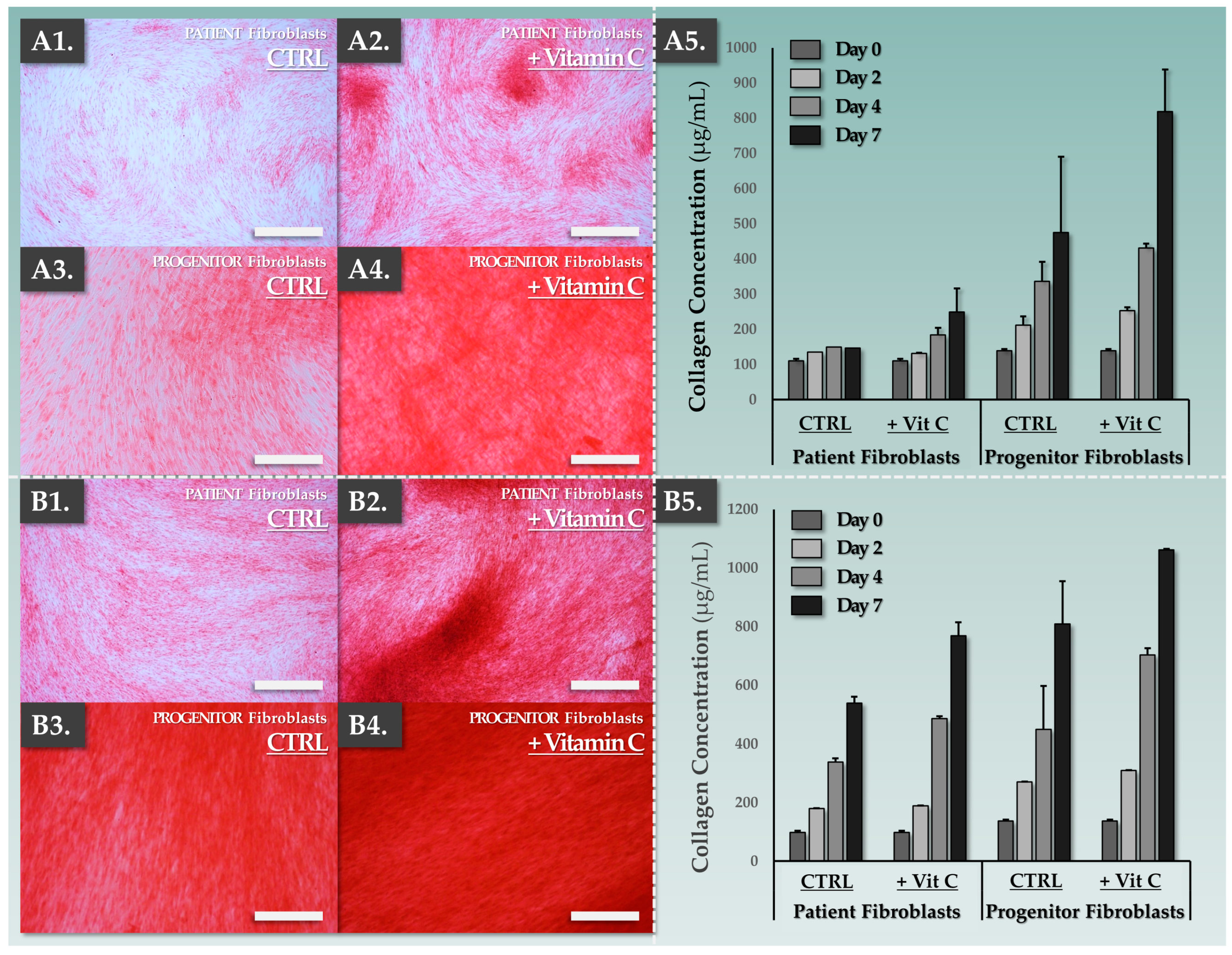
2.5. Dermal Template Preparation: In Vitro Collagen Synthesis Induction Conditions
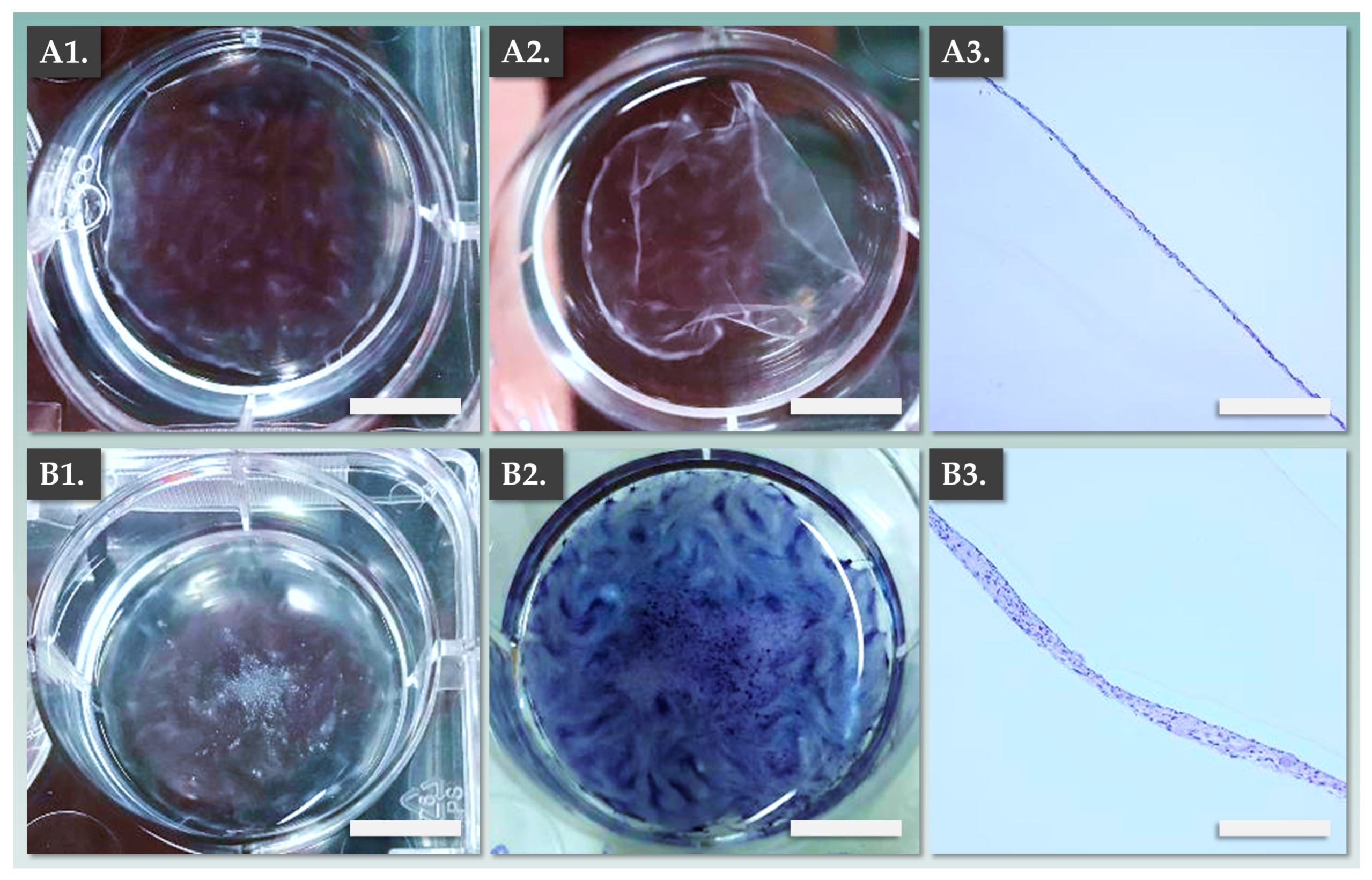
2.6. Allogeneic Dermal Template Preparation: In Vitro Manufacturing Optimization
2.7. Dermal Template Characterization Assay: Sirius Red Staining and Collagen Quantification
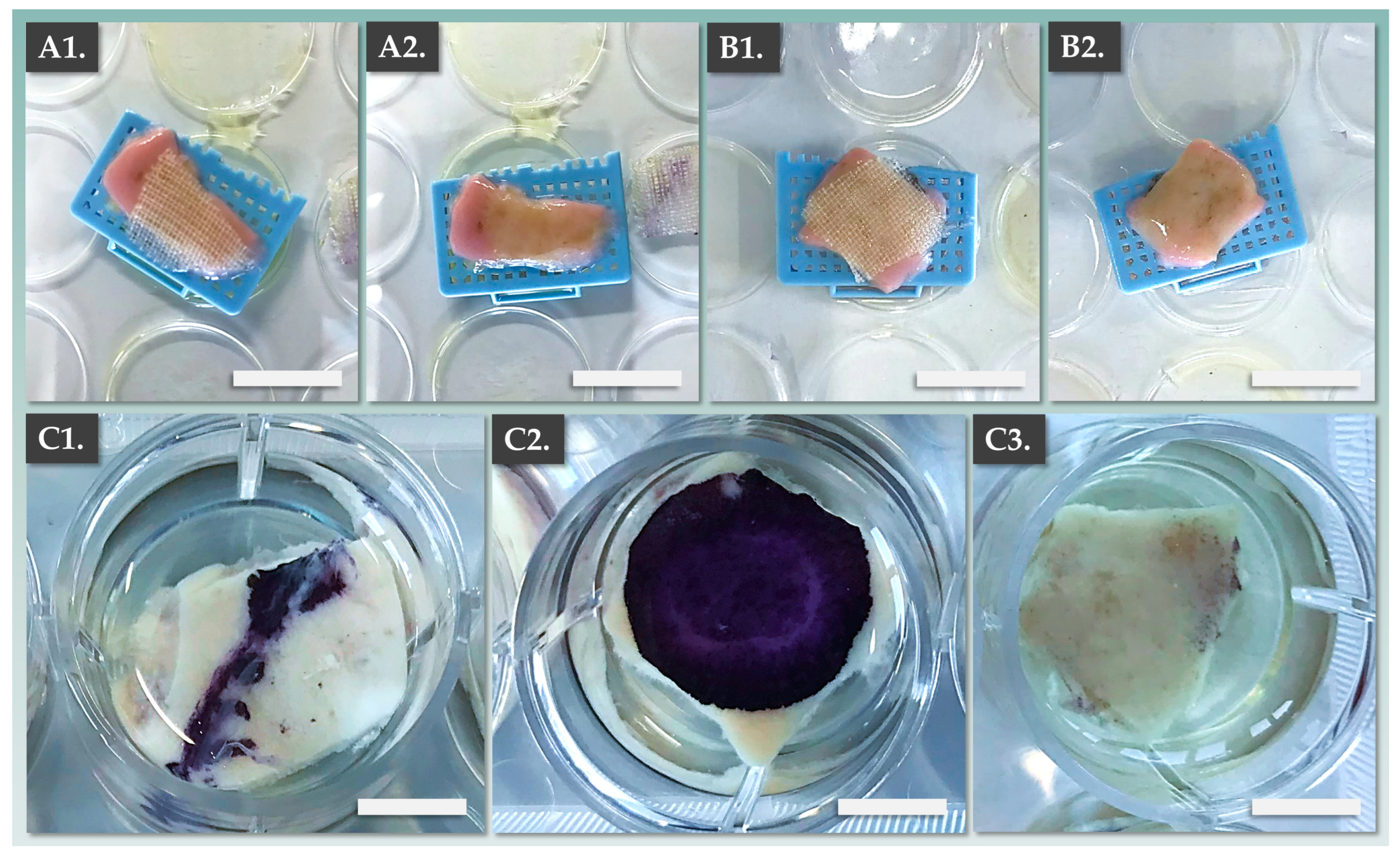
2.8. Combined DE-FE002-SK2 Construct Manufacturing Process
2.9. Combined DE-FE002-SK2 Construct Structural Characterization: Histology and Immunohistochemistry Assays
2.10. Combined DE-FE002-SK2 Construct Functional Characterization: Ex Vivo De-Epidermalized Dermis Model
2.11. Statistical Analyses and Data Presentation
3. Results
3.1. FE002-SK2 Primary Progenitor Fibroblasts Are Functionally Superior to Patient Fibroblasts for In Vitro Collagen Synthesis
3.2. DE-FE002-SK2 Constructs Can Be Manufactured for Clinical Use in Three Weeks
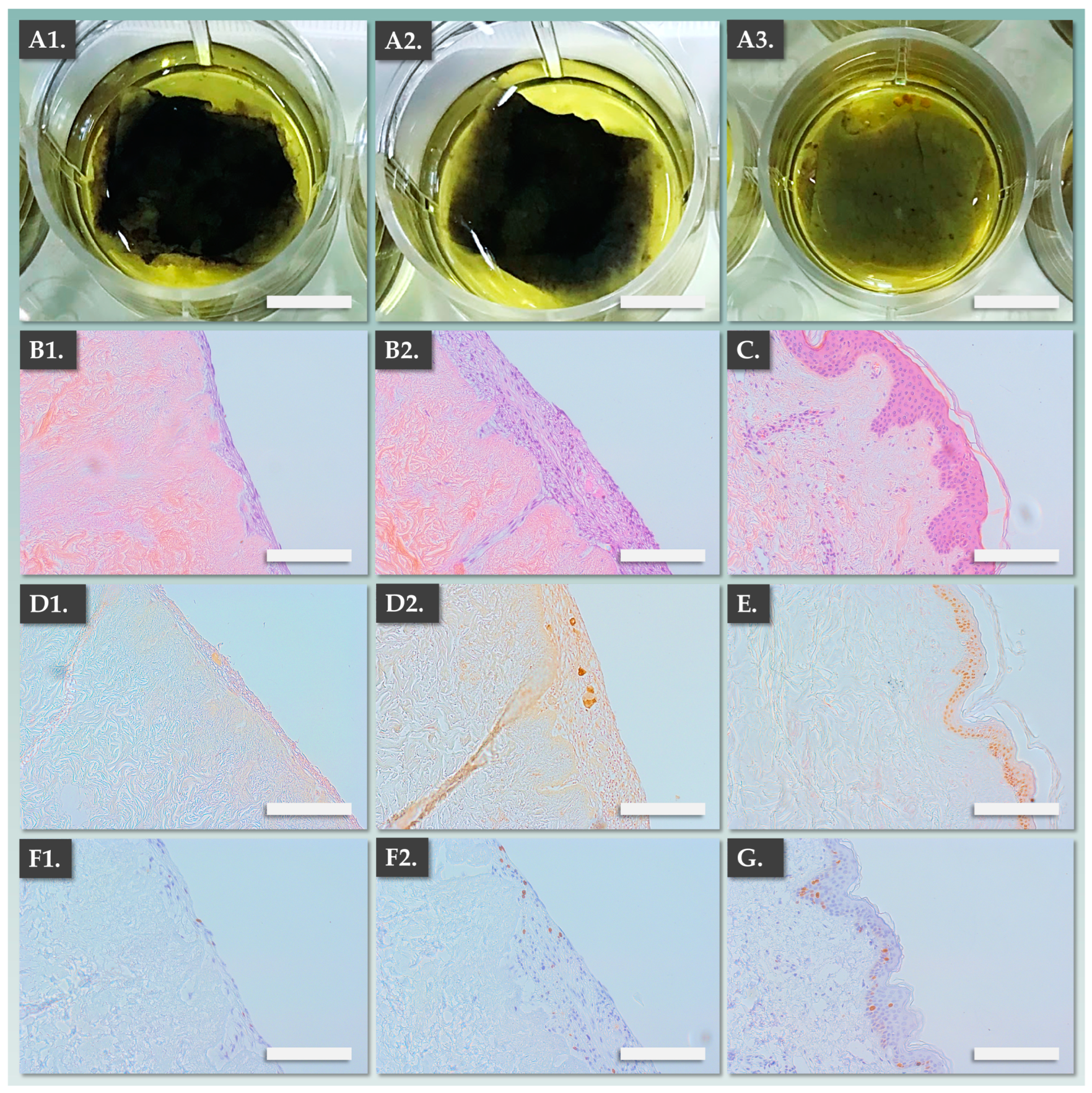
3.3. DE-FE002-SK2 Constructs Display Structural Attributes Which Are Equivalent or Superior to Fully Autologous CDEAs
3.4. DE-FE002-SK2 Constructs Display Enhanced Bio-Adhesive Functions on DED Compared to CEAs
4. Discussion
4.1. Historical Evolution and Technical Bottlenecks in Burn Patient Cytotherapeutic Care
4.2. Transitioning to Complex Cutaneous Grafts for Enhanced Burn Victim Clinical Outcomes
4.3. Dermal Components Are Necessary for High-Quality Closure of Extensive and Deep Burns
4.4. Allogeneic FE002-SK2 Primary Progenitor Fibroblasts Have Been Extensively Clinically Tested
4.5. DE-FE002-SK2 Constructs: Technically Enhanced Modern Alternatives to Standard CDEA Protocols
4.6. Study Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDEA | cultured dermo-epidermal autograft |
| CEA | cultured epithelial autograft |
| CHUV | Centre Hospitalier Universitaire Vaudois |
| DED | de-epidermalized dermis |
| DE-FE002-SK2 | bioengineered skin graft based on cultured allogeneic fibroblasts and autologous keratinocytes |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | dimethyl sulfoxide |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid |
| EOPCB | end of production cell bank |
| FBS | fetal bovine serum |
| FE002-SK2 | clinical grade primary progenitor fibroblast cell source |
| GMP | good manufacturing practices |
| H&E | hematoxylin and eosin |
| MCB | master cell bank |
| PBB | progenitor biological bandages |
| PBS | phosphate-buffered saline |
| PCB | parental cell bank |
| TBSA | total body surface area |
| UK | United Kingdom |
| USA | United States of America |
| WCB | working cell bank |
References
- Greenlagh, D.G. Management of burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef]
- Schlottmann, F.; Bucan, V.; Vogt, P.M.; Krezdorn, N. A short history of skin grafting in burns: From the gold standard of autologous skin grafting to the possibilities of allogeneic skin grafting with immunomodulatory approaches. Medicina 2021, 57, 225. [Google Scholar] [CrossRef]
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.-P.; Michetti, M.; Brunet, J.-F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn center organization and cellular therapy integration: Managing risks and costs. J. Burn Care Res. 2021, 42, 911–924. [Google Scholar] [CrossRef]
- Vogt, P.M.; Busche, M.N. Evaluation of infrastructure, equipment and training of 28 burn units/burn centers in Germany, Austria and Switzerland. Burns 2011, 37, 257–264. [Google Scholar] [CrossRef]
- Münzberg, M.; Ziegler, B.; Fischer, S.; Wölfl, C.G.; Grützner, P.A.; Kremer, T.; Kneser, U.; Hirche, C. In view of standardization: Comparison and analysis of initial management of severely burned patients in Germany, Austria and Switzerland. Burns 2015, 41, 33–38. [Google Scholar] [CrossRef]
- Lukish, J.R.; Eichelberger, M.R.; Newman, K.D. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J. Pediatr. Surg. 2001, 36, 1118–1121. [Google Scholar] [CrossRef]
- Kumar, R.J.; Kimble, R.M.; Boots, R.J.; Pegg, S.P. Treatment of partial-thickness burns: A prospective, randomized trial using TransCyte®. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf®) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef]
- Zuliani, T.; Saiagh, S.; Knol, A.C.; Esbelin, J.; Dréno, B. Fetal fibroblasts and keratinocytes with immunosuppressive properties for allogeneic cell-based wound therapy. PLoS ONE 2013, 8, e70408. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef]
- Climov, M.; Panayi, A.C.; Borah, G.; Orgill, D.P. The life-cycles of skin replacement technologies. PLoS ONE 2020, 15, e0229455. [Google Scholar] [CrossRef]
- Ramezankhani, R.; Torabi, S.; Minaei, N.; Madani, H.; Resaeiani, S.; Hassani, S.N.; Gee, A.P.; Dominici, M.; Silva, D.N.; Baharvand, H.; et al. Two decades of global progress in authorized Advanced Therapy Medicinal Products: An emerging revolution in therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 547653. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Rheinwald, J.G.; Green, H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977, 265, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef]
- O’Connor, N.E.; Mulliken, J.B.; Banks-Schlegel, S.; Kehinde, O.; Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981, 317, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Krupp, S.; Benathan, M.; Déglise, B.; Holzer, E.; Wiesner, L.; Delacrétaz, F.; Chioléro, R. Current concepts in pediatric burn care: Management of burn wounds with cultured epidermal autografts. Eur. J. Pediatr. Surg. 1992, 2, 210–215. [Google Scholar] [CrossRef]
- Braye, F.; Dumortier, R.; Bertin-Maghit, M.; Girbon, J.P.; Tissot, E.; Damour, O. Les cultures d’épiderme pour le traitement des grands brûlés. Etude sur deux ans (18 patients). Ann. Chir. Plast. Esthét. 2001, 46, 599–606. [Google Scholar] [CrossRef]
- Cuono, C.B.; Langdon, R.; Birchall, N.; Barttelbort, S.; McGuire, J. Composite autologous-allogeneic skin replacement: Development and clinical application. Plast. Reconstr. Surg. 1987, 80, 626–637. [Google Scholar] [CrossRef]
- Benathan, M.; Labidi-Ubaldi, F. Substituts épidermiques et dermo-épidermiques vivants pour le traitement des grands brûlés. Rev. Méd. Suisse Rom. 1998, 118, 149–153. [Google Scholar]
- Brockmann, I.; Ehrenpfordt, J.; Sturmheit, T.; Brandenburger, M.; Kruse, C.; Zille, M.; Rose, D.; Boltze, J. Skin-derived stem cells for wound treatment using cultured epidermal autografts: Clinical applications and challenges. Stem Cells Int. 2018, 2018, 4623615. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Li, Z.; Maitz, P.B. Cell therapy for severe burn wound healing. Burn. Trauma 2018, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Gobet, R.; Raghunath, M.; Altermatt, S.; Meuli-Simmen, C.; Benathan, M.; Dietl, A.; Meuli, M. Efficacy of cultured epithelial autografts in pediatric burns and reconstructive surgery. Surgery 1997, 121, 654–661. [Google Scholar] [CrossRef]
- Hartmann-Fritsch, F.; Marino, D.; Reichmann, E. About ATMPs, SOPs and GMP: The hurdles to produce novel skin grafts for clinical use. Transfus. Med. Hemother. 2016, 43, 344–352. [Google Scholar] [CrossRef]
- De Wilde, S.; Veltrop-Duits, L.; Hoozemans-Strik, M.; Ras, T.; Blom-Veenman, J.; Guchelaar, H.J.; Zandvliet, M.; Meij, P. Hurdles in clinical implementation of academic Advanced Therapy Medicinal Product: A national evaluation. Cytotherapy 2016, 18, 797–805. [Google Scholar] [CrossRef]
- Monnier, S.; Abdel-Sayed, P.; de Buys Roessingh, A.; Hirt-Burri, N.; Chemali, M.; Applegate, L.A.; Raffoul, W. Surgical management evolution between 2 massive burn cases at 17-year interval: Contribution of cell therapies in improving the surgical care. Cell Transplant. 2020, 29, 963689720973642. [Google Scholar] [CrossRef]
- Pontiggia, L.; Klar, A.; Böttcher-Haberzeth, S.; Biedermann, T.; Meuli, M.; Reichmann, E. Optimizing in vitro culture conditions leads to a significantly shorter production time of human dermo-epidermal skin substitutes. Pediatr. Surg. Int. 2013, 29, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Meuli, M.; Hartmann-Fritsch, F.; Hüging, M.; Marino, D.; Saglini, M.; Hynes, S.; Neuhaus, K.; Manuel, E.; Middelkoop, E.; Reichmann, E.; et al. A cultured autologous dermo-epidermal skin substitute for full-thickness skin defects: A Phase I, open, prospective clinical trial in children. Plast. Reconstr. Surg. 2019, 144, 188–198. [Google Scholar] [CrossRef]
- Moiemen, N.; Schiestl, C.; Hartmann-Fritsch, F.; Neuhaus, K.; Reichmann, E.; Löw, A.; Stenger, C.; Böttcher-Haberzeth, S.; Meuli, M. First time compassionate use of laboratory engineered autologous Zurich skin in a massively burned child. Burns Open 2021, 5, 113–117. [Google Scholar] [CrossRef]
- Schiestl, C.; Meuli, M.; Vojvodic, M.; Pontiggia, L.; Neuhaus, D.; Brotschi, B.; Reichmann, E.; Böttcher-Haberzeth, S.; Neuhaus, K. Expanding into the future: Combining a novel dermal template with distinct variants of autologous cultured skin substitutes in massive burns. Burns Open 2021, 5, 145–153. [Google Scholar] [CrossRef]
- Klama-Baryła, A.; Kitala, D.; Łabuś, W.; Kraut, M.; Glik, J.; Nowak, M.; Kawecki, M. Autologous and allogeneic skin cell grafts in the treatment of severely burned patients: Retrospective clinical study. Transplant. Proc. 2018, 50, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Coulomb, B.; Legreton, C.; Dubertret, L. Influence of human dermal fibroblasts on epidermalization. J. Investig. Dermatol. 1989, 92, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Lamme, E.N.; Van Leeuwen, R.T.J.; Brandsma, K.; Van Marle, J.; Middelkoop, E. Higher numbers of autologous fibroblasts in an artificial dermal substitute improve tissue regeneration and modulate scar tissue formation. J. Pathol. 2000, 190, 595–603. [Google Scholar] [CrossRef]
- Hohlfeld, J.; de Buys Roessingh, A.S.; Hirt-Burri, N.; Chaubert, P.; Gerber, S.; Scaletta, C.; Hohlfeld, P.; Applegate, L.A. Tissue engineered fetal skin constructs for paediatric burns. Lancet 2005, 366, 840–842. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive materials promote wound healing through modulation of cell behaviors. Adv. Sci. 2022, 9, e2105152. [Google Scholar] [CrossRef]
- Al-Dourobi, K.; Laurent, A.; Deghayli, L.; Flahaut, M.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; Waselle, L.; Simon, J.-P.; El Ezzi, O.; et al. Retrospective evaluation of Progenitor Biological Bandage use: A complementary and safe therapeutic management option for prevention of hypertrophic scarring in pediatric burn care. Pharmaceuticals 2021, 14, 201. [Google Scholar] [CrossRef]
- Mandla, S.; Davenport Huyer, L.; Radisic, M. Review: Multimodal bioactive material approaches for wound healing. APL Bioeng. 2018, 2, 021503. [Google Scholar] [CrossRef]
- Laurent, A.E.; Lin, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; De Buys Roessingh, A.S.; Raffoul, W.; She, B.-R.; Applegate, L.A. Bringing safe and standardized cell therapies to industrialized processing for burns and wounds. Front. Bioeng. Biotechnol. 2020, 8, 581. [Google Scholar] [CrossRef]
- Hata, R.-Y.; Senoo, H. L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J. Cell Physiol. 1989, 138, 8–16. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Jonkman, M.F.; Dijkman, R.; Ponec, M. Basement membrane reconstruction in human skin equivalents is regulated by fibroblasts and/or exogenously activated keratinocytes. J. Investig. Dermatol. 2005, 124, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.; Medawar, P.B. The fate of skin homografts in man. J. Anat. 1943, 77, 299–310. [Google Scholar]
- Medawar, P.B. Immunity to homologous grafted skin; The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948, 29, 58–69. [Google Scholar]
- Leto Barone, A.A.; Mastroianni, M.; Farkash, E.A.; Mallard, C.; Albritton, A.; Torabi, R.; Leonard, D.A.; Kurtz, J.M.; Sachs, D.H.; Cetrulo, C.L., Jr. Genetically modified porcine split-thickness skin grafts as an alternative to allograft for provision of temporary wound coverage: Preliminary characterization. Burns 2015, 41, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Dal Pra, I.; Armato, U. In vitro and in vivo characteristics of frozen/thawed neonatal pig split-skin strips: A novel biologically active dressing for areas of severe, acute or chronic skin loss. Int. J. Mol. Med. 2007, 19, 245–255. [Google Scholar] [CrossRef][Green Version]
- Hanna Luze, H.; Nischwitz, S.P.; Smolle, C.; Robert Zrim, R.; Kamolz, L.-P. The use of acellular fish skin grafts in burn wound management—A systematic review. Medicina 2022, 58, 912. [Google Scholar] [CrossRef]
- Júnior, E.M.L.; Filho, M.O.D.M.; Costa, B.A.; Fechine, F.V.; Vale, M.L.; Diógenes, A.K.D.L.; Neves, K.R.T.; Uchôa, A.M.D.N.; Soares, M.F.A.D.N.; de Moraes, M.E.A. Nile Tilapia fish skin–based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: A Phase III randomized controlled trial. Plast. Reconstr. Surg. 2021, 147, 1189–1198. [Google Scholar] [CrossRef]
- Yang, C.K.; Polanco, T.O.; Ii, J.C.L. A prospective, postmarket, compassionate clinical evaluation of a novel acellular fish-skin graft which contains Omega-3 fatty acids for the closure of hard-to-heal lower extremity chronic ulcers. Wounds 2016, 28, 112–118. [Google Scholar] [PubMed]
- Vogt, P.M.; Thompsont, S.; Andree, C.; Liu, P.; Breuing, K.; Hatzis, D.; Brown, H.; Mulligant, R.C.; Eriksson, E.; Murray, J.E. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc. Natl. Acad. Sci. USA 1994, 91, 9307–9311. [Google Scholar] [CrossRef]
- Sykulev, Y.; Joo, M.; Vturina, I.; Tsomides, T.J.; Eisen, H.N. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity 1996, 4, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Laurent, A.; Rey, M.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.; Hirt-Burri, N.; Applegate, L.A. Retrospectives on three decades of safe clinical experience with allogeneic dermal progenitor fibroblasts: High versatility in topical cytotherapeutic care. Pharmaceutics 2023, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Fisch, P.; Formica, F.A.; Gareus, R.; Linder, T.; Applegate, L.A.; Zenobi-Wong, M. A comparative study of cartilage engineered constructs in immunocompromised, humanized and immunocompetent mice. J. Immunol. Regen. Med. 2018, 2, 36–46. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.; Choi, W.H.; Park, S.R.; Choi, B.H.; Min, B.H. Immunophenotype and immune-modulatory activities of human fetal cartilage-derived progenitor cells. Cell Transplant. 2019, 28, 932–942. [Google Scholar] [CrossRef]
- Marino, D.; Luginbühl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra14. [Google Scholar] [CrossRef]
- Klar, A.S.; Böttcher-Haberzeth, S.; Biedermann, T.; Schiestl, C.; Reichmann, E.; Meuli, M. Analysis of blood and lymph vascularization patterns in tissue-engineered human dermo-epidermal skin analogs of different pigmentation. Pediatr. Surg. Int. 2014, 30, 223–231. [Google Scholar] [CrossRef]
- Hendrickx, B.; Verdonck, K.; Van den Berge, S.; Dickens, S.; Eriksson, E.; Vranckx, J.J.; Luttun, A. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cell 2010, 28, 1165–1177. [Google Scholar] [CrossRef]
- Böttcher-Haberzeth, S.; Biedermann, T.; Pontiggia, L.; Braziulis, E.; Schiestl, C.; Hendriks, B.; Eichhoff, O.M.; Widmer, D.S.; Meuli-Simmen, C.; Meuli, M.; et al. Human eccrine sweat gland cells turn into melanin-uptaking keratinocytes in dermo-epidermal skin substitutes. J. Investig. Dermatol. 2013, 133, 316–324. [Google Scholar] [CrossRef]
- Hirt-Burri, N.; Scaletta, C.; Gerber, S.; Pioletti, D.P.; Applegate, L.A. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Artif. Organs 2008, 32, 509–518. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bukovac, P.K.; Hauser, M.; Lottaz, D.; Marti, A.; Schmitt, I.; Schochat, T. The regulation of cell therapy and gene therapy products in Switzerland. Adv. Exp. Med. Biol. 2023, 1430, 41–58. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Values/Results | |||
|---|---|---|---|---|
| Cell seeding density (viable cells/cm2) | 1500 | 3000 | 6000 | 20,000 |
| Time to confluency (days) | 12 ± 2 | 10 ± 2 | 7 ± 1 | 4 ± 0.5 |
| Medium exchange procedures (n) | 5 | 4 | 3 | 2 |
| Seeding lot size 1 (106 cells) | 2.8 | 5.6 | 11.3 | 37.5 |
| Process Phase | Parameters | Targets | Methods | Gradings 1 | |
|---|---|---|---|---|---|
| CDEA Group | DE-FE002-SK2 Group | ||||
| 1. Dermal Template | Manufacturing timeframe | Two weeks of culture | Operator assessment | − | +++ |
| Fibroblast monolayer formation after expansion | Formation of a homogeneous cellular layer | Operator assessment; microscopy | ++ | +++ | |
| Dermal template sheet formation after vitamin C induction | Formation of a dermal template by collagen synthesis | Operator assessment; collagen staining | ++ | +++ | |
| Homogeneity of the dermal template sheet | Consistency of dermal template attributes over the whole surface | Operator assessment; histology | ++ | +++ | |
| Robustness of the dermal template sheet | Possibility to detach and manipulate the dermal template | Operator assessment; handling | + | +++ | |
| 2. Combined Construct | Manufacturing timeframe | One week of culture | Operator assessment | + | +++ |
| Compatibility between the dermal template and autologous keratinocytes | Passive combination of components in co-culture | Operator assessment; histology | ++ | +++ | |
| Stratification of the epidermal component | Formation of a stratified epidermal component | Operator assessment; histology | + | +++ | |
| Homogeneity of the construct | Consistency of construct attributes over the whole surface | Operator assessment; histology | + | ++ | |
| Robustness of the construct | Possibility to detach and manipulate the construct | Operator assessment; handling | + | ++ | |
| Attribute Type | Parameters | Targets | Methods | Gradings 1 | |
|---|---|---|---|---|---|
| CEA Group | DE-FE002-SK2 Group | ||||
| 1. Translational Attributes | Transfer of the construct to a Vaseline gauze | Possibility to manipulate and transport the construct using the gauze | Operator assessment | ++ | +++ |
| Application of the construct on the DED model | Simple topical application on the DED surface | Operator assessment | +++ | +++ | |
| Applied construct structural integrity maintenance | Maintenance of construct structural integrity on the DED model | Operator assessment | ++ | ++ | |
| 2. Functional Attributes | Initial adhesion of the construct to the DED model | Construct adheres rapidly to the DED model | Operator assessment | ++ | ++ |
| Endpoint homogeneous adhesion of the construct to the DED model | Construct adheres homogeneously to the DED model | Operator assessment; MTT | − | ++ | |
| Endpoint homogeneous metabolic activity throughout of the construct on the DED model | Cellular metabolic activity is significant and homogeneous throughout the construct on the DED model | Operator assessment; MTT | ± | +++ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Laurent, A.; Liao, Z.; Jaccoud, S.; Abdel-Sayed, P.; Flahaut, M.; Scaletta, C.; Raffoul, W.; Applegate, L.A.; Hirt-Burri, N. Cutaneous Cell Therapy Manufacturing Timeframe Rationalization: Allogeneic Off-the-Freezer Fibroblasts for Dermo-Epidermal Combined Preparations (DE-FE002-SK2) in Burn Care. Pharmaceutics 2023, 15, 2334. https://doi.org/10.3390/pharmaceutics15092334
Chen X, Laurent A, Liao Z, Jaccoud S, Abdel-Sayed P, Flahaut M, Scaletta C, Raffoul W, Applegate LA, Hirt-Burri N. Cutaneous Cell Therapy Manufacturing Timeframe Rationalization: Allogeneic Off-the-Freezer Fibroblasts for Dermo-Epidermal Combined Preparations (DE-FE002-SK2) in Burn Care. Pharmaceutics. 2023; 15(9):2334. https://doi.org/10.3390/pharmaceutics15092334
Chicago/Turabian StyleChen, Xi, Alexis Laurent, Zhifeng Liao, Sandra Jaccoud, Philippe Abdel-Sayed, Marjorie Flahaut, Corinne Scaletta, Wassim Raffoul, Lee Ann Applegate, and Nathalie Hirt-Burri. 2023. "Cutaneous Cell Therapy Manufacturing Timeframe Rationalization: Allogeneic Off-the-Freezer Fibroblasts for Dermo-Epidermal Combined Preparations (DE-FE002-SK2) in Burn Care" Pharmaceutics 15, no. 9: 2334. https://doi.org/10.3390/pharmaceutics15092334
APA StyleChen, X., Laurent, A., Liao, Z., Jaccoud, S., Abdel-Sayed, P., Flahaut, M., Scaletta, C., Raffoul, W., Applegate, L. A., & Hirt-Burri, N. (2023). Cutaneous Cell Therapy Manufacturing Timeframe Rationalization: Allogeneic Off-the-Freezer Fibroblasts for Dermo-Epidermal Combined Preparations (DE-FE002-SK2) in Burn Care. Pharmaceutics, 15(9), 2334. https://doi.org/10.3390/pharmaceutics15092334







