Autologous and Allogeneic Cytotherapies for Large Knee (Osteo)Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Consumables Used for the Study
2.2. Instruments and Equipment Used for the Study
2.3. Ethical Compliance of the Study and Regulatory Approval of Investigative Cytotherapeutic Protocols
2.4. Autologous Primary Chondrocyte Sourcing and Chondrogenic Cellular Active Substance Lot Manufacturing
2.5. Allogeneic FE002 Primary Chondroprogenitor Sourcing and Chondrogenic Cellular Active Substance Lot Manufacturing
2.6. FE002 Primary Chondroprogenitor Cellular Active Substance Characterization Assays
2.6.1. Allogeneic FE002 Cellular Active Substance Characterization: Cell Expansion Medium Selection
2.6.2. Allogeneic FE002 Cellular Active Substance Characterization: Multiplex Proteomic Analyses
2.6.3. Allogeneic FE002 Cellular Active Substance Qualification: β-Galactosidase Staining for In Vitro Senescence Assessment
2.6.4. Allogeneic FE002 Cellular Active Substance Qualification: Telomerase Activity Quantification for In Vitro Tumorigenicity Assessment
2.6.5. Allogeneic FE002 Cellular Active Substance Qualification: Soft Agarose Colony Formation Assay for In Vitro Semi-Quantitative Tumorigenicity Assessment
2.7. Cytotherapeutic Finished Product Manufacturing Process: Chondrogenic Induction of Cell-Bearing Chondro-Gide Constructs
2.7.1. Autologous Chondrocyte-Bearing Graft Manufacturing Process
2.7.2. Allogeneic FE002 Chondroprogenitor-Bearing Graft Manufacturing Process
2.8. Cytotherapeutic Finished Product Controls: Functional Validation
2.8.1. MTT Staining for Assessing Metabolic Activity and Cell Distribution throughout the Constructs
2.8.2. Evaluation of Chondrogenic Gene Induction in the Constructs via RT-PCR
2.8.3. DMMB Quantification for Assessment of ECM Synthesis and Deposition throughout the Constructs
2.8.4. Staining and Immunohistology for Specific ECM Component Visualization in the Constructs
2.9. Translational Qualification of the Allogeneic Cytotherapeutic Finished Products
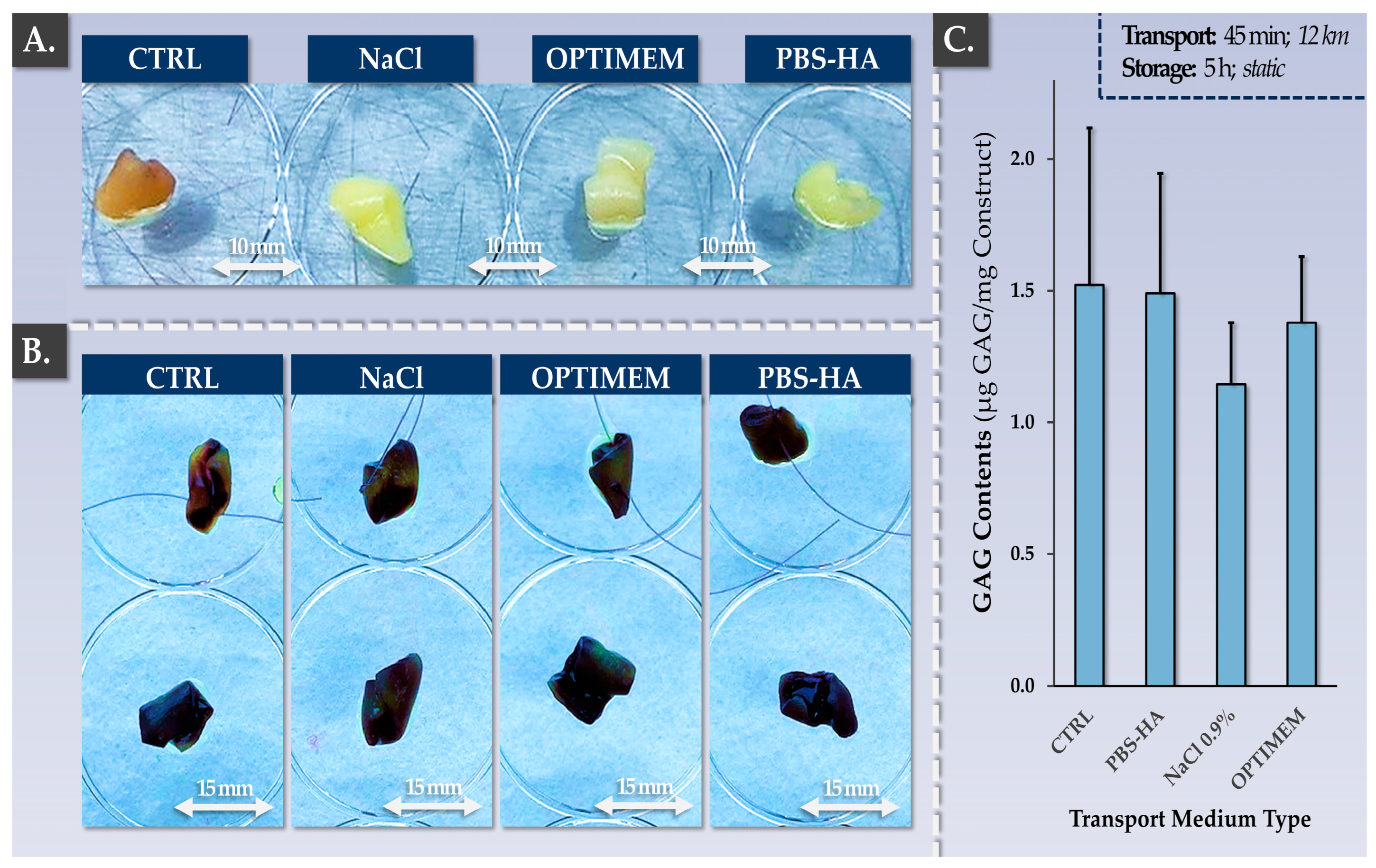
2.9.1. Qualification of Allogeneic Finished Product Transport Medium and Product Validity Period Validation
2.9.2. Validation of Allogeneic Finished Product Implantability via Suture Testing
2.10. Statistical Analysis and Data Presentation
3. Results
3.1. FE002 Primary Chondroprogenitors Possess Quality and Safety Attributes Compatible with Clinical Tissue Engineering
3.2. FE002 Primary Chondroprogenitors Possess Quality and Functionality Attributes Compatible with the Controlled GMP Manufacturing of Chondrogenic Cellular Active Substances
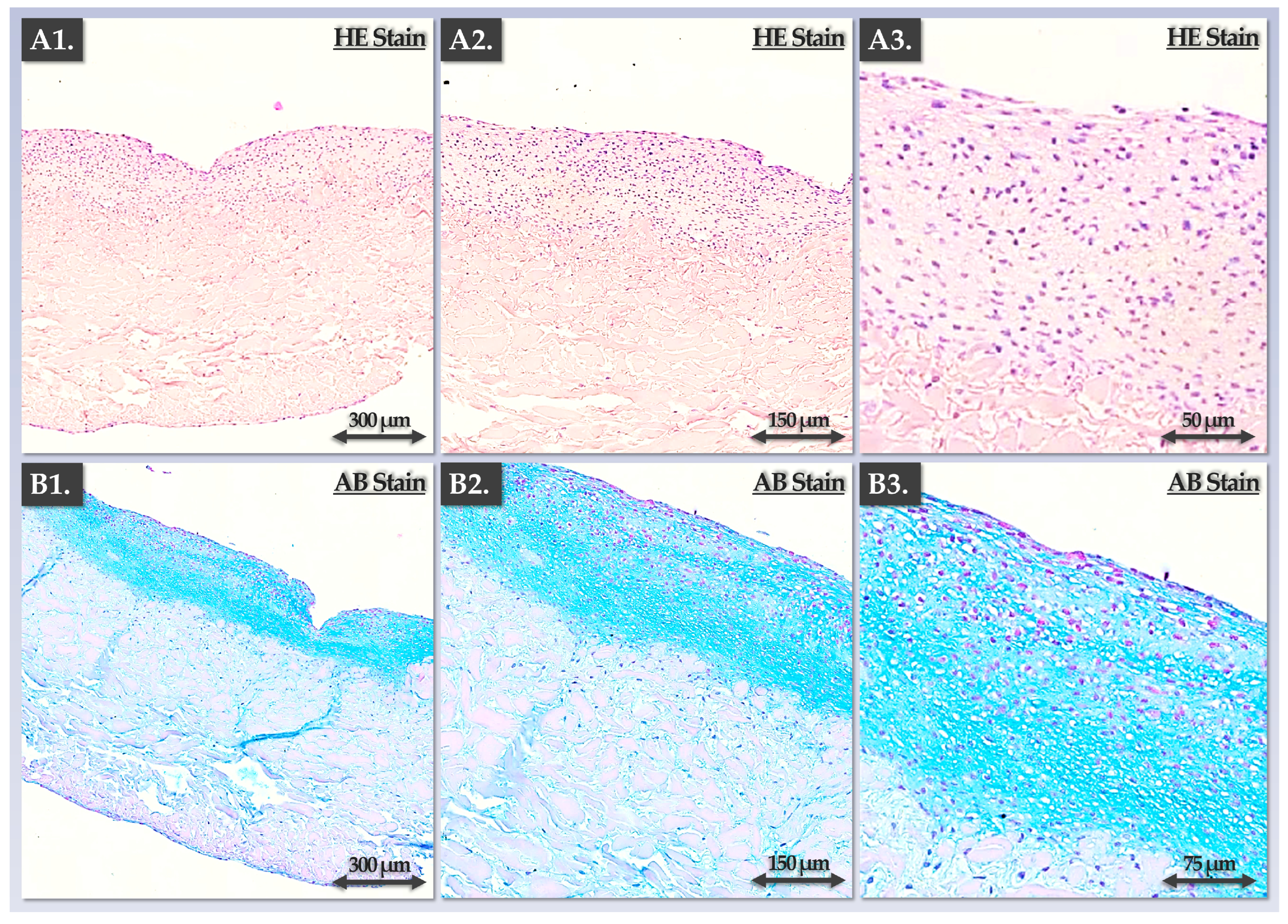
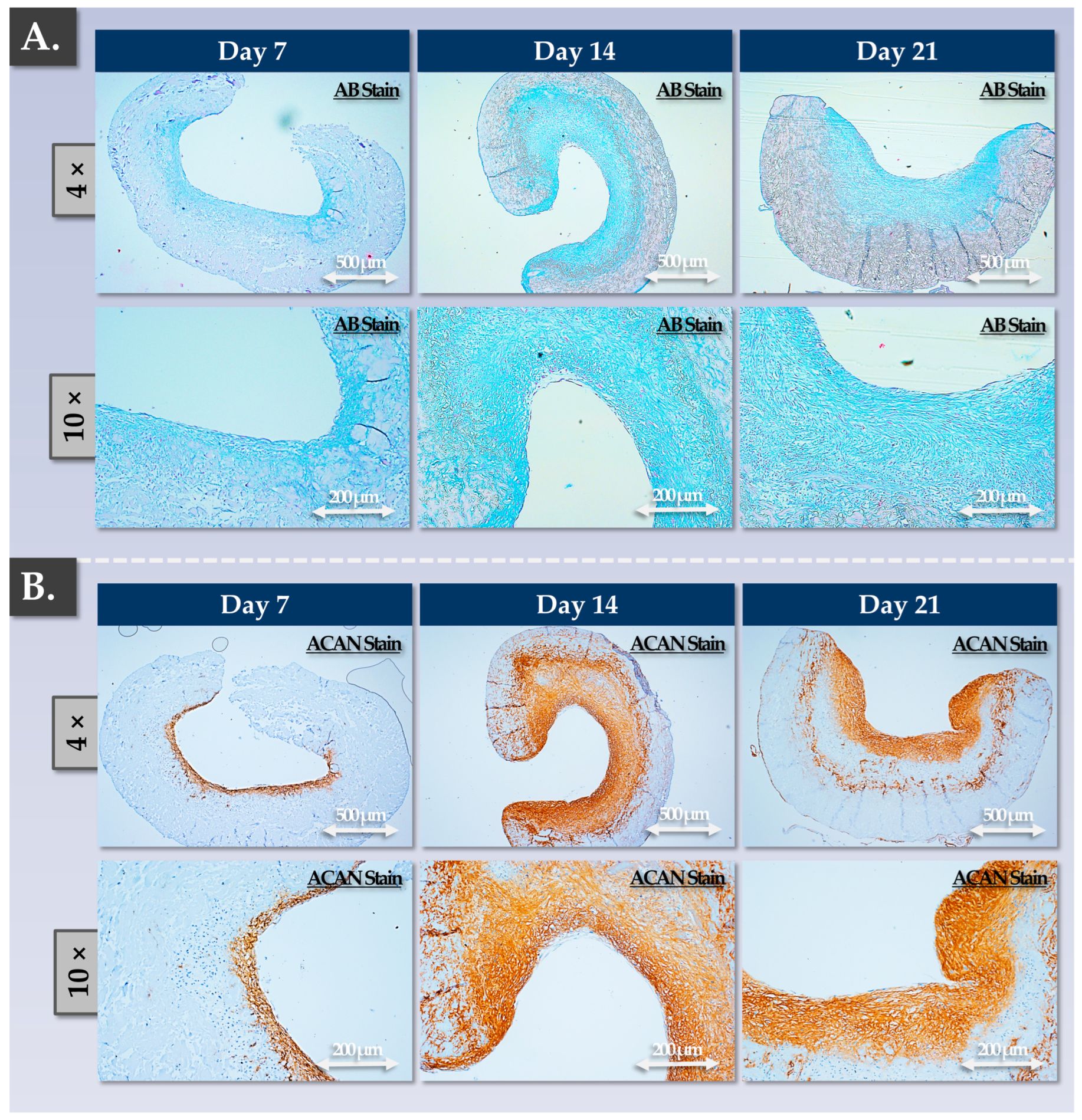
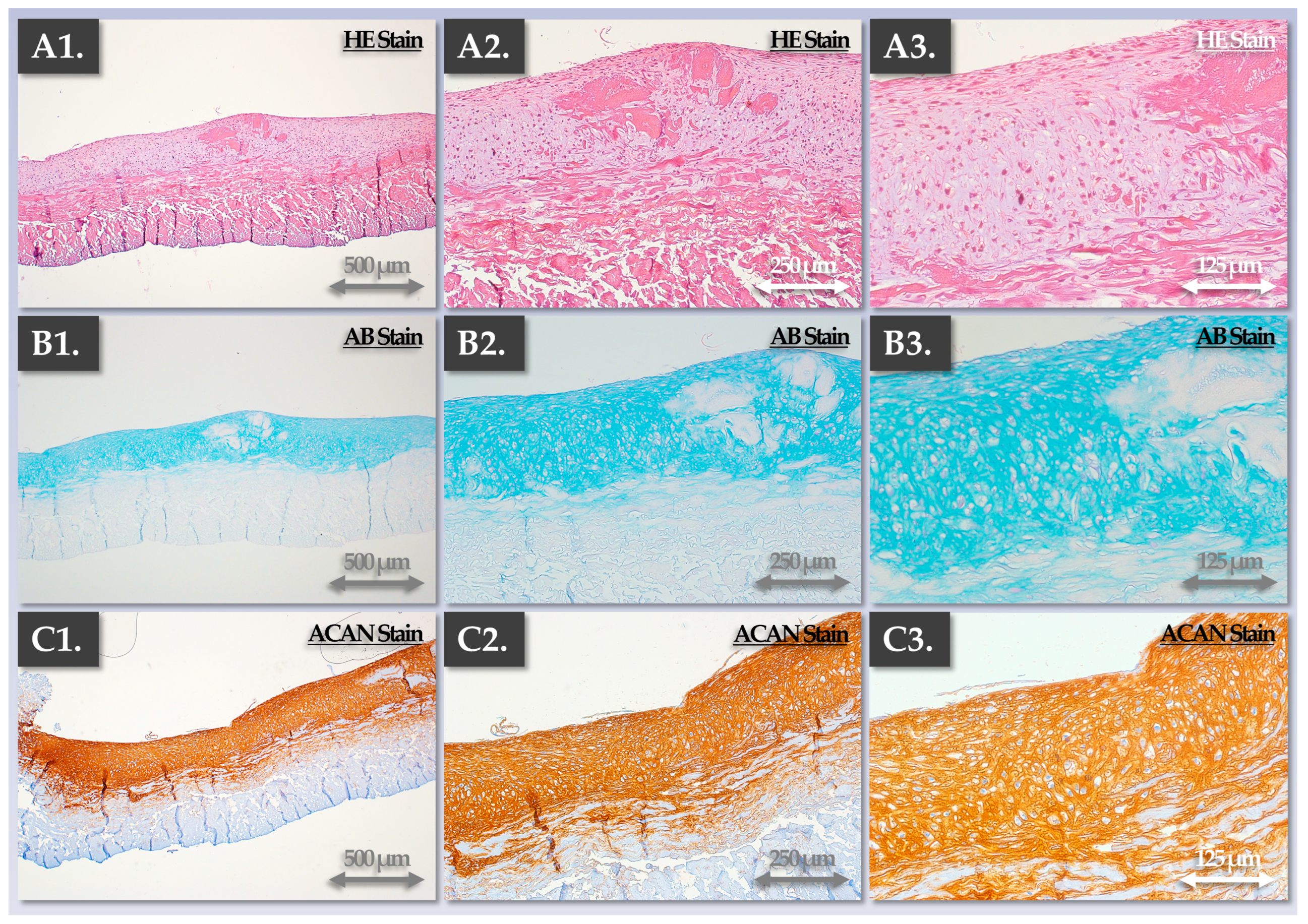
3.3. FE002 Primary Chondroprogenitors Possess Functional Attributes Comparable to Those of Clinical-grade HACs within Chondrogenically Induced Chondro-Gide Constructs
3.4. Allogeneic Finished Products Possess an Appropriate Pharmaceutical Form and Stability Attributes for Clinical Orthopedic Bioengineering
4. Discussion
4.1. Progressive Translational Development of Cell Therapies for Cartilage Repair/Regeneration: Extensive Manufacturing Experience and Long-Term Clinical Research on ACI
4.2. Safety, Quality, and Efficacy Attributes: FE002 Primary Chondroprogenitors Are Compatible with Modern Clinical Regenerative Medicine Requirements
4.3. FE002 Primary Chondroprogenitors Are Functionally Comparable to Clinical-grade HACs in Chondro-Gide Constructs
4.4. Autologous versus Allogeneic Approaches to Large (Osteo)-Chondral Defects of the Knee: Comparative Burden Analysis for Clinical Pathway Rationalization
4.5. Identified Study Limitations and Future Clinical Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niemeyer, P.; Hanus, M.; Belickas, J.; László, T.; Gudas, R.; Fiodorovas, M.; Cebatorius, A.; Pastucha, M.; Hoza, P.; Magos, K.; et al. Treatment of large cartilage defects in the knee by hydrogel-based autologous chondrocyte implantation: Two-year results of a prospective, multicenter, single-arm phase III trial. Cartilage 2022, 13, 19476035221085146. [Google Scholar] [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef]
- Brittberg, M.; Gomoll, A.H.; Canseco, J.A.; Far, J.; Lind, M.; Hui, J. Cartilage repair in the degenerative ageing knee. Acta Orthop. 2016, 87, 26–38. [Google Scholar] [CrossRef]
- Martin, R.; Laurent, A.; Applegate, L.A.; Philippe, V. Grands défects chondraux et ostéochondraux du genou: Traitement par greffe chondrocytaire autologue. Rev. Med. Suisse 2022, 18, 2384–2390. [Google Scholar] [CrossRef]
- Saris, D.B.; Vanlauwe, J.; Victor, J.; Almqvist, K.F.; Verdonk, R.; Bellemans, J.; Luyten, F.P.; TIG/ACT/01/2000&EXT Study Group. Treatment of symptomatic cartilage defects of the knee: Characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am. J. Sports Med. 2009, 37, 10S–19S. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Int. Med. 2011, 2, 205–212. [Google Scholar]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sports Med. 2010, 38, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Hoburg, A.; Niemeyer, P.; Laute, V.; Zinser, W.; Becher, C.; Kolombe, T.; Fay, J.; Pietsch, S.; Kuźma, T.; Widuchowski, W.; et al. Sustained superiority in KOOS subscores after matrix-associated chondrocyte implantation using spheroids compared to microfracture. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2482–2493. [Google Scholar] [CrossRef] [PubMed]
- Urlic, I.; Ivkovic, A. Cell sources for cartilage repair-Biological and clinical perspective. Cells 2021, 10, 2496. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.; Hoffman, L.; Zak, L.; Tiefenboeck, T.; Aldrian, S.; Albrecht, C. Clinical evaluation after matrix-associated autologous chondrocyte transplantation: A comparison of four different graft types. Bone Joint Res. 2021, 10, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kili, S.; Mills, S.; Levine, D.W. Tissue-engineered cartilage products: Clinical experience. In Principles of Tissue Engineering: Fourth Edition; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Davies, R.L.; Kuiper, N.J. Regenerative medicine: A review of the evolution of autologous chondrocyte implantation (ACI) therapy. Bioengineering 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.; Vasiliadis, H.S.; Brittberg, M.; Lindahl, A. Autologous chondrocyte implantation: A long-term follow-up. Am. J. Sports Med. 2010, 38, 1117–1124. [Google Scholar] [CrossRef]
- Brittberg, M.; Recker, D.; Ilgenfritz, J.; Saris, D.B.F.; SUMMIT Extension Study Group. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Five-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2018, 46, 1343–1351. [Google Scholar] [CrossRef]
- Peterson, L.; Minas, T.; Brittberg, M.; Nilsson, A.; Sjögren-Jansson, E.; Lindahl, A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin. Orthop. Rel. Res. 2000, 374, 212–234. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Oussedik, S.; Tsitskaris, K.; Parker, D. Treatment of articular cartilage lesions of the knee by microfracture or autologous chondrocyte implantation: A systematic review. Arthroscopy 2015, 31, 732–744. [Google Scholar] [CrossRef]
- Saris, D.; Price, A.; Widuchowski, W.; Bertrand-Marchand, M.; Caron, J.; Drogset, J.O.; Emans, P.; Podskubka, A.; Tsuchida, A.; Kili, S.; et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: Two-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2014, 42, 1384–1394. [Google Scholar] [CrossRef]
- Gille, J.; Kunow, J.; Boisch, L.; Behrens, P.; Bos, I.; Hoffmann, C.; Köller, W.; Russlies, M.; Kurz, B. Cell-laden and cell-free matrix-induced chondrogenesis versus microfracture for the treatment of articular cartilage defects: A histological and biomechanical study in sheep. Cartilage 2010, 1, 29–42. [Google Scholar] [CrossRef]
- Ibarra, C.; Villalobos, E.; Madrazo-Ibarra, A.; Velasquillo, C.; Martinez-Lopez, V.; Izaguirre, A.; Olivos-Meza, A.; Cortes-Gonzalez, S.; Perez-Jimenez, F.J.; Vargas-Ramirez, A.; et al. Arthroscopic matrix-assisted autologous chondrocyte transplantation versus microfracture: A 6-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2021, 49, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Basad, E.; Ishaque, B.; Bachmann, G.; Stürz, H.; Steinmeyer, J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: A 2-year randomised study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.C.; DeBerardino, T.M.; Williams, R.J., 3rd. NeoCart, an autologous cartilage tissue implant, compared with microfracture for treatment of distal femoral cartilage lesions: An FDA phase-II prospective, randomized clinical trial after two years. J. Bone Joint Surg. 2012, 94, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, D.; Staes, F.; Van Caspel, D.; Vanlauwe, J.; Bellemans, J.; Saris, D.B.; Luyten, F.P. Autologous chondrocyte implantation versus microfracture for knee cartilage injury: A prospective randomized trial, with 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 486–495. [Google Scholar] [CrossRef]
- Petri, M.; Broese, M.; Simon, A.; Liodakis, E.; Ettinger, M.; Guenther, D.; Zeichen, J.; Krettek, C.; Jagodzinski, M.; Haasper, C. CaReS (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: A retrospective matched-pair analysis. J. Orthop. Sci. 2013, 18, 38–44. [Google Scholar] [CrossRef]
- Cortese, F.; McNicholas, M.; Janes, G.; Gillogly, S.; Abelow, S.P.; Gigante, A.; Coletti, N. Arthroscopic delivery of matrix-induced autologous chondrocyte implant: International experience and technique recommendations. Cartilage 2012, 3, 156–164. [Google Scholar] [CrossRef]
- Abelow, S.P.; Guillen, P.; Ramos, T. Arthroscopic technique for matrix-induced autologous chondrocyte implantation for the treatment of large chondral defects in the knee and ankle. Oper. Tech. Orthop. 2006, 16, 257–261. [Google Scholar] [CrossRef]
- Bentley, G.; Biant, L.C.; Vijayan, S.; Macmull, S.; Skinner, J.A.; Carrington, R.W. Minimum ten-year results of a prospective randomised study of autologous chondrocyte implantation versus mosaicplasty for symptomatic articular cartilage lesions of the knee. J. Bone Joint Surg. 2012, 94, 504–509. [Google Scholar] [CrossRef]
- Biant, L.C.; Bentley, G.; Vijayan, S.; Skinner, J.A.; Carrington, R.W. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am. J. Sports Med. 2014, 42, 2178–2183. [Google Scholar] [CrossRef]
- Huang, B.J.; Hu, J.C.; Athanasiou, K.A. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials 2016, 98, 1–22. [Google Scholar] [CrossRef]
- Mumme, M.; Barbero, A.; Miot, S.; Wixmerten, A.; Feliciano, S.; Wolf, F.; Asnaghi, A.M.; Baumhoer, D.; Bieri, O.; Kretzcchmar, M.; et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: An observational first-in-human trial. Lancet 2016, 388, 1985–1994. [Google Scholar] [CrossRef]
- Vinardell, T.; Sheehy, E.J.; Buckley, C.T.; Kelly, D.J. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng. Part A 2012, 18, 1161–1170. [Google Scholar] [CrossRef]
- Almqvist, K.F.; Dhollander, A.A.; Verdonk, P.C.; Forsyth, R.; Verdonk, R.; Verbruggen, G. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sports Med. 2009, 37, 1920–1929. [Google Scholar] [CrossRef]
- Zak, L.; Albrecht, C.; Wondrasch, B.; Widhalm, H.; Vekszler, G.; Trattnig, S.; Marlovits, S.; Aldrian, S. Results 2 years after matrix-associated autologous chondrocyte transplantation using the Novocart 3D scaffold: An analysis of clinical and radiological data. Am. J. Sports Med. 2014, 42, 1618–1627. [Google Scholar] [CrossRef]
- Marcacci, M.; Berruto, M.; Brocchetta, D.; Delcogliano, A.; Ghinelli, D.; Gobbi, A.; Kon, E.; Pederzini, L.; Rosa, D.; Sacchetti, G.L.; et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin. Orthop. Rel. Res. 2005, 435, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, T.R.; Gallik, D.; Chevalier, Y.; Holzgruber, M.; Baur-Melnyk, A.; Müller, P.E.; Pietschmann, M.F. Effect of the defect localization and size on the success of third-generation autologous chondrocyte implantation in the knee joint. Internat. Orthop. 2021, 45, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.S.; Roberts, S. A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthritis Cart. 2013, 21, 2048–2057. [Google Scholar] [CrossRef]
- Vijayan, S.; Bartlett, W.; Bentley, G.; Carrington, R.W.; Skinner, J.A.; Pollock, R.C.; Alorjani, M.; Briggs, T.W. Autologous chondrocyte implantation for osteochondral lesions in the knee using a bilayer collagen membrane and bone graft: A two- to eight-year follow-up study. J. Bone Joint Surg. 2012, 94, 488–492. [Google Scholar] [CrossRef]
- Brix, M.O.; Stelzeneder, D.; Chiari, C.; Koller, U.; Nehrer, S.; Dorotka, R.; Windhager, R.; Domayer, S.E. Treatment of full-thickness chondral defects with Hyalograft C in the knee: Long-term results. Am. J. Sports Med. 2014, 42, 1426–1432. [Google Scholar] [CrossRef]
- Hossain, M.A.; Adithan, A.; Alam, M.J.; Kopalli, S.R.; Kim, B.; Kang, C.W.; Hwang, K.C.; Kim, J.H. IGF-1 facilitates cartilage reconstruction by regulating PI3K/AKT, MAPK, and NF-kB signaling in rabbit osteoarthritis. J. Infl. Res. 2021, 14, 3555–3568. [Google Scholar] [CrossRef]
- Kisiday, J.D. Expansion of chondrocytes for cartilage tissue engineering: A review of chondrocyte dedifferentiation and redifferentiation as a function of growth in expansion culture. Regen. Med. Front. 2020, 2, e200002. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Wen, Y.; Chen, J.; Lin, J.; Sheng, Z.; Zhou, W.; Sun, H.; An, C.; Chen, J.; et al. A high-resolution route map reveals distinct stages of chondrocyte dedifferentiation for cartilage regeneration. Bone Res. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Enochson, L.; Brittberg, M.; Lindahl, A. Optimization of a chondrogenic medium through the use of factorial design of experiments. BioRes Open Access 2012, 1, 306–313. [Google Scholar] [CrossRef]
- Martinez, I.; Elvenes, J.; Olsen, R.; Bertheussen, K.; Johansen, O. Redifferentiation of in vitro expanded adult articular chondrocytes by combining the hanging-drop cultivation method with hypoxic environment. Cell Transplant. 2008, 17, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J.; Zheng, M.H. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Tallheden, T.; Karlsson, C.; Brunner, A.; Van Der Lee, J.; Hagg, R.; Tommasini, R.; Lindahl, A. Gene expression during redifferentiation of human articular chondrocytes. Osteoarthritis Cart. 2004, 12, 525–535. [Google Scholar] [CrossRef]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.-F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef]
- Darwiche, S.E.; Scaletta, C.; Raffoul, W.; Pioletti, D.P.; Applegate, L.A. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012, 4, 23–32. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Ducrot, A.; Hirt-Burri, N.; Scaletta, C.; Jaccoud, S.; Nuss, K.; de Buys Roessingh, A.S.; Raffoul, W.; Pioletti, D.P.; et al. Development of standardized fetal progenitor cell therapy for cartilage regenerative medicine: Industrial transposition and preliminary safety in xenogeneic transplantation. Biomolecules 2021, 11, 250. [Google Scholar] [CrossRef]
- Studer, D.; Cavalli, E.; Formica, F.A.; Kuhn, G.A.; Salzmann, G.; Mumme, M.; Steinwachs, M.R.; Applegate, L.A.; Maniura-Weber, K.; Zenobi-Wong, M. Human chondroprogenitors in alginate-collagen hybrid scaffolds produce stable cartilage in vivo. J. Tissue Eng. Regen. Med. 2017, 11, 3014–3026. [Google Scholar] [CrossRef]
- Cavalli, E.; Fisch, P.; Formica, F.A.; Gareus, R.; Linder, T.; Applegate, L.A.; Zenobi-Wong, M. A comparative study of cartilage engineered constructs in immunocompromised, humanized and immunocompetent mice. J. Immunol. Regen. Med. 2018, 2, 36–46. [Google Scholar] [CrossRef]
- Levinson, C.; Lee, M.; Applegate, L.A.; Zenobi-Wong, M. An injectable heparin-conjugated hyaluronan scaffold for local delivery of transforming growth factor β1 promotes successful chondrogenesis. Acta Biomater. 2019, 99, 168–180. [Google Scholar] [CrossRef]
- Porcello, A.; Gonzalez-Fernandez, P.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Allémann, E.; et al. Thermo-responsive hyaluronan-based hydrogels combined with allogeneic cytotherapeutics for the treatment of osteoarthritis. Pharmaceutics 2023, 15, 1528. [Google Scholar] [CrossRef]
- Tritschler, H.; Fischer, K.; Seissler, J.; Fiedler, J.; Halbgebauer, R.; Huber-Lang, M.; Schnieke, A.; Brenner, R.E. New insights into xenotransplantation for cartilage repair: Porcine multi-genetically modified chondrocytes as a promising cell source. Cells 2021, 10, 2152. [Google Scholar] [CrossRef]
- Cherian, J.J.; Parvizi, J.; Bramlet, D.; Lee, K.H.; Romness, D.W.; Mont, M.A. Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-B1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cart. 2015, 23, 2109–2118. [Google Scholar] [CrossRef]
- Asnaghi, M.A.; Power, L.; Barbero, A.; Haug, M.; Köppl, R.; Wendt, D.; Martin, I. Biomarker signatures of quality for engineering nasal chondrocyte-derived cartilage. Front. Bioeng. Biotechnol. 2020, 8, 283. [Google Scholar] [CrossRef]
- Teo, A.Q.A.; Wong, K.L.; Shen, L.; Lim, J.Y.; Wei, S.T.; Lee, H.; Hui, J.H.P. Equivalent 10-year outcomes after implantation of autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation for chondral defects of the knee. Am. J. Sports Med. 2019, 47, 2881–2887. [Google Scholar] [CrossRef]
- Dhollander, A.A.; Verdonk, P.C.; Lambrecht, S.; Verdonk, R.; Elewaut, D.; Verbruggen, G.; Almqvist, K.F. Midterm results of the treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am. J. Sports Med. 2012, 40, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; Min, B.H.; Park, S.R.; Oh, H.J.; Truong, M.D.; Kim, M.; Choi, J.Y.; Park, I.S.; Choi, B.H. Engineered cartilage utilizing fetal cartilage-derived progenitor cells for cartilage repair. Sci. Rep. 2020, 10, 5722. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.-W.; Noh, M.J.; Choi, K.B.; Lee, K.H. Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 2012, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Pelttari, K.; Pippenger, B.; Mumme, M.; Feliciano, S.; Scotti, C.; Mainil-Varlet, P.; Procino, A.; von Rechenberg, B.; Schwamborn, T.; Jakob, M.; et al. Adult human neural crest-derived cells for articular cartilage repair. Sci. Transl. Med. 2014, 6, 251ra119. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, F.; Shafaei, H.; Soleimani Rad, J.; Rushangar, L.; Montaceri, A.; Jamshidi, M. High quality of infant chondrocytes in comparison with adult chondrocytes for cartilage tissue engineering. World J. Plast. Surg. 2017, 6, 183–189. [Google Scholar]
- Laurent, A.; Rey, M.; Scaletta, C.; Abdel-Sayed, P.; Michetti, M.; Flahaut, M.; Raffoul, W.; de Buys Roessingh, A.; Hirt-Burri, N.; Applegate, L.A. Retrospectives on three decades of safe clinical experience with allogeneic dermal progenitor fibroblasts: High versatility in topical cytotherapeutic care. Pharmaceutics 2023, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Laurent, A.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Martin, R. Human platelet lysate as an alternative to autologous serum for human chondrocyte clinical use. Cartilage 2021, 13, 509S–518S. [Google Scholar] [CrossRef] [PubMed]
- Chuma, H.; Mizuta, H.; Kudo, S.; Takagi, K.; Hiraki, Y. One day exposure to FGF-2 was sufficient for the regenerative repair of full-thickness defects of articular cartilage in rabbits. Osteoarthritis Cart. 2004, 12, 834–842. [Google Scholar] [CrossRef]
- Tonomura, H.; Nagae, M.; Takatori, R.; Ishibashi, H.; Itsuji, T.; Takahashi, K. The potential role of hepatocyte growth factor in degenerative disorders of the synovial joint and spine. Int. J. Mol. Sci. 2020, 21, 8717. [Google Scholar] [CrossRef]
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of cartilage regeneration: Emerging hypotheses and related therapeutic strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef]
- Stampoultzis, T.; Guo, Y.; Nasrollahzadeh, N.; Karami, P.; Pioletti, D.P. Mimicking loading-induced cartilage self-heating in vitro promotes matrix formation in chondrocyte-laden constructs with different mechanical properties. ACS Biomat. Sci. Eng. 2023, 9, 651–661. [Google Scholar] [CrossRef]
- Tran, N.T.; Truong, M.D.; Yun, H.W.; Min, B.H. Potential of secretome of human fetal cartilage progenitor cells as disease modifying agent for osteoarthritis. Life Sci. 2023, 324, 121741. [Google Scholar] [CrossRef]
- Haeusner, S.; Herbst, L.; Bittorf, P.; Schwarz, T.; Henze, C.; Mauermann, M.; Ochs, J.; Schmitt, R.; Blache, U.; Wixmerten, A.; et al. From single batch to mass production-Automated platform design concept for a phase II clinical trial tissue engineered cartilage product. Front. Med. 2021, 8, 712917. [Google Scholar] [CrossRef] [PubMed]
- Wixmerten, A.; Miot, S.; Bittorf, P.; Wolf, F.; Feliciano, S.; Hackenberg, S.; Häusner, S.; Krenger, W.; Haug, M.; Martin, I.; et al. Good Manufacturing Practice-compliant change of raw material in the manufacturing process of a clinically used advanced therapy medicinal product-A comparability study. Cytotherapy 2023, 25, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Hulme, C.H.; Garcia, J.; Mennan, C.; Perry, J.; Roberts, S.; Norris, K.; Baird, D.; Rix, L.; Banerjee, R.; Meyer, C.; et al. The up-scale manufacture of chondrocytes for allogeneic cartilage therapies. Tissue Eng. Part C 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kutaish, H.; Tscholl, P.M.; Cosset, E.; Bengtsson, L.; Braunersreuther, V.; Mor, F.M.; Laedermann, J.; Furfaro, I.; Stafylakis, D.; Hannouche, D.; et al. Articular cartilage repair after implantation of hyaline cartilage beads engineered from adult dedifferentiated chondrocytes: Cartibeads preclinical efficacy study in a large animal model. Am. J. Sports Med. 2023, 51, 237–249. [Google Scholar] [CrossRef]
- Marlovits, S.; Aldrian, S.; Wondrasch, B.; Zak, L.; Albrecht, C.; Welsch, G.; Trattnig, S. Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Am. J. Sports Med. 2012, 40, 2273–2280. [Google Scholar] [CrossRef]
- Nawaz, S.Z.; Bentley, G.; Briggs, T.W.; Carrington, R.W.; Skinner, J.A.; Gallagher, K.R.; Dhinsa, B.S. Autologous chondrocyte implantation in the knee: Mid-term to long-term results. J. Bone Joint Surg. 2014, 96, 824–830. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Joint Surg. 2010, 92, 2220–2233. [Google Scholar] [CrossRef]
- Aldrian, S.; Zak, L.; Wondrasch, B.; Albrecht, C.; Stelzeneder, B.; Binder, H.; Kovar, F.; Trattnig, S.; Marlovits, S. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: A prospective follow-up at a minimum of 10 years. Am. J. Sports Med. 2014, 42, 2680–2688. [Google Scholar] [CrossRef]
- Basad, E.; Wissing, F.R.; Fehrenbach, P.; Rickert, M.; Steinmeyer, J.; Ishaque, B. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: Clinical outcomes and challenges. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3729–3735. [Google Scholar] [CrossRef]
- Tosoratti, E.; Fisch, P.; Taylor, S.; Laurent-Applegate, L.A.; Zenobi-Wong, M. 3D-printed reinforcement scaffolds with targeted biodegradation properties for the tissue engineering of articular cartilage. Adv. Healthcare Mat. 2021, 10, e2101094. [Google Scholar] [CrossRef]
- Mumme, M.; Steinitz, A.; Nuss, K.M.; Klein, K.; Feliciano, S.; Kronen, P.; Jakob, M.; von Rechenberg, B.; Martin, I.; Barbero, A.; et al. Regenerative potential of tissue-engineered nasal chondrocytes in goat articular cartilage defects. Tissue Eng. Part A 2016, 22, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, V.P.; Muschler, G.F. Improved biological performance of human cartilage-derived progenitors in platelet lysate xenofree media in comparison to fetal bovine serum media. Cur. Res. Transl. Med. 2022, 70, 103353. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.G.; Kuiper, J.H.; Richardson, J.B.; Roberts, S.; Wright, K.T.; Kuiper, N.J. Impact of human platelet lysate on the expansion and chondrogenic capacity of cultured human chondrocytes for cartilage cell therapy. Eur. Cell Mater. 2018, 35, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, U.; Zachariah, S.M.; Thambaiah, A.; Tabasum, A.; Livingston, A.; Rebekah, G.; Srivastava, A.; Vinod, E. Comparison of human platelet lysate versus fetal bovine serum for expansion of human articular cartilage-derived chondroprogenitors. Cartilage 2021, 13, 107S–116S. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; bin Hassan, M.N.F.; Tang, Y.L.; Ng, M.H.; Law, J.X. Feasibility of human platelet lysate as an alternative to fetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021, 22, 1269. [Google Scholar] [CrossRef]
- Rikkers, M.; Levato, R.; Malda, J.; Vonk, L.A. Importance of timing of platelet lysate-supplementation in expanding or redifferentiating human chondrocytes for chondrogenesis. Front. Bioeng. Biotechnol. 2020, 8, 804. [Google Scholar] [CrossRef]
- Gaissmaier, C.; Fritz, J.; Krackhardt, T.; Flesch, I.; Aicher, W.K.; Ashammakhi, N. Effect of human platelet supernatant on proliferation and matrix synthesis of human articular chondrocytes in monolayer and three-dimensional alginate cultures. Biomaterials 2005, 26, 1953–1960. [Google Scholar] [CrossRef]
- Hildner, F.; Eder, M.J.; Hofer, K.; Aberl, J.; Redl, H.; van Griensven, M.; Gabriel, C.; Peterbauer-Scherb, A. Human platelet lysate successfully promotes proliferation and subsequent chondrogenic differentiation of adipose-derived stem cells: A comparison with articular chondrocytes. J. Tissue Eng. Regen. Med. 2015, 9, 808–818. [Google Scholar] [CrossRef]
- Textor, M.; Hoburg, A.; Lehnigk, R.; Perka, C.; Duda, G.N.; Reinke, S.; Blankenstein, A.; Hochmann, S.; Stockinger, A.; Resch, H.; et al. Chondrocyte isolation from loose bodies—An option for reducing donor site morbidity for autologous chondrocyte implantation. Int. J. Mol. Sci. 2023, 24, 1484. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Song, S.Y.; Kim, E. Advanced therapy medicinal products for autologous chondrocytes and comparison of regulatory systems in target countries. Regen. Ther. 2022, 20, 126–137. [Google Scholar] [CrossRef]
- Nordberg, R.C.; Otarola, G.A.; Wang, D.; Hu, J.C.; Athanasiou, K.A. Navigating regulatory pathways for translation of biologic cartilage repair products. Sci. Transl. Med. 2022, 14, eabp8163. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Orthopaedic gene therapy: Twenty-five years on. JBJS Rev. 2021, 9, e20. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.S.; Parvizi, J.; Ehiorobo, J.O.; Mont, M.A. The safety and efficacy of a novel cell-based gene therapy for knee osteoarthritis. Surg. Technol. Int. 2019, 35, 370–376. [Google Scholar]
- Sadri, B.; Tamimi, A.; Nouraein, S.; Bagheri Fard, A.; Mohammadi, J.; Mohammadpour, M.; Hassanzadeh, M.; Bajouri, A.; Madani, H.; Barekat, M.; et al. Clinical and laboratory findings following transplantation of allogeneic adipose-derived mesenchymal stromal cells in knee osteoarthritis, a brief report. Connect. Tissue Res. 2022, 63, 663–674. [Google Scholar] [CrossRef]
- Sadri, B.; Hassanzadeh, M.; Bagherifard, A.; Mohammadi, J.; Alikhani, M.; Moeinabadi-Bidgoli, K.; Madani, H.; Diaz-Solano, D.; Karimi, S.; Mehrazmay, M.; et al. Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: A phase II, triple-blinded, placebo controlled, randomized trial. Stem Cell Res. Ther. 2023, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Yano, K.; Watanabe, N.; Masamune, K.; Yamato, M. Non-clinical assessment design of autologous chondrocyte implantation products. Regen. Ther. 2015, 1, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Hamahashi, K.; Toyoda, E.; Ishihara, M.; Mitani, G.; Takagaki, T.; Kaneshiro, N.; Maehara, M.; Takahashi, T.; Okada, E.; Watanabe, A.; et al. Polydactyly-derived allogeneic chondrocyte cell-sheet transplantation with high tibial osteotomy as regenerative therapy for knee osteoarthritis. NPJ Regen. Med. 2022, 7, 71. [Google Scholar] [CrossRef]
- Chijimatsu, R.; Kobayashi, M.; Ebina, K.; Iwahashi, T.; Okuno, Y.; Hirao, M.; Fukuhara, A.; Nakamura, N.; Yoshikawa, H. Impact of dexamethasone concentration on cartilage tissue formation from human synovial derived stem cells in vitro. Cytotechnology 2018, 70, 819–829. [Google Scholar] [CrossRef]
- Oseni, A.O.; Butler, P.E.; Seifalian, A.M. Optimization of chondrocyte isolation and characterization for large-scale cartilage tissue engineering. J. Surg. Res. 2013, 181, 41–48. [Google Scholar] [CrossRef]
- Albrecht, C.; Tichy, B.; Nürnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: A comparative study. Osteoarthritis Cart. 2011, 19, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Dickinson, S.C.; Zavan, B.; Cortivo, R.; Hollander, A.P.; Abatangelo, G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: Relationship to symptomatology and time after implantation. Arthritis Res. Ther. 2008, 10, R132. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Martino, A.; Filardo, G.; Tetta, C.; Busacca, M.; Iacono, F.; Delcogliano, M.; Albisinni, U.; Marcacci, M. Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Europ. J. Radiol. 2011, 79, 382–388. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Brophy, R.H.; Lattermann, C.; Carey, J.L.; Flanigan, D.C. Failures, re-operations, and complications after autologous chondrocyte implantation—A systematic review. Osteoarthritis Cart. 2011, 19, 779–791. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Bukovac, P.K.; Hauser, M.; Lottaz, D.; Marti, A.; Schmitt, I.; Schochat, T. The regulation of cell therapy and gene therapy products in Switzerland. Adv. Exp. Med. Biol. 2023, 1430, 41–58. [Google Scholar] [CrossRef]
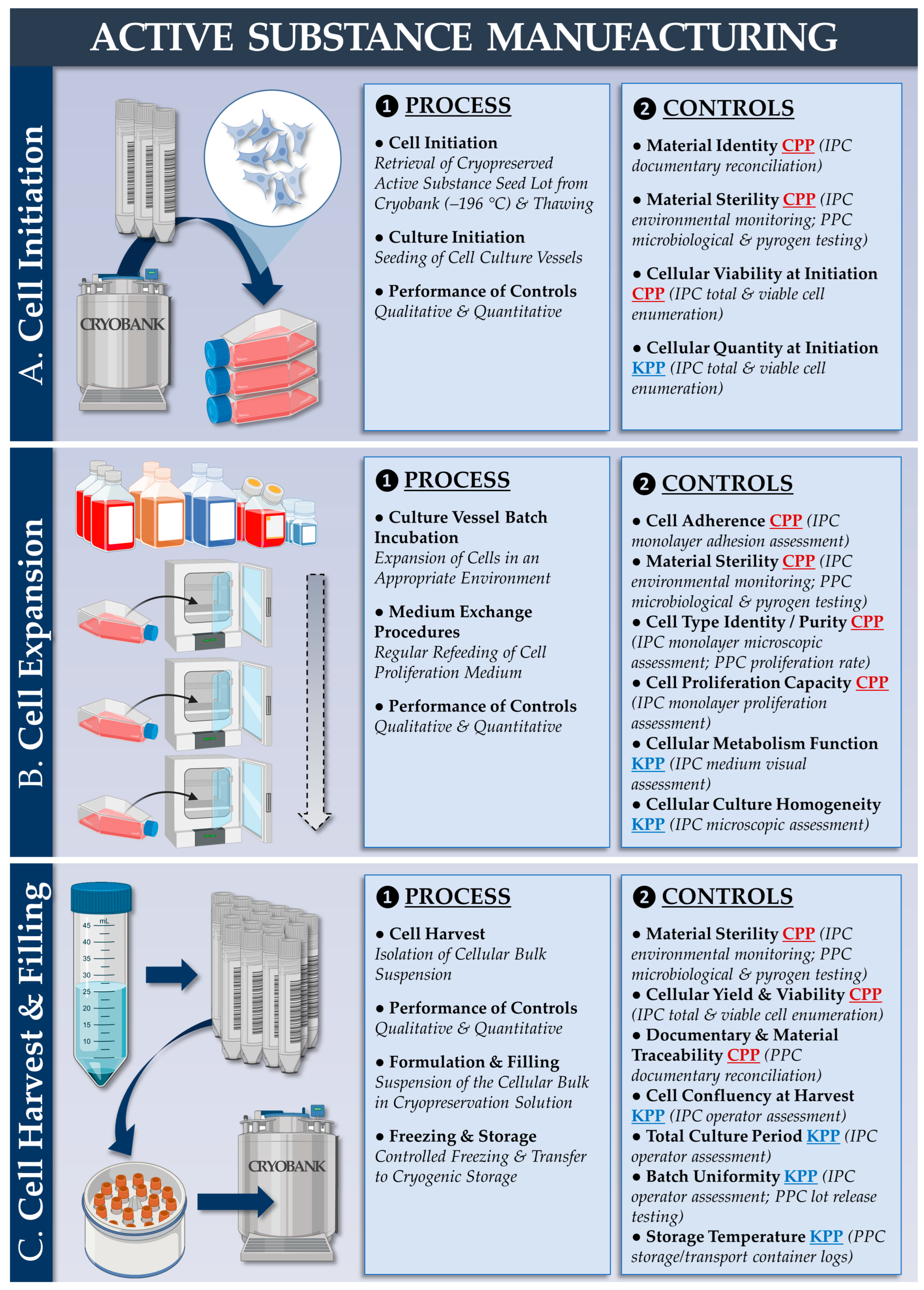
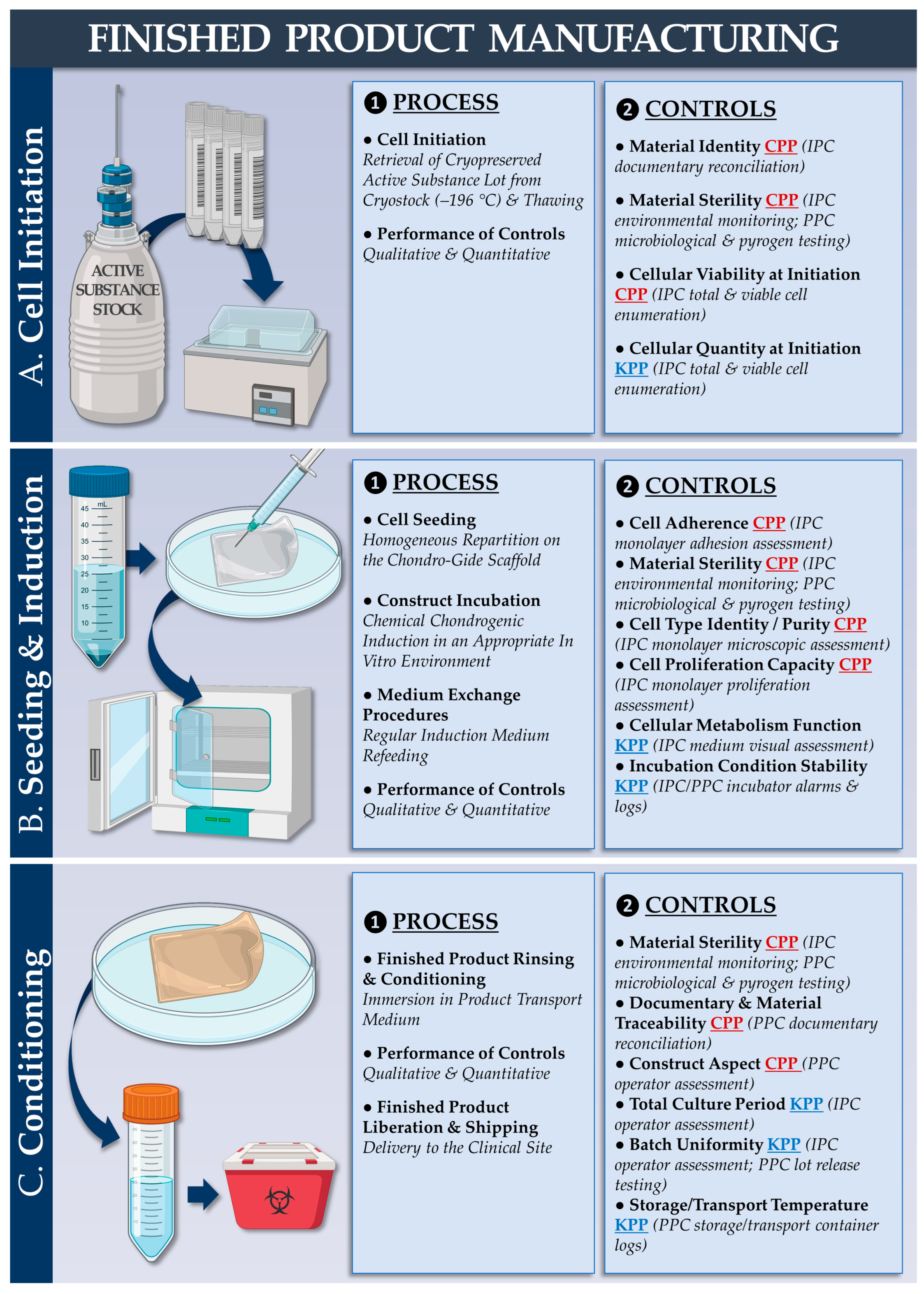
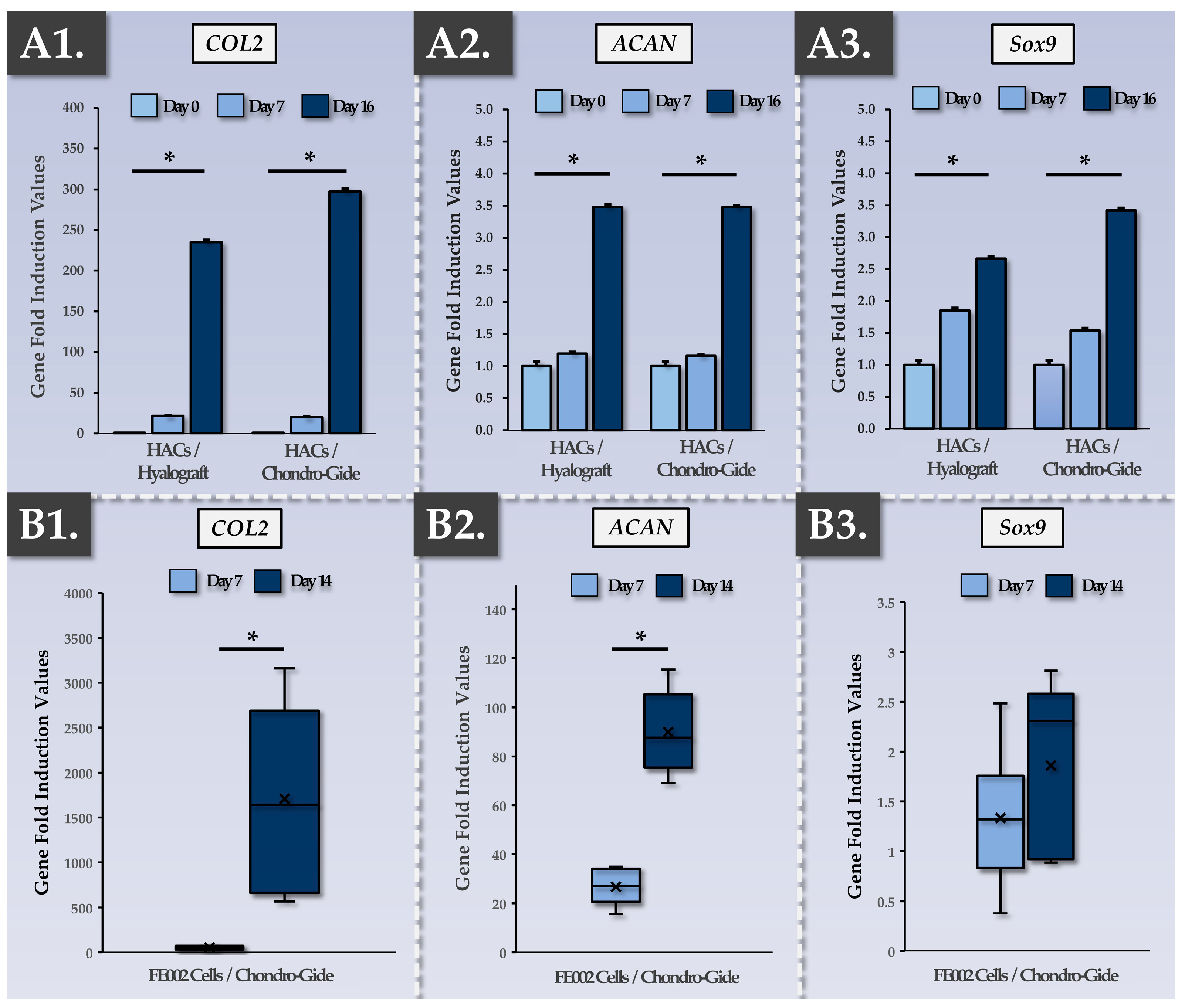
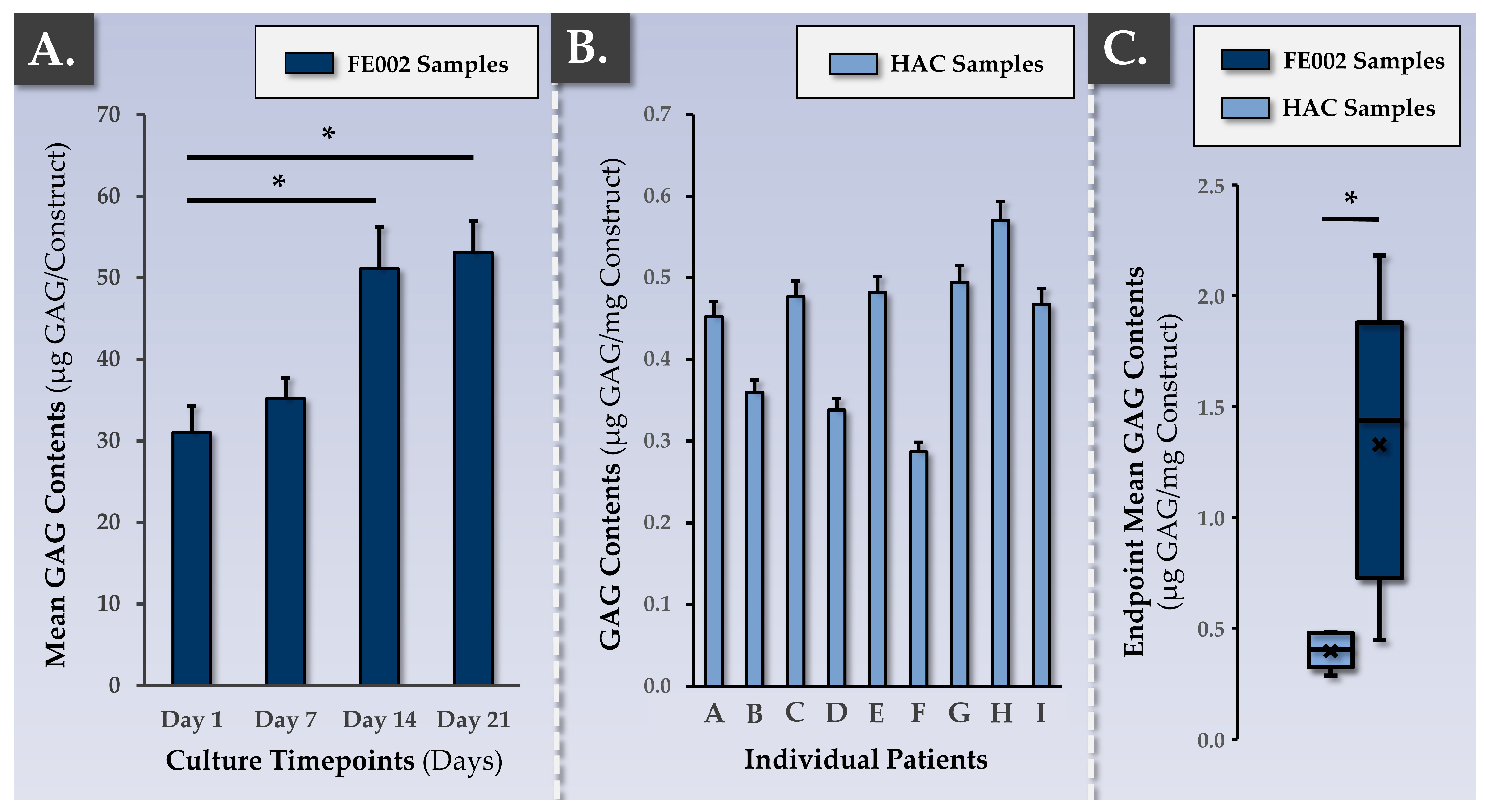
| Parameter/Specification | Autologous Protocol | Allogeneic Protocol | Purpose/Targets | Resource Requirements 1 (Allogeneic vs. Autologous) | |
|---|---|---|---|---|---|
| Regulatory Status of the Protocol for Clinical Investigational Use | Approved 2 (Swissmedic) | Pending Submission | NA | Increased | |
| 1. Cellular Active Substance | Cellular Active Substance Proliferation Medium | DMEM–Ham’s F12; L-glutamine; HPL 10% | DMEM; L-glutamine; FBS 10% | Obtention of ≥40 × 106 cells for a cellular active substance lot | Decreased |
| Cellular Active Substance Lot Cryopreservation | Yes; Liquid nitrogen | Yes; liquid nitrogen | Maintenance of appropriate biological functionalities | Conserved | |
| Cellular Active Substance Lot Processing for Scaffold Seeding | HACs expanded once in 2D before scaffold seeding | FE002 primary chondroprogenitors directly seeded after initiation | Use of cellular active substance materials with optimal quality and functionality attributes | Decreased | |
| 2. Finished Cytotherapeutic Product | Matrix/Scaffold | Chondro-Gide ≤ 20 cm2 | Chondro-Gide ≤ 20 cm2 | Use of cyto-/bio-compatible scaffold of appropriate dimensions with appropriate functionalities | Conserved |
| Cell Seeding Density on the Scaffold | [2 ± 0.5] × 106 cells/cm2 | [2 ± 0.5] × 106 cells/cm2 | Obtention of homogeneous scaffold cell seeding | Conserved | |
| Chondrogenic Induction Medium Composition | DMEM–Ham’s F12; HPL 10%; L-glutamine; ascorbic acid 0.025 mg/mL; TGF-β1 10 ng/mL; Insulin 10 μg/mL; dexamethasone 100 nM | DMEM; L-glutamine; ITS; TGF-β3 10 ng/mL; VitCp 82 µg/mL; dexamethasone 10 nM | Induction of chondrogenic genes and ECM deposition by viable cells | Conserved | |
| Chondrogenic Induction Time-Period | 16 ± 4 days | 14 ± 2 days | Induction of chondrogenic genes and ECM deposition by viable cells | Decreased | |
| Transport Medium Composition | NaCl 0.9%; AHS 20% | NaCl 0.9% | Maintenance of appropriate physical and biological functionalities | Decreased | |
| Finished Product Validity Period | 6 h after end of manufacture | 6 h after end of manufacture | Maintenance of appropriate physical and biological functionalities | Conserved | |
| Control/Assessment Type | Control Parameters | Control Methods | Targets/Acceptance Criteria | Autologous Protocol Assessment | Allogeneic Protocol Assessment | ||
|---|---|---|---|---|---|---|---|
| C | NC | C | NC | ||||
| 1. Endpoint Direct Assessment 1 of Finished Product Lot | Chondrogenic Gene Induction in 3D | RT-PCR | Induction of COL2 and ACAN | ✓ | - | ✓ | - |
| Cartilage GAG Presence (Total) in 3D | DMMB | Cartilage GAG presence ≥ 0.25 µg/mg | ✓ | - | ✓ | - | |
| Cartilage ECM Presence (Histology) in 3D | HE | Presence of staining | ✓ | - | ✓ | - | |
| ACAN | Presence of staining | ✓ | - | ✓ | - | ||
| AB | Presence of staining | ✓ | - | ✓ | - | ||
| Cellular Viability in 3D | MTT | Presence of MTT signal | ✓ | - | ✓ | - | |
| Homogeneity of Cell Presence Across the Construct | MTT signal homogeneity; Histology | Homogeneous staining of the whole surface of the construct | ✓ | - | ✓ | - | |
| Cells and Synthetized ECM Localized in 1 Layer of the Construct | MTT; Histology | Presence of cells and ECM in one layer of the construct (absent from the other layer) | ✓ | - | ✓ | - | |
| Cells Localized in Lacunae | Histology | Presence of cells in the lacunae | ✓ | - | ✓ | - | |
| Cellular Morphology | Histology | Rounded cellular morphology | ✓ | - | ✓ | - | |
| Homogeneous ECM Presence Across the Construct | Histology | Homogeneous presence of ECM across the construct | ✓ | - | ✓ | - | |
| Significant ECM Deposition Within the Construct | Histology | Significant ECM deposition in one layer of the construct | ✓ | - | ✓ | - | |
| 2. In-Process Indirect Assessment 2 of Finished Product Lot | Cell Viability in Monolayer Control Cultures | Cell enumeration; Operator assessment | Cellular viability ≥ 75% before control plate seeding; limited amounts of floating dead cells; induction medium consumption | ✓ | - | ✓ | - |
| Cellular Adhesion in Monolayer Control Cultures | Operator assessment | Presence of ≥60% adherent cells 24 h after seeding; absence of significant cellular detachment | ✓ | - | ✓ | - | |
| Cellular Proliferation in Monolayer Control Cultures | Operator assessment | Appropriate proliferative cellular morphology adoption, proliferation rate, and proliferation homogeneity in monolayer | ✓ | - | ✓ | - | |
| Cellular Population Purity in Monolayer Control Cultures | Operator assessment | Absence of observable cell sub-population presence | ✓ | - | ✓ | - | |
| Parameter Type | Control Parameters | IPC 1 | PPC 2 | Process Development /Validation 3 | Release Criterion |
|---|---|---|---|---|---|
| 1. Quality | Cell viability (monolayer recovery) | ✓ | - | ✓ | ✓ |
| Cell viability (3D) | - | ✓ | ✓ | ✓ | |
| Cell proliferation rate (monolayer recovery) | ✓ | - | ✓ | ✓ | |
| Endpoint cell yield (monolayer recovery) | ✓ | - | ✓ | - | |
| Cell morphology (3D) | - | ✓ | ✓ | - | |
| Localization of cells in the lacunae | - | ✓ | ✓ | - | |
| 2. Purity | Microscopic cell morphology assessment (monolayer recovery) | ✓ | - | ✓ | ✓ |
| Cell proliferation rate (monolayer recovery) | ✓ | - | ✓ | ✓ | |
| 3. Efficacy | Cell viability (3D) | - | ✓ | ✓ | ✓ |
| Chondrogenic gene induction (3D) | - | ✓ | ✓ | - | |
| Cartilage-specific ECM synthesis and deposition in 3D | - | ✓ | ✓ | - | |
| Macroscopic change in construct color and rigidity | ✓ | ✓ | ✓ | ✓ | |
| 4. Safety | In vitro and in vivo tumorigenicity assessment; telomerase activity quantification; cell type senescence assessment; karyotyping; literature review | - | - | ✓ | - |
| Microbiological safety (manufacturing system) | ✓ | ✓ | ✓ | ✓ | |
| Microbiological safety (raw/starting materials, retention samples) | ✓ | ✓ | ✓ | ✓ | |
| Microbiological safety (finished product lot) | - | ✓ | ✓ | ✓ | |
| 5. Stability | Finished product CQA maintenance after storage | - | ✓ | ✓ | - |
| Finished product CQA maintenance after transport | - | ✓ | ✓ | - |
| Parameter | Controls | Targets/Acceptance Criteria | Endpoint Construct Grading 1 | ||
|---|---|---|---|---|---|
| NaCl | OPTIMEM | HA-PBS | |||
| Cellular Viability | MTT | Presence of viable cells on the constructs | +++ | +++ | +++ |
| Cellular Repartition | MTT/HE | Homogeneous viable cell repartition on one side of the scaffold | +++ | +++ | +++ |
| Construct Morphology | Operator Assessment | Specific macroscopic change in construct color and rigidity | +++ | +++ | +++ |
| GAG Content | DMMB | Maintenance of total GAG contents | ++ | +++ | +++ |
| Aggrecan Presence | Histology | Maintenance of positive ACAN staining on one side of the construct | +++ | +++ | +++ |
| Alcian Blue Staining | Histology | Maintenance of positive AB staining on one side of the construct | +++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philippe, V.; Jeannerat, A.; Peneveyre, C.; Jaccoud, S.; Scaletta, C.; Hirt-Burri, N.; Abdel-Sayed, P.; Raffoul, W.; Darwiche, S.; Applegate, L.A.; et al. Autologous and Allogeneic Cytotherapies for Large Knee (Osteo)Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification. Pharmaceutics 2023, 15, 2333. https://doi.org/10.3390/pharmaceutics15092333
Philippe V, Jeannerat A, Peneveyre C, Jaccoud S, Scaletta C, Hirt-Burri N, Abdel-Sayed P, Raffoul W, Darwiche S, Applegate LA, et al. Autologous and Allogeneic Cytotherapies for Large Knee (Osteo)Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification. Pharmaceutics. 2023; 15(9):2333. https://doi.org/10.3390/pharmaceutics15092333
Chicago/Turabian StylePhilippe, Virginie, Annick Jeannerat, Cédric Peneveyre, Sandra Jaccoud, Corinne Scaletta, Nathalie Hirt-Burri, Philippe Abdel-Sayed, Wassim Raffoul, Salim Darwiche, Lee Ann Applegate, and et al. 2023. "Autologous and Allogeneic Cytotherapies for Large Knee (Osteo)Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification" Pharmaceutics 15, no. 9: 2333. https://doi.org/10.3390/pharmaceutics15092333
APA StylePhilippe, V., Jeannerat, A., Peneveyre, C., Jaccoud, S., Scaletta, C., Hirt-Burri, N., Abdel-Sayed, P., Raffoul, W., Darwiche, S., Applegate, L. A., Martin, R., & Laurent, A. (2023). Autologous and Allogeneic Cytotherapies for Large Knee (Osteo)Chondral Defects: Manufacturing Process Benchmarking and Parallel Functional Qualification. Pharmaceutics, 15(9), 2333. https://doi.org/10.3390/pharmaceutics15092333









