A Shortcut from Genome to Drug: The Employment of Bioinformatic Tools to Find New Targets for Gastric Cancer Treatment
Abstract
1. Introduction
2. Methodology
2.1. Selection of Microarray Transcriptome Studies
2.2. Data Acquisition and Processing
2.3. Metadata Construction
2.4. Gene Set Enrichment Analysis (GSEA)
2.5. Differential Expression and Survival Analysis from TCGA
2.6. Primer Designer
2.7. Cell Culture
2.8. Gene Expression by qRT-PCR
2.8.1. RNA Extraction
2.8.2. Conversion of mRNA to cDNA
2.8.3. Real-Time Quantitative PCR (qRT-PCR)
2.9. Three-Dimensional Structure Obtention
2.10. Virtual Screening
2.11. In Silico Toxicity Prediction
2.12. Prediction of ADME Parameters
2.13. Molecular Docking (MD) Assays
2.14. Protein–Protein Interaction Network
2.15. Re-Docking
2.16. Statistical Analysis
3. Results
3.1. Differential Gene Expression in Gastric Tumor Meta-Dataset
3.2. Gastric Tumor Group Involved in Multiple Cancer Progression Pathways
3.3. Expression and Prognostic Value of Genes Involved in Gastric Cancer Progression
3.4. Validation of Relevant Genes in Gastric Tumor Cell Lines Compared to Normal Gastric Cell Lines
3.5. In Silico High-Throughput Virtual Screening
3.6. Molecular Docking (MD) Validation
3.7. In Silico Pharmacokinetics Prediction
3.8. Protein–Protein Interaction Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ao, X.; Wang, Y.; Li, X.; Wang, J. Long Non-Coding RNA in Gastric Cancer: Mechanisms and Clinical Implications for Drug Resistance. Front. Oncol. 2022, 12, 841411. [Google Scholar] [CrossRef] [PubMed]
- INCA Estimativa 2023: Incidência de Câncer No Brasil; Instituto Nacional de Câncer: Brasília, Brazil, 2022; ISBN 9786588517093.
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy with S-1 versus Surgery Alone in Stage II or III Gastric Cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Leichman, L.; Silberman, H.; Leichman, C.G.; Spears, C.P.; Ray, M.; Muggia, F.M.; Kiyabu, M.; Radin, R.; Laine, L.; Stain, S.; et al. Preoperative Systemic Chemotherapy Followed by Adjuvant Postoperative Intraperitoneal Therapy for Gastric Cancer: A University of Southern California Pilot Program. J. Clin. Oncol. 1992, 10, 1933–1942. [Google Scholar] [CrossRef]
- Ajani, J.A.; Mansfield, P.F.; Lynch, P.M.; Pisters, P.W.; Feig, B.; Dumas, P.; Evans, D.B.; Raijman, I.; Hargraves, K.; Curley, S.; et al. Enhanced Staging and All Chemotherapy Preoperatively in Patients with Potentially Resectable Gastric Carcinoma. J. Clin. Oncol. 1999, 17, 2403–2411. [Google Scholar] [CrossRef]
- Guan, W.L.; He, Y.; Xu, R.H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Miller, Z.; Kim, K.S.; Lee, D.M.; Kasam, V.; Baek, S.E.; Lee, K.H.; Zhang, Y.Y.; Ao, L.; Carmony, K.; Lee, N.R.; et al. Proteasome Inhibitors with Pyrazole Scaffolds from Structure-Based Virtual Screening. J. Med. Chem. 2015, 58, 2036–2041. [Google Scholar] [CrossRef]
- Rohr, M.; Beardsley, J.; Nakkina, S.P.; Zhu, X.; Aljabban, J.; Hadley, D.; Altomare, D. A Merged Microarray Meta-Dataset for Transcriptionally Profiling Colorectal Neoplasm Formation and Progression. Sci. Data 2021, 8, 214. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Discovery and Ranking of the Most Robust Prognostic Biomarkers in Serous Ovarian Cancer. GeroScience 2023, 45, 1889–1898. [Google Scholar] [CrossRef]
- Maués, J.H.D.S.; Ribeiro, H.F.; Pinto, G.R.; de Oliveira Lopes, L.; Lamarao, L.M.; Pessoa, C.M.F.; de Fátima Aquino Moreira-Nunes, C.; de Carvalho, R.M.; Assumpção, P.P.; Rey, J.A. Gastric Cancer Cell Lines Have Different MYC-Regulated Expression Patterns but Share a Common Core of Altered Genes. Can. J. Gastroenterol. Hepatol. 2018, 2018, 5804376. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, I.; Tapia, O.; Espinoza, J.A.; Leal, P.; Buchegger, K.; Sandoval, A.; Bizama, C.; Araya, J.C.; Peek, R.M.; Roa, J.C. The Gene Expression Status of the PI3K/AKT/MTOR Pathway in Gastric Cancer Tissues and Cell Lines. Pathol. Oncol. Res. 2016, 22, 797–805. [Google Scholar] [CrossRef]
- Mesquita, F.P.; Lucena da Silva, E.; Souza, P.F.N.; Lima, L.B.; Amaral, J.L.; Zuercher, W.; Albuquerque, L.M.; Rabenhorst, S.H.B.; Moreira-Nunes, C.A.; Amaral de Moraes, M.E.; et al. Kinase Inhibitor Screening Reveals Aurora-a Kinase Is a Potential Therapeutic and Prognostic Biomarker of Gastric Cancer. J. Cell. Biochem. 2021, 122, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Beaulieu, J.-F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.B.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE Precis: Practical Implementation of Minimum Standard Guidelines for Fluorescence-Based Quantitative Real-Time PCR Experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- DeLano, W.L.; Lam, J. PyMOL: A Communications Tool for Computational Models. In Abstracts of Papers of the American Chemical Society; AMER CHEMICAL SOC: Washington, DC, USA, 2005. [Google Scholar]
- Kiss, R.; Sandor, M.; Szalai, F.A. http://Mcule.com: A Public Web Service for Drug Discovery. J. Cheminform. 2012, 4, 2012. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; AlAjmi, M.F.; Aatif, M.; Farhan, M.; Shafi, S. Identification of a Potential Inhibitor (MCULE-8777613195-0-12) of New Delhi Metallo-β-Lactamase-1 (NDM-1) Using In Silico and In Vitro Approaches. Molecules 2022, 27, 5930. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- De Magalhães, C.S.; Almeida, D.M.; Barbosa, H.J.C.; Dardenne, L.E. A Dynamic Niching Genetic Algorithm Strategy for Docking Highly Flexible Ligands. Inf. Sci. 2014, 289, 206–224. [Google Scholar] [CrossRef]
- Guedes, I.A.; Barreto, A.M.S.; Marinho, D.; Krempser, E.; Kuenemann, M.A.; Sperandio, O.; Dardenne, L.E.; Miteva, M.A. New Machine Learning and Physics-Based Scoring Functions for Drug Discovery. Sci. Rep. 2021, 11, 3198. [Google Scholar] [CrossRef]
- Inbar, Y.; Schneidman-Duhovny, D.; Halperin, I.; Oron, A.; Nussinov, R.; Wolfson, H.J. Approaching the CAPRI Challenge with an Efficient Geometry-Based Docking. Proteins Struct. Funct. Bioinform. 2005, 60, 217–223. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Caldarelli, I.; Basile, M.A.; Bencivenga, D.; Tramontano, A.; Perrotta, S.; della Ragione, F.; Oliva, A. The Tyrosine Kinase Inhibitor Dasatinib Induces a Marked Adipogenic Differentiation of Human Multipotent Mesenchymal Stromal Cells. PLoS ONE 2011, 6, e28555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, Y.; Xu, T.; Tan, Y.; Lv, W.; Zhao, C.; Wu, M.; Wu, Y.; Zhang, Q. CCDC69 Is a Prognostic Marker of Breast Cancer and Correlates with Tumor Immune Cell Infiltration. Front. Surg. 2022, 9, 879921. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, F.D.; López-López, E.; Eurídice Juárez-Mercado, K.; Medina-Franco, J.L. Computational Drug Design Methods—Current and Future Perspectives. In Silico Drug Design: Repurposing Techniques and Methodologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 19–44. [Google Scholar] [CrossRef]
- Anthony, L.F.W.; Kanding, B.; Selvan, R. Carbontracker: Tracking and Predicting the Carbon Footprint of Training Deep Learning Models. arXiv 2020, arXiv:2007.03051. [Google Scholar]
- Song, Y.; Ye, L.; Tan, Y.; Tong, H.; Lv, Z.; Wan, X.; Li, Y. Therapeutic Exosomes Loaded with SERPINA5 Attenuated Endometrial Cancer Cell Migration via the Integrin Β1/FAK Signaling Pathway. Cell. Oncol. 2022, 45, 861–872. [Google Scholar] [CrossRef]
- Wu, H.; Cui, M.; Li, C.; Li, H.; Dai, Y.; Cui, K.; Li, Z. Kaempferol Reverses Aerobic Glycolysis via MiR-339-5p-Mediated PKM Alternative Splicing in Colon Cancer Cells. J. Agric. Food Chem. 2021, 69, 3060–3068. [Google Scholar] [CrossRef]
- Jia, H.; Song, L.; Cong, Q.; Wang, J.; Xu, H.; Chu, Y.; Li, Q.; Zhang, Y.; Zou, X.; Zhang, C. The LIM Protein AJUBA Promotes Colorectal Cancer Cell Survival through Suppression of JAK1/STAT1/IFIT2 Network. Oncogene 2017, 36, 2655–2666. [Google Scholar] [CrossRef]
- Dommann, N.; Sánchez-Taltavull, D.; Eggs, L.; Birrer, F.; Brodie, T.; Salm, L.; Baier, F.A.; Medova, M.; Humbert, M.; Tschan, M.P. The LIM Protein AJUBA Augments Tumor Metastasis in Colon Cancer. Cancers 2020, 12, 1913. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Inoue, H.; Mori, M.; Akiyoshi, T. The Expression of Costimulatory Molecules CD80 and CD86 in Human Carcinoma Cell Lines: Its Regulation by Interferon Gamma and Interleukin-10. Cancer Immunol. Immunother. 1996, 43, 213–219. [Google Scholar] [CrossRef]
- Horn, L.A.; Long, T.M.; Atkinson, R.; Clements, V.; Ostrand-Rosenberg, S. Soluble CD80 Protein Delays Tumor Growth and Promotes Tumor-Infiltrating Lymphocytes. Cancer Immunol. Res. 2018, 6, 59–68. [Google Scholar] [CrossRef]
- Feng, X.Y.; Lu, L.; Wang, K.F.; Zhu, B.Y.; Wen, X.Z.; Peng, R.Q.; Ding, Y.; Li, D.D.; Li, J.J.; Li, Y.; et al. Low Expression of CD80 Predicts for Poor Prognosis in Patients with Gastric Adenocarcinoma. Future Oncol. 2019, 15, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Peggs, K.S.; Quezada, S.A. Ipilimumab: Attenuation of an Inhibitory Immune Checkpoint Improves Survival in Metastatic Melanoma. Expert Rev. Anticancer Ther. 2010, 10, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Wang, J.; Luo, X.; Ye, M.; Jin, X. Roles of NOLC1 in Cancers and Viral Infection. J. Cancer Res. Clin. Oncol. 2023, 149, 10593–10608. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Shang, Y.; Diao, X.; Huang, J.; Liu, H. Knockdown of NOLC1 Inhibits PI3K-AKT Pathway to Improve the Poor Prognosis of Esophageal Carcinoma. J. Oncol. 2021, 2021, 9944132. [Google Scholar] [CrossRef]
- L Garner, A.; D Janda, K. Protein-Protein Interactions and Cancer: Targeting the Central Dogma. Curr. Top. Med. Chem. 2010, 11, 258–280. [Google Scholar] [CrossRef]
- Lu, H.; Zhou, Q.; He, J.; Jiang, Z.; Peng, C.; Tong, R.; Shi, J. Recent Advances in the Development of Protein–Protein Interactions Modulators: Mechanisms and Clinical Trials. Signal Transduct. Target. Ther. 2020, 5, 213. [Google Scholar] [CrossRef]
- Bahmanyar, S.; Kaplan, D.D.; DeLuca, J.G.; Giddings, T.H.; O’Toole, E.T.; Winey, M.; Salmon, E.D.; Casey, P.J.; Nelson, W.J.; Barth, A.I.M. β-Catenin Is a Nek2 Substrate Involved in Centrosome Separation. Genes Dev. 2008, 22, 91–105. [Google Scholar] [CrossRef]

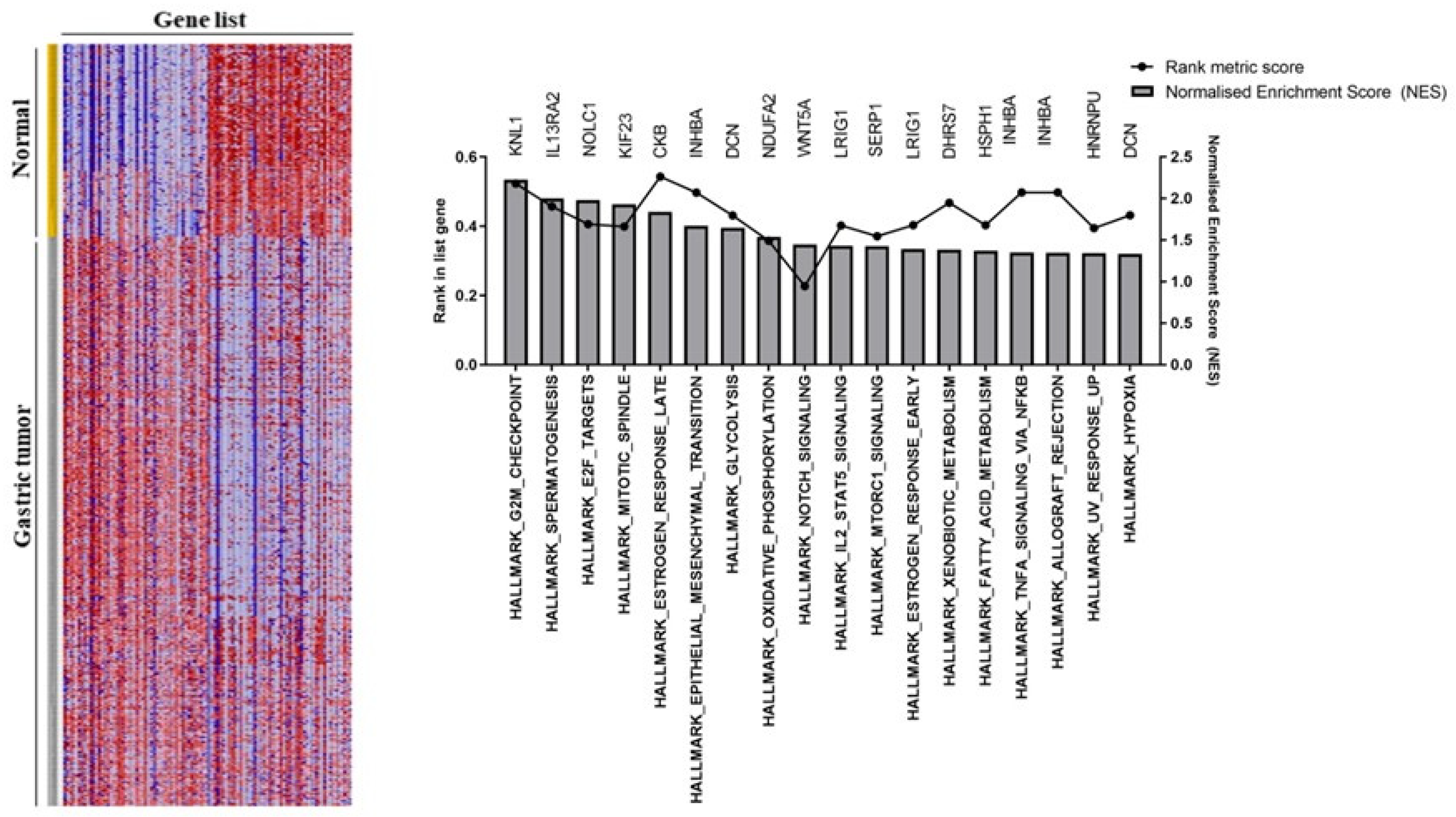

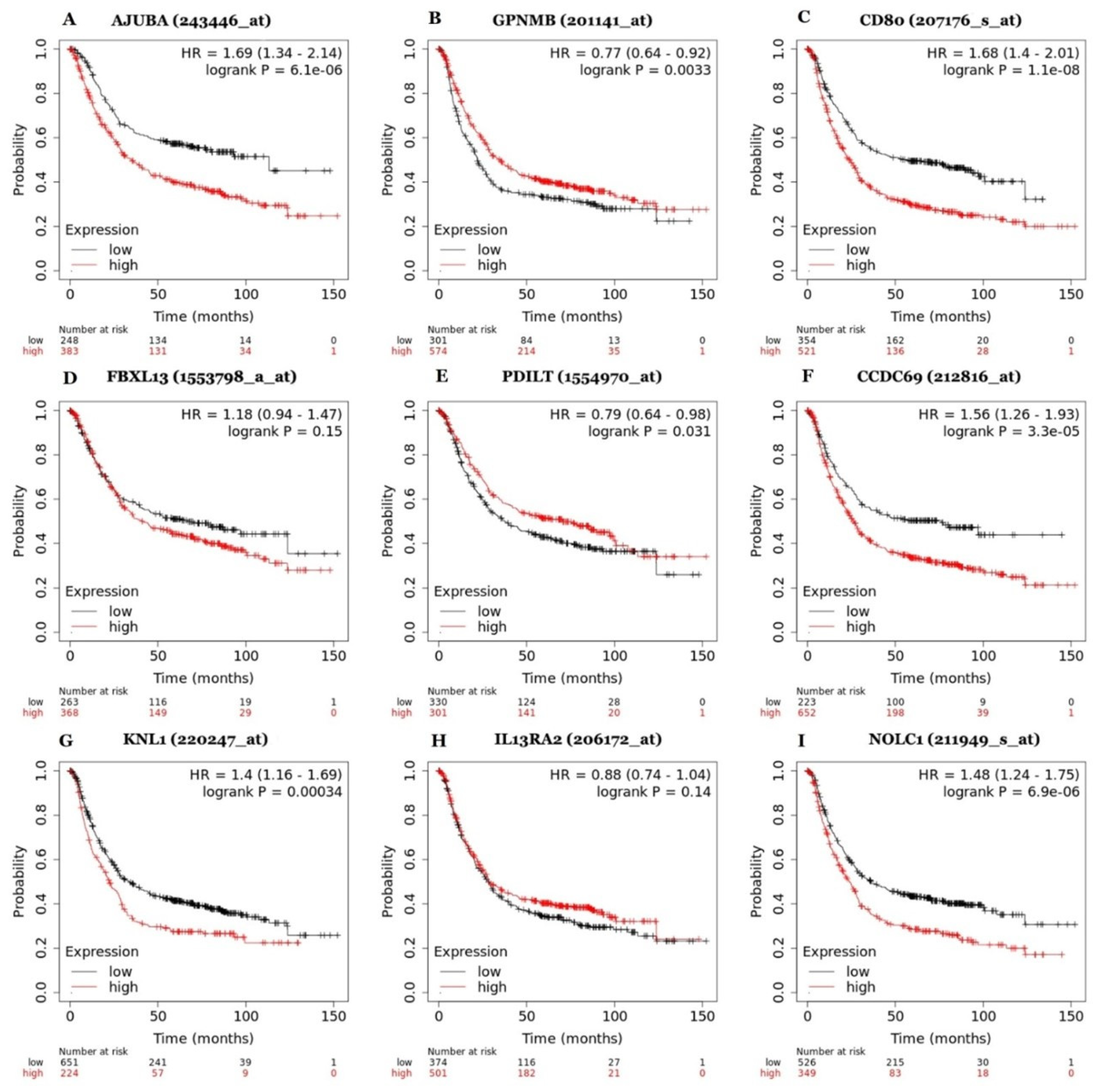
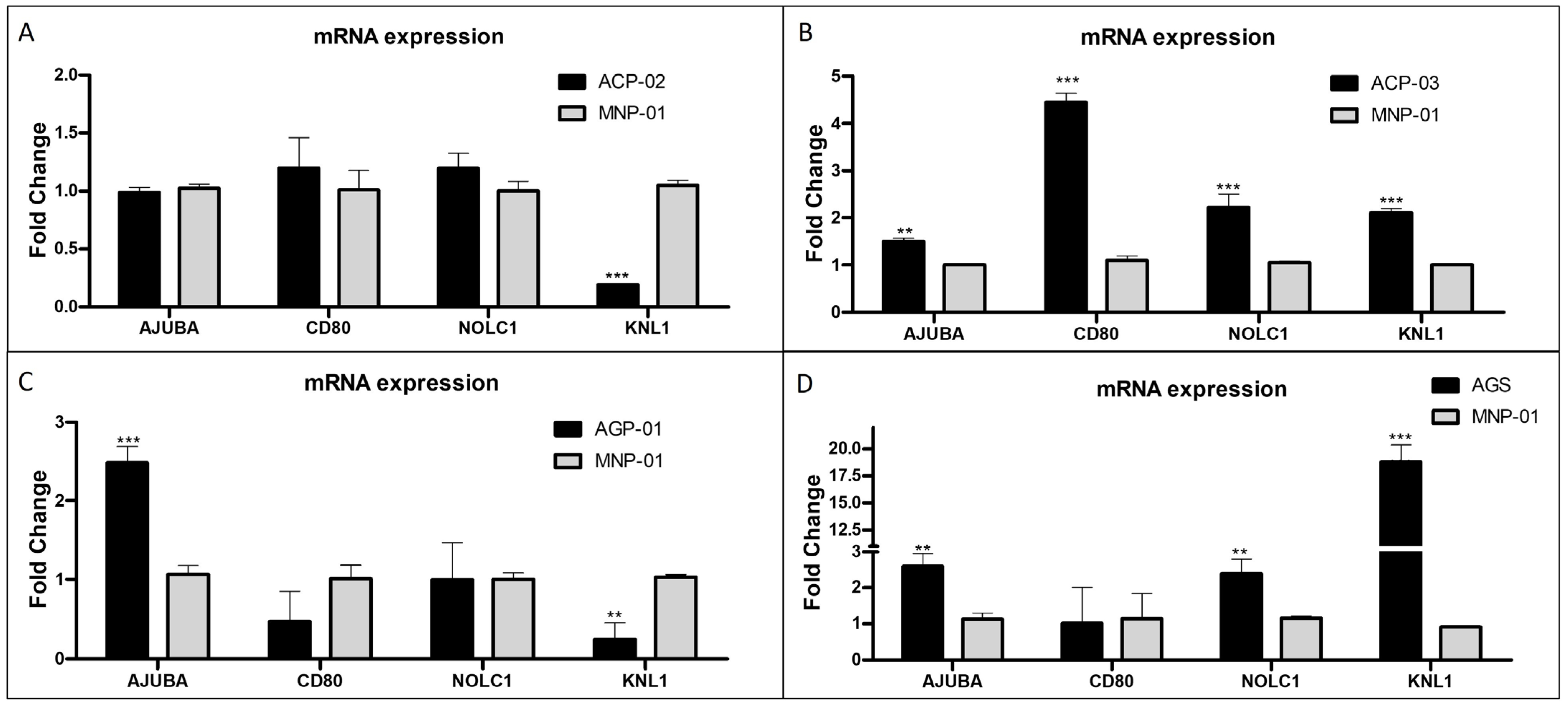
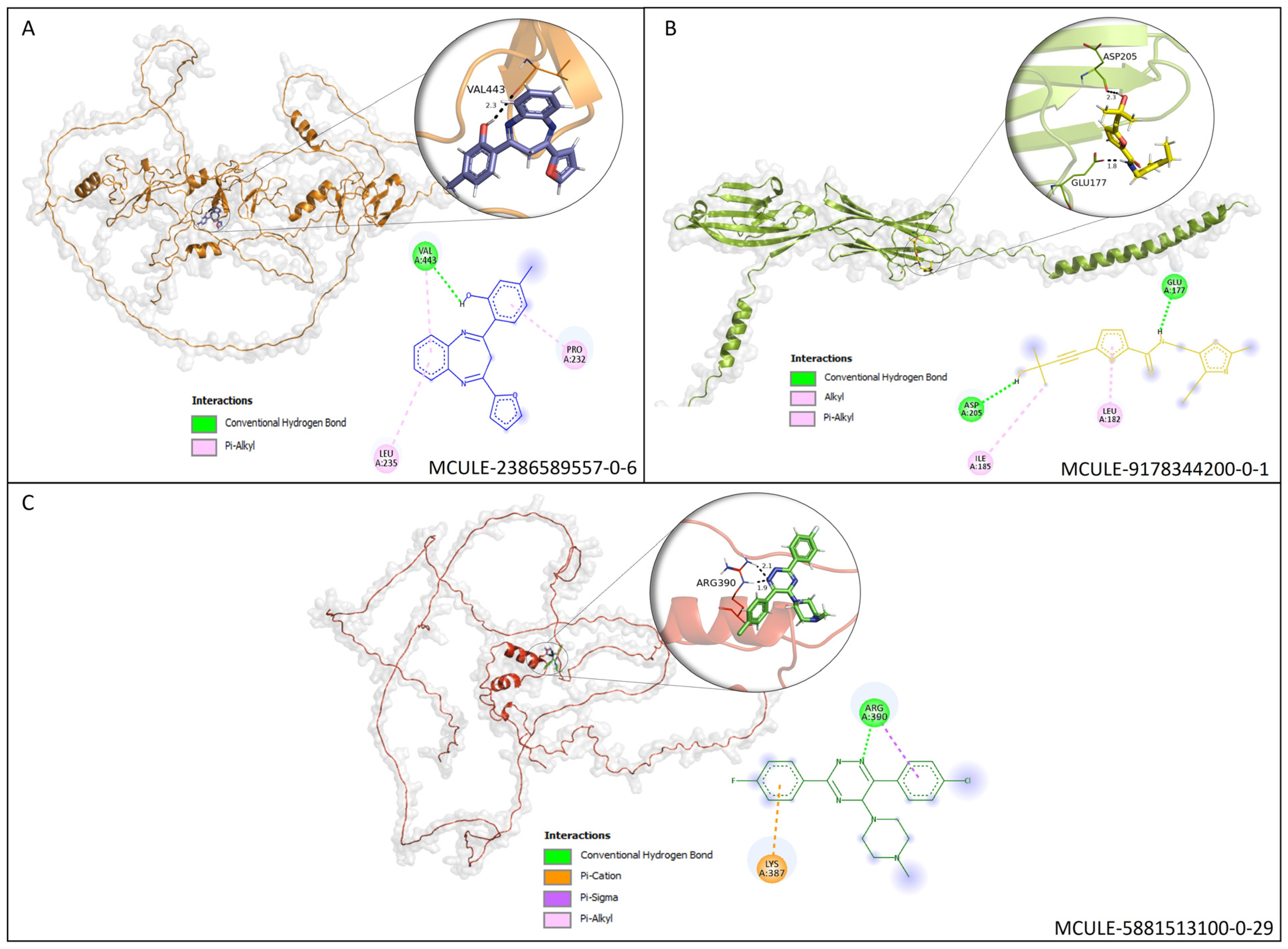


| Differentially Expressed Genes | |||||||
|---|---|---|---|---|---|---|---|
| Upregulated | Downregulated | ||||||
| Gene Symbol | LogFC | t | Adjusted p-Value | Gene Symbol | LogFC | t | Adjusted p-Value |
| AJUBA | 1.44 × 1014 | 7.80 | 7.78 × 1013 | FBXL13 | −1.56 × 1014 | −11.18 | 2.01 × 10−24 |
| GPNMB | 1.11 × 1014 | 6.20 | 1.53 × 108 | PDILT | −1.14 × 1014 | −9.20 | 2.98 × 10−17 |
| CD80 | 1.09 × 1014 | 7.84 | 5.74 × 10−13 | CCDC69 | −1.28 × 1014 | −6.83 | 3.98 × 10−10 |
| ANLN | 1.06 × 1014 | 4.82 | 1.61 × 10−5 | PDZK1IP1 | −1.24 × 1014 | −4.03 | 4.02 × 10−14 |
| ADGRG7 | 1.06 × 1014 | 4.75 | 2.22 × 10−5 | SCIN | −1.23 × 1014 | −7.05 | 1.02 × 10−10 |
| BICD1 | 1.04 × 1014 | 6.47 | 3.44 × 10−9 | ITIH5 | −1.21 × 1014 | −7.66 | 1.98 × 10−12 |
| KNL1 | 9.91 × 1014 | 11.01 | 8.37 × 10−5 | NKX2-3 | −1.20 × 1014 | −8.46 | 7.58 × 10−15 |
| ABCD3 | 9.71 × 1013 | 5.89 | 8.40 × 10−8 | ITGB1 | −1.17 × 1014 | −4.27 | 1.59 × 10−4 |
| CENPL | 9.54 × 1013 | 5.85 | 1.03 × 10−7 | SIGLEC11 | 1.16 × 1014 | −13.16 | 1.33 × 10−32 |
| PGTS2 | 9.26 × 1014 | 4.60 | 4.17 × 10−5 | PTCHD1 | −1.13 × 1014 | −7.52 | 5.07 × 10−12 |
| Ligand ID | Target | Chemical Formula | Mol. Wt. | 2D Structure |
|---|---|---|---|---|
| 1. MCULE-2386589557-0-6 | AJUBA | C20H16N2O2 | 316.352 |  |
| 2. MCULE-9178344200-0-1 | CD80 | C17H20N2O3S | 332.419 | 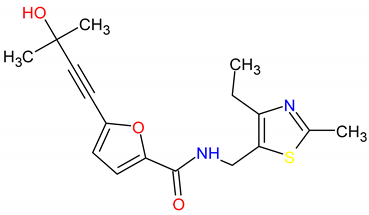 |
| 3. MCULE-5881513100-0-29 | NOLC1 | C20H19ClFN5 | 383.848 | 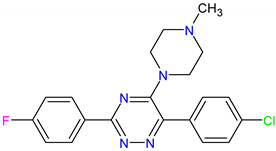 |
| Protein–Ligand Complex | Docking Score | Predicted Toxicity | |||
|---|---|---|---|---|---|
| Ligand ID | Target | DockThor | Mcule | LD50 | Class |
| MCULE-2386589557-0-6 | AJUBA | −8.4 | −7.3 | 2500 mg/kg | V |
| MCULE-9178344200-0-1 | CD80 | −7.7 | −5.6 | 600 mg/kg | IV |
| MCULE-5881513100-0-29 | NOLC1 | −7.2 | −7.5 | 640 mg/kg | IV |
| ADME Property | (1) | (2) | (3) |
|---|---|---|---|
| Molecular weight | 316.35 | 332.42 | 383.85 |
| TPSA | 58.09 | 103.60 | 45.15 |
| cLogP | 3.81 | 2.73 | 3.72 |
| LogS | −4.75 | −3.49 | −4.73 |
| GI absorption | High | High | High |
| BBB permeant | Yes | No | Yes |
| P-gp substrate | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, D.M.S.; Lima, O.G.; Mesquita, F.P.; da Silva, E.L.; de Moraes, M.E.A.; Burbano, R.M.R.; Montenegro, R.C.; Souza, P.F.N. A Shortcut from Genome to Drug: The Employment of Bioinformatic Tools to Find New Targets for Gastric Cancer Treatment. Pharmaceutics 2023, 15, 2303. https://doi.org/10.3390/pharmaceutics15092303
Brito DMS, Lima OG, Mesquita FP, da Silva EL, de Moraes MEA, Burbano RMR, Montenegro RC, Souza PFN. A Shortcut from Genome to Drug: The Employment of Bioinformatic Tools to Find New Targets for Gastric Cancer Treatment. Pharmaceutics. 2023; 15(9):2303. https://doi.org/10.3390/pharmaceutics15092303
Chicago/Turabian StyleBrito, Daiane M. S., Odnan G. Lima, Felipe P. Mesquita, Emerson L. da Silva, Maria E. A. de Moraes, Rommel M. R. Burbano, Raquel C. Montenegro, and Pedro F. N. Souza. 2023. "A Shortcut from Genome to Drug: The Employment of Bioinformatic Tools to Find New Targets for Gastric Cancer Treatment" Pharmaceutics 15, no. 9: 2303. https://doi.org/10.3390/pharmaceutics15092303
APA StyleBrito, D. M. S., Lima, O. G., Mesquita, F. P., da Silva, E. L., de Moraes, M. E. A., Burbano, R. M. R., Montenegro, R. C., & Souza, P. F. N. (2023). A Shortcut from Genome to Drug: The Employment of Bioinformatic Tools to Find New Targets for Gastric Cancer Treatment. Pharmaceutics, 15(9), 2303. https://doi.org/10.3390/pharmaceutics15092303







