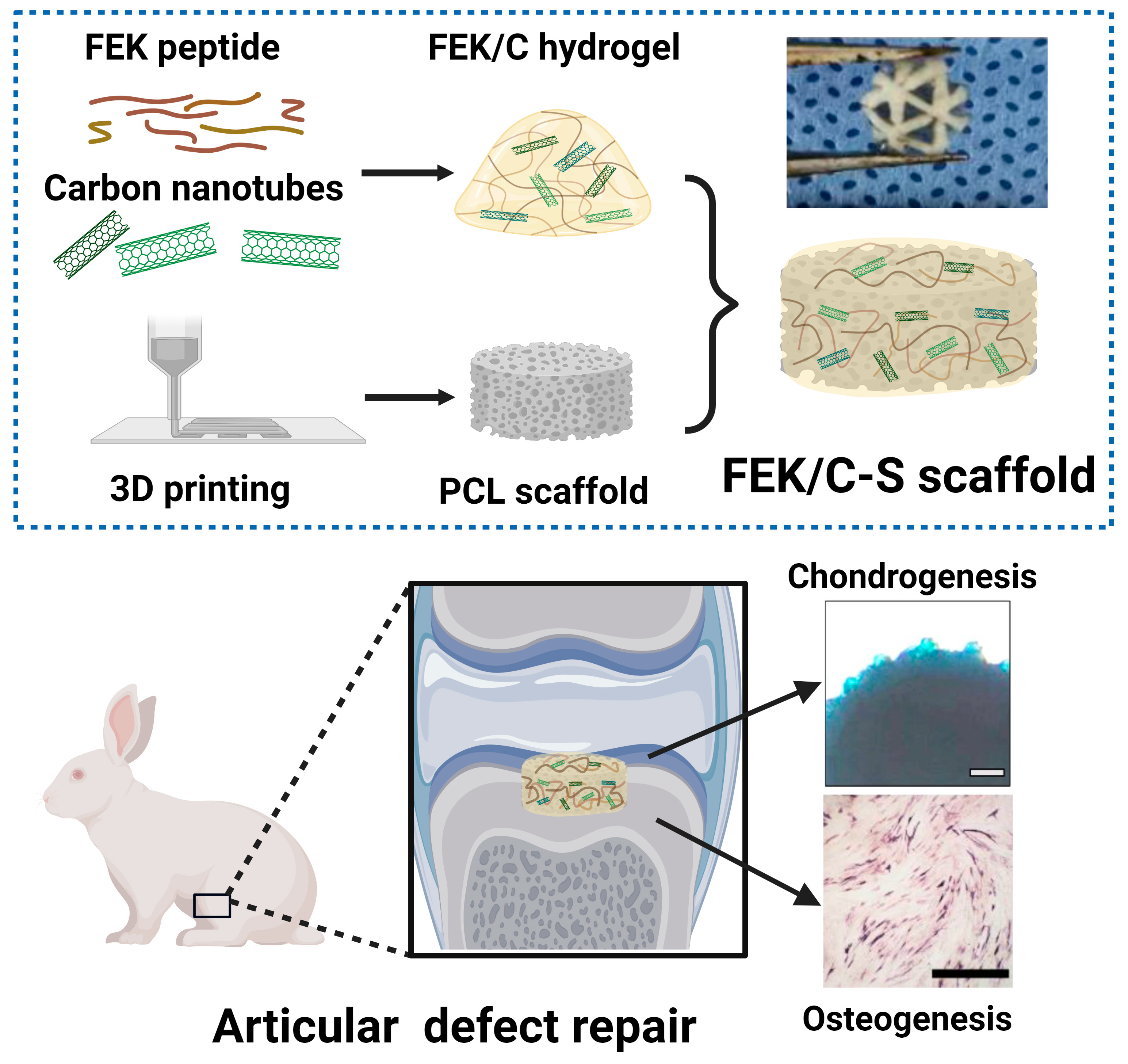
Enhanced Cartilage and Subchondral Bone Repair Using Carbon Nanotube-Doped Peptide Hydrogel–Polycaprolactone Composite Scaffolds
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Preparation of Octapeptide-CNT Hybrid Hydrogel (FEK/C)
2.3. Preparation of FEK/C Composite PCL Scaffold
2.4. Characterizations
2.5. Cell Culture
2.6. In Vitro Compatibility
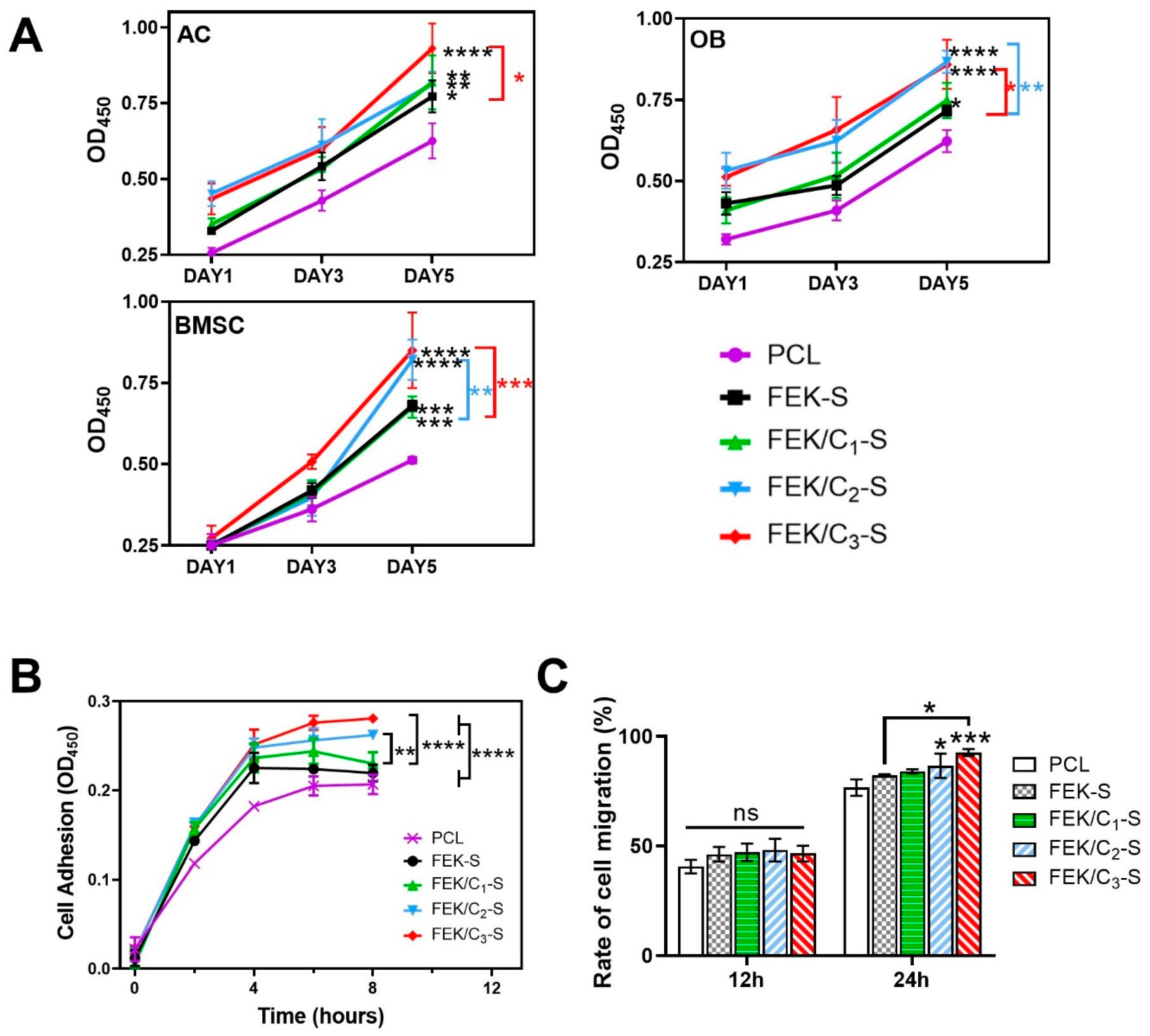
2.7. In Vitro Migration and Adhesion Assessment
2.8. Assessment of Osteogenesis
2.9. Assessment of Chondrogenesis
2.10. Animal Handling and Model Establishment
2.11. Micro-CT Imaging and Bone Mass Analysis
2.12. Histological Analysis
2.13. Statistical Analysis
3. Results
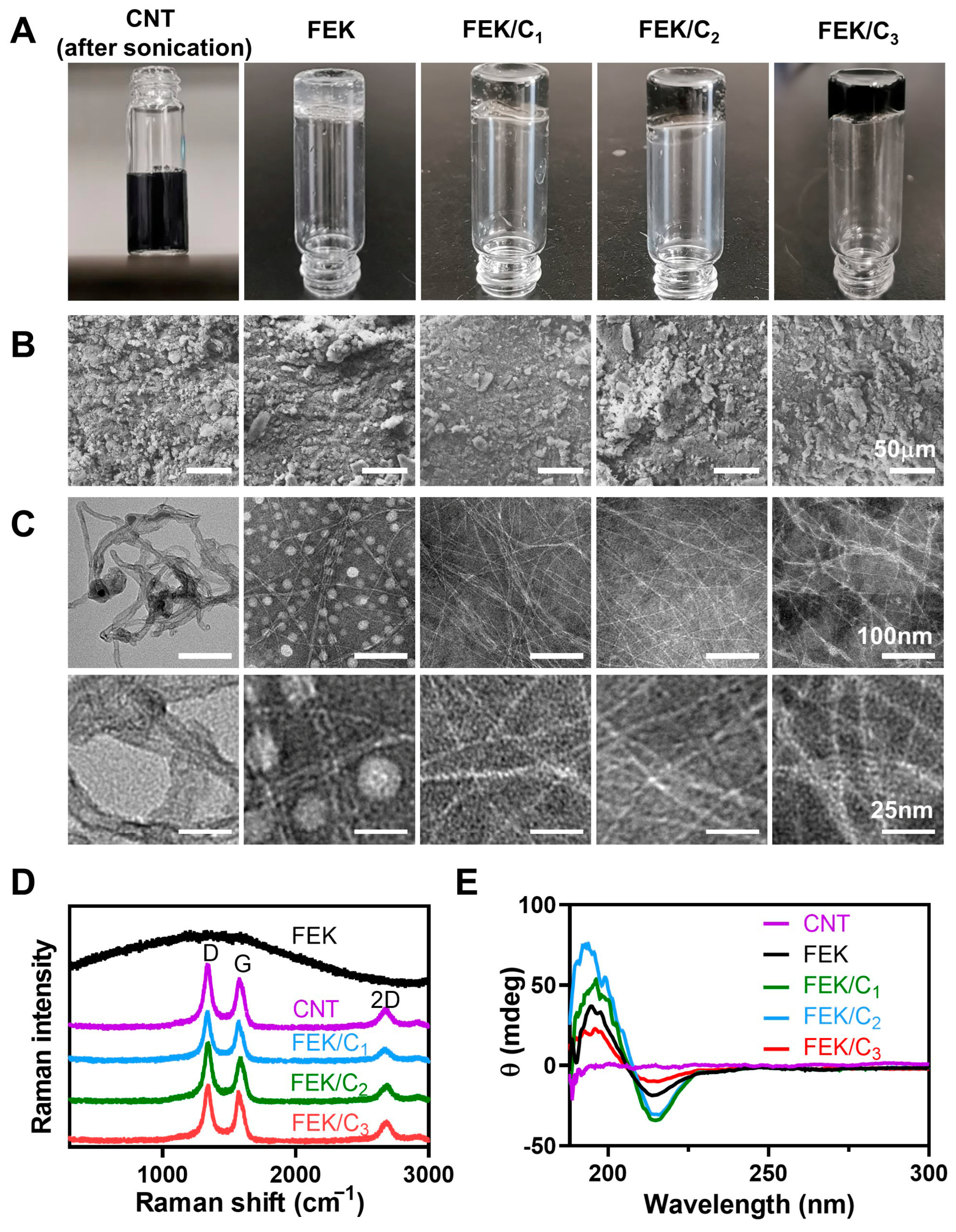
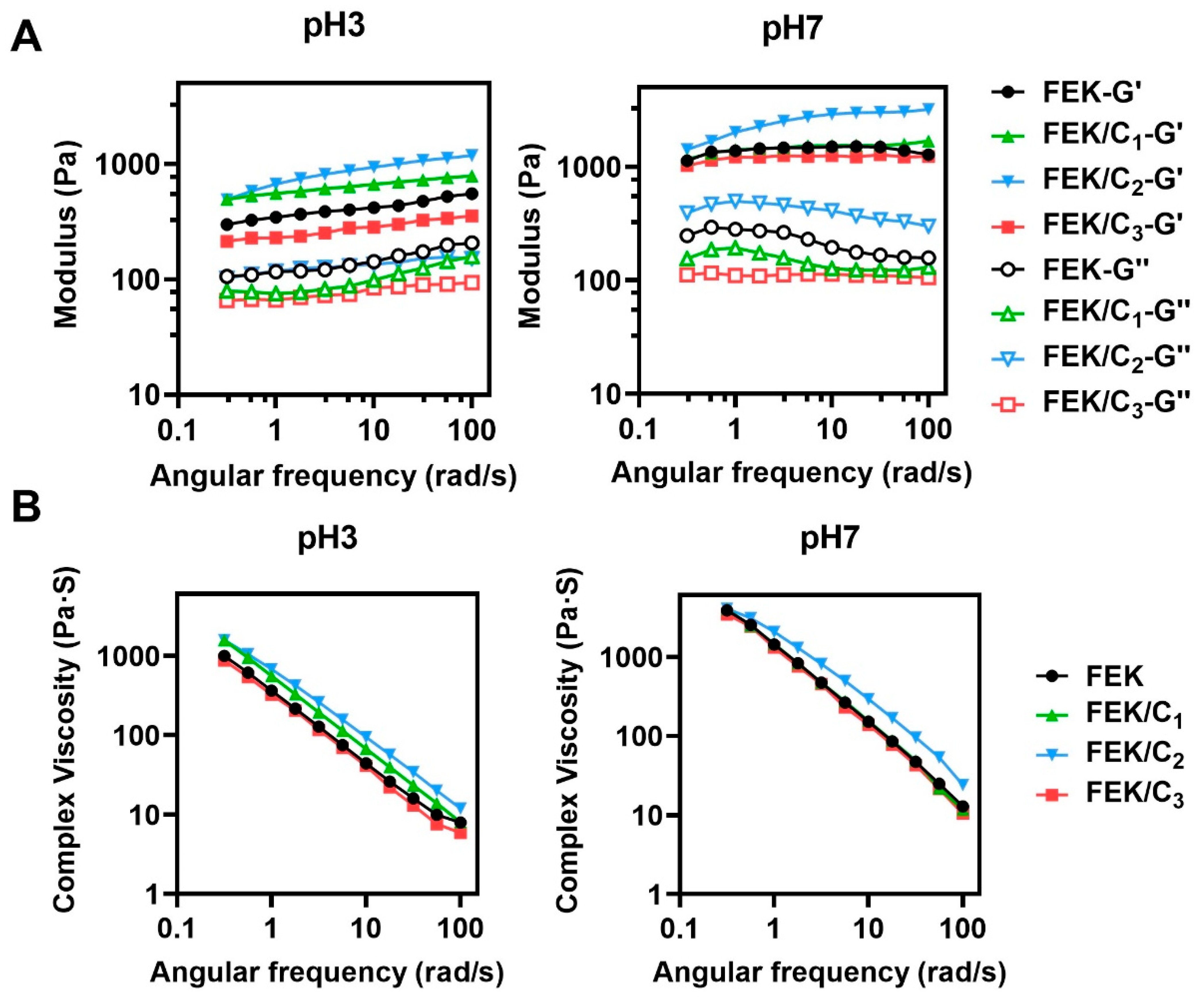
3.1. Characterization of FEK/C Hydrogel
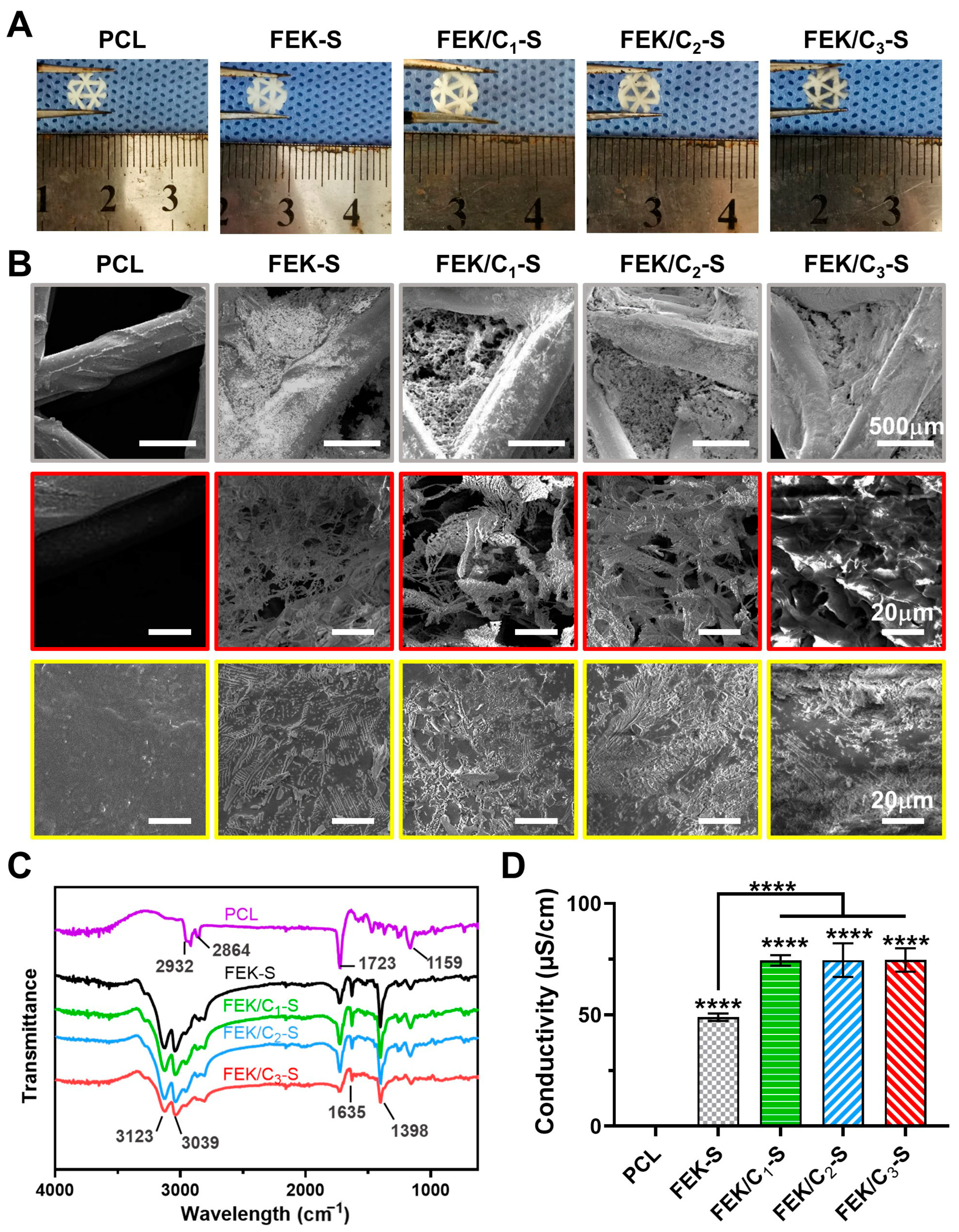
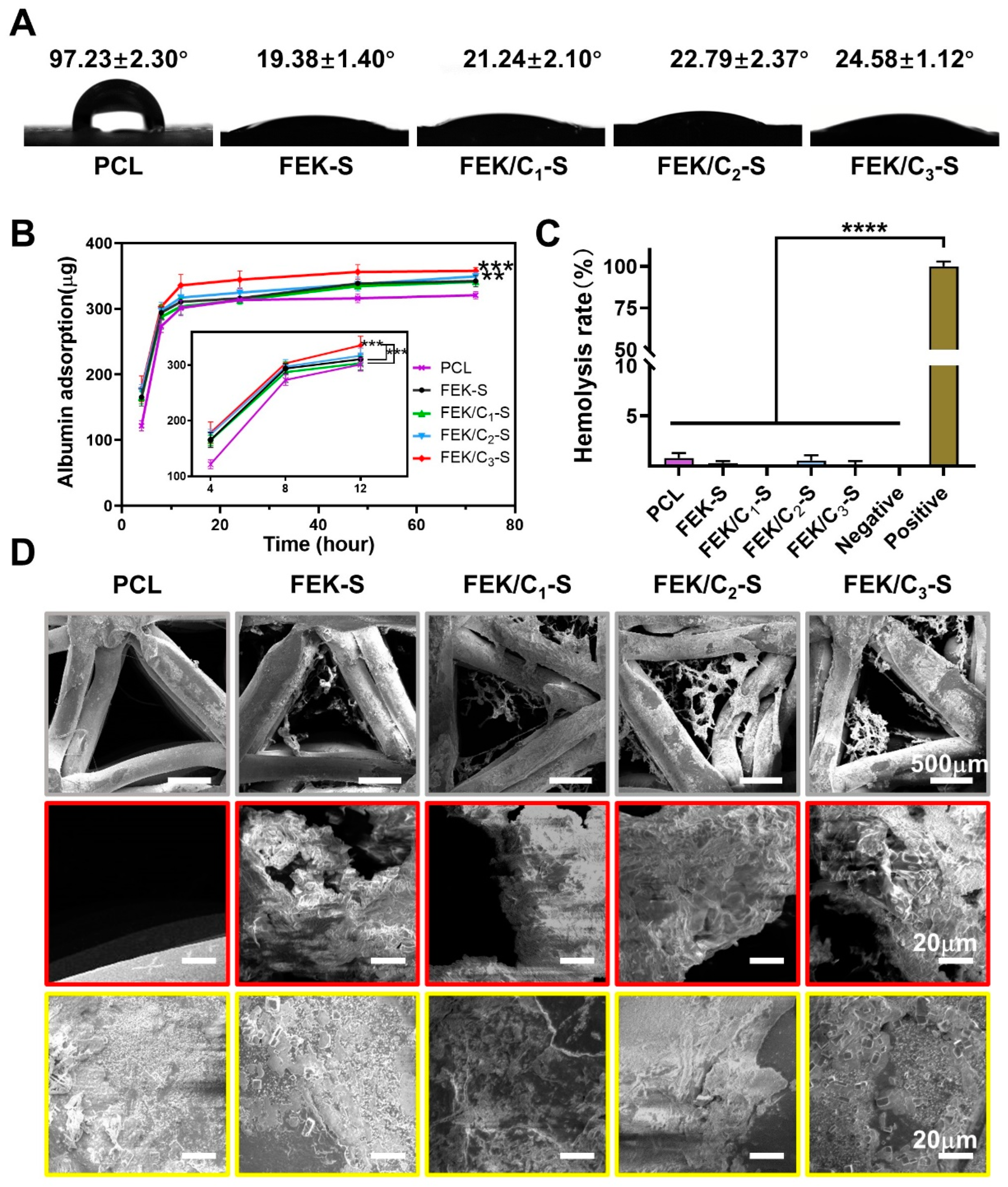
3.2. Characterization of FEK/C-S Scaffold
3.3. Effects of FEK/C-S Scaffold on Cellular Biocompatibility
3.4. Evaluation of Scaffold-Enabled Osteogenesis and Chondrogenesis
3.5. Evaluation of Bone Repair Using FEK/C-S Scaffold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tuan, R.S. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Mandelbaum, B.R.; Gerhardt, M.B.; Peterson, L. Autologous chondrocyte implantation of the talus. Arthroscopy 2003, 19 (Suppl. 1), 129–137. [Google Scholar] [CrossRef] [PubMed]
- Redondo, M.L.; Beer, A.J.; Yanke, A.B. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J. Knee Surg. 2018, 31, 231–238. [Google Scholar] [CrossRef]
- Richter, D.L.; Tanksley, J.A.; Miller, M.D. Osteochondral Autograft Transplantation: A Review of the Surgical Technique and Outcomes. Sports Med. Arthrosc. Rev. 2016, 24, 74–78. [Google Scholar] [CrossRef]
- Harris, J.D.; Siston, R.A.; Pan, X.; Flanigan, D.C. Autologous chondrocyte implantation: A systematic review. J. Bone Jt. Surg. Am. 2010, 92, 2220–2233. [Google Scholar] [CrossRef]
- Williams, R.; Khan, I.M.; Richardson, K.; Nelson, L.; McCarthy, H.E.; Analbelsi, T.; Singhrao, S.K.; Dowthwaite, G.P.; Jones, R.E.; Baird, D.M.; et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 2010, 5, e13246. [Google Scholar] [CrossRef]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone usage in additive manufacturing strategies for tissue engineering applications: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef]
- Dávila, J.L.; Freitas, M.S.; Inforçatti Neto, P.; Silveira, Z.C.; Silva, J.V.L.; d’Ávila, M.A. Fabrication of PCL/β-TCP scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. 2016, 133, 43031. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Nareswari, T.L.; Juniatik, M.; Aminatun, A.; Sari, M.; Utami, R.A.; Sari, Y.W.; Khairurrijal, K.; Yusuf, Y.; Suciati, T. A facile technique for overcoming seeding barriers of hydrophobic polycaprolactone/hydroxyapatite-based nanofibers for bone tissue engineering. J. Appl. Pharm. Sci. 2023, 13, 049–060. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Ji, C.; Qiu, M.; Ruan, H.; Li, C.; Cheng, L.; Wang, J.; Li, C.; Qi, J.; Cui, W.; Deng, L. Transcriptome Analysis Revealed the Symbiosis Niche of 3D Scaffolds to Accelerate Bone Defect Healing. Adv. Sci. 2022, 9, e2105194. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, P.; Prasopthum, A.; Parsons, A.J.; Ai, F.; Yang, J. Characterisation of bone regeneration in 3D printed ductile PCL/PEG/hydroxyapatite scaffolds with high ceramic microparticle concentrations. Biomater. Sci. 2021, 10, 138–152. [Google Scholar] [CrossRef]
- Li, P.; Fu, L.; Liao, Z.; Peng, Y.; Ning, C.; Gao, C.; Zhang, D.; Sui, X.; Lin, Y.; Liu, S.; et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials 2021, 278, 121131. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gao, R.; Liu, X.; Feng, Z.; Zhang, C.; Huang, P.; Dong, A.; Kong, D.; Wang, W. Biomimetic glycopeptide hydrogel coated PCL/nHA scaffold for enhanced cranial bone regeneration via macrophage M2 polarization-induced osteo-immunomodulation. Biomaterials 2022, 285, 121538. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Marchesan, S. Self-Assembled Peptide Nanostructures for ECM Biomimicry. Nanomaterials 2022, 12, 2147. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, Y.; Cao, Z.; Zhao, L.; Lv, J.; Li, J.; Xu, Y.; Zhang, P.; Liu, X.; Sun, Y.; et al. Cell-free and cytokine-free self-assembling peptide hydrogel-polycaprolactone composite scaffolds for segmental bone defects. Biomater. Sci. 2023, 11, 840–853. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Guo, J.; Zhang, H.; Zhang, X.; Yin, C.; Wang, L.; Zhu, Y.; Yao, Q. 3D molecularly functionalized cell-free biomimetic scaffolds for osteochondral regeneration. Adv. Funct. Mater. 2019, 29, 1807356. [Google Scholar] [CrossRef]
- Lim, Y.-B.; Lee, M. Nanostructures of β-sheet peptides: Steps towards bioactive functional materials. J. Mater. Chem. 2008, 18, 723–727. [Google Scholar] [CrossRef]
- Mujeeb, A.; Miller, A.F.; Saiani, A.; Gough, J.E. Self-assembled octapeptide scaffolds for in vitro chondrocyte culture. Acta Biomater. 2013, 9, 4609–4617. [Google Scholar] [CrossRef]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef]
- Usui, Y.; Aoki, K.; Narita, N.; Murakami, N.; Nakamura, I.; Nakamura, K.; Ishigaki, N.; Yamazaki, H.; Horiuchi, H.; Kato, H.; et al. Carbon Nanotubes with High Bone-Tissue Compatibility and Bone-Formation Acceleration Effects. Small 2008, 4, 240–246. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Fathi, M.; Barzegari, A.; Barar, J.; Omidian, H.; Omidi, Y. Recent advances in polymeric scaffolds containing carbon nanotube and graphene oxide for cartilage and bone regeneration. Mater. Today Commun. 2021, 26, 102097. [Google Scholar] [CrossRef]
- Wang, J.; Huang, C.; Wang, Y.; Chen, Y.; Ding, Z.; Yang, C.; Chen, L. Exploration of the single-walled carbon nanotubes’ influence for cartilage repair. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125520. [Google Scholar] [CrossRef]
- Khalid, P.; Suman, V.B. Carbon Nanotube-Hydroxyapatite Composite for Bone Tissue Engineering and Their Interaction with Mouse Fibroblast L929 In Vitro. J. Bionanosc. 2017, 11, 233–240. [Google Scholar] [CrossRef]
- Feng, J.-M.; Dai, Y.-J. Water-assisted growth of graphene on carbon nanotubes by the chemical vapor deposition method. Nanoscale 2013, 5, 4422–4426. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef]
- Tutak, W.; Chhowalla, M.; Sesti, F. The chemical and physical characteristics of single-walled carbon nanotube film impact on osteoblastic cell response. Nanotechnology 2010, 21, 315102. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtules of grahitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, L.; Liu, Z.; Shen, Z.; Jia, W.; Hou, P.; Sang, S. 3D printed-electrospun PCL/hydroxyapatite/MWCNTs scaffolds for the repair of subchondral bone. Regen. Biomater. 2023, 10, rbac104. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Chondrogenic Differentiation of Marrow Clots After Microfracture with BMSC-Derived ECM Scaffold In Vitro. Tissue Eng. Part A 2014, 20, 2646–2655. [CrossRef]
- Pinals, R.L.; Yang, D.; Rosenberg, D.J.; Chaudhary, T.; Crothers, A.R.; Iavarone, A.T.; Hammel, M.; Landry, M.P. Quantitative protein corona composition and dynamics on carbon nanotubes in biological environments. Angew. Chem. Int. Ed. 2020, 59, 23668–23677. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, M.; Yao, Y.; Ma, Y.; Pu, Y. MWCNT interactions with protein: Surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity. Int. J. Nanomed. 2019, 14, 993–1009. [Google Scholar] [CrossRef]
- Ahadian, S.; Yamada, S.; Estili, M.; Liang, X.; Banan Sadeghian, R.; Nakajima, K.; Shiku, H.; Matsue, T.; Khademhosseini, A. Carbon nanotubes embedded in embryoid bodies direct cardiac differentiation. Biomed. Microdevices 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Newman, P.; Minett, A.; Ellis-Behnke, R.; Zreiqat, H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1139–1158. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.W.; Zhang, Y.N.; Liu, Y.R.; Li, W.; Dou, S.X.; Wei, Y.; Wang, P.Y.; Li, H. Diffusion behaviors of Integrins in single cells altered by epithelial to mesenchymal transition. Small 2022, 18, 2106498. [Google Scholar] [CrossRef] [PubMed]
- Karimi, F.; O’Connor, A.J.; Qiao, G.G.; Heath, D.E. Integrin clustering matters: A review of biomaterials functionalized with multivalent integrin-binding ligands to improve cell adhesion, migration, differentiation, angiogenesis, and biomedical device integration. Adv. Healthc. Mater. 2018, 7, 1701324. [Google Scholar] [CrossRef]



| Name | Content |
|---|---|
| Octapeptide | A synthesized peptide with a sequence of FEFEFKFK |
| FEK | Self-assembled peptide hydrogel |
| FEK/C1 | Self-assembled peptide hydrogel containing 0.05 mg/mL CNT |
| FEK/C2 | Self-assembled peptide hydrogel containing 0.1 mg/mL CNT |
| FEK/C3 | Self-assembled peptide hydrogel containing 0.5 mg/mL CNT |
| FEK-S | PCL scaffold composited with FEK |
| FEK/C1-S | PCL scaffold composited with FEK/C1 |
| FEK/C2-S | PCL scaffold composited with FEK/C2 |
| FEK/C3-S | PCL scaffold composited with FEK/C3 |
| Content | IG | ID | I2D | IG/ID | I2D/IG |
|---|---|---|---|---|---|
| CNT | 371.25 | 453.75 | 168.75 | 0.82 | 0.45 |
| FEK/C1 | 302.5 | 366.25 | 122.5 | 0.83 | 0.40 |
| FEK/C2 | 323.75 | 421.25 | 140 | 0.77 | 0.43 |
| FEK/C3 | 363.75 | 405 | 165 | 0.90 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Wu, Y.; Cao, Z.; Liu, X.; Sun, Y.; Zhang, P.; Zhang, X.; Tang, K.; Cheng, M.; Yao, Q.; et al. Enhanced Cartilage and Subchondral Bone Repair Using Carbon Nanotube-Doped Peptide Hydrogel–Polycaprolactone Composite Scaffolds. Pharmaceutics 2023, 15, 2145. https://doi.org/10.3390/pharmaceutics15082145
Lv J, Wu Y, Cao Z, Liu X, Sun Y, Zhang P, Zhang X, Tang K, Cheng M, Yao Q, et al. Enhanced Cartilage and Subchondral Bone Repair Using Carbon Nanotube-Doped Peptide Hydrogel–Polycaprolactone Composite Scaffolds. Pharmaceutics. 2023; 15(8):2145. https://doi.org/10.3390/pharmaceutics15082145
Chicago/Turabian StyleLv, Jiayi, Yilun Wu, Zhicheng Cao, Xu Liu, Yuzhi Sun, Po Zhang, Xin Zhang, Kexin Tang, Min Cheng, Qingqiang Yao, and et al. 2023. "Enhanced Cartilage and Subchondral Bone Repair Using Carbon Nanotube-Doped Peptide Hydrogel–Polycaprolactone Composite Scaffolds" Pharmaceutics 15, no. 8: 2145. https://doi.org/10.3390/pharmaceutics15082145
APA StyleLv, J., Wu, Y., Cao, Z., Liu, X., Sun, Y., Zhang, P., Zhang, X., Tang, K., Cheng, M., Yao, Q., & Zhu, Y. (2023). Enhanced Cartilage and Subchondral Bone Repair Using Carbon Nanotube-Doped Peptide Hydrogel–Polycaprolactone Composite Scaffolds. Pharmaceutics, 15(8), 2145. https://doi.org/10.3390/pharmaceutics15082145






