Abstract
Biofilm formation and antimicrobial resistance pose significant challenges not only in clinical settings (i.e., implant-associated infections, endocarditis, and urinary tract infections) but also in industrial settings and in the environment, where the spreading of antibiotic-resistant bacteria is on the rise. Indeed, developing effective strategies to prevent biofilm formation and treat infections will be one of the major global challenges in the next few years. As traditional pharmacological treatments are becoming inadequate to curb this problem, a constant commitment to the exploration of novel therapeutic strategies is necessary. Light-triggered therapies have emerged as promising alternatives to traditional approaches due to their non-invasive nature, precise spatial and temporal control, and potential multifunctional properties. Here, we provide a comprehensive overview of the different biofilm formation stages and the molecular mechanism of biofilm disruption, with a major focus on the quorum sensing machinery. Moreover, we highlight the principal guidelines for the development of light-responsive materials and photosensitive compounds. The synergistic effects of combining light-triggered therapies with conventional treatments are also discussed. Through elegant molecular and material design solutions, remarkable results have been achieved in the fight against biofilm formation and antibacterial resistance. However, further research and development in this field are essential to optimize therapeutic strategies and translate them into clinical and industrial applications, ultimately addressing the global challenges posed by biofilm and antimicrobial resistance.
1. Introduction
Antimicrobial resistance (AMR) occurs when microbes (bacteria, viruses, fungi, and parasites) change over time and no longer respond to the action of drugs (antibiotics, antivirals, antifungals, and antiparasitics) that would normally kill them or limit their growth. In this regard, bacterial resistance to antibiotics is of paramount importance in the One Health context. Bacteria can indeed feature AMR, a process that is enabled by different mechanisms and can be classified into (I) intrinsic, (II) acquired, and (III) adaptive. While intrinsic AMR relates to all those inherent mechanisms that are adopted by bacteria to inhibit antimicrobial agents and that do not require any contact with the antimicrobial agents themselves, acquired resistance is the result of either an exchange of genetic material or mutations. This type of resistance is more common in biofilms due to cell proximity [1]. Moreover, altered gene expression can result from exposure to environmental stress [2]. It has also been suggested that adaptive resistance may arise from a change in the intrinsic resistance mechanisms, leading to an increase in AMR in response to environmental stress and mutations [3].
Antibiotics, which have been widely used for more than half a century to treat bacterial infections, have greatly contributed to the promotion of human health and life expectancy [4,5]. This significant impact of antibiotics has been undermined in recent years due to growing levels of antimicrobial resistance [6]. Persistent consumption of antibiotics, genetic variations, and exposure to infections in hospitals can favor the selection and spread of multidrug-resistant bacteria, which has enormous implications for worldwide healthcare delivery and population health [7,8]. According to the antimicrobial resistance report published in 2016, the number of deaths caused by pathogenic bacteria is anticipated to rise to 10 million by 2050 if no urgent action is taken to reverse this course [9]. To this end, many approaches are being explored by the scientific community, with variable success.
In this review, we describe light-triggered approaches that target different stages of biofilm formation and maturation. These include surface patterning, pharmacological interventions, and the use of smart materials. Moreover, we focus on those light-responsive materials and photo-cleavable or photoswitchable molecules that have been designed for antibacterial applications. In the last decades, the photopharmacology approach has been successfully applied to different biological targets, leading to a series of molecular tools for the functional modulation of ion channels [10,11,12], glutamate receptors [13], G-protein-coupled receptors [14,15,16], protein-protein interactions [17,18], and enzymes [19], all of which have been successfully tested in vitro and in vivo for different therapeutic applications [20,21,22,23].
The use of light as a trigger appears to be particularly promising, as several classes of organic and inorganic materials can respond to a broad spectrum of wavelengths [24,25,26,27,28,29]. Moreover, certain wavelengths of light are not harmful for humans or the environment. This approach allows a non-invasive, on/off regulation of the material properties and activity, ultimately leading to enhanced control of the irradiation site and dosage [26].
2. Biofilm Formation and Development
To survive harsh environmental conditions, some bacterial species can live in close proximity and form highly structured multicelluar communities called biofilms, which attach to surfaces and interfaces. Inside biofilms, bacterial cells show features that are distinct from those that they show in their planktonic state, such as reduced motility and metabolic activities, heterogeneity of gene expression, inter-communal division of labor, and enhanced tolerance to antibiotics [30] (Figure 1).
In the 1670s, biofilms as complicated bacterial structures were first recognized by the Dutch microscopist Anton Van Leeuwenhoek when working on dental plaques. With the advent of electron microscopy, it was later revealed that to anchor themselves at the infection site, bacterial communities form biofilms, in which the bacteria are embedded in self-secreted extracellular viscous polymeric substances (EPSs). In most cases, the biofilm matrix accounts for around 90% of the total biofilm mass, and it is composed of exopolysaccharides, amyloid-like proteins, lipids, and extracellular DNA (eDNA) [31,32,33]. Most exopolysaccharides are species-dependent and contain repeated sugar units of the same and different types that are responsible for their polycationic or polyanionic nature [34]. Those charged molecules are essential for water retention in the biofilms and to hydrate the environment, a feature required to protect the bacterial cells in the biofilm from desiccation due to water stress, hence keeping the non-rigid structure of the biofilm with different viscosities to allow cell movements in the matrix [35]. These properties of the EPS matrix provide mechanical support to protect the resident cells from external forces, such as fluid shear, and to ensure that the biofilm community remains attached to a surface. In the context of infectious biofilms, it is difficult for neutrophils to access biofilm-forming bacterial cells because, during phagocytosis, they can only exert stress up to 1 kPa, which is not enough to break the biofilm into small pieces. Moreover, neutrophils can only ingest pathogens smaller than 10 μm; therefore, living in clusters within biofilms eventually protects bacteria from being attacked [36].
Biofilms represent the main cause of infections, such as catheter-associated urinary tract infections. Unlike planktonic cells, the bacterial population in the biofilms exerts their action by reducing their motility and metabolic activities while, at the same time, upregulating their production of extracellular toxins at the site of infection, which ensures the maximum possible tissue damage. The overall result is an abundant release of nutrient supplies, leading to further cementation of biofilm [37]. At the same time, adjacent tissues are also colonized by the shedding of daughter planktonic cells. Eventually, this spread of infection and development of new biofilms would mature into a densely packed structure that is difficult to eradicate, hence triggering chronic and recurrent infections [38,39,40].
Bacteria switch from planktonic life to biofilm mode by utilizing a combination of van der Waals, electrostatic, and hydrophobic interactions to attach themselves reversibly to biotic or abiotic surfaces through fimbriae, pili, flagella, and glycocalyx. These attachments are easily affected by the substratum type, the hydrodynamics, and other characteristics of the aqueous medium. At some point, bacteria either commit themselves to the biofilm irreversibly or revert back to their planktonic lifestyle [41,42].
In cases of conducive conditions for growth and differentiation, a biofilm develops into spatially arranged 3D structures, interspersed with fluid-filled channels, where nutrients, oxygen, and essential substances can diffuse and circulate in each individual microenvironment for the embedded microbial cells to undergo coordinated community growth that leads to the formation of microcolonies [43]. In this way, bacteria display coordinated group behavior (secretion of virulence factors, formation of biofilm), which is based on a density-dependent signal called quorum sensing [44,45]. With the formation of biofilms, bacterial cells now have distinct features as compared to their planktonic lifestyle, such as the presence of an EPS matrix, increased nutrient supply, upregulated synthesis and secretion of extracellular material, and chemical and/or electrical interactions [46]. However, triggered by various environmental factors, the biofilm can lose its stability, either actively or passively, thus dispersing into its surroundings via the detachment of either single cells or large aggregates of cells [35,47,48], which can then land at new locations to initiate the formation of a new colony [49].
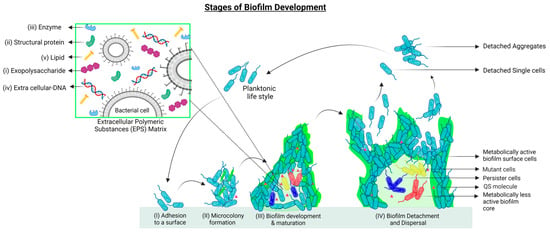
Figure 1.
Stages of biofilm development: (I) Attachment of free-living bacteria to a compatible surface using physical forces, cell appendages, and secreted adhesins. (II) Microcolony formation to maintain surface attachment is accompanied by the initial production of Extracellular Polymeric Substances (EPS, in light green). (III) Biofilm maturation is achieved through the establishment of a microenvironment suitable for cellular heterogeneity (depicted as red, blue, and yellow cells), the release of quorum sensing molecules (pink triangles), and enhanced EPS matrix production to combat environmental stress. (IV) At the time of nutrient depletion and accumulation of toxic compounds, dispersal of biofilm occurs. Cells can detach individually and in aggregates to start either planktonic life or grow into new biofilms. Zoomed section. Composition of the EPS matrix: The EPS matrix contains an array of biofunctional molecules such as (i) exopolysaccharides, essential for surface adhesion and structural integrity of biofilms; (ii) structural proteins, which connect the cells to the EPS and stabilize the biofilm architecture; and (iii) extracellular enzymes (mostly hydrolases and lyases) that facilitate the degradation of EPS molecules into simpler products to be used by the biofilms as sources of energy and carbon. The EPS degradation process is also important for biofilm dispersal and the formation of new biofilms. (iv) eDNA acts as an intercellular connector in the matrix and as a facilitator of Horizontal Gene Transfer (HGT). (v) Lipids are essential for bacterial adhesion to hydrophobic surfaces. The figure is partially adapted from [49].
Quorum Sensing
Quorum sensing (QS) is defined as a density-dependent microbial signal system that helps bacteria perceive and respond to temporal and contiguous environments (Figure 2). Quorum sensing depends on a network of autoinducer synthases, autoinducers (AIs), partner autoinducer receptors, and downstream signal transduction components [50]. AIs are innately produced at the basal level and gradually build up as microbial growth continues, leading to a positive feedback loop [51]. With the accumulation of critical concentrations of AIs, specific receptors become activated to start a signaling cascade of coordinated induction/repression of target genes within the bacterial population. This occurs under various environmental incentives, such as morphogenesis, biofilm formation, bioluminescence, drug resistance generation, regulation of the expression of virulence factors, dormancy generation, immune escape, and others [52,53,54]. Furthermore, it is important to consider that apart from quorum sensing as the determinant of cell density, there are other environmental signals (e.g., temperature, pH, osmolarity, oxidative stress, and nutrient deprivation) that bacteria must gather information about to determine their survival strategy [55]. QS-mediated regulation of virulence determinants has been found in both Gram-negative and Gram-positive bacteria [53,56].
The discovery of the first quorum sensing system dates back to the 1970s, when Vibrio fischeri, a bioluminescent marine bacterium, was found to colonize, symbiotically, the light organ of the Hawaiian squid Euprymna scolopes, establishing a positive correlation between the bacterial population density and the expression of genes responsible for bioluminescence in the host [57,58]. For the first time in 1994, the concept of the production of signal molecules by bacteria and their subsequent release into a specific environment was proposed as quorum sensing [59]. QS plays the most important role in biofilms formed by Salmonella spp., Escherichia coli, Campylobacter spp., Staphylococcus aureus, Listeria monocytogenes, and Bacillus cereus [60]. About 80% of microbial infections have been found to be related to QS-mediated biofilm formation [61].
There is great inter-population variation in the process of sensing signals, the type of signal molecules, the receptor of signal molecules, the mechanism of signal transduction, and the ultimate phenotype [62]. The AIs produced during QS range from molecules of low molecular weight to molecules of high molecular weight, such as oligopeptides [63]. Some examples of low molecular weight molecules involved in QS are N-acylhomoserine lactone (AHL or AI-1) [64], furanosyl borate diester (AI-2) [65], 4,5-dihydroxy-2,3-pentanedione (DPD) [66], 3-hydroxypalmitic acid methyl ester (3OH-PAME) [67], cis-11methyl-2-dodecenoic acid (diffusible signal factor, DSF) [68], 2-isocapryloyl-3R-hydroxymethyl-c-butyrolactone (A-factor) [69], diketopiperazines (DKP) [70], 2-heptyl 3-hydroxy-4-quinolone [71], and 4-hydroxy 2-heptylquinoline (HHQ) [72].
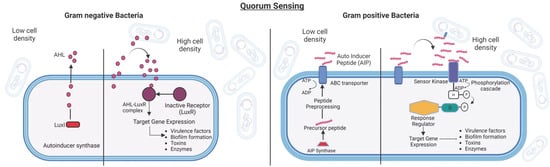
Figure 2.
(Left): Quorum Sensing (QS) in Gram-negative Bacteria. Gram-negative bacteria follow the LuxL-LuxR QS regulatory system. The autoinducer synthase (LuxI) secretes QS signaling molecules, AHLs (pink circles), that, upon reaching the threshold concentrations, enter the cells and activate the cognate AHL receptor (LuxR) and induce QS-regulated gene expression. (Right): Quorum Sensing (QS) in Gram-positive Bacteria. Gram-positive bacteria, on the other hand, use autoinducer peptides (AIPs) as signaling molecules. When they reach a certain concentration threshold, the autoinducers bind to the receptor kinase, which undergoes autophosphorylation and passes the phosphate group to the cytoplasmic response regulator. This activates the required genes in the quorum sensing regulon. The figure is partially adapted from [73].
3. Mechanism through Which Biofilms Combat Antibiotics
3.1. Metabolic Activity Heterogeneity and Tolerance Acquisition
The rate of metabolic activity inside a biofilm can vary significantly due to differences in the concentration of oxygen and nutrients that are available for the cells either at the surface or in the deep region of the biofilm. The bacterial subpopulations that feature the fastest growth rate are those residing on the surface of the biofilm, where oxygen availability is higher, limiting oxygen penetration to the slow- or non-growing populations occupying the inner zone of the biofilm. A study on the real-time detection of specific metabolites through fluorescent tags has revealed that the cells in the center of the biofilm are less active compared to the cells at the bulk liquid interface [74]. Another study focusing on the measurement of oxygen distribution at varying depths of biofilm using microelectrodes concluded that oxygen distribution strongly correlates with the biofilm structure and that it is depleted by as much as 30-fold in the core of the biofilm [75]. This gradient eventually leads to phenotypic and metabolic bacterial diversity, with a larger population displaying varied gene expression and phenotypes (i.e., susceptible, resistant, and tolerant cells) that co-evolve over time within the structure of the biofilm [76]. The low metabolic activity of these bacterial cells can be translated into low antibiotic target production and limited activity of such targets (i.e., enzymes involved in replication, protein formation, or peptidoglycan production). Unlike resistant cells, which, through genetic changes, develop long-term resistance to antibiotics, tolerant cells cannot grow or replicate during drug exposure but resume their growth when the antibiotic is removed. These cells are called persister cells, as they are able to outlive these unfavorable environmental conditions. This persistence is a transient phenotypic state rather than a genetic trait, as the same cells in planktonic form will become susceptible again. Persister cells are found in several human pathogens, including Staphylococcus aureus, Mycobacterium tuberculosis, Escherichia coli, Salmonella enterica subsp. enterica serovar Typhimurium, and Pseudomonas aeruginosa [77].
3.2. Adaptive Stress Responses
Biofilms are characterized by gradients of nutrients and oxygen that represent spatially organized stress conditions for the bacterial population, which in turn trigger adaptive responses such as the stringent response, the SOS response, and the general stress rpoS response, impairing the efficacy of antimicrobials and contributing to overall antibiotic tolerance [78]. Nutrient deficiency can also impact antimicrobial resistance as a consequence of the activation of stress responses that promote resistance by recruiting mechanisms of antioxidant activity and biofilm resistance or by modifying the cell surface to prevent the binding and entry of antimicrobials [79].
In stress conditions, when bacterial biofilms have populations with different growth rates (fast-growing, non-growing, or slow-growing cells), the availability of antibiotic targets is reduced in slow-growing cells. For example, β-lactams are effective on dividing bacterial cells only, which is evident in the large difference in minimum inhibitory concentration (MIC) and minimum biofilm inhibitory concentration (MBIC) of this antibiotic (1000 fold) in Pseudomonas aeruginosa [80]. Several clinically important antibiotics interfere with protein synthesis by binding at various functional centers of the ribosome, resulting in either freezing the ribosome in a particular conformation or hindering the binding of its ligands [81]. On slow-growing cells, antibiotics that bind the ribosome reversibly, such as tetracycline, have a bacteriostatic effect, while antibiotics that bind the ribosome irreversibly, like aminoglycosides, have a bactericidal effect. This selective effect of drugs on cells is due to cell growth’s dependence on ribosome abundance and protein synthesis [82]. DNA-binding quinolones are effective on non-growing cells but not as effective as on fast-growing cells [83].
The stringent response in persister cells is initiated through activation of the alarmone guanosine-5′-(tri)diphosphate-3′-diphosphate ((p)ppGpp), altering cellular physiology through transcriptional changes in populations with low metabolic activity, which consequently relocate cellular resources and ensure the survival of the bacterium [84]. Most antibiotics target active metabolic processes, and with this immediate shut-down of metabolism and growth, high levels of (p)ppGpp make bacteria insensitive to the actions of antibiotics [85]. A stringent response makes Pseudomonas aeruginosa biofilms tolerant to fluoroquinolones, meropenem, and gentamycin by preventing the accumulation of reactive oxidative species (ROS), which is considered a common mechanism by which antibiotics kill bacteria [86,87]. Polymyxins that target the membrane of Gram-negative bacteria are effective on non-growing populations, but metabolically active populations show adaptive resistance, impairing the penetration of the antibiotic [88]. However, the synergistic effect of combined antibiotics (tobramycin and colistin) was shown to successfully kill the metabolically active population of Pseudomonas aeruginosa on the surface of biofilms and the metabolically inactive population in the center of the biofilms, respectively [88,89].
Environmental stress also acts as a defining signal for recruitment of the multidrug efflux system as a stress response in many bacteria [79]. For example, the temporary activation of multidrug-resistant efflux pumps in Pseudomonas aeruginosa is triggered by adverse environmental conditions such as exposure to ROS (MexXY-OprM) [90], nitrosative stress (MexEF-OprJ) [91], or membrane-damaging agents (MDAs) [92]. An additional example is the temporary induction of β-lactamases and the activation of multidrug-resistant efflux pumps in Pseudomonas aeruginosa biofilms, which occurs only in the presence of β-lactam molecules. The induced β-lactamases are partially excreted by membrane vesicles into the matrix, where they inactivate β-lactam antibiotics before reaching cells [93].
The SOS response is a stress response to DNA damage that promotes tolerance to fluoroquinolones by inducing the expression of DNA repair mechanisms in Escherichia coli and Pseudomonas aeruginosa [94]. Sometimes, the common mechanism of antibiotics also triggers an SOS response in bacteria. For example, β-lactams, fluoroquinolones, or aminoglycosides all rely on the production of ROS. When ROS levels are not high enough, DNA oxidative damage (mutations) can occur, accompanied by the activation of the SOS response, repair of DNA damage, and the onset of antibiotic tolerance [95]. There is also selectivity in gene expression in planktonic and biofilm cells [96,97].
3.3. Antibiotic Resistance
Bacteria living in biofilms can exhibit a 10- to 1000-fold increase in antibiotic resistance as compared to similar bacteria living in planktonic states. For example, 100% of Staphylococcus epidermidis isolates were susceptible to vancomycin in a planktonic state, while 75% of the same bacteria were resistant when tested in a biofilm.
Many challenges are encountered by the antibiotic when it tries to penetrate the sticky, slimy membranes of the cells at the surface of biofilm. These are mostly represented by the complex biofilm architecture as well as the extracellular proteins and eDNA, which prevent the antibiotic from reaching its target. Meanwhile, the antibiotic may be deactivated even before it reaches its target. At the level of the microenvironment, metabolic byproducts, waste, and nutrients have started to accumulate, and oxygen supply from the surface may be greatly reduced, creating an anaerobic environment. The combination of all these factors can affect antibiotics in different ways, depending on their chemical structure and mechanism. For example, low oxygen levels reduce the bactericidal effects of tobramycin and ciprofloxacin, while pH changes can negatively influence aminoglycoside action. In such a situation, the resistant cells deep inside the biofilm enter the dormant state (persister cells), in which cell division is avoided. This preservation mechanism protects bacteria from the action of antibiotics, which usually require cells to be actively dividing. Interestingly, this dormancy is not permanent and reverses back to normal once cells are released from the biofilm [49].
3.4. Horizontal Gene Transfer (HGT)
Inside biofilms, horizontal gene transfer (HGT) is one way of driving the spread of antibiotic resistance genes (ARGs) due to the restricted motility of cells embedded in a matrix and eDNA as a means of intercellular contact. Moreover, as compared to natural transformation (transfer of chromosomal DNA and non-conjugative plasmids) and bacteriophage infection (transfer of bacteriophage genomic DNA), conjugation (transfer of conjugative plasmids and of integrative and conjugative elements (ICEs)) is the most common HGT mechanism in biofilms [98]. Due to their spatial organization, only those cellular subpopulations that reside in the core of biofilm can undergo HGT. However, biofilm growth promotes the persistence of plasmids carrying resistance genes. For example, in Staphylococcus aureus biofilms, conjugative plasmids were found to be 1600 times higher than in planktonic cultures. Another way of achieving HGT in Gram-negative bacterial biofilms is through integron-mediated acquisition/exchange of antibiotic resistance determinants by specific regulation of class 1 integron integrase [99,100].
3.5. Efflux Pumps in Biofilm Resistance
Efflux pumps are transport proteins involved in the removal of different metabolites, including antibiotics and secondary metabolites, to avoid toxic accumulation. They are thus implicated in antibiotic resistance and may promote biofilm antimicrobial resistance in several bacterial species [101], including Burkholderia cenocepacia [102], Escherichia coli [103], and Pseudomonas aeruginosa [104]. About twelve resistance-nodulation-division (RND) families of efflux pumps have been identified in Pseudomonas aeruginosa, four of which mediate antibiotic resistance [105]. MexAB-OprM, one of many efflux systems in bacteria, is most closely related to carbapenem resistance in Pseudomonas aeruginosa [106]. The regulatory genes mexR, nalD, and nalC14 negatively regulate the expression of MexAB-OprM, and any type of mutation in them may lead to the upregulation of MexAB-OprM, resulting in increased drug resistance in Pseudomonas aeruginosa [107].
4. Biofilm Disruption Strategies
Since biofilm formation contributes to bacterial pathogenicity and antibiotic resistance, various strategies have been employed to deal with this problem encountered in biofilm-infected tissues, tissue implants, and medical devices (Table 1).

Table 1.
Biofilm disruption strategies classified by mechanism of action.
4.1. Anti-Adhesion Strategies
Using anti-adhesion strategies, the exterior surface of the implanted medical device or biomaterial is altered, either directly or with the aid of a coating, to create a barrier that is not supportive of bacterial adhesion. Bacteria use cell appendages like pili or flagella along with physical factors like van der Waal’s forces, Brownian motion, or electrostatic interactions to adhere to biotic and abiotic surfaces. To colonize a host tissue surface, bacteria can adhere to host-produced components like fibrinogen, fibronectin, collagen, fibronectin-binding proteins (FnBPs), and fibrinogen-binding clumping factors (Clfs) [152].
Likewise, hydrophobic and non-polar surfaces also facilitate microbial binding. Coating the surfaces with antimicrobial agents such as metal-based nanoparticles, grafting the surface with cationic polymers, fabricating bionic antibacterial surfaces with a nano-scale structure, and using surfactants are good strategies to prevent bacterial adhesion on various surfaces. The strategy of inhibiting bacterial adhesion has been helpful in preventing biofilm-related infections on orthopedic implants [153]. Another study used glass, stainless steel, and silicon surfaces pretreated with dicephalic quaternary ammonium salts (QAS) to limit the adhesion of Staphylococcus epidermidis and Candida albicans cells [154]. Despite remarkable developments, anti-adhesion surface approaches featuring long-term stability need further investigation [153].
4.2. Quorum Quenching or Quorum Sensing Inhibition
QS regulates the expression of bacterial virulence factors, and therefore blocking QS can curb the virulence of bacteria. Novel therapeutic approaches interfering with QS, termed quorum sensing inhibition (QSI) or quorum quenching (QQ), have been introduced [155]. QS inhibitors (QSIs) interact with QS signaling systems in several ways, including (i) inhibition of synthesis; (ii) degradation; (iii) competition for receptor sites; (iv) inhibition of gene expression; and (v) removal of AIs. Since QSIs do not work through bactericidal or bacteristatic mechanisms to reduce bacterial virulence and biofilm formation, they pose less pressure for resistance selection in bacteria [156]. QSIs and quorum quenching (QQ) enzymes are the main QS inhibitors, and their functional targets include QS signaling molecules, receptors, and downstream signaling cascade components [157].
4.2.1. Targeting QS Signaling Molecules
Blocking the synthesis of QS molecules (AHLs and AIPs) in Gram-positive and Gram-negative bacteria or degrading them results in QS inhibition. AHL-lactonases, oxidoreductases, and antibodies are the major QS inhibitors. They target AI signaling molecules by disabling the enzymes that are responsible for their synthesis.
AHL-lactonase and AHL-acylase hydrolyze the lactone ring of AHL or cleave the acyl side chain of AHL in Gram-negative bacteria, which reduces AHL-LuxR binding and thus curbs QS signaling [158]. In Gram-negative bacteria, N-acyl homoserine lactone oxidoreductase, another QQ enzyme, modifies AIs and hinders their specific binding to receptors, resulting in reduced biofilm formation [138].
It has also been reported that antibodies play a role in the inhibition of QS signaling molecules [138]. In this regard, AIPs, which are produced by Gram-positive bacteria, are susceptible to antibody neutralization, as AHL bacterial molecules act as small-molecule toxins in mammalian cells, resulting in apoptosis and modulating NF-κB activity. For example, the XYD-11G2 antibody prevented the production of pyocyanin by Pseudomonas aeruginosa and neutralized the 3-oxo-C12-HSL signal [159]. A study on AIP-4 produced by Staphylococcus aureus observed its effective blocking by an anti-AI monoclonal antibody (AP4-24 H11) [160]. In addition, several naturally occurring brominated furanones have the ability to inhibit the LuxS enzyme in a concentration-dependent manner [161]. There are many reviews on QS molecules as targets for biofilm disruption [162,163].
4.2.2. Targeting Signaling Molecule Receptors
Inhibiting or competing for QS receptors is another strategy used by QS inhibitors. LuxR-AHL is a significant AI receptor protein found in Gram-negative bacteria. Therefore, disrupting the bond between signal receptors and AHLs with AHL analogs, structurally independent AHLs, and naturally occurring QS inhibitors is an effective alternative strategy for controlling QS. Several bulky groups have been added to the acyl side chain to create AHL analogs in Pseudomonas aeruginosa, Agrobacterium tumefaciens, and Vibrio fischeri, respectively, which have demonstrated the inhibition of LasR, TraR, and LuxR receptors [164].
Two types of naturally effective QS inhibitors are furanones and flavonoids, which can bind to the receptors of many pathogenic bacteria. Furanones are produced by the red alga Delisea pulchra and are known to regulate bacterial colonization and biofilm development through interference with the acylated homoserine lactone regulatory system in Gram-negative bacteria and the alternative AI-2 signaling system in Gram-negative and Gram-positive bacteria. Many furanones are now known as competitive inhibitors of LuxR-type receptors in Gram-negative bacteria by competing with AHL for binding to reduce QS signaling. Natural furanone ascorbic acid (vitamin C) is known to be a potent inhibitor of QS in Pseudomonas aeruginosa [165]. It has been shown to inhibit pyocyanin production, which supports cellular respiration and energy generation in oxygen-deficient conditions in Pseudomonas aeruginosa biofilms, thus affecting biofilm formation [166]. Libraries of synthetic furanones have also been developed, as they are potent anti-infectives and inhibit pathogenic phenotypes in Gram-negative and Gram-positive bacteria [167]. Flavonoids (e.g.,quercetins) are other natural QS inhibtors that are found in various plant parts (flowers, leaves, seeds) [168]. Quercetins are effective QS inhibitors in Pseudomonas aeruginosa, as they can inhibit biofilm formation and initial bacterial adherence and reduce virulence factor expression by competing with AHL for binding to the LasR receptor [169].
Another target for receptor binding competition is the competence stimulating peptide (CSP)-mediated QS system in Streptococcus pneumoniae, which uses two main CSP variants: CSP1 and CSP2, which bind to their corresponding histidine kinase receptors, ComD1 and ComD2, resulting in virulence and biofilm formation. Synthetic peptides like dominant-negative competence-stimulating peptides (dnCSPs) that compete with CSP for ComD binding have been used to reduce virulence factor expression in vitro and attenuate pneumococcus infections in mice [170,171]. To target the AI-2 QS system in Gram-positive bacteria, sulphone is among the many compounds that have shown an antagonistic effect on LuxP receptors in Vibrio harveyi [172]. There is detailed discussion of inhibitors targeting QS signal molecule receptors in several reviews [73,173,174].
4.2.3. Blocking the Signaling Cascade
Blocking the signaling cascade by deactivating the downstream response regulators or other regulatory factors is another strategy for QS inhibition. For example, triggered by upstream signaling, the downstream response regulator AgrA of Staphylococcus aureus is activated to induce the expression of QS-related genes. In this regard, Savarin, a known Staphylococcus aureus virulence inhibitor, can specifically target AgrA to stop the signaling cascade [73].
Virstatin, a small molecule, represses the expression of AnoR, which positively regulates LuxI-like synthase AnoI in Acinetobacter nosocomialis. This results in a reduced production of N-(3-hydroxy-dodecanoyl)-L-homoserine lactone (OH-dDHL), thus affecting the signaling cascade and reducing biofilm formation and motility [175]. There are several reviews on QS interfering mechanisms and their implications for bacterial pathogenecity [157,173,176].
4.2.4. Targeting the EPS Chemical Composition and Structure
There are several ways to target EPS matrix formation, mostly related to the inhibition of EPS production via the prevention of adhesin-mediated bacterial attachment to surfaces or to the degradation of EPS matrix in mature biofilms using mutants of enzymes that are produced by the bacteria themselves [177]. Despite being considered biofilm virulence factors, these enzymes can be engineered to initiate biofilm disassembly. Enzymes like glucano-hydrolases and glycoside hydrolases disrupt the viscosity and elasticity of the biofilms, which weakens biofilm cohesiveness and increases antibiotic penetration [178].
eDNA was found to play a vital role in the composition of bacterial biofilms in the context of HGT, and this paved the way to targeting eDNA with DNases. Dnase-I destroys eDNA through the hydrolysis of its phospholipid ester bonds [179]. Exogenous DNase I has shown inhibition of biofilms of many Gram-negative and Gram-positive bacteria, but it is more effective on young biofilms [179,180].
Bacteriophages have the ability to penetrate the tridimensional architecture of biofilms and eradicate bacterial biofilm. The novel phage F15 produces a polysaccharide depolymerase that hydrolyzes the EPS of Pseudomonas putida and inhibits biofilm formation [181]. To maintain biofilm adhesion properties and stability, extracellular proteins like DNA-binding proteins (DNABPs), functional amyloids/amyloid-like proteins (FA/ALPs), and other biofilm-associated proteins (Baps) are crucial [182]. Hence, proteases (e.g., Purified Esp [183], proteinase K [184], and cysteine proteases [185]) that can degrade EPS extracellular proteins have the potential to disperse a massive biofilm [177]. Recent research has proven curcumin, a distinctive yellow pigment and a major constituent of turmeric derived from the Curcuma longa plant, to be a potent anti-QS agent in many pathogens as it inhibits the production of QS-dependent factors such as exopolysaccharide and alginate [186]. In general, the combination of EPS synthesis inhibitors or EPS-degrading enzymes, which lack intrinsic antibacterial activity, with antimicrobial agents could be a good option for biofilm removal [187]. Many reviews on EPS degradation and synthesis inhibition have discussed this strategy in detail [188,189].
4.3. Targeting Persister Cells
There are several strategies to kill persister cells in biofilms: (1) the direct killing of metabolically dormant persister cells; (2) awakening the persister cells from metabolically inactive form to antibiotic-susceptible active form; (3) combining anti-persister drugs with conventional antibiotics; and (4) other indirect approaches such as interfering with the QS signaling circuit and genetic engineering of the metabolic pathways of persister cells [190]. Anti-persister agents, such as cationic antimicrobial peptides (AMPs), make pores on respiring cells as well as persister and dormant populations residing in the center of biofilms. They change the membrane potential through electrostatic interactions with oppositely charged cell membrane/wall components. A broad-spectrum antimicrobial peptide, TM5, can reduce planktonic and persister cells in biofilms formed by both Gram-positive and Gram-negative bacteria, but its in vivo clinical potency needs validation [191]. Arginine and tryptophan-containing cationic membrane-penetrating peptides have been shown to destroy the negatively charged lipopolysaccharide of the persistent Escherichia coli cell wall, resulting in membrane disruption and cell death [192]. Since AMPs have shown more biofilm inhibition than eradication potency, they have now been used in combination with antibiotics.
4.4. Targeting Efflux Pumps
Besides being a major factor in the development of antibiotic resistance, efflux pumps can influence the functions of biofilms directly or indirectly [193]. Upon exposure to tigecycline, Acinetobactor baumannii was shown to feature an attenuated tendency to form biofilm due to the downregulation of the adeG gene encoding for efflux pumps [194]. Many studies suggest that the bacterial QS mechanism is negatively affected if inhibitors hinder the efflux pump activity [195]. Another study on Acinetobactor baumannii demonstrated that with inhibition of the adeAB gene (for the MDR efflux pump belonging to the RND family) expression or deletion, hindrance of biofilm and QS systems occurs [196]. Chetri et al. [197] have thoroughly discussed the urgent need for improved efflux pump inhibitors.
5. Light-Based Antibiofilm Strategies
Owing to the high spatio-temporal control with which a light stimulus can be delivered, light-based strategies against bacterial proliferation and biofilm formation represent a rapidly expanding field of science. Compared to traditional pharmacotherapy, this distinct advantage makes light the perfect stimulus to confine the antibacterial action only when and where it is needed. Interestingly, light-triggered approaches against bacterial proliferation have been reported in the context of all developmental stages, starting from adhesion to biofilm formation, initiation, and maturation.
5.1. Bacterial Adhesion: Light-Triggered Control of Bacterial Adhesion
Smart antibacterial surfaces have been investigated for decades due to their potential to control bacterial attachment, biofilm formation, and dissolution. In 2018, Li and co-authors published a comprehensive review focusing on the design of smart antibacterial surfaces with antibiofouling and antimicrobial properties [28]. The idea behind the design of a smart surface with antimicrobial properties is to reduce the development of drug-resistant bacteria by means of a local and on-demand administration of the drug or to avoid bacterial adhesion and therefore biofilm formation. Different stimuli have been used to achieve controlled antimicrobial drug release, including pH, temperature, release of chemicals, use of charged polymers, and nano- and microstructured surfaces with biomimetic properties [198,199,200,201,202].
In this review, we make reference to those applications in which the antibacterial action is triggered by light. In this regard, the light stimulus can be addressed to control surface functionality, such as bacterial photolithography, or to control the bacteria’s adhesion properties, such as in optogenetic approaches (Figure 3).
Bacterial photolithography allows the control of biofilm patterning at a distance as small as 10 µm [203]. For biotechnological applications or to study complex bacterial communication circuits such as those involved in quorum sensing, the goal is the controlled patterning of the biofilm rather than its total eradication. Indeed, obtaining patterned biofilms on the microscale can allow the investigation of bacterial communication and biofilm formation [204], the exploration of the use of biofilms as living biomaterials [205], and the generation of more reliable results of drug tests due to the more controlled biofilm geometries [206]. A bottom-up approach to control bacteria colonies has been proposed. It involves a genetically-encoded biofilm patterning tool named “Biofilm Lithography”, which is able to control the expression of membrane adhesion proteins that are responsible for surface attachment [204]. This allowed the patterning of Escherichia coli biofilms with a 25-micrometer spatial resolution.
With the aim of patterning biofilm, Chen et al. [203] used a photolithography approach with Escherichia coli bacteria. The surface was functionalized with a mannoside group on a nonadhesive polyethylene glycol (PEG) coating (Figure 3, left-hand side). The α-D-mannoside group is recognized by the FimH bacteria receptor, and bacteria can adhere to this functionalized surface. The mannoside group is connected to the PEG coating by means of a photocleavable 2-nitrobenzyl linker. Upon exposure to UV light, the linker is cleaved, and the nonadhesive PEG becomes exposed. Photopatterning allows bacteria to adhere in non-illuminated regions and prevents them from adhering in illuminated regions and on bare PEG surfaces.
Sugar binding strategies were also used by Ma et al. [207], who developed a spiropyran- and galactose-decorated nanoplatform. These interactions were developed to image bacterial adhesion and eradicate the Pseudomonas aeruginosa biofilm from the surface.
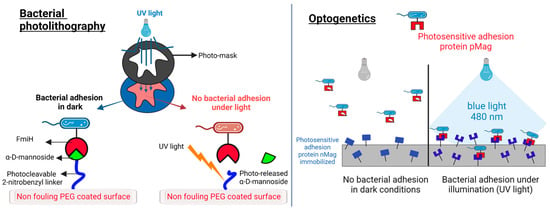
Figure 3.
Light-triggered strategies against bacterial adhesion. (Left): the photolithography approach to controlling bacteria adhesion [203]. The α-D-mannoside is immobilized on a PEG surface by means of a photocleavable 2-nitrobenzyl linker. The adhesive protein FimH in Escherichia coli binds to the sugar in dark conditions. Under UV light, the linker is cleaved, the sugar is released, and the surface becomes antiadhesive for the bacteria. The patterning of illuminated and non-illuminated regions can be as small as 10 µm. (Right): the optogenetic approach to controlling bacterial adhesion. Escherichia coli bacteria express on their surface a photoswitchable protein (pMag) that can heterodimerize with the nMag protein immobilized on the surface under blue light (480 nm). In dark conditions, the binding is impeded, and no bacterial adhesion can be obtained. Binding is reversible and can be repeated by illumination and dark cycles. The scheme is adapted from Chen et al. [208].
Bacterial binding properties can be controlled with an optogenetic approach. This technology is well established in neuroscience and provides interesting opportunities to control bacterial behavior at the cost of introducing genetic modifications in the pathogen [204,208,209,210,211].
Regarding the possibility of controlling bacterial adhesion and biofilm organization, Chen et al. [208] proposed the use of photoresponsive proteins (nMag and pMag), which heterodimerize under blue light (480 nm) and dissociate from each other in the dark. The author expressed pMag on the surface of Escherichia coli. This protein can interact under blue light with nMag immobilized on a glass substrate coated with PEG. In the dark, the adhesion is reversible. This allowed for blue-light switchable bacterial adhesion with high spatial and temporal resolution (Figure 3, right-hand side).
This optogenetic control has been recently scaled up from the surface of the material to the dynamically controlled bacteria-bacteria adhesion [212]. The ability to control photoswitchable adhesion between bacteria is important to regulate multicellular and associated bacterial behaviors such as aggregation, quorum sensing, biofilm formation, and metabolic processes. Chen et al. obtained this result by expressing pMag and nMag proteins on the surface of Escherichia coli to obtain bacteria that cluster when illuminated with blue light and disassemble in the dark.
Notably, in contrast to the photolithographic approach, which is not reversible as it involves the photocleavage of the linker on the surface, the optogenetic approach allows a reversible control on the bacterial adhesion since the adhesion can be repeatedly turned on and off.
5.2. Bacterial Communication: Photoswitchable Modulators of Quorum Sensing
As detailed in Section 2, quorum sensing is the communication system that bacteria use to organize into communities and develop biofilms. QS has been considered a target for synthetic biology strategies to control biofilm development [213]. In this regard, the possibility of using light to control and interfere with this communication system is extremely appealing. A tool that is able to control QS offers the possibility to control bacteria group biology, study QS circuits, inhibit biofilm formation, or promote biofilm dispersal on command, if needed [214]. The Feringa group investigated the possibility of using photoswitchable compounds to interfere with QS. Their molecular design relied on the modification of autoinducer molecules via a light-sensitive moiety. The introduction of an azobenzene unit in the N-Acyl homoserine lactones (AHLs) led to a small library of photoswitchable autoinducers for Gram-negative bacteria [215]. Their design, which was supported by previous SAR data and computational pharmacophore models of the AHL [164], led to the replacement of the alkyl chain of the lead compound with the azobenzene moiety. By means of bioluminescence assays measuring the LasQS-controlled bioluminescence in Escherichia coli, they obtained the opposite behavior for two of the tested molecules. Indeed, one of the molecules acquired QS-inducing activity while the other lost its activity upon trans-cis isomerization, proving that the photocontrol of the QS mechanism in bacteria is possible and that the contradictory behavior under light illumination can be ascribed to the geometry of the molecule. The active molecules have a more linear shape and therefore a better interaction with the receptor binding pocket than the less active conformations, which are more bent. Finally, by acting on the LasQS system, the authors were also able to photocontrol the production of virulence genes in Pseudomonas aeruginosa [215]. The gene expression of lasA was different for the irradiated and non-irradiated compounds, proving that it was possible to control the expression of virulence genes with light.
A recent follow-up study from this group [216] led to the development of photoswitchable autoinducers in Pseudomonas aeruginosa based on N-3-(oxo-dodecanoyl)-L-homoserine lactone (OdDHL) (Figure 4 on the top). In a bioluminescence assay in a QS reporter Escherichia coli strain, the authors evaluated the agonist or antagonist character of a library of photoswitchable compounds. One of the best-working compounds (AHL5) (Figure 4, bottom) showed a remarkable 700-fold difference in activity between the two forms, with a switching behavior between antagonist (non-irradiated) and agonist (irradiated with 365 nm) at 60 µM. This makes these compounds good candidates for QS studies.
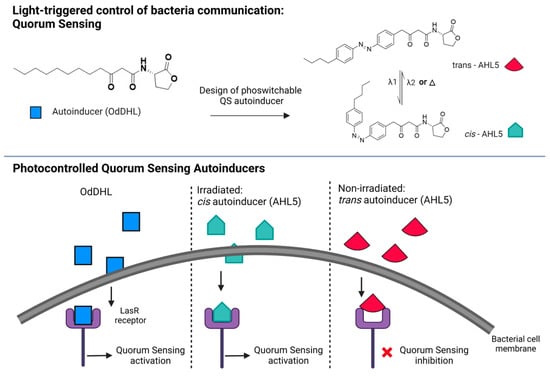
Figure 4.
Photoswitchable modulators of bacterial communication. Top panel: native QS autoinducer (OdDHL) and photoswitchable analogue design (AHL5) in trans and cis conformation. Trans-AHL5 is the thermodynamically stable form of the molecule, which can be switched to cis-AHL5 by UV light illumination (λ1 = 365 nm). A backward reaction from cis to trans can be obtained by exposure to visible light (λ2) or heat (Δ). Bottom panel: scheme of action of the autoinducer binding and activating the LasR receptor that triggers QS (native OdDHL and cis-AHL5) or acting as an antagonist (trans-AHL5) and inhibiting the receptor [216].
5.3. Biofilm Maturation and Planktonic Phase: Photocleavable and Photoswitchable Antibiotics
Two different strategies have been implemented to develop light-triggered antibiotics: (i) photocaged compounds and (ii) photoswitchable compounds (Figure 5). The molecular design is intrinsically different and has been recently reviewed [202]. In photocaged compounds, a photolabile moiety is attached to the drug scaffold, hindering its interaction with the biological target. This photolabile moiety is then removed by illumination, thus letting the drug interact with the biological target.
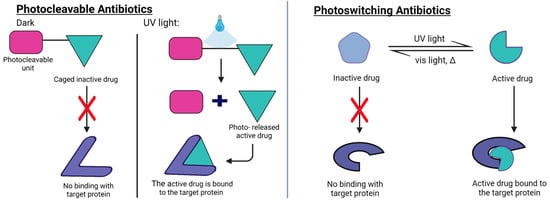
Figure 5.
Mechanisms of action of photocleavable antibiotics (Right) and photoswitchable antibiotics (Left). Photocleavable antibiotics consist of a drug molecule that is inhibited by the presence of a photocleavable moiety. Upon light-triggered cleavage of the impeding unit, the free antibiotic can interact with the target protein. Photoswitchable antibiotics feature a bistable form where only one of the conformations can interact with the binding protein and exert the pharmacological action. This strategy gives reversible control over the pharmacological action of the antibiotic, which can also be deactivated by switching back the molecule to its inactive form, either spontaneously with time or by using an appropriate wavelength of light.
The design of photoswitchable antibiotics involves the inclusion of a photoswitchable unit permanently attached to the antimicrobial drug. While, under dark conditions, the interaction with the biological target is hindered, the photoswitching of the light-sensitive moiety produces a conformational change that favors the interaction with the biological target. For photoswitchable antibiotics, the presence of a photoswitching unit can cause a reduced interaction with the biological target even in the case of the best performing isomer, thus decreasing drug potency. On the contrary, the presence of a photolabile element in caged antibiotics allows the release of the intact drug. However, the irreversibility of the photochemical reaction makes it impossible to deactivate the action of those drugs once the caged unit has been released. Photoswitchable antibiotics have, instead, the advantage that if the metastable form (i.e., the cis form) is the pharmacologically active conformation of the compound, it will deactivate spontaneously after the compound converts back to the thermally stable form (i.e., the trans form). This confers on the photoswitchable antibiotic a lower impact on the environment and a lower risk of triggering antibiotic resistance in bacteria.
The possible mechanisms of AMR development from photoswitchable drugs are also being studied. A 2021 study [217] analyzed the development of resistance in the Escherichia coli mutant strain CS1562 by the effect of trans/cis-tetra-ortho-chloroazobenzene-trimethoprim (TCAT) compounds. Both irradiated TCAT and thermally adapted TCAT were analyzed and compared to the reference analog trimethoprim (TMP). Interestingly, the photoswitchable compound had a different response to acquired resistance. The resistance mechanism to the photoactive compound appears to hinder the entry of the molecule into the cell, whereas the resistance to TMP involves changes in cell metabolism and alterations in the expression levels of enzymes associated with the biosynthesis of folate.
Hou et al. [218] developed CONBE, a photolabile ciprofloxacin compound. Their design strategy involved the conjugation of a photocleavable ortho-nitrobenzene to the 3-carbonyl of ciprofloxacin. After illumination with UV light (365 nm, 10 mW/cm2), leading to the photochemical cleavage of the cage element, the conversion of CONBE to ciprofloxacin was 91% efficient. The bactericidal activity obtained with this strategy was as potent as that of the parent drug. A light-activatable caged antibiotic based on vancomycin and cephalosporin was proposed by the Gademann group [219]. The photoreleased vancomycin was active against Gram-positive strains, and the uncaged cephalosporin was active against both Gram-positive and Gram-negative strains. To overcome the problem of using phototoxic UV light, vis-NIR activatable cleavage groups can be used. Contrera-Garcia et al. [220] proposed the design of a BODIPY photocage to protect quinolone-based antimicrobial compounds. This design strategy allowed the authors to use green (λmax = 520 nm), red (λmax = 635 nm), or far red (λmax = 730 nm) light for the photorelease.
Regarding photoswitchable antibiotics, their molecular design has been extensively reviewed in recent times [202]. Light-sensitive analogues of natural compounds, cystobactamids, have been proposed. Cystobactamids are active against a broad range of Gram-negative and Gram-positive pathogens by targeting bacterial gyrase [221]. Due to poor light penetration into biofilm colonies, the use of these compounds against the biofilm state is still difficult, and most of the studies focus on planktonic life styles. Researchers are exploring different chemical designs to gift azobenzenes with red and infrared absorption to allow antibiotic activity with deeper penetration [222].
Photosensitive surfactants based on photoswitchable compounds such as AzoTAB have been studied for their ability to perturb a lipid membrane and cause the disruption of vesicles [223]. This strategy can provide engineered compounds to target the bacteria’s membrane. This would produce photoswitchable antibiotic agents that reside in the membrane and, when activated by light, can cause its disruption, thus leading to the death of the bacteria [224]. An elegant evolution of this concept, albeit on cancer cells, has been proposed by Mutter et al. [225]. Fragaceatoxin (FraC) is a toxin able to form nanopores on the surface of sphingomyelin-rich cells, causing cell death. FraC has been decorated with azobenzene molecules with the aim of controlling the nanopore assembly with light. The authors obtained a system that is inactive in the dark but active upon illumination, causing cell lysis.
Finally, new targets for photoswitchable compounds have been explored, such as bacterial motility and bacterial membrane potential. Duchesne et al. [226] applied photoswitchable compounds to control the speed of Escherichia coli with light. They obtained different net effects (increase or decrease of speed) depending on the compound tested, proving that the design of such optical tools is very promising. De Souza-Guerriero et al. [227] explored the possibility of using a membrane-targeted azobenzene to photo-modulate the membrane potential in Gram-positive cells (Bacillus subtilis). These studies open the way to the use of optical tools to study bacteria spreading and biofilm electric signaling and develop new light-triggered strategies for bacterial infections and antimicrobial resistance.
6. Light-Based Materials Strategies to Tackle Bacterial Infections
A promising approach to counteracting bacterial adhesion and growth relies on the design of advanced functional materials, which may not only overcome some of the limitations encountered with photo-pharmacology strategies but also allow for a therapeutic effect without further reinforcing AMR mechanisms. The so-called “nanobiotics” harness the unique potential of their nanometric structure, shape, and tailorable surface chemistry to actively hinder bacterial processes and interfere with biofilm formation and adhesion [27,228]. Moreover, if loaded with bioactive compounds, such as bactericidal drugs (i.e., antibiotics and antimicrobial peptides) or biofilm dispersants (i.e., EPS enzymes and nitric oxide compound generators), the nanometric formulations grant a controlled and localized delivery of the cargo molecules, thus reducing the toll of potentially negative systemic side-effects [229].
Antibacterial materials are typically classified according to the stimulus that triggers their response, which can be either endogenous or exogenous. Endogenous stimuli include the acidic pH of the bacterial microenvironment and the secretion of bacteria metabolites and enzymes (such as lipases, proteases, and matrix metalloproteinases), while exogenous starters are generally physical-based, such as light, temperature, electricity, magnetic field, ion concentrations, and ultrasound [26,228]. Using light as a source of activation appears to be one of the most promising strategies, as several classes of organic and inorganic materials can respond to a broad spectrum of wavelengths. Moreover, light is not harmful to both humans and the environment; it allows a non-invasive, on/off regulation of the material’s properties and activity, and it can easily permit the control of the irradiation site and dosage [26].
Various light-activated antibacterial mechanisms have been proposed in the literature, encompassing permeation of the bacterial membrane, inhibition of enzyme activity, as well as the generation of reactive oxygen species (ROS), which in turn are responsible for toxic intracellular oxidation cascades, ultimately leading to DNA leakage, microorganism cell lysis, and biofilm disruption (Figure 6) [27,228]. Overall, the antibacterial strengths of those advanced, light-triggered material formulations reside in their ability to (i) respond on demand upon a specific luminous trigger; (ii) potentially leverage on multiple stimuli and activate distinct bacteriotoxic modus operandi; and (iii) physically breach crucial biological processes. On the other hand, conventional drugs are designed to interfere at a molecular scale but in a rather uncontrolled, systemic way, which, as a detrimental consequence, may favor the establishment of AMR.
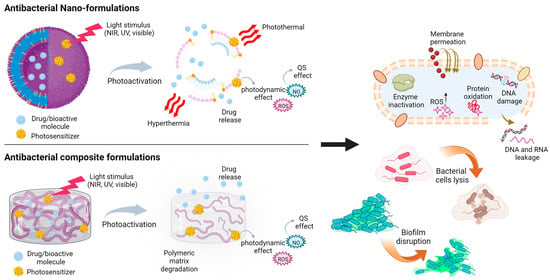
Figure 6.
Light-triggered formulations and their antibacterial effects. The photoactivation of antibacterial functional materials can spark a series of mechanisms, such as localized and controlled drug release, photodynamic effect, and photothermal effect, which can either act on each bacterial cell individually (via DNA damage, enzyme inactivation, protein oxidation, and cell lysis) or on the bacterial biofilm. Upon light stimulation, hybrid organic/inorganic composite formulations (here depicted as composite hydrogel as an example) may degrade or modify their matrix properties and release antibiotic cargos or photodynamic nanoparticles.
Depending on the targeted microorganisms or biomedical applications, a plethora of antibacterial light-triggered functional materials have been developed [230], either in the form of nanoformulations [26,229,231], functionalized surfaces [28,232], or hydrogel or polymer-based formulations [25,29].
6.1. Light-Triggered Nano-Formulations
Depending on the intended microorganisms or biomedical applications, a plethora of antibacterial light-triggered functional nanoformulations have been developed, such as micelles [233], liposomes [234], carbon quantum dots [235,236], silver- and gold-based nanoparticles [237], mesoporous silica particles [238,239], metallo-organic frameworks (MOFs), metal oxide-based nanostructures [240], up-conversion nanoparticles [241,242], and polymeric nanoparticles [243], either loaded with antimicrobial drugs or presenting at their surfaces an optically active compound. Table 2 reports an overview of some of the most recent studies, showing the material constituents, the light stimuli that trigger their mechanisms of action, as well as the targeted microorganisms and applications.

Table 2.
Light-triggered anti-bacterial nano-formulations.
What makes nano-formulations more versatile compared to traditional drugs or novel photo-pharmaceuticals is the different types of light sources that can be employed to prompt a specific antimicrobial mechanism, which span from near-infrared (NIR, 750–950 nm) to UV light (290–400 nm), passing through visible light (400–750 nm), and LED or laser sources. The light stimulus may either activate a nanoparticle photoactive core or trigger the plasmonic photothermal effect, which eventually leads to the generation of heat, a temperature increase at the nano-formulation surface and within its surroundings, and a necrotic cascade in the adjacent bacterial cells.
The plenitude of light-based stimuli may favor applicability in different settings, from the clinical/surgical environment to the point-of-care, personalized system. The presence of a high superficial area allows surface functionalization with photosensitizers able to enhance light absorption as well as chemical modification with targeting moieties useful for enzyme recognition or bacterial membrane/biofilm penetration. Moreover, the assorted shapes and sizes that can be easily obtained with the nanostructures can be leveraged to further widen the field of applications or the fabrication of multiple-responsive systems [256].
For example, Zhao and colleagues [233] developed NIR-activated liposomes composed of a thermosensitive phospholipid, distearoyl phosphatidylcholine (DSPC), and a quaternized cholesterol molecule, which were loaded with Tobramycin and functionalized with a cyanine dye (Cypate). Upon irradiation with NIR, the susceptible dye generated a temperature increase; when reaching 45 °C, the structure of the micelle was partially disrupted, and a localized antibiotic release of up to 80% could be obtained. Moreover, the heat generation mediated by the Cypate molecule induced enzyme denaturation, leading to cell death. The synergistic action between the photothermal therapy and the on-demand drug release caused a 7- to 8-fold increment in biofilm dispersion rate relative to the conventional, free antibiotic therapy. The photothermal effect (Figure 7), which is based on the absorption of light by electrons at the surface of conductive or thermo-responsive materials and on the consequent energy dissipation in the surroundings as heat, is widely used to liberate bioactive molecules from their vehicles or to cause permanent DNA damage via hyperthermia and bacterial cell lysis [228,241].
Several antibacterial nanostrategies are based on the use of metal oxide nanoparticles as photocatalytic agents to trigger extracellular and intracellular oxidation reactions. Bagchi and co-workers [240] presented a hybrid nanosystem composed of ZnO nanoparticles decorated with a photosensitive dye (squaraine, SQ) as an antibacterial coating for artificial implants. The well-known antimicrobial activity of ZnO nanostructures resides in the light-mediated electron transfer from the valence band to the conduction band of the material and the consequent generation of electron-hole pairs. These react with oxygen molecules in the surroundings and generate reactive oxygen species (ROS) such as hydroxyl radicals (●OH), superoxide anions (O2−), and singlet oxygen (1O2), which spark oxidation cascades, intracellular protein oxidation, bacterial membrane permeation, and genetic material leakage (Figure 7) [236,257]. The use of SQ in the hybrid construct is responsible for the interfacial electron transfer and more intense ROS generation (with treatment for 3 h at a NP concentration of 140 nM). Thanks to their nanometric size (~24 nm), SQ-ZnO NPs can be internalized in Staphylococcus aureus cells, disrupting the bacterial membrane and reducing biofilm adhesion. Nano-formulations that present dual photodynamic and photothermal effects to enhance their antimicrobial action have been extensively proposed in the literature (Table 3) [236,242,249,250].

Table 3.
Light-triggered anti-bacterial polymer-based or composite formulations.
Table 3.
Light-triggered anti-bacterial polymer-based or composite formulations.
| Materials | Formulation | Light | Mechanism | Target Microorganism | Application |
|---|---|---|---|---|---|
| PVA-Prussian blue nanoparticle hydrogel films | Nanoparticles in hydrogels | NIR | Localized photothermal therapy | Pseudomonas aeruginosa [258] | |
| Sodium alginate hydrogel loaded with Cu2O and Bi12O17Cl2 NPs | Nanoparticles in hydrogels | NIR | Hydrogel crosslinking, film formation, and ROS generation | Staphylococcus aureus, Escherichia coli, and Streptococcus mutans [259] | tooth whitening and biofilm removal |
| Upconversion nanoparticles (UCNPs) and porphyrinic MOFs (PCN-224) NPs doped with L-arginine and incorporated in PVDF electrospun fibers | Nanoparticles in nanofibers | NIR | ROS generation and nitric oxide-assisted photodynamic therapy | Staphylococcus aureus and Pseudomonas aeruginosa [260] | wound healing |
| Upconversion nanoparticles (UCNPs) incorporated in PVDF electrospun fibers | Nanoparticles in nanofibers | NIR | ROS generation | Staphylococcus aureus and Escherichia coli [261] | wound healing |
| PVA microneedles with a metal-organic framework and multifunctional porphyrin-like metal center NPs | Microneedles | NIR | Photothermal conversion and nanozyme/peroxidase properties of NPs | Staphylococcus aureus [260] | wound healing |
| Iodophilic MOF UiO-66 containing Au nanorods coated with SiO2 and embedded in PVP | Nanoparticles in films | NIR | Photoactive nanoparticles | Staphylococcus aureus and Escherichia coli [262] | nosocomial infections |
| Ag-sodium lignin sulfonate NPs and polypyrrole-polydopamine NPs in poly(ethylene glycol) diacrylate hydrogel | Nanoparticles in hydrogels | NIR | Photothermal activity and antibacterial Ag ion release | Staphylococcus aureus and Escherichia coli [263] | wound dressings |
| PLGA-PCL-methylene blue fibers | Nanofibers | Visible | Controlled matrix degradation and photosensitizer release, photodynamic therapy, and ROS generation | Escherichia coli and Streptococcus mutans [264] | |
| Conjugated polymer NPs + cell-penetrating peptides embedded in polyisocyanides hydrogel | Nanoparticles in hydrogels | White and NIR | Synergistic photodynamic and photothermal therapy | Staphylococcus aureus, Escherichia coli, and Aspergillus niger [248] | clinical infections |
| Light-responsive TiO2 nanotubes and thermo-responsive copolymer | Functionalized composite surface | UV | ROS generation | Staphylococcus aureus and Escherichia coli [265] | anti-adhesion |
| Porphyrin photosensitizer and PLGA-encapsulated bFGF nanospheres embedded in carboxymethyl chitosan-sodium alginate | Nanoparticles in hydrogels | Visible | Photodynamic chemotherapy | Staphylococcus aureus and MDR-Staphylococcus aureus [266] | burn wounds |
| ZnO incorporated with Ag NPs, embedded in carboxymethyl cellulose hydrogel | Nanoparticles in hydrogels | Visible | Ag and Zn ions are released and ROS generation occurs | Staphylococcus aureus and Escherichia coli [267] | |
| Porphyrin-based porous organic polymers | Nanoparticles in films | Visible | Photothermal effect and ROS generation | Methicillin-resistant Staphylococcus aureus [268] | wound healing |
| Riboflavin-modified PVC film | Functionalized composite surface | Blue light | ROS generation | Pseudomonas aeruginosa [269] | |
| Hydrogel of polyvinyl alcohol modified with chitosan, polydopamine, and NO release donor/red phosphorous nanofilm | Functionalized composite surface | NIR | Peroxynitrite (ONOO−) generation, controlled release, and hyperthermia | MDR-Staphylococcus aureus [270] | bone implants |
| PPy-poly dopamine NPs embedded in NIPAm/acrylic acid hydrogel | Nanoparticles in hydrogels | NIR | Light-triggered tunable hydrogel deformation and adhesion and photothermal therapy | Staphylococcus aureus and Escherichia coli [271] | wound healing |
| Ciprofloxacin-loaded PEG hydrogel | Hydrogel | UV | Light-triggered drug release and photo-cleavable molecular cage | Staphylococcus aureus [272] | wound healing |
| Dibenzaldehyde-grafted poly (ethylene glycol), lauric acid-terminated chitosan, and curcumin-loaded mesoporous polydopamine NPs | Nanoparticles in hydrogels | NIR | Light-triggered drug release, hyperthermia with cellular component leakage, and disruption of the bacterial membrane | Staphylococcus aureus and Escherichia coli [273] | wound healing |
| Polysaccharide hydrogel encapsulating ferric tannate NPs and vancomycin | Nanoparticles in hydrogels | NIR | Hyperthermia and light-triggered drug release | Staphylococcus aureus [274] | wound healing |
| Prussian blue and tannic acid-loaded polyacrylamide Hydrogel | Hydrogel | NIR | Photothermal therapy | Staphylococcus aureus [275] | wound healing |
| Curcumin-based metal-organic framework + vancomycin, and chitosan | Nanoparticles in hydrogels | NIR | Bacterial capturing, Zn ions, and antibiotic release | Staphylococcus aureus [276] | wound healing |
| TiO2 nanorod array | Functionalized composite surface | NIR | Hyperthermia, ROS generation, and bacterial membrane puncture | Staphylococcus aureus and Escherichia coli [277] | bone implants |
| Chitosan microspheres loaded with rose bengal and polypyrrole in PVA hydrogel | Nanoparticles in hydrogels | Visible and NIR | Photothermal and photodynamic therapy | Staphylococcus aureus and Escherichia coli [278] | wound healing |
| Aloe-Emodin/Carbon Nanoparticle Hybrid PEG hydrogel | Nanoparticles in hydrogels | NIR | ROS generation and drug release | Staphylococcus aureus and Escherichia coli [279] | wound healing |
| PVA-(GS-Linker-MPEG) hydrogel loaded with Cy3/Cy5-silica NPs and UCNPs | Nanoparticles in hydrogels | NIR | NIR-UV conversion and light-triggered antibiotic release | Staphylococcus aureus [280] | infected wounds |
| Rose bengal/graphene oxide/PVA/chitosan hybrid hydrogel | Nanoparticles in hydrogels | Visible and NIR | Photothermal therapy and ROS generation | Staphylococcus aureus and Escherichia coli [281] | wound healing |
| Photochromic low-MW supramolecular hydrogel, drug loaded | Hydrogel | Visible | Light-triggered hydrogel dissolution and drug release | Escherichia coli [282] | |
| Catechol-conjugated poly(vinylpyrrolidone) sulfobetaine/polyaniline | Polymer coating | NIR | Photothermal therapy | Staphylococcus aureus and Escherichia coli [283] | |
| Pectin—Ag/AgCl/ZnO plasmonic hybrid nanocomposites | Nanoparticles in hydrogels | Visible | Photocatalytic nanostructures, ROS generation, and Zn and Ag ion release | Staphylococcus aureus and Escherichia coli [284] | |
| Berberine-microalgae/carboxymethyl chitosan/sodium alginate hydrogel | Hydrogel | Visible | Light-triggered drug release, ROS generation, QS downregulation, and inhibition and destruction of the biofilm | Methicillin-resistant Staphylococcus aureus [285] | infected wounds |
| Chlorinated e6-methacrylated silk fibroin | Film | UV and NIR | Photodynamic therapy | Staphylococcus aureus [286] | surgical wounds |
| Dopamine-folic acid hydrogel loaded with transition metal ions + carbon quantum dot-decorated ZnO NPs | Nanoparticles in hydrogels | Visible and NIR | Photothermal therapy, ROS generation, Zn ion release, and bacteria wall penetration | Staphylococcus aureus and Escherichia coli [287] | wound healing |
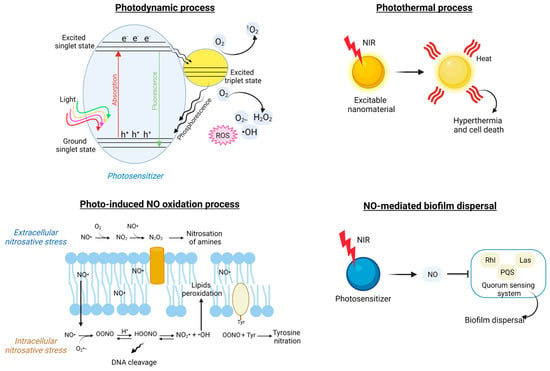
Figure 7.
Principal mechanisms involved in light-triggered formulations antibacterial action. The photodynamic process relies on the use of photosensitizers to produce cytotoxic ROS under visible, UV, or NIR light sources and in the presence of O2 (schematic liberally inspired from Wang et al. [231]. Upon light irradiation, electrons are promoted from the valence band to the conductive band, leaving positive holes behind. Both charge carriers can undergo energy transfer reactions with either water molecules or molecular O2 in their surroundings. The photothermal effect occurs as a result of NIR light absorption by atoms at the surface of conductive nanostructures, which convert the energy into heat, ultimately causing bacterial cell death. Photo-induced NO oxidation antibacterial mechanisms include extracellular and intracellular nitrosative stresses as a consequence of the production of HOONO, N2O3, and NO2●, which eventually lead to DNA cleavage, membrane lipid peroxidation, and membrane protein nitration (schematic adapted from Carpenter and Schoenfisch [288]). When released upon NIR stimulation from a photosensitizer precursor, nitric oxide has been shown to interfere with the quorum sensing system, promoting biofilm dispersal (schematic adapted from Zhao et al. [289]).
Another interesting mechanism of biofilm disruption is based upon the photo-induced release of nitric oxide (NO) and its derivatives in the EPS microenvironment [239,246,255,289]. Self-assembled micelles were formed with a diblock copolymer of poly-ethylene glycol and N-nitrosamine fragments, functionalized with a coumarin chromophore (PEO-b-PCouNO), and loaded with Ciprofloxacin [246]. The micelles were synthesized to respond to a visible light stimulus and simultaneously release the antibiotic compound and NO molecules to synergistically disperse Pseudomonas aeruginosa biofilm and kill the bacterial cells. The NO-antibacterial action is known to be multiple (Figure 7): (i) NO● can move across the bacterial membrane and initiate extra- and intracellular nitrosative stress cascades with the generation of oxidative by-products, lipid peroxidation, and nitration of membrane-bound proteins, which ultimately lead to RNA and DNA damage; (ii) NO can interfere with the quorum sensing pathway at a molecular level via nitrosylation of transcriptional regulators and enzymes (i.e., LasR, RhIR, MvfR, PqsD, and PqsE) [289] and, consequently, disperse bacterial biofilm.
6.2. Light-Responsive Hydrogels and Polymeric Composite Structures
Polymeric and hydrogel-based composite structures are promising antibacterial systems in applications such as wound healing, surgical sutures, tissue engineering, and dental procedures where good compliance is needed at the material and the tissue/organ interface. The light responsiveness of macromolecule-based materials may be due either to the presence of light-responsive moieties within the polymeric chain or to the integration of photoactivatable agents in the polymeric matrix. Table 3 reports a representative list of the most recent literature on the subject.
Hydrogels are defined as three-dimensionally cross-linked macromolecular networks able to swell as a result of intense water absorption. Depending on their mechanism of action, hydrogels may present intrinsic antibacterial properties (i.e., if formulated with antimicrobial peptides or with cationic polymers such as chitosan), or they can acquire a bactericidal effect when loaded with antibiotics, metal nanoparticles, or metal-organic frameworks (MOFs) [25,29,259,262,276]. The combination of active agents (either organic or inorganic) with a polymer- or hydrogel-like matrix appears to be the most interesting route, as it provides a tailorable platform with multi-functionality potential. Photo-activated antibacterial hydrogels exert their function upon irradiation under a specific wavelength that excites a photosensitizer (or a chromophore compound) embedded in the 3D network, which in turn triggers an oxidation cascade in the materials surrounding ROS generation (Figure 7) [29]. In parallel, the hydrogel matrix may undergo a photo-induced change in its mechanical properties and degrade or dissolve, thus slowly leaching out additional antibiotic cargos that perpetrate the antimicrobial action [271,272].
Qiao and colleagues [280] proposed a smart hydrogel with the dual purpose of monitoring the bacterial infection in wounds by means of a fluorescent light and releasing an antibiotic compound on demand upon NIR stimulation. The matrix of the hydrogel was constituted of polyvinyl alcohol (PVA) and an UV-cleavable polyprodrug (GS-Linker-MPEG); within this 3D network, multifunctionalities were included: (i) Cy3 and Cy5-modified silica nanoparticles (SNP-Cy3/Cy5) as sensing agents for bacterial infection; and (ii) up-conversion nanoparticles (UCNP) responsible for the prodrug UV-cleavage. Upon NIR irradiation, UCNPs were able to convert NIR light into UV light to activate the release of the GS drug from the polymeric network. In a different study, Feng et al. [271] developed a N-isopropylacrylamide and acrylic acid hydrogel that was able to tune its adhesion and deformation when subjected to NIR illumination. The hydrogel contained conductive polypyrrole and polydopamine PPy-PDA nanoparticles that can transfer NIR light energy into heat, reaching temperatures up to 58.7 °C within 10 min of stimulation and demonstrating photothermal converting ability. Moreover, as a consequence of the increased temperature in the material, water molecules rearrange within the 3D network while inter-polymer chain associations and hydrophobic interactions take place, thus causing an asymmetric shrinkage of the hydrogel and its self-deformation.
As a different mechanism of action, Hu and co-workers [285] targeted the biofilm QS of Methicillin-resistant Staphylococcus aureus (MRSA) with a composite hydrogel of microalgae Spirulina platensis and carboxymethyl chitosan/sodium alginate, loaded with berberine, a bioactive molecule known for its antibacterial effect and its ability to slow down some of the QS-associated processes. The berberine-loaded hydrogel promoted ROS formation in the wound microenvironment while suppressing biofilm formation and, more interestingly, down-regulating the expression of multi-resistant virulence.
The NIR-induced antimicrobial photothermal effect has also been widely documented in the literature for hydrogel-based systems, either alone or in combination with the photodynamic effect [231,275,283,287].
Table 3 reports other examples of light-triggered composite materials, such as microneedles [260], electrospun fibers [260,264], and functional coatings [270,283]. The range of shapes and 3D arrangements easily obtained when working with polymeric substrates enables the realization of multi-functional antibacterial strategies with spatial and temporal control of the photoactivation. Future developments must converge into hybrid approaches based on the combination of an organic matrix and inorganic compounds, which can benefit from the use of a broad spectrum of wavelengths to tackle the growth of microorganisms and the formation of biofilm.
7. Conclusions
The use of light to fight bacterial spreading and antimicrobial resistance can provide several advantages, such as: (i) the targeted action conferred by the light stimulus; (ii) the controlled release of drug action (both from photoswitchable molecules or light-responsive materials) in terms of dosing and timing without the risk of overusing antimicrobials or the risk of systemic effects associated with traditional antibiotic therapies; and (iii) a reduced risk of antimicrobial resistance occurrence since the photoswitchable drugs can be spread in their inactive form with a reduced environmental impact. Finally, light-triggered approaches can be coupled with other therapeutic strategies (i.e., conventional antibiotics) to provide a combination therapy approach to tackle the complex problem of bacterial infections and biofilm spreading.
However, there are also several challenges associated with the use of light-activated compounds for antimicrobial applications. One of the main challenges relates to the optimal delivery of compounds to the biofilm [290]. Biofilms are complex and heterogeneous structures, and the matrix that surrounds the bacteria can limit the penetration of compounds. To this end, the use of nanoparticles or other delivery systems can be of great help. Another challenge lies in the optimization of the activation process. UV light has a cytotoxic effect and should be avoided as a trigger for photosensitive compounds. Therefore, researchers are working to provide switchable compounds and materials that can be addressed with less harmful light triggers (i.e., near-infrared NIR light). Toxicity of light towards cells may happen in photothermal therapy, where the NIR light stimulation leads to an increase in the temperature of the material and, consequently, of its surroundings, causing necrotic effects within bacterial cells. When in contact with human/patient tissue, this can be a potential issue, which could be limited by developing strategies to localize the NIR illumination onto the light-responsive material or by decorating the light-responsive materials with targeting moieties for biofilm adhesion and penetration. Moreover, the light trigger needs to be tuned according to the chemical structure of the light-sensitive system, and the intensity and duration of light exposure may also need to be carefully tuned. Hence, it is important to develop methods for precisely controlling the activation of the compounds within the biofilm. Furthermore, the development of resistance to light-activated compounds is a potential concern. Bacteria may evolve mechanisms to avoid or resist the effects of such compounds, and it is therefore important to understand and address these mechanisms to ensure the long-term effectiveness of photopharmacological approaches. Finally, there are regulatory and safety considerations that need to be addressed when developing photopharmacological strategies for bacterial biofilm control. The safety of light-activated compounds and their potential impact on non-target organisms and the environment must be carefully evaluated.
In summary, the challenges of photopharmacology for bacterial biofilm control include optimizing the delivery and activation of the light-sensitive compounds, addressing the potential for resistance, and ensuring that regulatory and safety considerations are met.
As of today, the main drawbacks of light-triggered materials are related to their potential cytotoxicity, hemolysis, metabolic toxicity, and difficult body/tissue clearance when administered, for example, to human patients. Such safety issues may limit their applications in the biomedical field and should be overcome in order to implement nanotherapeutic strategies to efficiently battle microbial infections and bacterial resistance in clinical settings [26,236].
Author Contributions
A.K., E.P., G.S. and R.C. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
R.C. and E.P. acknowledge the European Regional Development Fund (ERDF) projects PhOAMS (No.1.1.1.5/21/A/003) and BioDrug (No. 1.1.1.5/19/A/004) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C 2020, 108, 110319. [Google Scholar] [CrossRef] [PubMed]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Baylay, A.J.; Piddock, L.J.; Webber, M.A. Molecular mechanisms of antibiotic resistance—Part I. In Bacterial Resistance to Antibiotics—From Molecules to Man; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 1–26. [Google Scholar]
- Shi, J.; Yan, Y.; Links, M.G.; Li, L.; Dillon, J.-A.R.; Horsch, M.; Kusalik, A. Antimicrobial resistance genetic factor identification from whole-genome sequence data using deep feature selection. BMC Bioinform. 2019, 20, 535. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Li, L.; Sun, Y.; Shi, L.; Li, W.; Zhang, J.; Wu, Y.; Xu, H.; Wang, M. Detection of multidrug resistant pathogenic bacteria and novel complex class 1 integrons in campus atmospheric particulate matters. Sci. Total Environ. 2023, 856, 158976. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Goverment of the United Kingdom: London, UK, 2016.
- Maleeva, G.; Wutz, D.; Rustler, K.; Nin-Hill, A.; Rovira, C.; Petukhova, E.; Bautista-Barrufet, A.; Gomila-Juaneda, A.; Scholze, P.; Peiretti, F.; et al. A photoswitchable GABA receptor channel blocker. Br. J. Pharmacol. 2019, 176, 2661–2677. [Google Scholar] [CrossRef]
- Gomila, A.M.J.; Rustler, K.; Maleeva, G.; Nin-Hill, A.; Wutz, D.; Bautista-Barrufet, A.; Rovira, X.; Bosch, M.; Mukhametova, E.; Petukhova, E.; et al. Photocontrol of Endogenous Glycine Receptors In Vivo. Cell Chem. Biol. 2020, 27, 1425–1433.e1427. [Google Scholar] [CrossRef]
- Castagna, R.; Maleeva, G.; Pirovano, D.; Matera, C.; Gorostiza, P. Donor-Acceptor Stenhouse Adduct Displaying Reversible Photoswitching in Water and Neuronal Activity. J. Am. Chem. Soc. 2022, 144, 15595–15602. [Google Scholar] [CrossRef]
- Reiner, A.; Isacoff, E.Y. Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat. Chem. Biol. 2014, 10, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Riefolo, F.; Matera, C.; Garrido-Charles, A.; Gomila, A.M.J.; Sortino, R.; Agnetta, L.; Claro, E.; Masgrau, R.; Holzgrabe, U.; Batlle, M.; et al. Optical Control of Cardiac Function with a Photoswitchable Muscarinic Agonist. J. Am. Chem. Soc. 2019, 141, 7628–7636. [Google Scholar] [CrossRef]
- Riefolo, F.; Sortino, R.; Matera, C.; Claro, E.; Preda, B.; Vitiello, S.; Traserra, S.; Jimenez, M.; Gorostiza, P. Rational Design of Photochromic Analogues of Tricyclic Drugs. J. Med. Chem. 2021, 64, 9259–9270. [Google Scholar] [CrossRef] [PubMed]
- Matera, C.; Calvé, P.; Casadó-Anguera, V.; Sortino, R.; Gomila, A.M.; Moreno, E.; Gener, T.; Delgado-Sallent, C.; Nebot, P.; Costazza, D. Reversible photocontrol of dopaminergic transmission in wild-type animals. Int. J. Mol. Sci. 2022, 23, 10114. [Google Scholar] [CrossRef] [PubMed]
- Nevola, L.; Varese, M.; Martin-Quiros, A.; Mari, G.; Eckelt, K.; Gorostiza, P.; Giralt, E. Targeted Nanoswitchable Inhibitors of Protein-Protein Interactions Involved in Apoptosis. ChemMedChem 2019, 14, 100–106. [Google Scholar] [CrossRef]
- Eli, S.; Castagna, R.; Mapelli, M.; Parisini, E. Recent Approaches to the Identification of Novel Microtubule-Targeting Agents. Front. Mol. Biosci. 2022, 9, 841777. [Google Scholar] [CrossRef]
- Matera, C.; Gomila, A.M.J.; Camarero, N.; Libergoli, M.; Soler, C.; Gorostiza, P. Photoswitchable Antimetabolite for Targeted Photoactivated Chemotherapy. J. Am. Chem. Soc. 2018, 140, 15764–15773. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef]
- Hull, K.; Morstein, J.; Trauner, D. In Vivo Photopharmacology. Chem. Rev 2018, 118, 10710–10747. [Google Scholar] [CrossRef]
- Fuchter, M.J. On the Promise of Photopharmacology Using Photoswitches: A Medicinal Chemist’s Perspective. J. Med. Chem. 2020, 63, 11436–11447. [Google Scholar] [CrossRef]
- Castagna, R.; Kolarski, D.; Durand-de Cuttoli, R.; Maleeva, G. Orthogonal Control of Neuronal Circuits and Behavior Using Photopharmacology. J. Mol. Neurosci. 2022, 72, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Fedatto Abelha, T.; Rodrigues Lima Caires, A. Light-Activated Conjugated Polymers for Antibacterial Photodynamic and Photothermal Therapy. Adv. NanoBiomed Res. 2021, 1, 2100012. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zou, L.; Wang, J.; Jin, Q.; Ji, J. Stimuli-responsive nanoplatforms for antibacterial applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1775. [Google Scholar] [CrossRef]
- Chakraborty, N.; Jha, D.; Roy, I.; Kumar, P.; Gaurav, S.S.; Marimuthu, K.; Ng, O.T.; Lakshminarayanan, R.; Verma, N.K.; Gautam, H.K. Nanobiotics against antimicrobial resistance: Harnessing the power of nanoscale materials and technologies. J. Nanobiotechnology 2022, 20, 375. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.; Chen, H.; Nan, K.; Jin, Y.; Sun, L.; Wang, B. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef]
- Pierau, L.; Versace, D.-L. Light and hydrogels: A new generation of antimicrobial materials. Materials 2021, 14, 787. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–biofilm interactions: The role of the EPS matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Kovach, K.; Davis-Fields, M.; Irie, Y.; Jain, K.; Doorwar, S.; Vuong, K.; Dhamani, N.; Mohanty, K.; Touhami, A.; Gordon, V.D. Evolutionary adaptations of biofilms infecting cystic fibrosis lungs promote mechanical toughness by adjusting polysaccharide production. NPJ Biofilms Microbiomes 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef]
- Slavkin, H.C. Biofilms, Microbial Ecology and Antoni van Leeuwenhoek. J. Am. Dent. Assoc. 1997, 128, 492–495. [Google Scholar] [CrossRef]
- Sedarat, Z.; Taylor-Robinson, A.W. A Consideration of antibacterial agent efficacies in the treatment and prevention of formation of Staphylococcus aureus biofilm. J. Microbiol. Infect. Dis. 2019, 9, 167–172. [Google Scholar] [CrossRef]
- Allcock, S.; Young, E.H.; Holmes, M.; Gurdasani, D.; Dougan, G.; Sandhu, M.S.; Solomon, L.; Török, M. Antimicrobial resistance in human populations: Challenges and opportunities. Glob. Health Epidemiol. Genom. 2017, 2, e4. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Petrova, O.E.; Sauer, K. Sticky situations: Key components that control bacterial surface attachment. J. Bacteriol. 2012, 194, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef] [PubMed]
- González-Rivas, F.; Ripolles-Avila, C.; Fontecha-Umaña, F.; Ríos-Castillo, A.G.; Rodríguez-Jerez, J.J. Biofilms in the spotlight: Detection, quantification, and removal methods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1261–1276. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Møretrø, T.; Heir, E.; Nesse, L.L.; Vestby, L.K.; Langsrud, S. Control of Salmonella in food related environments by chemical disinfection. Food Res. Int. 2012, 45, 532–544. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Lal, A. Quorum sensing: How bacteria talk to each other. Resonance 2009, 14, 866–871. [Google Scholar] [CrossRef]
- Atkinson, S.; Williams, P. Quorum sensing and social networking in the microbial world. J. R. Soc. Interface 2009, 6, 959–978. [Google Scholar] [CrossRef]
- Haque, S.; Yadav, D.K.; Bisht, S.C.; Yadav, N.; Singh, V.; Dubey, K.K.; Jawed, A.; Wahid, M.; Dar, S.A. Quorum sensing pathways in Gram-positive and-negative bacteria: Potential of their interruption in abating drug resistance. J. Chemother. 2019, 31, 161–187. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, F.; Ding, G.; Li, J.; Guo, X.; Zhu, J.; Zhou, L.; Cai, S.; Liu, X.; Luo, Y.; et al. Acyl homoserine lactone-based quorum sensing in a methanogenic archaeon. ISME J. 2012, 6, 1336–1344. [Google Scholar] [CrossRef]
- Withers, H.; Swift, S.; Williams, P. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 2001, 4, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Ziemichód, A.; Skotarczak, B. QS–systems communication of gram-positive bacterial cells. Acta Biol. 2017, 24, 51–56. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X. Effects of quorum sensing on the biofilm formation and viable but non-culturable state. Food Res. Int. 2020, 137, 109742. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Hastings, J. Bacterial bioluminescence: Its control and ecological significance. Microbiol. Rev. 1979, 43, 496–518. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Silva, L.R.; Giaouris, E.D.; Melo, L.F.; Simões, M. Quorum sensing in food spoilage and natural-based strategies for its inhibition. Food Res. Int. 2020, 127, 108754. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.R.; Dean, S.N.; Leary, D.H.; Walper, S.A. Response of lactobacillus plantarum WCFS1 to the gram-negative pathogen-associated quorum sensing molecule N-3-oxododecanoyl homoserine lactone. Front. Microbiol. 2019, 10, 715. [Google Scholar] [CrossRef]
- Ji, G.; Beavis, R.C.; Novick, R.P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 1995, 92, 12055–12059. [Google Scholar] [CrossRef]
- Eberhard, A.; Burlingame, A.L.; Eberhard, C.; Kenyon, G.L.; Nealson, K.H.; Oppenheimer, N. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 1981, 20, 2444–2449. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Dizin, E.; Hu, X.; Wavreille, A.-S.; Park, J.; Pei, D. S-Ribosylhomocysteinase (LuxS) is a mononuclear iron protein. Biochemistry 2003, 42, 4717–4726. [Google Scholar] [CrossRef] [PubMed]
- Flavier, A.B.; Ganova-Raeva, L.M.; Schell, M.A.; Denny, T.P. Hierarchical autoinduction in Ralstonia solanacearum: Control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 1997, 179, 7089–7097. [Google Scholar] [CrossRef]
- Wang, L.H.; He, Y.; Gao, Y.; Wu, J.E.; Dong, Y.H.; He, C.; Wang, S.X.; Weng, L.X.; Xu, J.L.; Tay, L. A bacterial cell–cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 2004, 51, 903–912. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Kameyama, S.; Onaka, H.; Horinouchi, S. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: Identification of a target gene of the A-factor receptor. Mol. Microbiol. 1999, 34, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Ram Chhabra, S.; De Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other gram-negative bacteria. Mol. Microbiol. 1999, 33, 1254–1266. [Google Scholar] [CrossRef]
- Diggle, S.P.; Winzer, K.; Chhabra, S.R.; Worrall, K.E.; Cámara, M.; Williams, P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol. Microbiol. 2003, 50, 29–43. [Google Scholar] [CrossRef]
- Déziel, E.; Lépine, F.; Milot, S.; He, J.; Mindrinos, M.N.; Tompkins, R.G.; Rahme, L.G. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc. Natl. Acad. Sci. USA 2004, 101, 1339–1344. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Ge, Y.; Zhu, X.; Pan, J. Regulatory mechanisms and promising applications of quorum sensing-inhibiting agents in control of bacterial biofilm formation. Front. Microbiol. 2020, 11, 589640. [Google Scholar] [CrossRef] [PubMed]
- Van Gennip, M.; Christensen, L.D.; Alhede, M.; Phipps, R.; Jensen, P.Ø.; Christophersen, L.; Pamp, S.J.; Moser, C.; Mikkelsen, P.J.; Koh, A.Y. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. Apmis 2009, 117, 537–546. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen distribution and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.; Alexandre, K.; Etienne, M. Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: Molecular mechanisms, antibiotic strategies and therapeutic perspectives. Front. Microbiol. 2020, 11, 2057. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. [Google Scholar] [CrossRef]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Poole, K. Stress responses as determinants of antimicrobial resistance in Pseudomonas aeruginosa: Multidrug efflux and more. Can. J. Microbiol. 2014, 60, 783–791. [Google Scholar] [CrossRef]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Vázquez-Laslop, N.; Mankin, A.S. Context-Specific Action of Ribosomal Antibiotics. Annu. Rev. Microbiol. 2018, 72, 185–207. [Google Scholar] [CrossRef]
- Greulich, P.; Scott, M.; Evans, M.R.; Allen, R.J. Growth-dependent bacterial susceptibility to ribosome-targeting antibiotics. Mol. Syst. Biol. 2015, 11, 796. [Google Scholar] [CrossRef]
- Levin, B.R.; Rozen, D.E. Non-inherited antibiotic resistance. Nat. Rev. Microbiol. 2006, 4, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Dalebroux, Z.D.; Swanson, M.S. ppGpp: Magic beyond RNA polymerase. Nat. Rev. Microbiol. 2012, 10, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Salzer, A.; Wolz, C. Role of (p)ppGpp in antibiotic resistance, tolerance, persistence and survival in Firmicutes. Microlife 2023, 4, uqad009. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.N.; Porse, A.; Abdelsamad, A.; Sommer, M.; Høiby, N.; Ciofu, O. Lack of the Major Multifunctional Catalase KatA in Pseudomonas aeruginosa Accelerates Evolution of Antibiotic Resistance in Ciprofloxacin-Treated Biofilms. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef]
- Nguyen, D.; Joshi-Datar, A.; Lepine, F.; Bauerle, E.; Olakanmi, O.; Beer, K.; McKay, G.; Siehnel, R.; Schafhauser, J.; Wang, Y.; et al. Active Starvation Responses Mediate Antibiotic Tolerance in Biofilms and Nutrient-Limited Bacteria. Science 2011, 334, 982–986. [Google Scholar] [CrossRef]
- Pamp, S.J.; Gjermansen, M.; Johansen, H.K.; Tolker-Nielsen, T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol. Microbiol. 2008, 68, 223–240. [Google Scholar] [CrossRef]
- Herrmann, G.; Yang, L.; Wu, H.; Song, Z.; Wang, H.; Høiby, N.; Ulrich, M.; Molin, S.; Riethmüller, J.; Döring, G. Colistin-tobramycin combinations are superior to monotherapy concerning the killing of biofilm Pseudomonas aeruginosa. J. Infect. Dis. 2010, 202, 1585–1592. [Google Scholar] [CrossRef]
- Fraud, S.; Poole, K. Oxidative stress induction of the MexXY multidrug efflux genes and promotion of aminoglycoside resistance development in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1068–1074. [Google Scholar] [CrossRef]
- Fetar, H.; Gilmour, C.; Klinoski, R.; Daigle, D.M.; Dean, C.R.; Poole, K. mexEF-oprN multidrug efflux operon of Pseudomonas aeruginosa: Regulation by the MexT activator in response to nitrosative stress and chloramphenicol. Antimicrob. Agents Chemother. 2011, 55, 508–514. [Google Scholar] [CrossRef]
- Fraud, S.; Campigotto, A.J.; Chen, Z.; Poole, K. MexCD-OprJ multidrug efflux system of Pseudomonas aeruginosa: Involvement in chlorhexidine resistance and induction by membrane-damaging agents dependent upon the AlgU stress response sigma factor. Antimicrob. Agents Chemother. 2008, 52, 4478–4482. [Google Scholar] [CrossRef]
- Askoura, M.; Mottawea, W.; Abujamel, T.; Taher, I. Efflux pump inhibitors (EPIs) as new antimicrobial agents against Pseudomonas aeruginosa. Libyan J. Med. 2011, 6, 5870. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Schurr, M.J.; Sauer, K. The MerR-like regulator BrlR confers biofilm tolerance by activating multidrug efflux pumps in Pseudomonas aeruginosa biofilms. J. Bacteriol. 2013, 195, 3352–3363. [Google Scholar] [CrossRef]
- Poudyal, B.; Sauer, K. The PA3177 gene encodes an active diguanylate cyclase that contributes to biofilm antimicrobial tolerance but not biofilm formation by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Król, J.E.; Wojtowicz, A.J.; Rogers, L.M.; Heuer, H.; Smalla, K.; Krone, S.M.; Top, E.M. Invasion of E. coli biofilms by antibiotic resistance plasmids. Plasmid 2013, 70, 110–119. [Google Scholar] [CrossRef]
- Usui, M.; Yoshii, Y.; Thiriet-Rupert, S.; Ghigo, J.-M.; Beloin, C. Intermittent antibiotic treatment of bacterial biofilms favors the rapid evolution of resistance. Commun. Biol. 2023, 6, 275. [Google Scholar] [CrossRef]
- Colizzi, S.E.; van Dijk, B.; Merks, R.M.H.; Rozen, D.E.; Vroomans, R.M.A. Evolution of genome fragility enables microbial division of labor. Mol. Syst. Biol. 2023, 19, e11353. [Google Scholar] [CrossRef]
- Soto, S.M. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence 2013, 4, 223–229. [Google Scholar] [CrossRef]
- Buroni, S.; Matthijs, N.; Spadaro, F.; Van Acker, H.; Scoffone, V.C.; Pasca, M.R.; Riccardi, G.; Coenye, T. Differential roles of RND efflux pumps in antimicrobial drug resistance of sessile and planktonic Burkholderia cenocepacia cells. Antimicrob. Agents Chemother. 2014, 58, 7424–7429. [Google Scholar] [CrossRef]
- Matsumura, K.; Furukawa, S.; Ogihara, H.; Morinaga, Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011, 16, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Brooun, A.; Liu, S.; Lewis, K. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2000, 44, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Das Talukdar, A.; Dutta Choudhury, M.; Maurya, A.P.; Paul, D.; Dhar Chanda, D.; Chakravorty, A.; Bhattacharjee, A. Transcriptional analysis of MexAB-OprM efflux pumps system of Pseudomonas aeruginosa and its role in carbapenem resistance in a tertiary referral hospital in India. PLoS ONE 2015, 10, e0133842. [Google Scholar] [CrossRef]
- Suresh, M.; Nithya, N.; Jayasree, P.; Vimal, K.; Manish Kumar, P. Mutational analyses of regulatory genes, mexR, nalC, nalD and mexZ of mexAB-oprM and mexXY operons, in efflux pump hyperexpressing multidrug-resistant clinical isolates of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2018, 34, 83. [Google Scholar] [CrossRef]
- Paxman, J.J.; Lo, A.W.; Sullivan, M.J.; Panjikar, S.; Kuiper, M.; Whitten, A.E.; Wang, G.; Luan, C.-H.; Moriel, D.G.; Tan, L. Unique structural features of a bacterial autotransporter adhesin suggest mechanisms for interaction with host macromolecules. Nat. Commun. 2019, 10, 1967. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Zhou, X.; Tian, S. Anti-biofilm and bacteriostatic effects of three flavonoid compounds on Streptococcus mutans. Biofouling 2023, 39, 245–256. [Google Scholar] [CrossRef]
- An, S.-J.; Namkung, J.-U.; Ha, K.-W.; Jun, H.-K.; Kim, H.Y.; Choi, B.-K. Inhibitory effect of d-arabinose on oral bacteria biofilm formation on titanium discs. Anaerobe 2022, 75, 102533. [Google Scholar] [CrossRef]
- Gong, H.; Hu, X.; Liao, M.; Fa, K.; Ciumac, D.; Clifton, L.A.; Sani, M.-A.; King, S.M.; Maestro, A.; Separovic, F. Structural disruptions of the outer membranes of gram-negative bacteria by rationally designed amphiphilic antimicrobial peptides. ACS Appl. Mater. Interfaces 2021, 13, 16062–16074. [Google Scholar] [CrossRef]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef]
- Huebinger, R.M.; Stones, D.H.; de Souza Santos, M.; Carlson, D.L.; Song, J.; Vaz, D.P.; Keen, E.; Wolf, S.E.; Orth, K.; Krachler, A.M. Targeting bacterial adherence inhibits multidrug-resistant Pseudomonas aeruginosa infection following burn injury. Sci. Rep. 2016, 6, 39341. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Jin, J.; Park, S.; Kim, J.-Y.; Lee, M.-J.; Sun, H.; Kwon, J.-S.; Lee, H.; Choi, S.-H.; Hong, J. Quantitative Interpretation of Hydration Dynamics Enabled the Fabrication of a Zwitterionic Antifouling Surface. ACS Appl. Mater. Interfaces 2020, 12, 7951–7965. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, S.; Lu, Z.; She, Y.; Xie, J.; Cong, Z.; Zhang, W.; Liu, R. Using In Vivo Assessment on Host Defense Peptide Mimicking Polymer-Modified Surfaces for Combating Implant Infections. ACS Appl. Bio Mater. 2021, 4, 3811–3829. [Google Scholar] [CrossRef]
- Zhu, J.; Uliana, A.; Wang, J.; Yuan, S.; Li, J.; Tian, M.; Simoens, K.; Volodin, A.; Lin, J.; Bernaerts, K.; et al. Elevated salt transport of antimicrobial loose nanofiltration membranes enabled by copper nanoparticles via fast bioinspired deposition. J. Mater. Chem. A 2016, 4, 13211–13222. [Google Scholar] [CrossRef]
- Ďureje, J.; Prošek, Z.; Trejbal, J.; Potocký, Š.; Tesárek, P. Influence of oxygen and argon plasma treatment on wettability and surface morphology of polypropylene microfibers. Acta Polytech. CTU Proc. 2022, 34, 11–14. [Google Scholar] [CrossRef]
- Eckhart, L.; Fischer, H.; Barken, K.B.; Tolker-Nielsen, T.; Tschachler, E. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 2007, 156, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis 2006, 114, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Ramesh, S. Cellulase inhibits Burkholderia cepacia biofilms on diverse prosthetic materials. Pol. J. Microbiol. 2013, 62, 327–330. [Google Scholar] [CrossRef]
- Kalpana, B.J.; Aarthy, S.; Pandian, S.K. Antibiofilm activity of α-amylase from Bacillus subtilis S8-18 against biofilm forming human bacterial pathogens. Appl. Biochem. Biotechnol. 2012, 167, 1778–1794. [Google Scholar] [CrossRef]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Dohare, S.; Sharma, D.; Narvi, S.S.; Agarwal, V. Designing quorum sensing inhibitors of Pseudomonas aeruginosa utilizing FabI: An enzymic drug target from fatty acid synthesis pathway. 3 Biotech 2019, 9, 40. [Google Scholar] [CrossRef]
- Storz, M.P.; Allegretta, G.; Kirsch, B.; Empting, M.; Hartmann, R.W. From in vitro to in cellulo: Structure–activity relationship of (2-nitrophenyl) methanol derivatives as inhibitors of PqsD in Pseudomonas aeruginosa. Org. Biomol. Chem. 2014, 12, 6094–6104. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, C. Biological activity and identification of a peptide inhibitor of LuxS from Streptococcus suis serotype 2. FEMS Microbiol. Lett. 2009, 294, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Gusti, A.R.; Zhang, Q.; Xu, J.-L.; Zhang, L.-H. Identification of quorum-quenching N-acyl homoserine lactonases from Bacillus species. Appl. Environ. Microbiol. 2002, 68, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-lactamase inhibitor boronic acid derivative SM23 as a new anti-Pseudomonas aeruginosa biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Ryu, E.-J.; Li, L.; Choi, B.-K.; Kim, B.M. New bicyclic brominated furanones as potent autoinducer-2 quorum-sensing inhibitors against bacterial biofilm formation. Eur. J. Med. Chem. 2017, 137, 76–87. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Chiarelli, L.R.; Makarov, V.; Brackman, G.; Israyilova, A.; Azzalin, A.; Forneris, F.; Riabova, O.; Savina, S.; Coenye, T. Discovery of new diketopiperazines inhibiting Burkholderia cenocepacia quorum sensing in vitro and in vivo. Sci. Rep. 2016, 6, 32487. [Google Scholar] [CrossRef]
- Li, T.; Mei, Y.; He, B.; Sun, X.; Li, J. Reducing quorum sensing-mediated virulence factor expression and biofilm formation in hafnia alvei by using the potential quorum sensing inhibitor L-carvone. Front. Microbiol. 2019, 9, 3324. [Google Scholar] [CrossRef]
- Czajkowski, R.; Krzyżanowska, D.; Karczewska, J.; Atkinson, S.; Przysowa, J.; Lojkowska, E.; Williams, P.; Jafra, S. Inactivation of AHLs by Ochrobactrum sp. A44 depends on the activity of a novel class of AHL acylase. Environ. Microbiol. Rep. 2011, 3, 59–68. [Google Scholar] [CrossRef]
- Yu, L.; Li, W.; Zhang, M.; Cui, Y.; Chen, X.; Ni, J.; Yu, L.; Shang, F.; Xue, T. Imidazole decreases the ampicillin resistance of an Escherichia coli strain isolated from a cow with mastitis by inhibiting the function of autoinducer 2. J. Dairy Sci. 2018, 101, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Für Naturforschung C 2003, 58, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Hu, Z.-Q.; Shimamura, T.; Hara, Y. Inhibition by epigallocatechin gallate (EGCg) of conjugative R plasmid transfer in Escherichia coli. J. Infect. Chemother. 2001, 7, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Goo, E.; Yu, S.; Choi, O.; Lee, J.; Kim, J.; Kim, H.; Igarashi, J.; Suga, H.; Moon, J.S. Small-molecule inhibitor binding to an N-acyl-homoserine lactone synthase. Proc. Natl. Acad. Sci. USA 2011, 108, 12089–12094. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; D’morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biswas, R. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Hentzer, M.; Wu, H.; Andersen, J.B.; Riedel, K.; Rasmussen, T.B.; Bagge, N.; Kumar, N.; Schembri, M.A.; Song, Z.; Kristoffersen, P. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J. 2003, 22, 3803–3815. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Singh, P.; Chenia, H.Y. Quorum sensing inhibitory potential and molecular docking studies of sesquiterpene lactones from Vernonia blumeoides. Phytochemistry 2016, 126, 23–33. [Google Scholar] [CrossRef]
- Vandeputte, O.M.; Kiendrebeogo, M.; Rasamiravaka, T.; Stevigny, C.; Duez, P.; Rajaonson, S.; Diallo, B.; Mol, A.; Baucher, M.; El Jaziri, M. The flavanone naringenin reduces the production of quorum sensing-controlled virulence factors in Pseudomonas aeruginosa PAO1. Microbiology 2011, 157, 2120–2132. [Google Scholar] [CrossRef]
- Wei, Z.; Li, T.; Gu, Y.; Zhang, Q.; Wang, E.; Li, W.; Wang, X.; Li, Y.; Li, H. Design, Synthesis, and Biological Evaluation of N-Acyl-Homoserine Lactone Analogs of Quorum Sensing in Pseudomonas aeruginosa. Front. Chem. 2022, 10, 948687. [Google Scholar] [CrossRef]
- Truchado, P.; Gimenez-Bastida, J.-A.; Larrosa, M.; Castro-Ibáñez, I.; Espín, J.C.; Tomas-Barberan, F.A.; García-Conesa, M.T.; Allende, A. Inhibition of quorum sensing (QS) in Yersinia enterocolitica by an orange extract rich in glycosylated flavanones. J. Agric. Food Chem. 2012, 60, 8885–8894. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, M. Analysis of the antibacterial effect of an Edwardsiella tarda LuxS inhibitor. Springerplus 2016, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Ryu, E.-J.; Sim, J.; Sim, J.; Lee, J.; Choi, B.-K. D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens. J. Microbiol. 2016, 54, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.M.; Russell, R.; Moreira, C.G.; Adebesin, A.M.; Wang, C.; Williams, N.S.; Taussig, R.; Stewart, D.; Zimmern, P.; Lu, B. QseC inhibitors as an antivirulence approach for Gram-negative pathogens. MBio 2014, 5, e02165-14. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
- El-Shaer, S.; Shaaban, M.; Barwa, R.; Hassan, R. Control of quorum sensing and virulence factors of Pseudomonas aeruginosa using phenylalanine arginyl β-naphthylamide. J. Med. Microbiol. 2016, 65, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Kitzenberg, D.A.; Lee, J.S.; Mills, K.B.; Kim, J.-S.; Liu, L.; Vázquez-Torres, A.; Colgan, S.P.; Kao, D.J. Adenosine Awakens Metabolism to Enhance Growth-Independent Killing of Tolerant and Persister Bacteria across Multiple Classes of Antibiotics. Mbio 2022, 13, e00480-22. [Google Scholar] [CrossRef]
- Koul, A.; Dendouga, N.; Vergauwen, K.; Molenberghs, B.; Vranckx, L.; Willebrords, R.; Ristic, Z.; Lill, H.; Dorange, I.; Guillemont, J.; et al. Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol. 2007, 3, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wood, T.K. Combatting persister cells with substituted indoles. Front. Microbiol. 2020, 11, 1565. [Google Scholar] [CrossRef]
- Palmqvist, N.; Foster, T.; Fitzgerald, J.R.; Josefsson, E.; Tarkowski, A. Fibronectin-binding proteins and fibrinogen-binding clumping factors play distinct roles in staphylococcal arthritis and systemic inflammation. J. Infect. Dis. 2005, 191, 791–798. [Google Scholar] [CrossRef]
- Yang, X.; Hou, J.; Tian, Y.; Zhao, J.; Sun, Q.; Zhou, S. Antibacterial surfaces: Strategies and applications. Sci. China Technol. Sci. 2022, 65, 1000–1010. [Google Scholar] [CrossRef]
- Paluch, E.; Piecuch, A.; Obłąk, E.; Lamch, Ł.; Wilk, K.A. Antifungal activity of newly synthesized chemodegradable dicephalic-type cationic surfactants. Colloids Surf. B Biointerfaces 2018, 164, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Piewngam, P.; Chiou, J.; Chatterjee, P.; Otto, M. Alternative approaches to treat bacterial infections: Targeting quorum-sensing. Expert Rev. Anti Infect. Ther. 2020, 18, 499–510. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Kang, Y.C.; Lee, J.-K. Quorum sensing inhibitors as antipathogens: Biotechnological applications. Biotechnol. Adv. 2019, 37, 68–90. [Google Scholar] [CrossRef]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum sensing inhibitors as Therapeutics: Bacterial biofilm inhibition. Bioorganic Chem. 2023, 136, 106551. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.D.L.; Xu, Y.; Meijler, M.M.; Janda, K.D. Antibody catalyzed hydrolysis of a quorum sensing signal found in Gram-negative bacteria. Bioorganic Med. Chem. Lett. 2007, 17, 1549–1552. [Google Scholar] [CrossRef]
- Park, J.; Jagasia, R.; Kaufmann, G.F.; Mathison, J.C.; Ruiz, D.I.; Moss, J.A.; Meijler, M.M.; Ulevitch, R.J.; Janda, K.D. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 2007, 14, 1119–1127. [Google Scholar] [CrossRef]
- Zang, T.; Lee, B.W.; Cannon, L.M.; Ritter, K.A.; Dai, S.; Ren, D.; Wood, T.K.; Zhou, Z.S. A naturally occurring brominated furanone covalently modifies and inactivates LuxS. Bioorg. Med. Chem. Lett. 2009, 19, 6200–6204. [Google Scholar] [CrossRef]
- Vani, S.; Vadakkan, K.; Mani, B. A narrative review on bacterial biofilm: Its formation, clinical aspects and inhibition strategies. Future J. Pharm. Sci. 2023, 9, 50. [Google Scholar] [CrossRef]
- Milly, T.A.; Tal-Gan, Y. Targeting peptide-based quorum sensing systems for the treatment of gram-positive bacterial infections. Pept. Sci. 2023, 115, e24298. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; O’Neill, J.C.; Miller, D.M.; Wezeman, R.J.; Mattmann, M.E.; Lin, Q.; Blackwell, H.E. Comparative analyses of N-acylated homoserine lactones reveal unique structural features that dictate their ability to activate or inhibit quorum sensing. Chembiochem 2008, 9, 389–400. [Google Scholar] [CrossRef]
- Chambial, S.; Dwivedi, S.; Shukla, K.K.; John, P.J.; Sharma, P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013, 28, 314–328. [Google Scholar] [CrossRef]
- Ren, D.; Bedzyk, L.A.; Ye, R.W.; Thomas, S.M.; Wood, T.K. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol. Bioeng. 2004, 88, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, Z.; Hentzer, M.; Andersen, J.B.; Molin, S.; Givskov, M.; Høiby, N. Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice. J. Antimicrob. Chemother. 2004, 53, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evidence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Zhu, L.; Lau, G.W. Inhibition of competence development, horizontal gene transfer and virulence in Streptococcus pneumoniae by a modified competence stimulating peptide. PLoS Pathog. 2011, 7, e1002241. [Google Scholar] [CrossRef]
- Koirala, B.; Lin, J.; Lau, G.W.; Tal-Gan, Y. Development of a Dominant Negative Competence-Stimulating Peptide (dnCSP) that Attenuates Streptococcus pneumoniae Infectivity in a Mouse Model of Acute Pneumonia. ChemBioChem 2018, 19, 2380–2386. [Google Scholar] [CrossRef]
- Peng, H.; Cheng, Y.; Ni, N.; Li, M.; Choudhary, G.; Chou, H.T.; Lu, C.D.; Tai, P.C.; Wang, B. Synthesis and evaluation of new antagonists of bacterial quorum sensing in Vibrio harveyi. ChemMedChem Chem. Enabling Drug Discov. 2009, 4, 1457–1468. [Google Scholar]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and control strategies of antibiotic resistance in pathological biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Mallo, N.; Otero-Casal, P.; Pose-Rodríguez, J.M.; Otero, A. Quorum sensing systems as a new target to prevent biofilm-related oral diseases. Oral Dis. 2022, 28, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Choi, C.H. Role of LuxIR homologue AnoIR in Acinetobacter nosocomialis and the effect of virstatin on the expression of anoR gene. J. Microbiol. Biotechnol. 2015, 25, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Shah, A.A.; Shahid, M.; Manzoor, I.; Aslam, B.; Rasool, M.H.; Saeed, M.; Ayaz, S.; Khurshid, M. Quorum sensing interfering strategies and their implications in the management of biofilm-associated bacterial infections. Braz. Arch. Biol. Technol. 2020, 63, e20190555. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting biofilms therapy: Current research strategies and development hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Baelo, A.; Levato, R.; Julián, E.; Crespo, A.; Astola, J.; Gavaldà, J.; Engel, E.; Mateos-Timoneda, M.A.; Torrents, E. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J. Control. Release 2015, 209, 150–158. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Banerjee, A.; Chowdhury, P.; Bauri, K.; Saha, B.; De, P. Inhibition and eradication of bacterial biofilm using polymeric materials. Biomater. Sci. 2023, 11, 11–36. [Google Scholar] [CrossRef]
- Cornelissen, A.; Ceyssens, P.-J.; T’syen, J.; Van Praet, H.; Noben, J.-P.; Shaburova, O.V.; Krylov, V.N.; Volckaert, G.; Lavigne, R. The T7-related Pseudomonas putida phage φ15 displays virion-associated biofilm degradation properties. PLoS ONE 2011, 6, e18597. [Google Scholar] [CrossRef]
- Fleming, D.; Rumbaugh, K.P. Approaches to dispersing medical biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rao, T.S. Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian J. Med. Res. 2017, 146, S1. [Google Scholar] [PubMed]
- Marx, C.; Gardner, S.; Harman, R.M.; Van de Walle, G.R. The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. Stem Cells Transl. Med. 2020, 9, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ortega-Ramirez, L.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Antibiofilm Action of Plant Terpenes in Salmonella Strains: Potential Inhibitors of the Synthesis of Extracellular Polymeric Substances. Pathogens 2023, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Eisha, S.; Park, S.; Morris, A.; Martin, I. Targeting Matrix Exopolysaccharides to Disrupt Pseudomonas aeruginosa Biofilms in Cystic Fibrosis. Med. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Wood, T.K. Combatting bacterial persister cells. Biotechnol. Bioeng. 2016, 113, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Bekale, L.A.; Molchanova, N.; Nielsen, J.E.; Wright, M.; Bacacao, B.; Diamond, G.; Jenssen, H.v.; Santa Maria, P.L.; Barron, A.E. Anti-persister and Anti-biofilm Activity of Self-Assembled Antimicrobial Peptoid Ellipsoidal Micelles. ACS Infect. Dis. 2022, 8, 1823–1830. [Google Scholar] [CrossRef]
- Necula, G.; Bacalum, M.; Radu, M. Interaction of Tryptophan-and Arginine-Rich Antimicrobial Peptide with E. coli Outer Membrane—A Molecular Simulation Approach. Int. J. Mol. Sci. 2023, 24, 2005. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, J.; Zhou, C.; Liu, H.; Zhang, X.; Zhou, T. Biofilm formation restrained by subinhibitory concentrations of tigecyclin in Acinetobacter baumannii is associated with downregulation of efflux pumps. Chemotherapy 2017, 62, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, A.; Khan, A.U. Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res. Lett. 2017, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Richmond, G.E.; Evans, L.P.; Anderson, M.J.; Wand, M.E.; Bonney, L.C.; Ivens, A.; Chua, K.L.; Webber, M.A.; Sutton, J.M.; Peterson, M.L. The Acinetobacter baumannii two-component system AdeRS regulates genes required for multidrug efflux, biofilm formation, and virulence in a strain-specific manner. MBio 2016, 7, e00430-16. [Google Scholar] [CrossRef] [PubMed]
- Chetri, S. The culmination of multidrug-resistant efflux pumps vs. meager antibiotic arsenal era: Urgent need for an improved new generation of EPIs. Front. Microbiol. 2023, 14, 1149418. [Google Scholar] [CrossRef]
- Kaur, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Schneider-Chaabane, A.; Bleicher, V.; Rau, S.; Al-Ahmad, A.; Lienkamp, K. Stimulus-Responsive Polyzwitterionic Surfaces Made from Itaconic Acid: Self-Triggered Antimicrobial Activity, Protein Repellency, and Cell Compatibility. ACS Appl. Mater. Interfaces 2020, 12, 21242–21253. [Google Scholar] [CrossRef]
- Liu, Z.; Yi, Y.; Song, L.; Chen, Y.; Tian, L.; Zhao, J.; Ren, L. Biocompatible mechano-bactericidal nanopatterned surfaces with salt-responsive bacterial release. Acta Biomater. 2022, 141, 198–208. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, W.; Qin, X.; Cui, M.; Guo, Y.; Wang, T.; Wang, K.; Shi, Z.; Zhang, C.; Li, W.; et al. Recent Progress on Bioinspired Antibacterial Surfaces for Biomedical Application. Biomimetics 2022, 7, 88. [Google Scholar] [CrossRef]
- Di Martino, M.; Sessa, L.; Di Matteo, M.; Panunzi, B.; Piotto, S.; Concilio, S. Azobenzene as Antimicrobial Molecules. Molecules 2022, 27, 5643. [Google Scholar] [CrossRef]
- Chen, F.; Ricken, J.; Xu, D.; Wegner, S.V. Bacterial Photolithography: Patterning Escherichia coli Biofilms with High Spatial Control Using Photocleavable Adhesion Molecules. Adv. Biosyst. 2019, 3, e1800269. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Riedel-Kruse, I.H. Biofilm Lithography enables high-resolution cell patterning via optogenetic adhesin expression. Proc. Natl. Acad. Sci. USA 2018, 115, 3698–3703. [Google Scholar] [CrossRef]
- Zhao, F.; Chavez, M.S.; Naughton, K.L.; Niman, C.M.; Atkinson, J.T.; Gralnick, J.A.; El-Naggar, M.Y.; Boedicker, J.Q. Light-Induced Patterning of Electroactive Bacterial Biofilms. ACS Synth. Biol. 2022, 11, 2327–2338. [Google Scholar] [CrossRef]
- Bruchmann, J.; Pini, I.; Gill, T.S.; Schwartz, T.; Levkin, P.A. Patterned SLIPS for the Formation of Arrays of Biofilm Microclusters with Defined Geometries. Adv. Healthc. Mater. 2017, 6, 1601082. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, J.; Bai, Y.; Zhang, Y.; Sun, H.; Zhang, X. A bacterial infection-microenvironment activated nanoplatform based on spiropyran-conjugated glycoclusters for imaging and eliminating of the biofilm. Chem. Eng. J. 2020, 399, 125787. [Google Scholar] [CrossRef]
- Chen, F.; Wegner, S.V. Blue Light Switchable Bacterial Adhesion as a Key Step toward the Design of Biofilms. ACS Synth. Biol. 2017, 6, 2170–2174. [Google Scholar] [CrossRef]
- Wei, J.; Jin, F. Illuminating bacterial behaviors with optogenetics. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101023. [Google Scholar] [CrossRef]
- Hoffman, S.M.; Tang, A.Y.; Avalos, J.L. Optogenetics Illuminates Applications in Microbial Engineering. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 373–403. [Google Scholar] [CrossRef]
- Lindner, F.; Diepold, A. Optogenetics in bacteria—Applications and opportunities. FEMS Microbiol. Rev. 2022, 46, fuab055. [Google Scholar] [CrossRef]
- Chen, F.; Wegner, S.V. Blue-Light-Switchable Bacterial Cell-Cell Adhesions Enable the Control of Multicellular Bacterial Communities. ACS Synth. Biol. 2020, 9, 1169–1180. [Google Scholar] [CrossRef]
- Fang, K.; Park, O.-J.; Hong, S.H. Controlling biofilms using synthetic biology approaches. Biotechnol. Adv. 2020, 40, 107518. [Google Scholar] [CrossRef]
- Hong, S.H.; Hegde, M.; Kim, J.; Wang, X.; Jayaraman, A.; Wood, T.K. Synthetic quorum-sensing circuit to control consortial biofilm formation and dispersal in a microfluidic device. Nat. Commun. 2012, 3, 613. [Google Scholar] [CrossRef]
- Van der Berg, J.; Velema, W.; Szymanski, W.; Driessen, A.; Feringa, B. Controlling the activity of quorum sensing autoinducers with light. Chem. Sci. 2015, 6, 3593–3598. [Google Scholar] [CrossRef]
- Hansen, M.J.; Hille, J.I.; Szymanski, W.; Driessen, A.J.; Feringa, B.L. Easily accessible, highly potent, photocontrolled modulators of bacterial communication. Chem 2019, 5, 1293–1301. [Google Scholar] [CrossRef]
- Lauxen, A.I.; Kobauri, P.; Wegener, M.; Hansen, M.J.; Galenkamp, N.S.; Maglia, G.; Szymanski, W.; Feringa, B.L.; Kuipers, O.P. Mechanism of resistance development in E. coli against TCAT, a trimethoprim-based photoswitchable antibiotic. Pharmaceuticals 2021, 14, 392. [Google Scholar] [CrossRef]
- Hou, D.; Wang, R.; Wang, Z.; Yang, G.; Xu, Z.; Zeng, Q.; Chen, Y. A light-activatable antibiotic with high activation efficiency and uncompromised bactericidal potency in the activated state. J. Leather Sci. Eng. 2021, 3, 8. [Google Scholar] [CrossRef]
- Shchelik, I.S.; Tomio, A.; Gademann, K. Design, Synthesis, and Biological Evaluation of Light-Activated Antibiotics. ACS Infect. Dis. 2021, 7, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Garcia, E.; Lozano, C.; Garcia-Iriepa, C.; Marazzi, M.; Winter, A.H.; Torres, C.; Sampedro, D. Controlling Antimicrobial Activity of Quinolones Using Visible/NIR Light-Activated BODIPY Photocages. Pharmaceutics 2022, 14, 1070. [Google Scholar] [CrossRef]
- Testolin, G.; Richter, J.; Ritter, A.; Prochnow, H.; Kohnke, J.; Bronstrup, M. Optical Modulation of Antibiotic Resistance by Photoswitchable Cystobactamids. Chemistry 2022, 28, e202201297. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.; Szymanski, W.; Feringa, B.L. Photocontrol of antibacterial activity: Shifting from UV to red light activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef] [PubMed]
- Diguet, A.; Yanagisawa, M.; Liu, Y.J.; Brun, E.; Abadie, S.; Rudiuk, S.; Baigl, D. UV-induced bursting of cell-sized multicomponent lipid vesicles in a photosensitive surfactant solution. J. Am. Chem. Soc. 2012, 134, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, W.; Beierle, J.M.; Kistemaker, H.A.; Velema, W.A.; Feringa, B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Mutter, N.L.; Volaric, J.; Szymanski, W.; Feringa, B.L.; Maglia, G. Reversible Photocontrolled Nanopore Assembly. J. Am. Chem. Soc. 2019, 141, 14356–14363. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, I.; Galstian, T.; Sorelli, L.; Rodrigue, D.; Fabbri, F.; Fafard, M. Photodynamic control of bacterial motility by means of azobenzene molecules. J. Photochem. Photobiol. 2021, 8, 100074. [Google Scholar] [CrossRef]
- de Souza-Guerreiro, T.C.; Bondelli, G.; Grobas, I.; Donini, S.; Sesti, V.; Bertarelli, C.; Lanzani, G.; Asally, M.; Paterno, G.M. Membrane Targeted Azobenzene Drives Optical Modulation of Bacterial Membrane Potential. Adv. Sci. 2023, 10, e2205007. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Ferreres, G.; Ivanova, K.; Ivanov, I.; Tzanov, T. Nanomaterials and Coatings for Managing Antibiotic-Resistant Biofilms. Antibiotics 2023, 12, 310. [Google Scholar] [CrossRef]
- Tallet, L.; Gribova, V.; Ploux, L.; Vrana, N.E.; Lavalle, P. New Smart Antimicrobial Hydrogels, Nanomaterials, and Coatings: Earlier Action, More Specific, Better Dosing? Adv. Healthc. Mater. 2021, 10, e2001199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shan, M.; Zhang, S.; Chen, X.; Liu, W.; Chen, J.; Liu, X. Stimuli-Responsive Antibacterial Materials: Molecular Structures, Design Principles, and Biomedical Applications. Adv. Sci. 2022, 9, e2104843. [Google Scholar] [CrossRef]
- Borzenkov, M.; Pallavicini, P.; Taglietti, A.; D’Alfonso, L.; Collini, M.; Chirico, G. Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms. Beilstein J. Nanotechnol. 2020, 11, 1134–1146. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, X.; Wei, X.; Yu, Y.; Chen, X.; Zhang, X.; Li, C. Near-Infrared Light-Activated Thermosensitive Liposomes as Efficient Agents for Photothermal and Antibiotic Synergistic Therapy of Bacterial Biofilm. ACS Appl. Mater. Interfaces 2018, 10, 14426–14437. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Wang, J.; Wang, Y.; Chen, A.; Wang, C.; Mo, W.; Li, Y.; Yuan, Q.; Zhang, Y. Photon-Responsive Antibacterial Nanoplatform for Synergistic Photothermal-/Pharmaco-Therapy of Skin Infection. ACS Appl. Mater. Interfaces 2019, 11, 300–310. [Google Scholar] [CrossRef]
- Dong, X.; Overton, C.M.; Tang, Y.; Darby, J.P.; Sun, Y.P.; Yang, L. Visible Light-Activated Carbon Dots for Inhibiting Biofilm Formation and Inactivating Biofilm-Associated Bacterial Cells. Front. Bioeng. Biotechnol. 2021, 9, 786077. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, B.; Zhang, Y.; Su, R.; Li, P.; Su, W. Fluorescent Carbon Dot-Curcumin Nanocomposites for Remarkable Antibacterial Activity with Synergistic Photodynamic and Photothermal Abilities. ACS Appl. Bio. Mater. 2021, 4, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Candreva, A.; De Rose, R.; Perrotta, I.D.; Guglielmelli, A.; La Deda, M. Light-induced clusterization of gold nanoparticles: A new photo-triggered antibacterial against E. coli proliferation. Nanomaterials 2023, 13, 746. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Sun, S.; Guo, P.; Wang, Y.; Qian, C.; Zhong, Y.; Yang, D. Wound therapy via a photo-responsively antibacterial nano-graphene quantum dots conjugate. J. Photochem. Photobiol. B 2020, 210, 111978. [Google Scholar] [CrossRef] [PubMed]
- García, A.; González, B.; Harvey, C.; Izquierdo-Barba, I.; Vallet-Regí, M. Effective reduction of biofilm through photothermal therapy by gold core@shell based mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 328, 111489. [Google Scholar] [CrossRef]
- Bagchi, D.; Rathnam, V.S.; Lemmens, P.; Banerjee, I.; Pal, S.K. NIR-light-active ZnO-based nanohybrids for bacterial biofilm treatment. ACS Omega 2018, 3, 10877–10885. [Google Scholar] [CrossRef]
- Qi, M.; Li, X.; Sun, X.; Li, C.; Tay, F.R.; Weir, M.D.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium. Dent. Mater. 2019, 35, 1665–1681. [Google Scholar] [CrossRef]
- Zhou, K.; Qiu, X.; Xu, L.; Li, G.; Rao, B.; Guo, B.; Pei, D.; Li, A.; He, G. Poly(selenoviologen)-Assembled Upconversion Nanoparticles for Low-Power Single-NIR Light-Triggered Synergistic Photodynamic and Photothermal Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 26432–26443. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Zhang, Y.; Zheng, Y.; Zhang, Y.; Meng, H.; Wu, G.; Hu, Y.; Gao, Y.; Huang, S.; et al. Synergy between Clinical Microenvironment Targeted Nanoplatform and Near-Infrared Light Irradiation for Managing Pseudomonas aeruginosa Infections. ACS Appl. Mater. Interfaces 2021, 13, 38979–38989. [Google Scholar] [CrossRef]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Cao, Q.; Wang, H.; Wang, X.; Han, H. pH-Responsive, Light-Triggered on-Demand Antibiotic Release from Functional Metal-Organic Framework for Bacterial Infection Combination Therapy. Adv. Funct. Mater. 2018, 28, 1800011. [Google Scholar] [CrossRef]
- Shen, Z.; He, K.; Ding, Z.; Zhang, M.; Yu, Y.; Hu, J. Visible-Light-Triggered Self-Reporting Release of Nitric Oxide (NO) for Bacterial Biofilm Dispersal. Macromolecules 2019, 52, 7668–7677. [Google Scholar] [CrossRef]
- Hong, L.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; Yeung, K.W.K. Rapid Biofilm Elimination on Bone Implants Using Near-Infrared-Activated Inorganic Semiconductor Heterostructures. Adv. Healthc. Mater. 2019, 8, 1900835. [Google Scholar] [CrossRef]
- Cui, Q.; Yuan, H.; Bao, X.; Ma, G.; Wu, M.; Xing, C. Synergistic Photodynamic and Photothermal Antibacterial Therapy Based on a Conjugated Polymer Nanoparticle-Doped Hydrogel. ACS Appl. Bio. Mater. 2020, 3, 4436–4443. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tao, B.; He, Y.; Mu, C.; Liu, G.; Zhang, J.; Liao, Q.; Liu, P.; Cai, K. Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 2019, 223, 119479. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Yang, Y.; Zhang, H.; Shi, J.; Yao, X.; Zhang, X. Near-Infrared Light-Triggered Therapy to Combat Bacterial Biofilm Infections by MoSe2/TiO2 Nanorod Arrays on Bone Implants. Adv. Mater. Interfaces 2020, 7, 1901706. [Google Scholar] [CrossRef]
- Huang, H.-H.; Anand, A.; Lin, C.-J.; Lin, H.-J.; Lin, Y.-W.; Harroun, S.G.; Huang, C.-C. LED irradiation of halogen/nitrogen-doped polymeric graphene quantum dots triggers the photodynamic inactivation of bacteria in infected wounds. Carbon 2021, 174, 710–722. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, J.; Qian, X.; An, L.; Fan, L. Using a visible light-triggered pH switch to activate nanozymes for antibacterial treatment. RSC Adv. 2020, 10, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Gan, H.Q.; Chen, G.R.; James, T.D.; Zhang, B.; Hu, Q.; Xu, F.; Hu, X.L.; He, X.P.; Mai, Y. Near-Infrared Light-Triggered Bacterial Eradication Using a Nanowire Nanocomposite of Graphene Nanoribbons and Chitosan-Coated Silver Nanoparticles. Front. Chem. 2021, 9, 767847. [Google Scholar] [CrossRef]
- Wang, K.; Zhan, S.; Sun, H.; Zhang, D.; Wang, J. Hollow porous core–shell ZnFe2O4/AgCl nanocubes coated with EDTA and Ag nanoparticles for enhanced photocatalytic performances of visible–light–driven. Chem. Eng. J. 2020, 400, 125908. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Liu, R.; Ma, D.; Xue, W. Dendritic Fe3O4@Poly(dopamine)@PAMAM Nanocomposite as Controllable NO-Releasing Material: A Synergistic Photothermal and NO Antibacterial Study. Adv. Funct. Mater. 2018, 28, 1707440. [Google Scholar] [CrossRef]
- Choi, S.K. Mechanistic basis of light induced cytotoxicity of photoactive nanomaterials. NanoImpact 2016, 3–4, 81–89. [Google Scholar] [CrossRef]
- Sivakumar, P.; Lee, M.; Kim, Y.S.; Shim, M.S. Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J. Mater. Chem. B 2018, 6, 4852–4871. [Google Scholar] [CrossRef] [PubMed]
- Borzenkov, M.; D’Alfonso, L.; Polissi, A.; Sperandeo, P.; Collini, M.; Dacarro, G.; Taglietti, A.; Chirico, G.; Pallavicini, P. Novel photo-thermally active polyvinyl alcohol-Prussian blue nanoparticles hydrogel films capable of eradicating bacteria and mitigating biofilms. Nanotechnology 2019, 30, 295702. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, J.; Xu, Y.; Liu, H.; Zhang, J.; Wang, Y.; Sun, Y.; Zhao, M.; Liao, L.; Wang, X. Fast Cross-Linked Hydrogel as a Green Light-Activated Photocatalyst for Localized Biofilm Disruption and Brush-Free Tooth Whitening. ACS Appl. Mater. Interfaces 2022, 14, 28427–28438. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Wang, H.; Li, S.; Pan, X.; Xu, B.; Yang, H.; Wu, Q.; Li, W.; Su, X.; et al. NIR Laser-Triggered Microneedle-Based Liquid Band-Aid for Wound Care. Adv. Funct. Mater. 2021, 31, 2100218. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, P.; Fan, Y.; Zhao, J.; Niu, S.; Song, L.; Ma, L.; Ren, L.; Ming, W. Near-infrared triggered antibacterial nanocomposite membrane containing upconversion nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109797. [Google Scholar] [CrossRef]
- Han, X.; Boix, G.; Balcerzak, M.; Moriones, O.H.; Cano-Sarabia, M.; Cortés, P.; Bastús, N.; Puntes, V.; Llagostera, M.; Imaz, I.; et al. Antibacterial Films Based on MOF Composites that Release Iodine Passively or Upon Triggering by Near-Infrared Light. Adv. Funct. Mater. 2022, 32, 2112902. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Zhao, W.; Zhao, C. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873. [Google Scholar] [CrossRef]
- Contreras, A.; Raxworthy, M.J.; Wood, S.; Tronci, G. Hydrolytic Degradability, Cell Tolerance and On-Demand Antibacterial Effect of Electrospun Photodynamically Active Fibres. Pharmaceutics 2020, 12, 711. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, J.; Wang, W.; Liu, K.; Zhou, C.; Liu, Z.; Kong, S.; Lin, S.; Deng, Y.; Guo, Z. Thermo and light-responsive strategies of smart titanium-containing composite material surface for enhancing bacterially anti-adhesive property. Chem. Eng. J. 2021, 407, 125783. [Google Scholar] [CrossRef]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/bFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Li, Z.; Feng, X.; Gao, S.; Jin, Y.; Zhao, W.; Liu, H.; Yang, X.; Hu, S.; Cheng, K.; Zhang, J. Porous Organic Polymer-Coated Band-Aids for Phototherapy of Bacteria-Induced Wound Infection. ACS Appl. Bio. Mater. 2019, 2, 613–618. [Google Scholar] [CrossRef]
- Munoz, M.; El-Khoury, A.; Eren Cimenci, C.; Gonzalez-Gomez, M.; Hunter, R.A.; Lomboni, D.; Variola, F.; Rotstein, B.H.; Vono, L.L.R.; Rossi, L.M.; et al. Riboflavin Surface Modification of Poly(vinyl chloride) for Light-Triggered Control of Bacterial Biofilm and Virus Inactivation. ACS Appl. Mater. Interfaces 2021, 13, 32251–32262. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.F.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; et al. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef]
- Feng, L.; Shi, W.; Chen, Q.; Cheng, H.; Bao, J.; Jiang, C.; Zhao, W.; Zhao, C. Smart Asymmetric Hydrogel with Integrated Multi-Functions of NIR-Triggered Tunable Adhesion, Self-Deformation, and Bacterial Eradication. Adv. Healthc. Mater. 2021, 10, e2100784. [Google Scholar] [CrossRef]
- Shi, Y.; Truong, V.X.; Kulkarni, K.; Qu, Y.; Simon, G.P.; Boyd, R.L.; Perlmutter, P.; Lithgow, T.; Forsythe, J.S. Light-triggered release of ciprofloxacin from an in situ forming click hydrogel for antibacterial wound dressings. J. Mater. Chem. B 2015, 3, 8771–8774. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Yuan, Z.; He, Y.; Chen, M.; Li, K.; Hu, J.; Yang, Y.; Xia, Z.; Cai, K. Near infrared light-triggered on-demand Cur release from Gel-PDA@ Cur composite hydrogel for antibacterial wound healing. Chem. Eng. J. 2021, 403, 126182. [Google Scholar] [CrossRef]
- Deng, H.; Sun, J.; Yu, Z.; Guo, Z.; Xu, C. Low-intensity near-infrared light-triggered spatiotemporal antibiotics release and hyperthermia by natural polysaccharide-based hybrid hydrogel for synergistic wound disinfection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111530. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Qian, H.; Lei, T.; Liu, W.; He, X.; Hu, Y.; Lei, P. Triggering Drug Release and Thermal-Disrupting Interface Induced Mitigation of Composite Photothermal Hydrogel Treating Infectious Wounds. Front. Bioeng. Biotechnol. 2021, 9, 796602. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, W.; Wei, W.; Zhao, Y.; Zhuang, P.; Wang, X.; Wang, Y.; Hu, Y.; Dai, H. Photothermal Hydrogel Encapsulating Intelligently Bacteria-Capturing Bio-MOF for Infectious Wound Healing. ACS Nano 2022, 16, 19491–19508. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Chai, M.; Yao, X.; Chen, W.; Chu, P.K. Synergistic antibacterial activity of physical-chemical multi-mechanism by TiO(2) nanorod arrays for safe biofilm eradication on implant. Bioact. Mater. 2021, 6, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Han, X.; Zhang, H.; Fan, A.; Yao, X.; Tang, B.; Zhang, X. Light-Triggered Antibacterial Hydrogels Containing Recombinant Growth Factor for Treatment of Bacterial Infections and Improved Wound Healing. ACS Biomater. Sci. Eng. 2021, 7, 1438–1449. [Google Scholar] [CrossRef]
- Xi, J.; Wu, Q.; Xu, Z.; Wang, Y.; Zhu, B.; Fan, L.; Gao, L. Aloe-Emodin/Carbon Nanoparticle Hybrid Gels with Light-Induced and Long-Term Antibacterial Activity. ACS Biomater. Sci. Eng. 2018, 4, 4391–4400. [Google Scholar] [CrossRef]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yang, Y.; Shi, J.; Zhang, H.; Yao, X.; Chen, W.; Zhang, X. A rose bengal/graphene oxide/PVA hybrid hydrogel with enhanced mechanical properties and light-triggered antibacterial activity for wound treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111447. [Google Scholar] [CrossRef]
- Karcher, J.; Pianowski, Z.L. Photocontrol of Drug Release from Supramolecular Hydrogels with Green Light. Chemistry 2018, 24, 11605–11610. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, E.B.; Jeong, C.J.; Sharker, S.M.; In, I.; Park, S.Y. Light controllable surface coating for effective photothermal killing of bacteria. ACS Appl. Mater. Interfaces 2015, 7, 15600–15606. [Google Scholar] [CrossRef]
- Yu, N.; Peng, H.; Qiu, L.; Wang, R.; Jiang, C.; Cai, T.; Sun, Y.; Li, Y.; Xiong, H. New pectin-induced green fabrication of Ag@AgCl/ZnO nanocomposites for visible-light triggered antibacterial activity. Int. J. Biol. Macromol. 2019, 141, 207–217. [Google Scholar] [CrossRef]
- Hu, H.; Zhong, D.; Li, W.; Lin, X.; He, J.; Sun, Y.; Wu, Y.; Shi, M.; Chen, X.; Xu, F.; et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today 2022, 42, 101368. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, Z.; Li, Q.; Tang, X.; Chen, X.; Ge, Y.; Ling, J. Light-triggered adhesive silk-based film for effective photodynamic antibacterial therapy and rapid hemostasis. Front. Bioeng. Biotechnol. 2022, 9, 1346. [Google Scholar] [CrossRef]
- Xiang, Y.; Mao, C.; Liu, X.; Cui, Z.; Jing, D.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; Zheng, Y.; et al. Rapid and Superior Bacteria Killing of Carbon Quantum Dots/ZnO Decorated Injectable Folic Acid-Conjugated PDA Hydrogel through Dual-Light Triggered ROS and Membrane Permeability. Small 2019, 15, e1900322. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, H.; Tao, X.; Xie, Y.; Yang, L.; Mao, Z.W.; Xia, W. Light-Triggered Nitric Oxide Release by a Photosensitizer to Combat Bacterial Biofilm Infections. Chemistry 2021, 27, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- Teirlinck, E.; Barras, A.; Liu, J.; Fraire, J.C.; Lajunen, T.; Xiong, R.; Forier, K.; Li, C.; Urtti, A.; Boukherroub, R. Exploring light-sensitive nanocarriers for simultaneous triggered antibiotic release and disruption of biofilms upon generation of laser-induced vapor nanobubbles. Pharmaceutics 2019, 11, 201. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).