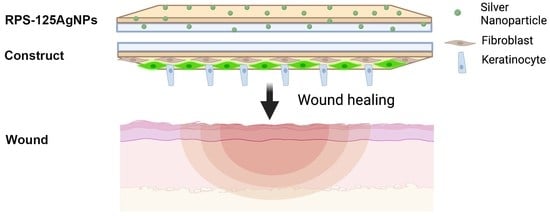
Radiosterilized Pig Skin, Silver Nanoparticles and Skin Cells as an Integral Dressing Treatment for Burns: Development, Pre-Clinical and Clinical Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Fibroblast, Keratinocytes, and Mesenchymal Stem Cells
2.2. Radiosterilized Pig Skin
2.3. Development and Study of the RPS-AgNPs Antibacterial Cover
2.3.1. Development of the Nanomaterial; RPS-AgNPs Antibacterial Cover
2.3.2. In Vitro Antibiofilm Capability
2.3.3. In Vivo Evaluation; Athymic Mouse Burn Model
Burn Wound Animal Model
Macroscopic Evaluation of Wound Closure
Microscopic Evaluation of Wound Repair
Determination of Potential Silver Bioaccumulation in Burned Skin and Organs in the Animal Model
2.4. Cellular Construct In Vitro Studies
2.4.1. Cellular Constructs
2.4.2. Cell Viability and Adhesion
2.4.3. Potential Cytotoxicity of RPS
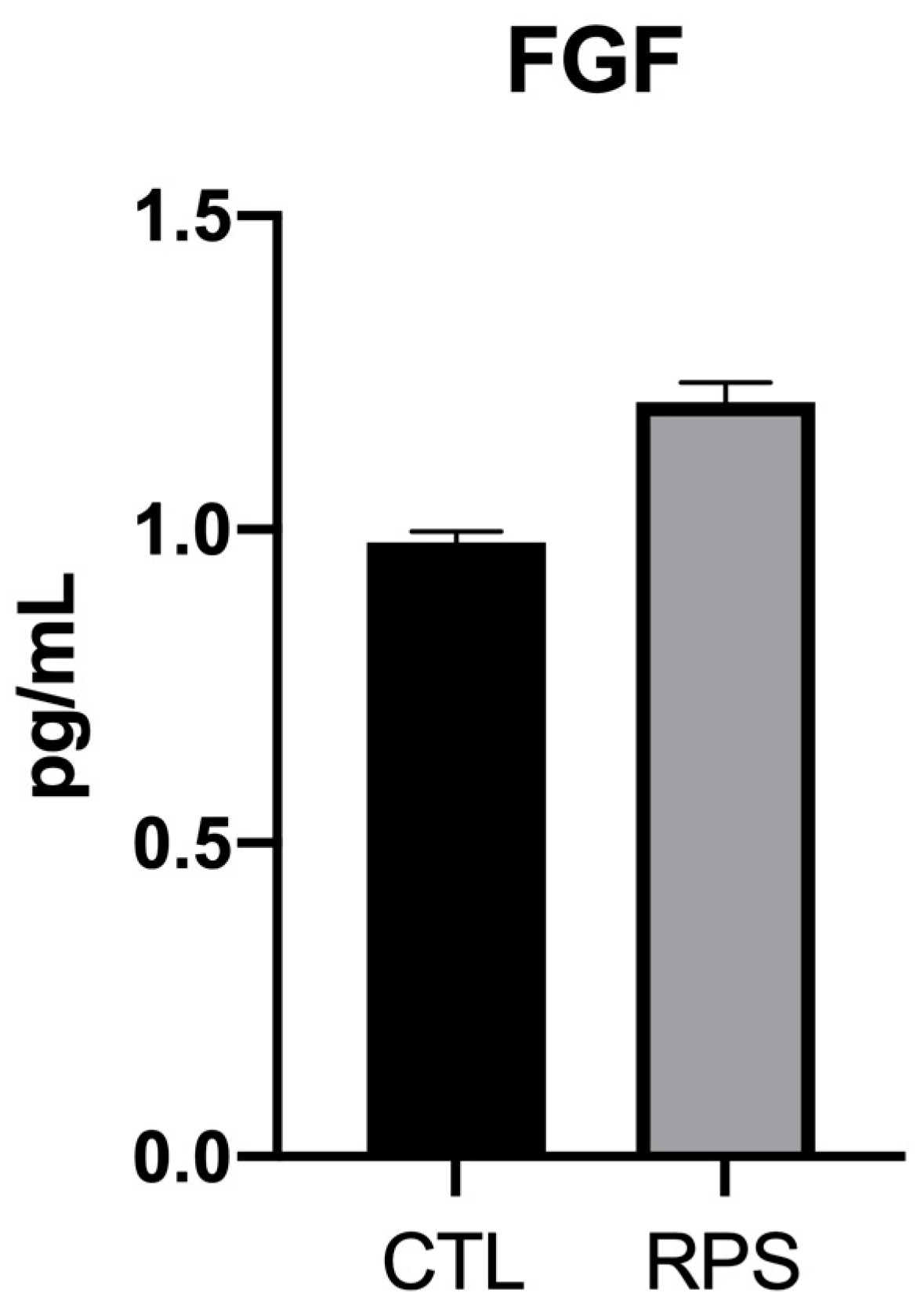
2.4.4. Cellular FGF Expression on RPS
2.5. Case of Study: Autologous Cellular Construct Dressed with Antibacterial Cover
2.5.1. Autologous Cellular Construct Generation
2.5.2. Cell Viability on Autologous Construct
2.5.3. Microbiological Test for the Construct to Be Implanted in the Patient
2.5.4. Implantation of the Autologous Cellular RPS-Fb-Kc Construct and Cover with the RPS-AgNPs125 Nanomaterial
2.5.5. Clinical Follow-Up of the Patient
3. Results
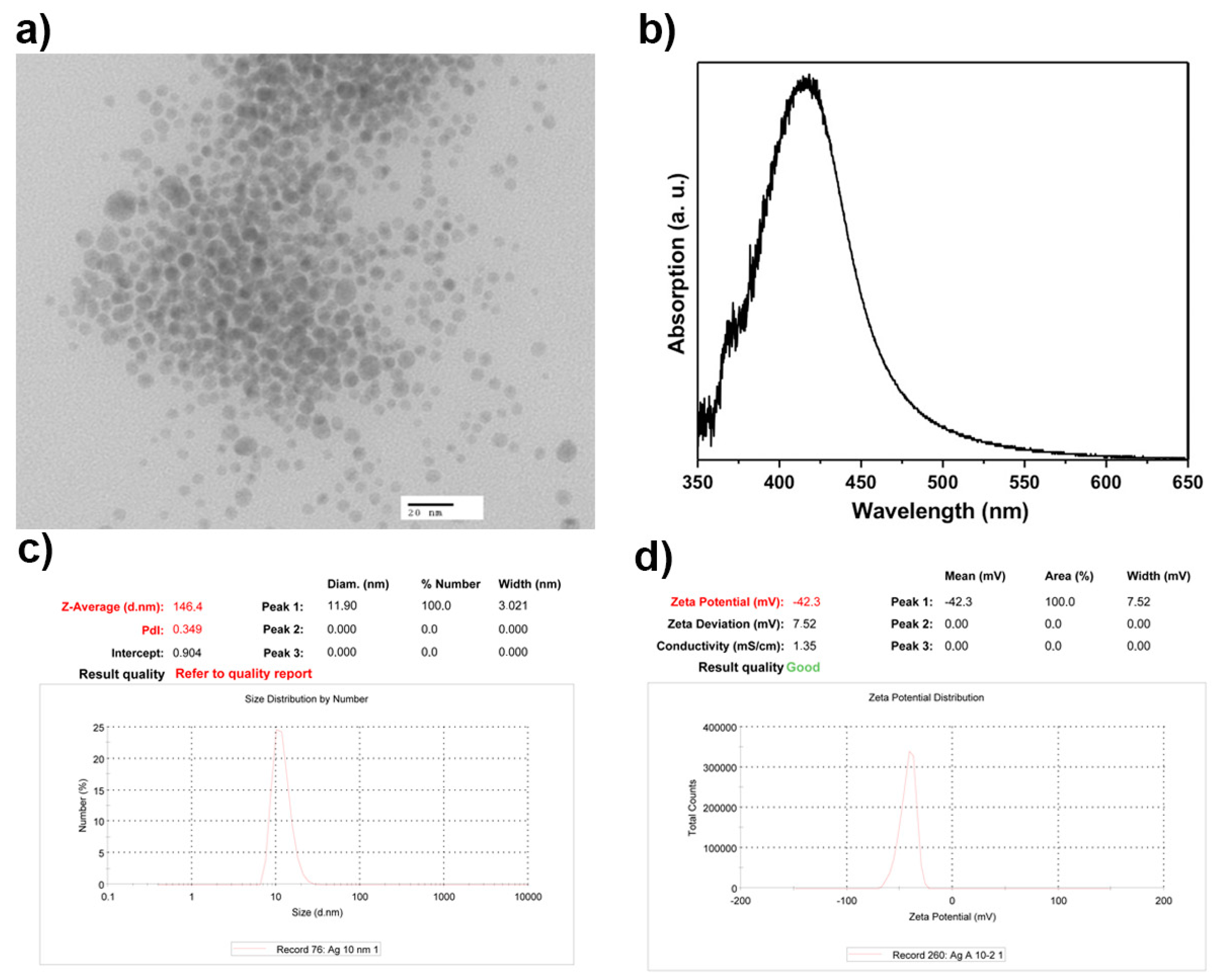
3.1. AgNPs Characterization
3.2. Antibacterial Effect of the Nanomaterial; RPS-AgNPs Covers
3.3. Characterization and Cell Viability Analysis of MSC on RPS-AgNPs125
3.4. RPS Is a Permissive Scaffold for Fibroblasts, Keratinocytes, and MSC Growth
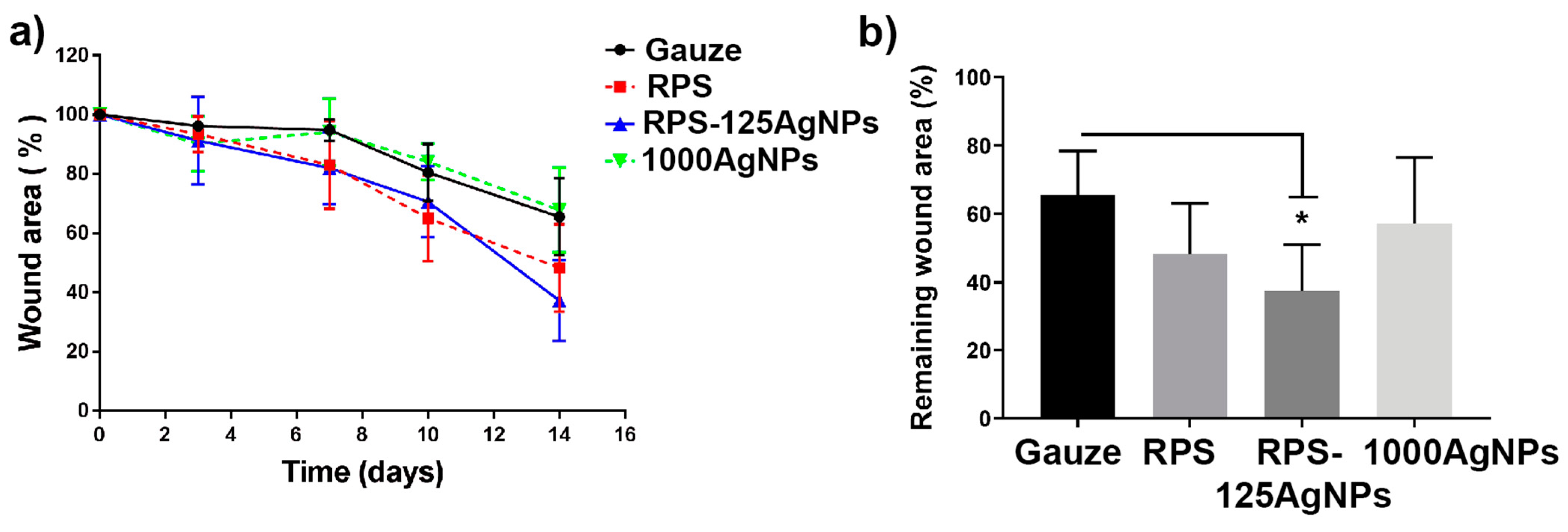
3.5. Radiosterilized Porcine Skin Impregnated with Silver Nanoparticles Promotes Wound Closure in a Burn Animal Model
3.6. Analysis of AgNPs Deposition on Burned Skin and Possible Ag Bioacumulation in Organs from Mice Treated Independently with the RPS-AgNPs125 Antibacterial Cover or AgNPs Solution at a Concentration of 1000 ppm
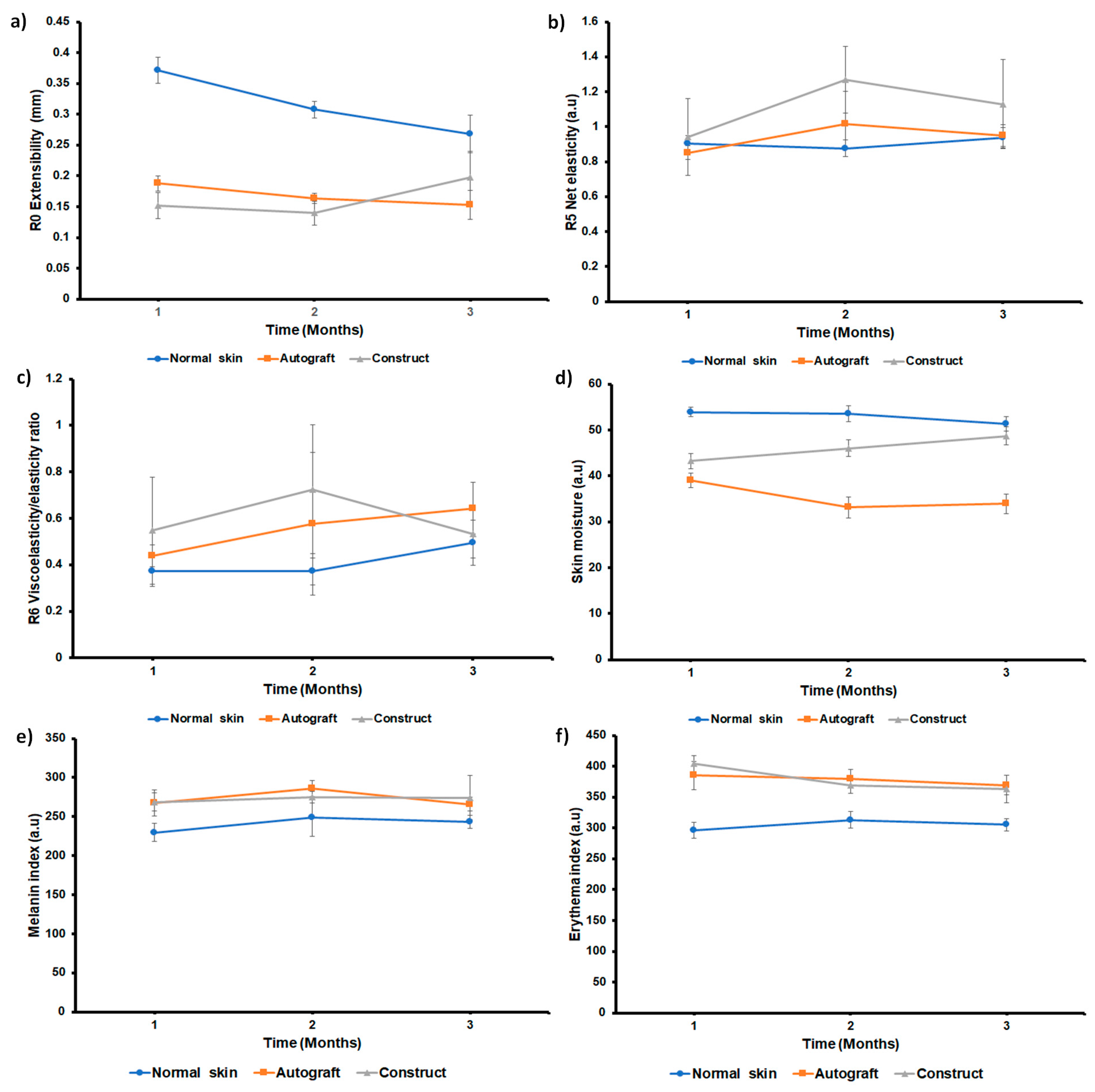
3.7. Implantation of the Construct and Nm Application in a Clinical Pilot Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rheinwatd, J.G.; Green, H. Serial Cultivation of Strains of Human Epidermal Keratinocytes: The Formation of Keratinizing Colonies from Single Cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.K.; Seifalian, A.M. Skin Regeneration Scaffolds: A Multimodal Bottom-up Approach. Trends Biotechnol. 2012, 30, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Böttcher-Haberzeth, S.; Biedermann, T.; Reichmann, E. Tissue Engineering of Skin. Burns 2010, 36, 450–460. [Google Scholar] [CrossRef]
- Klein, L.; Měřička, P.; Preis, J. Clinical Experience with Skin Xenografts in Burned Patients. In The Management of Burns and Fire Disasters: Perspectives 2000; Springer: Dordrecht, The Netherlands, 1995; pp. 343–345. [Google Scholar] [CrossRef]
- Artz, C.P.; Rittenbury, M.S.; Yarbrough, D.R. An Appraisal of Allografts and Xenografts as Biological Dressings for Wounds and Burns. Ann. Surg. 1972, 175, 934. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Brena-Molina, A.; Martínez-Lónez, V.; Melgarejo-Ramírez, Y.; De Dios, L.T.; Gómez-García, R.; De Lourdes Reyes-Frías, M.; Rodríguez-Rodríguez, L.; Garciadiego-Cázres, D.; Lugo-Martínez, H.; et al. Generation of Two Biological Wound Dressings as a Potential Delivery System of Human Adipose-Derived Mesenchymal Stem Cells. ASAIO J. 2015, 61, 718–725. [Google Scholar] [CrossRef][Green Version]
- Cabello-Arista, B.; Melgarejo-Ramírez, Y.; Retana-Flores, A.; Martínez-López, V.; Márquez-Gutiérrez, E.; Almanza-Pérez, J.; Lecona, H.; Reyes-Frías, M.L.; Ibarra, C.; Martínez-Pardo, M.E.; et al. Effects of Mesenchymal Stem Cell Culture on Radio Sterilized Human Amnion or Radio Sterilized Pig Skin in Burn Wound Healing. Cell Tissue Bank. 2022. [Google Scholar] [CrossRef]
- Groth, C.G. The Potential Advantages of Transplanting Organs from Pig to Man: A Transplant Surgeon’s View. Indian J. Urol. 2007, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Paterson, D.L. Multidrug Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. North Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Yougbaré, S.; Mutalik, C.; Okoro, G.; Lin, I.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Chang, C.C.; Kuo, T.R. Emerging Trends in Nanomaterials for Antibacterial Applications. Int. J. Nanomedicine 2021, 16, 5831–5867. [Google Scholar] [CrossRef]
- Monti, D.; Tampucci, S.; Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline Silver Dressings in Wound Management: A Review. Int. J. Nanomedicine 2006, 1, 441. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.A.; Silva-Bermudez, P.; Jiménez-López, B.; Martínez-López, V.; Melgarejo-Ramírez, Y.; Brena-Molina, A.; Ibarra, C.; Baeza, I.; Martínez-Pardo, M.E.; Reyes-Frías, M.L.; et al. Silver-Pig Skin Nanocomposites and Mesenchymal Stem Cells: Suitable Antibiofilm Cellular Dressings for Wound Healing. J. Nanobiotechnology 2018, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Ornelas-Flores, M.C.; García-López, J.; Melgarejo-Ramírez, Y.; Sánchez-Sánchez, R.; Leyva-Gómez, G.; Zacaula-Juárez, N.; González-Mendoza, O.; Manzo-Castrejón, H.A.; Ferreira-Aparicio, F.E.; Márquez-Gutiérrez, E.; et al. Implantation of a Heterologous Dermo-Epidermal Skin Substitute in a Patient with Deep Dermal Burn That Enhances Biomechanical and Functional Recovery: Case Report. Burn. Open 2018, 2, 144–153. [Google Scholar] [CrossRef]
- Anderl, J.N.; Zahller, J.; Roe, F.; Stewart, P.S. Role of Nutrient Limitation and Stationary-Phase Existence in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. [Google Scholar] [CrossRef]
- Goeres, D.M.; Loetterle, L.R.; Hamilton, M.A.; Murga, R.; Kirby, D.W.; Donlan, R.M. Statistical Assessment of a Laboratory Method for Growing Biofilms. Microbiology 2005, 151, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Zelver, N.; Hamilton, M.; Goeres, D.; Heersink, J. [24] Development of a Standardized Antibiofilm Test. Methods Enzymol. 2001, 337, 363–376. [Google Scholar] [CrossRef]
- Halkai, K.; Mudda, J.; Shivanna, V.; Patil, V.; Rathod, V.; Halkai, R. Cytotoxicity Evaluation of Fungal-Derived Silver Nanoparticles on Human Gingival Fibroblast Cell Line: An in Vitro Study. J. Conserv. Dent. 2019, 22, 160–163. [Google Scholar] [CrossRef]
- Galandáková, A.; Franková, J.; Ambrožová, N.; Habartová, K.; Pivodová, V.; Zálešák, B.; Šafářová, K.; Smékalová, M.; Ulrichová, J. Effects of Silver Nanoparticles on Human Dermal Fibroblasts and Epidermal Keratinocytes. Hum. Exp. Toxicol. 2016, 35, 946–957. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Deng, J.; Li, W.; Nie, X. Fibroblast Growth Factor in Diabetic Foot Ulcer: Progress and Therapeutic Prospects. Front. Endocrinol. 2021, 12, 1348. [Google Scholar]
- Zieger, M.A.J.; Ochoa, M.; Rahimi, R.; Campana, G.; Tholpady, S.; Ziaie, B.; Sood, R. Skin Regeneration Using Dermal Substrates That Contain Autologous Cells and Silver Nanoparticles to Promote Antibacterial Activity: In Vitro Studies. Mil. Med. 2017, 182, 376–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lankveld, D.P.K.; Oomen, A.G.; Krystek, P.; Neigh, A.; Troost—De Jong, A.; Noorlander, C.W.; Van Eijkeren, J.C.H.; Geertsma, R.E.; De Jong, W.H. The Kinetics of the Tissue Distribution of Silver Nanoparticles of Different Sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.B.; Hollman, P.C.H.; et al. Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef] [PubMed]
- Měřička, P. Brief History of the Tissue Bank, Charles University Hospital, Hradec Králové, Czech Republic. Cell Tissue Bank. 2000, 1, 17–25. [Google Scholar] [CrossRef]
- Greulich, C.; Kittler, S.; Epple, M.; Muhr, G.; Köller, M. Studies on the Biocompatibility and the Interaction of Silver Nanoparticles with Human Mesenchymal Stem Cells (HMSCs). Langenbeck’s Arch. Surg. 2009, 394, 495–502. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Silver Nanoparticles Do Not Influence Stem Cell Differentiation but Cause Minimal Toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Pastar, I.; Pellegrini, M.; Azzoni, P.; Vaschieri, C.; Pisciotta, A.; Carnevale, G.; Pellacani, G.; Magnoni, C. In Vitro Engineering of a Skin Substitute Based on Adipose-Derived Stem Cells. Cells Tissues Organs 2019, 207, 46–57. [Google Scholar] [CrossRef]
- Fife, C.E.; Horn, S.D.; Smout, R.J.; Barrett, R.S.; Thomson, B. Evaluation of Wound Closure Rates Using a Human Fibroblast-Derived Dermal Substitute Versus a Fetal Bovine Collagen Dressing: A Retrospective Study. Wound Manag. Prev. 2019, 65, 279–287. [Google Scholar] [CrossRef]
- Wasef, L.G.; Shaheen, H.M.; El-Sayed, Y.S.; Shalaby, T.I.A.; Samak, D.H.; Abd El-Hack, M.E.; Al-Owaimer, A.; Saadeldin, I.M.; El-mleeh, A.; Ba-Awadh, H.; et al. Effects of Silver Nanoparticles on Burn Wound Healing in a Mouse Model. Biol. Trace Elem. Res. 2020, 193, 456–465. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.S.; Song, K.S.; Ryu, H.R.; Sung, J.H.; Park, J.D.; Park, H.M.; Song, N.W.; Shin, B.S.; Marshak, D.; et al. Biopersistence of Silver Nanoparticles in Tissues from Sprague–Dawley Rats. Part. Fibre Toxicol. 2013, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-Biofilm Activity of Chitosan Gels Formulated with Silver Nanoparticles and Their Cytotoxic Effect on Human Fibroblasts. Mater. Sci. Eng. C 2016, 60, 317–323. [Google Scholar] [CrossRef]






| RPS-AgNPs Cover | Log Reduction * (±SD) |
|---|---|
| RPS-AgNPs1000 | 3.27 ± 0.12 |
| RPS-AgNPs500 | 2.28 ± 0.23 |
| RPS-AgNPs350 | 2.07 ± 0.20 |
| RPS-AgNPs250 | 1.9 ± 0.18 |
| RPS-AgNPs125 | 1.74 ± 0.24 |
| RPS-AgNPs125 | AgNPs Suspension at 1000 ppm | |
|---|---|---|
| Silver Concentration (ppm/g Tissue) | ||
| Heart | N.D. | N.D. |
| Liver | N.D. | N.D. |
| Spleen | N.D. | N.D. |
| Kidney | N.D. | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Sánchez, C.; Pérez-Díaz, M.; Melgarejo-Ramírez, Y.; Chopin-Doroteo, M.; Silva-Bermudez, P.; Martínez-López, V.; Zacaula-Juárez, N.; Zamudio-Cuevas, Y.; Hernández-Valencia, C.; López-Jácome, L.E.; et al. Radiosterilized Pig Skin, Silver Nanoparticles and Skin Cells as an Integral Dressing Treatment for Burns: Development, Pre-Clinical and Clinical Pilot Study. Pharmaceutics 2023, 15, 2105. https://doi.org/10.3390/pharmaceutics15082105
Ortega-Sánchez C, Pérez-Díaz M, Melgarejo-Ramírez Y, Chopin-Doroteo M, Silva-Bermudez P, Martínez-López V, Zacaula-Juárez N, Zamudio-Cuevas Y, Hernández-Valencia C, López-Jácome LE, et al. Radiosterilized Pig Skin, Silver Nanoparticles and Skin Cells as an Integral Dressing Treatment for Burns: Development, Pre-Clinical and Clinical Pilot Study. Pharmaceutics. 2023; 15(8):2105. https://doi.org/10.3390/pharmaceutics15082105
Chicago/Turabian StyleOrtega-Sánchez, Carmina, Mario Pérez-Díaz, Yaaziel Melgarejo-Ramírez, Mario Chopin-Doroteo, Phaedra Silva-Bermudez, Valentín Martínez-López, Noé Zacaula-Juárez, Yessica Zamudio-Cuevas, Carmen Hernández-Valencia, Luis Esaú López-Jácome, and et al. 2023. "Radiosterilized Pig Skin, Silver Nanoparticles and Skin Cells as an Integral Dressing Treatment for Burns: Development, Pre-Clinical and Clinical Pilot Study" Pharmaceutics 15, no. 8: 2105. https://doi.org/10.3390/pharmaceutics15082105
APA StyleOrtega-Sánchez, C., Pérez-Díaz, M., Melgarejo-Ramírez, Y., Chopin-Doroteo, M., Silva-Bermudez, P., Martínez-López, V., Zacaula-Juárez, N., Zamudio-Cuevas, Y., Hernández-Valencia, C., López-Jácome, L. E., Carlos-Martínez, A., Reyes-Medina, N., Tamez-Pedroza, L., Martínez-Pardo, M. E., Reyes-Frías, M. d. L., Lecona, H., Baeza, I., Martinez-Gutierrez, F., Márquez-Gutiérrez, E., ... Sánchez-Sánchez, R. (2023). Radiosterilized Pig Skin, Silver Nanoparticles and Skin Cells as an Integral Dressing Treatment for Burns: Development, Pre-Clinical and Clinical Pilot Study. Pharmaceutics, 15(8), 2105. https://doi.org/10.3390/pharmaceutics15082105