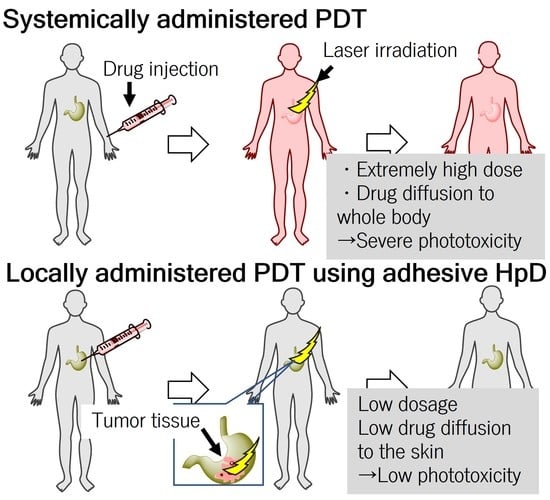
1. Introduction
Photodynamic therapy (PDT) is a non-invasive anti-tumor therapy that uses a combination of a photosensitizer and irradiation with light energy at a specific wavelength [
1,
2,
3]. The photosensitizer is systemically administered, and then the tumor tissue is irradiated with a light, which generates reactive oxygen species (ROS), mainly singlet oxygen (
1O
2) [
4,
5]. ROS can react with many biological molecules, including lipids, proteins, and nucleic acids, thereby killing cancer cells [
6]. As PDT can modulate ROS generation under the condition of light irradiation [
7], it is less invasive than surgical therapy, with less damage to normal tissue [
8]. In recent years, various nanomedicines such as metal–organic framework nanoparticles have also been developed for PDT [
9]. However, the systemically administered photosensitizers diffuse in the skin and eyes, which might cause phototoxicity to bright light and sunlight [
10]. Therefore, after PDT, patients must avoid exposure to light by shielding for a month [
11]. However, for elderly patients receiving PDT, intolerable stress from light-shielding management increases the risk of delirium and dementia and decreases the patient’s quality of life. In addition, for patients receiving PDT while working, this light-shielding management limits indoor as well as outdoor work.
To address skin phototoxicity after PDT, local administration of dihematoporphyrin ether has been previously investigated because local administration of photosensitizers can decrease the dose of photosensitizers [
12]. A phase 1 clinical trial has been conducted, although it has not been used for reasons unclear [
12]. We hypothesized that low molecular weight photosensitizers would be less effective because they would diffuse rapidly after administration. In this study, we investigated locally administered PDT using nano-adhesive porphyrin with polycations of quaternary ammonium salt groups, called adhesive porphyrin (aHP), as photosensitizers. aHP is composed of a copolymer of poly[2-(methacryloyloxy)ethyl]trimethylammonium chloride and poly[
N-(3-aminopropyl)methacrylamide hydrochloride] conjugated with hematoporphyrin dihydrochloride (HP), PMETAC-co-PAPMAA(HP). In a previous study, we reported the performance of aHP as a fluorescent tissue marking for surgery of gastrointestinal cancers in rats [
13]. Basically, low-molecular-weight fluorescent compounds cannot be used as fluorescent tissue marking agents because of their rapid diffusion. In fact, the fluorescent signal of hematoporphyrin cannot be detected even at 1 day after local injection of hematoporphyrin into the anterior wall of the stomach in the rats. In contrast, local injection of aHP shows a tissue-adhesive property and long-term retention for approximately one week due to the multi-valent electrostatic interactions between the positively charged moieties in aHP and negatively charged molecules in the tissue, such as chondroitin sulfate, heparan sulfate, and hyaluronan. From these results, we conceived the idea of locally administered PDT using aHP. In the case of gastrointestinal cancers such as esophageal, gastric, and colon cancers, photosensitizers can be injected into the submucosal layer near the tumor using an endoscope needle [
14]. If the drug injected into the submucosal layer of the gastrointestinal tract can diffuse and accumulate in the tumor, locally administered PDT with aHP may show an excellent therapeutic effect. In this study, the efficacy and safety of locally administered PDT with aHP were investigated using animal experiments in rodents for the treatment of gastrointestinal cancers.
2. Materials and Methods
2.1. Materials
Hematoporphyrin dihydrochloride (HP) was purchased from MedChem Express (San Diego, CA, USA). RPMI-1640 medium with L-glutamine and phenol red, methanol, and N-hydroxysuccinimide were purchased from FUJIFILM Wako Pure Chemical (Osaka, Japan). RPMI 1640 medium without phenol red was purchased from Thermo Fisher Scientific (San Jose, CA, USA). RIPA buffer was purchased from Nacalai Tesque (Kyoto, Japan). 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (WSCD-HCl) was purchased from Peptide Institute, Inc. (Osaka, Japan). Trypsin-ethylenediaminetetraacetic acid (EDTA), [2-(methacryloyloxy)ethyl]trimethylammonium chloride (METAC), 4,4′-azobis(4-cyanovaleric acid) (ACVA), and N-(3-aminopropyl)methacrylamide hydrochloride (APMAA) were purchased from Sigma-Aldrich (St Louis, MI, USA). 2,2,6,6-Tetramethyl-4-piperidinol (4-OH-TEMP) was purchased from the Tokyo Chemical Industry (Tokyo, Japan). Fetal bovine serum (FBS) was purchased from Gibco (Waltham, MA, USA). The LIVE/DEAD Cell Staining Kit was purchased from Dojindo (Kumamoto, Japan). The Apoptosis/Necrosis Assay Kit (ab176749) was purchased from Abcam (Cambridge, UK). 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Preparation of PMETAC-co-PAPMAA(HP)
PMETAC-co-PAPMAA(HP) was prepared as previously reported [
13]. Briefly, METAC (160 mg), APMAA (50 mg), and ACVA (10 mg) were dissolved in 2 mL of a 50% (
v/
v) EtOH–water mixture. After degassing with nitrogen for 10 min, polymerization was conducted at 60 °C for 24 h. The resulting PMETAC-co-PAPMAA copolymer was transferred into a pre-swollen membrane tube (Spectra/Por; molecular-weight cutoff size: 3500); dialyzed for 24 h against 2 L of water, which was changed after 2, 5, and 8 h; and then freeze-dried. The yield of the obtained polymer was 42.4% (89.5 mg). After 40 mg of the obtained PMETAC-co-PAPMAA was weighed into a 10 mL flask, 1 mL of phosphate buffer solution (pH 7.0) containing WSCD-HCl (15.5 mg), NHS (11.5 mg), and HP (2.5 mg) was added to the flask and stirred for 20 h at 25 °C. The mixture was transferred into a pre-swollen membrane tube (Spectra/Por; molecular-weight cutoff size: 3500) and dialyzed for 24 h against 2 L of water, which was changed after 2, 5, and 8 h, followed by freeze-drying. The yield of the obtained polymer was >99% (41.0 mg). The obtained PMETAC-co-PAPMAA(HP) is referred to as adhesive porphyrin (aHP). aHP was dissolved in PBS at a concentration of 2.5 mg/mL, in which the porphyrin concentration was 100 μM (59.8 μg/mL).
2.3. Characterizations
UV–vis spectra were collected using a UV-2600 UV-visible spectrophotometer (Shimadzu Corp., Kyoto, Japan). Fluorescence spectra were collected using an F-7000 fluorescence spectrophotometer (HITACHI, Tokyo, Japan). The size distribution and zeta potential of aHP was analyzed using dynamic light scattering (DLS, ELSZ-2000, Otsuka Electronics Co., Ltd., Tokyo, Japan).
2.4. Electron Spin Resonance (ESR) Analysis for Quantification of 1O2 Generation
The generation of 1O2 from HP and aHP was evaluated using ESR analysis using the spin trap agent 4-OH-TEMP. A stock solution of 4-OH-TEMP at the concentration of 4.5 M was dissolved in methanol. HP or aHP (200 μM porphyrin concentration, 90 μL) was mixed with 10 μL of 4-OH-TEMP stock solution, followed by light irradiation at 635 nm at 60 J cm−2. ESR spectra were measured using an X-band EPR spectrometer (FA-100; JEOL, Tokyo, Japan). The ESR measurements were conducted under the following conditions: frequency, 9.15 GHz; power, 5.0 mW; field, 325.6 ± 10 mT; sweep time, 0.5 min; modulation, 0.05 mT; and time constant, 0.03 s.
2.5. Intracellular Localization
Murine colon adenocarcinoma 26 (Colon 26) cells were purchased from Riken Cell Bank (RCB2657, Tsukuba, Japan), seeded in 35 mm glass bottom dish (Matsunami glass, Tokyo, Japan) at a density of 5 × 103 cells per glass dish, and incubated at 37 °C under a 5% CO2 atmosphere for 24 h. The medium was removed, 400 μL of fresh RPMI medium without FBS/phenol red containing HP or aHP (porphyrin concentration: 5 µM) was added, and the cells were incubated for 24 h at 37 °C under a 5% CO2 atmosphere. As polycations consisting of quaternary ammonium salt groups in the aHP interact with negatively charged macromolecules in FBS such as albumin via an electrostatic interaction, forming a precipitate in the cell culture medium, the medium without FBS was used when the drugs were applied to the cell culture. To observe the intracellular localization of photosensitizer, the cells were incubated for 5 min at 37 °C with Lysotracker Green (Invitrogen, 100 nM, from 0.1 mM stock in DMSO). The cells were examined using a laser scanning confocal fluorescence microscope (Zeiss LSM 900-Airyscan-2 microscope) with a 20× objective lens.
2.6. Cellular Uptake
Colon 26 cells were seeded in 24-well plates at 2.5 × 104 cells/well and incubated at 37 °C under a 5% CO2 atmosphere for 48 h. The medium was removed, 400 μL of fresh medium without FBS/phenol red containing HP or aHP (porphyrin concentration: 5 µM) was added, and the cells were incubated for 24 h at 37 °C under a 5% CO2 atmosphere. The cells were then rinsed with PBS three times and harvested using trypsin-EDTA. The number of cells was counted microscopically using a hemocytometer. One hundred microliters of RIPA buffer were added to the cell suspension with same cellular density. The fluorescence spectra of HP and aHP in cell lysates were measured using a fluorescence spectrometer (JASCO FP-6500, Tokyo, Japan). The excitation wavelength was 400 nm.
2.7. WST-1 Assay after Light Irradiation
Colon 26 cells were seeded into 48-well plates at a density of 3 × 104 cells/well. Cells were incubated at 37 °C in a 5% CO2 atmosphere for 24 h. The medium was removed, 200 μL of RPMI medium without FBS/phenol red containing HP or aHP (porphyrin concentration: 0, 1, 2.5, and 5 μM) was added, and the cells were incubated for 24 h at 37 °C in a 5% CO2 atmosphere. Cells were irradiated with 635 nm light at 14 J cm−2 (CivilLaser, Hangzhou, China). After irradiation, the medium was replaced with fresh RPMI containing FBS/phenol red, followed by incubation for 24 h. For the WST-1 assay, the medium was replaced with fresh medium containing 10% WST-1 and further incubated for 1 h. A microplate absorbance reader was used to determine the colorimetric absorbance of the dye solutions at 440 nm. All experiments were performed in triplicate. For LIVE/DEAD staining, the cells were stained with a LIVE/DEAD cell staining kit containing calcein-AM and propidium iodide (PI). Stained cells were observed under a fluorescence microscope (BZ-710; Keyence, Osaka, Japan).
2.8. Apoptosis/Necrosis Assay
Colon 26 cells were seeded into 48-well plates at a density of 3 × 104 cells/well. Cells were incubated at 37 °C in a 5% CO2 atmosphere for 24 h. The medium was removed, 200 μL RPMI medium without FBS/phenol red containing HP or aHP (porphyrin concentration: 5 µM) was added, and the cells were incubated for 24 h at 37 °C under a 5% CO2 atmosphere. The cells were irradiated with 635 nm light at 14 J cm−2. After irradiation, the medium was replaced with fresh RPMI medium containing FBS/phenol red, followed by incubation for 0, 3, 24 h. After removing the medium, the cells were washed thrice with RPMI medium without FBS/phenol red. Apoptosis and necrosis were detected using an Apoptosis/Necrosis Assay Kit. The fluorescence of the cells was examined using a fluorescence microscope (BZ-710; Keyence, Osaka, Japan).
2.9. ROS Generation in the Cells
Colon 26 cells were seeded into 48-well plates at a density of 3 × 104 cells/well. Cells were incubated at 37 °C in a 5% CO2 atmosphere for 24 h. The medium was removed and 200 μL RPMI medium without FBS/phenol red containing HP or aHP (porphyrin concentration: 5 µM) was added, and the cells were incubated for 24 h at 37 °C under a 5% CO2 atmosphere. DCFH-DA (0.1 M) was then added, and the cells were cultured for another 20 min. The cells were then washed three times with PBS to remove extracellular DCFH-DA. The cells were irradiated with light (635 nm, 14 J cm−2), and the fluorescence intensity in the cells was quantified using a BD Accuri™ C6 flow cytometer (BD Biosciences San Jose, CA, USA).
2.10. Animals
This study was conducted in strict accordance with the University of Tsukuba Guidelines for Animal Care and Laboratory Use, Japan (approval number: 21-476 and 21-477). Female BALB/c mice (six weeks old, Charles River Laboratories, Inc., Kanagawa, Japan) were used for experiments with locally administered PDT. Male Wistar rats (four weeks old, Charles River Laboratories, Inc., Kanagawa, Japan) were used for the experiments of skin phototoxicity. Animals were housed per cage and provided water and mouse chow ad libitum. The animals were maintained in a standard 12 h light–dark cycle.
2.11. Retention of aHP into the Injected Site after Subcutaneous Injection on the Tumor
Tumor-bearing mice were prepared by subcutaneous injection of Colon 26 cells (1 × 106 cells/mouse) into the right side of the back. When the tumor volume reached 30–40 mm3, the hair on the back near the tumor was dorsally shaved using electric clippers and removed using a local hair removal agent because the white hair of BALB/c mice exhibited autofluorescence. Mice were randomly divided into four groups (5 mice per group): aHP with light irradiation for three days, aHP without light irradiation, HP with light irradiation for three days, and HP without light irradiation. aHP and HP were subcutaneously injected into the tumor. At 30 min, 1 day, 2 days, and 3 days after injection, the fluorescent signals of HP and aHP were detected using an IVIS imaging system (PerkinElmer, MA, USA) (Ex/Em = 430/600 nm). At 30 min after IVIS measurement on days 0, 1, and 2, the tumor of light-irradiated groups was irradiated with light at 635 nm at 78 J cm−2.
2.12. In Vivo Anti-Cancer PDT by Local Administered aHP
Female BALB/c mice (6 weeks old, 14–16 g) were bred. Tumor-bearing mice were prepared by subcutaneous injection of Colon 26 cells (1 × 106 cells/mouse) into the right side of the back. When the tumor volume reached approximately 30–40 mm3, the hair of the mice was dorsally shaved using electric clippers. The mice were randomly divided into six groups (5 mice per group): aHP with light irradiation, aHP without light irradiation, HP with light irradiation, HP without light irradiation, PBS with light irradiation, and PBS without light irradiation. One hundred microliters of aHP, HP, or PBS was subcutaneously injected on the tumor. The porphyrin dose used for each injection was 300 µg kg−1. The light-irradiated groups received 635 nm light irradiation at 78 J cm−2. The tumor size was recorded, and the tumor volume was calculated as follows: tumor volume (V) = 0.52 × L × W2.
L and W are the long and short diameters of the tumor, respectively, as measured by a caliper.
2.13. Skin Phototoxicity
HP, aHP, and PBS were intraperitoneally injected into Wistar rats after depilation of the back skin at a porphyrin concentration of 2 mg kg−1. Twenty-four hours after drug injection, the back skin was irradiated with ultraviolet A (UVA) at 385 nm at 10 J cm−2. Twenty-four hours after UVA irradiation, the skin on the back was collected under anesthesia with isoflurane. The tissues were then fixed in 10% formalin buffer solution at pH 7.4, and tissue cross-sections were histologically observed using hematoxylin and eosin (HE) staining. The images were scanned using a digital slide scanner (NanoZoomer S210, Hamamatsu Photonics, Hamamatsu, Japan), and the hydrogel layer area was quantified using the NDP.view2 Viewing software U12388-01 (Hamamatsu Photonics, Hamamatsu, Japan).
2.14. Statistical Analysis
Differences between more than three groups were examined for statistical significance using one-way ANOVA followed by Tukey’s test (Kaleida Graph 4.5 J; Synergy Software, Reading, PA, USA). Using Student’s t-test, differences between the two groups were examined for statistical significance. A p < 0.05 was considered significant for all statistical analyses.
4. Discussion
PDT is a noninvasive treatment that combines a photosensitizer with specific wavelengths of light irradiation to generate ROS, which then destroy the cancer cells. Thus far, first-generation PDT with porfimer sodium, which is a complex mixture of porphyrin oligomers, and second-generation PDT with talaporfin sodium have been approved for health insurance coverage in Japan [
18]. First-generation PDT has high incidences of phototoxicity and long light-shielding time of 4 to 6 weeks [
18]. To avoid phototoxicity, low-molecular-weight talaporfin sodium, a tetrasodium salt of mono-
L-aspartyl chlorin e6 with a rapid metabolism, has been developed and used in hospital [
18]. However, after systemic administration, even talaporfin sodium requires light shielding for two weeks of hospitalization and avoidance of exposure to sunlight for two weeks after discharge from the hospital [
19]. In elderly patients receiving PDT, light shielding increases the risk of delirium and dementia and decreases the patient’s quality of life. In addition, for patients receiving PDT while working, this light-shielding management limits their work both outdoors and indoors.
To address the problem of severe phototoxicity after PDT, we investigated locally administered PDT using aHP as a photosensitizer. In the case of gastrointestinal cancers such as esophageal, gastric, and colon cancers, photosensitizers can be injected into the submucosal layer near the tumor using an endoscope needle [
14]. The endoscope is also equipped with a fiber-optic probe for laser and can perform PDT for gastrointestinal cancers. The treatment time is about 30 min; during PDT, as with gastroscopy, intravenous anesthesia is used, and the patient remains conscious but does not feel pain. If the drug injected into the submucosal layer of the gastrointestinal tract can diffuse and accumulate in the tumor, locally administered PDT would show an excellent therapeutic effect. As the gastrointestinal cancer model in mice cannot be used, in this study, photosensitizers aHP and HP were subcutaneously administered on the tumors of tumor-bearing mice to show a proof of concept for locally administered PDT using aHP as a photosensitizer.
First, we characterized aHP and compared it with HP. From the results of UV and FL spectra,
1O
2 generation, DLS, and π–π stacking of porphyrins in aHP occurs, resulting in a decrease in
1O
2 generation to 70% compared to HP. Interaction of porphyrin via π-stacking causes fluorescence quenching and the inhibition of
1O
2 generation, leading to light-to-heat energy conversion [
20]. However, a thermal increase was not detected in the current cell and animal experiments. We investigated the anti-tumor effects of PDT using aHP as a photosensitizer. From the cell experiment using colon 26 cells, we revealed the different localizations of low-molecular-weight HP and aHP. HP was localized in the cytoplasm, whereas aHP was localized in the endosomes (
Figure 2a). Tamura et al. reported that nanoparticles with quaternary ammonium salt groups accumulate in the endosome and/or lysosomes, which corresponds to our results [
21]. Low-molecular weight HP is incorporated into cancer cells by heme carrier protein 1 (HCP-1), followed by an accumulation in the mitochondria or the endoplasmic reticulum [
22]. In contrast, polycations adhere to cell membranes through electrostatic interactions [
23]. Therefore, aHP may have adhered to the cell membrane and entered the endosomes (
Figure 2a). Importantly, the fluorescence intensity of aHP in the cytoplasm is lower than that in the endosome and may not be incorporated into the cytoplasm because of the lack of an endosomal escape function. Low-molecular-weight HP is excreted via ABCG2 after intracellular uptake [
9], whereas aHP is retained in the cell because of its cell adhesive property [
23]. This difference in cell interactions may lead to a difference in the amount of intracellular fluorescence (
Figure 2c). When we evaluated the anti-tumor effect of aHP against colon 26 cells using WST-1 assay and LIVE/Dead staining, aHP exhibited a higher anti-tumor effect than HP, which exceeded the difference in the intracellular amount of porphyrin (
Figure 3). Previously, Kurokawa et al. reported that only 20% of cancer cells are dead following treatment with HP at 20 µM. Thus, low-molecular-weight HP is not effective even when used at a high concentration. On the other hand, aHP showed high anti-cancer activity even at the low concentration. To investigate the higher anti-tumor effect of aHP, we measured the amount of intracellular ROS. As shown in
Figure 4, aHP produces an approximately 30 times larger number of ROS than that of HP under light irradiation. In addition, after light irradiation, the aHP-treated cells swelled like balloons and induced apoptosis (
Figure 5). Although a detailed investigation is required for the analysis of the mechanism, oxidation of the cell membrane via aHP must be one of the causes. Thus, the adhesion of aHP to the cell membrane with light irradiation causes oxidation of the cell membrane, leading to cell swelling with large bubbles and membrane rupture. On oxidation, in addition, PS would be transferred to the outer cell membrane and exposed on the surface of apoptotic cells, which act as “eat-me” signals for the phagocytes [
24].
To examine the tissue adhesive property of aHP after subcutaneous injection in the tumor, aHP was administered subcutaneously to the tumor. As shown in
Figure 5, 20% of the injected aHP remained in the tumor even on day 3 after the local injection, whereas HP rapidly diffused and disappeared on day 1 after the injection. With light irradiation of the tumor tissue at 78 J cm
−2, no photobleaching occurred. Based on this result, locally administered PDT using aHP allows for light irradiation for three consecutive days. When light irradiation was performed for three days after subcutaneous injection of aHP on the tumor sites, the anti-tumor effect of aHP was higher than that of low-molecular-weight HP. This is not only due to the intratumor persistence of aHP but also due to its high anti-tumor efficacy and apoptotic activity.
Finally, we investigated the skin phototoxicity of aHP and HP following intraperitoneal injection. As the dosage in the gastrointestinal tract of rodents is limited, aHP was administered intraperitoneally to rats to prepare a photosensitivity model according to a previous study [
10]. For the skin phototoxicity test, skin thickness was measured 24 h after intraperitoneal injection of aHP and HP in rats and UVA irradiation to the skin of their backs with 385 nm light at 10 J cm
−2 [
10]. Phototoxicity causes skin thickness to increase due to edema and inflammation, which can be used as an indicator of phototoxicity [
10]. As shown in
Figure 8, the HP-treated group showed a significant increase in skin thickness after UVA irradiation. This indicates that low-molecular-weight photosensitizers diffuse and accumulate in the skin after intraperitoneal administration, resulting in skin phototoxicity. In HP-treated rats that were not exposed to UVA, skin thickness tended to increase, although the difference was not significant. This was probably due to the lighting in the breeding room every 12 h. As photosensitivity occurs in indoor light, patients must stay in a darkened room and wear long sleeves and sunglasses for several weeks. In contrast, the aHP-treated group showed no change in skin thickness, even after UVA irradiation. This is because of the high molecular weight of aHP compared to low-molecular-weight HP; the diffusion of aHP in the skin tissue is reduced. Thus, the administration of porphyrins with polycations not only significantly improves the efficacy of locally administered PDT but also avoids phototoxicity in animal models. The efficacy of locally administered PDT may be further improved by using different photosensitizers with polycations consisting of quaternary ammonium salt groups, which can be excited by near-infrared light in the biological optical transparency windows.