Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment
Abstract
1. Introduction
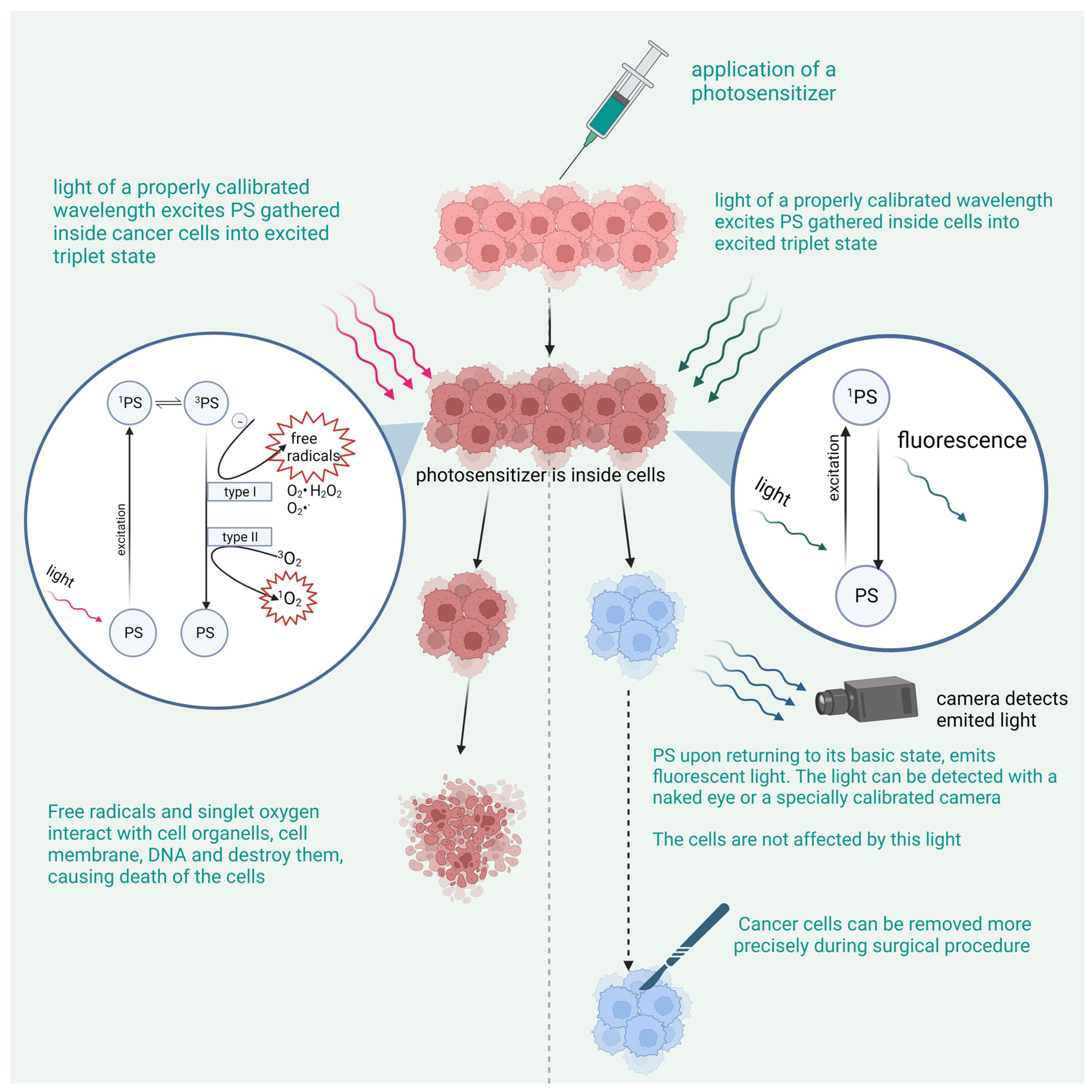
2. Photodynamic Therapy
2.1. The Principle of PDT
The Mechanism of the Cancer-Killing Process
2.2. Combining Photosensitizers with Nanotechnology Help Improve PDT
2.2.1. Quantum Dots and PDT
2.2.2. Nanoparticles and PDT
2.2.3. Liposomes and PDT
2.3. Combination of PDT with Different Treatment Methods
2.3.1. Immunotherapy
The Use of Antibodies to Accelerate the Affinity of the Photosensitizer to the Cancerous Cells
Combining Immunological Adjuvant and PDT
Combining PDT with a Check-Point Blockade to Stop the Tumor from Recruiting Lymphocytes for Its Microenvironment
The Use of PDT to Create Anticancer Vaccines
2.3.2. Radiotherapy
The Use of X-rays to Excite the Photosensitizer
The Combination of PDT and Radiotherapy
2.3.3. Chemotherapy
Combining Chemotherapy and PDT Enhances the Anticancer Properties of Chemotherapy
Combining Chemotherapy with PDT Enhances the Anticancer Effect of PDT
The Use of Drugs That Have Both PS and Cytostatic Properties
2.3.4. Surgery
2.4. Photodynamic Diagnostics
3. The Mechanism of PDD
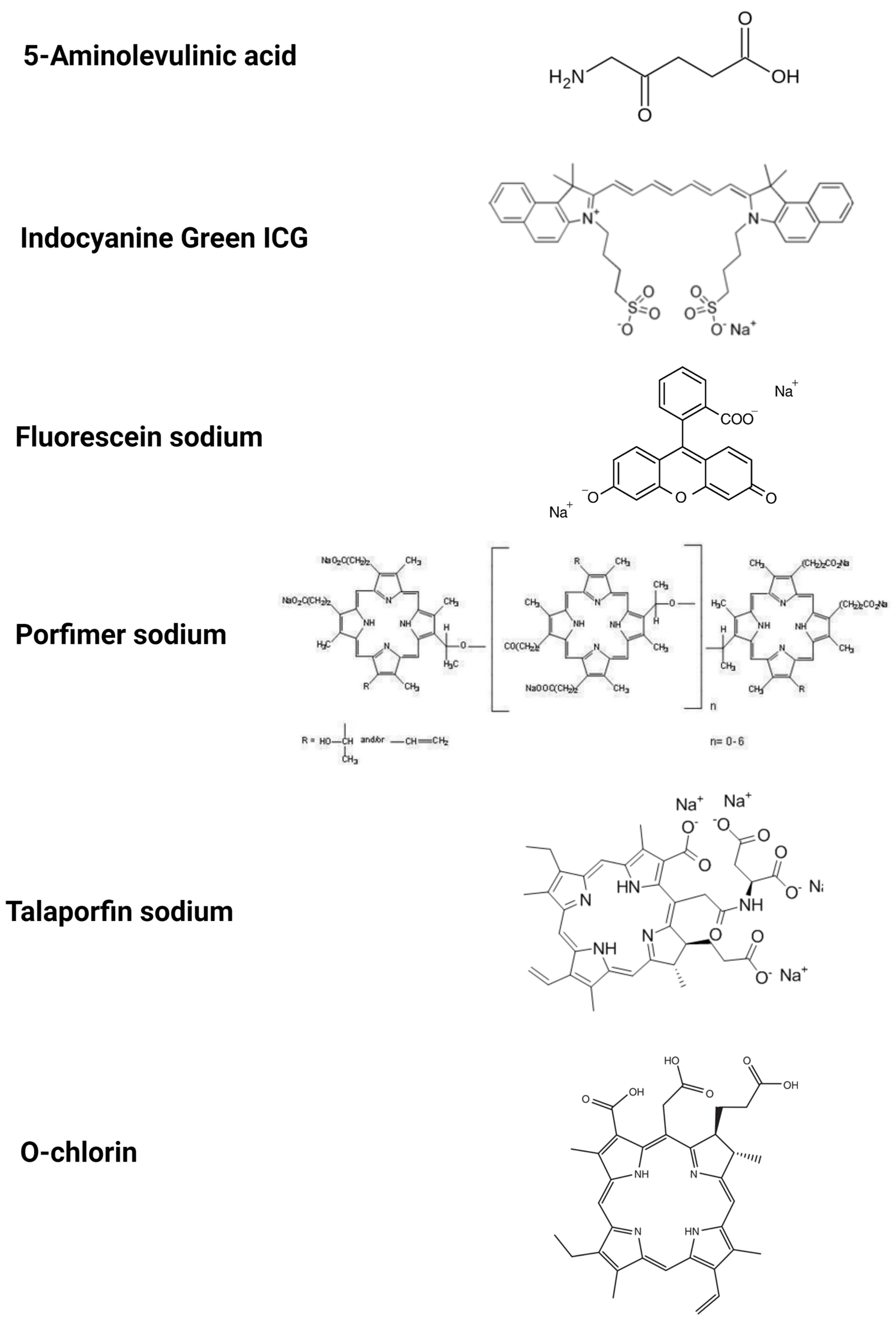
3.1. 5-Aminolevulinic Acid (5-ALA)
3.2. Indocyanine Green (ICG)
3.3. Fluorescein Sodium
3.4. Photofin (Porfimer Sodium)
3.5. Talaporfin Sodium
3.6. O-Chlorin
3.7. Raman Spectroscopy—A New Diagnostic Tool Employing Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manyak, M.J.; Russo, A.; Smith, P.D.; Glatstein, E. Photodynamic Therapy. J. Clin. Oncol. 1988, 6, 380–391. [Google Scholar] [CrossRef]
- Rkein, A.M.; Ozog, D.M. Photodynamic Therapy. Dermatol. Clin. 2014, 32, 415–425. [Google Scholar] [CrossRef]
- Didamson, O.C.; Abrahamse, H. Targeted Photodynamic Diagnosis and Therapy for Esophageal Cancer: Potential Role of Functionalized Nanomedicine. Pharmaceutics 2021, 13, 1943. [Google Scholar] [CrossRef]
- Kutwin, P.; Konecki, T.; Cichocki, M.; Falkowski, P.; Jabłonowski, Z. Photodynamic Diagnosis and Narrow-Band Imaging in the Management of Bladder Cancer: A Review. Photomed. Laser Surg. 2017, 35, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazonova, A.; Filonenko, E. Advantages of Combined Photodynamic Therapy in the Treatment of Oncological Diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer–A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic Therapy and Diagnosis: Principles and Comparative Aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy-Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Krupka, M.; Bartusik-Aebisher, D.; Strzelczyk, N.; Latos, M.; Sieroń, A.; Cieślar, G.; Aebisher, D.; Czarnecka, M.; Kawczyk-Krupka, A.; Latos, W. The Role of Autofluorescence, Photodynamic Diagnosis and Photodynamic Therapy in Malignant Tumors of the Duodenum. Photodiagnosis Photodyn. Ther. 2020, 32, 101981. [Google Scholar] [CrossRef]
- Matoba, Y.; Banno, K.; Kisu, I.; Aoki, D. Clinical Application of Photodynamic Diagnosis and Photodynamic Therapy for Gynecologic Malignant Diseases: A Review. Photodiagnosis Photodyn. Ther. 2018, 24, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Bartusik-Aebisher, D.; Osuchowski, F.; Aebisher, D. Photodynamic Therapy for Prostate Cancer-A Narrative Review. Photodiagnosis Photodyn. Ther. 2021, 33, 102158. [Google Scholar] [CrossRef]
- Gomer, C.J.; Ferrario, A.; Luna, M.; Rucker, N.; Wong, S. Photodynamic Therapy: Combined Modality Approaches Targeting the Tumor Microenvironment. Lasers Surg. Med. 2006, 38, 516–521. [Google Scholar] [CrossRef]
- Ahmad, J.; Garg, A.; Mustafa, G.; Ahmad, M.Z.; Aslam, M.; Mishra, A. Hybrid Quantum Dot as Promising Tools for Theranostic Application in Cancer. Electronics 2023, 12, 972. [Google Scholar] [CrossRef]
- Tabish, T.A.; Scotton, C.J.; J Ferguson, D.C.; Lin, L.; van der Veen, A.; Lowry, S.; Ali, M.; Jabeen, F.; Ali, M.; Winyard, P.G.; et al. Biocompatibility and Toxicity of Graphene Quantum Dots for Potential Application in Photodynamic Therapy. Nanomedicine 2018, 13, 1923–1937. [Google Scholar] [CrossRef]
- Pleskova, S.; Mikheeva, E.; Gornostaeva, E. Using of Quantum Dots in Biology and Medicine. Adv. Exp. Med. Biol. 2018, 1048, 323–334. [Google Scholar]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Zhang, W.; Yu, L.; Jiang, Y.; Guo, C. Phycocyanin-Functionalized Black Phosphorus Quantum Dots Enhance PDT/PTT Therapy by Inducing ROS and Irreparable DNA Damage. Biomater. Sci. 2021, 9, 5302–5318. [Google Scholar] [CrossRef]
- Hashemkhani, M.; Demirci, G.; Bayir, A.; Muti, A.; Sennaroglu, A.; Mohammad Hadi, L.; Yaghini, E.; Loizidou, M.; MacRobert, A.J.; Yagci Acar, H. Cetuximab-Ag2S Quantum Dots for Fluorescence Imaging and Highly Effective Combination of ALA-Based Photodynamic/Chemo-Therapy of Colorectal Cancer Cells. Nanoscale 2021, 13, 14879–14899. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, N.B.; Avatefi, M.; Karimi, M.; Mahmoudifard, M. Graphene Family in Cancer Therapy: Recent Progress in Cancer Gene/Drug Delivery Applications. J. Mater. Chem. B 2023, 11, 2568–2613. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, G.; Zang, W.; Zhou, X.; Shi, K.; Zhai, Y. Pure Drug Nano-Assemblies: A Facile Carrier-Free Nanoplatform for Efficient Cancer Therapy. Acta Pharm. Sin. B 2022, 12, 92–106. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Liu, B.; Tan, Y.N. Emerging Strategies in Enhancing Singlet Oxygen Generation of Nano-Photosensitizers Toward Advanced Phototherapy. Nanomicro Lett. 2022, 14, 123. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Song, X.; Dong, X.; Li, B. Nano-Photosensitizers for Enhanced Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 36, 102597. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, R.; Khorsandi, K. Methylene Blue, Curcumin and Ion Pairing Nanoparticles Effects on Photodynamic Therapy of MDA-MB-231 Breast Cancer Cell. Photodiagnosis Photodyn. Ther. 2017, 18, 284–294. [Google Scholar] [CrossRef]
- Guo, H.; Liu, F.; Liu, E.; Wei, S.; Sun, W.; Liu, B.; Sun, G.; Lu, L. Dual-Responsive Nano-Prodrug Micelles for MRI-Guided Tumor PDT and Immune Synergistic Therapy. J. Mater. Chem. B 2022, 10, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal Formulations of Photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y.; et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 8, 2100876. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Feron, O.; Amorim, C.A. Photodynamic Cancer Therapy Using Liposomes as an Advanced Vesicular Photosensitizer Delivery System. J. Control. Release 2021, 339, 75–90. [Google Scholar] [CrossRef]
- Plenagl, N.; Duse, L.; Seitz, B.S.; Goergen, N.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Photodynamic Therapy–Hypericin Tetraether Liposome Conjugates and Their Antitumor and Antiangiogenic Activity. Drug Deliv. 2019, 26, 23–33. [Google Scholar] [CrossRef]
- Woźniak, M.; Nowak, M.; Lazebna, A.; Więcek, K.; Jabłońska, I.; Szpadel, K.; Grzeszczak, A.; Gubernator, J.; Ziółkowski, P. The Comparison of in vitro Photosensitizing Efficacy of Curcumin-Loaded Liposomes following Photodynamic Therapy on Melanoma MUG-Mel2, Squamous Cell Carcinoma SCC-25, and Normal Keratinocyte HaCaT Cells. Pharmaceuticals 2021, 14, 374. [Google Scholar] [CrossRef]
- Feuser, P.E.; Cordeiro, A.P.; de Bem Silveira, G.; Borges Corrêa, M.E.A.; Lock Silveira, P.C.; Sayer, C.; de Araújo, P.H.H.; Machado-de-Ávila, R.A.; Dal Bó, A.G. Co-Encapsulation of Sodium Diethyldithiocarbamate (DETC) and Zinc Phthalocyanine (ZnPc) in Liposomes Promotes Increases Phototoxic Activity against (MDA-MB 231) Human Breast Cancer Cells. Colloids Surf. B Biointerfaces 2021, 197, 111434. [Google Scholar] [CrossRef]
- Cong, C.; He, Y.; Zhao, S.; Zhang, X.; Li, L.; Wang, D.; Liu, L.; Gao, D. Diagnostic and Therapeutic Nanoenzymes for Enhanced Chemotherapy and Photodynamic Therapy. J. Mater. Chem. B 2021, 9, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L. Photodynamic Combinational Therapy in Cancer Treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P.; et al. Photodynamic Immunotherapy of Cancers Based on Nanotechnology: Recent Advances and Future Challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Vandongen, G.; Visser, G.; Vrouenraets, M. Photosensitizer-Antibody Conjugates for Detection and Therapy of Cancer. Adv. Drug Deliv. Rev. 2004, 56, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low Dose Cyclophosphamide: Mechanisms of T Cell Modulation. Cancer Treat. Rev. 2016, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Mroz, P.; Chung, H.; Kawakubo, M.; Wolf, P.; Hamblin, M.R. Photodynamic Therapy plus Regulatory T-Cell Depletion Produces Immunity against a Mouse Tumour That Expresses a Self-Antigen. Br. J. Cancer 2013, 109, 2167–2174. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic Therapy plus Low-Dose Cyclophosphamide Generates Antitumor Immunity in a Mouse Model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Jiang, G.; Liang, M.; Chen, C.; Yang, Z.; Zou, L. Progress in Advanced Nanotherapeutics for Enhanced Photodynamic Immunotherapy of Tumor. Theranostics 2022, 12, 5272–5298. [Google Scholar] [CrossRef]
- Li, S.; Wang, D.; Cheng, J.; Sun, J.; Kalvakolanu, D.V.; Zhao, X.; Wang, D.; You, Y.; Zhang, L.; Yu, D. A Photodynamically Sensitized Dendritic Cell Vaccine That Promotes the Anti-Tumor Effects of Anti-PD-L1 Monoclonal Antibody in a Murine Model of Head and Neck Squamous Cell Carcinoma. J. Transl Med. 2022, 20, 505. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Z.; Pratx, G.; Chen, X.; Chen, H. Nanoscintillator-Mediated X-Ray Induced Photodynamic Therapy for Deep-Seated Tumors: From Concept to Biomedical Applications. Theranostics 2020, 10, 1296–1318. [Google Scholar] [CrossRef]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef]
- Chen, Y.S.; Peng, Y.B.; Yao, M.; Teng, J.P.; Ni, D.; Zhu, Z.J.; Zhuang, B.F.; Yang, Z.Y. Cisplatin and Photodynamic Therapy Exert Synergistic Inhibitory Effects on Small-Cell Lung Cancer Cell Viability and Xenograft Tumor Growth. Biochem. Biophys. Res. Commun. 2017, 487, 567–572. [Google Scholar] [CrossRef]
- Anand, S.; Honari, G.; Hasan, T.; Elson, P.; Maytin, E.V. Low-Dose Methotrexate Enhances Aminolevulinate-Based Photodynamic Therapy in Skin Carcinoma Cells in vitro and in vivo. Clin. Cancer Res. 2009, 15, 3333–3343. [Google Scholar] [CrossRef]
- Sinha, A.K.; Anand, S.; Ortel, B.J.; Chang, Y.; Mai, Z.; Hasan, T.; Maytin, E.V. Methotrexate Used in Combination with Aminolaevulinic Acid for Photodynamic Killing of Prostate Cancer Cells. Br. J. Cancer 2006, 95, 485–495. [Google Scholar] [CrossRef]
- Salva, K.A.; Wood, G.S. Epigenetically Enhanced Photodynamic Therapy (EPDT) Is Superior to Conventional Photodynamic Therapy for Inducing Apoptosis in Cutaneous T-Cell Lymphoma. Photochem. Photobiol. 2015, 91, 1444–1451. [Google Scholar] [CrossRef]
- Salva, K.A.; Kim, Y.H.; Rahbar, Z.; Wood, G.S. Epigenetically Enhanced PDT Induces Significantly Higher Levels of Multiple Extrinsic Pathway Apoptotic Factors than Standard PDT, Resulting in Greater Extrinsic and Overall Apoptosis of Cutaneous T-Cell Lymphoma. Photochem. Photobiol. 2018, 94, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, Y.; Zhao, Y.; Yu, H.; Zhou, H.; Chen, M. Polymeric Mixed Micelles Loaded Mitoxantrone for Overcoming Multidrug Resistance in Breast Cancer via Photodynamic Therapy. Int. J. Nanomed. 2017, 12, 6595–6604. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA Approval for Glioma Surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Pepa, G.M.D.; Menna, G.; Olivi, A. Technical Pearls to Effectively Use 5-ALA in Fluorescence-Guided Tumor Resection—5 Lessons from the Operating Room. Brain Sci. 2023, 13, 411. [Google Scholar] [CrossRef]
- Lakomkin, N.; Van Gompel, J.J.; Post, K.D.; Cho, S.S.; Lee, J.Y.K.; Hadjipanayis, C.G. Fluorescence Guided Surgery for Pituitary Adenomas. J. Neurooncol. 2021, 151, 403–413. [Google Scholar] [CrossRef]
- Cho, S.S.; Lee, J.Y.K. Intraoperative Fluorescent Visualization of Pituitary Adenomas. Neurosurg. Clin. N. Am. 2019, 30, 401–412. [Google Scholar] [CrossRef]
- Schupper, A.J.; Rao, M.; Mohammadi, N.; Baron, R.; Lee, J.Y.K.; Acerbi, F.; Hadjipanayis, C.G. Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery. Front. Neurol. 2021, 12, 682151. [Google Scholar] [CrossRef]
- Harada, Y.; Murayama, Y.; Takamatsu, T.; Otsuji, E.; Tanaka, H. 5-Aminolevulinic Acid-Induced Protoporphyrin IX Fluorescence Imaging for Tumor Detection: Recent Advances and Challenges. Int. J. Mol. Sci. 2022, 23, 6478. [Google Scholar] [CrossRef] [PubMed]
- Casas, A. Clinical Uses of 5-Aminolaevulinic Acid in Photodynamic Treatment and Photodetection of Cancer: A Review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Li, X.; Zhao, C.; Kou, H.; Zhu, F.; Yang, Y.; Lu, Y. PDD-Guided Tumor Excision Combined with Photodynamic Therapy in Patients with Extramammary Paget’s Disease. Photodiagnosis Photodyn. Ther. 2022, 38, 102841. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bulin, A.-L.; Hurbin, A.; Elleaume, H.; Coll, J.-L.; Broekgaarden, M. Photodynamic Diagnosis and Therapy for Peritoneal Carcinomatosis: Emerging Perspectives. Cancers 2020, 12, 2491. [Google Scholar] [CrossRef] [PubMed]
- Fayad, A.; Ansari, M.T.; Yang, H.; Ruddy, T.; Wells, G.A. Perioperative Diastolic Dysfunction in Patients Undergoing Noncardiac Surgery Is an Independent Risk Factor for Cardiovascular Events. Anesthesiology 2016, 125, 72–91. [Google Scholar] [CrossRef]
- Nompumelelo Simelane, N.W.; Kruger, C.A.; Abrahamse, H. Photodynamic Diagnosis and Photodynamic Therapy of Colorectal Cancer in vitro and in vivo. RSC Adv. 2020, 10, 41560–41576. [Google Scholar] [CrossRef]
- Fukuhara, H.; Yamamoto, S.; Karashima, T.; Inoue, K. Photodynamic Diagnosis and Therapy for Urothelial Carcinoma and Prostate Cancer: New Imaging Technology and Therapy. Int. J. Clin. Oncol. 2021, 26, 18–25. [Google Scholar] [CrossRef]
- Alekseeva, P.M.; Efendiev, K.T.; Shiryaev, A.A.; Rusakov, M.A.; Simonova, M.S.; Samoylova, S.I.; Fatyanova, A.S.; Reshetov, I.V.; Loschenov, V.B. Sublingual Administration of 5-Aminolevulinic Acid for Laser-Induced Photodiagnostics and Photodynamic Therapy of Oral Cavity and Larynx Cancers. Photodiagnosis Photodyn. Ther. 2021, 34, 102289. [Google Scholar] [CrossRef]
- Arens, C.; Reußner, D.; Woenkhaus, J.; Leunig, A.; Betz, C.S.; Glanz, H. Indirect Fluorescence Laryngoscopy in the Diagnosis of Precancerous and Cancerous Laryngeal Lesions. Eur. Arch. Otorhinolaryngol. 2007, 264, 621–626. [Google Scholar] [CrossRef]
- Fukuhara, H.; Inoue, K.; Kurabayashi, A.; Furihata, M.; Shuin, T. Performance of 5-Aminolevulinic-Acid-Based Photodynamic Diagnosis for Radical Prostatectomy. BMC Urol. 2015, 15, 78. [Google Scholar] [CrossRef]
- Ahmad, S.; Aboumarzouk, O.; Somani, B.; Nabi, G.; Kata, S.G. Oral 5-Aminolevulinic Acid in Simultaneous Photodynamic Diagnosis of Upper and Lower Urinary Tract Transitional Cell Carcinoma-A Prospective Audit. BJU Int. 2012, 110, E596–E600. [Google Scholar] [CrossRef]
- Wyss, P.; Degen, A.; Caduff, R.; Hornung, R.; Haller, U.; Fehr, M. Fluorescence Hysteroscopy Using 5-Aminolevulinic: A Descriptive Study. Lasers Surg. Med. 2003, 33, 209–212. [Google Scholar] [CrossRef]
- Regis, C.; Collinet, P.; Farine, M.O.; Mordon, S. Comparison of Aminolevulinic Acid- and Hexylester Aminolevulinate-Induced Protoporphyrin IX Fluorescence for the Detection of Ovarian Cancer in a Rat Model. Photomed. Laser Surg. 2007, 25, 304–311. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.D.; Keating, J.; Newton, A.; Corbett, C.; Xia, L.; Shin, M.; Frenzel Sulyok, L.; Deshpande, C.; Litzky, L.; Nie, S.; et al. A Clinical Trial of Intraoperative Near Infrared Imaging to Assess Tumor Extent and Identify Residual Disease during Anterior Mediastinal Tumor Resection. Cancer 2019, 125, 807. [Google Scholar] [CrossRef]
- Predina, J.D.; Newton, A.D.; Corbett, C.; Xia, L.; Shin, M.; Sulfyok, L.F.; Okusanya, O.T.; Cengel, K.A.; Haas, A.; Litzky, L.; et al. A Clinical Trial of Tumor Glow to Identify Residual Disease during Pleurectomy and Decortication. Ann. Thorac. Surg. 2019, 107, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.D.; Predina, J.D.; Nie, S.; Low, P.S.; Singhal, S. Intraoperative Fluorescence Imaging in Thoracic Surgery. J. Surg. Oncol. 2018, 118, 344. [Google Scholar] [CrossRef] [PubMed]
- Azari, F.; Kennedy, G.T.; Zhang, K.; Bernstein, E.; Maki, R.G.; Gaughan, C.; Jarrar, D.; Pechet, T.; Kucharczuk, J.; Singhal, S. Impact of Intraoperative Molecular Imaging after Fluorescent-Guided Pulmonary Metastasectomy for Sarcoma. J. Am. Coll. Surg. 2022, 234, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.K.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas during Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef]
- Pace, J.; Ivich, F.; Marple, E.; Niedre, M. Near-Infrared Diffuse in vivo Flow Cytometry. J. Biomed. Opt. 2022, 27, 097002. [Google Scholar] [CrossRef]
- Kennedy, G.T.; Azari, F.S.; Bernstein, E.; Nadeem, B.; Chang, A.; Segil, A.; Sullivan, N.; Encarnado, E.; Desphande, C.; Kucharczuk, J.C.; et al. Targeted Detection of Cancer Cells during Biopsy Allows Real-Time Diagnosis of Pulmonary Nodules. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4194–4204. [Google Scholar] [CrossRef] [PubMed]
- Sulek, J.E.; Steward, J.E.; Bahler, C.D.; Jacobsen, M.H.; Sundaram, A.; Shum, C.F.; Sandusky, G.E.; Low, P.S.; Sundaram, C.P. Folate-Targeted Intraoperative Fluorescence, OTL38, in Robotic-Assisted Laparoscopic Partial Nephrectomy. Scand. J. Urol. 2021, 55, 331–336. [Google Scholar] [CrossRef]
- Dindere, M.E.; Tanca, A.; Rusu, M.; Liehn, E.A.; Bucur, O. Intraoperative Tumor Detection Using Pafolacianine. Int. J. Mol. Sci. 2022, 23, 2842. [Google Scholar] [CrossRef]
- Predina, J.D.; Okusanya, O.; Newton, A.D.; Low, P.; Singhal, S. Standardization and Optimization of Intraoperative Molecular Imaging for Identifying Primary Pulmonary Adenocarcinomas. Mol. Imaging Biol. 2018, 20, 131–138. [Google Scholar] [CrossRef]
- Cramer Ahrens, L.; Green Krabbenhøft, M.; Würgler Hansen, R.; Mikic, N.; Pedersen, C.B.; Poulsen, F.R.; Korshoej, A.R. Effect of 5-Aminolevulinic Acid and Sodium Fluorescein on the Extent of Resection in High-Grade Gliomas and Brain Metastasis. Cancers 2022, 14, 617. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Nakamura, T.; Oinuma, T.; Yamagishi, H.; Masuyama, H.; Terano, A. Evaluation of a Novel High-Resolution Magnifying Videoendoscope That Is Capable of Photodynamic Diagnosis and Therapy for Gastric Cancer. Photodiagnosis Photodyn. Ther. 2015, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Hsiao, Y.C.; Chiang, Y.F.; Chang, C.J. Topical Application of Photofrin® for Photodynamic Diagnosis of Malignant Cutaneous Neoplasms. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J.; Fukami, S.; Ichikawa, M.; Nagai, K.; Kohno, M. Preliminary Report: Rapid Intraoperative Detection of Residual Glioma Cell in Resection Cavity Walls Using a Compact Fluorescence Microscope. J. Clin. Med. 2021, 10, 5375. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oinuma, T. Usefulness of Photodynamic Diagnosis and Therapy Using Talaporfin Sodium for an Advanced-Aged Patient with Inoperable Gastric Cancer (a Secondary Publication). Laser Ther. 2014, 23, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nishie, H.; Kataoka, H.; Yano, S.; Kikuchi, J.I.; Hayashi, N.; Narumi, A.; Nomoto, A.; Kubota, E.; Joh, T. A Next-Generation Bifunctional Photosensitizer with Improved Water-Solubility for Photodynamic Therapy and Diagnosis. Oncotarget 2016, 7, 74259–74268. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.; Dhillon, A.K.; Sharma, A.; Ahuja, T.; Siddhanta, S. Emergence of Raman Spectroscopy as a Probing Tool for Theranostics. Nanotheranostics 2023, 7, 216. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Khabibullin, V.R.; Usoltseva, L.O.; Vyrko, E.A.; Mikheev, I.V.; Volkov, D.S. Photothermal and Optoacoustic Spectroscopy: State of the Art and Prospects. Phys. Uspekhi 2022, 65, 270–312. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Surmacki, J.; Jablonska-Gajewicz, J.; Kordek, R. Raman “optical Biopsy” of Human Breast Cancer. Prog. Biophys. Mol. Biol. 2012, 108, 74–81. [Google Scholar] [CrossRef]
- Brozek-Pluska, B.; Kopec, M. Raman Microspectroscopy of Hematoporphyrins. Imaging of the Noncancerous and the Cancerous Human Breast Tissues with Photosensitizers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 169, 182–191. [Google Scholar] [CrossRef]
- Horgan, C.C.; Bergholt, M.S.; Nagelkerke, A.; Thin, M.Z.; Pence, I.J.; Kauscher, U.; Kalber, T.L.; Stuckey, D.J.; Stevens, M.M. Integrated Photodynamic Raman Theranostic System for Cancer Diagnosis, Treatment, and Post-Treatment Molecular Monitoring. Theranostics 2021, 11, 2006–2019. [Google Scholar] [CrossRef]
- Acunto, M.D. Detection of Intracellular Gold Nanoparticles: An Overview. Materials 2018, 11, 882. [Google Scholar] [CrossRef]

| Substance | The Stage of the Research | Types of Cancer |
|---|---|---|
| 5-Aminolevulinic acid | Registered or clinical use | High-grade glioma Bladder cancer (HAL) |
| Clinical trial | EV-tumor-derived extracellular vesicles Brain cancer other than HGG and metastasis to brain Upper urinary tract and prostate Liver cancers Gastric cancers Peritoneal metastasis Gastric cancer | |
| Indocyanine green (ICG) | Clinical trials | Hepatocellular carcinoma (HCC) Colorectal and pancreatic cancer Liver metastases Sentinel lymph nodes in breast cancer and melanoma |
| EC17 | Clinical trials | Glioma Ovarian cancer NSCLC Breast cancer Fr-alfa-rich cancerous cells |
| OTL38 | Registered for clinical use | Ovarian cancer (CYTALUX) |
| In vivo—animals | FR-positive metastatic cells etc.—circulating tumor cells Lung cancer Renal cancer Pituitary tumors Gastric cancer | |
| Fluorescein sodium | Off-label clinically used | Glioma |
| clinical trial | Brain metastases Meningiomas Lymphomas Pituitary tumors Pediatric brain stem Spinal cord tumors | |
| Porfimer sodium (Photofrin) | Clinical trial | Esophageal cancer Gastric cancer Skin cancer |
| Talaporfin sodium (contains Ce6) | Clinical trial | Malignant glioma Gastric cancer |
| O-chlorin | In vivo, in vitro | Yet to be determined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszowy, M.; Nowak-Perlak, M.; Woźniak, M. Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment. Pharmaceutics 2023, 15, 1712. https://doi.org/10.3390/pharmaceutics15061712
Olszowy M, Nowak-Perlak M, Woźniak M. Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment. Pharmaceutics. 2023; 15(6):1712. https://doi.org/10.3390/pharmaceutics15061712
Chicago/Turabian StyleOlszowy, Marta, Martyna Nowak-Perlak, and Marta Woźniak. 2023. "Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment" Pharmaceutics 15, no. 6: 1712. https://doi.org/10.3390/pharmaceutics15061712
APA StyleOlszowy, M., Nowak-Perlak, M., & Woźniak, M. (2023). Current Strategies in Photodynamic Therapy (PDT) and Photodynamic Diagnostics (PDD) and the Future Potential of Nanotechnology in Cancer Treatment. Pharmaceutics, 15(6), 1712. https://doi.org/10.3390/pharmaceutics15061712






