3.1. Density
Given that density is a fundamental property that can be easily measured and has the potential to serve as a predictor for other properties of small molecule-based in situ forming systems [
3,
7], such as injectability, viscosity, and matrix formation. Moreover, the density of the preparations was the crucial parameter for surface tension measurement. In addition, the density greater than the density of water is a confirmation that the formulation will settle down in the periodontal pocket throughout the administration. Such properties give the formulation a chance to adhere firmly in the periodontal pocket, resulting in effective treatment [
3]. The density of all formulations was measured and reported in
Table 3. The density of all formulations was greater than that of water (1.00 g/cm
3); thus, they are possible to firmly adhere in the pocket. The inclusion of borneol in the system resulted in a lower density compared to the solution comprising only the drug in NMP (control group). Typically, the density of ISM (solution state) is the sum of the densities of each component based on their respective mass fractions [
35]. The substitution of NMP with borneol addition was responsible for the reduced density of F1. Borneol was utilized as a matrix formation agent resulting in a comparable density to that of other small molecule-based in situ forming systems such as fatty acids-based in situ forming systems. The study demonstrated that the density of fatty acids-based in situ forming system with a concentration of 35%
w/
w varied between 1.0109 and 1.0240 g/cm
3, which depended on the length of the aliphatic chain. Specifically, the fatty acids with shorter aliphatic chains exhibited higher densities in comparison to those with longer chains [
7]. Moreover, This study conducted on the borneol-based ISM revealed that the density values varied with the amount of triacetin used. Namely, an increasing trend in density was observed with an increasing ratio of triacetin (
p < 0.05), which was attributed to the inherent density of triacetin and the reduced proportion of NMP in the formulations.
Furthermore, the correlation between density and the values of viscosity and surface tension was observed, with the exclusion of F1 which was non-ISM (control). This finding corroborates the prior research indicating that density may serve as a viable means of approximating the basic characteristics of any in situ forming system using small molecules as structural forming agents. Nevertheless, it presented a constraint. As mentioned before, density is a sum of density values for each of the components. Hence, in order to derive estimations for additional properties through the utilization of density, the variable factor should be limited, only one variable is allowed to be altered. It should be compared within the same system of preparation. The non-ISM solution (without matrix former) or ISM with a different matrix former should not be compared. For example, the inclusion of F1 in determining the relationship between density and other properties was found to be inadequate due to its inability to establish a correlation as it did not have an in situ forming system. Particularly, it had two variable factors: borneol concentration and triacetin concentration.
3.2. Viscosity
The viscosity of all ISM formulations and relative solvents is shown in
Table 3. Typically, NMP is a low viscous organic solvent; thus, ISM formulation containing NMP exhibited low viscosity. The viscosity of borneol-based ISM was rather higher than that of borneol-free formulation due to a strong hydrophilic interaction between borneol and solvent as well as the reduction of the solvent in the formulation [
3]. While Triacetin is a hydrophobic triglyceride ester with a viscosity of 25 cPs. Consequently, the addition of triacetin to the prepared ISM usually resulted in a slight increase in viscosity. The augmentation of the triacetin proportion resulted in a substantial rise in viscosity (
p < 0.05), suggesting the potential to regulate diverse ISM phenomena, including solvent-exchange, self-assembly, and drug release management. The ease of solvent and anti-solvent migration was influenced by viscosity. The formulation with high viscosity exhibited a slow solvent migration, leading to a decelerated self-assembly phenomenon [
11,
12]. In the presented borneol, the viscosity of the solution was increased [
3]. The elevated proportion of triacetin led to an increase in the viscosity of the solution, which was related to the inherent viscosity of triacetin. According to the H-bond interaction of each molecule, increase in viscosity was observed [
25]. Triacetin, which contains multiple functional groups capable of forming H-bonds, exhibited intermolecular interactions due to the high density of triacetin-loaded preparations.
Although the system viscosity was high by the increasing of triacetin, all preparations were deemed suitable for injection because their viscosity was less than 20 cPs [
11]. The developed system presented with a lower viscosity than the hydrogel-based injectable in situ forming system [
36]. Furthermore, the viscosity was lower than that of other injectable thermo-responsive in situ forming gel systems, such as carbopol-poloxamer gels, which have viscosities in the range of 19,000–36,000 cPs at 4 °C [
37,
38]. The low viscous of the borneol solution was due to the fact that it was low density solution and the borneol has a small molecular size with simple structure resulting in less molecular interactions. Rheological character was an important parameter for the ISM as it indicates ease of drug administration through a syringe [
1]. The exponential constant of F1, F2, F3, and F4 was 0.96, 0.98, 0.98, and 0.97, respectively. They were close to 1.00 indicating the viscosity of the formulation remains constant even with increasing shear rate. These indicated Newtonian flow behavior, which the shear rate was directly proportional to the shear stress. This flow behavior was suitable for ISM because the formulation requires force to be expelled from the syringe via a small needle. Namely, the force used for injection does not affect the viscosity change and leads to in ease of administration [
39]. Moreover, it was reported that the temperature did not change the flow behavior of polymer-based ISM but decreased shear stress in ranges of 25–37 °C [
40].
Hence, the fluid state of the developed systems, which was less viscous which refers to a high injectability, provided the potential to minimize the painful application that can occur during injection through the needle and the ease of use.
3.4. Self-Formation Ability of ISMs
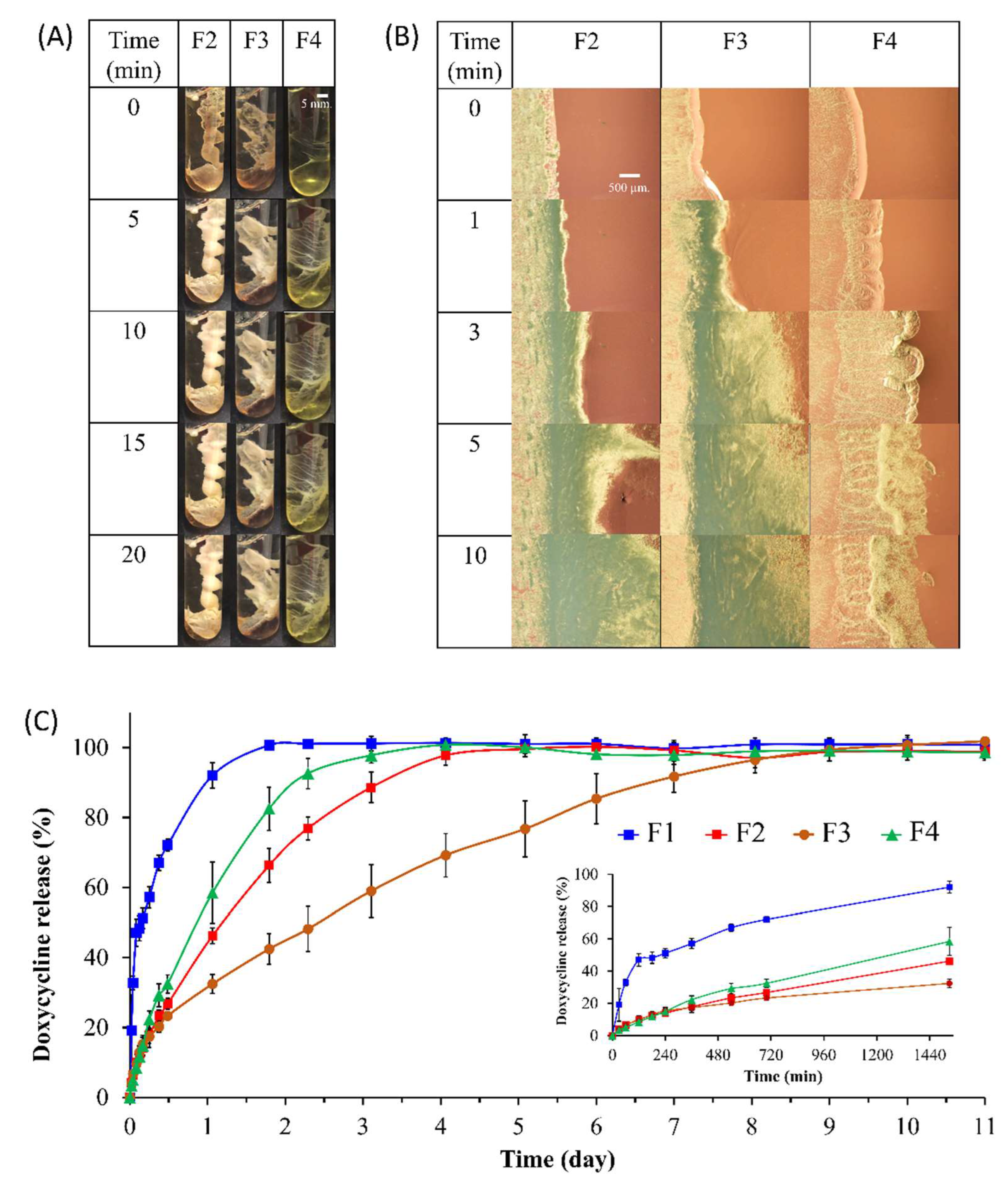
The self-formation behavior, which is the essential performance of the in situ forming system, was evaluated. The gel-like state of the prepared formulations upon injection into the PBS pH 6.8 buffer at several time points is shown in
Figure 2A where the opaque referred to the completeness of formation [
8]. Usually, the prepared borneol-based ISMs were homogeneous yellowish with clear solutions. As it was contacted with PBS pH 6.8, the yellowish ISM of the solution state was transformed into a white color indicating a gel-like white matrix formation. The white matrix was occurred from the white borneol crystals which precipitated through a phase separation process. However, the formulations that slow transformation rate or incomplete matrix formation may appear the yellowish color from the doxycycline. The gelling time of F2, F3, and F4 after transforming from the transparent ISM solution into the opaque white matrix was 5 min, 5 min, and >20 min, respectively. The borneol-based ISMs formulations had compact surfaces, whereas the increased triacetin formulations contained a soft surface and yellowish color indicating that triacetin could affect the formation behavior. Triacetin significantly delayed the completeness of ISMs matrix formation. The high triacetin-loaded ISM (F4) showed a slow matrix formation and could not transform to the solid-like matrix within 20 min. Whereas triacetin-free ISM (F2) and low triacetin-loaded ISM (F3) could change to the solid opaque matrix within 5 min, which tended to be possible to control the drug release. These are relevant to the viscosity value of the preparation, where the high viscosity resulted in the difficult migration of environment fluid inward and system fluid outward. The high triacetin ratio preparation (F4) showed limited formation due to the following reasons: Hydrophobic solvents, such as triacetin, have been observed to facilitate a slow phase inversion process [
41]. It was reported that solvents with a water solubility below 7%
w/
w have been demonstrated to cause a decrease in water uptake, leading to a slower drug release [
42]. The gel formation process was observed to be slowed down due to the hydrophobic nature of the triacetin (compared to NMP), which hindered water penetration. Moreover, at high levels of triacetin concentration, the solvent exchange was slower than the interfacial network formation. Subsequently, the liquid migration was blocked as the interfacial network of the initiated borneol matrix became denser with time with less porosity and high tortuosity. It was reported that the compact gelation structure resulted in a decrease in the coefficient of water diffusion [
43].
Given that fluid migration is prohibited, the solvent exchange mechanism is stopped. The self-formation of small molecule-based in situ forming systems stays in progress. The other mechanism, the continuous growth of crystals, occurred as a second mechanism. This rate of secondary self-formation was dependent on the ability of borneol crystallization in a given triacetin ratio at a given time. Theoretically, any unmixable object has an energy at its boundaries that enables nucleation and subsequently crystallization through a thermodynamic driving force [
44]. The thermodynamic stability of a system can be disrupted by a variety of factors, including the introduction of new interfacial energy from sources such as the initial crystal, aqueous surface, and bubble surface, as well as supersaturation resulting from solvent reduction. As the thermodynamics are disturbed, the system will stabilize itself by activating crystal formation [
45]. Therefore, surface tension which is related to the activating key of crystallization in thermodynamics term, surface free energy, was evaluated. Namely, the high surface tension results in ease of nucleation. As mentioned above, not only viscosity might affect the formation behavior, but also surface tension. The high portion of triacetin such as F4 showed a low surface tension which resulted in less thermodynamics driven energy. Subsequently, the nucleation was slower than that of less triacetin preparation including F2 and F3.
Not only how fast nucleation and crystallization need to be concerned, but also the number of nucleation origins at initial the time point. The high number of nucleation origin resulting in less porosity and high tortuosity. While the less nucleation origin showed less tortuosity [
25]. Finally, as the formation behavior changes, the release of drugs might be affected [
12]. However, it should be noted that the suitable formation speed depends on the purpose: burst control release, immediate release, sustaining release, etc. These statistics only provide management options. The suitable preparation properties are neither a fast formation nor a slow formation. For example, too fast formation may result in non-fitting with the target shape, while too slow formation may result in leakage from the target site. Hence, it is possible to predict the formation behavior for future research or industrial product development by utilizing essential physical properties such as viscosity and surface tension. The use of density is likewise relevant as it facilitates the prediction of both viscosity and surface tension.
3.5. Interfacial Network Formation of ISMs
To verify the impact of the interfacial network on the influx/outflux of relevant fluids and the formation rate. The boundary between preparations and PBS pH 6.8 (a representation of biological aqueous fluid) was tracked to achieve interfacial network formation as shown in
Figure 2B. The aqueous phase was placed on the right side, while the preparations were located on the left.
The absence of triacetin facilitated rapid networking at the interfacial boundary. As can be seen, the F2 formulation (triacetin-free ISM) had more formation at the third minute than the F3 formulation.
Solvent exchange was hampered when the self-networking at the interfacial boundary was occurred. However, the F2 formulation continued to undergo self-formation over time. This could be due to several reasons, including the presence of enough porous to allow the related fluid to exchange or the occurrence of a secondary mechanism such as crystal growth as mentioned. In practice, both of the aforementioned factors occurred concurrently. Particularly, the exchange cannot be completely blocked at the given time otherwise, the drug would be unable to be released from the system. Due to thermodynamic instability, borneol is precipitated. It is probable that both mechanisms were concomitant, albeit one mechanism may have exhibited greater salience at a particular time. Nonetheless, both mechanisms had an end-point. Regarding the first mechanism, solvent exchange, the solvent either underwent complete diffusion out of the system or encountered significant hindrance in its diffusion, leading to a state of negligible diffusivity [
46]. The second mechanism, crystal growth, terminated when the thermodynamics became stable [
44,
45]. These findings suggested that borneol-based ISMs had two mechanisms for self-formation.
The highest triacetin ratio preparation, F4, behaved very differently than the low triacetin formulation. The results were according to the previous discussion, the low surface tension formula (F4) showed less self-formation than the high surface tension formula (F2) due to the lower activating energy. The high viscosity of F4 was the other reason that resulted in less solvent exchange.
Relying solely on a standalone parameter for prediction can result in errors. Precisely, because the surface tension of F3 was lower than that of F2, the transformation of F2 should be faster. But the self-formation of F3 was greater than F2. This indicated that several parameters should be considered. The initial boundary network formation of F3 was faster than that of F2 because the viscosity of the solution and hydrophobicity of triacetin retarded the solvent exchange, resulting in a less dense network at a given time [
41]. The less dense initial network refers to the higher porosity, which allowed the processing of solvent exchange. Namely, at the initial time, the F3 possessed two mechanisms that synergized with each other to generate the surface network. While the less porosity of F2, the second mechanism dominated the first mechanism because the solvent exchange was prohibited.
3.6. In Vitro Drug Release
The formation of a matrix is an essential prerequisite for achieving a sustaining release of drugs from an in situ formation system. According to
Section 2.6, the standard curve of doxycycline was performed in the range of 1 to 50 µg/mL. The absorbance was linearly related to the concentration of doxycycline, with the equation of absorbance = 0.0248 × (concentration) + 0.002 and r
2 = 0.9999). The drug release study was performed in which 5%
w/
w doxycycline hyclate in NMP (Control, F1) and ISMs preparations (F2, F3, and F4) are selected. The release profile of all formulations are shown in
Figure 2C. The result indicated that the F1 exhibited characteristics of a drug release system that was non-sustained. Doxycycline hyclate in F1 was completely released within 2 h with a burst release at the initial phase of the drug, whereas F4, F2, and F3 presented a prolonged release up to 4, 5, and 10 h, respectively. The F3 showed none of the initial burst release as presented in
Figure 2C. These improvements corresponded to the formation of borneol matrix and the triacetin loading. Namely, the drug slowly migrated through the borneol solid matrix structure since the drug was entrapped in the formed matrix. Borneol has been used as matrix former for control the drug release [
3]. Currently, prolonging the release of drugs is a new challenge in the development of drug delivery systems. A possible approach for managing this issue is to diminish the hydrophilicity of ISM system by adding a hydrophilic substance [
47]. Triacetin has been used as a hydrophobic solvent in polymer-based ISM to slow phase inversion, limiting solvent diffusion and retard the drug release [
24,
47]. However, the proper amount of triacetin added is an important consideration [
24]. The different release profile from each matrix structure was due to the different interior structure, where the high tortuosity and less porosity provided the high ability to sustain the release of drugs. Because the high tortuosity and less porosity structure prolonged the migration of fluid, the drug diffusion with water was relied on this reason [
3,
25]. The release profile of F1, F2, and F4 was fitted well with the first order equation (r
2 = 0.9831, 0.9889, and 0.9943 respectively). Interestingly, F3 release profile showed none of the initial burst release and was fitted with the Higuchi equation (r
2 = 0.9969). The expedition of drug through the tortuosity of the triacetin added-hydrophobic borneol matrix, which accounted for the distance to the surface of the drug dissolution region, was claimed [
48]. Namely, the actives diffusion out was slowed by the internal structure of borneol matrix. Moreover, the F3 release profile also fits well with the Krosmeyer-Peppas equation (r
2 = 0.9974), where
n = 0.51 indicating the anomalous diffusion (
n = 0.45–0.89 for non-swelling system), where a mechanism of drug release is equally influenced by both of the diffusion and structure relaxation [
48]. Although, Fickian diffusion, which structure relaxation shows less effect on drug release ability, often found in a matrix sustained drug delivery system [
49]. The in situ forming system which required a structure formation process could result in a notably relaxation which influenced the release of drug than that of other sustaining drug delivery system.
Moreover, the hydrophobic fluid could retard the diffusion of the aqueous phase in which the drug diffusion was sustained, which corresponds to the report that clove oil could minimize the burst release of the drug [
8]. The deceleration of movement was similarly noted when employing alternative hydrophobic matrix formers, including bleached shellac and cholesterol [
9,
50]. As previously stated, however, the integration of the overall factor is crucial. The utilization of an inappropriate proportion of triacetin generated opposite outcomes. An excessive triacetin ratio delayed matrix formation, leading to a highly porous surface network. This, in turn, facilitated the free-floating movement of the drug with fluid migration, resulting in a burst release. The deficient matrix formation of excessive triacetin preparation exhibited worthlessness in ability to sustain drug release and control burst release effect, comparison to the non-triacetin formula. The non-triacetin formula, F2, showed a burst release due to the fact that the matrix formation occurred rapidly with no prohibition effect. Namely, the only way to escape this rapidly forming matrix barrier of F2 was to move directly and massively; as a result, a large uncomplicated tunnel was formed, resulting in a high burst release and less ability to sustain drugs release.
A large passage might be another reason. It was reported that the rapid crystal formation of crystal-based ISM resulted in a large passage in the matrix. This was due to the smaller number of nucleation origin, the crystal growth from less number of nuclei resulted in large passage. The growth of each crystallization origin that was accompanied by vignettes led to a reduction in porosity and an increase in tortuosity [
7]. F3, in contrast, which had triacetin, required more aqueous fluid influx to induce phase separation. Therefore, at the time point before the separation, a large aqueous-containing volume was obtained in this particular system. This high aqueous portion in the systems resulted in a high number of borneol nuclei, which coincided with the retarded crystal growth rate of each other, subsequently [
47]. Due to the high number of nucleation origins and slow crystallization rate, the F3 interior formed a structure was less porosity and high tortuosity. Hence the sustaining drug release ability was achieved.
3.7. Computational Results of MD Simulation
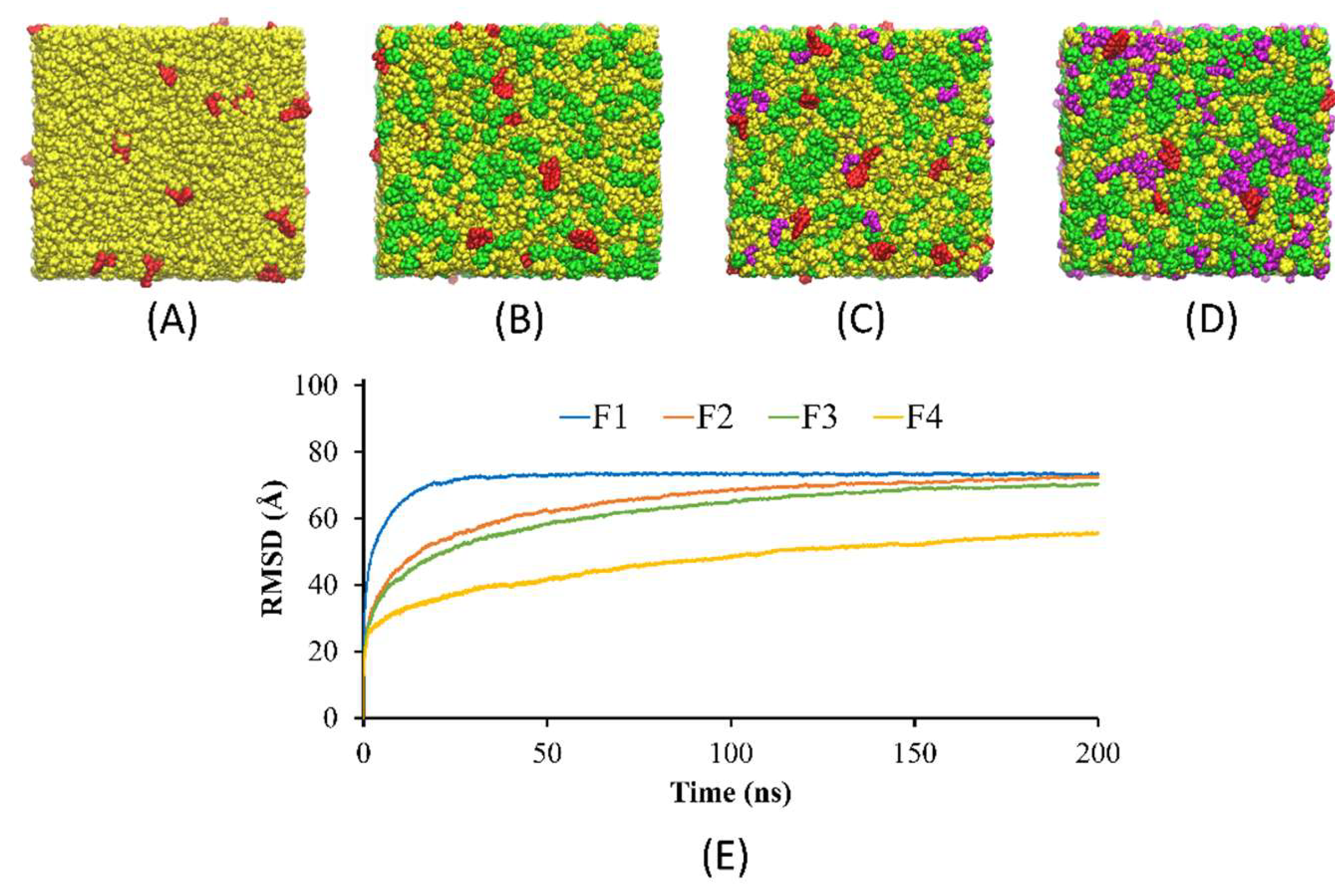
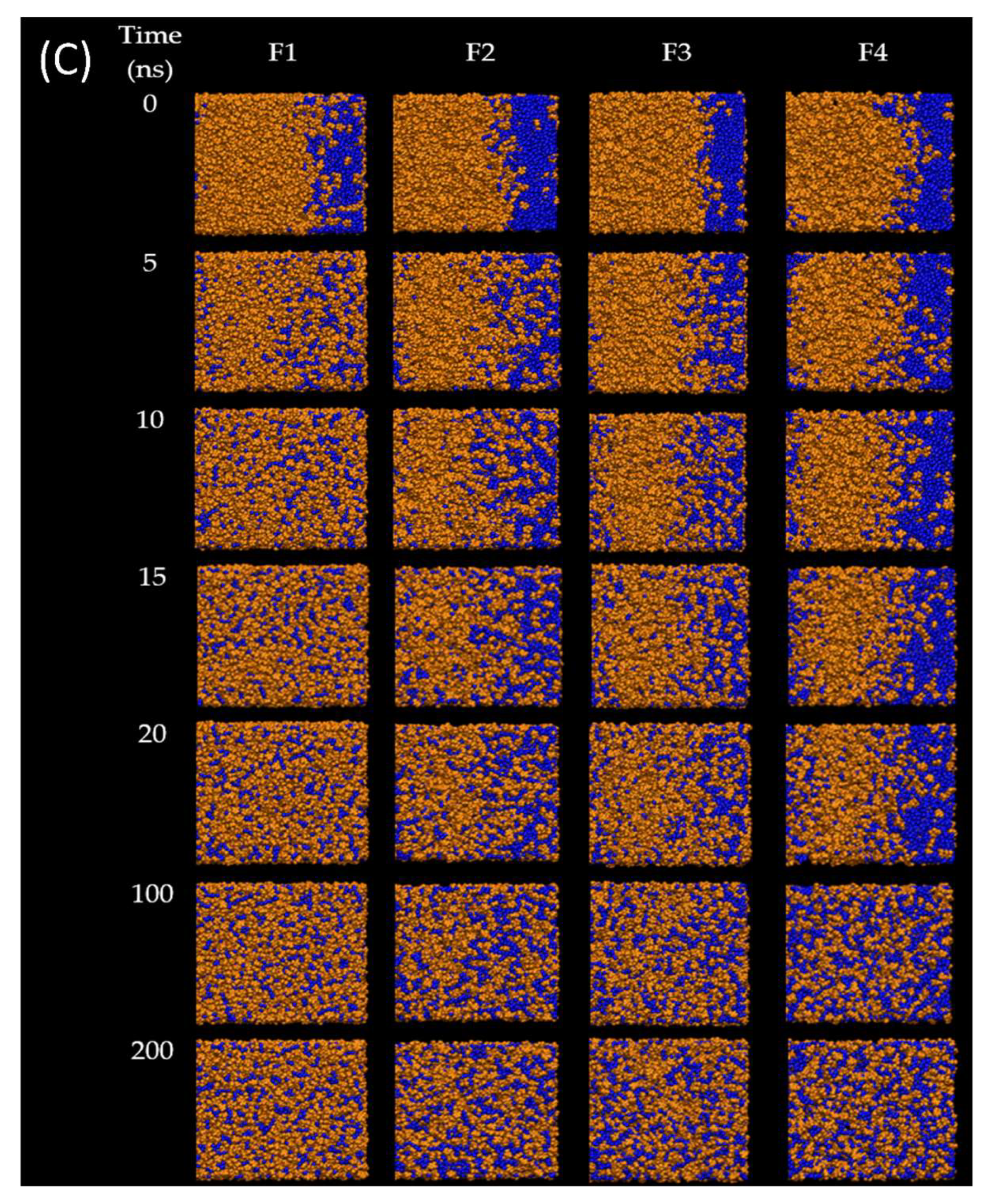
The present study aimed to investigate the solvent exchange mechanism of the borneol-based formulations (F1–F4) containing doxycycline, borneol, triacetin, and NMP molecules at the very first initial time after contact with water. First, the models of F1-F4 were rearranged, simulated, and analyzed. As shown in
Figure 3A–D, the components in the models mixed evenly within 200 ns of simulation time, indicating that all formulations exhibited homogenous formulations, which agreed with the experimental results showing that doxycycline hyclate dissolved and mixed well with other components in all formulations. Furthermore,
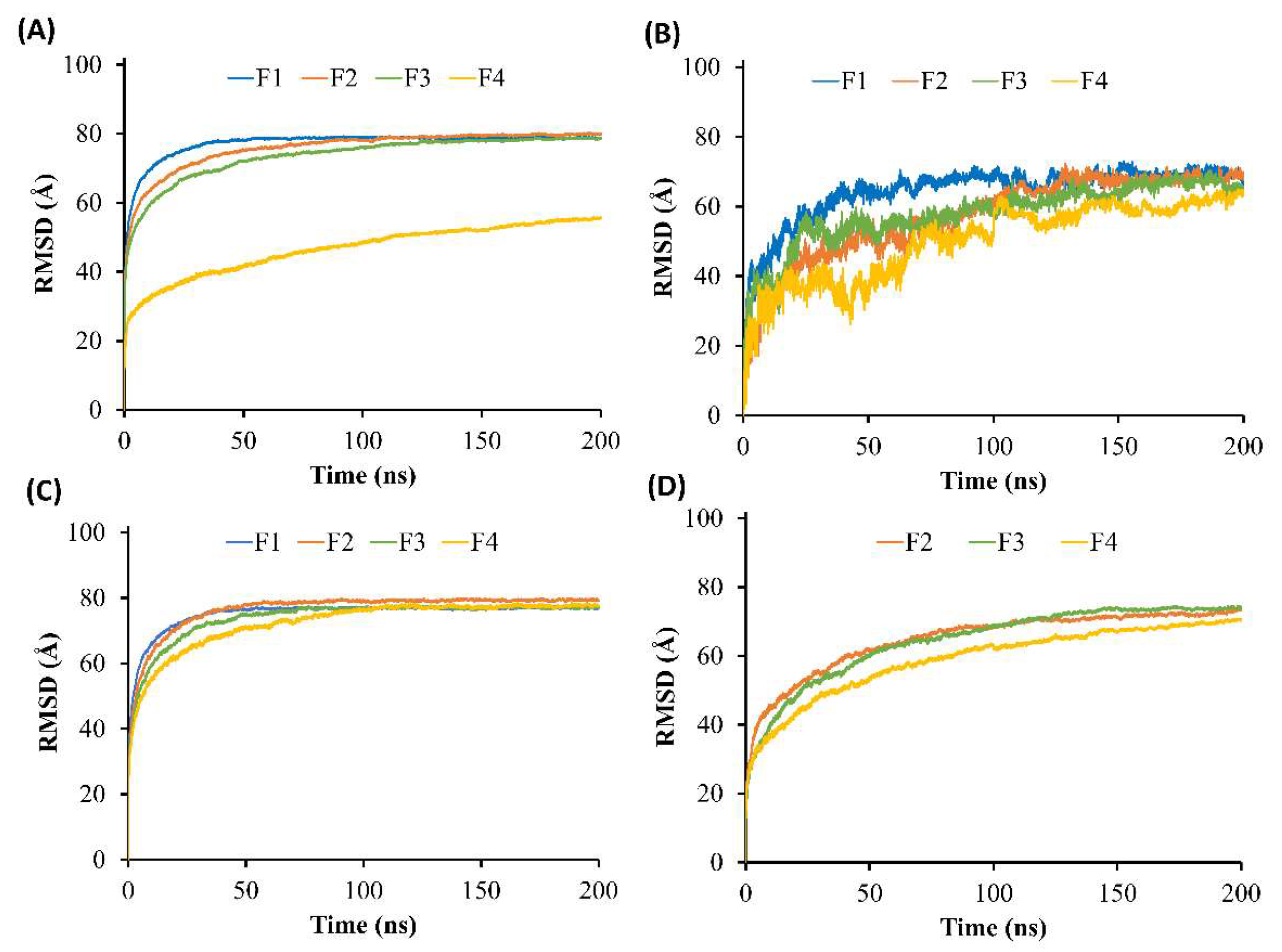
Figure 3E demonstrated that F1 equilibrated after 30 ns and maintained a level of 73 Å, while F2–F4 reached equilibrium after 125 ns and remained constant at 70, 68, and 55 Å, respectively. It can be noticed that F2–F4 took a longer time to equilibrate than F1, which could be due to the interference of the borneol molecules in the system. Moreover, the RMSD of F4 at equilibrium was much lower than those of F1–F3 due to the higher amount of triacetin and also a higher viscosity of F4.
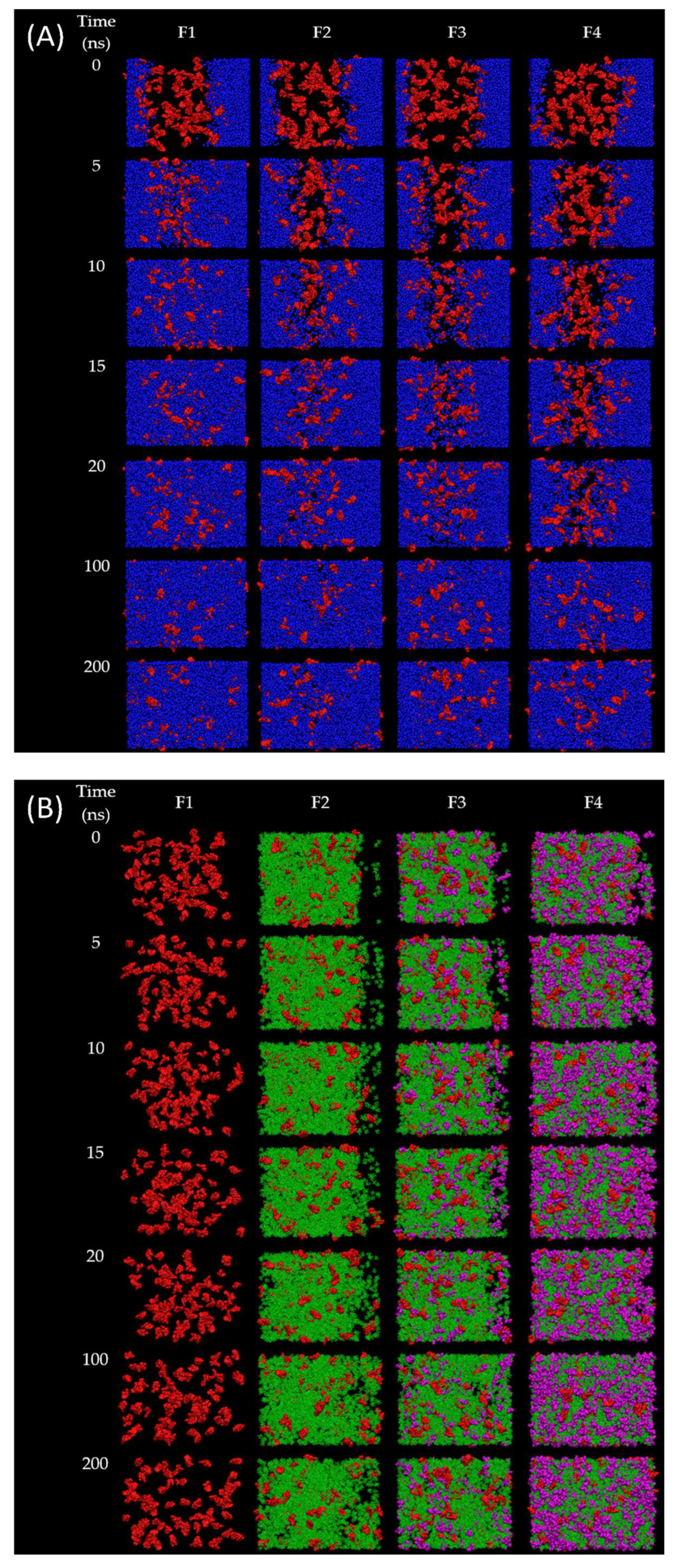
In order to observe the solvent exchanges of borneol-based formulations (F1–F4) upon the diffusion of water, the water-involved dynamic simulations of F1–F4 were performed for 200 ns. The snapshots during simulation time of 0, 5, 10, 15, 20, 100, and 200 ns of F1–F4 after contact with water were displayed in
Figure 4, and their RMSD were displayed in
Figure 5A. In the case of the doxycycline-loaded borneol-free formulation (F1), the water penetration occurred promptly within 5 ns as well as NMP, which mixed well with the water (
Figure 4A,C). The RMSD curve of F1 with water reached equilibrium at about 35 ns and remained at 78 Å (
Figure 5A). The absolute solvent exchange in which the drug was freely migrated suddenly occurred for F1 after contact with water due to a lack of matrix former in the formulation. For F2, water mixed well with NMP within 15 ns, and its RMSD became constant at 78 Å after 90 ns. Moreover, water in the system of F3 could be mixed well with NMP within 20 ns and F3 equilibrated at 78 Å after 125 ns. Whereas, the solvent exchange of F4 occurred within 100 ns and the RMSD of F4 with water increased slowly and attained the steady value of 55 Å in about 180 ns. It can be noticed that F4 with water used the highest simulation time to reach equilibrium, followed by F3, F2, and F1, implying that borneol and triacetin could slow the solvent exchange of the ISM formulation. Apparently, the rate of water infiltration was observed to decrease in correlation with the augmented quantity of triacetin. Moreover, borneol was employed as a matrix former in this study with the purpose of retard the solvent exchange mechanism. Regarding the previous experiments (
Section 3.4,
Section 3.5 and
Section 3.6), the borneol-based formulations, clearly demonstrated a slower matrix formation, resulting in slower migration between water and doxycycline molecules and enabling controlled drug release. Additionally, triacetin, a hydrophobic substance, was used as an additive solvent to interrupt the solvent exchange, as can also be seen from the results mentioned above.
The RMSD of doxycycline in the simulation of F1–F4 models after contact with water was also explored to observe the topography change over time of doxycycline in each system, as shown in
Figure 5B. The RMSD curve of F4 increased and fluctuated for the first 125 ns and became stable at 60 Å after 160 ns. Moreover, other formulations (F1–F3) with water had higher RMSD compared with F4 at the same time. Although the doxycycline in F1–F3 with water models reached equilibrium at a similar value of 69 Å, their duration times to became equilibrated were different; doxycycline in F1 with water took only 60 ns, followed by F2 and F3 (125 and 155 ns, respectively). A low and slowly increasing value of the RMSD reflects a tiny position change of molecules from the starting point compared to a high value of the RMSD. This indicated that the addition of borneol and triacetin in the formulation reflects the duration to reach equilibrium of doxycycline; hence, both of them have a direct effect on drug retardation. Additionally, the proportion of triacetin incorporated in ISM also affected the change in the position of the doxycycline molecular.
Typically, water molecules diffuse into the formulation after it contacts the crevicular fluid in the crevicular pocket. While the organic solvent from the formulation diffuses out into the environment, this process is known as a solvent exchange [
3,
50]. Therefore, the movement of NMP molecule as an organic solvent in this study was also important to observe to prove and understand the solvent exchange phenomenon. While the RMSD of the NMP conformation was analyzed and displayed in
Figure 5C. As shown in the results, this curve of F4 reached equilibrium at 77 Å after 110 ns, while those of F2 and F3 became constant after 60 and 75 ns, respectively. The effects of borneol and triacetin not only retarded the drug movement but also affected the movement of the solvent in the formulation. By comparison of the RMSD of doxycycline and NMP conformation, the movement of the NMP molecule was faster than that of doxycycline, resulting in faster reaching the equilibrium of NMP (
Figure 5C) because the molecular size of NMP is smaller than that of doxycycline. Although the RMSD of doxycycline and NMP conformation were constant within 200 ns, the RMSD of borneol conformation (
Figure 5D) was slightly increased even after 200 ns. From the results of this experiment and the previous experiments, it can be concluded that this phenomenon was caused by the borneol gradually forming a matrix. Whereas some molecules of borneol still moved and were gradually induced by water for matrix transformation. Especially, the F4 formulation containing 25% triacetin, showed a slowed down exchange of solvent and water, resulting in the slowest phase transformation of borenol, as expressed in
Figure 5D. Thus, the matrix transformation of the F4 formulation occurred gradually. Moreover, the diffusion constant of relevant molecules during the initial stages of matrix formation within nanoseconds was analyzed and displayed in
Table 4 to prove the aforementioned hypothesis. It was noticed that the addition of borneol and triacetin minimized the diffusion constant of doxycycline, and affected the amount of water/NMP migrated (
Figure 4A,C). Besides, the diffusion constant of NMP decreased upon the addition of borneol and/or triacetin. In comparison to F1, the impedetion of borneol clearly resulted in the decrease in the diffusion constant of both NMP and doxycycline in F2. The addition of triacetin to F3 also reduced the diffusion constant of both NMP and doxycycline. Although the addition of a hydrophobic solvent such as triacetin has the effect of ratarding solvent exchange, excessive addition of triacetin may result in incomplete or slow matrix formation because the solvent exchange process was interfered with by triacetin molecules. Eventually, the matrix was formed slowly (as discussed in
Section 3.4,
Section 3.5 and
Section 3.6) and could not slow down the solvent exchange process. Therefore, the diffusion constant in NMP of F4 was slightly higher than that of F3. The addition of triacetin (F3) also reduced the diffusion constant of both NMP and doxycycline. Although the addition of a hydrophobic solvent such as triacetin retarded the solvent exchange, resulting in sustained drug release, excessive addition of triacetin may result in different ways. Incomplete matrix formation or too slow matrix formation could occur because the solvent exchange process was interfered with triacetin molecules. Eventually, the matrix was formed slowly (as discussed in
Section 3.4,
Section 3.5 and
Section 3.6) and could not slow down the solvent exchange process. Therefore, the diffusion constant of NMP in F4 was slightly higher than that of F3. In addition, the diffusion constant of borneol decreased with the increasing amount of triacetin owing to the interfere of hydrophobic substance of triacetin. These results were consistent with MD and RMSD results (
Figure 4 and
Figure 5). In addition, the diffusion coefficient was also consistent with the matrix formation and drug release results, as shown in
Section 3.4,
Section 3.5 and
Section 3.6.
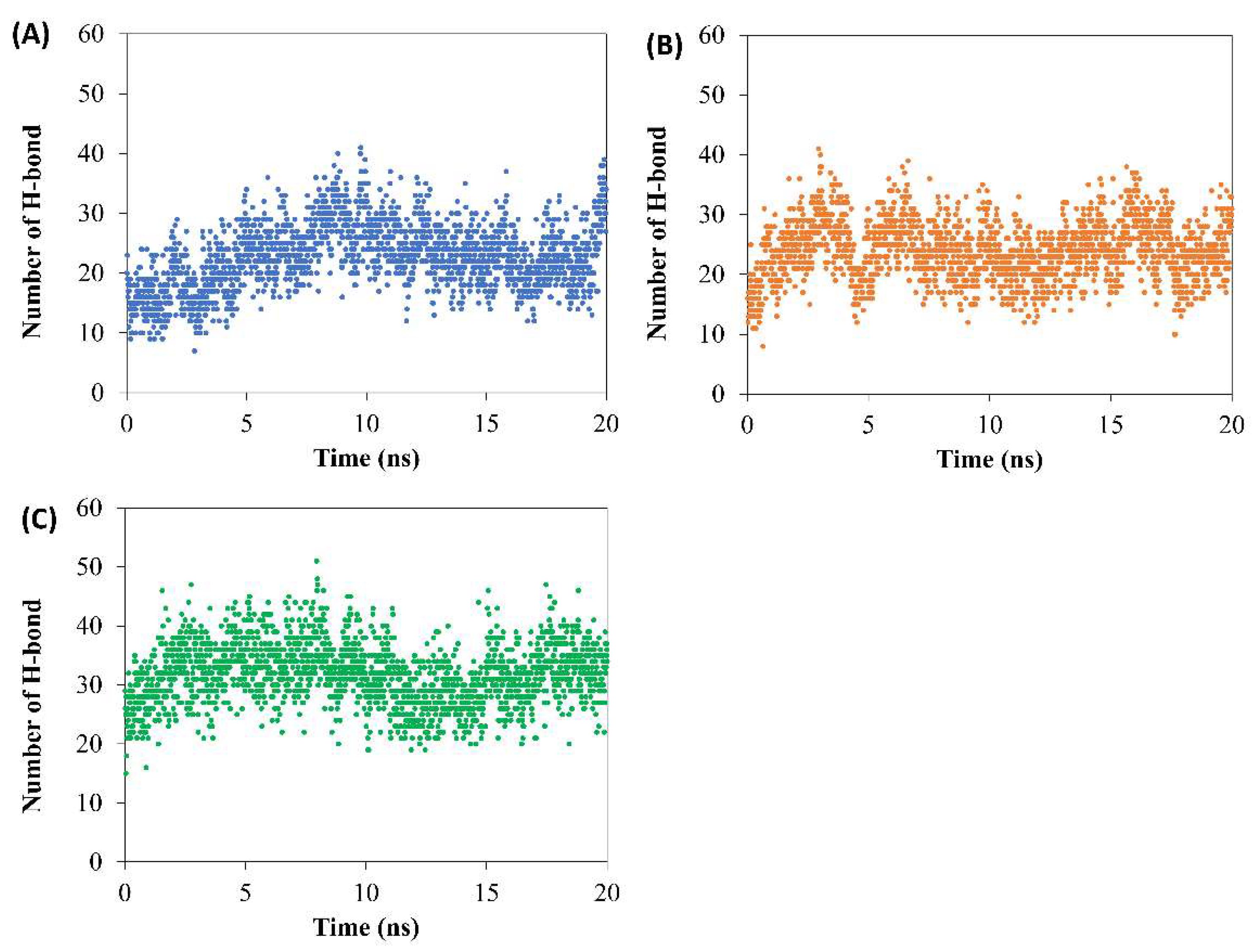
During 200 ns of simulation time, the number of H-bonds between doxycycline and the borneol molecule of F2–F4 after contact with water was evaluated using VMD software, with the distance between the acceptor and donor atoms being less than 3.5 Å at less than 60° angle [
33,
34]. Ostensibly, the H-bond formation between borneol and doxycycline (
Figure 6) in F4 had the highest trend value in the range of 20–40 NO., compared to those of the other two formulations that exhibited H-bonds in the range of 10–30 NO. Therefore, borneol in F4 had not yet self-aggregated, leaving more availability for H-bond formation with doxycycline compared to the other two formulations. This indirectly supported that the borneol matrix of F2 and F3 were better formed than that of F4. These simulations confirmed the authors’ claim on the aforementioned matrix formation from both macro/micro level studies [
7,
11,
12,
25].
According to the simulation results (MD), the borneol with or without triacetin retard both doxycycline and NMP migration. The addition of borneol and/or triacetin showed that matrices self-assembled of F2 and F3 were faster than that of F4 as shown in the following results: RMSD of NMP (
Figure 5C), RMSD of borneol (
Figure 5D), and the decrease of diffusion constant of borneol (
Table 4). In addition, the change in the number of hydrogen bonds between doxycycline and borneol molecule of F2–F4 indicated that the formation of the borneol matrix of F2 and F3 was better than that of F4. The results from molecular dynamic modelling, self-formation ability, interfacial network formation and in vitro release of ISMs supported each other. As a result, F3, a borneol-based formula containing 5% triacetin, could be a promising in situ forming system for drug delivery system due to its outstanding self-assembled time and ability to prolong drug release compared to others.