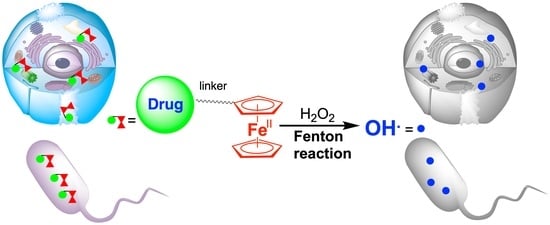
Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects †
Abstract
1. Introduction
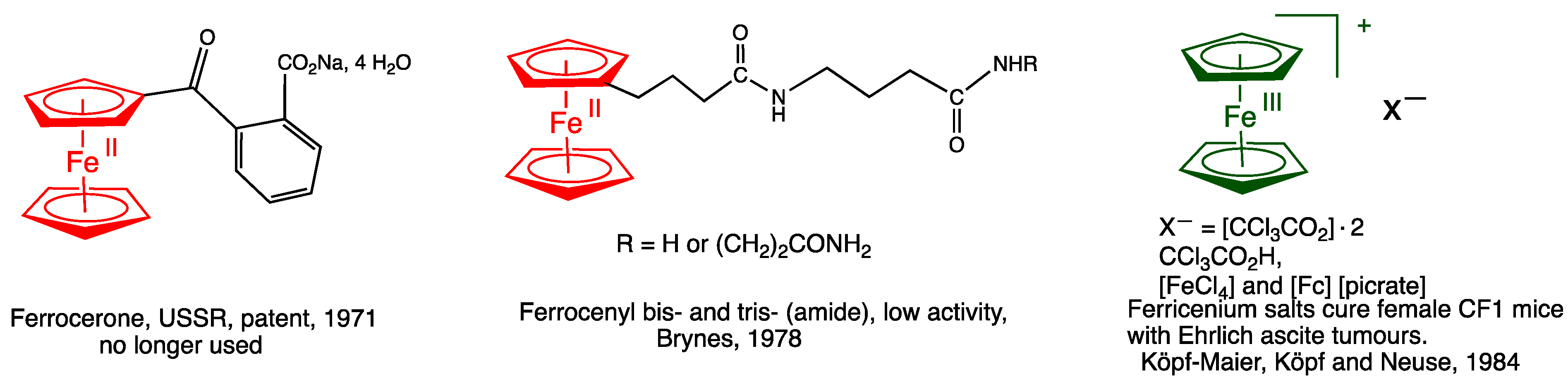
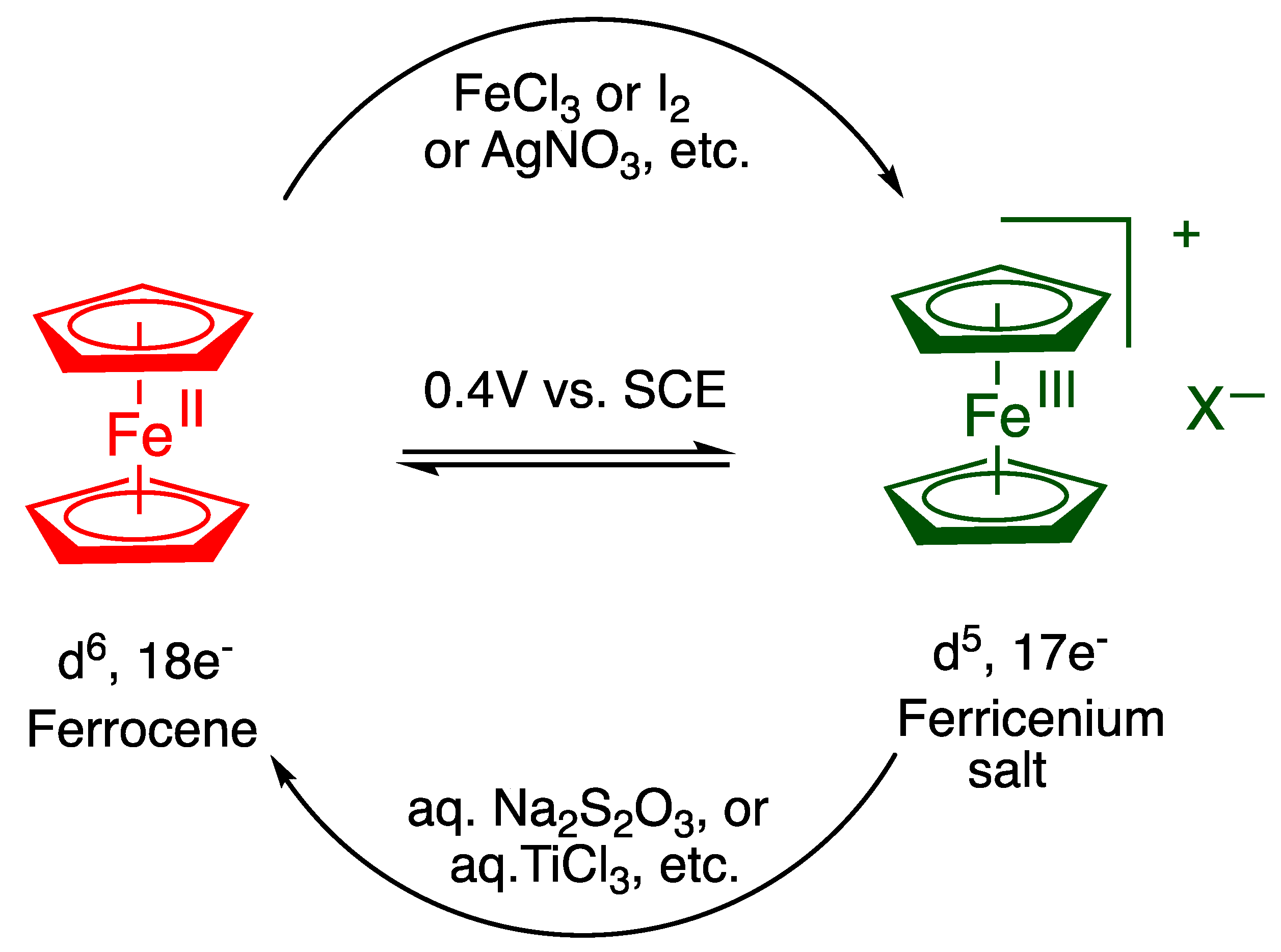
2. Pioneering and Early Studies
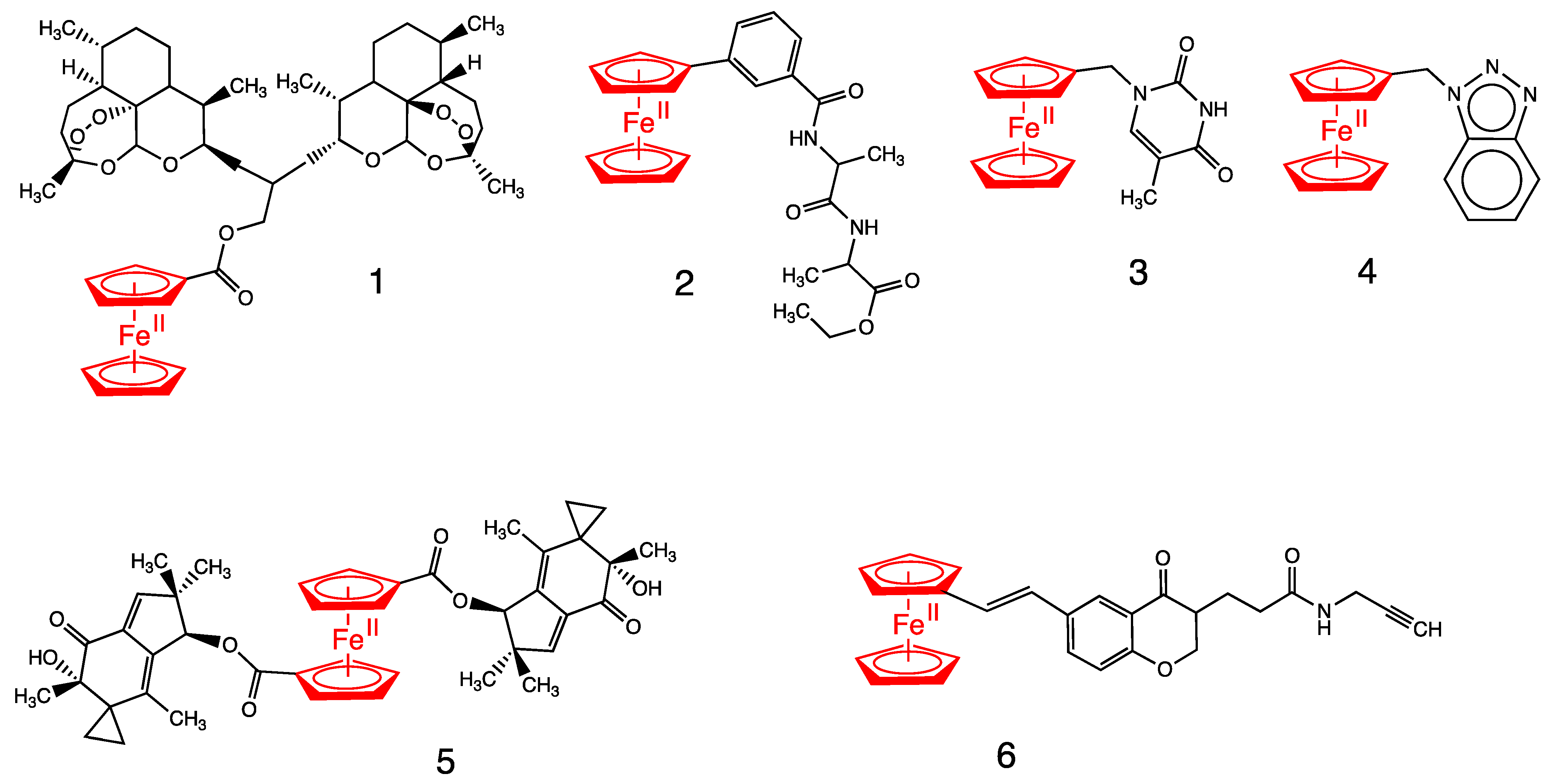
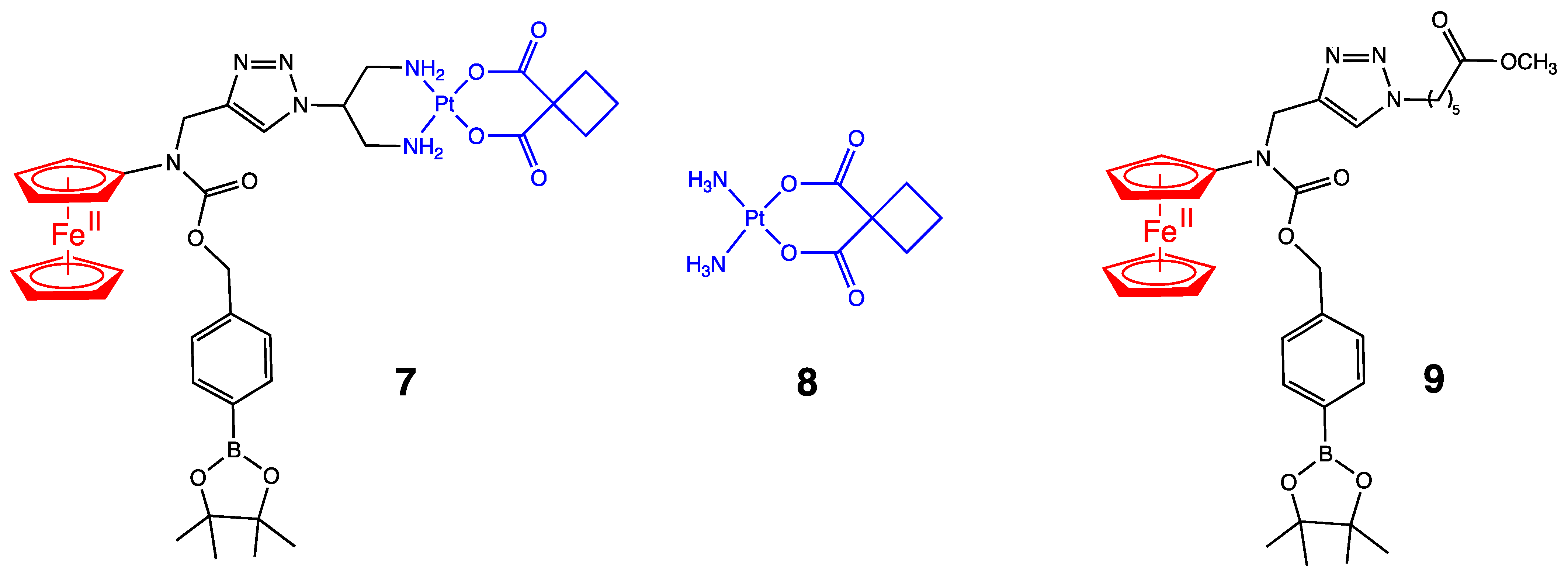
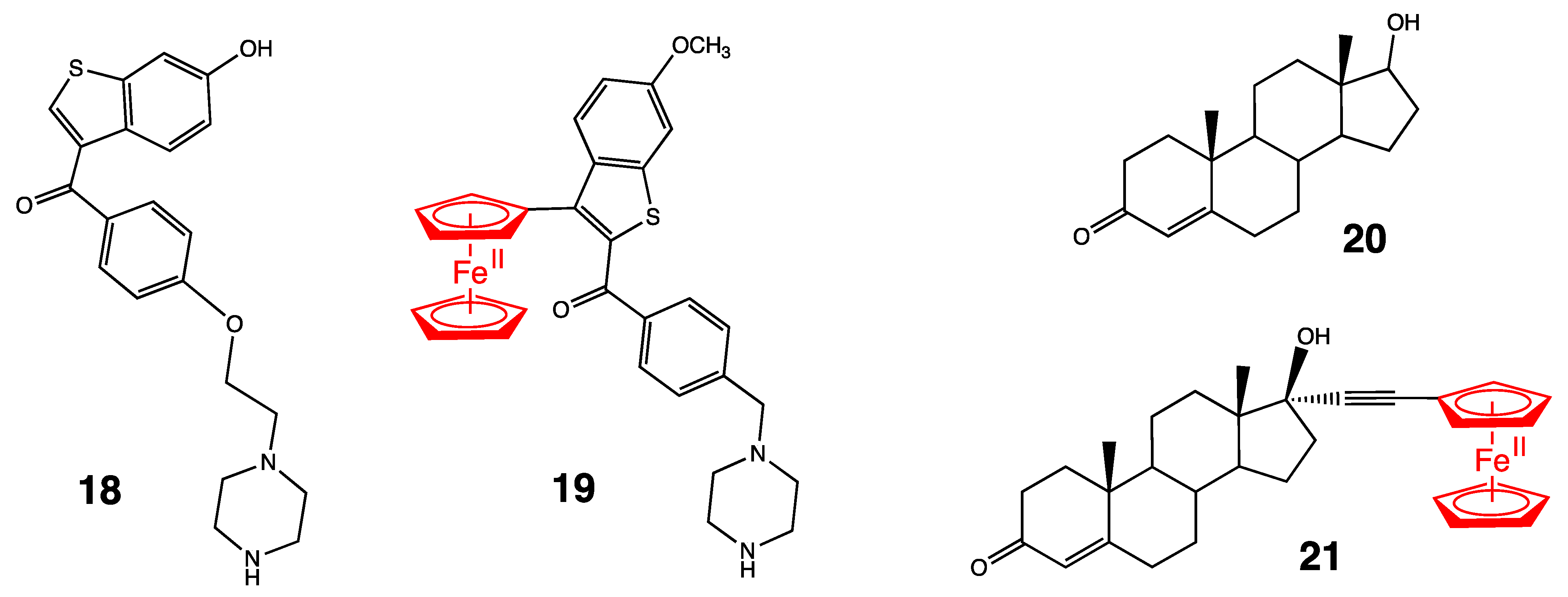
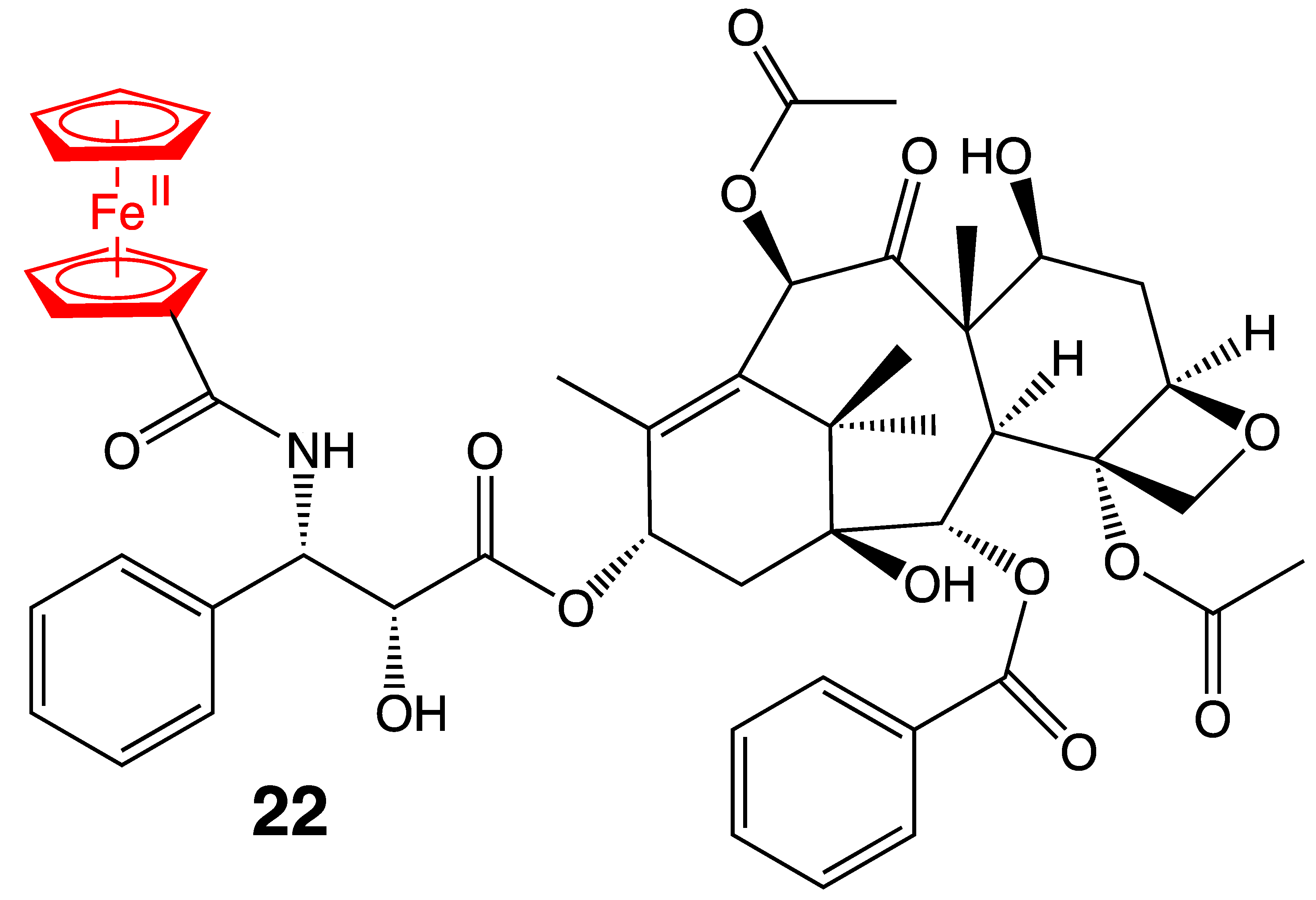
3. Antitumoral Ferrocenyl Conjugates
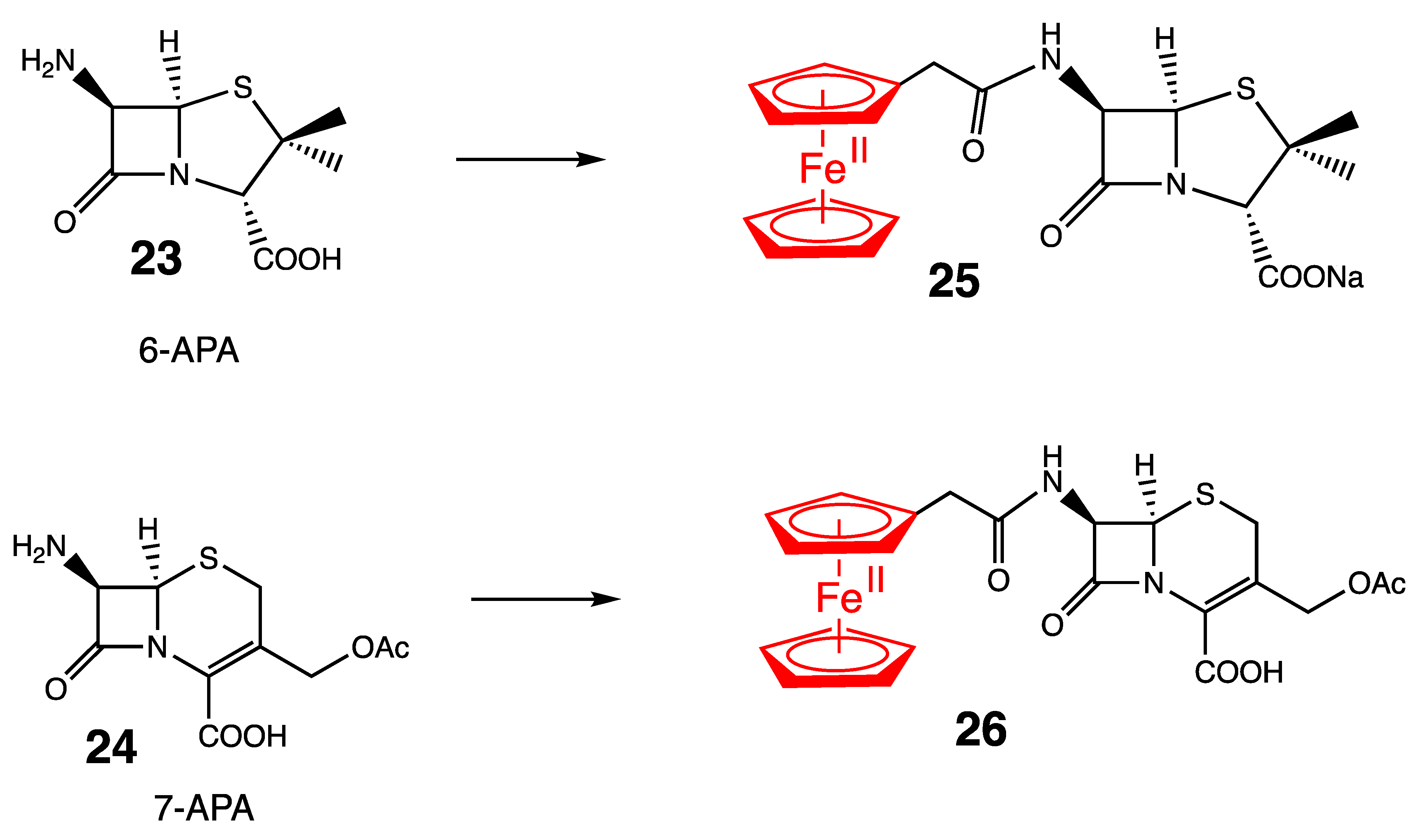
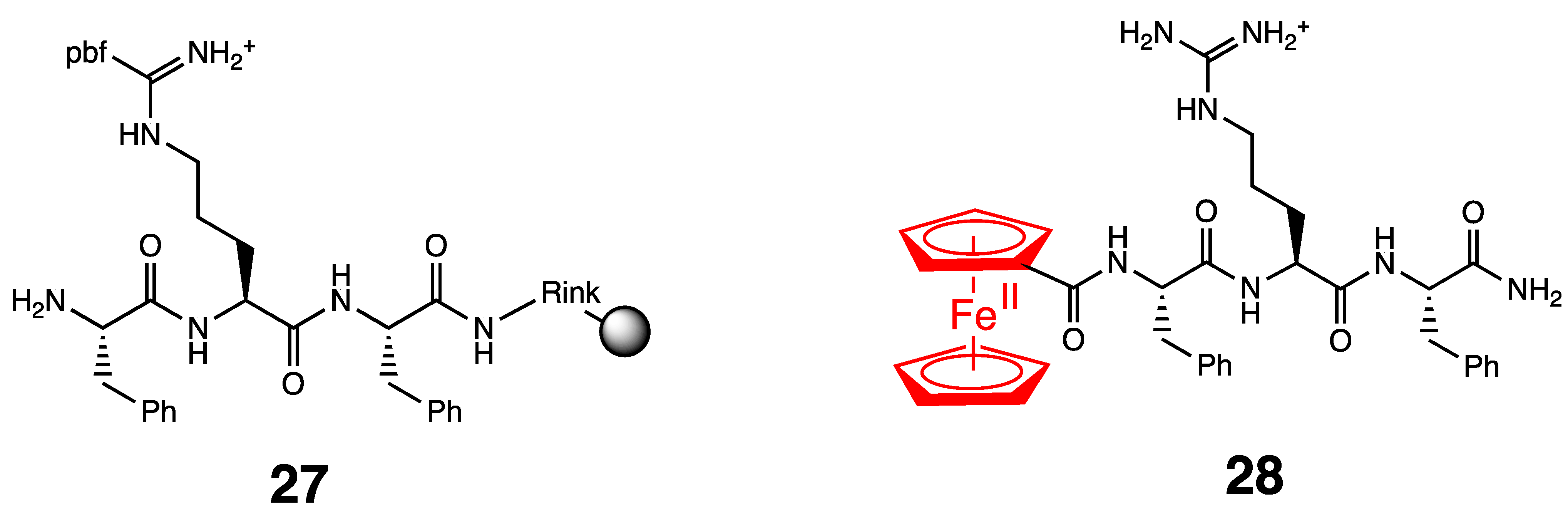
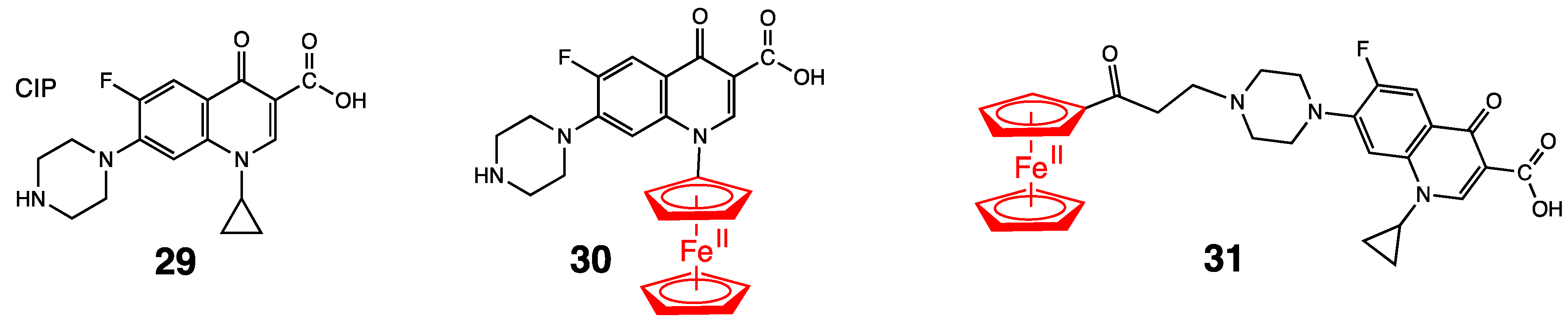
4. Antibacterial Ferrocenyl Conjugates
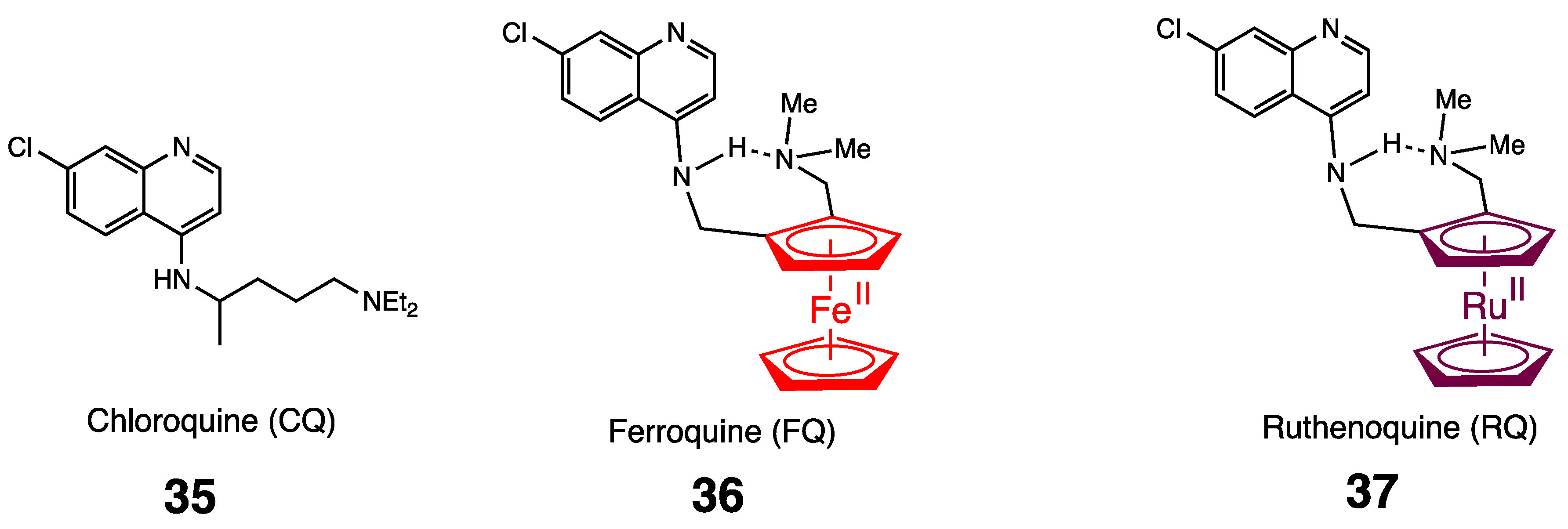
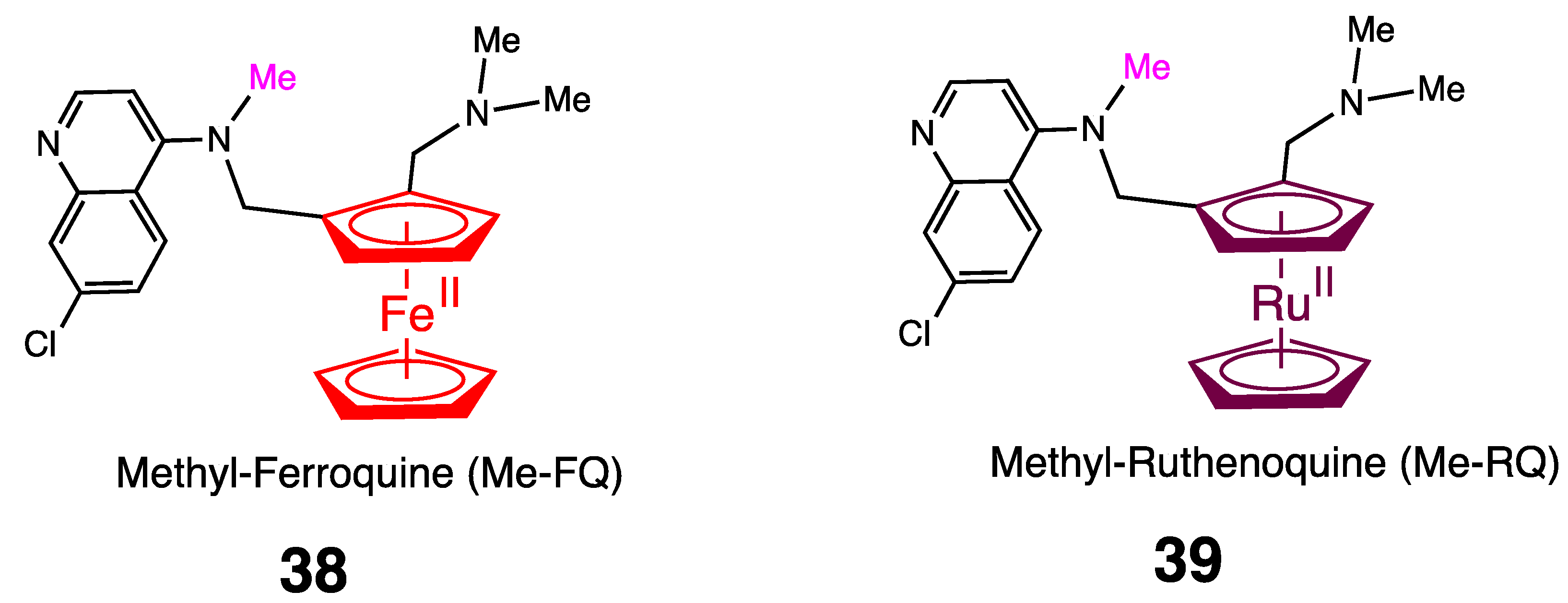
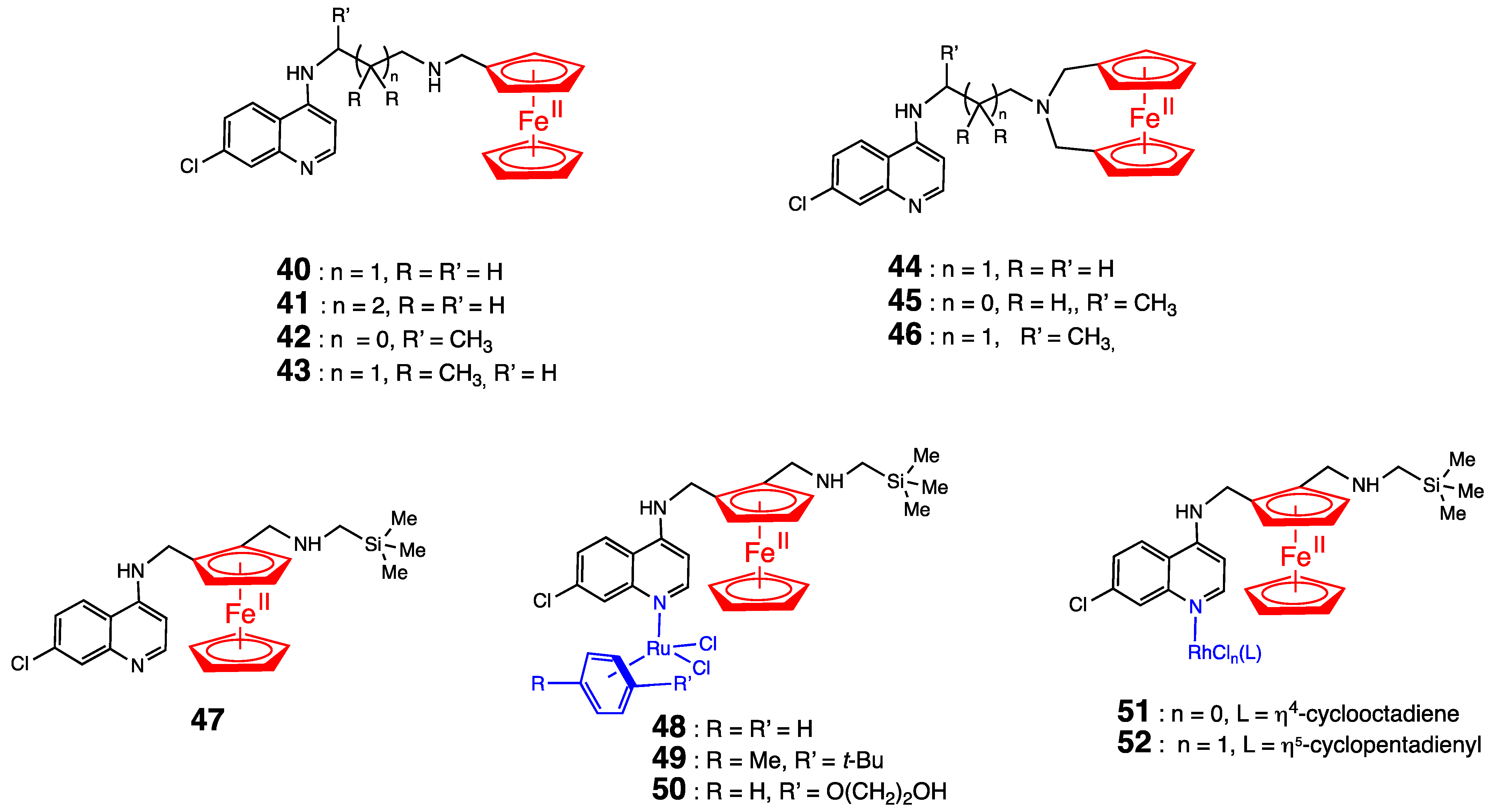
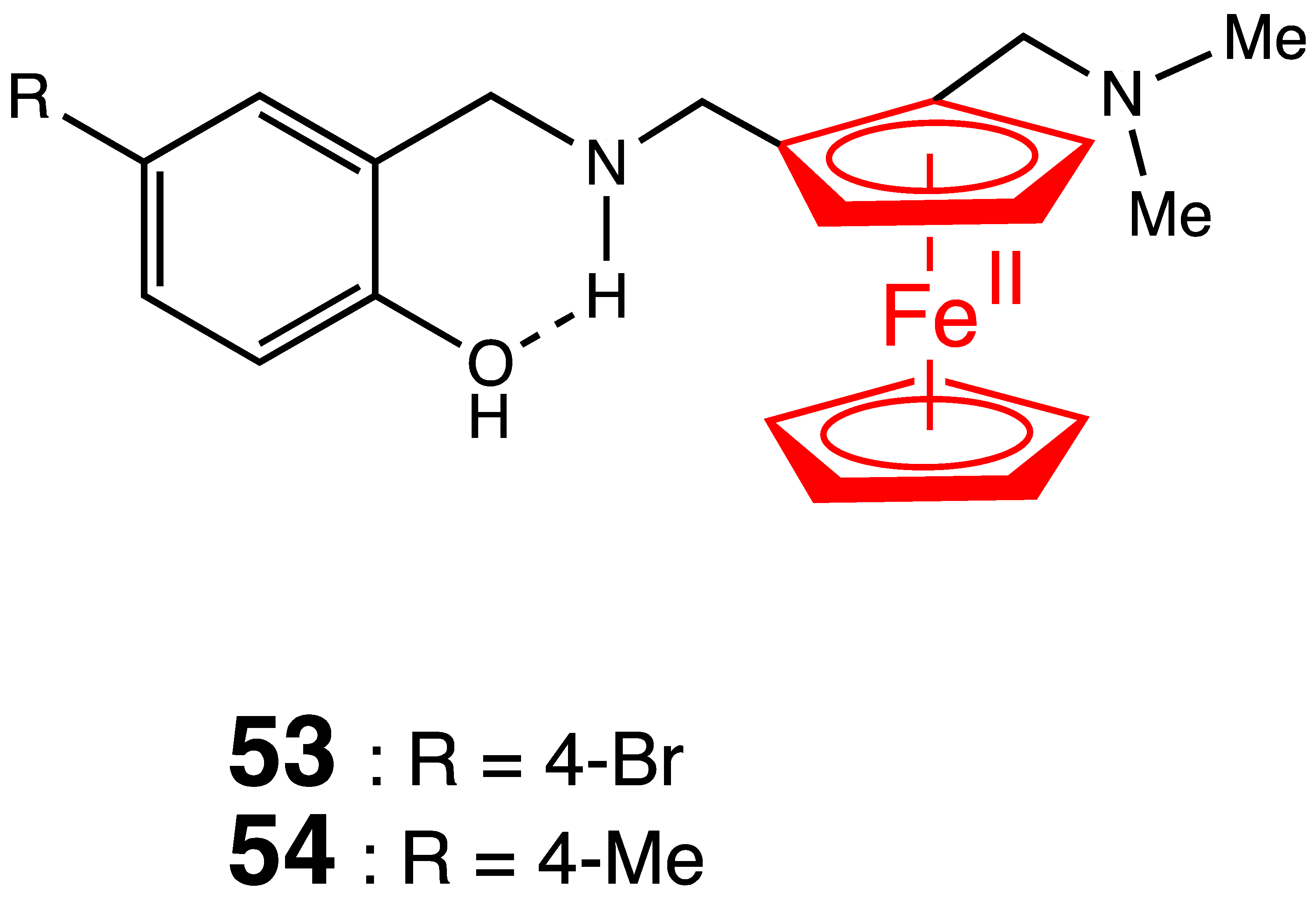
5. Antimalarial Ferrocenyl Conjugates
6. Antifungal Ferrocenyl Conjugates
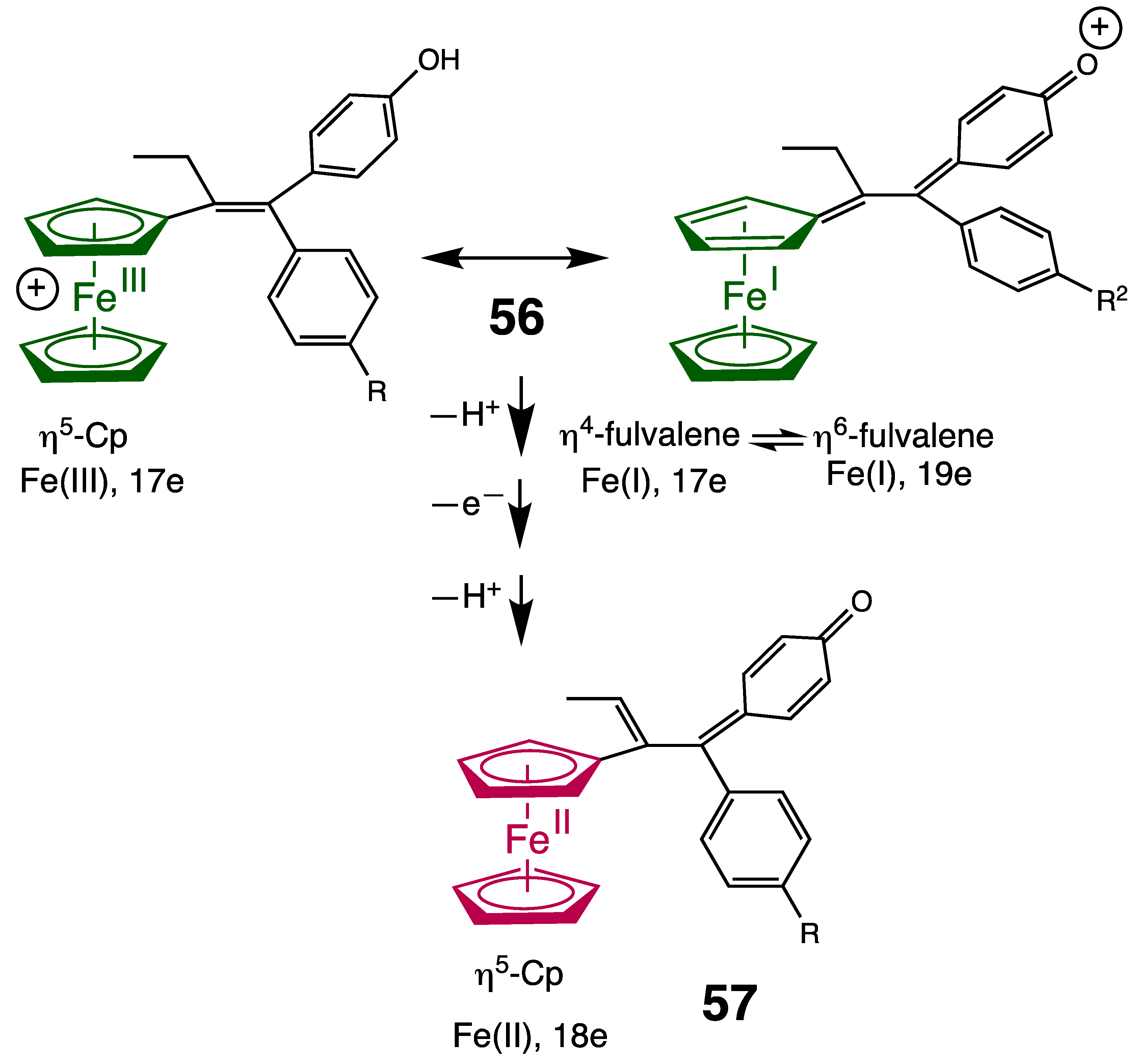
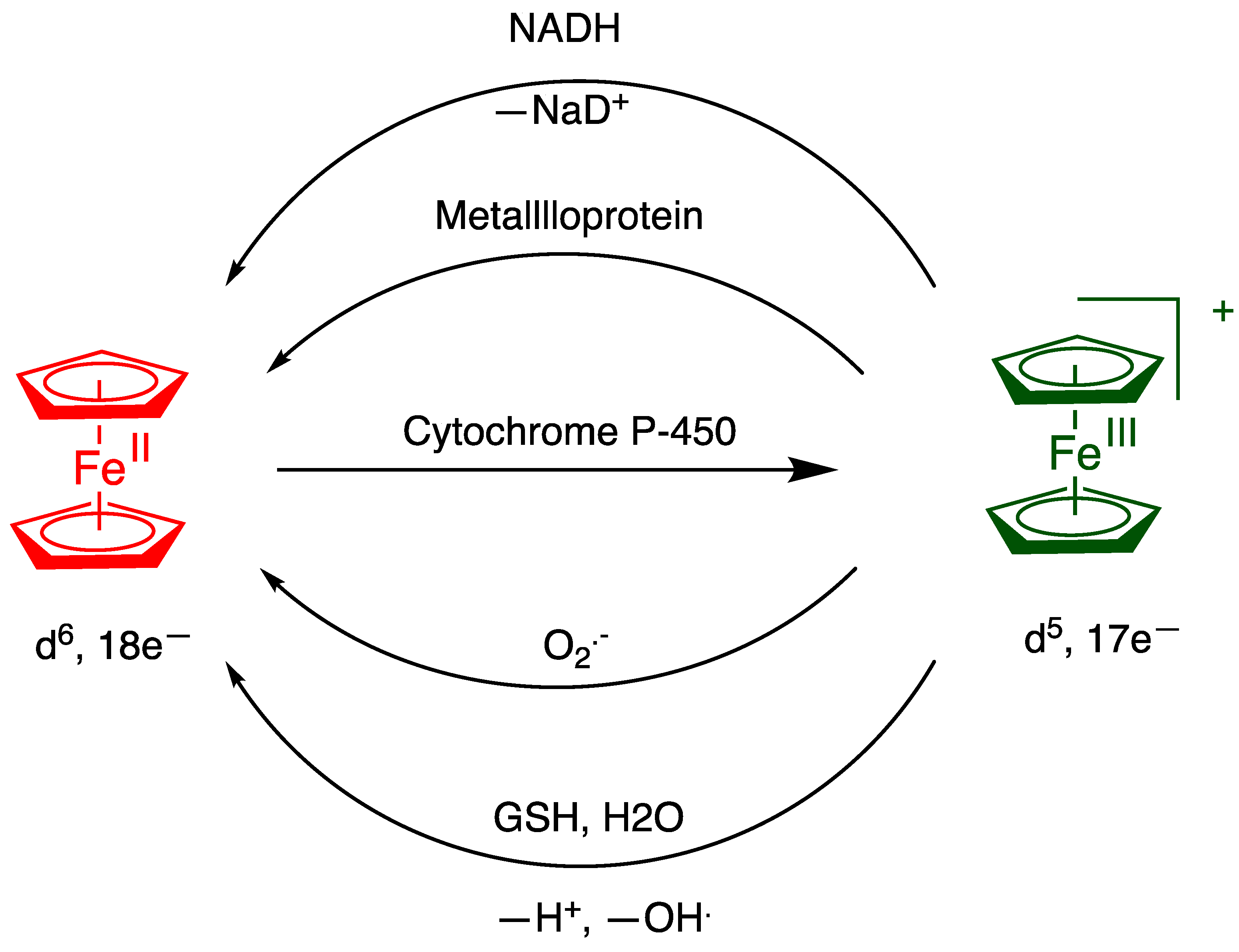
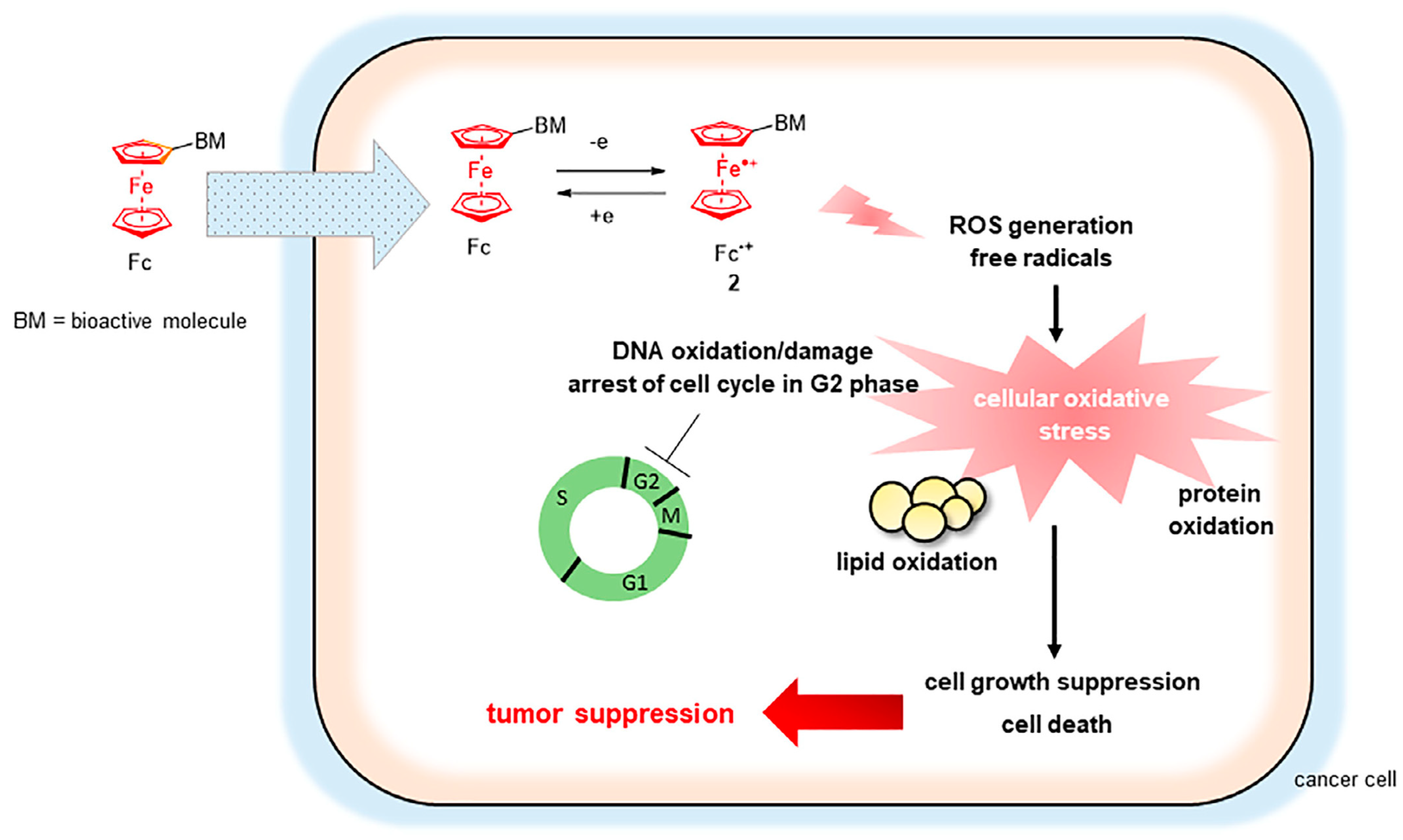
7. The Fenton Reaction with Fe2+ and Ferrocene Derivatives
8. Drug Delivery Using Ferrocene-Containing Nanomaterials
8.1. Introduction and Early Studies
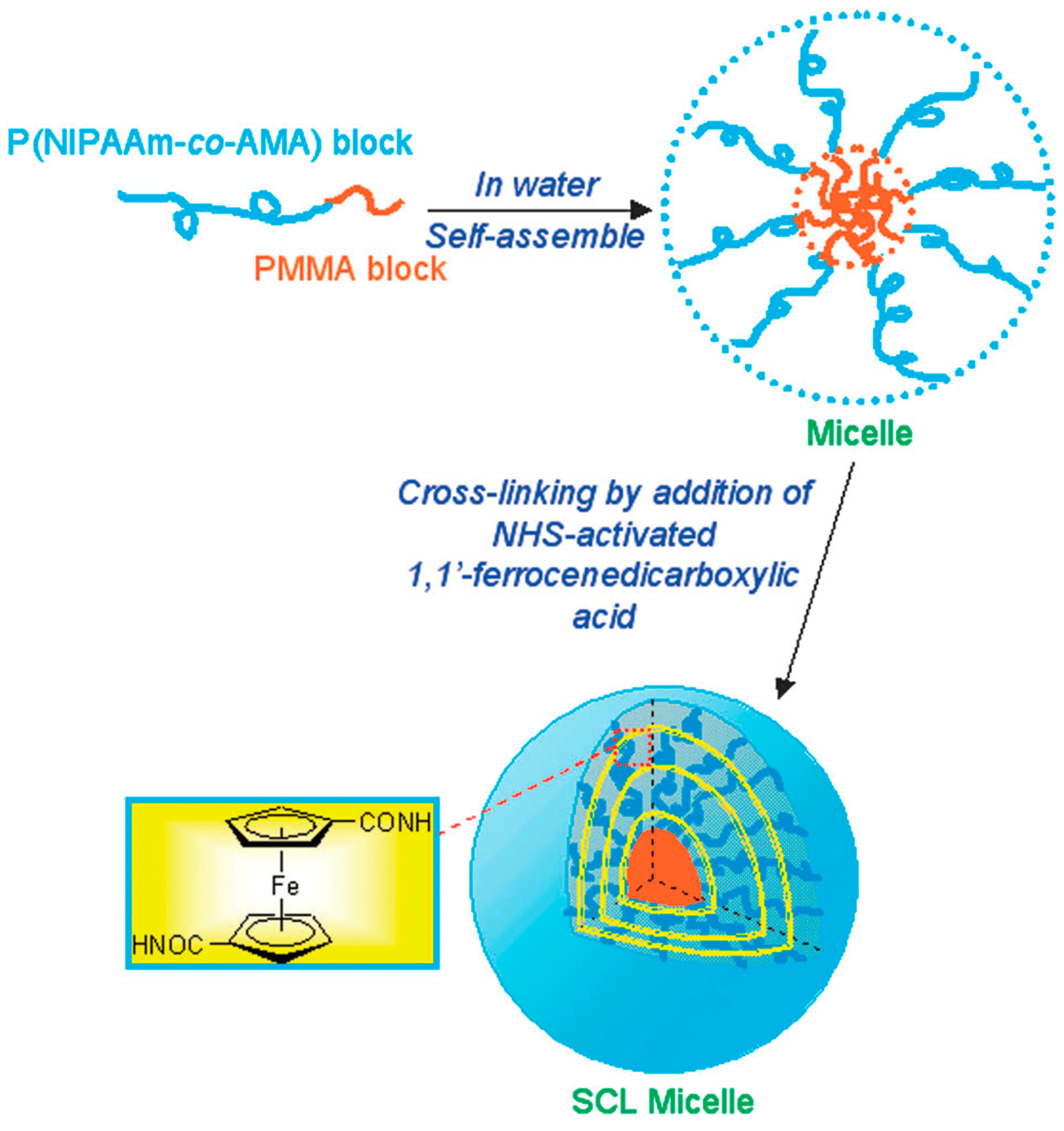
8.2. Polymers, Micelles, and Liposomes
8.3. Cyclodextrins and Pillar[6]arenes
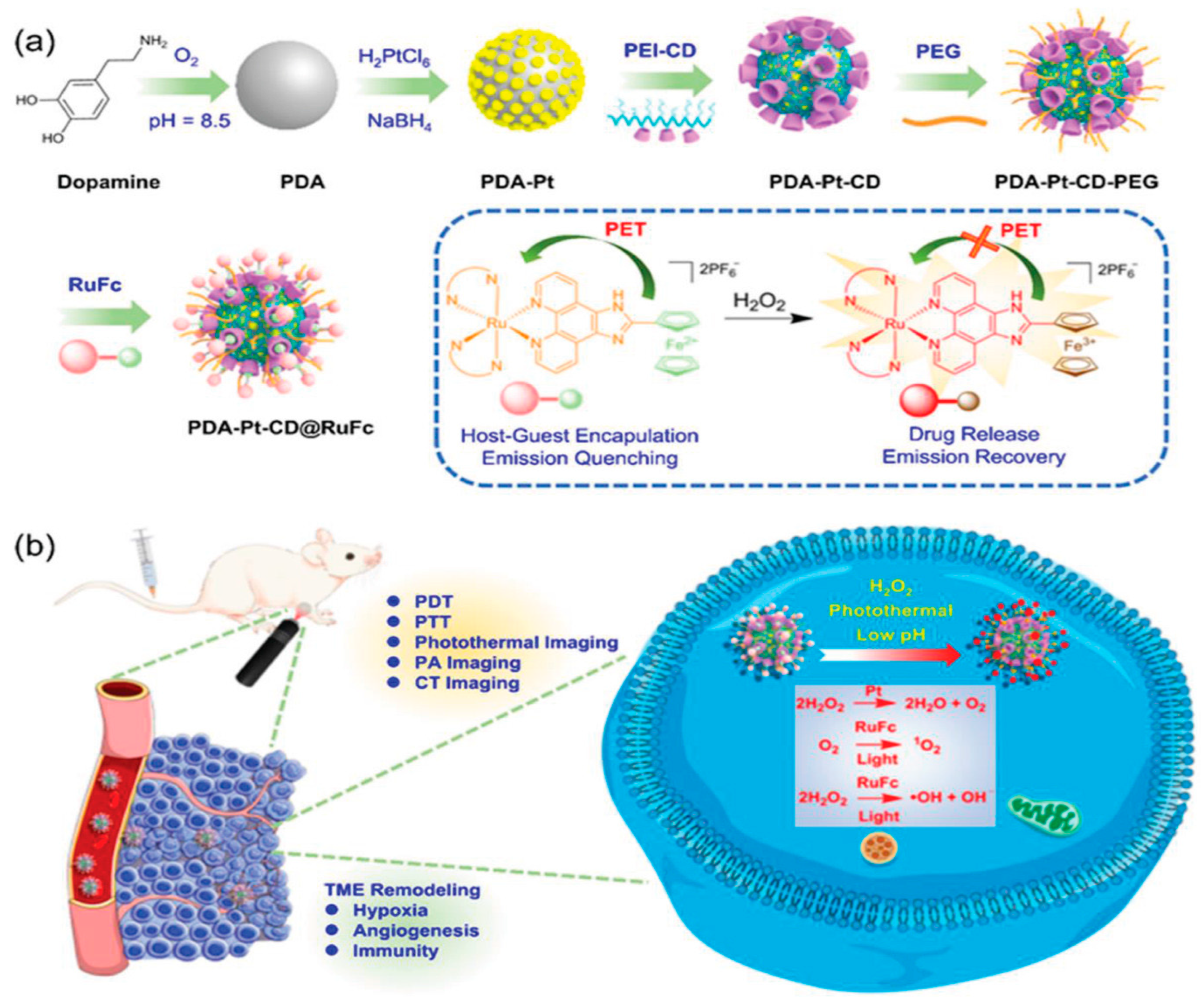
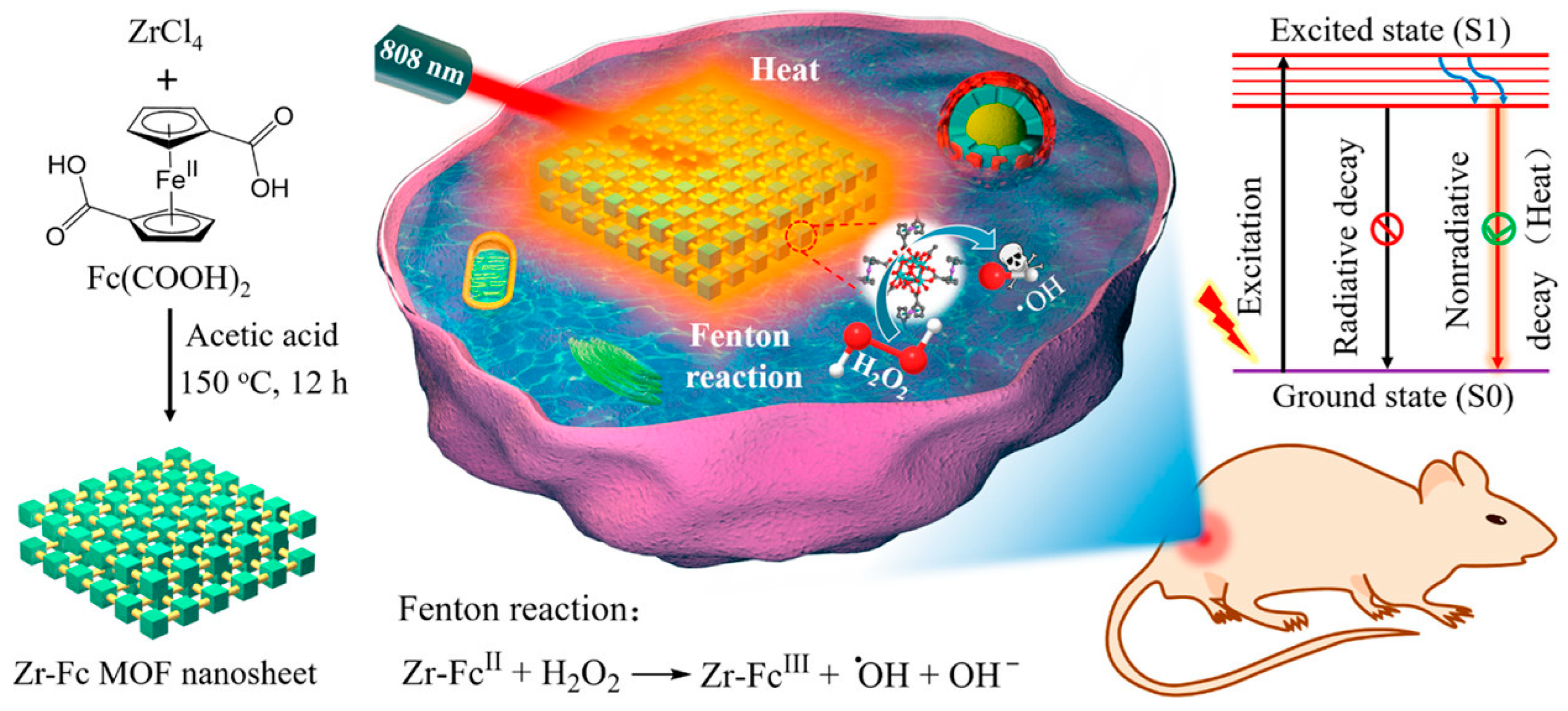
8.4. MOFs
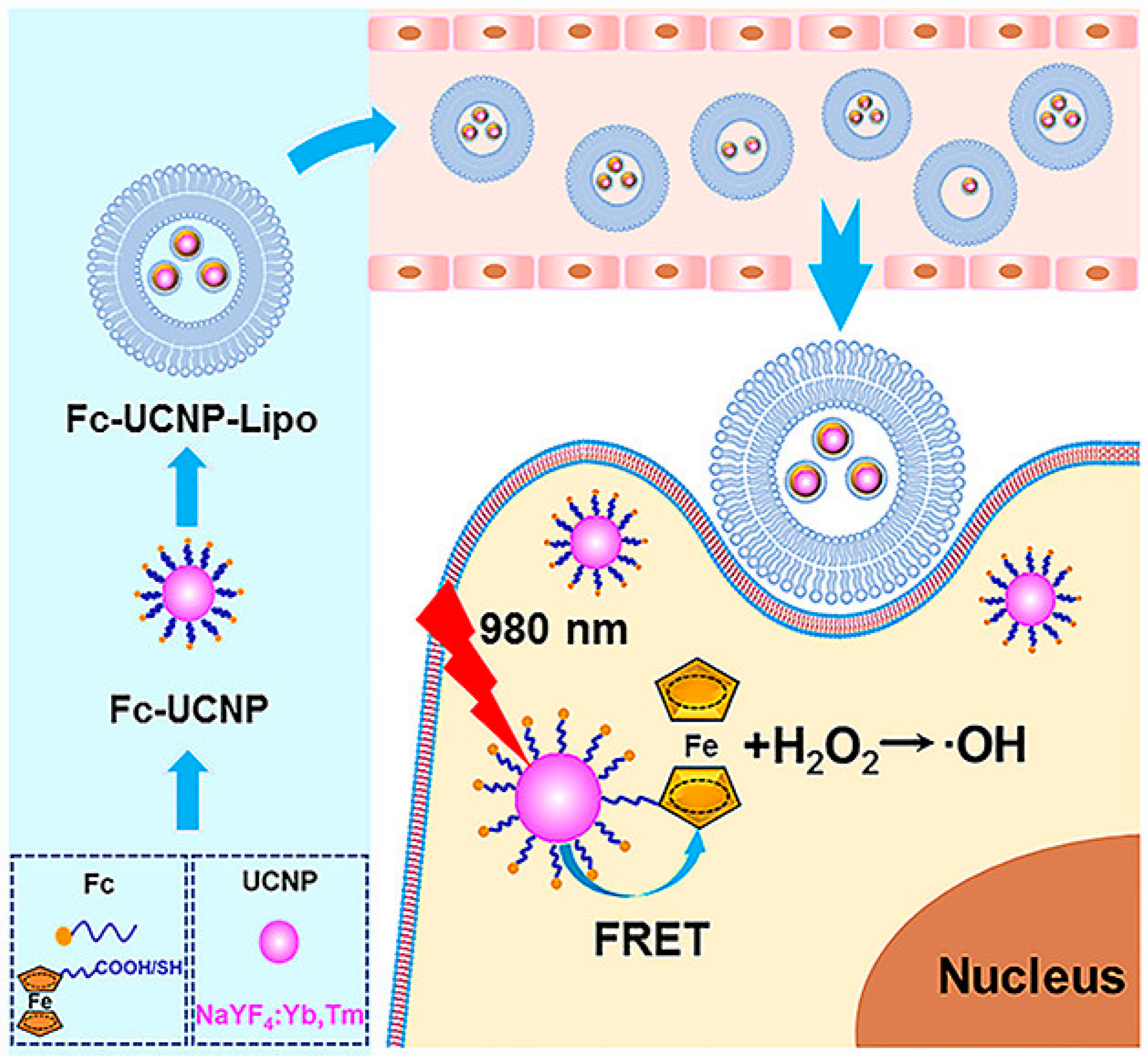
8.5. Dendrimers
9. Conclusions and Prospects
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial antimicrobiol resistance in 2019, A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 6–29. [Google Scholar] [CrossRef]
- Neuse, E.W. Macromolecular ferrocene compounds as cancer drug models. J. Inorg. Organomet. Polym. Mater. 2005, 15, 3–32. [Google Scholar] [CrossRef]
- Jaouen, G.; Salmain, M. (Eds.) Bioorganometallic Chemistry: Applications in Drug Discovery, Biocatalysis, and Imaging; Wiley-VCH: Weinheim, Germany, 2014; ISBN 978-3-527-33527-5. [Google Scholar]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Chem. Rev. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Braga, S.S.; Silva, A.M.S. A New Age for Iron: Antitumoral Ferrocenes. Organometallics 2013, 32, 5626–5639. [Google Scholar] [CrossRef]
- Koszytkowska-Stawińska, M.; Buchowicz, W. Ferrocene-triazole conjugates: Do we know why they are biologically active? Dalton Trans. 2023, 52, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, X.; Hou, A.; Cheng, C.; Wang, X.; Yue, Y.; Wu, Z.; Wu, H.; Liu, B.; Li, H.; et al. Growth of Cu2O Nanoparticles on Two-Dimensional Zr-Ferrocene- Metal-Organic Framework Nanosheets for Photothermally Enhanced Chemodynamic Antibacterial Therapy. Inorg. Chem. 2022, 61, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Singh, H.; Sonawane, P.; Paliwal, H.; Thareja, S.; Pathak, P.; Grishina, M.; Jaremko, M.; Emwas, A.-H.; et al. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15, 1071. [Google Scholar] [CrossRef]
- Raičević, V.; Radulović, N.; Sakač, M. Toward Selective Anticancer Agents: Ferrocene-Steroid Conjugates. Eur. J. Inorg. Chem. 2022, e202100951. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poonia, K. The redox mechanism of ferrocene and its phytochemical and biochemical compounds in anticancer therapy: A mini review. Inorg. Chem. Commun. 2022, 134, 109044. [Google Scholar] [CrossRef]
- Idlas, P.; Lepeltier, E.; Jaouen, G.; Passirani, C. Ferrocifen Loaded Lipid Nanocapsules: A Promising Anticancer Medication against Multidrug Resistant Tumors. Cancers 2021, 13, 2291. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, L.; Sun, J. The Antiproliferative Activity of Ferrocene Derivatives against Drug-Resistant Cancer Cell Lines: A Mini Review. Curr. Topics Med. Chem. 2021, 21, 1756–1772. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agent: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. Enhancing the Activity of Drugs by Conjugation to Organometallic Fragments. Chem. Eur. J. 2020, 26, 8676–8688. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.; Kong, F.; Gao, F. Current scenario of ferrocene-containing hybrids for antimalarial activity. Eur. J. Med. Chem. 2020, 185, 111791. [Google Scholar] [CrossRef]
- Mukaya, E.H.; Mbianda, X.Y. Macromolecular Prodrugs Containing Organoiron-Based Compounds in Cancer Research: A Review. Mini-Rev. Med. Chem. 2020, 20, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Aderibigbe, B.A. Ferrocene-Based Compounds with Antimalaria/Anticancer Activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.S.; Correia, J.D.; Kühn, F.E. Ferrocene derivatives as anti-infective agents. Coord. Chem. Rev. 2019, 396, 22–48. [Google Scholar] [CrossRef]
- Sierra, M.A.; Casarrubios, L.; De La Torre, M.C. Bio-Organometallic Derivatives of Antibacterial Drugs. Chem. Eur. J. 2019, 25, 7232–7242. [Google Scholar] [CrossRef]
- Albada, B.; Metzler-Nolte, N. Highly Potent Antibacterial Organometallic Peptide Conjugates. Acc. Chem. Res. 2017, 50, 2510–2518. [Google Scholar] [CrossRef]
- Chavain, N.; Biot, C. Organometallic Complexes: New Tools for Chemotherapy. Curr. Med. Chem. 2010, 17, 2729–2745. [Google Scholar] [CrossRef]
- Dive, D.; Biot, C. Ferrocene conjugates of chloroquine and other antimalarials: The development of ferroquine, a new antimalarial. ChemMedChem 2008, 3, 383–391. [Google Scholar] [CrossRef]
- Fouda, M.F.R.; Abd-Elzaher, M.M.; Abdelsamaia, R.A.; Labib, A.A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625. [Google Scholar] [CrossRef]
- Chantson, J.T.; Falzacappa, M.V.V.; Crovella, S.; Metzler-Nolte, N. Solid-phase synthesis, characterization, and antibacterial activities of metallocene-peptide bioconjugates. ChemMedChem 2006, 1, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- van Staveren, D.R.; Metzler-Nolte, N. Bioorganometallic chemistry of ferrocene. Chem. Rev. 2004, 104, 5931–5985. [Google Scholar] [CrossRef] [PubMed]
- Fiorina, V.J.; Dubois, R.J.; Brynes, S. Ferrocenyl Polyamines as Agents for the Chemoimmunotherapy of Cancer. J. Med. Chem. 1976, 21, 393. [Google Scholar] [CrossRef] [PubMed]
- Neuse, E.W.; Köpf-Maier, P.; Köpf, H. Ferricenium Complexes: A New Type of Water-Soluble Antitumor Agent. J. Cancer Clin. Oncol. 1984, 108, 336–340. [Google Scholar] [CrossRef]
- Hiroshi, T.; Musahiro, M. DNA cleaving activity and cytotoxic activity of ferricenium cations. Chem. Lett. 1997, 26, 1177–1178. [Google Scholar] [CrossRef]
- Osella, D.; Ferrali, M.; Zanello, P.; Laschi, F.; Fontani, M.; Nervi, C.; Cavigiolio, G. On the mechanism of the antitumor activity of ferrocenium derivatives. Inorganica Chim. Acta 2000, 306, 42–48. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospect. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Astruc, D. Electron-Transfer Processes in Dendrimers and their Implication in Biology, Catalysis, Sensing and Nanotechnology. Nat. Chem. 2012, 4, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Aranzaes, J.R.; Daniel, M.-C.; Astruc, D. Metallocenes as references for the determination of redox potentials by cyclic voltammetry: Permethylated iron and cobalt sandwich complexes, inhibition by polyamine dendrimers and the role of hydroxy-containing ferrocenes. Can. J. Chem. 2006, 84, 288–299. [Google Scholar] [CrossRef]
- Neuse, E.W.; Kanzawa, F. Evaluation of the Activity of Some Water-Soluble Ferrocene and Ferricenium Compounds Against Carcinoma of the Lung by the Human Tumor Clonogenic-Assay. Appl. Organomet. Chem. 1990, 4, 19–26. [Google Scholar] [CrossRef]
- Langer, R. Polymeric Delivery Systems for Controlled Drug Release. Chem. Eng. Commun. 1980, 6, 1–48. [Google Scholar] [CrossRef]
- Langer, R.S.; Peppas, N.A. Present and Future Applications of Biomaterials in Controlled Drug DeLivery Systems. Biomaterials 1981, 2, 201–214. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Caldwell, G.; Meirim, M.G.; Neuse, E.W.; van Rensburg, C.E.J. Antineoplastic activity of polyaspartamide-ferrocene conjugates. Appl. Organomet. Chem. 1998, 12, 793–799. [Google Scholar] [CrossRef]
- Allard, E.; Passirani, C.; Garcion, E.; Pigeon, P.; Vessières, A.; Jaouen, G.; Benoit, J.-P. Lipid nanocapsules loaded with an organometallic tamoxifen derivative as a novel drug-carrier system for experimental malignant gliomas. J. Control. Release 2008, 130, 146–153. [Google Scholar] [CrossRef]
- Petrovski, Z.; de Matos, M.R.N.; Braga, S.S.; Pereira, C.C.; Matos, M.L.; Gonçalves, I.S.; Pillinger, M.; Alves, P.M.; Romão, C.C. Synthesis, characterization and antitumor activity of 1,2-disubstituted ferrocenes and cyclodextrin inclusion complexes. J. Organomet. Chem. 2008, 693, 675–684a. [Google Scholar] [CrossRef]
- Edwards, E.I.; Epton, R.; Marr, G. The synthesis and Reactions of Homonuclear Ferrocene Acid Anhydrides and their Use in the Preparation of Ferrocenyl-Penicilins and Cephalosporins. J. Organomet. Chem. 1979, 168, 259–272. [Google Scholar] [CrossRef]
- Scutaru, D.; Tataru, L.; Mazilu, I.; Vata, M.; Lixandru, T.; Simionescu, C. Contributions to the synthesis of some ferrocene-containg antibiotics. Appl. Organomet. Chem. 1993, 7, 225–231. [Google Scholar] [CrossRef]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Millet, P.; Georges, A.J.; Abessolo, H.; Dive, D.; et al. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene−chloroquine analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef]
- Biot, C.; François, N.; Maciejewski, L.; Brocard, J.; Poulain, D. Synthesis and Antifungal Activity of a Ferrocene-fluconazole Analogue. Biorg. Med. Chem. Lett. 2000, 10, 839–841. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Praveen, M. Synthesis, characterization and antibacterial properties of symmetric 1,1-ferrocene derived Schiff-base ligands and their Co(II), Cu(II), Ni(II) and Zn(II) chelates. Appl. Organomet. Chem. 2000, 14, 376–382. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton Trans. 2012, 41, 6350–6358. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Wong, J.W.; Griffin, R.C.; Doery, J.C. Artemisinin and Its Derivatives: A potential treatment for leukemia. Anti-Cancer Drugs 2019, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- XSun, X.; Yan, P.; Zou, C.; Wong, Y.; Shu, Y.; Lee, Y.M.; Zhang, C.; Yang, N.; Wang, J.; Zhang, J. Targeting autophagy enhances the anticancer effect of artemisinin and its derivatives. Med. Res. Rev. 2019, 39, 2172–2193. [Google Scholar] [CrossRef]
- Lal, B.; Badshah, A.; Altaf, A.A.; Khan, N.; Ullah, S. Miscellaneous applications of ferrocene-based peptides/amides. Appl. Organomet. Chem. 2011, 25, 843–855. [Google Scholar] [CrossRef]
- Simenel, A.A.; Morozova, E.A.; Snegur, L.V.; Zykova, S.I.; Kachala, V.V.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M. Simple Route to Ferrocenylalkyl Nucleobases. Antitumor Activity In Vivo. Appl. Organomet. Chem. 2009, 23, 219–224. [Google Scholar] [CrossRef]
- Snegur, L.V.; Nekrasov, Y.S.; Sergeeva, N.S.; Zhilina, Z.V.; Gumenyuk, V.V.; Starikova, Z.A.; Simenel, A.A.; Morozova, N.B.; Sviridova, I.K.; Babin, V.N. Ferrocenylalkylazoles: Bioactivity, synthesis, structure. Appl. Organomet. Chem. 2008, 22, 139–147. [Google Scholar] [CrossRef]
- Knauer, S.; Biersack, B.; Zoldakova, M.; Effenberger, K.; Milius, W.; Schobert, R. Melanoma-Specific Ferrocene Esters of the Fungal Cytotoxin Illudin M. Anti-Cancer Drugs 2009, 20, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Hikisz, P.; Szczupak, Ł.; Therrien, B.; Koceva-Chyła, A. Ferrocenyl and Dicobalt Hexacarbonyl Chromones—New Organometallics Inducing Oxidative Stress and Arresting Human Cancer Cells in G2/M Phase. Eur. J. Med. Chem. 2014, 81, 289–300. [Google Scholar] [CrossRef]
- Reshetnikov, V.; Daum, S.; Janko, C.; Karawacka, W.; Tietze, R.; Alexiou, C.; Paryzhak, S.; Dumych, T.; Bilyy, R.; Tripal, P.; et al. ROS-Responsive N-Alkylaminoferrocenes for Cancer-Cell-Specific Targeting of Mitochondria. Angew. Chem. Int. Ed. 2018, 57, 11943–11946. [Google Scholar] [CrossRef]
- Osborne, C.K. Drug therapy—Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef]
- Top, S.; Tang, J.; Vessières, A.; Carrez, D.; Provot, C.; Jaouen, G. Ferrocenyl hydroxytamoxifen: A prototype for a new range of oestradiol receptor site-directed cytotoxics. Chem. Commun. 1996, 8, 955–956. [Google Scholar] [CrossRef]
- McMurry, J.E. Carbonyl-Coupling Reactions Using Low-Valent Titanium. Chem. Rev. 1989, 89, 1513–1524. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Leclercq, G.; Quivy, J.; Tang, J.; Vaissermann, J.; Huché, M.; Jaouen, G. Synthesis, biochemical properties and molecular modelling studies of organometallic specific estrogen receptor modulators (SERMs), the ferrocifens and hydroxyferrocifens: Evidence for an antiproliferative effect of hydroxyferrocifens on both hormone-dependent and hormone-independent breast cancer cell lines. Chem. Eur. J. 2003, 9, 5223–5236. [Google Scholar] [CrossRef] [PubMed]
- Görmen, M.; Pigeon, P.; Top, S.; Hillard, E.A.; Huché, M.; Hartinger, C.G.; de Montigny, F.; Plamont, M.-A.; Vessières, A.; Jaouen, G. Synthesis, Cytotoxicity, and Compare Analysis of Ferrocene and [3]Ferrocenophane Tetrasubstituted Olefin Derivatives against Human Cancer Cells. ChemMedChem 2010, 5, 2039–2050. [Google Scholar] [CrossRef]
- de Oliveira, A.C.; Hillard, E.A.; Pigeon, P.; Rocha, D.D.; Rodrigues, F.A.; Montenegro, R.C.; Costa-Lotufo, L.V.; Goulart, M.O.; Jaouen, G. Biological evaluation of twenty-eight ferrocenyl tetrasubstituted olefins: Cancer cell growth inhibition, ROS production and hemolytic activity. Eur. J. Med. Chem. 2011, 46, 3778–3787. [Google Scholar] [CrossRef]
- Plażuk, D.; Vessières, A.; Hillard, E.A.; Buriez, O.; Labbé, E.; Pigeon, P.; Plamont, M.-A.; Amatore, C.; Zakrzewski, J.; Jaouen, G. A [3]Ferrocenophane Polyphenol Showing a Remarkable Antiproliferative Activity on Breast and Prostate Cancer Cell Lines. J. Med. Chem. 2009, 52, 4964–4967. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Buriez, O.; Chau, F.; Labbé, E.; Ganguly, R.; Amatore, C.; Jaouen, G.; Vessières, A.; Leong, W.K.; Top, S. Synthesis, Characterization, and Biological Properties of Osmium-Based Tamoxifen Derivatives—Comparison with Their Homologues in the Iron and Ruthenium Series. Eur. J. Inorg. Chem. 2015, 4217–4226. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Jaouen, G. Organometallic Antitumor Compounds: Ferrocifens as Precursors to Quinone Methides. Angew. Chem. Int. Ed. 2015, 54, 10230–10233. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Wang, Y.; Top, S.; Najlaoui, F.; Alvarez, M.C.G.; Bignon, J.; McGlinchey, M.J.; Jaouen, G. A New Series of Succinimido-ferrociphenols and Related Heterocyclic Species Induce Strong Antiproliferative Effects, Especially against Ovarian Cancer Cells Resistant to Cisplatin. J. Med. Chem. 2017, 60, 8358–8368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dansette, P.M.; Pigeon, P.; Top, S.; McGlinchey, M.J.; Mansuy, D.; Jaouen, G. A new generation of ferrociphenols leads to a great diversity of reactive metabolites, and exhibits remarkable antiproliferative properties. Chem. Sci. 2018, 9, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Gaschard, M.; Othman, M.; Salmain, M.; Jaouen, G. α-Hydroxylactams as Efficient Entries to Diversely Functionalized Ferrociphenols: Synthesis and Antiproliferative Activity Studies. Molecules 2022, 27, 4549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pigeon, P.; Li, W.; Yan, J.; Dansette, P.M.; Othman, M.; McGlinchey, M.J.; Jaouen, G. Diversity-oriented synthesis and bioactivity evaluation of N-substituted ferrocifen compounds as novel antiproliferative agents against TNBC cancer cells. Eur. J. Med. Chem. 2022, 234, 114202. [Google Scholar] [CrossRef]
- Garcia, J.S.; Gaschard, M.; Navizet, I.; Sahihi, M.; Top, S.; Wang, Y.; Pigeon, P.; Vessières, A.; Salmain, M.; Jaouen, G. Inhibition of Cathepsin B by Ferrocenyl Indenes Highlights a new Pharmacological Facet of Ferrocifens. Eur. J. Inorg. Chem. 2022, e202101075. [Google Scholar] [CrossRef]
- Ferreira, A.P.; da Silva, J.L.F.; Duarte, M.T.; da Piedade, M.F.M.; Robalo, M.P.; Harjivan, S.G.; Marzano, C.; Gandin, V.; Marques, M.M. Synthesis and Characterization of New Organometallic Benzo[b]thiophene Derivatives with Potential Antitumor Properties. Organometallics 2009, 28, 5412–5423. [Google Scholar] [CrossRef]
- Top, S.; Thibaudeau, C.; Vessières, A.; Brulé, E.; Le Bideau, F.; Joerger, J.-M.; Plamont, M.-A.; Samreth, S.; Edgar, A.; Marrot, J.; et al. Synthesis and Structure Activity Relationship of Organometallic Steroidal Androgen Derivatives. Organometallics 2009, 28, 1414–1424. [Google Scholar] [CrossRef]
- Carmona-Negrón, J.A.; Santana, A.; Rheingold, A.L.; Meléndez, E. Synthesis, structure, docking and cytotoxic studies of ferrocene–hormone conjugates for hormone-dependent breast cancer application. Dalton Trans. 2019, 48, 5952–5964. [Google Scholar] [CrossRef]
- Narváez-Pita, X.; Rheingold, A.L.; Meléndez, E. Ferrocene-steroid conjugates: Synthesis, structure and biological activity. J. Organomet. Chem. 2017, 846, 113–120. [Google Scholar] [CrossRef]
- Raičević, V.; Radulović, N.; Jovanović, L.; Rodić, M.; Kuzminac, I.; Jakimov, D.; Wrodnigg, T.; Knedel, T.; Janiak, C.; Sakač, M. Ferrocenylmethylation of estrone and estradiol: Structure, electrochemistry, and antiproliferative activity of new ferrocene–steroid conjugates. Appl. Organomet. Chem. 2020, 34, e5889. [Google Scholar] [CrossRef]
- Huang, X.-F.; Wang, L.-Z.; Tang, L.; Lu, Y.-X.; Wang, F.; Song, G.-Q.; Ruan, B.-F. Synthesis, characterization and antitumor activity of novel ferrocene derivatives containing pyrazolyl-moiety. J. Organomet. Chem. 2014, 749, 157–162. [Google Scholar] [CrossRef]
- Smit, F.J.; Bezuidenhout, J.J.; Bezuidenhout, C.C.; N’da, D.D. Synthesis and in vitro biological activities of ferrocenyl–chalcone amides. Med. Chem. Res. 2016, 25, 568–584. [Google Scholar] [CrossRef]
- Panaka, S.; Trivedi, R.; Jaipal, K.; Giribabu, L.; Sujitha, P.; Kumar, C.G.; Sridhar, B. Ferrocenyl chalcogeno (sugar) triazole conjugates: Synthesis, characterization and anticancer properties. J. Organomet. Chem. 2016, 813, 125–130. [Google Scholar] [CrossRef]
- Wei, J.-N.; Jia, Z.-D.; Zhou, Y.-Q.; Chen, P.-H.; Li, B.; Zhang, N.; Hao, X.-Q.; Xu, Y.; Zhang, B. Synthesis, characterization and antitumor activity of novel ferrocene-coumarin conjugates. J. Organomet. Chem. 2019, 902, 120968. [Google Scholar] [CrossRef]
- Plażuk, D.; Wieczorek, A.; Ciszewski, W.M.; Kowalczyk, K.; Błauż, A.; Pawlędzio, S.; Makal, A.; Eurtivong, C.; Arabshahi, H.J.; Reynisson, J.; et al. Synthesis and in vitro Biological Evaluation of Ferrocenyl Side-Chain-Functionalized Paclitaxel Derivatives. ChemMedChem 2017, 12, 1882–1892. [Google Scholar] [CrossRef]
- Ismail, M.K.; Khan, Z.; Rana, M.; Horswell, S.L.; Male, L.; Nguyen, H.V.; Perotti, A.; Romero-Canelón, I.; Wilkinson, E.A.; Hodges, N.J. Effect of Regiochemistry and Methylation on the Anticancer Activity of a Ferrocene-Containing Organometallic Nucleoside Analogue. ChemBioChem 2020, 21, 2487–2494. [Google Scholar] [CrossRef]
- Tsypysheva, I.P.; Koval’skaya, A.V.; Petrova, P.R.; Lobov, A.N.; Erastov, A.S.; Zileeva, Z.R.; Vakhitov, V.; Vakhitova, Y.V. Synthesis of conjugates of (−)-cytisine derivatives with ferrocene-1-carbaldehyde and their cytotoxicity against HEK293, Jurkat, A549, MCF-7 and SH-SY5Y cells. Tetrahedron 2020, 76, 130902. [Google Scholar] [CrossRef]
- Gómez, J.; Sierra, D.; Ojeda, C.; Thavalingam, S.; Miller, R.; Guzmán, F.; Metzler-Nolte, N. Solid-phase synthesis and evaluation of linear and cyclic ferrocenoyl/ruthenocenoyl water-soluble hexapeptides as potential antibacterial compounds. J. Biol. Inorg. Chem. 2021, 26, 599–615. [Google Scholar] [CrossRef]
- Costa, N.C.S.; Piccoli, J.P.; Santos-Filho, N.A.; Clementino, L.; Fusco-Almeida, A.M.; De Annunzio, S.R.; Fontana, C.R.; Verga, J.B.M.; Eto, S.F.; Pizauro-Junior, J.M.; et al. Antimicrobial activity of RP-1 peptide conjugate with ferrocene group. PLoS ONE 2020, 15, e0228740. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Wenzel, M.; Merz, K.; Bandow, J.E.; Metzler-Nolte, N. Synthesis and Biological Evaluation of Ferrocene-Containing Bioorganometallics Inspired by the Antibiotic Platensimycin Lead Structure. Organometallics 2019, 29, 4312–4319. [Google Scholar] [CrossRef]
- Albada, B.; Metzler-Nolte, N. Organometallic-peptide bioconjugates: Synthetic strategies and medicinal applications. Chem. Rev. 2016, 116, 11797–11839. [Google Scholar] [CrossRef] [PubMed]
- Hess, J. Rational approaches towards inorganic and organometallic antibacterials. J. Biol. Chem. 2021, 403, 363–375. [Google Scholar] [CrossRef]
- Castro, W.; Navarro, M.; Biot, C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013, 5, 81–96. [Google Scholar] [CrossRef]
- Dubar, F.; Anquetin, G.; Pradines, B.; Dive, D.; Khalife, J.; Biot, C. Enhancement of the Antimalarial Activity of Ciprofloxacin Using a Double Prodrug/Bioorganometallic Approach. J. Med. Chem. 2009, 52, 7954–7957. [Google Scholar] [CrossRef]
- Szczupak, Ł.; Kowalczyk, A.; Trzybiński, D.; Woźniak, K.; Mendoza, G.; Arruebo, M.; Steverding, D.; Stączek, P.; Kowalski, K. Organometallic ciprofloxacin conjugates with dual action: Synthesis, characterization, and antimicrobial and cytotoxicity studies. Dalton Trans. 2020, 49, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Tirkey, V.; Mishra, S.; Dash, H.R.; Das, S.; Nayak, B.P.; Mobin, S.M.; Chatterjee, S. Synthesis, characterization and antibacterial studies of ferrocenyl and cymantrenyl hydrazone compounds. J. Organomet. Chem. 2013, 732, 122–129. [Google Scholar] [CrossRef]
- Stringer, T.; Seldon, R.; Liu, N.; Warner, D.F.; Tam, C.; Cheng, L.W.; Land, K.M.; Smith, P.J.; Chibale, K.; Smith, G.S. Antimicrobial activity of organometallic isonicotinyl and pyrazinyl ferrocenyl-derived complexes. Dalton Trans. 2017, 46, 9875–9885. [Google Scholar] [CrossRef] [PubMed]
- Yagnam, S.; Trivedi, R.; Krishna, S.; Giribabu, L.; Praveena, G.; Prakasham, R.S. Bioactive isatin (oxime)-triazole-thiazolidinedione ferrocene molecular conjugates: Design, synthesis and antimicrobial activities. J. Organomet. Chem. 2021, 937, 121716. [Google Scholar] [CrossRef]
- Lewandowski, E.M.; Szczupak, Ł.; Kowalczyk, A.; Mendoza, G.; Arruebo, M.; Jacobs, L.M.C.; Stączek, P.; Chen, Y.; Kowalski, K. Metallocenyl 7-ACA Conjugates: Antibacterial Activity Studies and Atomic-Resolution X-ray Crystal Structure with CTX-M beta-Lactamase. ChemBioChem 2020, 21, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Koçak, E.; Özkul, C.; Önel, G.T.; Nemutlu, E.; Kir, S. Screening the antimicrobial effect of ferrocene-boronic acid on Pseudomonas aeruginosa using proteomics and metabolomics approach. J. Res. Pharm. 2020, 24, 812–820. [Google Scholar] [CrossRef]
- Biegański, P.; Szczupak, Ł.; Arruebo, M.; Kowalski, K. Brief survey on organometalated antibacterial drugs and metal-based materials with antibacterial activity. Chem. Biol. 2021, 2, 368–386. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Biot, C. Ferrocene-Based Antimalarials. Future Med. Chem. 2012, 4, 783–797. [Google Scholar] [CrossRef]
- Domarle, O.; Blampain, G.; Agnaniet, H.; Nzadiyabi, T.; Lebibi, J.; Brocard, J.; Maciejewski, L.; Biot, C.; Georges, A.J.; Millet, P. In vitro antimalarial activity of a new organometallic analog, ferrocene-chloroquine. Antimicrob. Agents Chemother. 1998, 42, 540–544. [Google Scholar] [CrossRef]
- Delhaes, L.; Biot, C.; Berry, L.; Delcourt, P.; Maciejewski, L.A.; Camus, D.; Brocard, J.S.; Dive, D. Synthesis of ferroquine enantiomers: First investigation of effects of metallocenic chirality upon antimalarial activity and cytotoxicity. ChemBioChem 2002, 3, 418–423. [Google Scholar] [CrossRef]
- Dubar, F.; Egan, T.J.; Pradines, B.; Kuter, D.; Ncokazi, K.K.; Forge, D.; Paul, J.-F.; Pierrot, C.; Kalamou, H.; Khalife, J.; et al. The Antimalarial Ferroquine: Role of the Metal and Intramolecular Hydrogen Bond in Activity and Resistance. ACS Chem. Biol. 2011, 6, 275–287. [Google Scholar] [CrossRef]
- Dive, D.; Biot, C. Ferroquine as an oxidative shock antimalarial. Curr. Top. Med. Chem. 2014, 14, 1684–1692. [Google Scholar] [CrossRef]
- Navarro, M.; Castro, W.; Biot, C. Bioorganometallic Compounds with Antimalarial Targets: Inhibiting Hemozoin Formation. Organometallics 2012, 31, 5715–5727. [Google Scholar] [CrossRef]
- Li, Y.; de Kock, C.; Smith, P.J.; Chibale, K.; Smith, G.S. Synthesis and evaluation of a carbosilane congener of ferroquine and its corresponding half-sandwich ruthenium and rhodium complexes for antiplasmodial and b-hematin inhibition activity. Organometallics 2014, 33, 4345–4348. [Google Scholar] [CrossRef]
- Abid, M.; Singh, S.; Egan, T.J.; Joshi, M.C. Structural-activity Relationship of Metallo-aminoquines as Next Generation Antimalarials. Curr. Top. Med. Chem. 2022, 22, 436–472. [Google Scholar] [CrossRef] [PubMed]
- Chopin, N.; Bosson, J.; Iikawa, S.; Picot, S.; Bienvenu, A.-L.; Lavoignat, A.; Bonnot, G.; Riou, M.; Beaugé, C.; Guillory, V.; et al. Evaluation of ferrocenyl-containing g-hydroxy- g -lactam-derived tetramates as potential antiplasmodials. Eur. J. Med. Chem. 2022, 243, 114735. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; Dingle, L.M.K.; Swart, T.; Cash, D.; Laming, D.; de la Mare, J.-A.; Taylor, D.; Hoppe, H.C.; Biot, C.; Edkins, A.L.; et al. The in Vitro Antiplasmodial and Antiproliferative Activity of New Ferrocene-Based α-Aminocresols Targeting Hemozoin Inhibition and DNA Interaction. ChemBioChem 2020, 21, 2643–2658. [Google Scholar] [CrossRef]
- Karagöz, A.; Leidenberger, M.; Hahn, F.; Hampel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T.; et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- de Lange, C.; Coertzen, D.; Smit, F.; Wentzel, J.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; N’Da, D.D. Synthesis, in vitro antimalarial activities and cytotoxicities of amino-artemisinin-ferrocene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 289–292. [Google Scholar] [CrossRef]
- de Lange, C.; Coertzen, D.; Smit, F.; Wentzel, J.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; N’Da, D.D. Synthesis, antimalarial activities and cytotoxicities of amino-artemisinin-1,2-disubstituted ferrocene hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 3161–3163. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, C.; Li, W.; Luo, Y.; Wu, Y.; Ling, H. The current scenario on anticancer activity of artemisinin metal complexes, hybrids, and dimers. Arch. Pharm. 2022, 355, e2200086. [Google Scholar] [CrossRef]
- Rubbiani, R.; Weil, T.; Tocci, N.; Mastrobuoni, L.; Jeger, S.; Moretto, M.; Ng, J.; Lin, Y.; Hess, J.; Ferrari, S.; et al. In vivo active organometallic-containg antimycotic agents. RSC Chem. Biol. 2021, 2, 1263–1273. [Google Scholar] [CrossRef]
- Shunmugaperumal, S.; Dhasarathan, S.; Selvaraj, P.K.; Kaliappan, I. Multi Metal Ion Recognizing Unsymmetrical tetra dentate Schiff bases Associated with Antifungal activity. Orient. J. Chem. 2021, 37, 833–846. [Google Scholar] [CrossRef]
- Cilli, E.M.; Costa, N.C.; Santos-Filho, N.A.; Piccoli, J.P.; Fusco-Almeida, A.M.; Santos, C.T.; de Souza, J.O.; Zanini, C.L.; Aguiar, A.C.C.; Oliva, G.; et al. New Strategies for Novel Drugs: Antimicrobial Peptides Containing Ferrocene with Improved Antifungal and Antiplasmodial Biological Activity. Protein Pept. Lett. 2022, 29, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Patujo, J.; Azeem, M.; Khan, M.; Muhammad, H.; Raheel, A.; Fatima, S.; Mirza, B.; Hussain, Z.; Badshah, A. Assessing the biological potential of new symmetrical ferrocene based bisthiourea analogues. Biorg. Chem. 2021, 106, 104180. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Arora, A.; Kalra, P.; Maurya, I.K.; Ruizc, C.E.; Estebanc, M.A.; Sinha, S.; Goyal, K.; Sehgal, R. A strategic approach to the synthesis of ferrocene appended chalcone linked triazole allied organosilatranes: Antibacterial, antifungal, antiparasitic and antioxidant studies. Bioorg. Med. Chem. 2019, 27, 188–195. [Google Scholar] [CrossRef]
- Fenton, H.J.H. Oxidation of tartric acid in the presence of iron. J. Chem. Soc. 1894, 65, 899. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Zepp, R.G.; Faust, B.C.; Hoigne, J. Hydroxyl Radical formation in aqueous Reactions (pH 3-8) of Iron (II) with Hydrogen Peroxide- The Photo-Fenton Reaction. Environ. Sci. Technol. 1992, 26, 313–319. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Lai, C.-S.; Piette, L.H. Further evidence for OH radical production in Fenton’s reagent. Tetrahedron Lett. 1979, 9, 775–778. [Google Scholar] [CrossRef]
- Gallard, H.; de Laat, J.; Legube, B. Effect of pH on the oxidation rate of organic compounds by Fe-II/H2O2. Mechanisms and simulation. New. J. Chem. 1998, 22, 263–268. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental Implications of Hydroxyl Radicals. Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide anion radical (O2−), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef]
- Hamon, J.R.; Astruc, D. Dramatic Salt Effects on the Basic and Nucleophilic Properties of Superoxide Radical Anion Generated from O2 and Iron(I) “Electron Reservoir Complexes”. J. Am. Chem. Soc. 1983, 105, 5951–5953. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Transition metal sandwiches as reservoirs of electrons, protons, hydrogen atoms and hydrides: Molecular activation and electronics. New J. Chem. 1992, 16, 305–328. [Google Scholar]
- Cable, E.E.; Isom, H.C. Metabolism of 3,5,5-trimethylhexanoyl-ferrocene by rat liver: Release of iron from 3,5,5-trimethylhexanoyl-ferrocene by a microsomal, phenobarbital-inducible cytochrome P-450. Drug. Metab. Dispos. 1999, 27, 255–260. [Google Scholar] [PubMed]
- Connelly, N.G.; Geiger, W.E. Chemical redox agents for organometallic chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Astruc, D. Electron Transfer and Radical Processes in Transition Metal Chemistry; VCH: New York, NY, USA, 1995; ISBN 1-56081-566-3. [Google Scholar]
- Věžník, J.; Konhefr, M.; Fohlerová, Z.; Lacina, K. Redox-dependent cytotoxicity of ferrocene derivatives and ROS-activated prodrugs based on ferrocenyliminoboronates. J. Inorg. Biochem. 2021, 224, 111561. [Google Scholar] [CrossRef]
- Astruc, D.; Hamon, J.-R.; Althoff, G.; Román, E.; Batail, P.; Michaud, P.; Mariot, J.-P.; Varret, F.; Cozak, D. Design, Stabilization and Efficiency of Organometallic “Electron Reservoirs”. 19-Electron Sandwichs h5-C5R5Fe(I)h6-C6R6, a Key Class Active in Redox Catalysis. J. Am. Chem. Soc. 1979, 101, 5445–5447. [Google Scholar] [CrossRef]
- Rajasekharan, M.V.; Giesinski, S.; Ammeter, J.H.; Ostwald, N.; Michaud, P.; Hamon, J.-R.; Astruc, D. Electron Paramagnetic ESR Studies of the Electronic Structure and Dynamic Jahn-Teller Effect in Fe(I) Sandwich Compounds. J. Am. Chem. Soc. 1982, 104, 2400–2407. [Google Scholar] [CrossRef]
- Huynh, M.H.V.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2007, 107, 5004–5064. [Google Scholar] [CrossRef]
- Vessières, A.; Wang, Y.; McGlinchey, M.J.; Jaouen, G. Multifaceted chemical behaviour of metallocene (M = Fe, Os) quinone methides. Their contribution to biology. Coord. Chem. Rev. 2021, 430, 213658. [Google Scholar] [CrossRef]
- Fomin, F.M.; Zaitseva, K.S. Mechanism of the oxidation of ferrocene and its derivatives with hydrogen peroxide: A new effect. Russ. J. Phys. Chem. 2014, 88, 466–470. [Google Scholar] [CrossRef]
- Astruc, D. Transition Metal Radicals: Chameleon Structure and Catalytic Function. Acc. Chem. Res. 1991, 24, 36. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive Oxygen Species in Cancer: A Dance with the Devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef]
- Hall, E.J.; Wuu, C.-S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Rad. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.-F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Translat. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Mizutani, H.; Tada-Oikawa, S.; Hiraku, Y.; Kojima, M.; Kawanishi, S. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005, 76, 1439–1453. [Google Scholar] [CrossRef]
- Lin, L.-S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous Fenton-like Ion Delivery and Glutathione Depletion by MnO2-Based Nanoagent to Enhance Chemodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 4902–4906. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomedicine Meets Fenton Chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug. Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Turrin, C.-O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Rolland, O.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P. Dendrimers and nanomedicine: Multivalency in action. New J. Chem. 2009, 33, 1809–1824. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, C.-Y. Toward Biocompatible Semiconductor Quantum Dots: From Biosynthesis and Bioconjugation to Biomedical Application. Chem. Rev. 2015, 115, 11669–11717. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug. Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Salmon, L.; Ruiz, J.; Astruc, D. How to very efficiently functionalize gold nanoparticles by “click” chemistry. Chem. Commun. 2008, 44, 5788–5790. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal-Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Wei, H.; Quan, C.-Y.; Chang, C.; Zhang, X.-Z.; Zhuo, R.-X. Preparation of Novel Ferrocene-Based Shell Cross-Linked Thermoresponsive Hybrid Micelles with Antitumor Efficacy. J. Phys. Chem. B 2010, 114, 5309–5314. [Google Scholar] [CrossRef]
- Allard, E.; Jarnet, D.; Vessières, A.; Vinchon-Petit, S.; Jaouen, G.; Benoit, J.-P.; Passirani, C. Local Delivery of Ferrociphenol Lipid Nanocapsules Followed by External Radiotherapy as a Synergistic Treatment Against Intracranial 9L Glioma Xenograft. Pharm. Res. 2010, 27, 56–64. [Google Scholar] [CrossRef]
- Yang, K.; Yu, G.; Yang, Z.; Yue, L.; Zhang, X.; Sun, C.; Wei, J.; Rao, L.; Chen, X.; Wang, R. Supramolecular Polymerization-Induced Nanoassemblies for Self-Augmented Cascade Chemotherapy and Chemodynamic Therapy of Tumor. Angew. Chem. Int. Ed. 2021, 60, 17570–17578. [Google Scholar] [CrossRef]
- Gu, H.; Mu, S.; Qiu, G.; Liu, X.; Zhang, L.; Yuan, Y.; Astruc, D. Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release. Coord. Chem. Rev. 2018, 364, 51–85. [Google Scholar] [CrossRef]
- Su, H.; Cui, Y.; Wang, F.; Zhang, W.; Zhang, C.; Wang, R. Theranostic supramolecular polymers formed by the self-assembly of a metal-chelating prodrug. Biomater. Sci. 2021, 9, 463–470. [Google Scholar] [CrossRef]
- Huo, Y.; He, Z.; Wang, C.; Zhang, L.; Xuan, Q.; Wei, S.; Wang, Y.; Pan, D.; Dong, B.; Wei, R.; et al. The recent progress of synergistic supramolecular polymers: Preparation, properties and applications. Chem. Commun. 2021, 57, 1413–1429. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, Z.; Hou, C.; Liu, J. Protein-based supramolecular polymers: Progress and prospect. Chem. Commun. 2014, 50, 9997–10007. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.-Q.; Yi, Y.; Wei, Z.-Y.; Chen, H.; Xu, J.-F.; Wang, H.; Zhao, Y.; Zhang, X. Supramolecular Peptide Therapeutics: Host–Guest Interaction-Assisted Systemic Delivery of Anticancer Peptides. CCS Chem. 2020, 2, 739–748. [Google Scholar] [CrossRef]
- Chen, X.-M.; Chen, Y.; Yu, Q.; Gu, B.-H.; Liu, Y. Supramolecular Assemblies with Near-Infrared Emission Mediated in Two Stages by Cucurbituril and Amphiphilic Calixarene for Lysosome-Targeted Cell Imaging. Angew. Chem. Int. Ed. 2018, 57, 12519–12523. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhao, X.; Zhou, J.; Mao, Z.; Huang, X.; Wang, Z.; Hua, B.; Liu, Y.; Zhang, F.; He, Z.; et al. Supramolecular Polymer-Based Nanomedicine: High Therapeutic Performance and Negligible Long-Term Immunotoxicity. J. Am. Chem. Soc. 2018, 140, 8005–8019. [Google Scholar] [CrossRef]
- Liu, L.; Rui, L.; Gao, Y.; Zhang, W. Self-assembly and disassembly of a redox-responsive ferrocene-containing amphiphilic block copolymer for controlled release. Polym. Chem. 2014, 6, 1817–1829. [Google Scholar] [CrossRef]
- Ni, M.; Zhang, N.; Xia, W.; Wu, X.; Yao, C.; Liu, X.; Hu, X.-Y.; Lin, C.; Wang, L. Dramatically Promoted Swelling of a Hydrogel by Pillar[6]arene-Ferrocene Complexation with Multistimuli Responsiveness. J. Am. Chem. Soc. 2016, 138, 6643–6649. [Google Scholar] [CrossRef]
- Tan, J.; Li, H.; Hu, X.; Abdullah, R.; Xie, S.; Zhang, L.; Zhao, M.; Luo, Q.; Li, Y.; Sun, Z.; et al. Size-Tunable Assemblies Based on Ferrocene-Containing DNA Polymers for Spatially Uniform Penetration. Chem 2019, 5, 1775–1792. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, C.; Ma, Y.; Zhu, L.; Lu, B.; Wang, Y.; Wang, J.; Dong, C.-M.; Yao, Y. Tumor microenvironment responsive polypeptide-based supramolecular nanoprodrugs for combination therapy. Acta Biomater. 2022, 146, 396–405. [Google Scholar] [CrossRef]
- Lou, H.; Fang, H.; Wang, T.; Wang, D.; Han, Q.; Zhou, W.; Song, Y.; Tan, W.; Zhou, B. Biodegradable Porous Polymeric Drug with pH-Stimuli-Responsive Delivery Capacity for Combined Cancer Therapy. ACS Appl. Polym. Mater. 2021, 4, 714–724. [Google Scholar] [CrossRef]
- Lu, X.; Wang, X.; Li, A.; Zhou, T.; Zhang, L.; Qu, J.; Mao, Z.; Gu, X.; Zhang, X.; Jing, S. Ferrocenylseleno-dopamine functionalized carbon dots for redox-gated imaging and drug delivery in cancer cells. Dyes Pigm. 2022, 205, 110586. [Google Scholar] [CrossRef]
- Park, S.-C.; Kim, N.-H.; Yang, W.; Nah, J.-W.; Jang, M.-K.; Lee, D. Polymeric micellar nanoplatforms for Fenton reaction as a new class of antibacterial agents. J. Control. Release 2016, 221, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wei, P.; Yang, M.; Chen, W.; Zhao, B.; Li, W.; Wang, J.; Qiu, L.; Chen, J. Redox-sensitive hyaluronic acid-ferrocene micelles delivering doxorubicin for enhanced tumor treatment by synergistic chemo/chemodynamic therapy. J. Drug Deliv. Sci. Technol. 2022, 77, 103851. [Google Scholar] [CrossRef]
- Yang, F.; Song, C.; Ji, R.; Song, X.; Yang, B.; Lv, Y.; Wei, Z. Increasing the Production of Reactive Oxygen Species through a Ferroptosis Pathway Disrupts the Redox Balance of Tumor Cells for Cancer Treatment. ACS Appl. Polym. Mater. 2022, 4, 5001–5011. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, X.; Cheng, Q.; Wang, Y.; Wang, R.; Cheng, X.; Xu, J.; Liu, K.; Li, L.; Li, X.; et al. Ferrocene Functionalized Upconversion Nanoparticle Nanosystem with Efficient Near-Infrared-Light-Promoted Fenton-Like Reaction for Tumor Growth Suppression. Inorg. Chem. 2020, 59, 9177–9187. [Google Scholar] [CrossRef] [PubMed]
- Thiem, H.J.; Brandl, M.; Breslow, R. Molecular modeling calculations on the acylation of beta-cyclodextrin by ferrocenylacrylate esters. J. Am. Chem. Soc. 1988, 110, 8612–8616. [Google Scholar] [CrossRef]
- Moreno, S.; Hübner, H.; Effenberg, C.; Boye, S.; Ramuglia, A.; Schmitt, D.; Voit, B.; Weidinger, I.M.; Gallei, M.; Appelhans, D. Redox- and pH-Responsive Polymersomes with Ferrocene Moieties Exhibiting Peroxidase-like, Chemoenzymatic Activity and H2O2-Responsive Release Behavior. Biomacromolecules 2022, 23, 4655–4667. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, W.; Ke, W.; Chen, W.; He, C.; Ge, Z. Multifunctional Polymeric Micelles with Amplified Fenton Reaction for Tumor Ablation. Biomacromolecules 2018, 19, 1990–1998. [Google Scholar] [CrossRef]
- Xing, C.; Chen, H.; Guan, Y.; Zhang, S.; Tong, T.; Ding, N.; Luo, T.; Kang, Y.; Pang, J. Cyclodextrin-based supramolecular nanoparticles break the redox balance in chemodynamic therapy-enhanced chemotherapy. J. Colloid Interface Sci. 2022, 628, 864–876. [Google Scholar] [CrossRef]
- Liang, J.; Zheng, Y.; Wu, X.; Tan, C.; Ji, L.; Mao, Z. A Tailored Multifunctional Anticancer Nanodelivery System for Ruthenium-Based Photosensitizers: Tumor Microenvironment Adaption and Remodeling. Adv. Sci. 2020, 7, 1901992. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Meng, C.; Zhu, P.; Liu, X.; Qian, J.; Ling, S.; Zhang, Y.; Ling, Y. Pillar[6]arene-Based Supramolecular Nanocatalysts for Synergistically Enhanced Chemodynamic Therapy by the Intracellular Cascade Reaction. ACS Appl. Mater. Interfaces 2021, 13, 53574–53585. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, H.; Wang, L.; Liu, X.; Lin, T.; Haq, F.; Vatsadze, S.Z.; Lemenovskiy, D.A. Lemenovskiy, Ferrocene-contained metal organic frameworks: From synthesis to applications. Chem. Rev. 2021, 430, 213737. [Google Scholar] [CrossRef]
- Fang, C.; Deng, Z.; Cao, G.; Chu, Q.; Wu, Y.; Li, X.; Peng, X.; Han, G. Co–Ferrocene MOF/Glucose Oxidase as Cascade Nanozyme for Effective Tumor Therapy. Adv. Funct. Mater. 2020, 30, 1910085. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, H.; Wang, L.; Liu, J.; Shea, K.J. Ferrocene-based metal–organic framework nanosheets loaded with palladium as a super-high active hydrogenation catalyst. J. Mater. Chem. A 2019, 7, 15975–15980. [Google Scholar] [CrossRef]
- Ma, J.; Wong-Foy, A.G.; Matzger, A.J. The Role of Modulators in Controlling Layer Spacings in a Tritopic Linker Based Zirconium 2D Microporous Coordination Polymer. Inorg. Chem. 2015, 54, 4591–4593. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.-J.; Peng, X. One Stone Two Birds: Zr-Fc Metal–Organic Framework Nanosheet for Synergistic Photothermal and Chemodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20321–20330. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst dendrimers- Molecular-level control of size, shape, surface chemistry, topology and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons. In Concepts, Syntheses, Applications; Wiley-VCH: Wenheim, Germany, 2001; ISBN 3-527-2997-1. [Google Scholar]
- Caminade, A.-M.; Turin, C.-O.; Laurent, R.; Ouali, A.; Delavaut-Nicot, B. Dendrimers. In Towards Catalytic, Material and Biomedical Uses; Wiley: Chichester, UK, 2011; ISBN 978-0-470-74881-7. [Google Scholar]
- Lee, C.C.; Mackay, J.A.; Frechet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Boas, U.; Heegaard, P.M.H. Dendrimers in drug research. Chem. Soc. Rev. 2004, 33, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D. Research avenues on dendrimers towards molecular biology: From biomimetism to medicinal engineering. C. R. Acad. Sci. Ser. B 1996, 322, 757–766. [Google Scholar]
- Guo, Y.; Zhang, X.; Sun, W.; Jia, H.-R.; Zhu, Y.-X.; Zhang, X.; Zhou, N.; Wu, F.-G. Metal-Phenolic Network-Based Nanocomplexes that Evoke Ferroptosis by Apoptosis: Promoted Nuclear Drug Influx and Reversed Drug Resistance of Cancer. Chem. Mater. 2019, 31, 10071–10084. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Fan, Y.; Chen, J.; Wang, H.; Shen, M.; Shi, X. Metal-Phenolic-Network-Coated Dendrimer-Drug Conjugates for Tumor MR Imaging and Chemo/Chemodynamic Therapy via Amplification of Endoplasmic Reticulum Stress. Adv. Mater. 2022, 34, 2107009. [Google Scholar] [CrossRef]
- Casado, C.M.; Cuadrado, I.; Morán, M.; Alonso, B.; García, B.; González, B.; Losada, J. Redox-active ferrocenyl dendrimers and polymers in solution and immobilised on electrode surfaces. Coord. Chem. Rev. 1999, 185–186, 53–79. [Google Scholar] [CrossRef]
- Daniel, M.-C.; Ruiz, J.; Blais, J.-C.; Daro, N.; Astruc, D. Synthesis of five generations of redox-stable pentamethylamidoferrocenyl dendrimers and comparison of amidoferrocenyl- and pentamethylamidoferrocenyl dendrimers as electrochemical exoreceptors. Chem. Eur. J. 2003, 9, 4371–4379. [Google Scholar] [CrossRef]
- Ornelas, C.; Aranzaes, J.R.; Cloutet, E.; Alves, S.; Astruc, D. Click assembly of 1,2,3-triazole-linked dendrimers, including ferrocenyl dendrimers, which sense both oxo anions and metal cations. Angew. Chem. Int. Ed. 2007, 46, 872–877. [Google Scholar] [CrossRef]
- Ornelas, C.; Ruiz, J.; Belin, C.; Astruc, D. Giant Dendritic Molecular Electrochrome Batteries with Ferrocenyl and Pentamethylferrocenyl Termini. J. Am. Chem. Soc. 2009, 131, 590–601. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Liu, S.; Feng, G.; Tang, B.Z.; Liu, B. Recent advances of AIE light-up probes for photodynamic therapy. Chem. Sci. 2021, 12, 6488–6506. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Klajnert-Maculewicz, B.; Johnson, K.A.-M.; Brinkman, H.F.; Janaszewska, A.; Hedstrand, D.M. Non-Traditional Intrinsic Luminescence: Inexplicable Blue Fluorescence Observed For Dendrimers, Macromolecules And Small Molecular Structures Lacking Traditional/Conventional Luminophores. Prog. Polym. Sci. 2019, 90, 35–117. [Google Scholar] [CrossRef]
- Studzian, M.; Działak, P.; Pułaski, Ł.; Hedstrand, D.M.; Tomalia, D.A.; Klajnert-Maculewicz, B. Synthesis, Internalization and Visualization of N-(4-Carbomethoxy) Pyrrolidone Terminated PAMAM [G5:G3-TREN] Tecto(dendrimers) in Mammalian Cells. Molecules 2020, 25, 4406. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, K.T.; Sakthivel, T.; Florence, A.T. Solubilisation And Transformation of Amphipathic Lipidic Dendron Vesicles (Dendrisomes) Into Mixed Micelles. Colloids Surf. A 2005, 268, 52–59. [Google Scholar] [CrossRef]
- Xiao, Q.; Rubien, J.D.; Wang, Z.; Reed, E.H.; Hammer, D.A.; Sahoo, D.; Heiney, P.A.; Yadavalli, S.S.; Goulian, M.; Wilner, S.E.; et al. Self-Sorting and Coassembly of Fluorinated, Hydrogenated, and Hybrid Janus Dendrimers into Dendrimersomes. J. Am. Chem. Soc. 2016, 138, 12655–12663. [Google Scholar] [CrossRef]
- Perli, G.; Wang, Q.; Braga, C.B.; Bertuzzi, D.L.; Fontana, L.A.; Soares, M.C.P.; Ruiz, J.; Megiatto, J.D.; Astruc, D.; Ornelas, C. Self-Assembly of a Triazolylferrocenyl Dendrimer in Water Yields Nontraditional Intrinsic Green Fluorescent Vesosomes for Nanotheranostic Applications. J. Am. Chem. Soc. 2021, 143, 12948–12954. [Google Scholar] [CrossRef]
- Bertuzzi, D.L.; Braga, C.B.; Perli, G.; Ornelas, C. Water-Soluble Well-Defined Bifunctional Ferrocenyl Dendrimer with Anti-Cancer Activity. Eur. J. Inorg. Chem. 2022, e202101084. [Google Scholar] [CrossRef]
- Ornelas, C. Brief Timelapse on Dendrimer Chemistry: Advances, Limitations, and Expectations. Macromol. Chem. Phys. 2016, 217, 149–174. [Google Scholar] [CrossRef]
- Baartzes, N.; Szabo, C.; Cenariu, M.; Imre-Lucaci, F.; Dorneanu, S.A.; Fischer-Fodor, E.; Smith, G.S. In vitro antitumour activity of two ferrocenyl metallodendrimers in a colon cancer cell line. Inorg. Chem. Commun. 2018, 98, 75–79. [Google Scholar] [CrossRef]
- Konkoľová, E.; Janočková, J.; Perjési, P.; Vašková, J.; Kožurková, M. Selected ferrocenyl chalcones as DNA/BSA-interacting agents and inhibitors of DNA topoisomerase I and II activity. J. Organomet. Chem. 2018, 861, 1–9. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Snegur, L.V. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics 2022, 10, 226. [Google Scholar] [CrossRef]
- Peters, D.G.; Purnell, C.J.; Haaf, M.P.; Yang, Q.X.; Connor, J.R.; Meadowcroft, M.D. Dietary lipophilic iron accelerates regional brain iron-load in C57BL6 mice. Brain Struct. Funct. 2018, 223, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Kumar, V. Has ferrocene really delivered its role in accentuating the bioactivity of organic scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef]
- Abbas, G.; Irfan, A.; Ahmed, I.; Al-Zeidaneen, F.K.; Muthu, S.; Fuhr, O.; Thomas, R. Synthesis and investigation of anti-COVID-19 ability of ferrocene Schiff base derivatives by quantum chemical and molecular docking. J. Mol. Struct. 2022, 1253, 132242. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, Y.; An, N.; Zhang, H.; Wang, C.; Tian, W.; Huang, T. Host-guest interactions based supramolecular complexes self-assemblies for amplified chemodynamic therapy with H2O2 elevation and GSH consuption properties. Chin. Chem. Lett. 2023, 34, 107552. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- McNerney, M.P.; Doiron, K.E.; Ng, T.L.; Chang, T.Z.; Silver, P.A. Theranostic cells: Emerging clinical applications of synthetic biology. Nat. Rev. Genet. 2021, 22, 730–746. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ornelas, C.; Astruc, D. Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects. Pharmaceutics 2023, 15, 2044. https://doi.org/10.3390/pharmaceutics15082044
Ornelas C, Astruc D. Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects. Pharmaceutics. 2023; 15(8):2044. https://doi.org/10.3390/pharmaceutics15082044
Chicago/Turabian StyleOrnelas, Catia, and Didier Astruc. 2023. "Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects" Pharmaceutics 15, no. 8: 2044. https://doi.org/10.3390/pharmaceutics15082044
APA StyleOrnelas, C., & Astruc, D. (2023). Ferrocene-Based Drugs, Delivery Nanomaterials and Fenton Mechanism: State of the Art, Recent Developments and Prospects. Pharmaceutics, 15(8), 2044. https://doi.org/10.3390/pharmaceutics15082044