Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation
Abstract
:1. Introduction
- The level of nitrites in the formulation;
- The percentage of conversion of nitrites into nitrosamines.
2. Experimental Design
2.1. Case Studies on the Impact of Excipient Type and Supplier on Nitrosamine Formation
- The level of nitrite in each excipient;
- The MW of the nitrosamine that is expected to form;
- The tablet dose weight;
- The expected percentage of conversion of nitrite into nitrosamine;
- The dosage form composition.
2.1.1. Theoretical Nitrosamine Formation per Excipient
2.1.2. Total Theoretical Formation of Nitrosamine in the Tablet
2.2. Evaluation of Impact of Nitrosamine MW, Tablet Dose Weight, and Percentage of Conversion on Nitrosamine Formation
3. Results
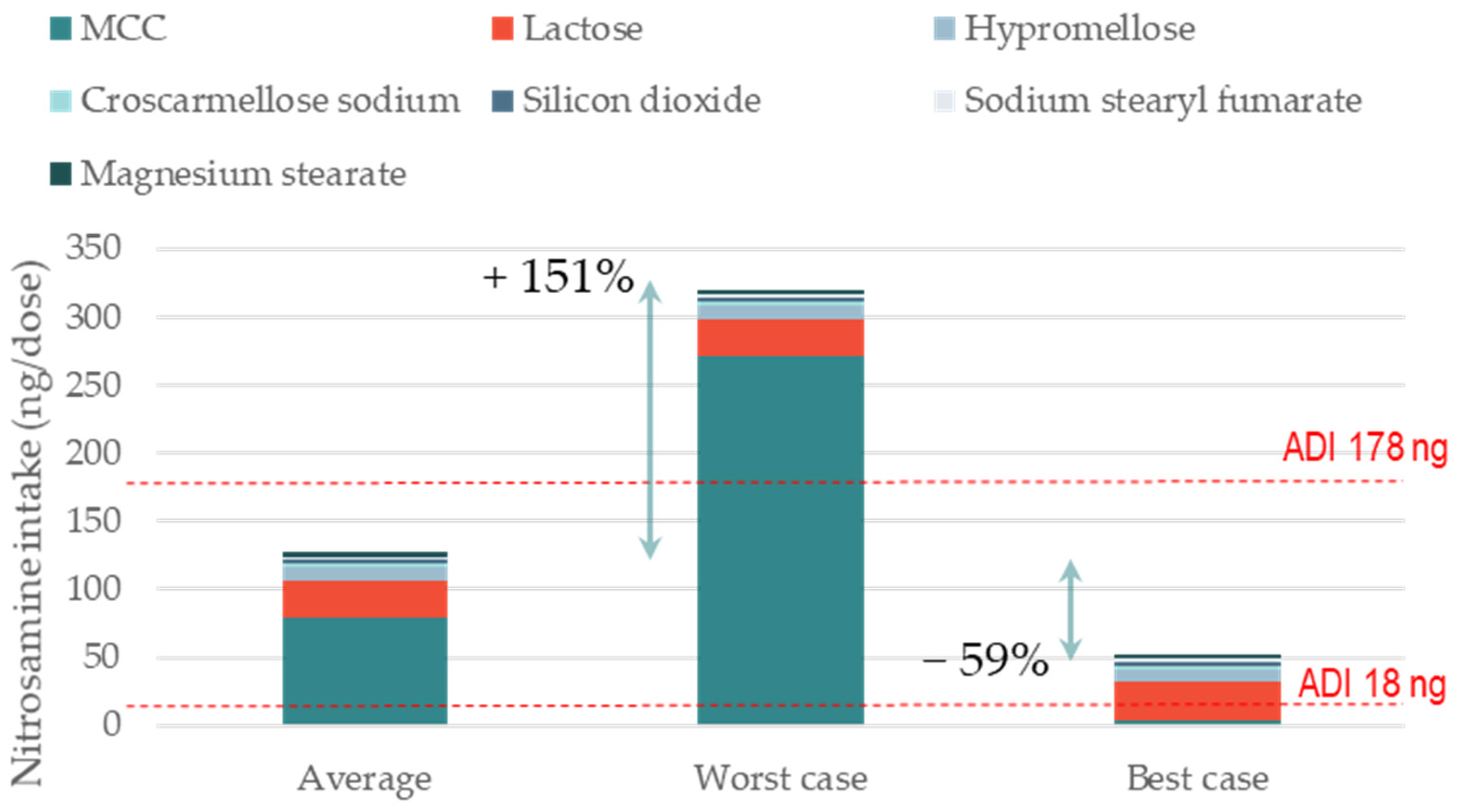
3.1. Impact of Excipient Supplier Selection on the Formation of Nitrosamine Drug Substance-Related Impurities (NDSRIs)
3.2. Impact of Re-Formulation on NDSRI Formation
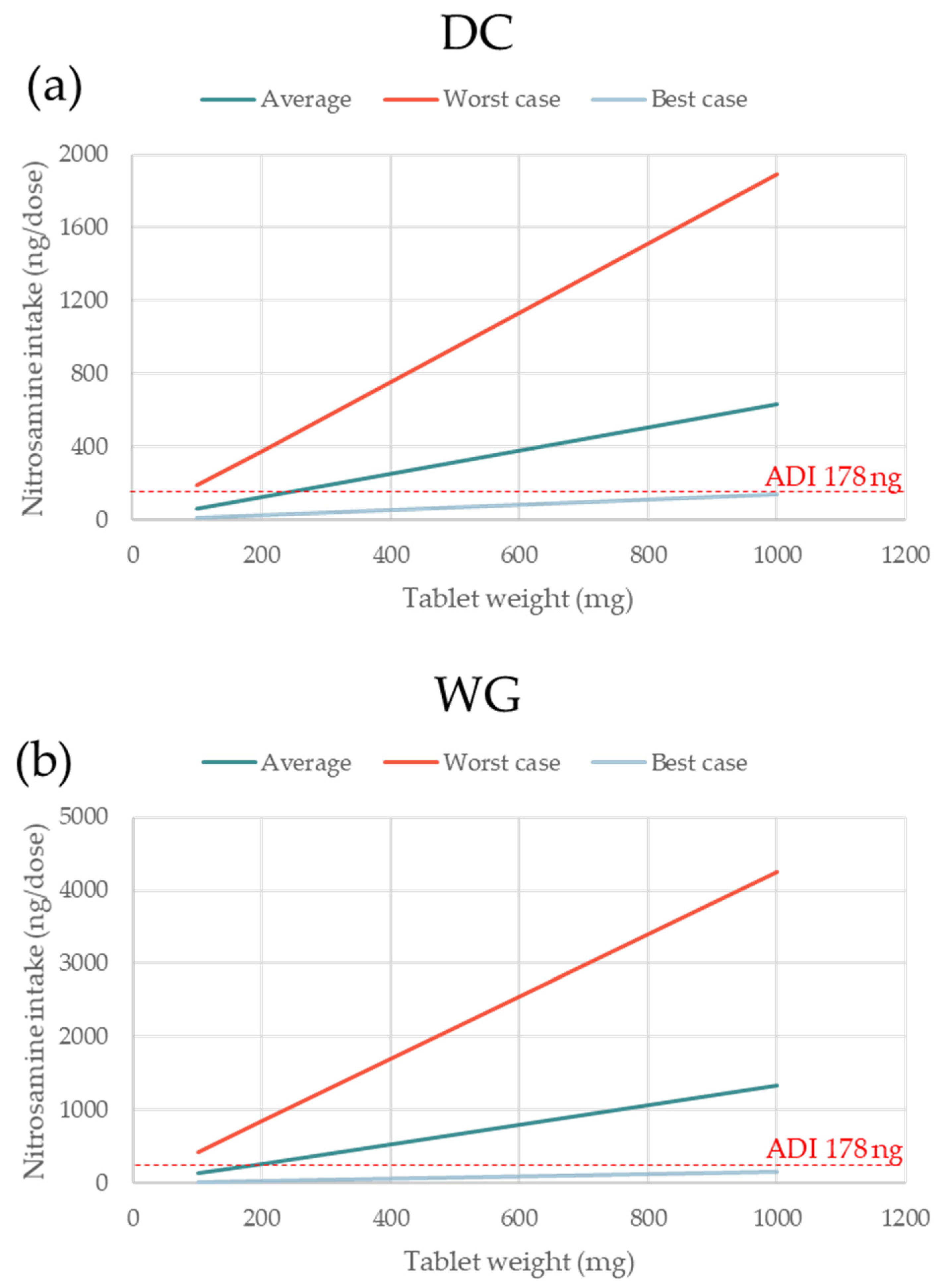
3.3. Impact of Nitrosamine MW and Tablet Dose Weight on Nitrosamine Formation
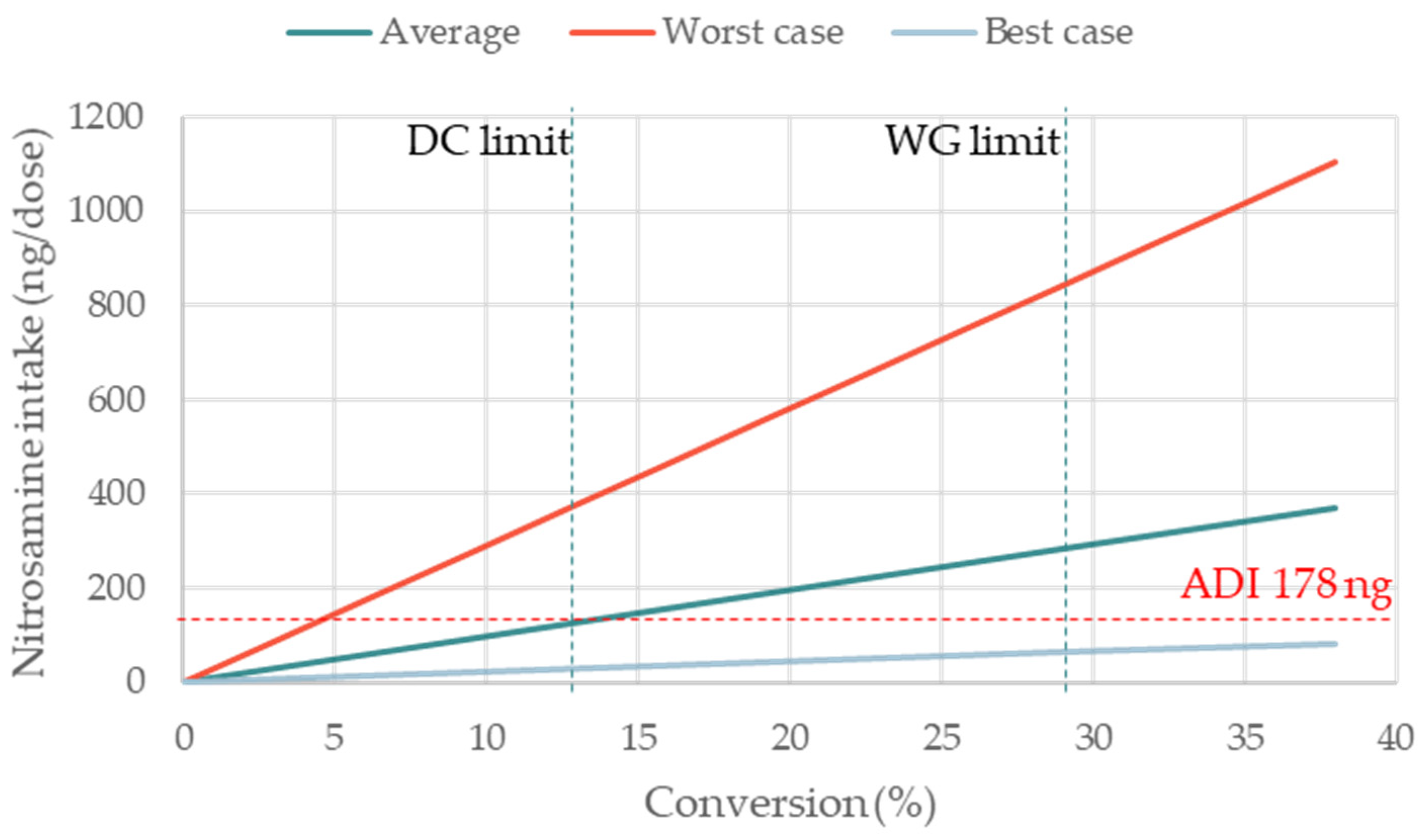
3.4. Impact of the Percentage of Conversion on NDSRIs Formation
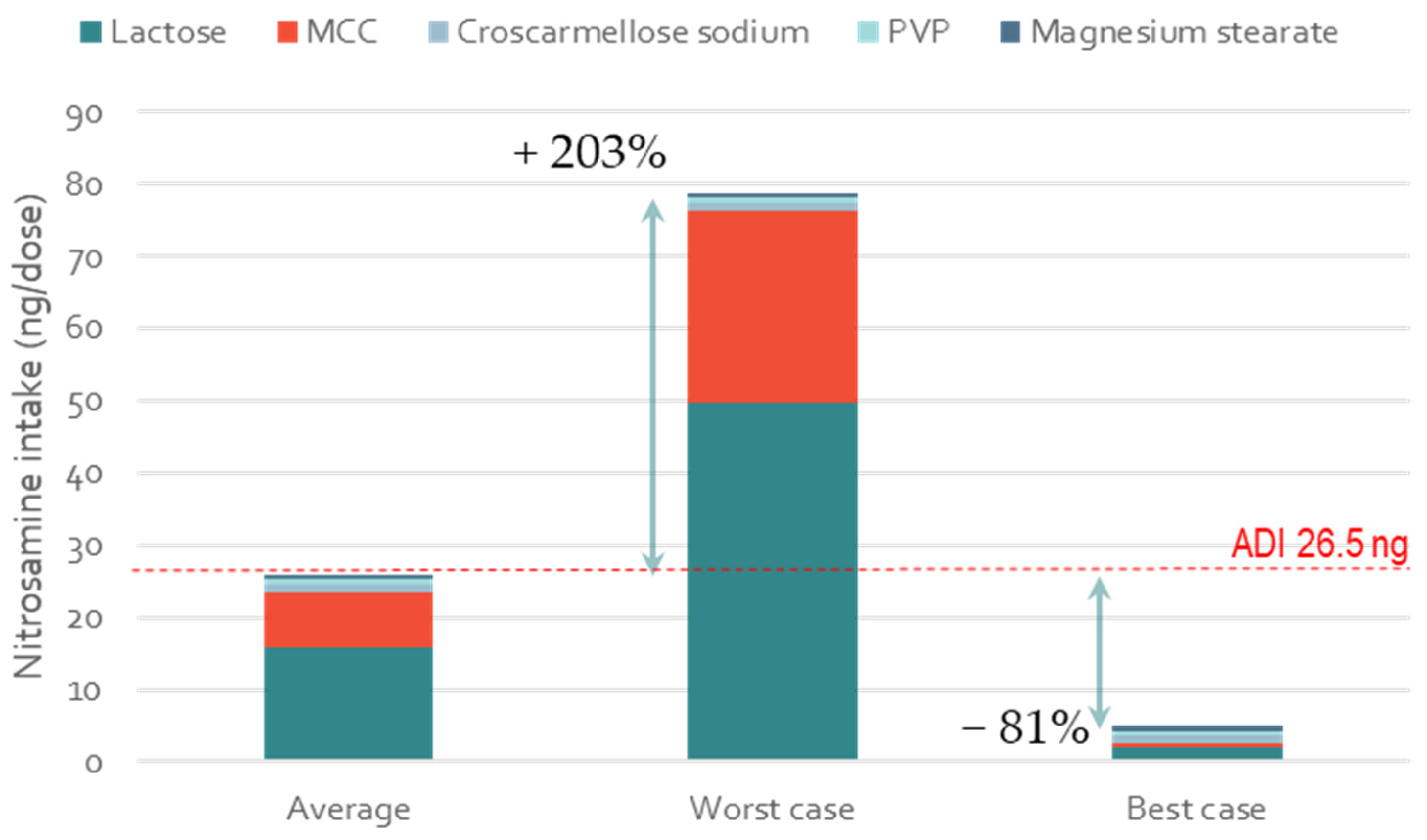
3.5. Impact of Excipient Supplier Selection on the Formation of Low-Molecular-Weight Nitrosamines
3.6. A Tool for the Calculation of Nitrosamine Formation in Oral Solid Dosage
- The tablet dose weight;
- The dosage form composition (in percentage);
- The level of nitrite in each ingredient;
- The MW of the nitrosamine that is expected to form;
- The expected percentage of conversion of nitrite into nitrosamines.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jireš, J.; Douša, M.; Gibala, P.; Kubelka, T. N-Nitrosation in the absence of nitrosating agents in pharmaceuticals? J. Pharm. Biomed. Anal. 2022, 218, 114872. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, G.; Martelli, A. Genotoxic and carcinogenic risk to humans of drug–nitrite interaction products. Mutat. Res. Mol. Mech. Mutagen. 2007, 635, 17–52. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, A. Mutagenic Impurities: Strategies for Identification and Control; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Schlingemann, J.; Burns, M.J.; Ponting, D.J.; Avila, C.M.; Romero, N.E.; Jaywant, M.A.; Smith, G.F.; Ashworth, I.W.; Simon, S.; Saal, C.; et al. The Landscape of Potential Small and Drug Substance Related Nitrosamines in Pharmaceuticals. J. Pharm. Sci. 2022, 112, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Tennant, R.E.; Oliveira, A.A.F.; Ponting, D.J. What Makes a Potent Nitrosamine? Statistical Validation of Expert-Derived Structure–Activity Relationships. Chem. Res. Toxicol. 2022, 35, 1997–2013. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.C.; Swager, T.M. An Organic Chemist’s Guide to N-Nitrosamines: Their Structure, Reactivity, and Role as Contaminants. J. Org. Chem. 2021, 86, 2037–2057. [Google Scholar] [CrossRef] [PubMed]
- Cross, K.P.; Ponting, D.J. Developing structure-activity relationships for N-nitrosamine activity. Comput. Toxicol. 2021, 20, 100186. [Google Scholar] [CrossRef] [PubMed]
- Homšak, M.; Trampuž, M.; Naveršnik, K.; Kitanovski, Z.; Žnidarič, M.; Kiefer, M.; Časar, Z. Assessment of a Diverse Array of Nitrite Scavengers in Solution and Solid State: A Study of Inhibitory Effect on the Formation of Alkyl-Aryl and Dialkyl N-Nitrosamine Derivatives. Processes 2022, 10, 2428. [Google Scholar] [CrossRef]
- Hao, G.; Hu, R.; Wang, X.; Gao, P.; Wang, L.; Jiang, M.; Xin, L.; Tan, G.; Zhao, Y.; Sun, F.; et al. N-Nitrosodimethylamine formation in metformin hydrochloride sustained-release tablets: Effects of metformin and hypromellose used in drug product formulation. J. Pharm. Biomed. Anal. 2023, 222, 115066. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Control of Nitrosamine Impurities in Human Drugs: Guidance for Industry; Center for Drug Evaluation and Research: Silver Spring, MD, USA, 2021. [Google Scholar]
- Boetzel, R.; Schlingemann, J.; Hickert, S.; Korn, C.; Kocks, G.; Luck, B.; Blom, G.; Harrison, M.; François, M.; Allain, L.; et al. A Nitrite Excipient Database: A Useful Tool to Support N-Nitrosamine Risk Assessments for Drug Products. J. Pharm. Sci. 2022, 112, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.; Ashworth, I.W.; Harris, L.; Hillier, M.C.; Nanda, K.K.; Scrivens, G. N-Nitrosamine Formation in Pharmaceutical Solid Drug Products: Experimental Observations. J. Pharm. Sci. 2023, 112, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Schlingemann, J.; Boucley, C.; Hickert, S.; Bourasseau, L.; Walker, M.; Celdran, C.; Chemarin, T.; Pegues, C.; Fritzsche, M.; Keitel, J.; et al. Avoiding N-nitrosodimethylamine formation in metformin pharmaceuticals by limiting dimethylamine and nitrite. Int. J. Pharm. 2022, 620, 121740. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Updates on Possible Mitigation Strategies to Reduce the Risk of Nitrosamine Drug Substance-Related Impurities in Drug Products. Internet 2021. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/updates-possible-mitigation-strategies-reduce-risk-nitrosamine-drug-substance-related-impurities (accessed on 16 May 2023).
- DFE Pharma. Wet Granulation of Milled Lactose. Available online: https://dfepharma.com/media/v0uaffeh/wet-granulation-of-milled-lactose.pdf (accessed on 16 May 2023).
- EMA. Questions and Answers for Marketing Authorisation Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral on Nitrosamine Impurities in Human Medicinal Products. EMA/409815/2020 Rev11. Published Online 2022. Available online: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (accessed on 16 May 2023).
- Carloni, L.-E.; Lochner, S.; Sterckx, H.; Van Daele, T. Solid State Kinetics of Nitrosation Using Native Sources of Nitrite. J. Pharm. Sci. 2023, 112, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Bayne, A.-C.V.; Misic, Z.; Stemmler, R.T.; Wittner, M.; Frerichs, M.; Bird, J.K.; Besheer, A. N-nitrosamine Mitigation with Nitrite Scavengers in Oral Pharmaceutical Drug Products. J. Pharm. Sci. 2023, 112, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- EMA. Questions and Answers for Marketing Authorisation Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral on Nitrosamine Impurities in Human Medicinal Products. EMA/409815/2020 Rev16. Published Online 2023. Available online: https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders/applicants-chmp-opinion-article-53-regulation-ec-no-726/2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (accessed on 15 July 2023).




| Nitrosamine Studied | Case Study of Replacement | Nitrosamine MW | Tablet Dose Weight (mg) | Amine Type | Amine Form | Manufacturing | Expected Percentage of Conversion |
|---|---|---|---|---|---|---|---|
| Nitrosamine drug substance-related impurity (NDSRI) | MCC supplier | 400 | 200 | 2nd | Salt | DC | 13% |
| Lactose supplier | 400 | 200 | 2nd | Salt | WG | 29% | |
| Superdisintegrant type | 400 | 200 | 2nd | Salt | WG | 29% | |
| MCC + lactose supplier + Superdisintegrant type and supplier | 400 | 200 | 2nd | Salt | WG | 29% | |
| Small nitrosamine N-Nitrosodiethylamine (NDEA) | MCC + lactose supplier | 102 | 1000 | 2nd | Salt | WG | 2.2% |
| NDSRI Case Studies | NDEA Case Study | ||||
|---|---|---|---|---|---|
| MCC Supplier | Lactose Supplier | Superdisintegrant Type | MCC + Lactose Supplier + Superdisintegrant Type and Supplier | MCC + Lactose Supplier | |
| Manufacturing | DC | WG | WG | WG | WG |
| Ingredients | |||||
| API | 15% | 10% | 10% | 10% | 10% |
| MCC | 50% | 22.5% | 22.5% | 22.5% | 22.5% |
| Lactose | 22.5% | 60% | 60% | 60% | 60% |
| Croscarmellose sodium | 3% | 5% | 5% (either/or) | 5% (either/or) | 5% |
| Crospovidone | - | - | - | ||
| Hypromellose | 5% | - | - | - | - |
| Povidone | - | 2% | 2% | 2% | 2% |
| Silicon dioxide | 1% | - | - | - | - |
| Sodium stearyl fumarate | 3% | - | - | - | - |
| Magnesium stearate | 0.5% | 0.5% | 0.5% | 0.5% | 0.5% |
| Parameter | Impact of Nitrosamine MW | Impact of Dose Weight | Impact of Percentage of Conversion |
|---|---|---|---|
| Nitrosamine MW (g/mol) | Variable | 400 | 400 |
| Tablet dose weight (mg) | 200 | Variable | 200 |
| Amine type | 2nd | 2nd | Variable |
| Amine form | Salt | Salt | Variable |
| Manufacturing | DC/WG | DC/WG | Variable |
| Percentage of conversion | 13%/29% | 13%/29% | Variable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berardi, A.; Jaspers, M.; Dickhoff, B.H.J. Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation. Pharmaceutics 2023, 15, 2015. https://doi.org/10.3390/pharmaceutics15082015
Berardi A, Jaspers M, Dickhoff BHJ. Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation. Pharmaceutics. 2023; 15(8):2015. https://doi.org/10.3390/pharmaceutics15082015
Chicago/Turabian StyleBerardi, Alberto, Maarten Jaspers, and Bastiaan H. J. Dickhoff. 2023. "Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation" Pharmaceutics 15, no. 8: 2015. https://doi.org/10.3390/pharmaceutics15082015
APA StyleBerardi, A., Jaspers, M., & Dickhoff, B. H. J. (2023). Modeling the Impact of Excipients Selection on Nitrosamine Formation towards Risk Mitigation. Pharmaceutics, 15(8), 2015. https://doi.org/10.3390/pharmaceutics15082015






