An In Vivo Electroencephalographic Analysis of the Effect of Riluzole against Limbic and Absence Seizure and Comparison with Glutamate Antagonists
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
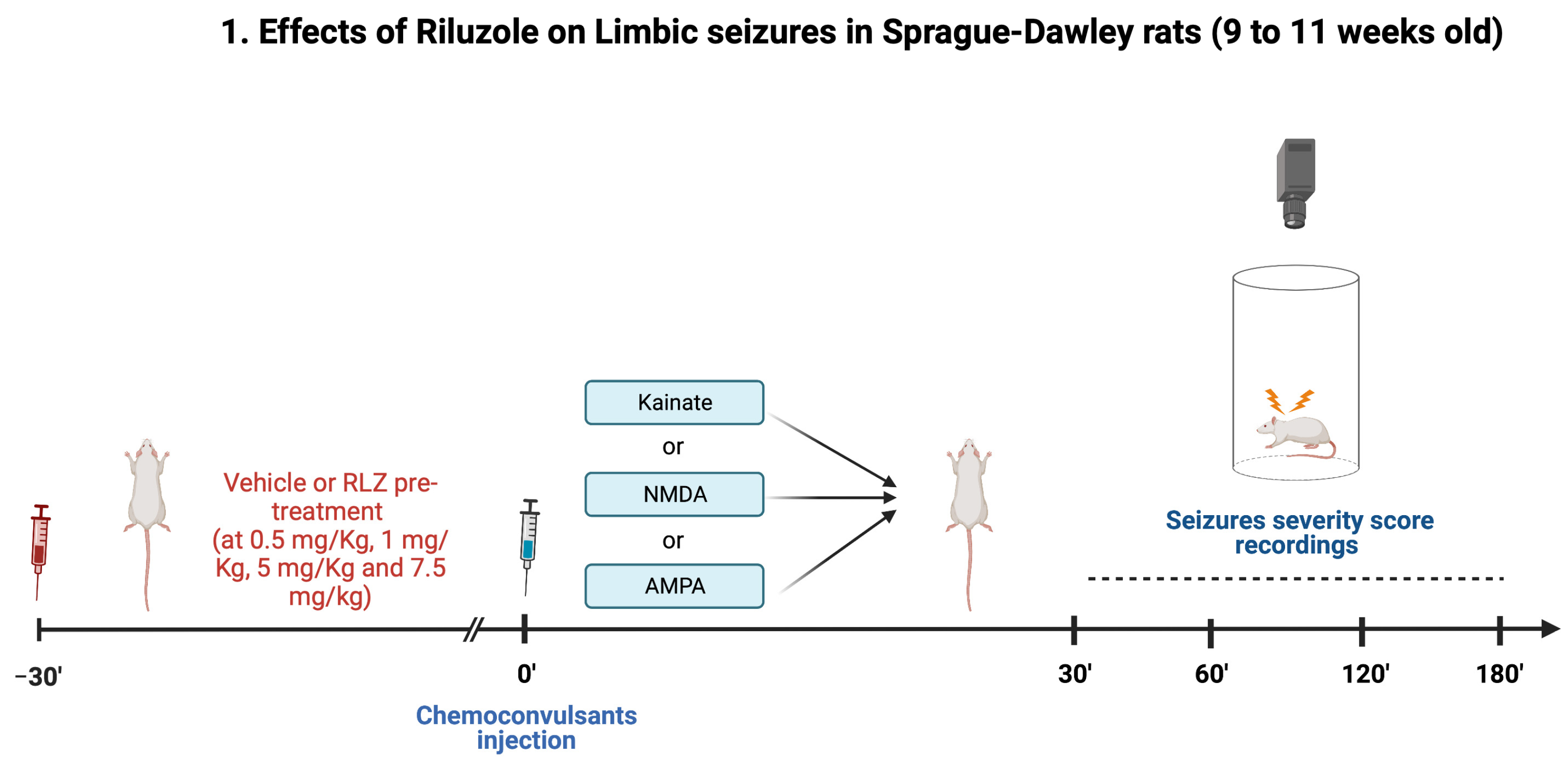
2.3. Experimental Protocol in Sprague–Dawley Rats
- (1)
- vehicle + KA;
- (2)
- RLZ 0.5 mg/kg + KA;
- (3)
- RLZ 1 mg/kg + KA;
- (4)
- RLZ 5 mg/kg + KA;
- (5)
- RLZ 7.5 mg/kg + KA.
- (1)
- vehicle + AMPA or NMDA;
- (2)
- RLZ 0.5 mg/kg + AMPA or NMDA;
- (3)
- RLZ 1 mg/kg + AMPA or NMDA;
- (4)
- RLZ 5 mg/kg + AMPA or NMDA;
- (5)
- RLZ 7.5 mg/kg + AMPA or NMDA.
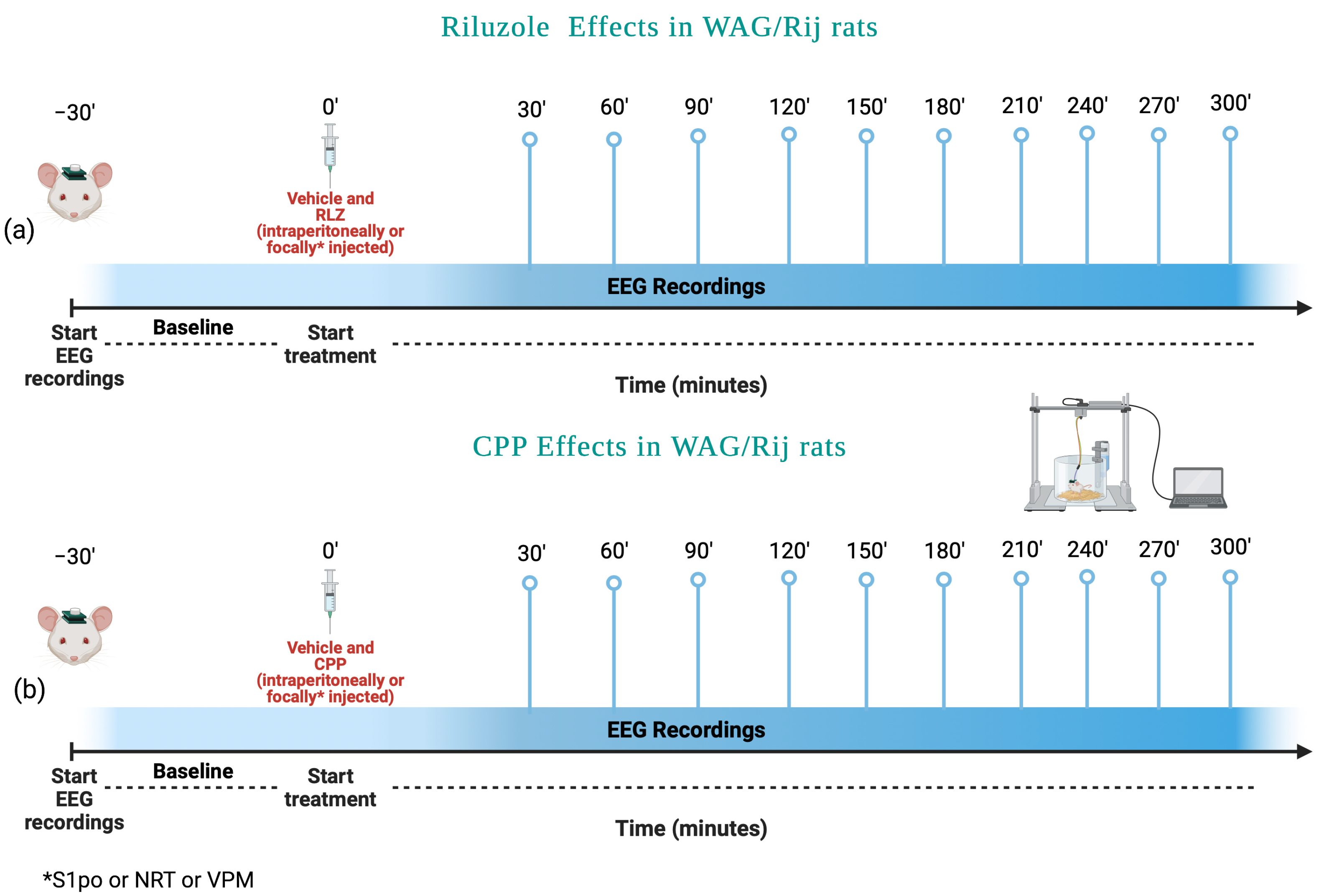
2.4. Experimental Protocol in WAG/Rij Rats
- (1)
- Vehicle;
- (2)
- RLZ 0.5 mg/kg;
- (3)
- RLZ 1 mg/kg;
- (4)
- RLZ 5 mg/kg;
- (5)
- RLZ 7.5 mg/kg.
- (1)
- Vehicle;
- (2)
- RLZ 0.25 μg/0.5 μL;
- (3)
- RLZ 0.5 μg/0.5 μL;
- (4)
- RLZ 1 μg/0.5 μL.
- (1)
- Vehicle;
- (2)
- CPP 5 mg/kg;
- (3)
- CPP 10 mg/kg;
- (4)
- CPP 15 mg/kg.
- (1)
- Vehicle;
- (2)
- CPP 5 pmol/μL;
- (3)
- CPP 10 pmol/μL;
- (4)
- CPP 20 pmol/μL.
Animal Surgery and EEG Recording
2.5. Statistical Analysis
3. Results
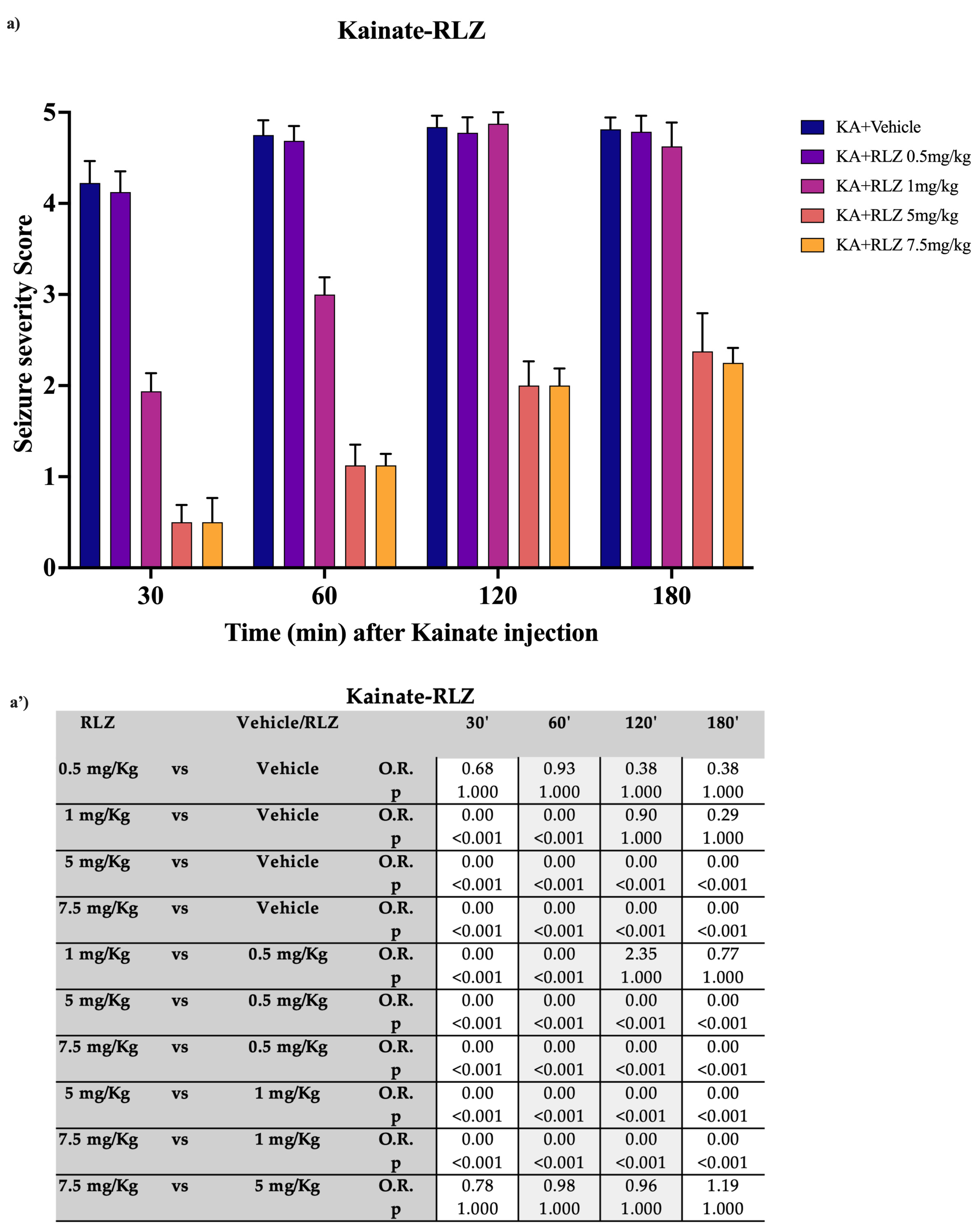
3.1. Effects of RLZ on KA-Induced Seizure in Sprague–Dawley Rats
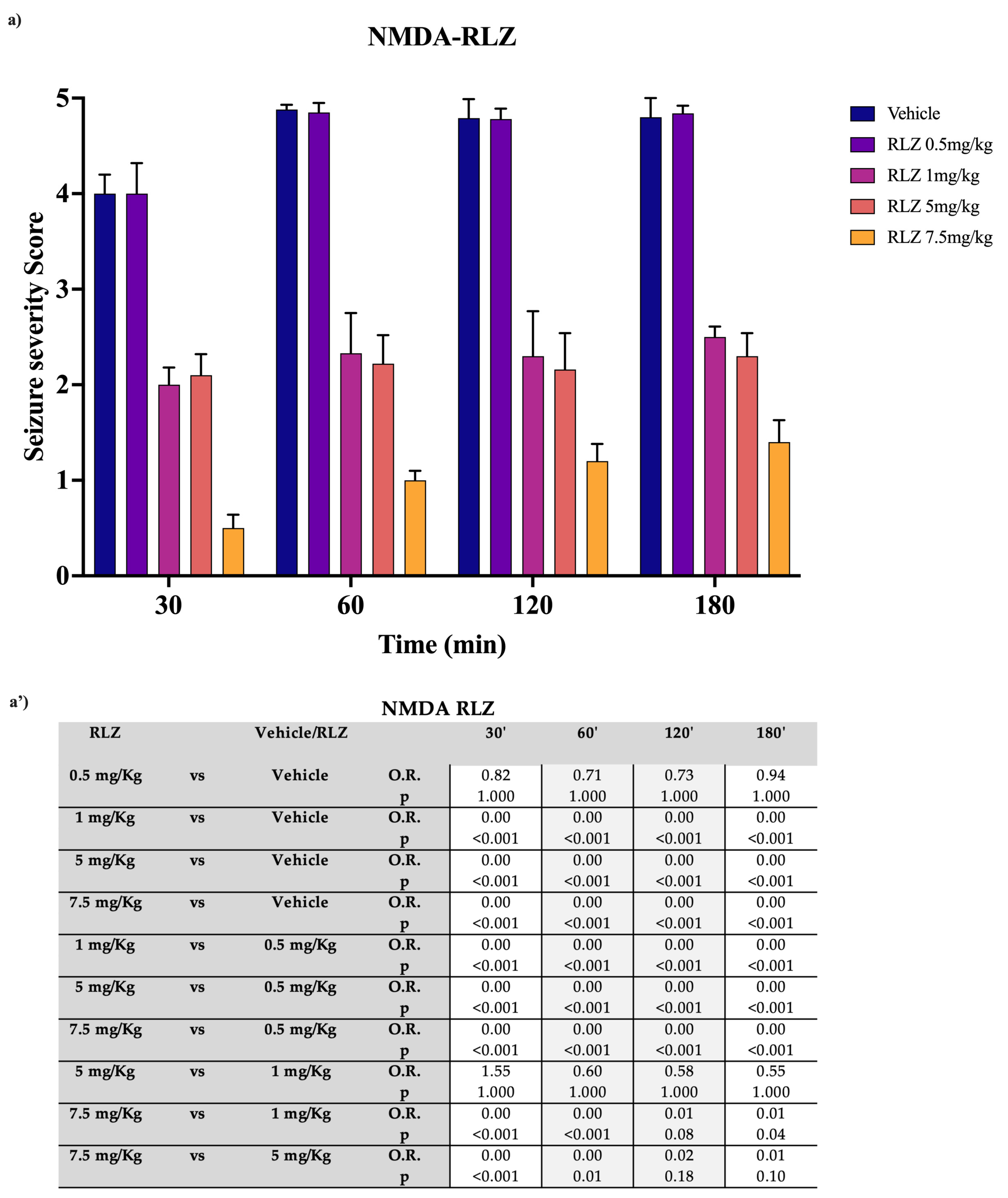
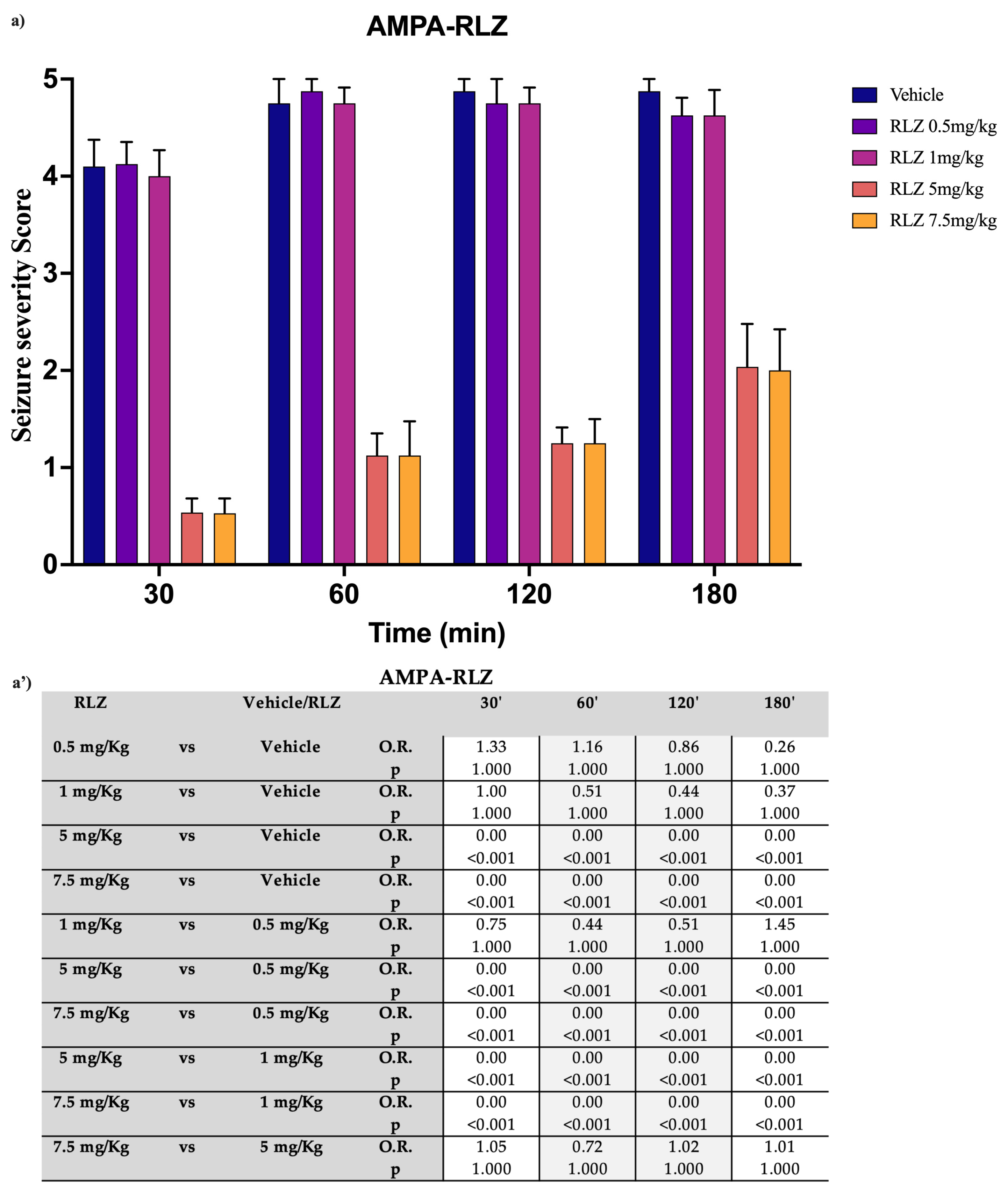
3.2. Effects of RLZ on Seizure NMDA or AMPA-Induced in Sprague–Dawley Rats
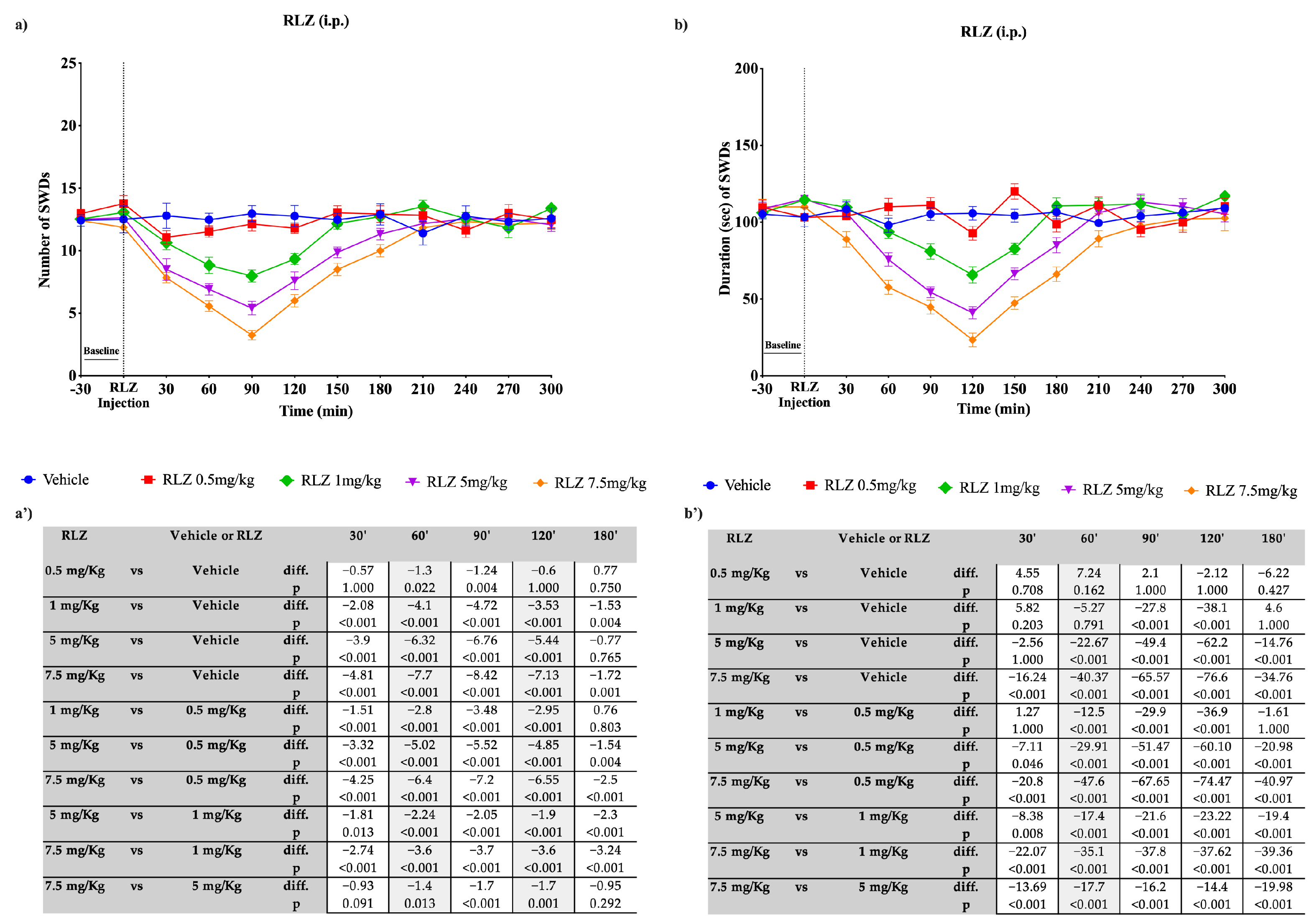
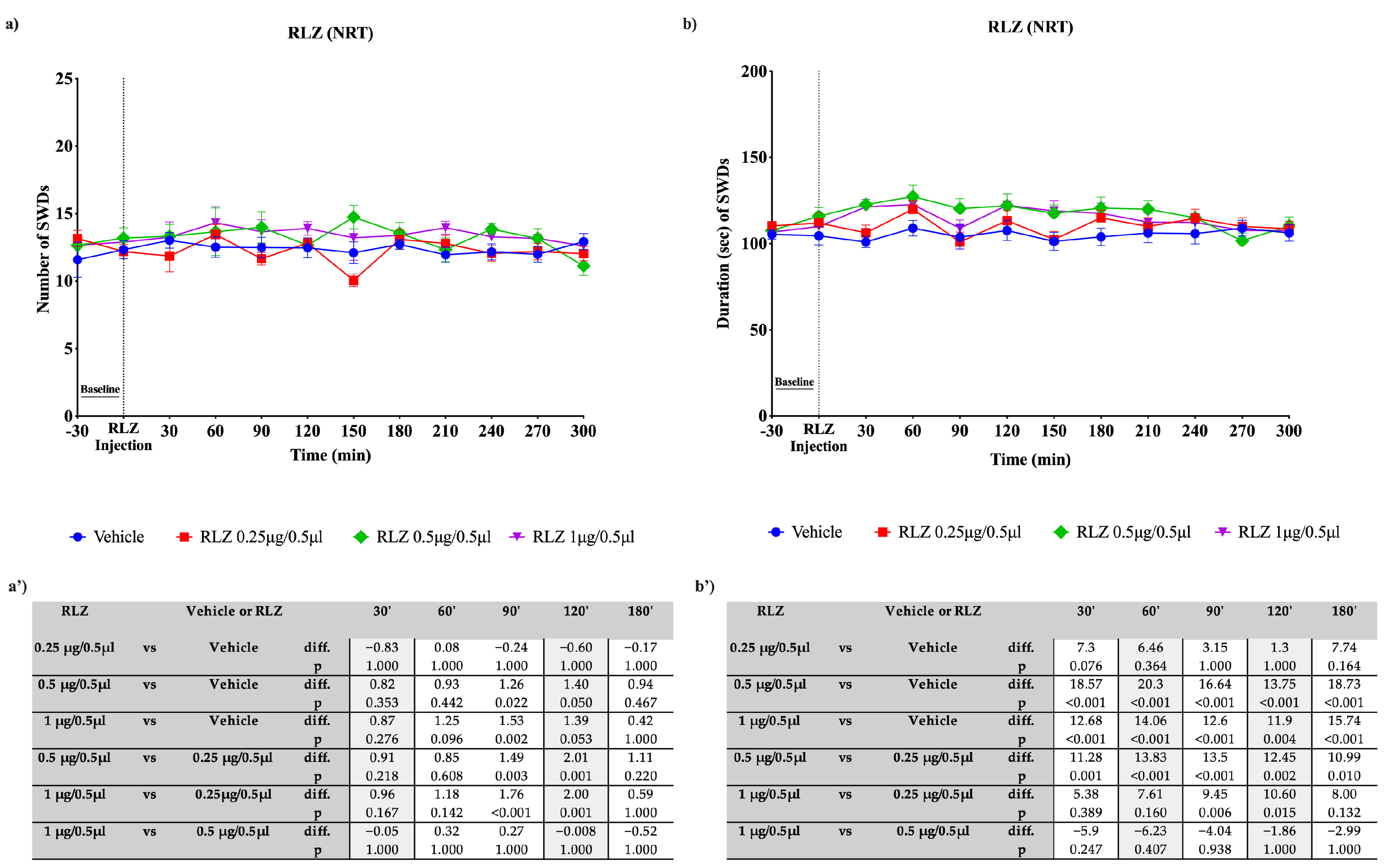
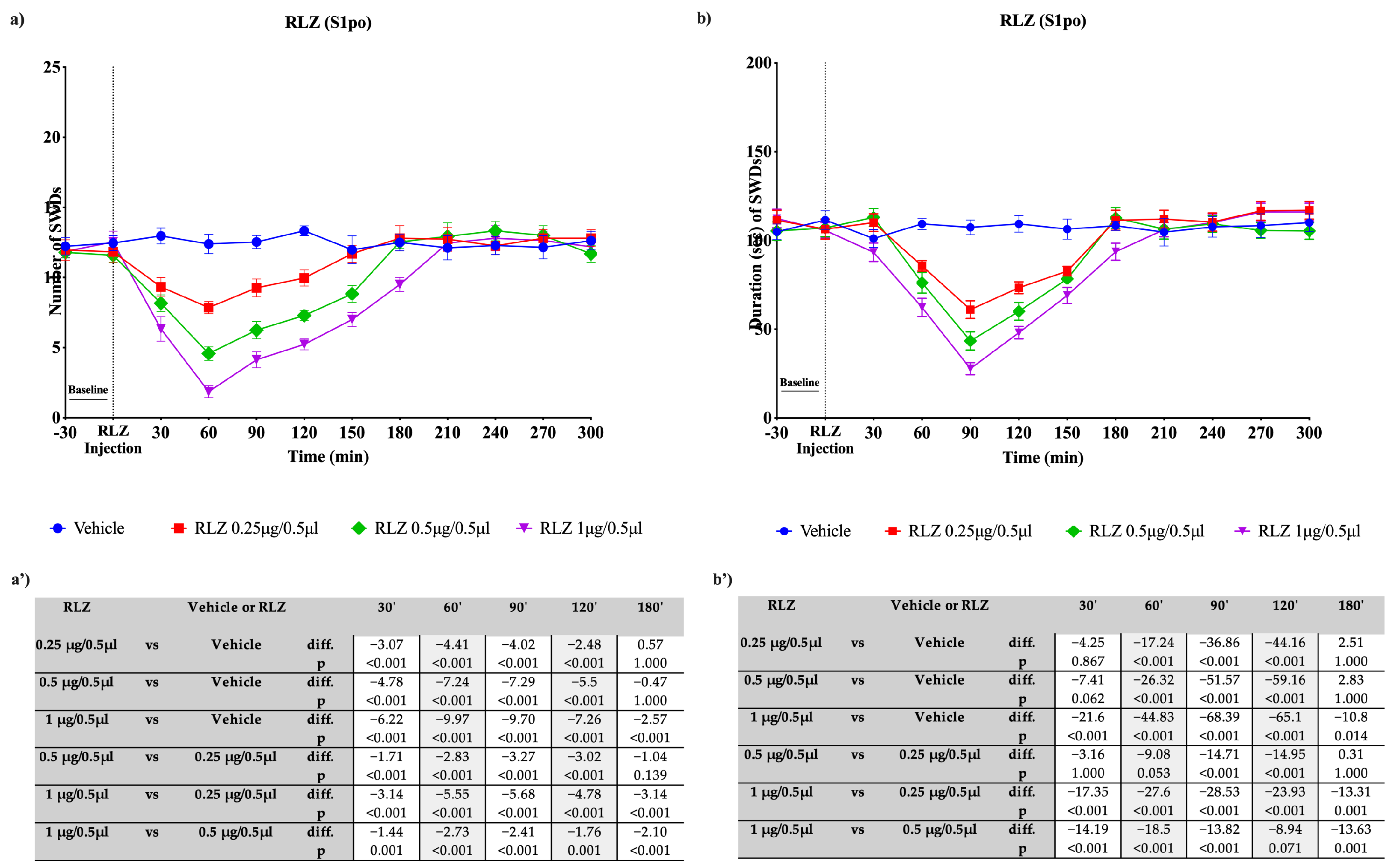
3.3. Effects of RLZ, i.p. and Focally Administered on Absence Seizures in WAG/Rij Rats
3.4. Effects of CPP on Absence Seizures in WAG/Rij Rats
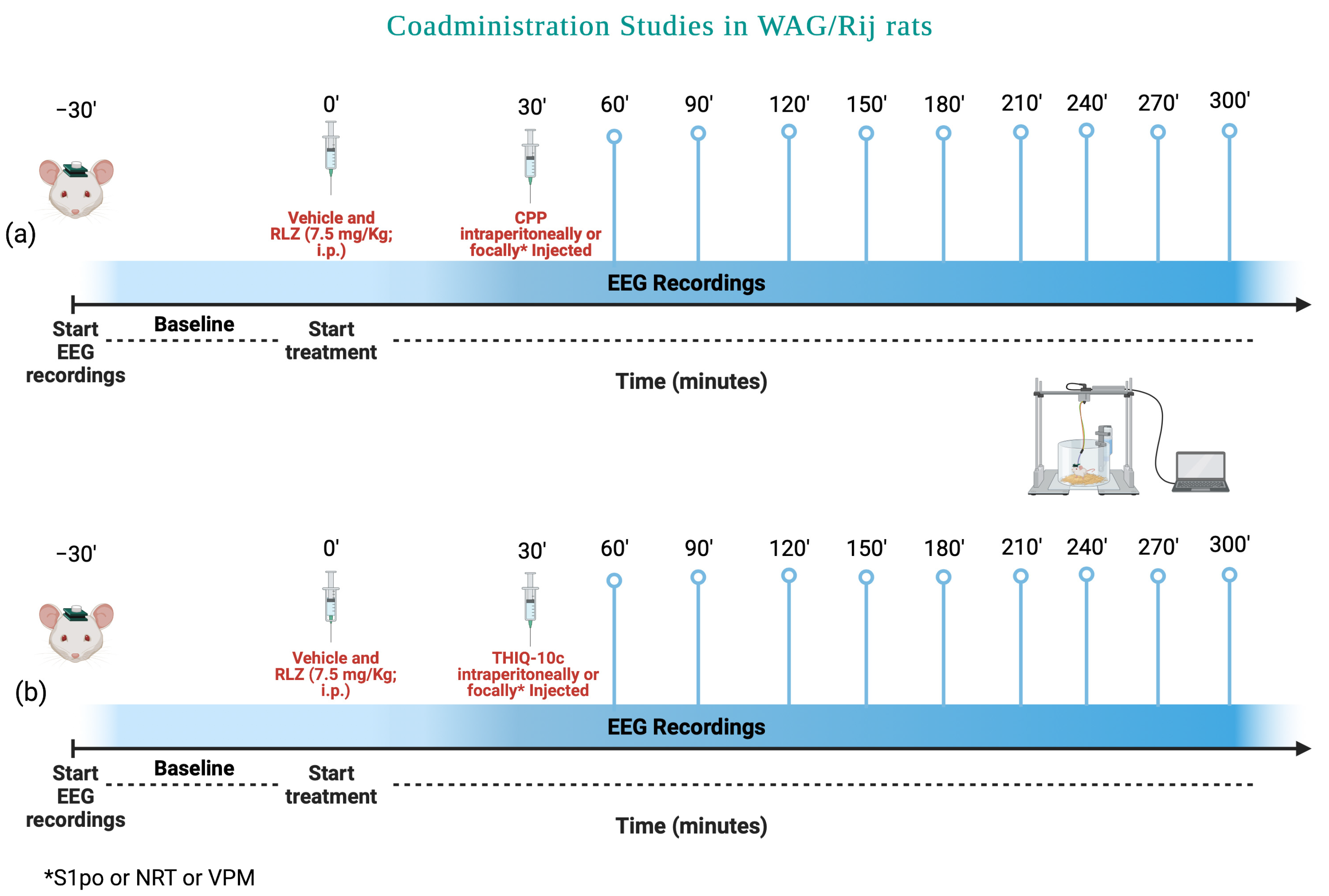
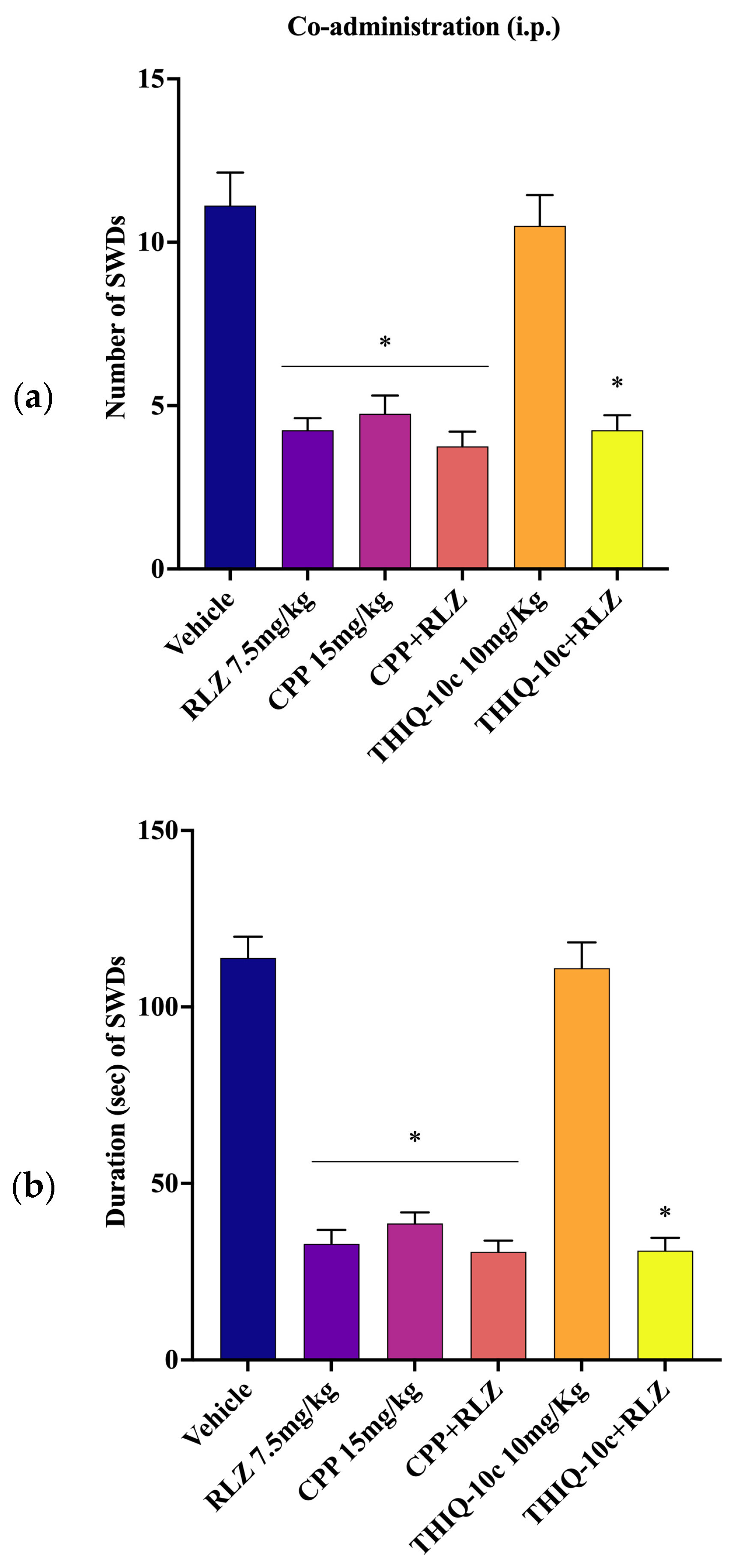
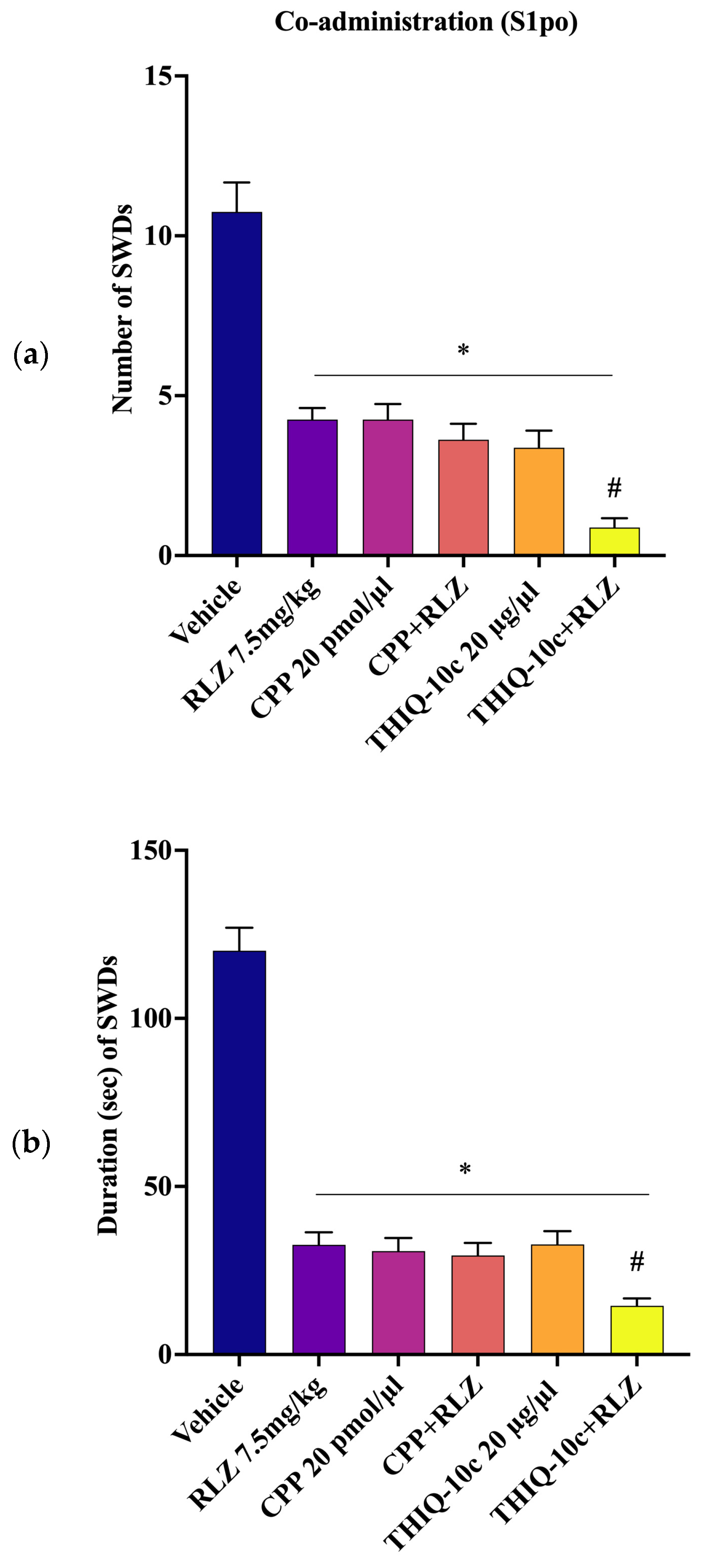
3.5. Effects of Combined Administration of RLZ with Glutamate Receptor Antagonists in WAG/Rij Rats
4. Discussion
4.1. Effects of RLZ against Limbic Seizure in Sprague–Dawley Rats
4.2. Effects of RLZ against Absence Seizure in WAG/Rij Rats
4.3. Effects of Co-Administration of RLZ with NMDA/AMPA Antagonist Receptors in WAG/Rij Rats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, J.A.; Jackson, C.E.; Heiman-Patterson, T.D.; Bettica, P.; Brooks, B.R.; Pioro, E.P. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Nikbakht, A.; Kargar-soleimanabad, S.; Siahposht-Khachaki, A.; Farzin, D. The effect of Riluzole on neurological outcomes, blood-brain barrier, brain water and neuroinflammation in traumatic brain injury. Brain Disord. 2022, 8, 100052. [Google Scholar] [CrossRef]
- Matthews, D.C.; Mao, X.; Dowd, K.; Tsakanikas, D.; Jiang, C.S.; Meuser, C.; Andrews, R.D.; Lukic, A.S.; Lee, J.; Hampilos, N.; et al. Riluzole, a glutamate modulator, slows cerebral glucose metabolism decline in patients with Alzheimer’s disease. Brain 2021, 144, 3742–3755. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N. The efficacy and safety of riluzole for neurodegenerative movement disorders: A systematic review with meta-analysis. Drug Deliv. 2018, 25, 43–48. [Google Scholar] [CrossRef]
- Lazarevic, V.; Yang, Y.; Ivanova, D.; Fejtova, A.; Svenningsson, P. Riluzole attenuates the efficacy of glutamatergic transmission by interfering with the size of the readily releasable neurotransmitter pool. Neuropharmacology 2018, 143, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, M.C. A Review of the Neural Mechanisms of Action and Clinical Efficiency of Riluzole in Treating Amyotrophic Lateral Sclerosis: What have we Learned in the Last Decade? CNS Neurosci. Ther. 2011, 17, 4–31. [Google Scholar] [CrossRef]
- Kyllo, T.; Singh, V.; Shim, H.; Latika, S.; Nguyen, H.M.; Chen, Y.J.; Terry, E.; Wulff, H.; Erickson, J.D. Riluzole and novel naphthalenyl substituted aminothiazole derivatives prevent acute neural excitotoxic injury in a rat model of temporal lobe epilepsy. Neuropharmacology 2023, 224, 109349. [Google Scholar] [CrossRef]
- He, Y.; Benz, A.; Fu, T.; Wang, M.; Covey, D.F.; Zorumski, C.F.; Mennerick, S. Neuroprotective agent riluzole potentiates postsynaptic GABAA receptor function. Neuropharmacology 2002, 42, 199–209. [Google Scholar] [CrossRef]
- Jahn, K.; Schlesinger, F.; Jin, L.J.; Dengler, R.; Bufler, J.; Krampfl, K. Molecular mechanisms of interaction between the neuroprotective substance riluzole and GABAA-receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2008, 378, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; O’Brien, T.J.; Jette, N.; Scheffer, I.E.; De Curtis, M.; Perucca, P. Epilepsy; Monash University: Clayton, Australia, 2018; Volume 4, p. 18024. [Google Scholar]
- Hanada, T. Ionotropic glutamate receptors in epilepsy: A review focusing on ampa and nmda receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef]
- Leo, A.; Giovannini, G.; Russo, E.; Meletti, S. The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia 2018, 59, 1098–1108. [Google Scholar] [CrossRef]
- Rusina, E.; Bernard, C.; Williamson, A. The kainic acid models of temporal lobe epilepsy. eNeuro 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, A.; Bonci, A.; Mercuri, N.B.; Bernardi, G. Effects of riluzole on rat cortical neurones: An in vitro electrophysiological study. Br. J. Pharmacol. 1997, 120, 225–230. [Google Scholar] [CrossRef]
- Spadoni, F.; Hainsworth, A.H.; Mercuri, N.B.; Caputi, L.; Martella, G.; Lavaroni, F.; Bernardi, G.; Stefani, A. Lamotrigine derivatives and riluzole inhibit INa,P in cortical neurons. Neuroreport 2002, 13, 1167–1170. [Google Scholar] [CrossRef]
- Diao, L.; Hellier, J.L.; Uskert-Newsom, J.; Williams, P.A.; Staley, K.J.; Yee, A.S. Diphenytoin, riluzole and lidocaine: Three sodium channel blockers, with different mechanisms of action, decrease hippocampal epileptiform activity. Neuropharmacology 2013, 73, 48–55. [Google Scholar] [CrossRef]
- Zona, C.; Cavalcanti, S.; De Sarro, G.; Siniscalchi, A.; Marchetti, C.; Gaetti, C.; Costa, N.; Mercuri, N.; Bernardi, G. Kainate-induced currents in rat cortical neurons in culture are modulated by riluzole. Synapse 2002, 43, 244–251. [Google Scholar] [CrossRef]
- De Sarro, G.; Siniscalchi, A.; Ferreri, G.; Gallelli, L.; De Sarro, A. NMDA and AMPA/kainate receptors are involved in the anticonvulsant activity of riluzole in DBA/2 mice. Eur. J. Pharmacol. 2000, 408, 25–34. [Google Scholar] [CrossRef]
- Zgrajka, W.; Nieoczym, D.; Czuczwar, M.; Kiś, J.; Brzana, W.; Wlaź, P.; Turski, W.A. Evidences for pharmacokinetic interaction of riluzole and topiramate with pilocarpine in pilocarpine-induced seizures in rats. Epilepsy Res. 2010, 88, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, D.S.; Kwak, S.E.; Choi, H.C.; Song, H.K.; Choi, S.Y.; Kwon, O.S.; Kim, Y.I.; Kang, T.C. Anti-glutamatergic effect of riluzole: Comparison with valproic acid. Neuroscience 2007, 147, 136–145. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Sekowski, A.; Drelewska, E.; Czuczwar, S.J. Riluzole enhances the antiseizure action of conventional antiepileptic drugs against pentetrazole-induced convulsions in mice. Pol. J. Pharmacol. 2004, 56, 187–193. [Google Scholar] [PubMed]
- Yoshida, M.; Noguchi, E.; Tsuru, N.; Ohkoshi, N. Effect of riluzole on the acquisition and expression of amygdala kindling. Epilepsy Res. 2001, 46, 101–109. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Constanti, A.; Leo, A.; Lüttjohann, A.; van Luijtelaar, G.; De Sarro, G. Upholding WAG/Rij rats as a model of absence epileptogenesis: Hidden mechanisms and a new theory on seizure development. Neurosci. Biobehav. Rev. 2016, 71, 388–408. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Tallarico, M.; Iannone, M.; Fedosova, E.; Nesci, V.; De Sarro, G.; Sarkisova, K.; Russo, E. Cognitive impairment in the WAG/Rij rat absence model is secondary to absence seizures and depressive-like behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109652. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Dong, X.; Fan, J.; Lin, D.; Wang, X.; Kuang, H.; Gong, L.; Chen, C.; Jiang, J.; Xia, N.; He, D.; et al. Captopril alleviates epilepsy and cognitive impairment by attenuation of C3-mediated inflammation and synaptic phagocytosis. J. Neuroinflamm. 2022, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Nesci, V.; Tallarico, M.; Amodio, N.; Gallo Cantafio, E.M.; De Sarro, G.; Constanti, A.; Russo, E.; Citraro, R. IL-6 Receptor Blockade by Tocilizumab Has Anti-absence and Anti-epileptogenic Effects in the WAG/Rij Rat Model of Absence Epilepsy. Neurotherapeutics 2020, 17, 2004–2014. [Google Scholar] [CrossRef]
- De Sarro, G.; Ibbadu, G.F.; Marra, R.; Rotiroti, D.; Loiacono, A.; Donato Di Paola, E.; Russo, E. Seizure susceptibility to various convulsant stimuli in dystrophin-deficient mdx mice. Neurosci. Res. 2004, 50, 37–44. [Google Scholar] [CrossRef]
- Citraro, R.; Russo, E.; Gratteri, S.; Di Paola, E.D.; Ibbadu, G.F.; Curinga, C.; Gitto, R.; Chimirri, A.; Donato, G.; De Sarro, G. Effects of non-competitive AMPA receptor antagonists injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology 2006, 51, 1058–1067. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; De Fazio, S.; Marra, R.; Gitto, R.; Chimirri, A.; De Sarro, G.; Paola, E.D. Di Enhancement of anti-absence effects of ethosuximide by low doses of a noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist in a genetic animal model of absence epilepsy. Epilepsy Behav. 2008, 13, 295–299. [Google Scholar] [CrossRef]
- Tallarico, M.; Leo, A.; Guarnieri, L.; Zito, M.C.; De Caro, C.; Nicoletti, F.; Russo, E.; Constanti, A.; De Sarro, G.; Citraro, R. N-acetylcysteine aggravates seizures while improving depressive-like and cognitive impairment comorbidities in the WAG/Rij rat model of absence epilepsy. Mol. Neurobiol. 2022, 59, 2702–2714. [Google Scholar] [CrossRef] [PubMed]
- Palkovits, M. The rat brain in stereotaxic coordinates. Neuropeptides 1983, 3, 319. [Google Scholar] [CrossRef]
- Leo, A.; Citraro, R.; Amodio, N.; De Sarro, C.; Gallo Cantafio, M.E.; Constanti, A.; De Sarro, G.; Russo, E. Fingolimod Exerts only Temporary Antiepileptogenic Effects but Longer-Lasting Positive Effects on Behavior in the WAG/Rij Rat Absence Epilepsy Model. Neurotherapeutics 2017, 14, 1134–1147. [Google Scholar] [CrossRef] [PubMed]
- Van Luijtelaar, E.L.J.M.; Coenen, A.M.L. Two types of electrocortical paroxysms in an inbred strain of rats. Neurosci. Lett. 1986, 70, 393–397. [Google Scholar] [CrossRef]
- Andersen, P.K. Regression Modeling Strategies with: Applications to Linear Models, Logistic Regression and Survival Analysis; Frank, E., Harrell, J., Eds.; Springer: New York, NY, USA, 2001; p. 568. ISBN 0-387-95232-2. Stat. Med. 2003, 22, 2531–2532. [Google Scholar] [CrossRef]
- Chiu, K.M.; Wu, C.C.; Wang, M.J.; Lee, M.Y.; Wang, S.J. Protective effects of bupivacaine against kainic acid-induced seizure and neuronal cell death in the rat hippocampus. Biol. Pharm. Bull. 2015, 38, 522–530. [Google Scholar] [CrossRef]
- Russo, E.; Constanti, A.; Ferreri, G.; Citraro, R.; De Sarro, G. Nifedipine affects the anticonvulsant activity of topiramate in various animal models of epilepsy. Neuropharmacology 2004, 46, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Romettino, S.; Lazdunski, M.; Gottesmann, C. Anticonvulsant and sleep-waking influences of riluzole in a rat model of absence epilepsy. Eur. J. Pharmacol. 1991, 199, 371–373. [Google Scholar] [CrossRef]
- De Sarro, G.B.; De Sarro, A. Anticonvulsant properties of non-competitive antagonists of the N-methyl-D-aspartate receptor in genetically epilepsy-prone rats: Comparison with CPPene. Neuropharmacology 1993, 32, 51–58. [Google Scholar] [CrossRef]
- De Sarro, G.; De Sarro, A. Anticonvulsant activity of competitive antagonists of NMDA receptor in genetically epilepsy-prone rats. Eur. J. Pharmacol. 1992, 215, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; dos Santos, W.F.; Fontana, A.C.K. Role of glutamate excitotoxicity and glutamate transporter EAAT2 in epilepsy: Opportunities for novel therapeutics development. Biochem. Pharmacol. 2021, 193, 114786. [Google Scholar] [CrossRef]
- Andersen, J.V.; Schousboe, A. Glial Glutamine Homeostasis in Health and Disease. Neurochem. Res. 2022, 48, 1100–1128. [Google Scholar] [CrossRef]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef]
- Bissaro, M.; Moro, S. Rethinking to riluzole mechanism of action: The molecular link among protein kinase CK1δ activity, TDP-43 phosphorylation, and amyotrophic lateral sclerosis pharmacological treatment. Neural Regen. Res. 2019, 14, 2083–2085. [Google Scholar]
- Debono, M.W.; Le Guern, J.; Canton, T.; Doble, A.; Pradier, L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 1993, 235, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Doble, A. The pharmacology and mechanism of action of riluzole. Neurology 1996, 47, 233S–241S. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, B.D.; Kratzer, U.; Schmidt, W.J. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998, 358, 181–190. [Google Scholar] [CrossRef]
- Lamanauskas, N.; Nistri, A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur. J. Neurosci. 2008, 27, 2501–2514. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.J.; Schlesinger, F.; Song, Y.P.; Dengler, R.; Krampfl, K. The interaction of the neuroprotective compounds riluzole and phenobarbital with AMPA-type glutamate receptors: A patch-clamp study. Pharmacology 2010, 85, 54–62. [Google Scholar] [CrossRef]
- Centonze, D.; Calabresi, P.; Pisani, A.; Marinelli, S.; Marfia, G.A.; Bernardi, G. Electrophysiology of the neuroprotective agent riluzole on striatal spiny neurons. Neuropharmacology 1998, 37, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Chapman, A.G. Acute and chronic anticonvulsant effects of d(-)CPPene in genetically epilepsy-prone rats. Epilepsy Res. 1993, 15, 193–199. [Google Scholar] [CrossRef]
- Faingold, C.L.; Randall, M.E.; Naritoku, D.K.; Boersma Anderson, C.A. Noncompetitive and competitive NMDA antagonists exert anticonvulsant effects by actions on different sites within the neuronal network for audiogenic seizures. Exp. Neurol. 1993, 119, 198–204. [Google Scholar] [CrossRef]
- De Sarro, C.; Tallarico, M.; Pisano, M.; Gallelli, L.; Citraro, R.; De Sarro, G.; Leo, A. Liraglutide chronic treatment prevents development of tolerance to antiseizure effects of diazepam in genetically epilepsy prone rats. Eur. J. Pharmacol. 2022, 928, 175098. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Wang, K.Y.; Wang, W.C. Mechanisms underlying the riluzole inhibition of glutamate release from rat cerebral cortex nerve terminals (synaptosomes). Neuroscience 2004, 125, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Thompson, M.A.; Nadler, J.V. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur. J. Pharmacol. 1993, 250, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Dorst, J.; Ludolph, A.C.; Huebers, A. Disease-modifying and symptomatic treatment of amyotrophic lateral sclerosis. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617734734. [Google Scholar] [CrossRef]
- Peeters, B.W.M.M.; van Rijn, C.M.; Vossen, J.M.H.; Coenen, A.M.L. Involvement of NMDA receptors in non-convulsive epilepsy in WAG/Rij rats. Life Sci. 1990, 47, 523–529. [Google Scholar] [CrossRef]
- Peeters, B.W.M.M.; Ramakers, G.M.J.; Vossen, J.M.H.; Coenen, A.M.L. The WAG/Rij Rat Model for Nonconvulsive Absence Epilepsy: Involvement of NonNMDA Receptors. Brain Res. Bull. 1994, 33, 709–713. [Google Scholar] [CrossRef]
- Kaminski, R.M.; Van Rijn, C.M.; Turski, W.A.; Czuczwar, S.J.; Van Luijtelaar, G. AMPA and GABA(B) receptor antagonists and their interaction in rats with a genetic form of absence epilepsy. Eur. J. Pharmacol. 2001, 430, 251–259. [Google Scholar] [CrossRef]
- Jakus, R.; Graf, M.; Ando, R.D.; Balogh, B.; Gacsalyi, I.; Levay, G.; Kantor, S.; Bagdy, G. Effect of two noncompetitive AMPA receptor antagonists GYKI 52466 and GYKI 53405 on vigilance, behavior and spike-wave discharges in a genetic rat model of absence epilepsy. Brain Res. 2004, 1008, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Citraro, R.; Leo, A.; Franco, V.; Marchiselli, R.; Perucca, E.; De Sarro, G.; Russo, E. Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive-like behavior. Epilepsia 2017, 58, 231–238. [Google Scholar] [CrossRef]
- Operto, F.F.; Orsini, A.; Sica, G.; Scuoppo, C.; Padovano, C.; Vivenzio, V.; de Simone, V.; Rinaldi, R.; Belfiore, G.; Mazza, R.; et al. Perampanel and childhood absence epilepsy: A real life experience. Front. Neurol. 2022, 13, 952900. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.L.; Van Luijtelaar, E.L.J.M. Genetic Animal Models for Absence Epilepsy: A Review of the WAG/Rij Strain of Rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Peeters, B.W.; van Rijn, C.M.; Van Luijtelaar, E.L.; Coenen, A.M. Antiepileptic and behavioural actions of MK-801 in an animal model of spontaneous absence epilepsy. Epilepsy Res. 1989, 3, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.W.M.M.; Ramakers, G.M.J.; Ellenbroek, B.A.; Vossen, J.M.H.; Coenen, A.M.L. Interactions between NMDA and NonNMDA Receptors in Nonconvulsive Epilepsy in the WAG/Rij Inbred Strain. Brain Res. Bull. 1994, 33, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Meeren, H.; Van Luijtelaar, G.; Lopes Da Silva, F.; Coenen, A. Evolving concepts on the pathophysiology of absence seizures: The cortical focus theory. Arch. Neurol. 2005, 62, 371–376. [Google Scholar] [CrossRef]
- Touret, M.; Parrot, S.; Denoroy, L.; Belin, M.F.; Didier-Bazes, M. Glutamatergic alterations in the cortex of genetic absence epilepsy rats. BMC Neurosci. 2007, 8, 69. [Google Scholar] [CrossRef]
- Karimzadeh, F.; Soleimani, M.; Mehdizadeh, M.; Jafarian, M.; Mohamadpour, M.; Kazemi, H.; Joghataei, M.T.; Gorji, A. Diminution of the NMDA receptor NR2B subunit in cortical and subcortical areas of WAG/Rij rats. Synapse 2013, 67, 839–846. [Google Scholar] [CrossRef]
- Van de Bovenkamp-Janssen, M.C.; van der Kloet, J.C.; van Luijtelaar, G.; Roubos, E.W. NMDA-NR1 and AMPA-GluR4 receptor subunit immunoreactivities in the absence epileptic WAG/Rij rat. Epilepsy Res. 2006, 69, 119–128. [Google Scholar] [CrossRef]
- Zavvari, F.; Mousavi, S.M.M.; Ejlali, M.; Barfi, S.; Karimzadeh, F. Glutamate signaling pathway in absence epilepsy: Possible role of ionotropic ampa glutamate receptor type 1 subunit. Iran. J. Pharm. Res. 2020, 19, 410–418. [Google Scholar]
- D’Antuono, M.; Inaba, Y.; Biagini, G.; D’Arcangelo, G.; Tancredi, V.; Avoli, M. Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures. Genes Brain Behav. 2006, 5, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011, 11, 56–63. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citraro, R.; Bosco, F.; Di Gennaro, G.; Tallarico, M.; Guarnieri, L.; Gallelli, L.; Rania, V.; Siniscalchi, A.; De Sarro, G.; Leo, A. An In Vivo Electroencephalographic Analysis of the Effect of Riluzole against Limbic and Absence Seizure and Comparison with Glutamate Antagonists. Pharmaceutics 2023, 15, 2006. https://doi.org/10.3390/pharmaceutics15072006
Citraro R, Bosco F, Di Gennaro G, Tallarico M, Guarnieri L, Gallelli L, Rania V, Siniscalchi A, De Sarro G, Leo A. An In Vivo Electroencephalographic Analysis of the Effect of Riluzole against Limbic and Absence Seizure and Comparison with Glutamate Antagonists. Pharmaceutics. 2023; 15(7):2006. https://doi.org/10.3390/pharmaceutics15072006
Chicago/Turabian StyleCitraro, Rita, Francesca Bosco, Gianfranco Di Gennaro, Martina Tallarico, Lorenza Guarnieri, Luca Gallelli, Vincenzo Rania, Antonio Siniscalchi, Giovambattista De Sarro, and Antonio Leo. 2023. "An In Vivo Electroencephalographic Analysis of the Effect of Riluzole against Limbic and Absence Seizure and Comparison with Glutamate Antagonists" Pharmaceutics 15, no. 7: 2006. https://doi.org/10.3390/pharmaceutics15072006
APA StyleCitraro, R., Bosco, F., Di Gennaro, G., Tallarico, M., Guarnieri, L., Gallelli, L., Rania, V., Siniscalchi, A., De Sarro, G., & Leo, A. (2023). An In Vivo Electroencephalographic Analysis of the Effect of Riluzole against Limbic and Absence Seizure and Comparison with Glutamate Antagonists. Pharmaceutics, 15(7), 2006. https://doi.org/10.3390/pharmaceutics15072006






